Abstract
Arylalkylamine N-acetyltransferase (AANAT) plays a crucial role in synchronizing internal biological functions to circadian and circannual changes. Generally speaking, only one copy of AANAT gene has been found in mammals, however, three independent duplications of this gene were detected in several cetartiodactyl lineages (i.e., Suidae, Hippopotamidae, and Pecora), which originated in the middle Eocene, a geological period characterized with the increased climate seasonality. Lineage-specific expansions of AANAT and the associated functional enhancement in these lineages strongly suggest an improvement in regulating photoperiodic response to adapt to seasonal climate changes. In contrast, independent inactivating mutations or deletions of the AANAT locus were identified in the four pineal-deficient clades (cetaceans, sirenians, xenarthrans, and pangolins). Loss of AANAT function in cetaceans and sirenians could disrupt the sleep-promoting effects of pineal melatonin, which might contribute to increasing wakefulness, adapting these clades to underwater sleep. The absence of AANAT and pineal glands in xenarthrans and pangolins may be associated with their body temperature maintenance. The present work demonstrates a far more complex and intriguing evolutionary pattern and functional diversity of mammalian AANAT genes than previously thought and provides further evidence for understanding AANAT evolution as driven by rhythmic adaptations in mammals.
Keywords: AANAT, gene duplication, gene loss, rhythmic adaptions, cetartiodactyls
Introduction
The circadian and circannual rhythms of organisms reflect significant adaptations for coping with the daily and annual fluctuations of external environments (Yerushalmi and Green 2009; Gwinner 2012), and daylength (photoperiod) is the most powerful environmental cue (Bradshaw and Holzapfel 2007). The circadian system is responsible for measuring and transducing of photoperiod alterations, whereas the circannual mechanism fulfils the important function of the timely prediction of the changes in the food supply, weather conditions, and predator activity, etc. By helping anticipate environmental changes, endogenous rhythms allow for synchronizing major biological functions, including sleep, migration, and reproduction to environmental cycles, thus enabling organisms to maximize survival and use of resources (Hut and Beersma 2011).
The pineal organ and its hormone melatonin play a pivotal role in the transduction of photoperiod information to synchronize daily and seasonal rhythms (Falcón et al. 2009). As a hormone of darkness, melatonin is characterized by having circulating levels that are much higher at night than in the day (Utiger 1992; Hut and Beersma 2011). Moreover, melatonin acts as a critical input signal of timing system, involving in the circadian organization of physiological status as well as in photoperiodic measurement (Kumar 2017). Furthermore, the duration and amplitude of nocturnal melatonin levels vary with seasonal photoperiodic variations. The changing duration of melatonin provides a dominant time cue for seasonal behavioral cycles (Bartness and Goldman 1989; Lincoln 2006). Additionally, the rhythmicity of pineal melatonin is a hallmarker of internal time in mammals, but some taxa such as cetaceans, sirenians, pangolins, and xenarthrans (i.e., anteaters, sloths, and armadillos) seem to lack pineal glands (Ralph 1975; Kappers 1983; Panin et al. 2012).
The regulation of melatonin rhythm primarily depends on the expression level and enzyme activity of arylalkylamine N-acetyltransferase (AANAT), the rate-limiting enzyme in melatonin biosynthesis (Klein 2007; Pevet and Challet 2011). The dramatic increase in the expression and activity of AANAT at nighttime leads to a significant elevation in melatonin levels, whereas weak activity during the daytime severely limits the amount of substrates available for conversion to melatonin (Klein et al. 1996; Ganguly et al. 2002). Animals exposed to light at night experience a rapid decline in AANAT activity, followed by a large decline in melatonin levels (Klein and Weller 1970). Therefore, AANAT has become the master regulator for adjusting the endogenous melatonin levels in response to external photoperiodic changes and thus plays a crucial part in regulating rhythmic processes (Klein 2007; Kumar 2017). It was found that a knockout of the AANAT gene in zebrafish caused a lack of melatonin and the abolishment of circadian sleep regulation (Gandhi et al. 2015).
Preliminary investigations have shown that kinetic differences and the different temperature–activity relationships of multiple AANATs in teleosts were likely to represent evolutionary responses to different environmental pressures (Zilberman-Peled et al. 2004, 2011). The evolution of avian AANAT regions was potentially driven by the selection for nocturnality (Fidler et al. 2004). As for mammals, it has been well established that only one copy of an AANAT gene has been conserved within mammalian genomes (Saha et al. 2019). However, recent analyses identified double AANAT-like sequences in three artiodactyl species, including the hippopotamus (Hippopotamus amphibius), cattle (Bos taurus), and goat (Capra hircus) (Kim et al. 2016; Lopes-Marques et al. 2019). By contrast, manatees and several cetaceans have been reported to have lost the AANAT gene (Huelsmann et al. 2019; Lopes-Marques et al. 2019). Thus, the evolution of the AANAT locus across mammals remains an alluring area of inquiry. Similarly, our understanding of the association between AANAT evolution and rhythmic adaptations remains limited.
In the present study, a comprehensive survey of the AANAT locus in 256 mammalian lineages was conducted to uncover the evolutionary characteristics of the AANAT gene across mammalian phylogeny. Interestingly, we observed the duplications of AANAT in certain artiodactyl clades and inactivations of AANAT in pineal-deficient clades, which was in contrast to only one functional AANAT gene in other mammals. Gene duplication and loss are widely considered as important genetic sources for functional innovations and evolutionary adaptations (Zhang et al. 2002; Sharma, Hecker, et al. 2018). Therefore, we further explored what evolutionary forces have shaped the diversification of mammalian AANAT genes. Phylogenetic trees and the ancestral state of all AANAT sequences were first reconstructed to elucidate gene duplication events within Cetartiodactyla, and we then evaluated whether selection pressures relaxed in lineages with inactivated AANAT genes. Finally, AANAT activity assays for represented species were performed to explore the potential functional modifications of AANAT genes in mammals with different evolutionary patterns.
Results
Evolutionary Characteristics of AANAT Genes in Mammals
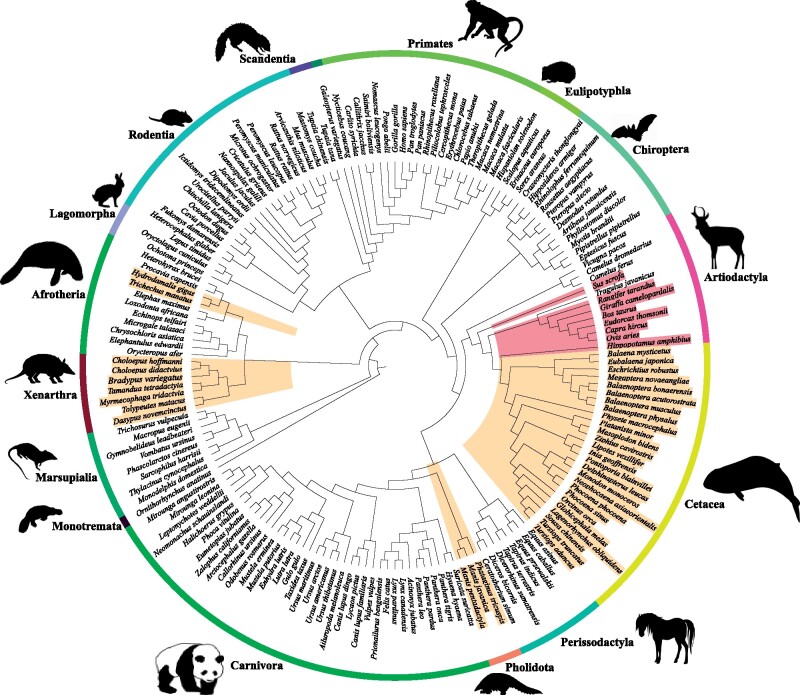
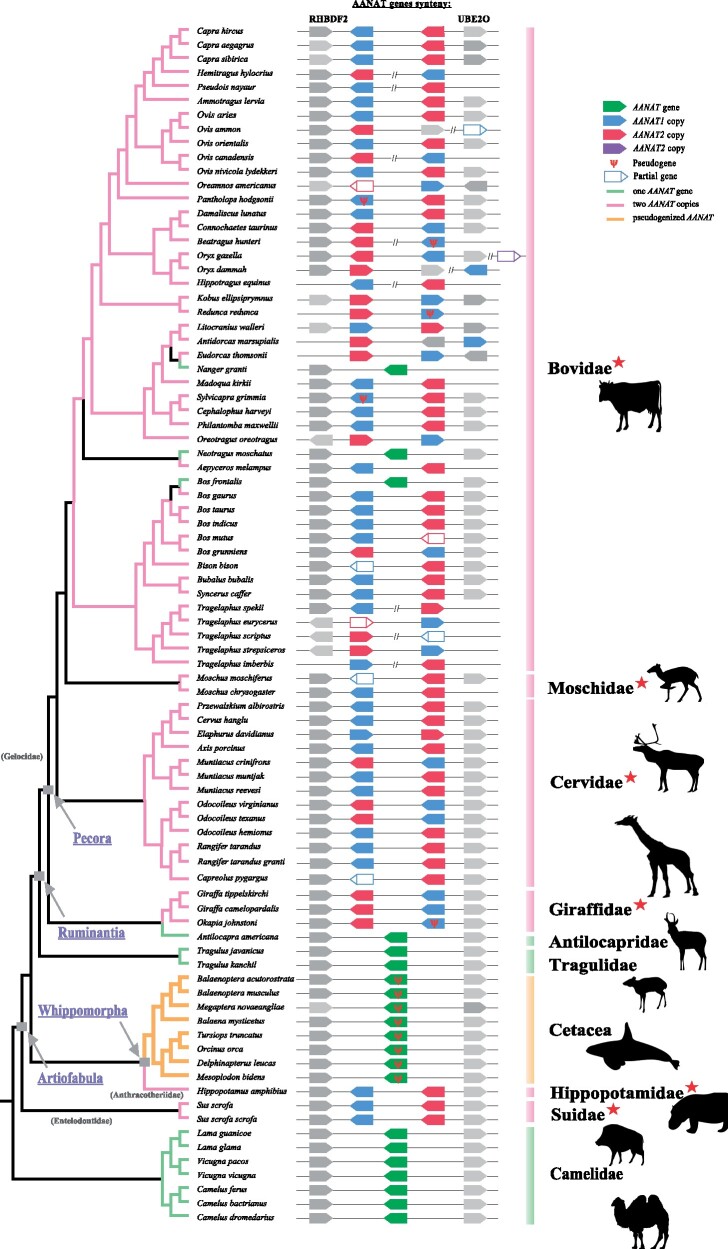
To obtain a broader picture of AANAT evolution in mammals, we identified AANAT sequences from 174 mammalian species with high-quality genome assemblies, including 12 artiodactyl species. Most mammals were found to have a single copy of the AANAT gene. Phylogenetic trees of AANAT sequences showed a major congruence with commonly acknowledged phylogeny, indicating that AANAT genes were orthologous (supplementary fig. S1, Supplementary Material online). Interestingly, besides AANAT gene duplications identified in three artiodactyls (i.e., hippopotamus, cattle, and goat) which were reported previously, two AANAT sequences were also detected in other five artiodactyl species (fig. 1), including pig (Sus scrofa) and four ruminants (reindeer Rangifer tarandus, Thomson’s gazelle Eudorcas thomsonii, giraffe Giraffa camelopardalis, and sheep Ovis aries). To further explore the evolution characteristics of the AANAT gene in artiodactyls, additional 82 artiodactyl species covering all major families were added to our analyses. The results showed that one copy of AANAT gene was only observed in three families, including Camelidae, Tragulidae, and Antilocapridae, whereas two adjacent AANAT copies could be identified in the vast majority of ruminants, hippopotamus, and two species of Suidae (fig. 2). Significant sequence similarity (a range of 82.8–99.2%) was found between two AANAT copies within each species. However, nonsynonymous substitution sites were also identified, most of which caused radical amino acid changes and were located within or adjacent to important functional domains, and may lead to functional divergence. For example, pairwise comparion of cattle AANAT sequences showed a total of 10 amino acid changes and two of them (i.e., E43K and Q71R) were located in the binding pocket of AANAT.
Fig. 1.
Phylogenetic tree of mammals showing species with different evolution patterns of AANAT. Tree topology is based on multiple studies of mammalian phylogeny (Meredith et al. 2011; Song et al. 2012; Kumar et al. 2017; Upham et al. 2019). Gene duplication and loss events are mapped to the species tree, indicated by red and yellow, respectively. All silhouettesare reproduced from PHYLOPIC (http://www.phylopic.org/, last accessed May 21, 2021)
Fig. 2.
The repertoires and synteny of AANAT genes in cetartiodactyl species with available genome sequences. Topology of the tree is based on previous studies (Hassanin et al. 2012; Bibi 2013; Chen et al. 2019). AANAT genes synteny is schematically shown by different colored polygons pointing out the orientation in the genome, and the conserved RHBDF2 upstream and UBE2O downstream genes are shown in gray. Unfilled polygons indicate target region could not be obtained completely. Distances between genes or gene length are not drawn to scale.
Furthermore, three copies of AANAT were observed in two species of Bovidae, including Père David’s deer Elaphurus davidianus (NCBI: GCA_002443075.1) and gemsbok Oryx gazelle (GCA_006410575.1). However, only two copies were identified in Père David’s deer when using another independently sequenced genome (http://animal.nwsuaf.edu.cn/code/index.php/RGD, last accessed May 21, 2021), which left open the possibility that the different copy numbers may be sequencing errors or artifacts arising from genome assembly. Furthermore, PCR amplification and sequencing further corroborated that Père David’s deer had only two copies of the AANAT gene. Furthermore, the premature stop codons were detected in five artiodactyls (chiru Pantholops hodgsonii, okapi Okapia johnstoni, hirola Beatragus hunteri, Bohar reedbuck Redunca redunca, and bush duiker Sylvicapra grimmia), rendering one of the two AANAT copies putatively nonfunctional, while these genes did not exhibit any shared inactivation mutations and their closely related species retained two functional copies.
Three Independent Duplications of the AANAT Gene in Artiodactyls
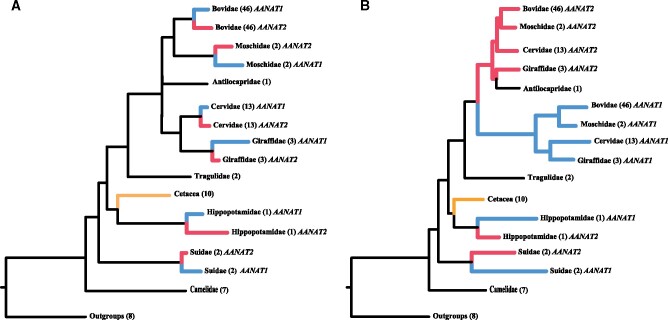
As AANAT gene duplications were identified specifically in artiodactyl species, we focused on the evolutionary relationships of artiodactyl AANAT genes to infer duplication events. Similar tree topologies were generated when phylogenetic trees of the AANAT gene were reconstructed using the maximum likelihood (ML) and Bayesian inference (BI) (fig. 3). Phylogenetic reconstructions for coding and noncoding sequences both showed that AANAT copies in pig and hippopotamus formed their respective clades, supporting the independent duplications in these two clades (fig. 3). However, the coding and noncoding trees presented different patterns for AANAT evolution in pecorans. In the phylogenetic tree based on coding sequences, duplicated AANAT copies formed respective monophyletic clades within multiple species (fig. 3A and supplementary fig. S2, Supplementary Material online), implying gene duplications after pecoran species divergence. By contrast, in the noncoding tree, pecoran AANAT copies were clearly divided into two distinct groups (fig. 3B and supplementary fig. S3, Supplementary Material online), suggesting one single-gene duplication event occurred in the common ancestor of all pecoran ruminants. Actually, the findings based on noncoding sequences were corroborated by the phylogenetic analyses based on concatenated coding and noncoding sequences (supplementary fig. S4, Supplementary Material online), which split pecoran AANAT1/AANAT2 copies into two separate groups with high nodal supports (BS/PP = 100/1). Furthermore, we also detected that gene conversion might have affected the coding regions of AANAT copies in most artiodactyls using the software RDP4 (supplementary table S4, Supplementary Material online), which could have led to the phylogenetic confusion of some lineages in the coding analyses. Thus, according to the phylogenetic trees of noncoding sequences and concatenated coding and noncoding sequences, three independent duplications were clearly identified in artiodactyls, that is, the ancestral lineages of Suidae (i.e., Entelodontidae), Hippopotamidae (i.e., Anthracotheriidae), and pecorans (i.e., Gelocidae).
Fig. 3.
Schematic phylogeny trees of AANAT sequences based on coding region (A) and noncoding region (B). ML and BI analyses produced nearly identical tree topology. See supplementary figures S2 and S3, Supplementary Material online, for the detailed tree with species and bootstrapping supports.
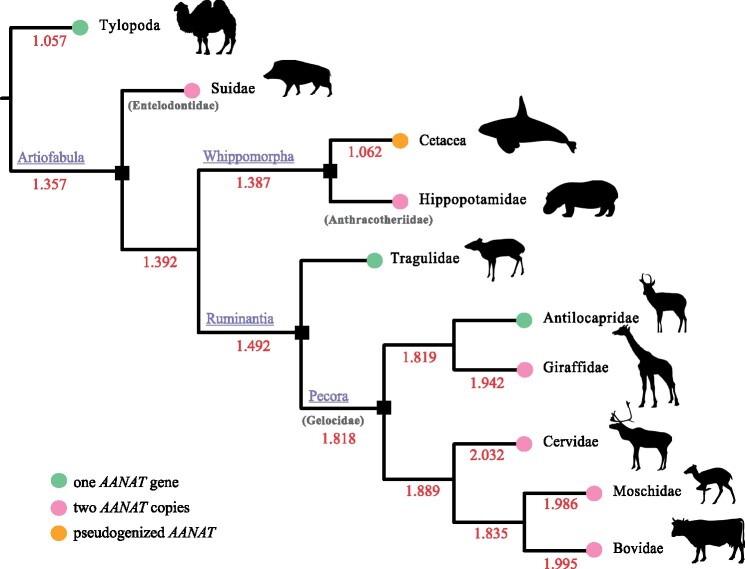
The ancestral character of the copy number at the relevant nodes was estimated using the ML approach (fig. 4 and supplementary fig. S5, Supplementary Material online). For the node of Artiofabula, as well as the node of Whippomorpha and the ancestral node for cetacean clades, the estimated copy numbers were 1.357 (95% CI: 0.695–2.019), 1.387 (95% CI: 0.741–2.033), and 1.107 (95% CI: 0.537 - 1.678), respectively, implying that no AANAT duplications had occurred in these ancestral nodes. Notably, the earliest increased copy number was recorded in the ancestral node of Pecora, with an ancestral character estimate of 1.818 (95% CI: 1.412–2.225), implying that expansions of the AANAT copy number occurred before the radiation of pecoran ruminants. Additionally, we estimated the ages of divergence between AANAT copies using BI implemented in the software package BEAST (supplementary fig. S7, Supplementary Material online). It was indicated that the divergence times for AANAT sequences in Suidae and Pecora were 46.94 and 47.60 Ma, respectively, which were congruent with the appearance of their ancestors during the Middle Eocene (37.2–48.6 Ma), whereas the age for the divergence of hippopotamus AANAT copies was estimated to be 22.77 Ma, which was in the early Miocene (15.9–23 Ma).
Fig. 4.
Reconstruction copy numbers of AANAT gene across cetartiodactyl phylogeny. The time-calibrated phylogenetic tree of Cetartiodactyla was derived from Timetree (Kumar et al. 2017). The estimated numbers of ancestral nodes are shown on each branch in red. The copy number of AANAT in extant cetartiodactyl species are shown in colored circles.
Inactivations of the AANAT Gene in All Four Pineal-Deficient Mammalian Lineages
Our genome alignments revealed that one or more inactivating mutations (premature stop codons, frameshift indels, altered start codons, or splice site mutations) were presented in all placental taxa without detectable pineal gland (i.e., cetaceans, sirenians, pangolins, and xenarthrans) (fig. 1). Inactivation mutations were found to be located in the first exon of the AANAT gene (three coding exons, 207 residue protein in humans), which may have led to a functional loss in these lineages. For cetaceans, all species had one nonsense mutation at the end of exon 1 that led to premature stop codons, and additional inactivation mutations were also detected in the most toothed whales, with even the sperm whale Physeter microcephalus showing the deletion of entire exon 1 (supplementary fig. S8, Supplementary Material online). These disruptive mutations in AANAT genes were further confirmed by polymerase chain reaction (PCR) amplification and sequencing of AANAT genes from six cetacean species. Similar results were also found in two species of Sirenia. For example, the Florida manatee Trichechus manatus had an ACG initiation codon mutation and a 13-bp frameshift deletion in exon 3, and Steller’s sea cow Hydrodamalis gigas had a 22-bp frameshift deletion in exon 1 (supplementary fig. S9, Supplementary Material online). For Pholidota, two premature stop codons were detected in all three pangolins, of which one premature stop codon was located at positions 28–30 in exon 1 and another was located at positions 211–213 in exon 2 (supplementary fig. S10, Supplementary Material online). Furthermore, the largest number of frameshift mutations were found in xenarthran species, including the nine-banded armadillo Dasypus novemcinctus (13), the three-banded armadillo Tolypeutes matacus (9), the Hoffmann’s two-fingered sloth Choloepus hoffmanni (7), the Brown-throated sloth Bradypus variegatus (5), and the southern two-toed sloth Choloepus didactylus (4). These mutations spread across all exons of AANAT and inactivated its reading frame (supplementary fig. S11, Supplementary Material online). Finally, Blast searches failed to show any evidence for the presence of AANAT in two anteaters (giant anteater Myrmecophaga tridactyla and southern tamandua T. tetradactyla), whereas the entire sequence for conserved flanking genes (RHBDF2, UBE2O) could be recovered.
Next, selection analyses were performed to test whether AANAT evolved neutrally in the lineages with inactivating mutations (table 1). Indeed, an ω value of 0.382 for all four gene-loss clades was significantly higher than the ω value of 0.170 observed for other background branches with intact sequences (P < 0.001), suggesting that AANAT evolved under relaxed selection in these pseudogenized branches. The values for crown Cetacea and crown Xenarthra were 0.840 (P = 0.820) and 1.504 (P = 0.700), respectively, which were both close to the expected value of 1.000 for the neutrally evolving in crown branches subsequent to inactivation of AANAT gene on the stem branches. The crown Pholidota had an ω value of 0.471 that was significantly higher relative to the background value (P < 0.001) but was different from the expected value for neutral evolution (P = 0.046). As for crown Sirenia, the slightly elevated ω of 0.155 was not significantly different from the background value of 0.148 (P = 0.485), suggesting inactivation of AANAT gene was a young event.
Table 1.
LRTs of Various Models on the Selective Pressures on AANAT.
| Models | Ω | −ln L | np | Models Compared | 2Δ(ln L) | P Values | |
|---|---|---|---|---|---|---|---|
| Data set I | A: all branches have one ω | 0.175 | 20,201.823 | 320 | |||
| B: all branches have one ω = 1 | 1.000 | 21,458.178 | 319 | A vs. B | 2,512.710 | 0 | |
| C: all branches with pseudogenized AANAT has ω2 others have ω1 | ω1 = 0.170, ω2 = 0.382 | 20,192.811 | 321 | A vs. C | 18.024 | 2.18137E−05 | |
| D: all branches with pseudogenized AANAT has ω2 = 1 others have ω1 | ω1 = 0.169, ω2 = 1.000 | 20,203.316 | 320 | C vs. D | 21.010 | 4.56892E−06 | |
| E: each branch has its own ω | Variable ω by branch | 19,922.015 | 318 | C vs. E | 541.592 | 4.6171E−117 | |
| Data set II | A: all branches have one ω | 0.159 | 17,906.143 | 300 | |||
| B: all branches have one ω = 1 | 1.000 | 19,149.248 | 299 | A vs. B | 2,486.210 | 0 | |
| C: the crown Cetacea has ω2 others have ω1 | ω1 = 0.158, ω2 = 0.840 | 17,902.075 | 301 | A vs. C | 8.136 | 0.004 | |
| D: the crown Cetacea has ω2 = 1 others have ω1 | ω1 = 0.158, ω2 = 1.000 | 17,902.101 | 300 | C vs. D | 0.052 | 0.820 | |
| Data set III | A: all branches have one ω | 0.148 | 16,542.044 | 252 | |||
| B: all branches have one ω = 1 | 1.000 | 17,811.403 | 251 | A vs. B | 2,538.718 | 0 | |
| C: the crown Sirenia branch has ω2 others have ω1 | ω1 = 0.148, ω2 = 0.155 | 16,541.800 | 253 | A vs. C | 0.488 | 0.485 | |
| D: the crown Sirenia branch has ω2 = 1 others have ω1 | ω1 = 0.148, ω2 = 1.000 | 16,551.960 | 252 | C vs. D | 20.320 | 6.55122E−06 | |
| Data set IV | A: all branches have one ω | 0.154 | 16,877.636 | 254 | |||
| B: all branches have one ω = 1 | 1.000 | 18113.721 | 253 | A vs. B | 2472.170 | 0 | |
| C: the crown Pholidota has ω2 others have ω1 | ω1 = 0.152, ω2 = 0.471 | 16871.939 | 255 | A vs. C | 11.394 | 0.000736817 | |
| D: the crown Pholidota has ω2 = 1 others have ω1 | ω1 = 0.152, ω2 = 1.000 | 16,873.920 | 254 | C vs. D | 3.962 | 0.046 | |
| Data set V | A: all branches have one ω | 0.158 | 17,621.820 | 258 | |||
| B: all branches have one ω = 1 | 1.000 | 18,865.766 | 257 | A vs. B | 2,487.892 | 0 | |
| C: the crown Xenarthra has ω2 others have ω1 | ω1 = 0.156, ω2 = 1.504 | 17,616.249 | 259 | A vs. C | 11.142 | 0.000843948 | |
| D: the crown Xenarthra has ω2 = 1 others have ω1 | ω1 = 0.156, ω2 = 1.000 | 17,616.323 | 258 | C vs. D | 0.148 | 0.700 |
We further estimated the inactivation time for AANAT in each lineage (supplementary table S5, Supplementary Material online). The results revealed that the cetacean inactivation of AANAT occurred around 42.2–45.5 Ma in the common ancestor of the crown Cetacea. For crown Xenarthra, the loss was estimated to have occurred in the ancestor of xenarthrans that lived 60–72 Ma. Within Pholidota, we estimate that AANAT was inactivated around 27.3–30.4 Ma. By contrast, inactivation dates for crown Sirenia were estimated in the more recent range of 0.5–0.6 Ma, allowing for the possibility that there has been a lag time between the cessation of purifying selection and the accumulation of the first inactivating mutation in this lineage.
Enzyme Assay for AANAT Genes in Different Mammalian Lineages
To determine whether different evolutionary characteristics of AANAT were accompanied by functional changes, we performed in vitro enzymatic assays for nine species from three categories, including those with single AANAT genes (C3H/HeJ mice C3, dromedary Camelus dromedarius, and Java mouse-deer Tragulus javanicus), two AANAT copies (pig, hippopotamus, cattle, and reindeer), and mutant AANAT genes (minke whale Balaenoptera acutorostrata and C57BL/6J mice B6) (fig. 6). The kinetic constants of the recombinant AANATs were determined using serotonin as a substrate and calculated using the Michaelis–Menten equation (supplementary fig. S12 and table S6, Supplementary Material online). We found that the AANATs from the four artiodactyls with two AANAT copies showed significantly higher activity than those from the three species with a single copy of AANAT (P = 0.036). For AANATs from species with the two copies, AANAT2s were found to exhibit significantly higher activity than AANAT1s (P = 0.029). In addition, enzyme activities were significantly different among the four artiodactyl species with AANAT duplicates, with reindeer showing the strongest activities for acetylating substrates, whereas hippopotamus showed the weakest activities. Furthermore, in the three species with single-copy AANAT, dromedary, and lesser mouse-deer showed higher catalytic capacities than C3 mice (P = 0.033). In contrast, the minke whale, which has a pseudogenized AANAT gene, showed nearly undetectable AANAT activity, whereas B6 mice with a truncated AANAT protein had a relatively weak activity.
Discussion
In the present study, we scanned the AANAT gene against a total of 256 mammalian species and provided a comprehensive evolutionary analysis of this gene across the entire mammalian phylogeny. Most mammalian lineages have only one copy of AANAT, however, besides previously identified AANAT duplication events in hippopotamus, cattle, and goat, novel duplications in three clades (i.e., Suidae, Hippopotamidae, and Pecora) of Artiodactyla were also identified in the present study. And intriguingly, besides cetaceans and sirenians, pangolins and xenarthrans also possessed inactivation mutations in AANAT genes. These four mammalian taxa with inactivated AANAT genes all lack pineal glands and show particular biological rhythms. Enzyme assays further corroborated the functional diversification of AANAT across mammalian lineages. Our study provides some novel insights into gene duplication and loss in mammals driven by rhythmic regulation to adapt to seasonal climate changes and specific ecological niches.
Three Independent Duplications of AANAT in Artiodactyls in Response to Seasonal Climate Changes
A complicated evolutionary pattern of AANAT was identified in artiodactyl species, with two copies found in pig, hippopotamus, and pecoran lineages, whereas other lineages had one copy. Phylogenetic trees and copy number of ancestral state reconstructions in artiodactyl AANAT sequences suggested that three independent duplication events had occurred along the ancestral lineages, respectively, of Suidae (i.e., Entelodontidae), Hippopotamidae (i.e., Anthracotheriidae), and pecorans (i.e., Gelocidae). Fossil records revealed that three ancestors with duplicated AANAT all appeared in the Middle Eocene (Prothero and Foss 2007; Vislobokova 2008; Hackmann and Spain 2010; Scherler et al. 2019), a period characterized by global cooling and increased climate seasonality (Agustí et al. 2003; Mosbrugger et al. 2005). This was supported by the present estimates of time for the AANAT gene duplication in Suidae and Pecora which well coincided with the period of Middle Eocene. By contrast, the divergence of hippopotamus AANAT copies was estimated to occur 22.77 Ma, not in the Middle Eocene but in the Early Miocene, a period with gradually increased climate seasonality (Bruch et al. 2007, 2011). The appearance of seasonal climates drove the spread of deciduous forests and thus brought about the beginning of seasonal differences in the availability and abundance of vegetation (Janis 1989; Woodburne 2004). Consequently, herbivores were faced with big challenges to survive during the annual food shortage periods. Through detecting and transducing photoperiodic alterations, the timing mechanisms mediated by melatonin allow for triggering physiological behaviors at the appropriate period, providing the organisms with time to prepare and survive in advance of climate changes (Kumar 2017). Furthermore, accurate anticipation is crucial for determining favorable breeding time to optimize the survival of offspring. For instance, the timing of artiodactyl parturition generally coincides with seasonal plant growth (Grzimek 2003; Martin et al. 2004). Therefore, the expansion of the AANAT gene, together with enhanced regulation of photoperiodic responses may thus confer advantages in dealing with the predictable climate changes to conquer unfavorable resource conditions. This is congruent with the hypothesis that duplications of the AANAT gene contributed to better seasonal adaptation as revealed in insects and teleosts (García‐Allegue et al. 2001; Barberà et al. 2013).
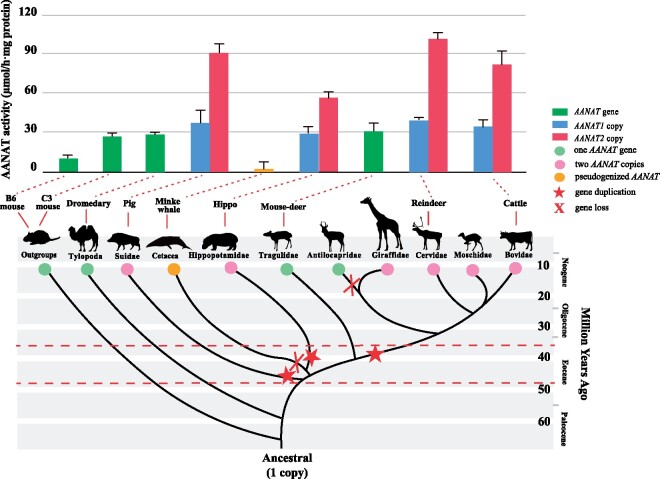
The AANATs of artiodactyls showed significantly higher activities than those of mice, and furthermore, duplicated AANAT copies (i.e., AANAT1 and AANAT2) showed enhanced activity relative to single-copy AANAT genes (fig. 5). In general, AANAT activity exhibited a close positive relationship with the amount of produced melatonin (Ebihara et al. 1997; Klein 2006; Byeon and Back 2016). This increased enzyme activity of AANAT duplicates strongly suggested that artiodactyls might have an enhanced capacity for melatonin secretion and improved their ability to cope with seasonal changes. In light of previous studies that gene conversion often promoted the beneficial increase in dosage of genes that mediate the interaction between organism and environment (Innan and Kondrashov 2010; Meslin et al. 2011), the extensive gene conversion events identified in the evolution of artiodactyl AANAT genes could further supported a potentially important roles of an increased amount of AANAT gene expression in artiodactyles. In addition, it was also noticed that AANAT activity differed between artiodactyls with two copies, such as reindeer, which showed the highest AANAT activity in this study, whereas hippopotamus showing the lowest. Although reindeers live in polar photic conditions, they can still determine seasonal timing accurately by monitoring the indiscernible photoperiod changes in their environments (Skogland 1989; Stokkan et al. 2007), which was supported, to some degree, by the substantially high activity of their two AANAT copies that would be beneficial for quickly adjusting melatonin level. In contrast, the hippopotamus, an example of species inhabiting the tropics with a less marked seasonality, mainly used the rainfall as a more reliable seasonal marker (Estes 2012; Ogutu et al. 2014), which might have led to decreased activities of the reserved two copies.
Fig. 5.
Gene gain and loss events and enzyme activities of AANAT gene across the cetartiodactyl phylogeny. Red stars in the branches of the phylogenetic tree are indicating putative duplication event. The red X shows the presumed occurrences of gene loss event. The red dotted lines in the bottom part of the diagram indicate the middle Eocene (48.6–37.2 Ma). Bars in the upper part represent the maximum enzyme activity of AANAT proteins in corresponding species (indicated by the red dotted lines). The approximate divergence times were obtained from multiple resources (Boisserie et al. 2005; McGowen et al. 2009; Janis and Theodor 2014; Zurano et al. 2019). For details, see Materials and Methods.
Inactivation of AANAT and Pineal-Deficiency Contribute to Escaping from the Circadian Regulation
Pineal gland, the primary source of circulating melatonin, is a major endocrine component in the regulation of circadian behaviors. Surgical removal of the pineal gland in animals can abolish the circadian behavior and reduce sleep duration (Kocher et al. 2006; Trivedi et al. 2016). During the mammalian evolution, four taxa (cetaceans, sirenians, pangolins, and xenarthrans) have been reported to lack of discernable pineal glands (Ralph 1975; Kappers 1983), and correspondingly, independent inactivating mutations or missing of AANAT locus were identified in these four clades in the present study. This result may have been associated with their particular features in circadian rhythm to adapt to specific ecological niches. Cetaceans and sirenians, as fully aquatic marine mammals, have evolved an irregular circadian organization of activity and sleep. Most obviously, they adopt a unique unihemispheric slow-wave sleep, allowing one cerebral hemisphere to remain awake enough while the other one still benefits from sleep (Lyamin et al. 2008; Aulsebrook et al. 2016). This is favorable for maintaining active states in water to achieve the necessity to breathe at the surface and more efficient monitoring of the environment. The inactivation mutations of cetacean AANAT and its undetectable enzyme activity indicated the functional loss of the AANAT gene in this lineage. Knockout of the AANAT gene in zebrafish significantly reduces almost half of their nighttime sleep (Gandhi et al. 2015). The inactivation of AANAT and the loss of pineal glands in cetaceans and sirenians disrupt the production of melatonin that can promote sleep, thus contributing to increased wakefulness to adapt to completely aquatic environments. In contrast, xenarthrans and pangolins display markedly convergent traits, featuring predominantly by nocturnality, imperfect temperature regulatory systems, and low, labile body temperatures (McNab 1980; Gilmore et al. 2001). They have synchronized their activity rhythm primarily to fluctuations in ambient temperature rather than photoperiodic changes to achieve thermal balance (Giné et al. 2015; Attias et al. 2018). Besides modulating biological rhythms, the increased circulating levels of melatonin have been shown to decrease core body temperature through enhancing distal heat loss (Saarela and Reiter 1994; Kräuchi et al. 2006). Thus, the absence of AANAT and pineal glands may be associated with adaptive changes in circadian rhythmicity, affecting activity patterns and body temperature maintenance (Cagnacci et al. 1992). Overall, the disruptions of AANAT function together with a deficiency of pineal glands would be accordingly advantageous for escaping from the circadian regulation of daily activity to accommodate adaptations for specific ecological niches and life history traits.
Conclusion
The present study demonstrated a more complex and intriguing evolutionary pattern of mammalian AANAT genes than previously recognized, which yielded some new insights into the evolution and function of this gene. Remarkably, we identified and characterized new AANAT gene sequences from artiodactyls based on sequence analyses and functional activity assays. Our findings further revealed independent gene duplications in three ancestral lineages of artiodactyls, including Suidae, Hippopotamidae, and Pecora. The coincidence between gene duplication and the enhanced seasonal climate during the Middle Eocene, in combination with the increased activity of duplicated AANAT copies, suggested that AANAT gene duplication is an advantage for artiodactyls to evolve photoperiodic response to adapt to seasonal climate changes. Additionally, it is interesting to find gene deletion or inactivation in multiple mammalian lineages that have lost pineal glands and exhibit irregular circadian features, including fully aquatic cetaceans and sirenians, pangolins, and xenarthrans. This study could provide some novel insights into mammalian AANAT evolution as driven by or associated with rhythmic adaptations in mammals.
Materials and Methods
Gene Identification
Using previously published intact AANAT gene sequences from human, mouse, panda as queries, we conducted Blastn (version 2.5.0) (Camacho et al. 2009) searches in local databases constructed using 174 mammalian genome sequences, including 12 artiodactyl species, retrieved from the National Center for Biotechnology Information (NCBI) website (https://www.ncbi. nlm.nih.gov/, last accessed May 21, 2021). The coding sequences of AANAT were determined manually after removing all introns following the canonical AG/GT rule for splicing (Burset et al. 2000). To further explore the evolution of AANAT in Artiodactyla, we then identified the AANAT gene sequences from 82 additional artiodactyl genomes retrieved from the NCBI and Ruminant Genome Database (http://animal.nwsuaf.edu.cn/code/index.php/RGD, last accessed May 21, 2021) using the camel AANAT gene sequence (XM_010983838.2) as a query. Detailed information concerning each genome assembly is described in supplementary table S1, Supplementary Material online. All the identified AANAT genes were classified into three categories as follows: intact genes, partial genes, and pseudogenes, according to amino acid alignment and Blast results. The Blast hits with a length of <200 bp were discarded. If a target gene was not found, the genomic region between the four flanking genes (SPHK1, UBE2O, RHBDF2, and CYGB) was inspected, manually to ensure the continuity or disruption of each genomic region (with presence or absence of N’s). We failed to detect AANAT sequences and recover the continuous flanking sequences in three artiodactyls, which may have resulted from either incomplete genome sequencing or poor genome assembly. The unavailable AANAT fragments from 14 artiodactyl species were excluded from subsequent analysis (supplementary table S1, Supplementary Material online). Phylogenetic relationships and divergence times of species were obtained from the Timetree website (www.timetree.org, last accessed May 21, 2021) (Kumar et al. 2017) and multiple references (Boisserie et al. 2005; McGowen et al. 2009; Meredith et al. 2011; Song et al. 2012; Janis and Theodor 2014; Upham et al. 2019; Zurano et al. 2019).
To validate the inactivation mutations of AANAT in cetaceans and the copy number of AANAT genes in artiodactyls, we additionally sequenced the AANAT gene from six cetacean species (one mysticetes and five odontocetes) and three artiodactyl species: minke whale, baji Lipotes vexillifer, finless porpoise Neophocaena asiaeorientalis, Indo-pacific humpbacked dolphin Sousa chinensis, striped dolphin Stenella coeruleoalba, short-beaked common dolphin Delphinus delphis, cattle, sheep, and Père David’s deer. Each sample used for this study was collected from dead individuals in the wild and sampling was conducted systematically in accordance with all the ethical guidelines and legal requirements in China. Genomic DNA was extracted from muscle samples using standard phenol/chloroform extraction protocols followed by ethanol precipitation. The entire sequences of AANAT genes were PCR amplified using a suite of forward (F) and reverse (R) primers (supplementary tables S2 and S3, Supplementary Material online). PCR reactions were conducted with 2 × Phanta Master Mix (Vazyme, Nanjing, China) according to the manufacturer’s specifications. PCR products were purified using the MiniBEST DNA Fragment Purification Kit (TaKaRa) and then sequenced directly for both strands using an ABI 3730 automated genetic analyzer. To isolate different copies of AANAT genes in artiodactyl species, the purified PCR products were cloned into the pMD19‐T vector (TaKaRa). For each PCR product, at least six positive clones were picked for sequencing using the M13 universal primer pair. The resulting sequences were assembled at 99% similarity level to allow for 1% mutations by considering both Taq polymerase‐derived errors and allelic variations, aiming to avoid inclusion of the same AANAT multiple times. Each assembled sequence was counted as one copy.
Phylogenetic Reconstruction
Coding, noncoding, and combined phylogenetic trees were constructed through the ML approach with IQ-TREE (Trifinopoulos et al. 2016) and Bayesian methods implemented in Mr.Bayes v.3.2.3 (Ronquist et al. 2012). The upstream/downstream regions with a length of <1,000 bp were discarded in the noncoding and combined analyses. The optimal model of sequence substitution was determined by MrModeltest 2.3 (Nylander 2004) using the Akaike Information Criterion. BI analysis was run for 30,000,000 iterations of a Markov chain Monte Carlo (MCMC) algorithm, with six simultaneous chains, and trees were sampled every 1,000 generations. Support for the nodes and parameter estimates were derived from a majority rule consensus of the last 15,000 trees sampled after convergence. For the ML analyses, the best tree was reconstructed with 10,000 bootstrap replications. Gene conversion events were also detected using five methods implemented in the RDP4 software package (Martin et al. 2015).
The ancestral states for the number of AANAT gene copies were reconstructed based on the ML approach using the fastAnc function in the phytools R package (Revell 2012). The estimated ancestral states were mapped on the phylogeny using the contMap function in phytools (supplementary fig. S5, Supplementary Material online), while the 95% confidence intervals (CIs) for point estimates of ancestral states were plotted on a traitgram using the fancyTree function from the same package (supplementary fig. S6, Supplementary Material online).
The noncoding data were used for estimating the divergence times of two AANAT copies using Bayesian method implemented in BEAST v1.10.4 (Suchard et al. 2018). In BEAUti (from BEAST package), nucleotide substitution model was set to “HKY,” and the clock model was set to “uncorrelated relaxed molecular clock” (Drummond et al. 2006) with “lognormal” distribution and “Yule Process” of speciation (Gernhard 2008) was set as Tree prior. MCMC was run independently for five times with each run for ten million generations with sampling for every 1,000 generations. LogCombiner v1.10.4 was used for removing a burnin of 2,000 from each of the five tree files and for generating a combined tree file. A best tree was generated with all the annotations incorporated using TreeAnnotator v1.10.4. The combined log file was imported to Tracer v1.7.1 for assessing the effective sample size (ESS). It was found to be >200 for all the parameters. The final tree and divergence times were visualized using FigTree v1.4.4. Four calibration points were used in the present study: the divergent time of Ruminantia set as 38–50 Ma (Hassanin et al. 2012; Bibi 2013), the split between dog Canis familiaris and giant panda Ailuropoda melanoleuca set at 42–48 Ma (Sato et al. 2009; dos Reis et al. 2012), the split between large flying fox Pteropus vampyrus and greater horseshoe bat Rhinolophus ferrumequinum set at 55.6–60.5 Ma (Eick et al. 2005; Yu et al. 2014), and that between human Homo sapiens and Rhesus monkey Macaca mulatta set at 27.95–31.35 Ma (Wildman et al. 2003; Yang and Rannala 2006).
Selective Pressure Analyses
The rate ratios (ω) of nonsynonymous (dN) to synonymous (dS) substitutions were calculated using codon-based ML models implemented in the CODEML program of PAML 4.8a (Yang 2007). All frameshift insertions in the alignments were deleted, and frameshift deletions and nonsense mutations were recoded as missing data prior to analysis. First, we used one-ratio (A) model that assumed a uniform ω ratio for all branches. Next, we tested two-ratio (C) model that allowed two ω ratios that differed between the background branches and the target branches. Then, one-ratio (B) model with a fixed ω= 1.0 for all branches and two-ratio (D) model with a fixed ω = 1 on the branches of interest were used as null hypotheses. Finally, we tested a free-ratio (E) model that allowed an independent ω value for each branch. A likelihood ratio test (LRT) with a χ2 distribution was used to evaluate which models were statistically different from the null model at a threshold of P < 0.05.
Estimation of Inactivation Times
To date the loss of AANAT in different lineages, we used the methods described in previous studies (Sharma, Hecker, et al. 2018; Sharma, Lehmann, et al. 2018). The inactivation time (Tn) was estimated by the following equation:
where T represents the time since the split from the last common ancestor. We used the lower and upper bound of the CI for the species divergence time T from TimeTree (Kumar et al. 2017). The Ka/Ks value estimated for pseudogenic branches was referred to as K, whereas the Ka/Ks value for mammals with a functional AANAT was referred to as Ks ⋅ Ka is the number of nonsynonymous substitutions per nonsynonymous site, whereas Ks is the number of synonymous substitutions per synonymous site. The Ka/Ks values were determined by the CODEML program.
Recombinant Protein Preparation
The recombinant enzymes from seven cetartiodactyl species, including cattle, hippopotamus, pig, reindeer, dromedary, Java mouse-deer, and minke whale were expressed and purified in the present study. Mice are extensively used in transgenic models and biology studies including sleep, circadian rhythm, and melatonin research (Uz and Manev 2001; Sharma, Sahota, et al. 2018). Moreover, it has been demonstrated that there are two kinds of laboratory mouse strains, melatonin-deficient (B6) and melatonin-proficient strains (C3). B6 mice have a naturally truncated AANAT protein as a result of aberrant splicing, whereas C3 mice retain an intact and functional AANAT gene (Roseboom et al. 1998). Thus, we chose AANAT genes from these two mouse strains as control groups in this study. Coding regions were inserted into the bacterial expression vector pGEX4T1 and verified by Sanger sequencing. This allowed for the construction of AANAT proteins fused to glutathione S-transferase. The way of expression, production, and purification of AANATs has been described previously (Pavlicek et al. 2008; Falcón et al. 2014).
Enzymatic Activity Assays
The colorimetric assay for the AANAT enzyme activity was based on the quantification of Coenzyme A generated during acetyl transfer (Falcón et al. 2014; Paulin et al. 2015). Typically, reactions were performed in 96-well plates, where each well contained 0.5 µg of AANAT enzyme in a 100 µl final volume of phosphate buffer (0.1 M, pH 6.8) solution including acetyl-Coenzyme-A 0.5 mM, EDTA 2 mM, BSA 0.05 mg/ml, and serotonin concentrations ranging from 0.05 to 15 mM. Incubation was 30 min at 37 °C for all enzymes. Each reaction was terminated by the addition of 150 µl of stop solution (0.2 M phosphate buffer pH 6.1, containing 1 mM DTNB, 10 mM EDTA, and 3 M guanidine hydrochloride) and the optical density in each well was measured at 405 nm after 5 min of incubation at room temperature. Controls were run using a reaction medium that contained no or heat‐treated (65 °C, 5 min) enzyme. The kinetics constant values obtained were calculated by nonlinear fitting (Prism8 from GraphPad) according to the Michaelis–Menten equation.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (grant nos. 32030011 to G.Y., 31772448 to S.X., 31630071 to G.Y., and 32070409 to S.X.), the Qinglan Project of Jiangsu Province to S.X., National Key Programme of Research and Development, Ministry of Science and Technology (grant no. 2016YFC0503200 to G.Y. and S.X.), and the Priority Academic Program Development of Jiangsu Higher Education Institutions to G.Y. and S.X.
Author Contributions
G.Y. and S.X. conceptualized and supervised the study. D.Y., M.Y., and Y.C. collected the data and conducted the bioinformatics analyses. D.Y. and R.Z. performed AANAT activity experiments. D.Y. prepared the original draft, and G.Y. and S.X. revised the manuscript. All authors read and approved the final manuscript.
Data Availability
There are no data to be archived. All data were included in supplementary materials for readers to validate.
References
- Agustí J, De Siria AS, Garcés M.. 2003. Explaining the end of the hominoid experiment in Europe. J Hum Evol. 45(2):145–153. [DOI] [PubMed] [Google Scholar]
- Attias N, Oliveira-Santos LGR, Fagan WF, Mourão G.. 2018. Effects of air temperature on habitat selection and activity patterns of two tropical imperfect homeotherms. Anim Behav. 140:129–140. [Google Scholar]
- Aulsebrook AE, Jones TM, Rattenborg NC, Roth IIT, Lesku JA.. 2016. Sleep ecophysiology: integrating neuroscience and ecology. Trends Ecol Evol. 31(8):590–599. [DOI] [PubMed] [Google Scholar]
- Barberà M, Mengual B, Collantes-Alegre JM, Cortés T, González A, Martínez-Torres D.. 2013. Identification, characterization and analysis of expression of genes encoding arylalkylamine N-acetyltransferases in the pea aphid Acyrthosiphon pisum. Insect Mol Biol. 22(6):623–634. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Goldman BD.. 1989. Mammalian pineal melatonin: a clock for all seasons. Experientia 45(10):939–945. [DOI] [PubMed] [Google Scholar]
- Bibi F.2013. A multi-calibrated mitochondrial phylogeny of extant Bovidae (Artiodactyla, Ruminantia) and the importance of the fossil record to systematics. BMC Evol Biol. 13(1):166–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisserie J-R, Lihoreau F, Brunet M.. 2005. The position of Hippopotamidae within Cetartiodactyla. Proc Natl Acad Sci U S A. 102(5):1537–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM.. 2007. Evolution of animal photoperiodism. Annu Rev Ecol Evol Syst. 38(1):1–25. [Google Scholar]
- Bruch AA, Uhl D, Mosbrugger V.. 2007. Miocene climate in Europe: patterns and evolution: a first synthesis of NECLIME. Palaeogeogr Palaeocl. 253(1–2):1–7. [Google Scholar]
- Bruch AA, Utescher T, Mosbrugger V.. 2011. Precipitation patterns in the Miocene of Central Europe and the development of continentality. Palaeogeogr Palaeocl. 304(3–4):202–211. [Google Scholar]
- Burset M, Seledtsov IA, Solovyev VV.. 2000. Analysis of canonical and non-canonical splice sites in mammalian genomes. Nucleic Acids Res. 28(21):4364–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byeon Y, Back K.. 2016. Low melatonin production by suppression of either serotonin N-acetyltransferase or N-acetylserotonin methyltransferase in rice causes seedling growth retardation with yield penalty, abiotic stress susceptibility, and enhanced coleoptile growth under anoxic conditions. J Pineal Res. 60(3):348–359. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Elliott JA, Yen SS.. 1992. Melatonin: a major regulator of the circadian rhythm of core temperature in humans. J Clin Endocrinol Metab. 75(2):447–452. [DOI] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL.. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10(1):421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Qiu Q, Jiang Y, Wang K, Lin Z, Li Z, Bibi F, Yang Y, Wang J, Nie W, et al. 2019. Large-scale ruminant genome sequencing provides insights into their evolution and distinct traits. Science 364(6446):eaav6202. [DOI] [PubMed] [Google Scholar]
- dos Reis M, Inoue J, Hasegawa M, Asher RJ, Donoghue PC, Yang Z.. 2012. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc R Soc B Biol Sci. 279(1742):3491–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Ho SY, Phillips MJ, Rambaut A.. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol. 4(5):e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara S, Adachi A, Hasegawa M, Nogi T, Yoshimura T, Hirunagi K.. 1997. In vivo microdialysis studies of pineal and ocular melatonin rhythms in birds. Biol Signals. 6(4–6):233–240. [DOI] [PubMed] [Google Scholar]
- Eick GN, Jacobs DS, Matthee CA.. 2005. A nuclear DNA phylogenetic perspective on the evolution of echolocation and historical biogeography of extant bats (Chiroptera). Mol Biol Evol. 22(9):1869–1886. [DOI] [PubMed] [Google Scholar]
- Estes RD.2012. The behavior guide to African mammals: including hoofed mammals, carnivores, primates. Berkeley: University of California Press. [Google Scholar]
- Falcón J, Besseau L, Fuentès M, Sauzet S, Magnanou E, Boeuf G.. 2009. Structural and functional evolution of the pineal melatonin system in vertebrates. Ann N Y Acad Sci. 1163(1):101–111. [DOI] [PubMed] [Google Scholar]
- Falcón J, Coon SL, Besseau L, Cazaméa-Catalan D, Fuentès M, Magnanou E, Paulin C-H, Boeuf G, Sauzet S, Jørgensen EH, et al. 2014. Drastic neofunctionalization associated with evolution of the timezyme AANAT 500 Mya. Proc Natl Acad Sci U S A. 111(1):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler AE, Kuhn S, Gwinner E.. 2004. Convergent evolution of strigiform and caprimulgiform dark-activity is supported by phylogenetic analysis using the arylalkylamine N-acetyltransferase (Aanat) gene. Mol Phylogenet Evol. 33(3):908–921. [DOI] [PubMed] [Google Scholar]
- Gandhi AV, Mosser EA, Oikonomou G, Prober DA.. 2015. Melatonin is required for the circadian regulation of sleep. Neuron 85(6):1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Coon SL, Klein DC.. 2002. Control of melatonin synthesis in the mammalian pineal gland: the critical role of serotonin acetylation. Cell Tissue Res. 309(1):127–137. [DOI] [PubMed] [Google Scholar]
- García-Allegue R, Madrid JA, Sánchez-Vázquez FJ.. 2001. Melatonin rhythms in European sea bass plasma and eye: influence of seasonal photoperiod and water temperature. J Pineal Res. 31(1):68–75. [DOI] [PubMed] [Google Scholar]
- Gernhard T.2008. The conditioned reconstructed process. J Theor Biol. 253(4):769–778. [DOI] [PubMed] [Google Scholar]
- Gilmore DP, Da Costa CP, Duarte DP.. 2001. Sloth biology: an update on their physiological ecology, behavior and role as vectors of arthropods and arboviruses. Braz J Med Biol Res. 34(1):9–25. [DOI] [PubMed] [Google Scholar]
- Giné GAF, Cassano CR, de Almeida SS, Faria D.. 2015. Activity budget, pattern and rhythm of maned sloths (Bradypus torquatus): responses to variations in ambient temperature. Mamm Biol. 80(6):459–467. [Google Scholar]
- Grzimek B.2003. Artiodactyla (Even-toed ungulates). In: Grzimek B, editor. Grzimek’s animal life encyclopaedia. Bingley: Emerald Group Publishing. p. 263–417. [Google Scholar]
- Gwinner E.2012. Circannual rhythms: endogenous annual clocks in the organization of seasonal processes. New York: Springer Science & Business Media. [Google Scholar]
- Hackmann TJ, Spain JN.. 2010. Invited review: ruminant ecology and evolution: perspectives useful to ruminant livestock research and production. J Dairy Sci. 93(4):1320–1334. [DOI] [PubMed] [Google Scholar]
- Hassanin A, Delsuc F, Ropiquet A, Hammer C, Jansen van Vuuren B, Matthee C, Ruiz-Garcia M, Catzeflis F, Areskoug V, Nguyen TT, et al. 2012. Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. CR Biol. 335(1):32–50. [DOI] [PubMed] [Google Scholar]
- HuelsmannM, , HeckerN, , SpringerMS, , GatesyJ, , SharmaV, , Hiller M.. 2019. Genes lost during the transition from land to water in cetaceans highlight genomic changes associated with aquatic adaptations. Sci Adv. 5(9):eaaw6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut RA, Beersma DGM.. 2011. Evolution of time-keeping mechanisms: early emergence and adaptation to photoperiod. Philos Trans R Soc Lond B Biol Sci. 366(1574):2141–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innan H, Kondrashov F.. 2010. The evolution of gene duplications: classifying and distinguishing between models. Nat Rev Genet. 11(2):97–108. [DOI] [PubMed] [Google Scholar]
- Janis CM.1989. A climatic explanation for patterns of evolutionary diversity in ungulate mammals. Palaeontology 32(3):463–481. [Google Scholar]
- Janis CM, Theodor JM.. 2014. Cranial and postcranial morphological data in ruminant phylogenetics. Zitteliana 32:15–31. [Google Scholar]
- Kappers JA.1983. Comparative gross and fine morphology of the mammalian pineal gland. In: Axelrod J, Fraschini F, Velo GP, editors. The pineal gland and its endocrine role. Boston: Springer. p. 37–59. [Google Scholar]
- Kim W, Park H, Seo S.. 2016. Global metabolic reconstruction and metabolic gene evolution in the cattle genome. PLoS One 11(3):e0150974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Roseboom PH, Coon SL.. 1996. New light is shining on the melatonin rhythm enzyme: the first postcloning view. Trends Endocrinol Metab. 7(3):106–112. [DOI] [PubMed] [Google Scholar]
- Klein DC.2007. Arylalkylamine N-acetyltransferase:“the Timezyme”. J Biol Chem. 282(7):4233–4237. [DOI] [PubMed] [Google Scholar]
- Klein DC.2006. Evolution of the vertebrate pineal gland: the AANAT hypothesis. Chronobiol Int. 23(1–2):5–20. [DOI] [PubMed] [Google Scholar]
- Klein DC, Weller JL.. 1970. Indole metabolism in the pineal gland: a circadian rhythm in N-acetyltransferase. Science 169(3950):1093–1095. [DOI] [PubMed] [Google Scholar]
- Kocher L, Brun J, Borson-Chazot F, Gonnaud PM, Claustrat B.. 2006. Increased REM sleep associated with melatonin deficiency after pinealectomy: a case study. Chronobiol Int. 23(4):889–901. [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Cajochen C, Pache M, Flammer J, Wirz-Justice A.. 2006. Thermoregulatory effects of melatonin in relation to sleepiness. Chronobiol Int. 23(1–2):475–484. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Suleski M, Hedges SB.. 2017. TimeTree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol. 34(7):1812–1819. [DOI] [PubMed] [Google Scholar]
- Kumar V.2017. Biological timekeeping: clocks, rhythms and behaviour. New Delhi: Springer. [Google Scholar]
- Lincoln GA.2006. Melatonin entrainment of circannual rhythms. Chronobiol Int. 23(1–2):301–306. [DOI] [PubMed] [Google Scholar]
- Lopes-Marques M, Ruivo R, Alves LQ, Sousa N, Machado AM, Castro LFC.. 2019. The singularity of Cetacea behavior parallels the complete inactivation of melatonin gene modules. Genes 10(2):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, Siegel JM.. 2008. Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav Rev. 32(8):1451–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DP, Murrell B, Golden M, Khoosal A, Muhire B.. 2015. RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol. 1(1):vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Rodger J, Blache D.. 2004. Nutritional and environmental effects on reproduction in small ruminants. Reprod Fertil Dev. 16(4):491–501. [DOI] [PubMed] [Google Scholar]
- McGowen MR, Spaulding M, Gatesy J.. 2009. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Mol Phylogenet Evol. 53(3):891–906. [DOI] [PubMed] [Google Scholar]
- McNab BK.1980. Energetics and the limits to a temperate distribution in armadillos. J Mammal. 61(4):606–627. [Google Scholar]
- Meredith RW, Janečka JE, Gatesy J, Ryder OA, Fisher CA, Teeling EC, Goodbla A, Eizirik E, Simão TLL, Stadler T, et al. 2011. Impacts of the Cretaceous Terrestrial Revolution and KPg extinction on mammal diversification. Science 334(6055):521–524. [DOI] [PubMed] [Google Scholar]
- Meslin C, Brimau F, Nagnan-Le Meillour P, Callebaut I, Pascal G, Monget P.. 2011. The evolutionary history of the SAL1 gene family in Eutherian mammals. BMC Evol Biol. 11(1):148–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbrugger V, Utescher T, Dilcher DL.. 2005. Cenozoic continental climatic evolution of Central Europe. Proc Natl Acad Sci U S A. 102(42):14964–14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander J.2004. MrModeltest v2. Program distributed by the author. Uppsala (Sweden: ): Uppsala University, Evolutionary Biology Centre. [Google Scholar]
- Ogutu JO, Piepho H-P, Dublin HT.. 2014. Reproductive seasonality in African ungulates in relation to rainfall. Wildl Res. 41(4):323–342. [Google Scholar]
- Panin M, Gabai G, Ballarin C, Peruffo A, Cozzi B.. 2012. Evidence of melatonin secretion in cetaceans: plasma concentration and extrapineal HIOMT-like presence in the bottlenose dolphin Tursiops truncatus. Gen Comp Endocrinol. 177(2):238–245. [DOI] [PubMed] [Google Scholar]
- Paulin C-H, Cazaméa-Catalan D, Zilberman-Peled B, Herrera-Perez P, Sauzet S, Magnanou E, Fuentès M, Gothilf Y, Muñoz-Cueto JA, Falcón J, et al. 2015. Subfunctionalization of arylalkylamine N‐acetyltransferases in the sea bass Dicentrarchus labrax: two‐ones for one two. J Pineal Res. 59(3):354–364. [DOI] [PubMed] [Google Scholar]
- Pavlicek J, Coon SL, Ganguly S, Weller JL, Hassan SA, Sackett DL, Klein DC.. 2008. Evidence that proline focuses movement of the floppy loop of arylalkylamine N-acetyltransferase (EC 2.3. 1.87). J Biol Chem. 283(21):14552–14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevet P, Challet E.. 2011. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris. 105(4–6):170–182. [DOI] [PubMed] [Google Scholar]
- Prothero DR, Foss SE.. 2007. The evolution of artiodactyls. Baltimore (MD: ): Johns Hopkins University Press. [Google Scholar]
- Ralph CL.1975. The pineal gland and geographical distribution of animals. Int J Biometeorol. 19(4):289–303. [DOI] [PubMed] [Google Scholar]
- Revell LJ.2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol. 3(2):217–223. [Google Scholar]
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP.. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RoseboomPH, , NamboodiriM, , ZimonjicDB, , PopescuNC, , R. RodriguezI, , GastelJA, , Klein DC.. 1998. Natural melatonin `knockdown' in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Mol Brain Res. 63(1):189–197. [DOI] [PubMed] [Google Scholar]
- Saarela S, Reiter RJ.. 1994. Function of melatonin in thermoregulatory processes. Life Sci. 54(5):295–311. [DOI] [PubMed] [Google Scholar]
- Saha S, Singh KM, Gupta BBP.. 2019. Melatonin synthesis and clock gene regulation in the pineal organ of teleost fish compared to mammals: similarities and differences. Gen Comp Endocrinol. 279:27–34. [DOI] [PubMed] [Google Scholar]
- Sato JJ, Wolsan M, Minami S, Hosoda T, Sinaga MH, Hiyama K, Yamaguchi Y, Suzuki H.. 2009. Deciphering and dating the red panda’s ancestry and early adaptive radiation of Musteloidea. Mol Phylogenet Evol. 53(3):907–922. [DOI] [PubMed] [Google Scholar]
- Scherler L, Lihoreau F, Becker D.. 2019. To split or not to split Anthracotherium? A phylogeny of Anthracotheriinae (Cetartiodactyla: Hippopotamoidea) and its palaeobiogeographical implications. Zool J Linn Soc-Lond. 185(2):487–510. [Google Scholar]
- SharmaR, , SahotaP, , Thakkar MM.. 2018. Melatonin promotes sleep in mice by inhibiting orexin neurons in the perifornical lateral hypothalamus. J Pineal Res. 65(2):e12498. [DOI] [PubMed] [Google Scholar]
- Sharma V, Hecker N, Roscito JG, Foerster L, Langer BE, Hiller M.. 2018. A genomics approach reveals insights into the importance of gene losses for mammalian adaptations. Nat Commun. 9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Lehmann T, Stuckas H, Funke L, Hiller M.. 2018. Loss of RXFP2 and INSL3 genes in Afrotheria shows that testicular descent is the ancestral condition in placental mammals. PLoS Biol. 16(6):e2005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogland T.1989. Comparative social organization of wild reindeer in relation to food, mates and predator avoidance. Berlin (Germany: ): Paul Parey. [Google Scholar]
- Song S, Liu L, Edwards SV, Wu S.. 2012. Resolving conflict in eutherian mammal phylogeny using phylogenomics and the multispecies coalescent model. Proc Natl Acad Sci U S A. 109(37):14942–14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Van Oort BE, Tyler NJ, Loudon AS.. 2007. Adaptations for life in the Arctic: evidence that melatonin rhythms in reindeer are not driven by a circadian oscillator but remain acutely sensitive to environmental photoperiod. J Pineal Res. 43(3):289–293. [DOI] [PubMed] [Google Scholar]
- Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A.. 2018. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 4(1):vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ.. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44(W1):W232–W235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi AK, Malik S, Rani S, Kumar V.. 2016. Pinealectomy abolishes circadian behavior and interferes with circadian clock gene oscillations in brain and liver but not retina in a migratory songbird. Physiol Behav. 156:156–163. [DOI] [PubMed] [Google Scholar]
- Upham NS, Esselstyn JA, Jetz W.. 2019. Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17(12):e3000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utiger RD.1992. Melatonin-the hormone of darkness. N Engl J Med. 327(19):1377–1379. [DOI] [PubMed] [Google Scholar]
- UzT, , Manev H.. 2001. Prolonged swim-test immobility of serotonin N-acetyltransferase (AANAT)-mutant mice. J Pineal Res. 30(3):166–170. [DOI] [PubMed] [Google Scholar]
- Vislobokova I.2008. The oldest representative of Entelodontoidea (Artiodactyla, Suiformes) from the Middle Eocene of Khaichin Ula II, Mongolia, and some evolutionary features of this superfamily. Paleontol J. 42(6):643–654. [Google Scholar]
- Wildman DE, Uddin M, Liu G, Grossman LI, Goodman M.. 2003. Implications of natural selection in shaping 99.4% nonsynonymous DNA identity between humans and chimpanzees: enlarging genus Homo. Proc Natl Acad Sci U S A. 100(12):7181–7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburne MO.2004. Late Cretaceous and Cenozoic mammals of North America: biostratigraphy and geochronology. New York: Columbia University Press. [Google Scholar]
- Yang Z.2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 24(8):1586–1591. [DOI] [PubMed] [Google Scholar]
- Yang Z, Rannala B.. 2006. Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Mol Biol Evol. 23(1):212–226. [DOI] [PubMed] [Google Scholar]
- Yerushalmi S, Green RM.. 2009. Evidence for the adaptive significance of circadian rhythms. Ecol Lett. 12(9):970–981. [DOI] [PubMed] [Google Scholar]
- Yu W, Wu Y, Yang G.. 2014. Early diversification trend and A sian origin for extent bat lineages. J Evol Biol. 27(10):2204–2218. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang YP, Rosenberg HF.. 2002. Adaptive evolution of a duplicated pancreatic ribonuclease gene in a leaf-eating monkey. Nat Genet. 30(4):411–415. [DOI] [PubMed] [Google Scholar]
- Zilberman-Peled B, Benhar I, Coon SL, Ron B, Gothilf Y.. 2004. Duality of serotonin-N-acetyltransferase in the gilthead seabream (Sparus aurata): molecular cloning and characterization of recombinant enzymes. Gen Comp Endocrinol. 138(2):139–147. [DOI] [PubMed] [Google Scholar]
- Zilberman-Peled B, Bransburg-Zabary S, Klein DC, Gothilf Y.. 2011. Molecular evolution of multiple arylalkylamine N-acetyltransferase (AANAT) in fish. Mar Drugs. 9(5):906–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurano JP, Magalhães FM, Asato AE, Silva G, Bidau CJ, Mesquita DO, Costa GC.. 2019. Cetartiodactyla: updating a time-calibrated molecular phylogeny. Mol Phylogenet Evol. 133:256–262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are no data to be archived. All data were included in supplementary materials for readers to validate.