Abstract
This study investigated basal and reciprocal relationships between implicit power motivation (n Power), a preference for having impact and dominance over others, and both salivary estradiol and testosterone in women. 49 participants completed the Picture Story Exercise, a measure of n Power. During a laboratory contest, participants competed in pairs on a cognitive task and contest outcome (win vs. loss) was experimentally varied. Estradiol and testosterone levels were determined in saliva samples collected at baseline and several times post-contest , including one day post-contest. n Power was positively associated with basal estradiol concentrations. The positive correlation between n Power and basal estradiol was stronger in single women, women not taking oral contraceptives, or for women with low-CV estradiol samples than in the overall sample of women. Women's estradiol responses to a dominance contest were influenced by the interaction of n Power and contest outcome: Estradiol increased in power-motivated winners but decreased in power-motivated losers. For power-motivated winners, elevated levels of estradiol were still present the day after the contest. Lastly, n Power and estradiol did not correlate with self-reported dominance and correlated negatively with self-reported aggression. Self-reported dominance and aggression did not predict estradiol changes as a function of contest outcome. Overall, N Power did not predict basal testosterone levels or testostosterone changes as a function of dominance contest outcome.
Keywords: Salivary estradiol, Salivary testosterone, Implicit power motivation, Dominance, Women, Contest, Aggression, Questionnaires, Measurement Error, Oral Contraceptives, Relationship Status
Introduction
In mammals and many non-mammalian species, estradiol has well-documented effects on reproductive physiology, behavior, learning, and memory (Beach 1981, Becker et al. 2002). It has also been suggested that it plays a role in dominance behaviors ranging from dominant posturing to physical aggression, particularly in primates (Michael & Zumpe 1993, Zumpe & Michael 1989). But, with few exceptions, its role in female dominance in humans remains largely unexplored (Cashdan 2003). A facilitating role of testosterone in human dominance, on the other hand, has been documented extensively for men (Mazur & Booth 1998). But, as Mazur and Booth (1998) have pointed out, research findings have not documented a consistent role for testosterone in women's dominance striving (see also Bateup et al. 2002, Booth & Dabbs 1995, Cashdan 1995, Gladue 1991, Kemper 1990, Kivlighan et al. 2005, Purifoy & Koopmans 1979). In the present research, we therefore tested the assumption that estradiol plays a critical role for women's dominance motivation, just as testosterone has been shown to do for men's dominance motivation.
More specifically, we explored the relationship between estradiol and the degree to which women have a nonconscious preference for dominance (implicit need for power motivation, or n Power). Implicit power motivation, a personality measure of dominance in humans, is defined as an enduring preference for having impact on and dominating others (Schultheiss 2001, Winter 1973). From a motivational perspective, women high in n Power are more likely to be aroused by affectively charged cues associated with an opportunity to have dominance, to engage in dominance behaviors once aroused by the predictive cue, and to be rewarded by having impact or dominance (Schultheiss et al. 2005a, 2005b). n Power is implicit in the sense that it operates outside of individuals’ conscious awareness, is assessed indirectly with the Picture Story Exercise (PSE) (Smith 1992), and does not correlate with self-report measures of dominance (McClelland 1987, McClelland et al. 1989, Schultheiss, in press). In numerous studies, the PSE measure of n Power has been found to predict individuals’ testosterone, cortisol, and norepinephrine responses to a variety of dominance challenges and outcomes (McClelland 1982, McClelland et al. 1980, 1985, Schultheiss & Rohde 2002, Schultheiss et al. 2004, Schultheiss et al. 2005a, Wirth et al. 2006).
In exploring the relationship between n Power and estradiol in women, we examined both basal and dynamic relationships between these measures. Regarding the basal relationship, we expected n Power and estradiol to be positively correlated. If high levels of estradiol facilitate a greater preference for dominance, this may also facilitate greater access to mates. High or rising levels of estradiol have also been shown to increase sexual motivation and activity (Adams et al. 1978, Grammer et al. 2004, Haselton et al. 2007, Udry & Morris 1968). The combination of estradiol-facilitated dominance preference and sexual motivation could potentially lead to greater reproductive success. Preliminary support for this hypothesis comes from Schultheiss, Dargel, and Rohde (2003a), who reported a positive relationship between menstrual-cycle-phase estradiol and n Power in single women. Although this relationship is consistent with the hypothesis that estradiol is linked to n Power in women, it requires replication.
Moreover, despite the fact that oral contraceptives reduce endogenous estradiol levels, Schultheiss et al. (2003a) failed to find an effect of oral contraceptive use on the relationship between n Power and estradiol, perhaps due to small sample size. However, other studies examining the link between estradiol and behavioral measures of dominance report a stronger correlation between estradiol and outcome measures for normally cycling women than for women using oral contraceptives (e.g. Grammer et al. 2004). We therefore expected the hypothesized basal relationship between n Power and estradiol to be stronger for normally cycling women than for women on the pill.
Finally, because free estradiol concentrations in saliva and serum are extremely low, measurement error tends to be higher than for other steroid hormones, with average intra- and inter-assay CV's in the 10 to 20% range (Lipson & Ellison 1996, Schultheiss et al. 2003a, Yang et al. 2004). Because measurement error can attenuate the true relationship between two variables, we also examined whether the hypothesized relationship between n Power and basal estradiol would be stronger for saliva samples with low measurement error than for samples with high measurement error.
In the present research, we also examined for the first time dynamic relationships between n Power and estradiol in humans, using a dominance-contest paradigm that has been exploited successfully in studies on the relationship between dominance and testosterone in human males (e.g., Mazur & Booth, 1998; Schultheiss & Rohde, 2002). Research on testosterone has shown that its relationship with dominance is not restricted to a basal correlation, but testosterone concentrations also change as a function of dominance interactions and contests, with winners showing increases, and losers decreases, in testosterone (Booth et al. 1989, Mazur 1985, Rose et al. 1975). Often, post-contest hormone changes also depend on an individual's motivation to attain dominance (Sapolsky 1987). In men and women, testosterone and cortisol post-dominance contest changes are the function of an interaction between the individual's n Power and the contest outcome (Schultheiss et al. 2005a, Wirth et al. 2006; cf. Josephs et al., 2006, for related findings). For men, power-motivated winners have increases in post-contest testosterone, whereas power-motivated losers have decreases in testosterone. However, this pattern does not hold for women. Among female participants, both power-motivated winners and losers shows increases in testosterone, a finding that Schultheiss et al. (2005a) explained as a by product of adrenal activation during the contest.
Extrapolating the hypothesis that estradiol is critically involved in power motivation in women, we hypothesized that in power-motivated women, estradiol changes induced by the outcome of a dominance contest outcome would mirror those of testosterone in power-motivated men. We reasoned that surging levels of estradiol after a dominance victory will prime the power-motivated individual to further assert her dominance, whereas falling estradiol levels after a defeat may serve to transiently decrease dominance motivation and thereby the likelihood of further losses (for similar arguments related to the functional role of contest-induced testosterone changes see Mazur 1985). We therefore expected that n Power would predict estradiol increases in dominance contest winners and estradiol decreases in dominance contest losers. In men, power-motivation-dependent effects of dominance contests on testosterone changes are observable 15 minutes post-contest, but not before or after (Schultheiss 2007). To explore whether similar time-course changes can be observed in women's post-contest estradiol levels, we measured salivary estradiol repeatedly after the contest, including one measurement the day after the contest.
As Mazur and Booth (1998) have pointed out, self-report measures of dominance motivation frequently fail to predict testosterone levels and testosterone-related outcomes in men. In contrast, indirect measures of dominance motivation like the PSE n Power measure reliably predict both basal testosterone levels and testosterone responses to dominance contest outcomes in men (Schultheiss 2007). In the present research, we explored whether this dissociation between self-report and indirect measures of dominance motivation also holds for the hypothesized link between dominance motivation and estradiol in women.
Finally, we also chose to assay salivary testosterone to test discriminant effects of n Power on basal estradiol and testosterone as well as changes in estradiol and testosterone as a function of dominance contest outcome. The inclusion of both testosterone and estradiol provides a more complete picture of the basal and dynamic relationships between the putative dominance hormones and n Power in women.
Methods
Subjects
Data were collected from a subsample of 53 female graduate and undergraduate students at the University of Michigan (19.96 ± 0.27 years old) who participated in a larger study on the effects of power motivation on hormonal responses to dominance contests (see Wirth, Welsh, & Schultheiss (2006, Study 2) for details on the full sample and findings related to contest-dependent cortisol changes). Four participants’ data were incomplete and were omitted from the analysis; hence, N = 49 for all analyses. Students majoring in Psychology were not allowed to participate. On average, participants reported to be 17.47 days past the onset of their last menstruation. Fourteen women reported taking hormonal oral contraceptives. Participants refrained from eating and oral hygiene for at least one hour prior to the start of the study.
Design
For the following analyses, basal salivary estradiol, basal salivary testosterone, post-contest changes in salivary estradiol, and post-contest changes in salivary testosterone were the dependent variables and implicit power motivation, experimentally-varied contest outcome (win versus lose), and self-reported needs for dominance and aggression were the independent variables.
Procedure
Sessions were run by a single male or female experimenter. As part of hypotheses unrelated to those reported here, participants were administered, in a double-blind fashion, 200 mg caffeine or placebo (vitamin C) at the beginning of the study. Caffeine condition did not have an effect on any of the analyses reported here. In the pre-contest phase, participants provided a saliva sample (T1, at 0 min), then completed a PSE (25 min duration), a questionnaire containing dominance and aggression scales, and other tasks. Next, the experimenter announced that participants would compete against each other in a contest based on a serial response task (SRT), described below. Participants then provided a second saliva sample (T2, at 52 min), listened to a 10-min tape-recorded goal imagery exercise vividly describing the course of the ensuing contest from the winner's perspective (cf. Schultheiss 2001), and provided a third saliva sample (T3, at 64 min) while working on another task.
During the subsequent contest phase, participants competed against each other on 10 rounds of the SRT, with a total duration of 10 min. The SRT required participants to quickly and accurately respond to asterisks (*) presented sequentially in four different screen positions by pressing one of four response keys mapped to those screen positions. The experimenter explained to participants that after each round, the computers would calculate their performance scores based on their speed and accuracy on the SRT and then compare their results to determine the winner of a round. Each round started with a screen announcing the round number, followed by a countdown. Participants then worked on the SRT for 50 sec. After that, they saw a black screen featuring the words “Calculating and comparing scores...” for 2 sec, followed by either a green screen with the words “You have won this round” and accompanied by a jubilant jingle or a red screen with the words “You have lost this round” and accompanied by a low-frequency snarling tone for 2 sec, followed by a blank screen that retained the color of the feedback screen (3 sec). Participants in the winning condition “won” all rounds except for the second and the fifth, and participants in the losing condition correspondingly “lost” all rounds except for the second and the fifth.
In the post-contest phase, participants collected fourth, fifth and sixth saliva samples (T4, at 78 min, 0 min post-contest; T5, at 93 min, 15 min post-contest; T6, at 108 min, 30 min post-contest) while completing other tasks unrelated to the results reported here. Finally, they completed a background-data questionnaire, and a suspicion check in the form of an open-ended questionnaire asking for anything they had noted about the study. Participants returned the following day and provided a seventh saliva sample (T7). No participants demonstrated awareness that the contest outcome was experimentally manipulated. Participants were paid for their participation and fully debriefed about the study's hypotheses and manipulations at the end of the second session.
Implicit Power Motivation (n Power)
Implicit power motivation was assessed with the PSE with instructions specified by Schultheiss and Pang (2007). Participants were given five minutes to write creative stories in response to pictures. Five pictures were chosen for the PSE stories: women in laboratory, ship captain, boxer, protester hurling a stone, and bicycle race (for further description of these pictures see Schultheiss & Pang, 2007). PSE stories were coded for motivation Power in accordance with Winter's (1994) Manual for Scoring Motive Imagery in Running Text by a trained coder. The themes in participant's PSE stories coded for implicit power motivation include strong, forceful actions that have impact over others, controlling others, influencing or persuading others, offering unsolicited help or advice, impressing others, fame, prestige, reputation, and actions that elicit a strong emotional response in others. The trained coder had reached 85% agreement with expert coding in the Winter Manual training materials. On average when added together, participants’ five PSE stories were 553 ± 17 words long and contained 5.62 ± 0.33 power images. PSE word count was significantly correlated with n Power scores (p <.05) and were therefore corrected for PSE word count through regression. Power motive residuals were converted to z scores, which were used in subsequent analysis.
Self-reported dominance and aggression
We measured self-reported needs for dominance and aggression using the correspondingly named scales from the Personality Research Form (PRF, Form E; Jackson 1984), a self-report inventory of motivational needs. Each scale had 16 likert-scaled items, and each scale had 8 reverse-coded items. “I have been known to fly into a rage if things didn't go as I had planned” is an example item from the aggression scale and “I seldom feel like hitting anyone” is an example of a reverse-coded item from the aggression scale. “I would like to be an executive with power over others” is an example item from the dominance scale and “I avoid positions of power over other people” is an example of a reverse-coded item from the dominance scale. These scales were chosen for their similarity to the coding categories for n Power, enabling us to directly compare implicit and explicit measures of dominance motivation in our analyses. In validation samples, both the dominance and aggression scales showed satisfactory internal consistency, Cronsbach's αs = 0.67 and 0.63, respectively (Jackson 1984).
Salivary sampling
For each of the seven salivary samples participants provided, participants used a stick of sugar-free chewing gum to collect up to 7.5 ml saliva in a sterile polypropylene vial and then discarded the chewing gum (Dabbs 1991). Participants sealed the vials immediately after each collection. The experimenter placed the vials in frozen storage immediately after the experimental session was complete. Samples were freed from mucopolysaccarides and other residuals by three freeze thaw cycles followed by centrifugation. Salivary 17ß-Estradiol levels were assessed with solid-phase Coat-A-Count 125I radioimmunoassays for 17ß-Estradiol (TKE2) provided by Diagnostic Products Corporation (Los Angeles).
Salivary estradiol
To determine salivary estradiol concentrations, we prepared water-based 1:80 dilutions of all standards (with a resulting range of 0.625 to 20 pg/mL) and controls (see Schultheiss et al. 2003a, for validation data). 800 uL of the saliva samples, standards, and controls were pipetted into antibody-coated tubes and allowed to incubate overnight. Next, 1 ml radiotracer was added to each tube and allowed to incubate overnight. Finally, tubes were aspirated and counted for 3 minutes. Assay reliability was evaluated by including control samples with known hormone concentrations in each assay (Bio-Rad Lyphochecks from Bio-Rad Laboratories, Hercules, CA). The assay manufacturer documents that its assay does not cross-react with estrogens in oral contraceptives. Analytical sensitivity (B0 −3 SD) was at 0.20 pg/mL. Analytical recovery was 91.67% for low (2.25 pg/mL) and 107.33% for high (5.14 pg/mL) estradiol levels. Inter-assay CV for these measurements was 5.07% and 5.11%, respectively. Participants’ seven saliva samples were counted in duplicate and had a mean intra-assay CV of 24.62%.
Salivary testosterone
To determine salivary testosterone concentrations, we prepared water-based dilutions of all standards (with a resulting range of 5 to 400 pg/mL) and controls. 400 uL of the saliva samples, standards, and controls were pipetted into antibody-coated tubes and allowed to incubate overnight. Next, 1 ml radiotracer was added to each tube and allowed to incubate overnight. Finally, tubes were aspirated and counted for 3 minutes. Assay reliability was evaluated by including control samples with known hormone concentrations in each assay (Bio-Rad Lyphochecks from Bio-Rad Laboratories, Hercules, CA). Analytical sensitivity (B0 −3 SD) was at 2.44 pg/mL. Analytical recovery was 113.41% for low (8.20 pg/mL) and 92.44% for high (18.20 pg/mL) testosterone levels. Inter-assay testosterone CV was 13.02% for saliva pools collected from female participants. Participants’ seven saliva samples were counted in duplicate and had a mean intra-assay CV of 12.29%.
Data Analysis
SYSTAT 10.0 statistical software was used for all analyses, including regression. Descriptive statistics are shown as mean (± SEM). See Table 1 for sample characteristics of n Power, PRF scales, salivary estradiol, salivary testosterone, and age.
Table 1.
Sample characteristics for n Power (word-count corrected z scores), PRF dominance scale, PRF aggression scale, salivary estradiol (in pg/mL), salivary testosterone (in pg/mL), and age (in years)
| Mean | SEM | CV | |
|---|---|---|---|
| n Power | 0.00 | 0.14 | |
| PRF Aggression | 7.73 | 0.34 | |
| PRF Dominance | 9.60 | 0.64 | |
| Salivary estradiol | |||
| T1 | 2.08 | 0.22 | 21.92% |
| T2 | 2.01 | 0.22 | 27.41% |
| T3 | 1.88 | 0.21 | 24.29% |
| T4 | 2.03 | 0.25 | 20.45% |
| T5 | 1.99 | 0.24 | 25.07% |
| T6 | 2.20 | 0.28 | 18.60% |
| T7 | 2.25 | 0.28 | 24.70% |
| Salivary testosterone | |||
| T1 | 19.62 | 1.69 | 10.90% |
| T2 | 15.01 | 1.21 | 12.98% |
| T3 | 15.36 | 1.13 | 13.49% |
| T4 | 15.75 | 1.15 | 13.16% |
| T5 | 15.42 | 1.18 | 13.44% |
| T6 | 14.87 | 1.06 | 12.08% |
| T7 | 20.40 | 1.35 | 9.97% |
| Age | 19.96 | 0.27 |
Results
Estradiol measurement stability at times 1 & 2
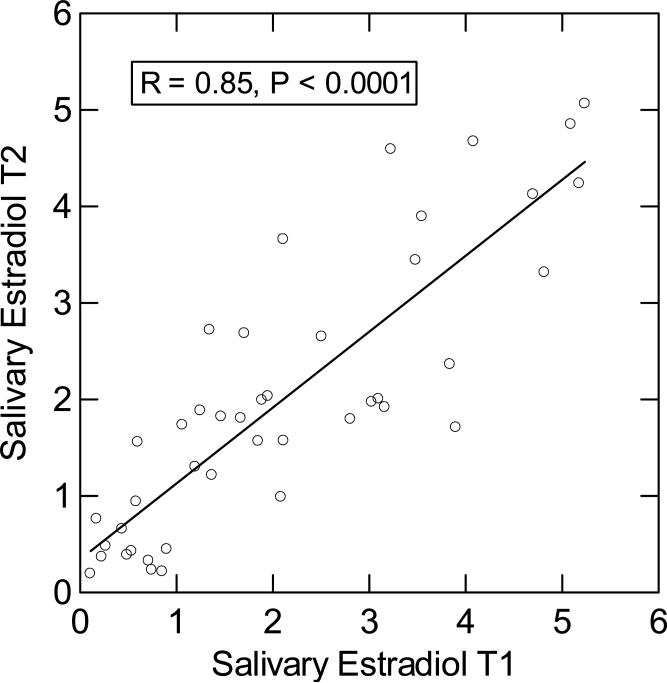
Due to the high average intra-assay CV, we first determined the stability of the estradiol measurements for the 43 participants that had samples at both T1 and T2. We found a significant and highly positive correlation between the first two estradiol measurements (see Figure 1), suggesting high retest stability of our estradiol measure. Given this high stability, we averaged the first two estradiol measurements to increase reliability for the basal estradiol analysis. Participants’ mean basal estradiol concentration (average T1 and T2) was 2.02 ± 0.33 pg/mL.
Figure 1.
Correlation of basal salivary estradiol measurements at T1 (0 min) and T2 (52 min).
Regression of n Power and basal estradiol
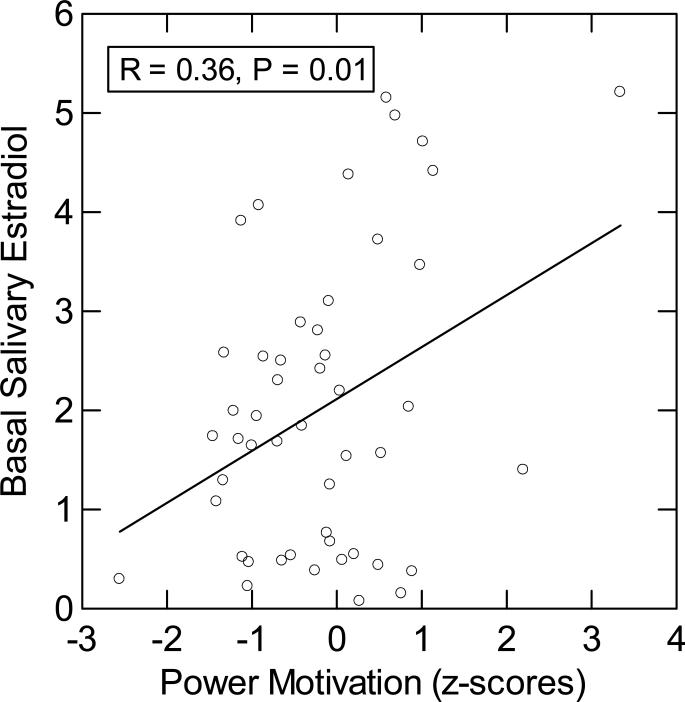
A regression of estradiol on n Power scores revealed a highly significant effect of n Power on basal estradiol (see Figure 2). In this analysis of all 49 participants, we included six participants for whom we had data from only one saliva sample due to insufficient saliva volume on the other.
Figure 2.
Correlation between n Power and basal salivary estradiol (average of T1 and T2).
Testing effects of CV levels on the nPower-estradiol correlation
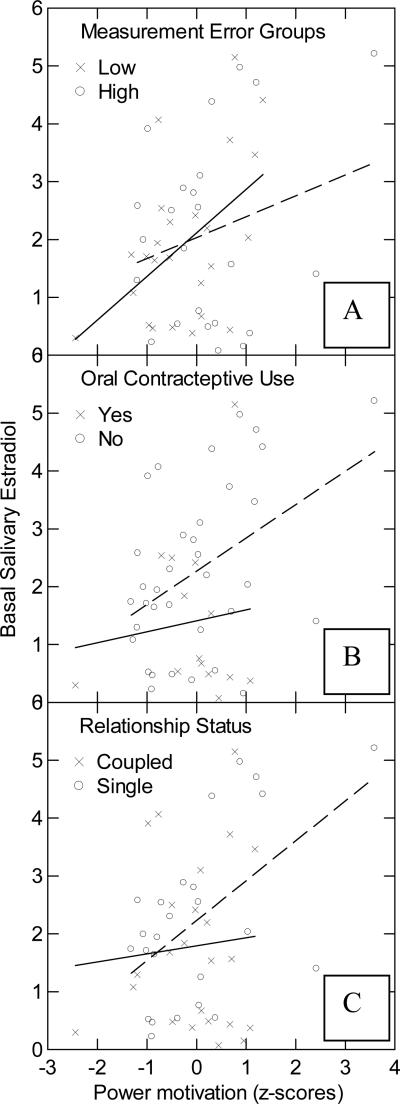
Next, we split the sample at the median average coefficient of variation (CV) for basal estradiol into a low-CV (10.22% ± 1.06) and a high-CV group (40.92% ± 5.43) to examine the effect of measurement error on the association between n Power and estradiol. The regression of estradiol on n Power scores for the low-CV group showed a stronger correlation with n Power (R = 0.50, P = 0.01, N = 25) than for the high-CV group (R = 0.24, ns, N = 24; see Figure 3, Panel A). This finding indicates that high measurement error can attenuate the association between n Power and estradiol.
Figure 3.
Correlations between n Power and basal salivary estradiol (average of T1 and T2). Panel A depicts the correlations for groups of high (dashed line) and low (solid line) estradiol measurement error (CV). Panel B depicts the correlations for women who do (solid line) and do not (dashed line) use oral contraceptives. Panel C depicts the correlations for the single women (dashed line) and for women in close relationships (solid line).
Testing effects of oral contraceptives on the nPower-estradiol correlation
Analysis of hormonal contraceptive use showed that the positive relationship between n Power and estradiol is stronger in normally cycling women (R = 0.44, P = 0.009, N = 35) than in women who are taking hormonal contraceptives (R = 0.12, P = ns, N = 14; see Fig. 3, Panel B).
Testing effects of relationship status on the nPower-estradiol correlation
Schultheiss and colleagues (2003a) reported a significant positive relationship between n Power and estradiol in single women, but not in women in close relationships. This study replicated their finding. When grouping the women into those who were in close relationships and those who were not, we found that there is a highly significant, positive correlation between n Power and estradiol for single women (R = 0.55, P = 0.004, N = 25), but not for women in a close relationship (R = 0.08, ns, N = 24; see Figure 3, Panel C). In this sample, women in a close relationship were significantly more likely to use oral contraceptives (χ2(1, N = 49) = 6.87, p < 0.01).
Testing effects of contest outcome and n Power on estradiol changes
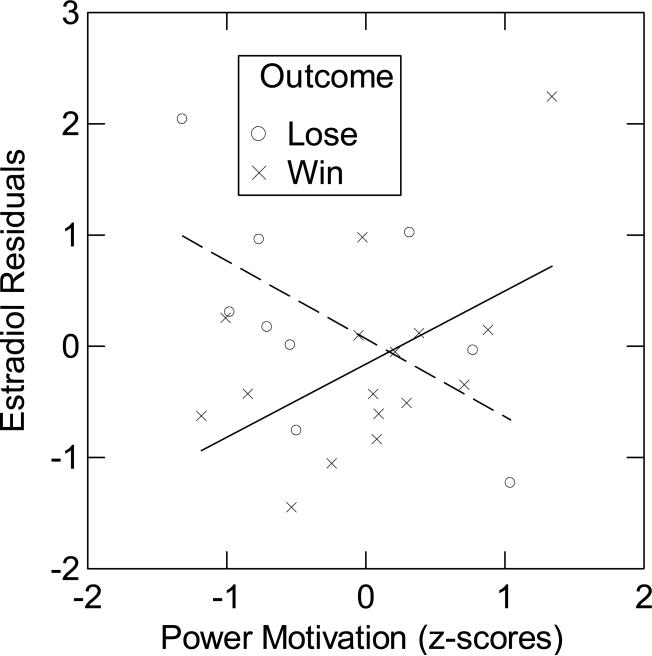
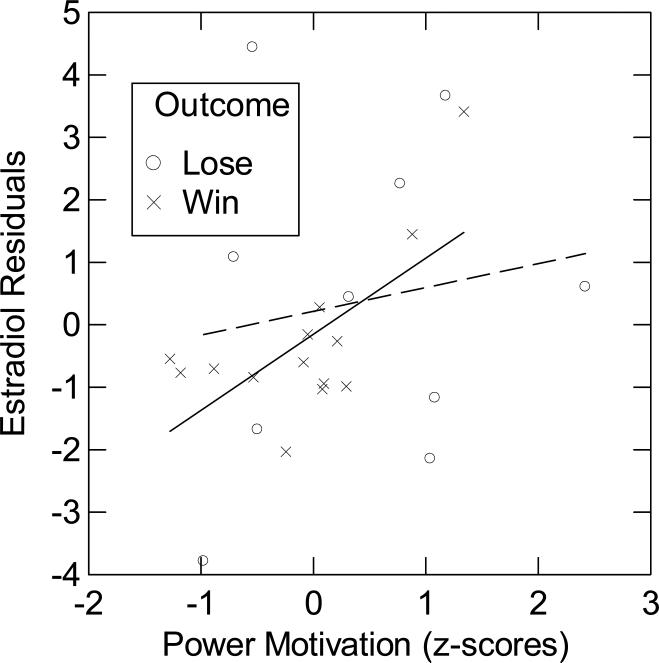
Because measurement error was an important factor in the correlation between n Power and basal salivary estradiol, we continued to divide the sample into high CV and low CV groups for the dominance contest analysis to see if measurement error continued play a role in the relationship between n Power and estradiol. We split participants into two groups at the median of the averaged CV of combined pre-contest (T3) and post-contest (T4, T5, T6) estradiol samples. Examining the low-CV group (12.47% ± 0.70) using ANCOVA, we did not find significant main effects of n Power or contest outcome, Ps < 0.10. But there was a highly significant interaction of n Power and contest outcome on residualized average estradiol levels (variance in post-contest estradiol after controlling for pre-contest estradiol) (B = 1.38, SE = 0.48, t (1,20) = 2.84 , P = 0.01). The within-subjects, Contest Outcome × n Power × Time interaction on estradiol residuals was not significant documenting that the effect is not driven by differential changes across post-estradiol levels. This allowed us to average the three post-contest estradiol measurements (T4, T5, T6) (2.64 pg/mL ± 0.29). For winners, the positive correlation between n Power and residualized average estradiol was significant (R = 0.35, P = 0.05, N = 16). For losers, we found a negative correlation between n Power and estradiol residuals that approached the level of a trend (R = −0.42, P = 0.125, N = 9; cf. Fig. 4). There were no effects for the high-CV group (32.30% ± 2.76; N = 24). Despite the fact that time was not a significant factor, we also ran the analyses for each post-contest time point. After controlling for pre-contest estradiol, the n Power × Contest Outcome interaction was significant at T4 (B = 1.25, SE = 0.43, t (1,20) = 2.93 , P < 0.01), T5 (B = 1.07, SE = 0.39, t (1,20) = 1.95 , P = 0.065), and T6 (B = 1.79, SE = 0.80, t (1,20) = 2.25 , P = 0.036). At each time point, the direction of the correlation between n Power and residualized estradiol for the groups of winners and losers was the same as in the analyses that aggregated over timepoints T4, T5, and T6. These findings suggest that the n Power × Contest Outcome effect on post-contest estradiol did not vary substantially from immediately after the contest to 30 min post-contest.
Figure 4.
Estradiol residuals (average post-contest estradiol (T4, T5, & T6) adjusted for pre-contest estradiol (T3)) as a function of n Power and contest outcome for participants in the low-CV group (N = 25). The contest losers are represented by the dashed line and the winners by the solid line.
For the analysis on estradiol changes as a result of the contest outcome from immediately before the contest (T3), to the day after the contest (T7), we split the sample at the averaged median CV of combined estradiol samples T3 and T7. Examining the group with low estradiol CV (14.91% ± 2.60; N = 24), there was still a significant, positive correlation in winners between n Power and the estradiol residuals (variance in day-after estradiol (T7) controlling for pre-contest estradiol (T3)) (R = 0.44, P = 0.02, N = 14; cf. Fig. 5). This effect is similar in direction and magnitude as the effect showing estradiol changes immediately post-contest. For losers, there was no relationship between n Power and estradiol residuals (R = 0.07, ns, N = 10). There were no day-after effects for the high-CV group (34.41% ± 3.59; N = 25).
Figure 5.
Estradiol residuals (day-after contest estradiol (T7) adjusted for pre-contest estradiol (T3)) as a function of n Power and contest outcome for participants in the low-CV group (N = 24). The contest losers are represented by the dashed line and the winners by the solid line.
Discriminant prediction of basal and dynamic estradiol concentrations by n Power and self-report measures of dominance
The last set of analyses, for which results are presented in Table 2, compared the validity of n Power against PRF questionnaire scales of dominance and aggression in predicting basal estradiol and contest-induced estradiol changes. The correlation between the questionnaire measure of dominance and basal estradiol was not significant. However, analyses did reveal a negative trend between the questionnaire scale of aggression and estradiol. We also examined the relationships between n Power and the scales and found that n Power was unrelated to the questionnaire measure of dominance, but that higher levels of n Power were significantly related to lower PRF aggression scores. Just as in the n Power analyses, we tried to predict changes in post-contest estradiol as a function of contest outcome and self-reported dominance or aggression for the low estradiol CV group. Using ANCOVA, neither the dominance (B = −0.48, SE = 0.87, t (1,20) = −1.35, ns) nor the aggression scales (B = −0.11, SE = 0.17, t (1,20) = −.68, ns), in interaction with contest outcome, significantly predicted post-contest estradiol residuals using pre-contest estradiol (T3) as a covariate.
Table 2.
Correlations between n Power (z scores), PRF dominace scale, PRF aggression scale, basal salivary estradiol (average of T1 and T2), and basal salivary testosterone (average of T1 and T2)
| n Power | PRF Aggression | PRF Dominance | Basal salivary estradiol | |
|---|---|---|---|---|
| n Power | - | |||
| PRF Aggression | −0.36*** | - | ||
| PRF Dominance | −0.21 | 0.19 | - | |
| Basal salivary estradiol | 0.36*** | −0.27* | 0.07 | - |
| Basal salivary testosterone | −0.04 | 0.07 | 0.08 | 0.44**** |
P-values
.10
*.05
.01
< .01
Testing basal and dynamic relationships between testosterone and n Power
To test associations between n Power and salivary testosterone in the same sample in which we had evaluated the associations between n Power and salivary estradiol, we yoked the analyses by using the same participant groups (divided by low versus high estradiol CV) for the testosterone analyses. Participants’ mean basal testosterone concentration (average T1 and T2) was 17.25 ± 1.38 pg/mL. We found a highly significant positive correlation between basal testosterone (T1 & T2) and basal estradiol (see Table 2). However, basal testosterone was not correlated with n Power (see Table 2). We found that there was not a significant relationship between n Power and basal testosterone for women with low estradiol CVs (R = 0.02, ns, N = 25) or women with high estradiol CVs (R = −0.20, ns, N = 24). For single women, we found that there was not a significant relationship between n Power and basal testosterone (R = 0.16, ns, N = 25), but that for women in close relationships, there was a trend negative correlation between basal testosterone and n Power (R = −0.39, P = .06, N = 24). For both normally-cycling women and women taking oral contraceptives, there was not a significant relationship between n Power and basal testosterone, R = −0.02, ns, N = 35 and R = −0.40, ns, N = 14, respectively. For women with low estradiol CVs, the interaction of n Power and contest outcome was not significant for averaged post-contest testosterone levels (B = 2.34, SE = 2.57, t (1,20) = 0.91, ns) and for day-after testosterone levels (B = 2.76, SE = 3.25, t (1,19) = 0.85, ns), after controlling for pre-contest testosterone in each case.
We also employed bi-partial correlation analysis, which measures the co-variation between post-contest (T4, T5, T6) estradiol and testosterone after controlling for pre-contest (T3) levels of estradiol and testosterone, to determine the relationship between post-contest estradiol changes and post-contest testosterone changes, independent of n Power. Post-contest estradiol and post-contest testosterone did not covary (R = −0.01, ns).
Discussion
Our hypothesis that women's n Power and basal estradiol levels would be positively correlated was confirmed. Across all participants, a higher nonconscious preference for dominance was associated with higher basal estradiol levels. This finding is consistent with research from the primate literature that documents a link between estradiol and dominance (Michael & Zumpe 1993, Zumpe & Michael 1989). It also replicates an earlier finding by Schultheiss et al. (2003a), who found a positive correlation between n Power and estradiol. However, like in that earlier study, we found that the positive correlation is particularly strong in single women, but not in women engaged in close relationships.
Past research has linked high levels of n Power to sexual activity (McClelland 1975, Winter 1973), and this finding holds both for men and for women (Schultheiss et al. 2003b). Moreover, high or rising levels of estradiol have also been found to be associated with behavioral indicators of mate pursuit (Grammer et al. 2004, Haselton et al. 2007) and increased sexual activity in women (Adams et al. 1978, Udry & Morris 1968). We therefore speculate that the link between n Power and estradiol we observed in the present research may account for the greater likelihood of high-n Power women to engage in sexual activity. The difference between single women and women engaged in close relationships may suggest that in single women, n Power is closely aligned with estradiol's role in attracting a sexual partner (cf. Grammer et al. 2004), but that this link is less important for women who have already found a partner (for related findings from the literature on testosterone and relationship status in men, see Booth & Dabbs 1993, Gray et al. 2002, Mazur & Michalek 1998, McIntyre et al. 2006, van Anders & Watson 2006).
The current study also expanded upon past research by showing that, as expected, measurement error and oral contraceptive use are important factors to consider when examining the relationship between n Power and salivary estradiol. When we divided our sample at the median CV for the basal estradiol measurement, we found that the low-CV group showed a significant positive correlation between n Power and estradiol, while the high-CV group did not, suggesting that salivary estradiol measurement error can mask the association. A similar finding emerged from our analyses of dynamic relationships between n Power and estradiol in the context of a dominance contest: the hypothesized interaction between n Power and contest outcome on post-contest estradiol changes emerged only in the low-CV group, but not in the high-CV group. Finally, women with normal cycles had a stronger positive correlation between n Power and basal estradiol, suggesting that suppression of endogenous estradiol production might also mask the link between motivational dispositions and hormone levels. However, oral contraceptive use and relationship status are highly confounded in the present study, and further studies need to disentangle the respective effects of these variables.
Consistent with our prediction of a dynamic relationship between n Power and estradiol, we also found that estradiol changes after winning or losing a dominance contest depend on women's n Power. Winners high in n Power had post-contest increases in estradiol and losers high in n Power had decreases in estradiol, a result that closely resembles the pattern of testosterone responses in high-n Power men to victory and defeat in dominance contests (Schultheiss & Rohde, 2002; Schultheiss et al. 2005a). Moreover, elevated estradiol levels in high-n Power winners could even be documented one day after the contest. This latter finding parallels past work by Mazur and colleagues (1992), who reported that male winners of chess competitions showed elevated testosterone levels for weeks.
What is the mechanism driving the quick estradiol responses to the dominance contest we observed in high-n Power women in the present study? Our results do not appear to fit the adrenal-activation model proposed by Schultheiss and colleagues (2005a) to account for high-n Power women's testosterone responses to dominance contests (see introduction). Wirth et al. (2006) found in the larger sample from which we drew our female subject pool that high n Power predicts decreased cortisol after a contest victory and increased cortisol after a defeat, a pattern that is the exact opposite of the increased estradiol in high n Power winners and decreased estradiol in high n Power losers we observed in the present study. We therefore think it is unlikely that the changes in estradiol we documented in the present study represent a byproduct of adrenal steroid production.
One possible mechanism that may account for rapid situation-induced changes in gonadal steroid production has been proposed by Sapolsky (1987) for the case of male testosterone responses to dominance challenges. Sapolsky found that dominant baboons respond to a challenge by releasing norepinephrine, which stimulates testicular steroid release within minutes. In contrast, non-dominant baboons respond to similar challenges with increased cortisol levels, which inhibit steroid release from the testes equally quickly. While Sapolsky's model is very suitable for explaining the effects of n Power on men's testosterone responses to winning and losing a contest (Schultheiss 2007), the question to what extent sympathetic vs. HPA-axis responses to dominance challenges can have similar effects on the female ovaries and hence on estradiol release have, to our knowledge, not been explored so far. It is interesting to note, however, that estradiol changes could be under sympathetic control through direct vagal innervation and stimulation of the ovaries, which would allow rapid modulation of estradiol production (Gerendai & Halasz 2001). Moreover, consistent with our observation that in high-n Power individuals estradiol and cortisol change in opposite directions, gonadal steroid release is inversely related to adrenal activation (e.g. Viau 2002). It remains to be determined, though, whether the mechanisms involved in this inverse relationship can account for the rapid estradiol changes we observed in the present research.
Yet another possible mechanism is suggested by recent evidence that shows that social interactions, such as dominance contests, can rapidly modulate aromatase activity driving fast changes in estradiol concentrations in the CNS (Balthazart & Ball 2006). However, this effect takes place locally in target tissues of the brain and it remains unclear to what extent this effect could be detected peripherally (e.g., in saliva).
We also sampled salivary testosterone in our study to examine the degree to which testosterone and estradiol had similar effects. We found that testosterone and estradiol were positively related at baseline. However, testosterone was not related to n Power at baseline, and testosterone changes were not predicted by n Power in interaction with contest outcome. Thus, in this sample, estradiol was the hormone that was related to a preference for dominance, and estradiol was also the hormone that was responsive to dominance contest outcomes in interaction with individuals’ preference for dominance. Additionally, despite the baseline correlation between the two hormones, we found that changes in estradiol and testosterone as a function of the contest outcome were not related. Thus, our data suggest that the two hormones have non-overlapping functions with regard to dominance in women. Our data provide support for the hypothesis that estradiol may be more closely related to dominance in women than testosterone.
Finally, in the present research we also tested the hypothesis that implicit and self-report measures of dominance motivation are differentially associated with salivary estradiol measures. As hypothesized, n Power was not related to the self-report PRF dominance scale, and this self-report measure did not predict estradiol concentrations. Moreover, while n Power predicted estradiol changes in interaction with contest outcome, neither self-reported dominance or aggression predicted estradiol changes in interaction with contest outcome. These findings further corroborate the idea that implicit measures of dominance motivation are better predictors of endocrine processes than self-report measures of dominance. Schultheiss (2007) argued that the influence of sex steroids on motivation and behavior exists in many species without any need for conscious awareness and that conscious beliefs about one's motivational needs are not “read-outs” of, or identical with, the output of core motivational brain systems. Other research groups have also shown that even in humans, steroid hormones can cause physiological and behavioral changes outside of conscious awareness (VanHonk et al. 1999, 2001, 2005).
The negative correlation between estradiol and self-reported aggression replicates earlier research by Cashdan (2003) and Gladue (1991). However, in humans, aggression is typically not a socially acceptable outlet for the need for dominance. Winter (1988) reported that successful outlets for n Power in women are often subtle forms of dominance that are shaped by social responsibility, education, and socialization and that these expressions of n Power rarely include physical aggression. Moreover, in humans, aggressive behavior may reflect defensiveness and not dominance, and it is notable that in animals, defensive aggression is not dependent on circulating levels of gonadal steroids whereas dominant aggression is (cf. Albert et al., 1992; Schultheiss & Wirth, in press). The current findings, along with those of Cashdan (2003) and Gladue (1991), might point to the possibility that those high in n Power and estradiol have learned successfully to channel their dominance motivation into socially acceptable outlets and not into aggression.
To sum up, we have found that estradiol, but not testosterone, and a nonconscious need for dominance are positively related in women. This positive relationship is strongest in single women, women not taking oral contraceptives, and in the absence of high salivary estradiol measurement error. Additionally, we have documented for the first time that changes in estradiol after a dominance contest depend on the interplay between the outcome of the contest and individual's power motivation.
Acknowledgements
This research was supported by a National Institute of Mental Health grant (R03 MH63069-01/02) and the Rackham Predoctoral Fellowship from the University of Michigan. We are grateful to Baxter Allen, Nicolette Jones, and Kathrin Riebel for their assistance in data collection and processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DB, Gold AR, Burt AD. Rise in female-initiated sexual activity at ovulation and its suppression by oral contraceptives. New England Journal of Medicine. 1978;299(21):1145–1150. doi: 10.1056/NEJM197811232992101. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Jonik RH, Walsh ML. Hormone-dependent aggression in male and female rats: experiential, hormonal, and neural foundations. Neuroscience and Biobehavioral Reviews. 1992;16(2):177–192. doi: 10.1016/s0149-7634(05)80179-4. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends in Neurosciences. 2006;29(5):241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Bateup, Helen S, Booth A, Shirtcliff EA, Granger DA. Testosterone, cortisol, and women's competition. Evolution and Human Behavior. 2002;23:181–192. [Google Scholar]
- Beach FA. Historical origins of modern research on hormones and behavior. Hormones and Behavior. 1981;15:325–376. doi: 10.1016/0018-506x(81)90001-5. [DOI] [PubMed] [Google Scholar]
- Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. MIT Press; Cambridge: 2002. [Google Scholar]
- Booth A, Shelley G, Mazur A, Tharp G, Kittok R. Testosterone, and winning and losing in human competition. Hormones and Behavior. 1989;23:556–571. doi: 10.1016/0018-506x(89)90042-1. [DOI] [PubMed] [Google Scholar]
- Booth A, Dabbs JM. Testosterone and men's marriages. Social Forces. 1993;72:463–477. [Google Scholar]
- Booth A, Dabbs JM. Cortisone, testosterone, and competition among women. Pennsylvania State Press; University Park: 1993. [Google Scholar]
- Cashdan E. Hormones, sex, and status in women. Hormones and Behavior. 1995;29:354–366. doi: 10.1006/hbeh.1995.1025. [DOI] [PubMed] [Google Scholar]
- Cashdan E. Hormones and competitive aggression in women. Aggressive Behavior. 2003;29:107–115. [Google Scholar]
- Dabbs JM. Salivary testosterone measurements: collecting, storing, and mailing slaiva samples. Physiology and Behavior. 1991;49:815–817. doi: 10.1016/0031-9384(91)90323-g. [DOI] [PubMed] [Google Scholar]
- Gerendai I, Halasz B. Asymmetry of the neuroendocrine system. News in Physiological Science. 2001;16:92–95. doi: 10.1152/physiologyonline.2001.16.2.92. [DOI] [PubMed] [Google Scholar]
- Gladue BA. Aggressive behavioral characteristics, hormones, and sexual orientation in men and women. Aggressive Behavior. 1991;17:313–326. [Google Scholar]
- Grammer K, Renninger L, Fischer B. Disco clothing, female sexual motivation, and relationship status: is she dressed to impress? Journal of Sex Research. 2004;41:66–74. doi: 10.1080/00224490409552214. [DOI] [PubMed] [Google Scholar]
- Gray PB, Kahlenberg SM, Barrett ES, Lipson SF, Ellison PT. Marriage and fatherhood are associated with lower testosterone levels in males. Evolution and Human Behavior. 2002;23:193–201. [Google Scholar]
- Haselton MG, Mortezaie M, Pillsworth EG, Bleske-Rechek A, Frederick DA. Ovulatory shifts in human female ornamentation: Near ovulation, women dress to impress. Hormones and Behavior. 2007;51:40–45. doi: 10.1016/j.yhbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Jackson DN. Personality Research Form. 3rd ed. Sigma Assessment Systems, Inc.; Port Huron: 1984. [Google Scholar]
- Josephs RA, Sellers JG, Newman ML, Mehta PH. The mismatch effect: when testosterone and status are at odds. Journal of Personality and Social Psychology. 2006;90(6):999–1013. doi: 10.1037/0022-3514.90.6.999. [DOI] [PubMed] [Google Scholar]
- Kemper TD. Social structure and testosterone: explorations of the socio-bio-social chain. Rutgers University Press; New Brunswick: 1990. [Google Scholar]
- Kivlighan KT, Granger DA, Booth A. Gender differences in testosterone and cortisol response to competition. Psychoneuorendocrinology. 2005;30:58–71. doi: 10.1016/j.psyneuen.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Lipson SF, Eillison PT. Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Human Reproduction. 1996;11(10):2090–2096. doi: 10.1093/oxfordjournals.humrep.a019055. [DOI] [PubMed] [Google Scholar]
- Mazur A. A biosocial model of status in face-to-face primate groups. Social Forces. 1985;64:377–402. [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behavioral and Brain Sciences. 1998;21:353–397. [PubMed] [Google Scholar]
- Mazur A, Booth A, Dabbs J. Testosterone and chess competition. Social Psychology Quarterly. 1992;55:70–77. [Google Scholar]
- Mazur A, Michalek J. Marriage, divorce, and male testosterone. Social Forces. 1998;77:315–330. [Google Scholar]
- McClelland DC. The need for power, sympathetic activation, and illness. Motivation and Emotion. 1982;6:31–41. [Google Scholar]
- McClelland DC. Power: The inner experience. Irvington Publishers; New York: 1975. [Google Scholar]
- McClelland DC. Human Motivation. Cambridge University Press; New York: 1987. [Google Scholar]
- McClelland DC, Floor E, Davidson RJ, Saron C. Stressed power motivation, sympathetic activation, immune function, and illness. Journal of Human Stress. 1980;6:11–19. doi: 10.1080/0097840X.1980.9934531. [DOI] [PubMed] [Google Scholar]
- McClelland DC, Ross G, Patel V. The effect of an academic examination on salivary norepinephrine and immunoglobulin levels. Journal of Human Stress. 1985;11:52–59. doi: 10.1080/0097840X.1985.9936739. [DOI] [PubMed] [Google Scholar]
- McClelland DC, Koestner R, Weinberger, Joel How do self-attributed and implicit motives differ? Psychological Review. 1989;96:690–702. [Google Scholar]
- McIntyre M, Gangestad SW, Gray PB, Chapman JF, Burnham TC, O'Rourke MT, Thornhill R. Romantic involvement often reduces men's testosterone levels – but not always: the moderating role of extrapair sexual interest. Journal of Personality and Social Psychology. 2006;91:642–651. doi: 10.1037/0022-3514.91.4.642. [DOI] [PubMed] [Google Scholar]
- Michael RP, Zumpe D. A review of hormonal factors influencing the sexual and aggressive behavior of macaques. American Journal of Primatology. 1993;30:213–241. doi: 10.1002/ajp.1350300306. [DOI] [PubMed] [Google Scholar]
- Purifoy FE, Koopmans LH. Androstenedione, testosterone, and free testosterone concentration in women f various occupations. Social Biology. 1979;26:179–188. doi: 10.1080/19485565.1979.9988376. [DOI] [PubMed] [Google Scholar]
- Rose RM, Bernstein IS, Gordon T. Consequences of social conflict on plasma testosterone levels in rhesus monkeys. Psychosomatic Medicine. 1975;37:50–61. doi: 10.1097/00006842-197501000-00006. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, social status, and reproductive physiology in free-living baboons. In: Crews D, editor. Psychobiology and reproductive behavior: An evolutionary perspective. Prentice Hall; Englewood Cliffs: 1987. pp. 291–322. [Google Scholar]
- Schultheiss OC. An information processing account of implicit motive arousal. In: Maehr ML, Pintrich P, editors. Advances in motivation and achievement. Vol. 12. JAI Press; Greenwich: 2001. pp. 1–41. New directions in measures and methods. [Google Scholar]
- Schultheiss OC. A biobehavioral model of implicit power motivation: arousal, reward, and frustration. In: Harmon-Jones E, Winkielman P, editors. Social Neuroscience: Integrating biological and psychological explanations of social behavior. Guilford Press; New York: 2007. pp. 176–196. [Google Scholar]
- Schultheiss OC. Implicit motives. In: John OP, Robins RW, Pervin LA, editors. Handbook of Personality: Theory and Research. 3rd edition. Guilford Press; New York: (in press) [Google Scholar]
- Schultheiss OC, Rohde W. Implicit power motivation predicts men's testosterone changes and implicit learning in a contest situation. Hormones and Behavior. 2002;41:195–202. doi: 10.1006/hbeh.2001.1745. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Pang JS. Measuring implicit motives. In: Robins RW, Fraley RC, Krueger R, editors. Handbook of Research Methods in Personality Psychology. Guilford Press; New York: 2007. pp. xx–xx. [Google Scholar]
- Schultheiss OC, Wirth MM. Biopsychological aspects of motivation. In: Heckhausen J, Heckhausen H, editors. Motivation and action. 2nd edition. Cambridge University Press; New York: (in press) [Google Scholar]
- Schultheiss OC, Dargel A, Rohde W. Implicit motives and gonadal steroid hormones: Effects of menstrual cycle phase, oral contraceptive use, and relationship status. Hormones and Behavior. 2003a;43:293–301. doi: 10.1016/s0018-506x(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Dargel A, Rohde W. Implicit Motives and sexual motivation and behavior. Journal of Research in Personality. 2003b);37:224–230. [Google Scholar]
- Schultheiss OC, Wirth MM, Stanton SJ. Effects of affiliation and power motivation arousal on salivary progesterone and testosterone. Hormones and Behavior. 2004;46(5):592–599. doi: 10.1016/j.yhbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Wirth MM, Torges CM, Pang JS, Villacorta MA, Welsh KM. Effects of implicit power motivation on men's and women's implicit learning and testosterone changes after social victory or defeat. Journal of Personality and Social Psychology. 2005a;88:174–188. doi: 10.1037/0022-3514.88.1.174. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Pang JS, Torges CM, Wirth MM, Treynor W. Perceived facial expressions of emotion as motivational incentives: Evidence from a differential implicit learning paradigm. Emotion. 2005b;5(1):41–54. doi: 10.1037/1528-3542.5.1.41. [DOI] [PubMed] [Google Scholar]
- Smith CP, editor. Motivation and personality: Handbook of thematic content analysis. Cambridge University Press; New York: 1992. [Google Scholar]
- Udry JR, Morris NM. Distribution of coitus in the menstrual cycle. Nature. 1968;220(167):593–596. doi: 10.1038/220593a0. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Watson NV. Relationship status and testosterone in North American heterosexual and non-heterosexual men and women: Cross-sectional and longitudinal data. Psychoneuroendocrinology. 2006;31:715–723. doi: 10.1016/j.psyneuen.2006.01.008. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, Verbaten R, van den Hout M, Koppeschaar H, Thijssen J, et al. Correlations among salivary testosterone, mood, and selective attention to threat in humans. Hormones and Behavior. 1999;36(1):17–24. doi: 10.1006/hbeh.1999.1521. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, Hermans E, Putman P, Koppeschaar H, Thijssen J, Verbaten R, van Doornen L. A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Behavioral Neuroscience. 2001;115:238–242. doi: 10.1037/0735-7044.115.1.238. [DOI] [PubMed] [Google Scholar]
- van Honk J, Peper JS, Schutter DJ. Testosterone reduces unconscious fear but not consciously experienced anxiety: implications for the disorders of fear and anxiety. Biological Psychiatry. 2005;58(3):218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic–pituitary– gonadal and –adrenal axes. Journal of Neuroendocrinology. 2002;14(6):506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Winter DG. The power motive. Free Press; New York: 1973. [Google Scholar]
- Winter DG. The power motive in women – and men. Journal of Personality and Social Psychology. 1988;54:510–519. [Google Scholar]
- Winter DG. 4th ed. Department of Psychology, University of Michigan; Ann Arbor: 1994. Manual for scoring motive imagery in running text. Unpublished manuscript. [Google Scholar]
- Wirth MM, Welsh KM, Schultheiss OC. Salivary cortisol changes in humans after winning or losing a dominance contest depend on implicit power motivation. Hormones and Behavior. 2006;49:346–352. doi: 10.1016/j.yhbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Yang DT, Owen WE, Ramsay CS, Xie H, Roberts WL. Performance Characteristics of Eight Estradiol Immunoassays. American Journal of Clinical Pathology. 2004;122:332–337. doi: 10.1309/5N2R-4HT4-GM0A-GPBY. [DOI] [PubMed] [Google Scholar]
- Zumpe D, Michael RP. Female dominance rank and behavior during artificial menstrual cycles in social groups if rhesus monkeys (Macaca mulatta). American Journal of Primatology. 1989;17:287–304. doi: 10.1002/ajp.1350170404. [DOI] [PubMed] [Google Scholar]