Abstract
Purpose
Carbapenem-resistant Klebsiella pneumoniae (CRKP) infections have become a serious threat with high morbidity and mortality. Early identification of risk factors for CRKP infections is important, but these factors are still controversial. Therefore, we aimed to identify the risk factors and clinical outcomes of CRKP infections.
Patients and Methods
The retrospective, single-center study was carried out in the respiratory intensive care unit of the Chinese People’s Liberation Army General Hospital from 2017 to 2020. Patients infected with K. pneumoniae were included and categorized into the CRKP group and carbapenem-sensitive K. pneumoniae (CSKP) group based on the susceptibility to carbapenems. The independent risk factors were investigated by univariate analysis and multivariate logistic regression analysis. The clinical outcomes were also evaluated between the two groups.
Results
A total of 138 eligible patients were included in our study, with a median age of 80.5 years (interquartile range: 62.0–86.3), and 78.3% of them were males. Of the 138 patients, there were 97 patients in the CRKP group, and the other 41 were assigned into the CSKP group. Multivariate analysis showed that exposure to ≥three types of comorbidities (OR = 5.465, P = 0.003), previous hospitalization (OR = 4.279, P = 0.006), use of quinolones (OR = 5.872, P = 0.012), and indwelling urinary catheter (OR = 5.035, P = 0.000) were independent risk factors for CRKP infections. The in-hospital mortality rate of the CRKP group was 42.1%, which was higher compared with the CSKP group (17.5%, P = 0.006).
Conclusion
Exposure to ≥three types of comorbidities, previous hospitalization, use of quinolones, and indwelling urinary catheter were independent risk factors for CRKP infections, which had higher mortality compared with CSKP infections. Early detection of high-risk patients and timely control measures should be implemented to prevent the emergence of CRKP infections and thereby improve the clinical outcomes.
Keywords: carbapenems, Klebsiella pneumoniae, resistance, risk factors, prognosis
Introduction
With the rapidly increased prevalence of carbapenem-resistant Klebsiella pneumoniae (CRKP), infections caused by CRKP have become a serious threat to global public health with limited treatment options, leading to heavy economic costs.1 Currently, the production of carbapenemase enzyme is the main mechanism mediating K. pneumoniae resistance to carbapenems,2 which are categorized into class A (represented by KPC), class B (such as NDM, VIM, and IMP), and class D (mainly OXA-48).3 In China, KPC (74%), followed by NDM (17%), has become the most common carbapenemase in CRKP since it was firstly reported in 2007.4 The overexpression of efflux pumps and loss of outer membrane porins also contribute to the development of resistance. Patients infected by CRKP are usually associated with higher mortality (42.14%) compared with carbapenem-sensitive K. pneumoniae (CSKP) infections (21.16%).5 The Centers for Disease Control and Prevention (CDC) has prioritized CRKP as an “urgent public health threat” requiring immediate and aggressive actions in 2019.6,7 Therefore, it is crucially necessary to evaluate the risk factors of CRKP infections and thereby take reasonable actions to intervene in early stage, which can be helpful to reduce the occurrence of CRKP and improve the outcomes of high-risk patients.
Single comorbidities, such as diabetes and renal dysfunction,8,9 have been proved to be the risk factors for CRKP infections, while little information is available on the relationship between exposure to ≥three types of comorbidities and such infections. Many studies have suggested that special attention should be paid to those patients with ≥three types of comorbidities,10 which has a significant influence on reducing the physiologic reserve of patients and consequently increases the risk of adverse outcomes.10–12 Therefore, we would investigate whether exposure to ≥three types of comorbidities could be a predictive indicator for CRKP infections in the present study.
Here, we carried out a retrospective descriptive cohort study and aimed to explore the independent risk factors and evaluate the clinical outcomes of CRKP infections, with the hope to provide a theoretical basis for scientific prevention and control of such infections in clinical practice.
Methods
Ethics Statement
The study was approved by the Medical Ethics Committee of Chinese People’s Liberation Army (PLA) General Hospital (approval number: S2018-192-01) and complied with the principles of the Declaration of Helsinki. The informed consent form (ICF) was not required because it was a retrospective study, and the personal data of involved subjects were anonymized and maintained with confidentiality.
Study Design and Population
The retrospective descriptive cohort study was conducted among K. pneumoniae infected inpatients at the respiratory intensive care unit (ICU) of Chinese PLA General Hospital from Jan. 2017 to Jun. 2020. Patients included in the study were categorized into the CRKP group (case group) and CSKP group (control group). The clinical data of the two groups were analyzed to identify the risk factors and prognosis of CRKP infections. The inclusion criteria were set as follows: 1) 18 years of age or older; 2) hospitalized patients; 3) patients having clinical cultures positive for K. pneumoniae and infected by K. pneumoniae; 4) patients with existing antimicrobial susceptibilities to carbapenems; 5) patients having a diagnosis for nosocomial infections, which was defined as the first positive culture obtained >48 h after hospital admission, and the patient did not have clinical signs of infection at the time of admission.13 Exclusion criteria were set as follows: 1) patients were just colonized by K. pneumoniae but without such infections, which was mainly determined by the clinical judgment of treating physicians, as well as the quality of specimens and microbiological data; and 2) the medical records of patients were not complete. When there were more than one sample from a single patient, only the records of the first positive sample would be analyzed.
Antimicrobial Susceptibility Test
Antimicrobial susceptibilities of K. pneumoniae isolates were determined by the microdilution broth method based on the guidelines of the 2016 Clinical and Laboratory Standards Institute (CLSI). CRKP was defined as a strain that was resistant to at least one of carbapenems, with the criteria of minimum inhibitory concentration (MIC) of doripenem ≥4 μg/mL, ertapenem ≥2 μg/mL, imipenem ≥4 μg/mL, or meropenem ≥4 μg/mL. Escherichia coli ATCC 25922 was used as the quality control strain.
Data Collection
All clinical data were extracted from the medical record database by two independent reviewers (HZ and MY). Any controversy was judged by the third reviewer (JW). The following data of each patient were collected: 1) Demographic characteristics: patient ID, admission diagnosis, age, sex, height, and weight. 2) Comorbidity: hypertension, diabetes, cardiovascular diseases, hepatic dysfunction, renal dysfunction, lung diseases, digestive system diseases, nervous diseases, malignancy, fracture, hypoalbuminemia, and anemia. Co-occurrence of three or more comorbidities mentioned above in the same patient was defined as exposure to ≥three types of comorbidities, which was also recorded as a possible risk factor included in the subsequent analysis. 3) The previous hospitalization within 90 days. 4) Previous drug use (≥7 days) within 30 days: glucocorticoids, β-lactam/β-lactamase inhibitors, carbapenems, aminoglycosides, macrolides, tetracyclines, glycopeptides, quinolones, sulfonamides, linezolid, antifungal agents, etc. 5) Clinical biochemical indexes within 7 days: hemoglobin (Hb), white blood cell (WBC) count, neutrophil ratio (ANC), platelet (PLT), serum albumin (ALB), glutamic pyruvic transaminase (ALT), glutamic oxaloacetic transaminase (AST), serum creatinine (Scr), etc. 6) Etiological examination: date of K. pneumoniae isolation, the type of specimens, the susceptibilities against carbapenems, etc. 7) Previous invasive procedures within 7 days: mechanical ventilation, peripheral venous catheterization, central venous catheterization, indwelling drainage catheter, indwelling gastric catheter, and indwelling urinary catheter. 8) Clinical outcomes: improved, worsened, and death. Improved was defined as resolution or partial resolution of clinical symptoms and signs of infections, as well as gradual normalization of laboratory findings (such as WBC count, C-reactive protein, and procalcitonin). Worsened was defined as worsening of presenting symptoms or signs of infections and persistently abnormal laboratory findings without any tendency of improvement. Death was defined as all-cause in-hospital mortality during the whole hospitalization.
Statistical Analysis
All data were analyzed by SPSS version 20.0. Statistical significance was defined as P<0.05. To identify the potential risk factors of CRKP infections, the univariate analysis was performed using different statistical methods according to the types of data. Normally distributed quantitative variables were described as mean ± standard deviation (S.D.), which were compared by Student’s t-test, while the non-normally distributed ones were expressed as median (interquartile range) [M (IQR)] and analyzed by Wilcoxon Rank Sum Test. For qualitative data, they were expressed as number and percentage [n (%)] and analyzed by Pearson Chi-square test or Fisher’s exact test with theoretical total numbers less than 5. Variables with P≤0.1 in the univariate analysis were candidates of binary logistic regression for multivariate analysis. To identify the independent risk factors, the logistic regression was carried out by the likelihood ratio test (forward: LR, defaulted inclusion criteria P≤0.05, defaulted exclusion criteria: P≥0.1). The odds ratio (OR) and 95% confidence interval (CI) of each independent risk factor were calculated to evaluate the strength of association between CRKP infections and exposure. In the analysis of the prognosis of CRKP infections, the mortality between the CRKP and CSKP groups was compared using the Pearson Chi-square test, and the survival curve was drawn by the Kaplan-Meier method (Log rank test).
Results
Characteristics of Patients
A total of 138 patients were enrolled in the study after patients who were just colonized by K. pneumoniae were excluded based on the pre-defined exclusion criteria. As shown in Table 1, there were 97 (70.3%) patients infected by CRKP, and 41 (29.7%) were infected by CSKP. The median age was 80.5 years (interquartile range: 62.0–86.3), and most of them were males (n=108, 78.3%). The most frequently detected infection was pneumonia (n=130, 94.2%), followed by bloodstream infections (n=31, 22.5%). Strains were isolated from various clinical specimens, where both CRKP and CSKP strains were recovered mainly from sputum (50.5% and 58.5%, respectively) and tracheal aspirates (26.8% and 26.8%, respectively).
Table 1.
Characteristics of Patients in the CRKP and CSKP Groups
| Variable | Total (n=138) | CRKP (n=97) | CSKP (n=41) |
|---|---|---|---|
| Demographic dataa | |||
| Age (year) | 80.5 (62.0–86.3) | 84.0 (74.0–88.0) | 77.5 (55.8–87.0) |
| Male (n, %) | 108 (78.3) | 75 (77.3) | 33 (80.5) |
| Height (cm) | 170.0 (165.0–173.0) | 170.0 (162.0–173.5) | 170.0 (167.5–173.3) |
| Weight (kg) | 64.6±12.0 | 65.3±12.3 | 63.3±11.7 |
| BMI (kg/m2) | 22.8±4.0 | 23.2±4.0 | 22.1±4.1 |
| Distribution of specimens, n (%) | |||
| Sputum | 73 (52.9) | 49 (50.5) | 24 (58.5) |
| Tracheal aspirates | 37 (26.8) | 26 (26.8) | 11 (26.8) |
| Urine | 8 (5.8) | 7 (7.2) | 1 (2.4) |
| Catheterb | 6 (4.3) | 6 (6.2) | 0 (0.0) |
| Blood | 6 (4.3) | 5 (5.2) | 1 (2.4) |
| Bronchoalveolar lavage fluid | 6 (4.3) | 2 (2.1) | 4 (9.8) |
| Othersc | 2 (1.4) | 2 (2.1) | 0 (0.0) |
| Type of infections, n (%) | |||
| Pneumonia | 130 (94.2) | 94 (96.9) | 36 (87.8) |
| Bloodstream infections | 31 (22.5) | 24 (24.7) | 7 (17.1) |
| Urinary tract infections | 24 (17.4) | 22 (22.7) | 2 (4.9) |
| Catheter-related infections | 9 (6.5) | 8 (8.2) | 1 (2.4) |
| Othersd | 2 (1.4) | 2 (2.1) | 0 (0.0) |
Notes: aThe normal distribution of quantitative data was judged by Kolmogorov–Smirnov test. For normally distributed quantitative data (weight and BMI in this study), they were described as mean ±S.D., while the non-normally distributed ones (age and height) were expressed as median (IQR). bThe specimen of the catheter was referred as endotracheal, venous, drainage, or urinary catheter. cOther specimens were referred to one wound secretion and one pleural fluid. dOther infections were referred to one skin and soft tissue infection and empyema.
Abbreviations: n, sample size; %, the percentage; CRKP, carbapenem-resistant Klebsiella pneumoniae; CSKP, carbapenem-sensitive Klebsiella pneumoniae.
Univariate Analysis of Risk Factors for CRKP Infections
Table 2 presents the potential risk factors of CRKP infections. Regarding the demographic characteristics, we found that the age of patients infected with CRKP was higher compared with those infected with CSKP (P=0.022), and there was no statistical difference between the two groups in terms of gender, height, weight, and body mass index (BMI). Clinical biochemical indexes exhibited that the CRKP group had a significantly lower Hb level (P=0.001) and higher AST level at the time of positive culture compared with the CSKP group (P=0.021). A significantly statistical difference was also found in some comorbidities: cardiovascular diseases (P=0.025), renal dysfunction (P=0.031), anemia (P=0.042), and exposure to ≥three types of comorbidities (P=0.001). Besides, a larger proportion of patients with CRKP infections had a previous hospitalization (P=0.011) and ≥30 days of hospitalization (P=0.037) compared with patients infected with CSKP. The previous drug use (≥7 days) in 30 days was similar between the two groups except for carbapenem (P=0.005) and quinolones (P=0.014). In addition, a statistically significant difference was found in many previous invasive procedures in 7 days: mechanical ventilation (P=0.001), peripheral venous catheterization (P=0.045), central venous catheterization (P=0.002), indwelling gastric catheter (P=0.014), and indwelling urinary catheter (P=0.000).
Table 2.
Univariate Analysis of Risk Factors for Infections Caused by CRKP
| Variable | CRKP (n=97) | CSKP (n=41) | χ2/z/ tc | P |
|---|---|---|---|---|
| Demographic characteristicsa | ||||
| Age (year) | 84.0 (74.0–88.0) | 77.5 (55.8–87.0) | −2.287 | 0.022 |
| Male (n, %) | 75 (77.3) | 33 (80.5) | 0.170 | 0.680 |
| Height (cm) | 170.0 (162.0–173.5) | 170.0 (167.5–173.3) | −0.376 | 0.707 |
| Weight (kg) | 65.3±12.3 | 63.3±11.7 | −0.668 | 0.506 |
| BMI (kg/m2) | 23.2±4.0 | 22.1±4.1 | −1.136 | 0.259 |
| Clinical biochemical indexesa | ||||
| Hb (g/L) | 90.5±21.4 | 104.2±22.1 | 3.395 | 0.001 |
| WBC (109/L) | 10.0 (7.5–13.3) | 8.3 (6.3–11.5) | −0.261 | 0.794 |
| ANC (%) | 85.1 (76.5–90.8) | 80.8 (76.3–91.0) | −0.042 | 0.966 |
| PLT (109/L) | 134.0 (81.0–182.5) | 187.5 (83.0–262.8) | −1.542 | 0.123 |
| ALB (g/L) | 34.6±5.1 | 34.4±5.9 | −0.160 | 0.873 |
| ALT (U/L) | 13.6 (8.2–28.1) | 16.2 (12.3–22.3) | −1.154 | 0.249 |
| AST (U/L) | 26.5 (16.6–57.3) | 20.4 (15.3–33.1) | −2.301 | 0.021 |
| Scr (μmol/L) | 84.6 (58.5–162.5) | 81.7 (50.6–114.3) | −1.499 | 0.134 |
| Comorbidityb | ||||
| Hypertension | 42 (43.3) | 11 (26.8) | 3.305 | 0.069 |
| Diabetes | 29 (29.9) | 6 (14.6) | 3.546 | 0.060 |
| Cardiovascular diseases | 58 (59.8) | 16 (39.0) | 4.999 | 0.025 |
| Hepatic dysfunction | 44 (45.4) | 18 (43.9) | 0.025 | 0.875 |
| Renal dysfunction | 40 (41.2) | 9 (22.0) | 4.681 | 0.031 |
| Lung diseases | 35 (36.1) | 13 (31.7) | 0.243 | 0.622 |
| Digestive diseases | 26 (26.8) | 7 (17.1) | 1.500 | 0.221 |
| Nervous diseases | 48 (49.5) | 17 (41.5) | 0.744 | 0.388 |
| Malignancy | 15 (15.5) | 3 (7.3) | 1.686 | 0.194 |
| Fracture | 8 (8.2) | 7 (17.1) | 1.496 | 0.221 |
| Hypoalbuminemia | 54 (55.7) | 19 (46.3) | 1.007 | 0.316 |
| Anemia | 44 (45.3) | 11 (26.8) | 4.129 | 0.042 |
| ≥three types of comorbidities | 87 (89.7) | 27 (65.9) | 11.398 | 0.001 |
| Previous hospitalization in 90 daysb | ||||
| Hospitalization | 38 (39.2) | 7 (17.1) | 6.406 | 0.011 |
| Hospitalization ≥30 days | 18 (18.6) | 2 (4.9) | 4.351 | 0.037 |
| Previous drugs use (≥7 days) in 30 daysb | ||||
| Glucocorticoids | 17 (17.5) | 4 (9.8) | 1.348 | 0.246 |
| β-lactam/β-lactamase inhibitors | 28 (28.9) | 10 (24.4) | 0.289 | 0.591 |
| Carbapenems | 38 (39.2) | 6 (14.6) | 7.992 | 0.005 |
| Aminoglycosides | 5 (5.2) | 2 (4.9) | 0.000 | 1.000 |
| Macrolides | 16 (16.5) | 4 (9.8) | 1.056 | 0.304 |
| Tetracyclines | 5 (5.2) | 4 (9.8) | 0.388 | 0.533 |
| Glycopeptides | 20 (20.6) | 7 (17.1) | 0.230 | 0.631 |
| Quinolones | 25 (25.8) | 3 (7.3) | 6.070 | 0.014 |
| Sulfonamides | 7 (7.2) | 0 (0.0) | 1.798 | 0.180 |
| Linezolid | 16 (16.5) | 5 (12.2) | 0.413 | 0.520 |
| Antifungal agents | 30 (30.9) | 7 (17.1) | 2.819 | 0.093 |
| Previous invasive procedures in 7 daysb | ||||
| Mechanical ventilation | 68 (70.1) | 16 (39.0) | 11.687 | 0.001 |
| Peripheral venous catheterization | 33 (34.0) | 7 (17.1) | 4.021 | 0.045 |
| Central venous catheterization | 49 (50.5) | 9 (22.0) | 9.651 | 0.002 |
| Indwelling drainage catheter | 4 (4.1) | 1 (2.4) | 0.000 | 1.000 |
| Indwelling gastric catheter | 53 (54.6) | 13 (31.7) | 6.073 | 0.014 |
| Indwelling urinary catheter | 72 (74.2) | 17 (41.5) | 13.509 | 0.000 |
Notes: Bold P<0.05 indicates statistical significance. aKolmogorov–Smirnov test was used to judge the normal distribution of quantitative data. For normally distributed quantitative data (weight, BMI, Hb, and ALB in this study), they were described as mean ±S.D., while the non-normally distributed ones were expressed as median (IQR); bQualitative data were expressed as number and percentage (n (%)). cTo compare the difference of variables between the two groups, the qualitative data were analyzed by Pearson Chi-square test (χ2) or Fisher’s exact test with theoretical total numbers less than 5. Similarly, the Student’s t-test (t) was used for quantitative data with normal distribution. Otherwise, the Wilcoxon Rank Sum Test (z) was used.
Abbreviations: CRKP, carbapenem-resistant Klebsiella pneumoniae; CSKP, carbapenem-sensitive Klebsiella pneumoniae; n, sample size; BMI, Body Mass Index; Hb, hemoglobin; WBC, white blood cell count; ANC, neutrophil ratio; PLT, platelet; ALB, serum albumin; ALT, glutamic pyruvic transaminase; AST, glutamic oxaloacetic transaminase; Scr, serum creatinine.
Multivariate Analysis of Risk Factors for CRKP Infections
All the variables with statistical significance in univariate analysis were included in the binary logistic regression for multivariate analysis. In addition, we also introduced variables with P≤0.1 (hypertension, diabetes, and antifungal agents) to avoid omitting potential risk factors. Results showed that more than three types of comorbidities (OR=5.465, P=0.003), hospitalization (OR=4.279, P=0.006), quinolones (OR=5.872, P=0.012), and indwelling urinary catheter (OR=5.035, P=0.000) were identified as independent risk factors for CRKP infections (Table 3).
Table 3.
Multivariate Logistic Regression Analysis of Risk Factors for Infections Caused by CRKP
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age (year) | – | – | 0.295 |
| Hb | – | – | 0.178 |
| AST | – | – | 0.465 |
| Hypertension | – | – | 0.559 |
| Diabetes | – | – | 0.196 |
| Cardiovascular diseases | – | – | 0.449 |
| Renal dysfunction | – | – | 0.130 |
| Anemia | – | – | 0.336 |
| ≥three types of comorbidities | 5.465 | 1.773–16.848 | 0.003 |
| Hospitalization | 4.279 | 1.517–12.072 | 0.006 |
| Hospitalization ≥30 days | – | – | 0.198 |
| Carbapenems | – | – | 0.090 |
| Quinolones | 5.872 | 1.466–23.516 | 0.012 |
| Antifungal agents | – | – | 0.545 |
| Mechanical ventilation | – | – | 0.816 |
| Peripheral venous catheterization | – | – | 0.558 |
| Central venous catheterization | – | – | 0.260 |
| Indwelling gastric catheter | – | – | 0.671 |
| Indwelling urinary catheter | 5.035 | 2.067–12.264 | 0.000 |
Note: Bold P<0.05 indicates statistical significance.
Abbreviations: CRKP, carbapenem-resistant Klebsiella pneumoniae; OR, odds ratio; CI, confidence interval; Hb, hemoglobin; AST, glutamic oxaloacetic transaminase.
Prognostic Analysis of CRKP Infections
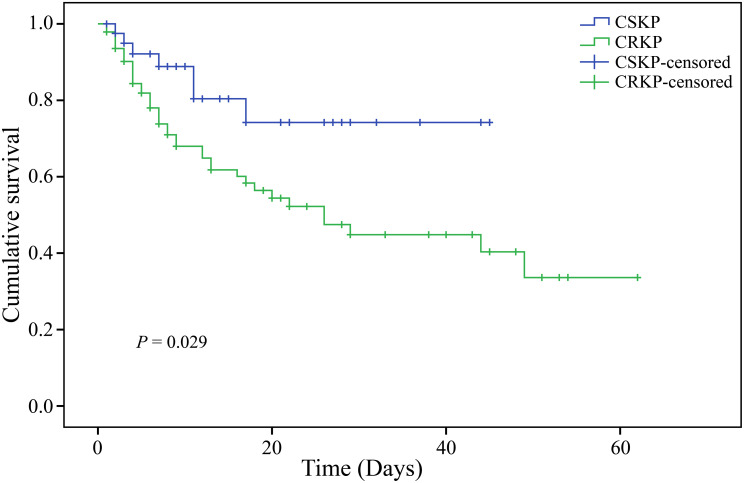
The in-hospital mortality rate of patients infected with CRKP was 42.1%, which was higher compared with those infected with CSKP (17.5%, P=0.006) (Table 4). Similarly, the Kaplan-Meier survival curve also indicated a statistically significant difference in in-hospital mortality (Log rank test, P=0.029), where the survival rate of the CRKP group was significantly lower compared with the CSKP group (Figure 1).
Table 4.
Analysis of Clinical Outcomes for CRKP and CSKP Patients
| Clinical Outcomes (n, %) | CRKP (n=95) | CSKP (n=40) | χ2 | P |
|---|---|---|---|---|
| Improved | 55 (57.9) | 33 (82.5) | 7.509 | 0.006 |
| Worsened and death | 40 (42.1) | 7 (17.5) |
Notes: The difference in clinical outcomes between the CRKP and CSKP groups was analyzed by the Pearson Chi-square test (χ2), with P<0.05 indicating statistical significance. Improved was defined as resolution or partial resolution of clinical symptoms and signs of infections, as well as gradual normalization of laboratory findings. Worsened was defined as worsening of presenting symptoms or signs of infections and persistently abnormal laboratory findings without any tendency of improvement. Death was defined as all-cause in-hospital mortality during the whole hospitalization.
Abbreviations: CRKP, carbapenem-resistant Klebsiella pneumoniae; CSKP, carbapenem-sensitive Klebsiella pneumoniae; n, sample size; %, the percentage of specific clinical outcome in the CRKP or CSKP group.
Figure 1.
Kaplan-Meier survival curves for CRKP and CSKP patients (Log rank test, P=0.029).
Note: P<0.05 indicates statistical significance.
Abbreviations: CRKP, carbapenem-resistant Klebsiella pneumoniae; CSKP, carbapenem-sensitive Klebsiella pneumoniae.
Discussion
In this retrospective study, we identified that previous use of quinolones, indwelling urinary catheter, hospitalization, and exposure to ≥three types of comorbidities were independent risk factors of CRKP infections, which had higher mortality compared with patients infected with CSKP. Early intervention of these risk factors could be helpful to prevent patients from carbapenem resistance, thereby improving the clinical outcomes of K. pneumoniae infections in clinical practice.
Although many studies have investigated the risk factors for CRKP infections, it still remains controversial whether previous use of quinolones is an independent risk factor or not.14–18 Exposure to quinolones can increase the selective pressure of K. pneumoniae,19 which not only induces the resistance to quinolones but also simultaneously increases the risk of carbapenem resistance through various mechanisms. For example, quinolones can up-regulate the expression of MexEF-OprN (an efflux pump of many antibiotics) and trigger the deficiency of OprD (an outer membrane porin), inducing the resistance to multiple antibiotics, such as carbapenems.18 Meanwhile, the plasmids mediating quinolone resistance may encode carbapenemase genes simultaneously, such as the KPC gene, which also leads to the emergence of strains resistant to carbapenems.20 As another strong risk factor, indwelling urinary catheter can not only provide a suitable place for CRKP to colonize,21 but also be prone to damage the urethra mucosal barrier, eventually augmenting the invasion possibility of conditional bacteria.18 In addition, exposure to ≥three types of comorbidities was another independent risk factor for CRKP infections, which was defined as the co-occurrence of three or more comorbidities in the same patient. Previous studies have found that patients with ≥three types of comorbidities are associated with worse clinical outcomes, such as longer hospital length of stays, higher rate of ICU admission, and even mortality.10–12 Those patients are usually under poor physical conditions, have an inferior function of the immune system, and consequently are more likely to fail to defend themselves against resistant and serious infections.20,22 Special attention should be paid to these above-mentioned patients, with careful medical observations and intensified nursing care.
As indicated in this study, the mortality of patients infected with CRKP was 42.1%, which was significantly higher compared with those infected with CSKP (17.5%, P<0.05), which might be attributed to the limited treatment options. Indeed, it has been proved that resistance to carbapenems is an independent risk factor of death caused by K. pneumoniae.23 Previous studies show that the mortality of CRKP infections varies from 33.2% to 50.1% based on geographical location, and it is 44.8% in Asia, which is consistent with our results.5
There were some limitations in this study. Firstly, it was a retrospective cohort, where potential information and selection bias were unavoidable, and thereby it could not provide a firm strength of evidence for the relationship between risk factors and CRKP infections. Secondly, we excluded some patients with K. pneumoniae due to incomplete medical records. It might also increase the risk of selection bias. Finally, the sample size of our study was relatively small, which might hamper the power of statistical analysis.
Conclusions
Our retrospective study suggested that exposure to ≥three types of comorbidities, previous hospitalization, quinolones, and indwelling urinary catheter were independent risk factors for CRKP infections, which were associated with higher mortality compared with CSKP infections. However, the above-mentioned conclusions still need to be further verified by randomized controlled trials with higher quality in the future due to several limitations in the current study.
Acknowledgments
We express our sincere appreciation to the involved patients and hospital.
Funding Statement
This work was supported by the National Natural Science Foundations of China (81770004 and 82073894) and Cultivation Project of PLA General Hospital for Distinguished Young Scientists (2020-JQPY-004).
Ethical Approval
The study was approved by Medical Ethics Committee of PLA General Hospital (approval number: S2018-192-01) and adhered to the principles of the Declaration of Helsinki. The informed consent form (ICF) was not required.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Reyes J, Aguilar AC, Caicedo A. Carbapenem-resistant klebsiella pneumoniae: microbiology key points for clinical practice. Int J Gen Med. 2019;12(2):437–446. doi: 10.2147/IJGM.S214305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. 2017;41(3):252–275. doi: 10.1093/femsre/fux013 [DOI] [PubMed] [Google Scholar]
- 3.Brink AJ. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr Opin Infect Dis. 2019;32(6):609–616. doi: 10.1097/QCO.0000000000000608 [DOI] [PubMed] [Google Scholar]
- 4.Zhang R, Liu L, Zhou H, et al. Nationwide surveillance of clinical carbapenem-resistant enterobacteriaceae (CRE) strains in China. EBioMedicine. 2017;19:98–106. doi: 10.1016/j.ebiom.2017.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi: 10.1186/s12941-017-0191-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States [EB/OL]. USA: Centers for Disease Control and Prevention; November13, 2019[June 08, 2021]. Available from:https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed August11, 2021. [Google Scholar]
- 7.Suay-Garcia B, Perez-Gracia MT. Present and future of carbapenem-resistant enterobacteriaceae (CRE) Infections. Antibiotics (Basel). 2019;8(3):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang G, Zhang M, Sun F, et al. Epidemiology, mortality and risk factors for patients with K. pneumoniae bloodstream infections: clinical impact of carbapenem resistance in a tertiary university teaching hospital of Beijing. J Infect Public Health. 2020;13(11):1710–1714. doi: 10.1016/j.jiph.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Yi H, Fang J, et al. Antimicrobial resistance and risk factors for mortality of pneumonia caused by Klebsiella pneumoniae among diabetics: a retrospective study conducted in Shanghai, China. Infect Drug Resist. 2019;12(2):1089–1098. doi: 10.2147/IDR.S199642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XD, Zhao YS, Li YF, et al. Medical comorbidities at admission is predictive for 30-day in-hospital mortality in patients with acute myocardial infarction: analysis of 5161 cases. J Geriatr Cardiol. 2011;8(1):31–34. doi: 10.3724/SP.J.1263.2011.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fakhry SM, Leon S, Derderian C, et al. Intensive care unit bounce back in trauma patients: an analysis of unplanned returns to the intensive care unit. J Trauma Acute Care Surg. 2013;74(6):1528–1533. doi: 10.1097/TA.0b013e31829247e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan MC, Roman SA, Sosa JA. Clinical and economic outcomes of thyroid surgery in elderly patients: a systematic review. J Thyroid Res. 2012;2012:615846. doi: 10.1155/2012/615846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng C, Cai J, Liu H, et al. Clinical characteristics and risk factors in mixed-enterococcal bloodstream infections. Infect Drug Resist. 2019;12:3397–3407. doi: 10.2147/IDR.S217905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuo Y, Zhao D, Song G, et al. Risk factors, molecular epidemiology, and outcomes of carbapenem-resistant klebsiella pneumoniae infection for hospital-acquired pneumonia: a matched case-control study in Eastern China during 2015–2017. Microb Drug Resist. 2021;27(2):204–211. doi: 10.1089/mdr.2020.0162 [DOI] [PubMed] [Google Scholar]
- 15.Yuan Y, Wang J, Yao Z, et al. Risk factors for carbapenem-resistant klebsiella pneumoniae bloodstream infections and outcomes. Infect Drug Resist. 2020;13:207–215. doi: 10.2147/IDR.S223243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He G, Huang J, Huang S, et al. Risk factors affecting clinical outcome in patients with carbapenem-resistant K. pneumoniae: a retrospective study. Med Sci Monit. 2020;26:e925693. doi: 10.12659/MSM.925693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang H, Wei J, Zhou W, et al. Risk factors and mortality for patients with bloodstream infections of Klebsiella pneumoniae during 2014–2018: clinical impact of carbapenem resistance in a large tertiary hospital of China. J Infect Public Health. 2020;13(5):784–790. doi: 10.1016/j.jiph.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Qin RR, Huang L, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection and mortality of Klebsiella pneumoniae infection. Chin Med J (Engl). 2018;131(1):56–62. doi: 10.4103/0366-6999.221267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girijan SK, Paul R, Vj JR, et al. Investigating the impact of hospital antibiotic usage on aquatic environment and aquaculture systems: a molecular study of quinolone resistance in Escherichia coli. Sci Total Environ. 2020;748:141538. doi: 10.1016/j.scitotenv.2020.141538 [DOI] [PubMed] [Google Scholar]
- 20.Zhu WM, Yuan Z, Zhou HY. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9(1):23. doi: 10.1186/s13756-020-0686-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geladari A, Simitsopoulou M, Antachopoulos C, et al. Immunomodulatory effects of colistin on host responses against carbapenem-resistant Klebsiella pneumoniae biofilms. Int J Antimicrob Agents. 2020;56(6):106182. doi: 10.1016/j.ijantimicag.2020.106182 [DOI] [PubMed] [Google Scholar]
- 22.Fang L, Xu H, Ren X, et al. Epidemiology and risk factors for carbapenem-resistant Klebsiella pneumoniae and subsequent MALDI-TOF MS as a tool to cluster KPC-2-producing Klebsiella Pneumoniae, a retrospective study. Front Cell Infect Microbiol. 2020;10:462. doi: 10.3389/fcimb.2020.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamma PD, Goodman KE, Harris AD, et al. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant enterobacteriaceae bacteremia. Clin Infect Dis. 2017;64(3):257–264. doi: 10.1093/cid/ciw741 [DOI] [PMC free article] [PubMed] [Google Scholar]