Abstract
Background and Objective
The term myxedema psychosis (MP) was introduced to describe the occurrence of psychotic symptoms in patients with untreated hypothyroidism, but the optimal assessment and treatment of this condition are unclear. We aimed to synthesize data from the literature to characterize the clinical presentation and management of MP.
Methods
We performed a systematic review according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines in PubMed (Medline), Embase, Google Scholar, and Cochrane databases, including observational studies, case series, and case reports published from 1/1/1980 to 31/12/2019 in the English language. Descriptive statistics along with univariate and multivariate analysis were used for data synthesis.
Results
Out of 1583 articles screened, 71 case reports met our inclusion criteria providing data on 75 MP cases. The median age at diagnosis was 42 years [32–56]. About 53% had no prior hypothyroidism diagnosis. Delusions occurred in 91%, with a predominance of persecutory ideas (84%), while hallucinations occurred in 78%. Physical symptoms and signs of hypothyroidism were absent in 37% and 26%, respectively. If symptoms occurred, nonspecific fatigue was seen most frequently (63%). The median thyroid-stimulating hormone value was 93 mIU/L [60–139]. Thyroid peroxidase antibodies were found positive in 75% (23/33) of reported cases. Creatinine kinase was reported abnormal in seven cases. Cranial imaging (CT or MRI) and electroencephalogram were normal in 89%, 75%, and 73% of the cases reported. The majority of patients were treated orally with thyroxine in combination with short-term antipsychotics. More than 90% of them showed complete recovery. Univariate analysis revealed a trend towards a shorter duration of psychosis with IV thyroid hormone therapy (p= 0.0502), but the effect was not consistent in a multivariate analysis.
Conclusion
While we identified a substantial lack of published research on MP, our pooled analysis of case observations suggests that the condition presents a broad spectrum of psychiatric and physical symptoms lending support to the value of screening for thyroid dysfunction in patients with first-ever psychosis.
Prospero Registration Number
CRD42020160310.
Keywords: psychosis, hypothyroidism, madness, myxedema, depression, neuropsychiatric
Background
Hypothyroidism is a common disease with an estimated global prevalence of 0.1–3.6%.1–4 The Committee on Myxedema of the Clinical Society of London issued the first report that described the development of delusions and hallucinations in almost half of hypothyroid patients (109 patients).5 Sixty years later, in 1949, Asher et al reexamined this relationship in fourteen patients who had psychosis and clinical evidence of hypothyroidism. The patients received thyroid hormones supplements, with nine patients achieving full recovery.6 He labeled this association “myxedema madness,” which later was renamed “myxedema psychosis” (MP).6 MP is a secondary psychotic disorder resulting from other medical conditions according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).7
The underlying pathophysiology is poorly understood. Previous research linked hypothyroidism to changes in neurometabolic activity that might contribute to MP, including;8 tyrosine hydroxylase imbalance in the anterior locus coeruleus;9 abundance of T3 receptors in the amygdala and the hippocampus;10 altered serotonin-mediated neurotransmission11,12 and attenuation of cerebral regional blood flow and glucose metabolism.13,14
Diagnostic discrimination between MP and other secondary psychoses is clinically relevant as the management differs according to the exact etiology. An important differential diagnosis of psychosis in hypothyroidism patients is Hashimotos’ encephalopathy (HE), also called steroids responsive encephalopathy with autoimmune thyroiditis.15 While the pathophysiological mechanism underlying MP is related to brain neurochemical alterations accompanying thyroid hormones deficiency, neuropsychiatric changes in HE are caused by an autoimmune response not directly linked to hypothyroidism. This explains the excellent response to steroids in most HE cases.15
Seventy years have elapsed since Asher’s description, yet, little is known about MP, likely due to the paucity of available literature.8 Thus, we aimed to review the literature and synthesize data on its clinical symptomatology, diagnosis, management strategies, and clinical outcomes.
Methods
This systematic review complied with preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.16 The review protocol was registered at PROSPERO (registration number: CRD42020160310) and was published.17
Eligibility Criteria
Observational studies, case series, and case reports providing data on patients diagnosed with MP were eligible for inclusion. We only included reports on adult patients (18 years or older) with confirmed hypothyroidism (thyroid stimulating hormone> normal range plus low thyroid hormones, or clinical evidence of hypothyroidism) and psychotic features meeting the DSM-5 criteria of psychosis due to a general medical condition in whom myxedema psychosis was the likely diagnosis as per the treating physician. We excluded cases of Hashimoto’s encephalopathy (HE), thyroxine-induced mania, subclinical hypothyroidism, secondary hypothyroidism (due to possible confounding by other hormonal imbalances or mass effect), or cases with alternative diagnoses more likely than MP.
Search Strategy and Information Source
We conducted a comprehensive search in the following databases; PubMed, Medline, EMBASE, Google Scholar (first 300 hits), and Cochrane databases for studies published from 1/1/1980 to 31/12/2019. We included articles labeled “letter to the editor” if they were, in fact, case reports. We limited our search to articles written in the English language only. We used combinations of free text, keywords, Emtree, and MesH-terms, including psychosis, psycho, madness, psychiatric, hypothyroid, myxedema, myxoedema. Search strings used in each database are detailed in the supplement. (Supplementary 1) We also performed a snowball search in bibliographies of identified full‐text articles and relevant review articles.
Screening, Data Extraction, and Quality Assessment
Two independent reviewers (MFHM) and (SS) performed the literature search and screening. First, the titles and abstracts were screened. Subsequently, the full text of potentially eligible articles was reviewed and assessed for inclusion. At each step, the two reviewers discussed discrepancies noted, and if consensus could not be reached, a third reviewer (MD) settled the discrepancy per protocol. We used a web-based literature screening application (Rayvan; http://rayyan.qcri.org) to conduct article screening and duplicate removals.18
We extracted general data on included publications such as type, author, year, and journal as well as demographic data of the patients reported such as sex, age, gender, history of psychosis, history of hypothyroidism, and causes of hypothyroidism. Moreover, data on clinical presentation data was extracted, including psychiatric presentation, duration of psychosis, hypothyroidism symptoms or signs, associated rhabdomyolysis, cranial imaging finding; electroencephalography (EEG) findings; thyroid stimulating hormone level; thyroid hormone levels; anti-thyroid peroxidase status; creatinine kinase levels. We used the tool proposed by Murad et al to adjudicate the quality of included case reports and series.19 The tool comprises eight questions assessing four domains (selection, ascertainment, causality, and reporting). We generated an overall score, and we then graded the quality as either good (> 5), fair (4–5), or poor (< 3).
Statistical Analysis
We used the Jamovi 1.1.9 software for statistical analysis.20 Descriptive statistics were applied to summarize data using the median (IQR) for continuous variables and frequencies for categorical variables. We used (n/N) and percentage values for presenting numbers of cases with a specific characteristic amongst cases that reported either the presence or the absence of this characteristic. Acknowledging the subjective nature of reporting in case studies, the two reviewers had to agree on whether a specific characteristic was present in any given case before inclusion in the final analysis. Exploratory multivariate logistic regression including potentially clinically relevant variables (gender, age, symptoms duration, TSH level, FreeT4, antipsychotic drugs duration, IV thyroid hormone therapy, and starting thyroxine dose) associated with recovery (resolution of psychosis) or rapid recovery (less than 2 weeks) was also performed.
Results
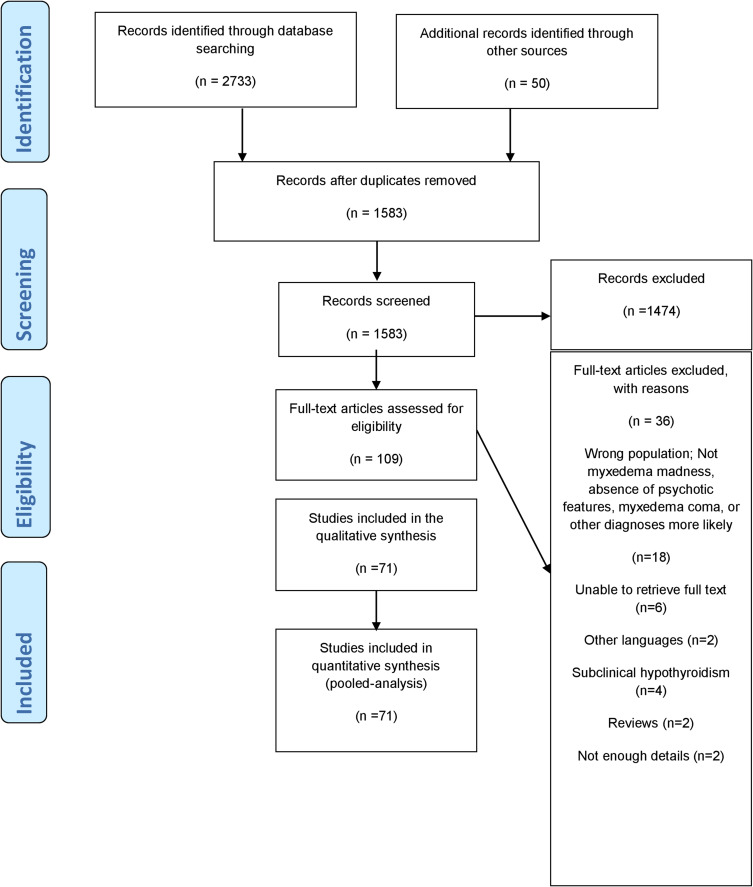
The initial search retrieved 2733 articles; 50 additional articles were identified through other means, of which 71 references describing 75 cases were included for the final analysis.21–91 The PRISMA flow diagram is shown in Figure 1. All included studies were case reports due to the absence of other forms of evidence (Table 1). Quality assessment utilizing the methodological quality and synthesis of case series and case reports tool revealed fair to good quality of most of the included cases (Supplement 2).
Figure 1.
PRISMA flow diagram.
Note: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. Creative Commons.93
Table 1.
Summary of Included Case Studies
| Case Study | Gender | Symptom Duration [Days] | Psychiatric Symptoms Reported | TSH Level | Antipsychotic Treatment [Yes/No], Duration [Weeks] | T4 Starting Dose (mcg) | T4 Maintenance Dose (mcg) | Recovery Outcome | Duration to Outcome in Weeks | Follow Up Duration [Weeks] |
|---|---|---|---|---|---|---|---|---|---|---|
| Reddy 201952 | Male, 37 | 15 | Delusions with no hallucinations | 100 | No | 100 | 300 | Complete recovery | 2 | 2 |
| Mohamed 201921 | Male, 44 | 14 | Hallucinations and delusions | 100 | Yes, 20 | 300 | 100 | Complete recovery | 2 | 24 |
| Singh 201976 | Female, 30 | 365 | Hallucinations, but delusions not explicitly mentioned | 60 | Yes, NS | NS | NS | Complete recovery | 3 | 3 |
| Fernandes 201955 | Male, 33 | 90 | Hallucinations with no delusions | 350 | Yes, 8 | 100 | 100 | Complete recovery | 2 | 13 |
| Todorov 201960 | Female, 43 | 7 | Hallucinations and delusions | 152 | Yes, 4 | 50 | 125 | Complete recovery | 1.5 | 8 |
| Natarajan 201967 | Female, 30 | 90 | Hallucinations and delusions | 100 | No | 100 | 100 | Complete recovery | 2 | 2 |
| Mavroson 201724 | Male, 31 | 4 | Hallucinations and delusions | 306 | Yes, 0.5 | 150 | 125 | Complete recovery | 0.5 | 2 |
| Philip 201777 | Female, 51 | 365 | Hallucinations and delusions | 109 | NS | 100 | 100 | Complete recovery | 2 | 2 |
| Gupta 201737 | Female, 44 | 21 | Delusions with no hallucinations | 100 | Yes, 4 | 300 | 100 | Complete recovery | 0.6 | 4 |
| Rizvi 201778 | Female, 35 | 15 | Hallucinations and delusions | 70.7 | Yes, 5 | 75 | 75 | Complete recovery | 1.7 | 13 |
| Zorkin 201758 | Male, 40 | 7 | Delusions with no hallucinations | 100 | Yes, NS | 100 | 112 | Complete recovery | 2 | 2 |
| Das 201769 | Male, 68 | 14 | Delusions with no hallucinations | 55 | Yes, 39 | 88 | 88 | Complete recovery | 3 | 52 |
| O’Hanlon 201772 | Female, 63 | NS | Delusions with no hallucinations | NS | Yes, NS | NS | NS | Complete recovery | 0.5 | NS |
| Shlykov 201630 | Female, 65 | 60 | Hallucinations and delusions | 61 | Yes, 1 | 50 | 100 | Complete recovery | 3 | 12 |
| Er 201634 | Female, 60 | 7 | Hallucinations and delusions | 45 | Yes, 1.3 | 25 | 100 | Complete recovery | 1 | 28 |
| Nazou 201639 | Female, 48 | 14 | Hallucinations and delusions | 145 | Yes, 0.6 | 75 | 75 | Complete recovery | 3 | 52 |
| Agachanli 201679 | Male, 31 | 45 | Hallucinations and delusions | 105.9 | Yes, 3 | 150 | 150 | Complete recovery | 1.6 | 14 |
| Morgado 201649 | Male, 36 | 2 | Hallucinations and delusions | 98 | Yes, 26 | 100 | 100 | Complete recovery | 2 | 104 |
| Mehta 201659 | Female, 21 | 730 | Delusions, but hallucinations not explicitly mentioned | 200 | Yes, NS | 100 | 100 | Recovery with other cognitive deficits | 4 | 4 |
| Larouche 201545 | Female, 29 | 7 | Delusions with no hallucinations | 100 | Yes, 0.5 | 200 | 100 | Complete recovery | 1 | 26 |
| Ueno 201523 | Male, 90 | 2 | Hallucinations and delusions | 105 | Yes, NS | 50 | 75 | Partial recovery | 2 | 7 |
| Hines 201526 | Female, 48 | 14 | Delusions with no hallucinations | 93 | Yes, 0.1 | 50 | 50 | Complete recovery | 0.3 | 0.4 |
| Bel Feki 201527 | Female, 60 | NS | Hallucinations and delusions | 45 | NS | NS | NS | Complete recovery | NS | |
| Amdouni 201529 | Female, 36 | NS | Hallucinations and delusions | 135 | NS | NS | NS | NS | NS | |
| Berkowitz 201532 | Female, 28 | NS | Not specified | 20 | NS | 50 | 75 | Complete recovery | 0.4 | 104 |
| Hynicka 201535 | NS | NS | Hallucinations and delusions | 60 | Yes, NS | 50 | 88 | Complete recovery | 1.6 | 2 |
| Morosán 201463 | Female, 62 | 7 | Hallucinations and delusions | 62.9 | Yes, 8 | 200 | 150 | Complete recovery | 0.6 | 24 |
| Baziki 201480 | Female, 54 | 90 | Delusions with no hallucinations | 85 | Yes, NS | 200 | NS | Complete recovery | 1.4 | 3 |
| Juneja 201447 | Female, 34 | NS | Hallucinations and delusions | 100 | Yes, NS | NS | NS | Complete recovery | 6 | 6 |
| Parikh 201464 | Female, 30 | 547 | Hallucinations and delusions | 63.7 | Yes, 1 | 100 | 100 | Complete recovery | 1 | 6 |
| Islam 201381 | Female, 39 | 7 | Hallucinations and delusions | 87 | Yes, 1.6 | NS | NS | Complete recovery | 0.6 | 39 |
| Tuman 201382 | Female, 56 | 180 | Hallucinations and delusions | Yes, NS | NS | NS | Complete recovery | 1 | 1 | |
| Lazaro 201328 | Female, 45 | 60 | Delusions with no hallucinations | 70 | Yes, 39 | NS | 75 | Complete recovery | 4 | 77 |
| Lin CL 201342 | Female, 41 | 7 | Hallucinations and delusions | 18.7 | Yes, 7 | 90 | 75 | Complete recovery | 3 | 104 |
| Dastjerdi 201356 | Male, 53 | NS | Hallucinations and delusions | 32 | Yes, NS | 150 | NS | Complete recovery | 2 | 104 |
| Hyams 201357 | Female, 26 | 14 | Delusions with no hallucinations | 38 | Yes, 0.4 | 100 | 100 | Complete recovery | 2 | 26 |
| Atilan 201383 | Male, 25 | NS | Hallucinations and delusions | 150 | Yes, NS | 150 | 150 | Complete recovery | 0.4 | NS |
| Weston 201370 | Male, 60 | NS | Hallucinations and delusions | 150 | NS | NS | 150 | Complete recovery | 3 | 22 |
| Sharma 201371 | Female, 24 | 1100 | Hallucinations and delusions | 60 | Yes, NS | NS | NS | NS | NS | NS |
| Neal 201222 | Male, 32 | 6 | Hallucinations, but delusions not explicitly mentioned | 98 | No | 150 | 150 | Complete recovery | 0.4 | NS |
| Martell 201268 | Female, 36 | 21 | Hallucinations and delusions | 209 | Yes, NS | 12.5 | 137 | Complete recovery | 5 | 8 |
| Leung 201184 | Female, 38 | NS | Hallucinations and delusions | 220 | Yes, NS | 150 | NS | Complete recovery | 1.85 | NS |
| Kumar 201185 | Male, 83 | 14 | Hallucinations with no delusions | 233 | NS | NS | NS | Complete recovery | 2 | 2 |
| Manea 201136 | Female, 42 | 4 | Not specified | 75 | Yes, NS | NS | NS | Complete recovery | 4 | 52 |
| Khemka 201146 | Female, 56 | 60 | Hallucinations and delusions | 14 | Yes, NS | 25 | 25 | Complete recovery | NS | NS |
| Khemka 201146 | Female, 77 | NS | Hallucinations and delusions | 18 | Yes, NS | 25 | 100 | Complete recovery | 2.4 | NS |
| Azzopardi 201075 | Male, 59 | NS | Delusions with no hallucinations | 100 | NS | NS | NS | Partial recovery | NS | NS |
| Nielsen 201065 | Male, 47 | 90 | Hallucinations with no delusions | 47 | No | 112 | NS | Complete recovery | 6 | 6 |
| Kandukuri 201066 | Male, 23 | NS | Hallucinations and delusions | 200 | Yes, NS | NS | NS | Recovery with other cognitive deficits | NS | 104 |
| Greene 200986 | Female, 39 | NS | Hallucinations and delusions | 53 | Yes, NS | NS | NS | Complete recovery | NS | NS |
| Sathya 200974 | Female, 47 | 3 | Hallucinations and delusions | 63 | Yes, 4 | 25 | 100 | Complete recovery | 1 | 4 |
| Moeller 200938 | Female, 51 | NS | Hallucinations and delusions | 176 | Yes, 0.1 | 100 | 100 | Complete recovery | 0.7 | 1 |
| Selvaraj 200854 | Male, 65 | 21 | Delusions, but hallucinations not explicitly mentioned | 60 | No | NS | NS | Complete recovery | NS | |
| Tor 200733 | Female, 72 | 60 | Hallucinations and delusions | 79 | Yes, NS | 50 | 50 | Complete recovery | 2 | 13 |
| Khaldi 200650 | Female, 53 | 10 | Delusions with no hallucinations | 387 | Yes, 0.9 | NS | 100 | Complete recovery | 0.8 | 6 |
| Stowell 200543 | Female, 35 | 14 | Delusions with no hallucinations | 150 | Yes, 0.9 | 200 | 150 | Complete recovery | 0.5 | NS |
| Heinrich 200387 | Female, 73 | 14 | Hallucinations with no delusions | 53 | Yes, 2 | NS | NS | Complete recovery | 2 | NS |
| Benvenga 200388 | Female, 55 | 90 | Hallucinations with no delusions | 30 | Yes, 21 | 100 | 100 | Complete recovery | 0.6 | 625 |
| Chari 200289 | Male, 28 | 180 | Delusions with no hallucinations | 70 | Yes, 8 | 25 | 150 | Complete recovery | NS | 26 |
| Nathan 199725 | Male, 43 | 30 | Hallucinations and delusions | 46.6 | NS | NS | NS | Complete recovery | 1 | 20 |
| Westphal 199761 | Male, 46 | 5 | Hallucinations and delusions | 139 | Yes, 0.5 | 50 | 50 | Complete recovery | 0.5 | 26 |
| Westphal 199761 | Female, 29 | NS | Hallucinations with no delusions | 1500 | Yes, 1.4 | 100 | NS | Complete recovery | 1.3 | 52 |
| Ward 199490 | Female, 73 | 21 | Hallucinations and delusions | 61 | Yes, NS | NS | NS | Complete recovery | NS | 14 |
| Pearce 199153 | Female, 86 | 5 | Hallucinations and delusions | 60 | Yes, NS | NS | NS | Complete recovery | 2 | NS |
| Rao 199062 | Female, 28 | 3 | Hallucinations, but delusions not explicitly mentioned | 40 | NS | NS | NS | Complete recovery | 4 | 4 |
| Darko 198931 | Male, 24 | NS | Hallucinations and delusions | 353 | Yes, NS | 50 | 150 | Recovery with other cognitive deficits | 3 | 25 |
| Davis 198991 | Male, 40 | 21 | Hallucinations and delusions | 370 | Yes, 0.1 | NS | NS | Complete recovery | 1 | 52 |
| Santiago 198741 | Female, 36 | 7 | Hallucinations and delusions | 110 | NS | NS | NS | Complete recovery | 1.6 | 8 |
| Cook 198644 | Male, 25 | NS | Delusions with no hallucinations | 97 | NS | NS | NS | Complete recovery | 0.3 | |
| Cook 198644 | Male, 42 | NS | Hallucinations and delusions | 29 | NS | NS | NS | Complete recovery | 0.4 | |
| Shaw 198573 | Female, 53 | 730 | Hallucinations and delusions | NS | Yes, NS | 100 | NS | Complete recovery | 2 | 26 |
| Samuel 198448 | Female, 32 | 365 | Hallucinations and delusions | 24 | Yes, NS | 100 | 300 | NS | 2 | NS |
| Hall 198251 | Female, 34 | NS | Hallucinations and delusions | NS | NS | NS | NS | Complete recovery | NS | 208 |
| Hall 198251 | Female, 35 | NS | Hallucinations and delusions | NS | NS | NS | NS | Complete recovery | NS | 13 |
| Madakasira 198140 | Female, 68 | 14 | Delusions with no hallucinations | NS | NS | NS | NS | Complete recovery | NS | NS |
Note: Partial recovery means improvement of psychosis symptoms with no complete resolution.
Abbreviations: MP, myxedema psychosis; T3 triiodothyronine; T4 thyroxine, NS; not specified.
Baseline Characteristics
The female-to-male ratio was 2:1. The median age was 42 [32–56] years, with the oldest case reported aged 90 years. The majority of cases were Caucasians, 44%, followed by Asians, 36%. 53% of patients had no prior history of hypothyroidism, and 82% had no prior psychosis history. Autoimmune thyroiditis was the most common reported cause of hypothyroidism 51%.
Clinical Features
The median duration of psychotic symptoms was 14.5 [7–82.5] days ranging from two days to three years. Delusions were present in 91%. The most common form of delusions was paranoid/persecutory 84%. Hallucinations were present in 77.5%, with auditory hallucinations being the most prevalent 77.6%. Manic symptoms accompanied psychosis more than depressive symptoms, 52% and 36%, respectively (Table 2). Hypothyroidism symptoms and signs were not always reported, and when presented, often lacking sufficient details. Hypothyroidism symptoms were present in 63% (26/41) of the cases. Only 22 cases described the nature of hypothyroidism symptoms. Fatigue occurred in 63% (14/22), weight gain 36%, cold intolerance 36% (7/22), and hoarse voice was seen in 18% of the cases (4/22). Hypothyroidism signs were present in 75% of the cases (39/52). The most common abnormal findings were dry skin 60% (23/43), facial or pretibial edema 52% (20/43), delayed relaxation, or diminished deep tendon reflexes (DTR) 47% (18/38), while hoarseness of voice occurred 26% (10/38).
Table 2.
Summary of Baseline Characteristics, Clinical Features, and Diagnostic Workup of Included Cases
| Basel line Characteristic | Frequency (n/N) % | Clinical Feature | Frequency (n/N) % | Laboratory and Diagnostic Workup | Frequency (n/N) % |
|---|---|---|---|---|---|
| Female | (49/74) 66% | Delusion | (46/70) 91% | Normal EEG | (11/15) 73% |
| Male | (25/74) 33% | Hallucination | (55/71) 77.5% | Normal CT head | (24/27) 89% |
| African | (5/25) 20% | Hypothyroid symptoms | (26/41) 63% | ||
| Asian | (9/25) 36% | Hypothyroidism signs | (39/53) 74% | Normal MRI head | (12/16) 75% |
| Caucasian | (11/25) 44% | ||||
| Hypothyroidism history | (34/72) 47% | Manic symptoms | (23/44) 52% | Normal CSF | (4/6) 67% |
| Previous psychotic episode | (13/71) 18% | ||||
| Previous psychotic episode likely related to hypothyroidism | (9/13) 69% | Depression symptoms | (16/43) 37% | Normal Creatinine Kinase | (0/7) 0% |
| Family history of Psychotic disorder | (3/29) 10% |
Notes: (n/N) refers to the crude number of a certain characteristic divided by the number of cases where this specific feature was assessed as reported (either present or absent) by the two reviewers.
Laboratory Testing
The median thyroid-stimulating hormone median value was 93 mIU/L [60–93]. The median-free T4 was 0.2 ng/dl [0.13–0.39]. The median thyroid peroxidase value was 138 IU/L [82.5–323]. In 7 of the reported cases, creatinine kinase was observed abnormal with a median value of 4490 [1767–9485] IU/L. Lumbar puncture was reported in six patients. Analysis of cerebrospinal fluid revealed mild protein elation in two cases (33%) and was found normal in four cases (67%).
Diagnostic Imaging
Cranial magnetic resonance imaging was normal in 75% (12/16) of the cases and showed structural brain changes in four patients, including crescent-shaped foci of T2 hyperintensity visualized as slight effusion below the dura matter (n=1), nonspecific white matter changes (n=1), and age-related atrophic changes (n=1). Similarly, patients who underwent cranial computed tomography displayed normal brain scans in 89% of cases (24/27). The electroencephalogram was normal in 73% (11/15) and showed generalized slowing without a focal change in 27% of cases (4/15) (Supplementary 3).
Antipsychotic Medications
Antipsychotic medications were utilized in the treatment of 92% (n=55/60) of the cases. The median duration of antipsychotic use was 1.8 [0.6–8] weeks. The longest antipsychotic treatment duration was 39 weeks.
Thyroid Hormone Supplementation
The median initiating and maintenance dose of thyroxine was 100 mcg [50–141]. Intravenous thyroid hormone therapy was administered in 10% of the patients (5/50). In 85% (44/52) of the cases, no thyroxine loading was given. Triiodothyronine was administered to 8% (n=5/58) of the cases.
Steroids
Steroids were administered in 3% of the cases (2/67). The median dose used was equivalent to 50 mg of prednisolone that was used for periods of three days and two weeks, respectively.
Outcome and Follow-Up
Clinical outcome data were reported for 96% of the cases (72/75). The majority of patients, 97% (66/68), required hospitalization, and 93% demonstrated remission. However, two patients (3%) showed no improvement or residual psychosis,23,75 while another three cases (4%) displayed recovery of psychosis with persisting residual deficits in cognition, memory, orientation, attention.31,66 The duration-to outcome occurrence was reported in 83% (62/75) of the cases. The median duration to the outcome (recovery) was 1.93 [0.8–2] weeks. The use of intravenous thyroid hormone supplementation (4/46) was associated with faster recovery compared to oral administration (0.55 [0.5–0.85] weeks vs 2.0 [1–2.85] weeks (p= 0.022). The univariate analysis also revealed a trend towards a shorter duration of psychosis (p= 0.0502) with IV thyroid hormone therapy. However, this effect could not be confirmed in the multivariate analysis (Table 3). Recovery duration did not differ between patients who received triiodothyronine and those who did not 0.5 [0.37–1.95] weeks vs 2 [1–2] weeks, p= 0.2). In the multivariate analysis (Table 3), gender (p= 0.33), age (p=0.46), symptoms duration (p=0.98) TSH level (p=0.29), FreeT4 (p=0.32), antipsychotic drugs duration (p=0.22), IV thyroid hormone therapy, and starting thyroxine dose (0.52) were not associated with shorter duration of psychosis (<2 weeks).
Table 3.
Table Summarizing the Result of the Multivariate Analysis
| Outcome Duration | Odds Ratio | Standard Error | Z | P value | 95% Confidence Interval |
|---|---|---|---|---|---|
| Gender | 0630998 | 0.1818115 | −0.96 | 0.338 | 0.0002226–17.89051 |
| Age | 1.059715 | 0.0844381 | 0.73 | 0.467 | 0.906495–1.238833 |
| Symptoms duration | 0.9985847 | 0.0087879 | −0.16 | 0.872 | 0.9815084–1.015958 |
| TSH Level | 1.011948 | 0.0115271 | 1.04 | 0.297 | 0.9896059–1.034795 |
| Free T4 level | 23.3286 | 74.00553 | 0.99 | 0.321 | 0.0465172–11,699.39 |
| Antipsychotic duration | 1.352324 | 0.3359208 | 1.22 | 0.224 | 0.8310751–2.200501 |
| Intravenous thyroid hormones | 1 | (omitted) | |||
| Thyroxine starting dose | 1.011141 | 0176096 | 0.64 | 0.525 | 0.9772096–1.046251 |
| _cons | 0.0018515 | 0095248 | −1.22 | 0.221 | 7.74e-08-44.30106 |
Notes: Log likelihood = −6.5106511 Pseudo R2 = 0.3718. The outcome of interest is a shorter duration of psychosis recovery (< 2 weeks).
Abbreviations: TSH, thyroid stimulating hormone; T4, thyroxine.
Discussion
The major finding of this systematic review is that evidence on the pathophysiology as well as the clinical course and management of MP is limited to case reports. Descriptive pooling of extracted data from these reports and exploratory analysis indicates that MP can manifest with a wide range of psychiatric and physical symptoms and is commonly treated with antipsychotics and thyroid hormone supplementation. Although the vast majority of patients needed to be hospitalized, very few displayed persisting residual deficits after treatment. Prospective research is urgently needed to improve our understanding of MP and identify factors that may modulate clinical outcomes in order to design standardized diagnostic and therapeutic regimens.
The prevalence of MP was not primarily studied. Therefore, it can only be indirectly estimated from studies evaluating psychotic symptoms in patients with hypothyroidism. Based on the Committee on Myxedema of the Clinical Society of London report, the prevalence of psychotic symptoms was around 50% in hypothyroid patients in the late nineteenth century,5 and was less than 2% in 1965 based on a study of four-hundred hypothyroid patients in which 2% of patients were described to have hypothyroidism associated mental changes (this study did not provide details about the nature of psychic changes, which could be non-psychotic or hypothyroidism unrelated).92
In this review, we identified reports of MP in all adult age groups with a slightly higher proportion of reports on younger patients. Symptoms of hypothyroidism were observed in half of the cases, indicating that the absence of a prior history of hypothyroidism does not rule out MP. The majority of the cases did not have a personal nor family history of psychosis, supporting the direct link between thyroid pathology and psychosis. Although the pooled data from reported cases do not constitute a representative population, we observed a possible pattern in the clinical manifestation of the predominance of paranoid/persecutory delusions and auditory hallucinations. Manic symptoms tended to accompany MP more often than symptoms of depression. Fatigue was the most frequent somatic symptom; that is, however, nonspecific and can be seen in both primary and secondary psychiatric disorders. Although interesting, the predominance of manic symptoms and fatigue can be due to under-reporting as many clinical features were reported in few cases only. Interestingly, almost 40% of the cases did not have any physical hypothyroidism symptoms, while TSH was elevated in all cases with low thyroid hormones supporting the value of screening for thyroid dysfunction in patients with first-ever psychosis. Cranial imaging data did not suggest any relevant changes in brain structure related to MP. Almost all patients needed hospital admission. Antipsychotic medications were given to most of the patients (92%) with remission following short-term administration. Whether this indicates that termination of antipsychotic therapy after remission of psychotic symptoms is safe in patients with MP remains to be answered by prospective research.
Our pooled data of MP cases showed that administration of thyroxin was performed either intravenously or orally. Explorative comparison of synthesized data did not show the superiority of one administration route over the other, although unadjusted comparison showed a possible trend toward faster recovery after IV administration. However, this observation is solely based on cumulative case reports and needs to be viewed in conjunction with the potential increased risk of arrhythmias associated with IV thyroxin.94,95 Administration of triiodothyronine in MP was reported small fraction of the cases and should be investigated in prospective research. In most cases, steroids were not needed, questioning the value of immunosuppressive medication in MP, contrary to myxedema coma and HE. Most cases recovered completely, and only a few cases were left with residual psychosis or cognitive deficits. The etiology of partial improvement or residual cognitive deficits could be either a chronic and irreversible metabolic effect induced by hypothyroidism, as hypothesized by Asher et al or a primary psychiatric illness precipitated by hypothyroidism. All cases that recovered improved within two weeks on average, not exceeding six weeks at most. However, not all cases had long-term follow-up details to study recurrence of psychosis in the presence or absence of dysthyroid status.
Our review excluded two reports of patients with mania that did not exert psychotic features.96,97 Although the reported cases would be classified under the initial term “Myxedema Madness,” we have excluded them as they would not fit within the term “Myxedema psychosis.”
Prior systematic reviews examining HE,98–100 showed that only 20–25% of patients had clinical hypothyroidism, and most of them were euthyroid. It has been stated that psychosis occurs in around a third or less of the patients (26–36%) and is usually accompanied by other features such as; seizures in up to two-thirds (59–66%) and myoclonus (36–42%). These features were not seen in our patient population and their presence may help distinguish HE from MP, especially if the patients are euthyroid. Thyroperoxidase antibodies were found positive in almost all HE cases (86–100%), while our review revealed that 50% of MP cases had autoimmune thyroiditis. This may support the diagnostic value of a negative, not a positive, thyroperoxidase antibody test to differentiate MP from HE. Elevated protein in the cerebrospinal fluid is another characteristic feature of HE occurring in 71–78%, while electroencephalogram is usually abnormal (80–98%).15 These may also help differentiate it from MP as shown in our review, abnormal CSF and or EEG are not common in MP cases. Moreover, the small number of MP cases with abnormal CSF or EEG as depicted by our review could have been simply misdiagnosed HE cases.
Our systematic review has strengths, including a comprehensive literature search following PRISMA guidelines after a priori registration and publication of the predefined review protocol. We were able to pool data from a wide variety of sources identified in the published literature. We provided data on the demographics, diagnosis, and management of myxedema psychosis. Our pooled analysis is solely based on case reports constituting a non-representative population. However, our work clearly shows a substantial research gap, substantiating an urgent need for prospective well-designed research to characterize the clinical course and characteristics of myxedema and to test tailored diagnostic and therapeutic strategies. Publication and reporting bias cannot be ruled since cases considered not interesting or those with adverse outcomes could have been under-reported. Moreover, incomplete reporting of many clinical symptoms or details’ reporting within individual cases may have biased the final conclusion with regards to background data, outcomes, and follow-up duration. Furthermore, the diagnosis of MP was a clinical diagnosis as deemed likely by the treating physicians. We limited our search to case reports starting from 1980. By doing this, we may have missed including a small number of cases; however, considering that the differential diagnosis of HE was first described in 1966,101 we intended to collect comparable cases in order to differentiate between both conditions. Another reason for limiting our search to the time period since 1980 was changes in presentation and symptomatology of hypothyroidism due to improved diagnosis and disease management over time.
Conclusion
Our systematic review and pooled analysis identified a substantial lack of published research on MP. Available case observations indicate that patients with MP present with a broad spectrum of psychiatric and physical symptoms lending support to the value of screening for thyroid dysfunction in patients with first-ever psychosis.
Acknowledgments
This work is the publication of a Master’s thesis of the Master’s program in Clinical Research, Center for Clinical Research and Management Education, Division of Health Care Sciences, Dresden International University, Dresden, Germany.
Ethical Approval
Ethical approval is not required for this article as it is a secondary synthesis of publicly available data.
Disclosure
Dr. Timo Siepmann reports being a editorial board member at Neuropsychiatric Disease and Treatment. He received grants from Michael J. Fox Foundation, the German Federal Ministry of Health, Kurt Goldstein Institute and the German Parkinson Association that were not related to this work. He received royalties from Dresden International University for serving as teacher and program director of the Master’s Program in Clinical Research (MPCR) and from AstraZeneca for serving as advisor. Dr. Barlinn and Dr. Siepmann are editorial board members of Neuropsychiatric Disease and Treatment. The authors report no other conflicts of interest in this work.
References
- 1.Hunter I, Greene SA, MacDonald TM, Morris AD. Prevalence and aetiology of hypothyroidism in the young. Arch Dis Child. 2000;83(3):207–210. doi: 10.1136/adc.83.3.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoogendoorn EH, Hermus AR, De Vegt F, et al. Thyroid function and prevalence of anti-thyroperoxidase antibodies in a population with borderline sufficient iodine intake: influences of age and sex. Clin Chem. 2006;52(1):104–111. doi: 10.1373/clinchem.2005.055194 [DOI] [PubMed] [Google Scholar]
- 3.Aoki Y, Belin RM, Clickner R, et al. Total T 4 in the United States population and their association with participant characteristics: national health and nutrition examination survey (NHANES 1999–2002). Thyroid. 2007;17(12):1211–1223. doi: 10.1089/thy.2006.0235 [DOI] [PubMed] [Google Scholar]
- 4.Mehran L, Amouzegar A, Rahimabad PK, Tohidi M, Tahmasebinejad Z, Azizi F. Thyroid function and metabolic syndrome: a population-based thyroid study. Horm Metab Res. 2017;49(3):192–200. doi: 10.1055/s-0042-117279 [DOI] [PubMed] [Google Scholar]
- 5.Report of a committee of the Clinical Society Of London. Available from:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1288618/. Accessed February8, 2020.
- 6.Asher R. Myxoedematous madness. Br Med J. 1949;2(4627):555–562. doi: 10.1136/bmj.2.4627.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regier DA, Kuhl EA, Kupfer DJ, The DSM-5. Classification and criteria changes. World Psychiatry. 2013;12(2):92–98. doi: 10.1002/wps.20050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sardar S, Habib M-B, Sukik A, et al. Myxedema psychosis: neuropsychiatric manifestations and rhabdomyolysis unmasking hypothyroidism. Case Rep Psychiatry. 2020;2020:7801953. doi: 10.1155/2020/7801953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crocker AD, Overstreet DH, Crocker JM. Hypothyroidism leads to increased dopamine receptor sensitivity and concentration. Pharmacol Biochem Behav. 1986;24(6):1593–1597. doi: 10.1016/0091-3057(86)90491-0 [DOI] [PubMed] [Google Scholar]
- 10.Claustre J, Balende C, Pujol JF. Influence of the thyroid hormone status on tyrosine hydroxylase in central and peripheral catecholaminergic structures. Neurochem Int. 1996;28(3):277–281. doi: 10.1016/0197-0186(95)00088-7 [DOI] [PubMed] [Google Scholar]
- 11.Ruel J, Faure R, Dussault JH. Regional distribution of nuclear T3 receptors in rat brain and evidence for preferential localization in neurons1. J Endocrinol Investig off J Ital Soc Endocrinol. 1985;8(4):343–348. doi: 10.1007/BF03348511 [DOI] [PubMed] [Google Scholar]
- 12.Bauer M, Heinz A, Whybrow PC. Thyroid hormones, serotonin and mood: of synergy and significance in the adult brain. Mol Psychiatry. 2002;7(2):140–156. doi: 10.1038/sj.mp.4000963 [DOI] [PubMed] [Google Scholar]
- 13.Bauer M, Silverman DHS, Schlagenhauf F, et al. Brain glucose metabolism in hypothyroidism: a positron emission tomography study before and after thyroid hormone replacement therapy. J Clin Endocrinol Metab. 2009;94(8):2922–2929. doi: 10.1210/jc.2008-2235 [DOI] [PubMed] [Google Scholar]
- 14.Constant EL, de Volder AG, Ivanoiu A, et al. Cerebral blood flow and glucose metabolism in hypothyroidism: a positron emission tomography study. J Clin Endocrinol Metab. 2001;86(8):3864–3870. doi: 10.1210/jcem.86.8.7749 [DOI] [PubMed] [Google Scholar]
- 15.Mocellin R, Walterfang M, Velakoulis D. Hashimoto’s encephalopathy: epidemiology, pathogenesis and management. CNS Drugs. 2007;21(10):799–811. doi: 10.2165/00023210-200721100-00002 [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(7716):332–336. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed MFH, Siepmann T, Suwileh S, et al. Myxedema psychosis: a protocol for a systematic review and a pooled analysis. Medicine (Baltimore). 2020;99(26):e20778. doi: 10.1097/MD.0000000000020778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Med. 2018;23(2):60–63. doi: 10.1136/BMJEBM-2017-110853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Available from:https://www.jamovi.org/download.html. Accessed February9, 2020.
- 21.Mohamed MFH, Mahgoub AB, Sardar S, Elzouki AN. Acute psychosis and concurrent rhabdomyolysis unveiling diagnosis of hypothyroidism. BMJ Case Rep. 2019;12:10. doi: 10.1136/bcr-2019-231579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neal JM, Yuhico RJO. “Myxedema madness” associated with newly diagnosed hypothyroidism and obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):717–718. doi: 10.5664/jcsm.2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueno S, Tsuboi S, Fujimaki M, et al. Acute psychosis as an initial manifestation of hypothyroidism: a case report. J Med Case Rep. 2015;9(1):1–4. doi: 10.1186/s13256-015-0744-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavroson MM, Patel N, Akker E. Myxedema psychosis in a patient with undiagnosed Hashimoto thyroiditis. J Am Osteopath Assoc. 2017;117(1):50–54. doi: 10.7556/jaoa.2017.007 [DOI] [PubMed] [Google Scholar]
- 25.Nathan R, Rix K, Kent J. Myxoedematous madness and grievous bodily harm. J Clin Forensic Med. 1997;4(2):85–90. doi: 10.1016/S1353-1131(97)90079-1 [DOI] [PubMed] [Google Scholar]
- 26.Hines A, Stewart JT, Catalano G. A case of capgras syndrome related to hypothyroidism. J Psychiatr Pract. 2015;21(6):445–448. doi: 10.1097/PRA.0000000000000108 [DOI] [PubMed] [Google Scholar]
- 27.Bel Feki M, Derouiche S, Kammoun R, Mziou O, Mnif L, Melki W. Myxœdema madness: case report. Eur Psychiatry. 2015;30:1869. doi: 10.1016/s0924-9338(15)31433-4 [DOI] [Google Scholar]
- 28.Lazaro PCF, Loureiro JC, Banzato CEM. Psychosis associated with methimazoleinduced hypothyroidism: a case report. J Bras Psiquiatr. 2013;62(2):171–173. doi: 10.1590/S0047-20852013000200012 [DOI] [Google Scholar]
- 29.AmdouniI F, Abid Y, Ben Khedher M, Ghachem R. Acute psychosis in the setting of severe hypothyroidism. Eur Psychiatry. 2015;30:1270. doi: 10.1016/s0924-9338(15)30994-9 [DOI] [Google Scholar]
- 30.Shlykov MA, Rath S, Badger A, Winder GS. “Myxoedema madness” with Capgras syndrome and catatonic features responsive to combination olanzapine and levothyroxine. BMJ Case Rep. 2016;2016:bcr2016215957. doi: 10.1136/bcr-2016-215957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darko DF, Krull A, Dickinson M, Gillin JC, Risch SC. The diagnostic dilemma of myxedema and madness, axis I and axis II: a longitudinal case report. Int J Psychiatry Med. 1988;18(3):263–270. doi: 10.2190/y6ym-9f5w-d24l-34ak [DOI] [PubMed] [Google Scholar]
- 32.Berkowitz MR. Resolution of hypothyroidism after correction of somatovisceral reflex dysfunction by refusion of the cervical spine. J Am Osteopath Assoc. 2015;115(1):46–49. doi: 10.7556/jaoa.2015.007 [DOI] [PubMed] [Google Scholar]
- 33.Tor PC, Lee HY, Fones CSL. Late-onset mania with psychosis associated with hypothyroidism in an elderly Chinese lady. Singapore Med J. 2007;48(4):354–357. [PubMed] [Google Scholar]
- 34.Er C, Anil Sule A. Late onset radioiodine-induced hypothyroidism presenting with psychosis 14 years after treatment: a rare case. Oxford Med Case Rep. 2016;2016(4):68–70. doi: 10.1093/omcr/omw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hynicka LM. Myxedema madness: a case for short-term antipsychotics? Ann Pharmacother. 2015;49(5):607–608. doi: 10.1177/1060028015570089 [DOI] [PubMed] [Google Scholar]
- 36.Manea M, Rusanu V, Patrichi BE, et al. P03-563 - Psychotic disorder after radioactive iodine treatment in a 42-year-old woman thyroidectomized for papillary thyroid carcinoma: case report. Eur Psychiatry. 2011;26:1733. doi: 10.1016/s0924-9338(11)73437-x [DOI] [Google Scholar]
- 37.Gupta A, Bastiampillai T, Disha TI, Lam DH. Rapid response to loading dose levothyroxine in myxedema psychosis. Prim Care Companion CNS Disord. 2017;19(1). doi: 10.4088/PCC.16l01974 [DOI] [PubMed] [Google Scholar]
- 38.Moeller KE, Goswami R, Larsen LM. Myxedema madness rapidly reversed with levothyroxine. J Clin Psychiatry. 2009;70(11):1607–1608. doi: 10.4088/JCP.08l04958yel [DOI] [PubMed] [Google Scholar]
- 39.Nazou M, Parlapani E, Nazlidou EI, Athanasis P, Bozikas VP. Psychotic episode due to Hashimoto’s thyroiditis. Psychiatrike. 2016;27(2):144–147. doi: 10.22365/jpsych.2016.272.144 [DOI] [PubMed] [Google Scholar]
- 40.Madakasira S, Hall TB. Capgras syndrome in a patient with myxedema. Am J Psychiatry. 1981;138(11):1506–1508. doi: 10.1176/ajp.138.11.1506 [DOI] [PubMed] [Google Scholar]
- 41.Santiago JM, Stoker DL, Beigel A, et al. Capgras’ syndrome in a myxedema patient. Hosp Community Psychiatry. 1987;38(2):199–201. [DOI] [PubMed] [Google Scholar]
- 42.Lin CL, Yang SN, Shiah IS. Acute mania in a patient with hypothyroidism resulting from Hashimoto’s Thyroiditis. Gen Hosp Psychiatry. 2013;35(6):683.e1–683.e2. doi: 10.1016/j.genhosppsych.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 43.Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics. 2005;46(3):259–261. doi: 10.1176/appi.psy.46.3.259 [DOI] [PubMed] [Google Scholar]
- 44.Cook DM, Boyle PJ. Rapid reversal of myxedema madness with triiodothyronine. Ann Intern Med. 1986;104(6):893–894. doi: 10.7326/0003-4819-104-6-893_2 [DOI] [PubMed] [Google Scholar]
- 45.Larouche V, Snell L, Morris DV. Iatrogenic myxoedema madness following radioactive iodine ablation for Graves’ disease, with a concurrent diagnosis of primary hyperaldosteronism. Endocrinol Diabetes Metab Case Rep. 2015;2015. doi: 10.1530/edm-15-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khemka D, Ali JA, Koch CA. Primary hypothyroidism associated with acute mania: case series and literature review. Exp Clin Endocrinol Diabetes. 2011;119(8):513–517. doi: 10.1055/s-0031-1277137 [DOI] [PubMed] [Google Scholar]
- 47.Juneja V, Nance M. Treatment of hypothyroidism and psychosis. Aust N Z J Psychiatry. 2014;48(8):780. doi: 10.1177/0004867414526285 [DOI] [PubMed] [Google Scholar]
- 48.Samuel W, Maniam T. Paranoid psychosis in myxoedema: a case report. Med J Malaysia. 1984;39(2):156–158. [PubMed] [Google Scholar]
- 49.Morgado P, Mendonça-Gonçalves M. Acute mania induced by hypothyroidism in a male patient after thyroidectomy. J Neuropsychiatry Clin Neurosci. 2016;28(1):e21–e22. doi: 10.1176/appi.neuropsych.15090219 [DOI] [PubMed] [Google Scholar]
- 50.Khaldi S, Dan B, Basiaux P, De Nutte N, Kornreich C. Manic episode precipitated by withdrawal of hormone replacement therapy in severe hypothyroidism. J Psychiatr Pract. 2006;12(6):409–410. doi: 10.1097/00131746-200611000-00010 [DOI] [PubMed] [Google Scholar]
- 51.Hall RCW, Popkin MK, Devaul R, Hall AK, Gardner ER, Beresford TP. Psychiatric manifestations of Hashimoto’s thyroiditis. Psychosomatics. 1982;23(4):337–342. doi: 10.1016/S0033-3182(82)73397-3 [DOI] [PubMed] [Google Scholar]
- 52.Reddy KS, Rao GP. Hypothyroidism presenting as acute mania-rare case report. Indian J Psychiatry. 2019;61(9):S527–S529. [Google Scholar]
- 53.Pearce MJ, Walbridge DG. Myxoedema madness: a case report. Int J Geriatr Psychiatry. 1991;6(3):189–190. doi: 10.1002/gps.930060313 [DOI] [Google Scholar]
- 54.Selvaraj V, Padala PR. Thyroid myopathy with rhabdomyolysis presenting as agitation: a case report. Prim Care Companion J Clin Psychiatry. 2008;10(4):328. doi: 10.4088/pcc.v10n0411a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandes S, Safeekh A, Shetty S, Chandini S. Hypothyroidism presenting as hallucinosis: a clinical masquerade. Arch Med Heal Sci. 2019;7(1):90. doi: 10.4103/amhs.amhs_65_19 [DOI] [Google Scholar]
- 56.Dastjerdi G. Delusional type psychosis associated with hypothyroidism: a case report. Procedia - Soc Behav Sci. 2013;84:1050–1052. doi: 10.1016/j.sbspro.2013.06.697 [DOI] [Google Scholar]
- 57.Hyams C, Joshi P, Foster P, Katz J. Acute psychosis caused by hypothyroidism following radioactive iodine treatment of Graves’ disease. JRSM Short Rep. 2013;4(4):1–4. doi: 10.1177/2042533313476858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandes VC. Severe hypothyroidism presenting with acute mania and psychosis: a case report and literature review. Bipolar Disord. 2017;3:116. doi: 10.4172/2472-1077.1000116 [DOI] [Google Scholar]
- 59.Mehta R, Nd S, Ry M. A case report of psychosis in a patient of myxedema: myxedema madness. Available from:www.scholarena.com. Accessed December29, 2019.
- 60.Todorov L, Boudaoud AA, De Raykeer RP, et al. A case of violent suicide attempt in a context of myxedema psychosis following radioiodine treatment in a patient with graves’ disease. Case Rep Psychiatry. 2019;2019. doi: 10.1155/2019/4972760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westphal SA. Unusual presentations of hypothyroidism. Am J Med Sci. 1997;314(5):333–337. doi: 10.1097/00000441-199711000-00011 [DOI] [PubMed] [Google Scholar]
- 62.Rao AC, Bhat VK, Kini S. Myxoedema presenting with psychosis. Indian J Psychiatry. 1990;32(3):287–289. [PMC free article] [PubMed] [Google Scholar]
- 63.Morosán Allo YJ, Rosmarin M, Urrutia A, et al. Myxedema madness complicating postoperative follow-up of thyroid cancer. Arch Endocrinol Metab. 2015;59(4):359–364. doi: 10.1590/2359-3997000000090 [DOI] [PubMed] [Google Scholar]
- 64.Parikh N, Sharma P, Parmar C. A case report on myxedema madness: curable psychosis. Indian J Psychol Med. 2014;36(1):80–81. doi: 10.4103/0253-7176.127260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nielsen JM. I see dead people. Hypothyroid myopathy and hypothyroid psychosis. J Miss State Med Assoc. 2010;51(5):135–136. [PubMed] [Google Scholar]
- 66.Kandukuri RC, Khan MA, Soltys SM. Nonadherence to medication in hypothyroidism: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(3). doi: 10.4088/PCC.09m00863gre [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natarajan MN. Hypothyroidism presenting as psychosis - a case report n m natarajan. Univ J Med Med Specialities. 2020. Available from:http://ejournal-tnmgrmu.ac.in/index.php/medicine/article/view/11655. [Google Scholar]
- 68.Martell T, Matuszak J. Mania and psychosis in a receiving interferon. Psychiatr Ann. 2012;42(9):312–313. doi: 10.3928/00485713-20120906-02 [DOI] [Google Scholar]
- 69.Das S, Doval N, Moun V. Autoimmune thyroiditis presenting as psychosis. Shanghai Arch Psychiatry. 2017;29(3):174–176. doi: 10.11919/j.issn.1002-0829.216059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weston SN, Weston C. The mysterious case of the lost pituitary: amiodarone-induced hypothyroidism. Hosp Med. 2000;61(1):64–65. doi: 10.12968/hosp.2000.61.1.1869 [DOI] [PubMed] [Google Scholar]
- 71.Natarajan MNM. Hypothyroidism presenting as psychosis: a case report. Indian J Psychiatry. 2013;55(S99). [Google Scholar]
- 72.O’Hanlon S, Kingston T. That sneaking suspicion, a case of “myxoedema madness. Ir J Med Sci. 2017;186:171–280. doi: 10.1007/s11845-017-1629-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shaw E, Halper J, Yi PE, Asch S. Diagnosis of “myxedema madness”. Am J Psychiatry. 1985;142(5):655. doi: 10.1176/ajp.142.5.655b [DOI] [PubMed] [Google Scholar]
- 74.Sathya A, Radhika R, Mahadevan S, Sriram U. Mania as a presentation of primary hypothyroidism. Singapore Med J. 2009;50(2):e65–e67. [PubMed] [Google Scholar]
- 75.Azzopardi L, Murfin C, Sharda A, De Silva N. Myxoedema madness. BMJ Case Rep. 2010;2010(sep16 1):bcr0320102841–bcr0320102841. doi: 10.1136/bcr.03.2010.2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh G, Choudhary S, Agarwal S, Yadav A. Severe hypothyroidism playing a major cause of psychiatric illness. Indian J Psychiatry. 2019;61(9):S554–S555. [Google Scholar]
- 77.Philip D, Bauman AJ, Comi RJ. Reversible mania: an unusual presentation of hypothyroidism. Endocr Rev. 2017;38(3). [Google Scholar]
- 78.Rizvi A, Shaan F, Fatima N. Hashimoto’s thyroiditis presenting as acute psychosis with religious delusion: a case report. Sri Lanka J Psychiatry. 2017;8(2):29. doi: 10.4038/sljpsyc.v8i2.8158 [DOI] [Google Scholar]
- 79.Agachanli R, Balaban OD, Sila M, Eradamlar N. Psychosis related with Hashimoto thyroiditis: a case report. Dusunen Adam. 2016;29(2):181–186. doi: 10.5350/DAJPN2016290212 [DOI] [Google Scholar]
- 80.Psychotic depression due to Hashimoto thyroiditis. Klin Psikofarmakol Bul. 2014;24:S290–S291. [Google Scholar]
- 81.Islam L, Selle V, Masu A, Gambini O. Latrogenic hypothyroidism leading to an acute psychotic episode. Int Clin Psychopharmacol. 2011;26:e153. doi: 10.1097/01.yic.0000405898.34742.e5 [DOI] [Google Scholar]
- 82.Hypothyroidism induced psychosis: a case report. Klin Psikofarmakol Bul. 2013;23:S128–S129. [Google Scholar]
- 83.Klinik psikofarmakoloji bülteni-bulletin of clinical psychopharmacology; 2016. doi: 10.1080/10177833.2013.11790875 [DOI]
- 84.Leung JG. Severe hypothyroidism presenting as psychosis: a case of myxedema madness. J Pharm Pract. 2011;24(2):249. doi: 10.1177/0897190011403437 [DOI] [Google Scholar]
- 85.Kumar A, Ramaraju D, Veera RL. “Seeing things with hypothyroidism”-a case report of amiodarone induced hypothyroidism presenting with hallucinations. J Am Geriatr Soc. 2011;59:S117. doi: 10.1111/j.1532-5415.2011.03416.x [DOI] [Google Scholar]
- 86.Post-partum thyroiditis with myxedema madness. Thyroid. 2009;19:S85. doi: 10.1089/thy.2009.1589 [DOI] [Google Scholar]
- 87.Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis. Prim Care Companion J Clin Psychiatry. 2003;05(06):260–266. doi: 10.4088/pcc.v05n0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benvenga S, Lapa D, Trimarchi F. Don’t forget the thyroid in the etiology of psychoses. Am J Med. 2003;115(2):159–160. doi: 10.1016/S0002-9343(03)00298-5 [DOI] [PubMed] [Google Scholar]
- 89.Chari S. The lesson from a yellow psychotic patient. Hosp Med. 2002;63(6):370–371. doi: 10.12968/hosp.2002.63.6.2010 [DOI] [PubMed] [Google Scholar]
- 90.Ward A, Bergmann K. Myxoedematous madness - or not?. Int J Geriatr Psychiatry. 1994;9(5):419–420. [Google Scholar]
- 91.Davis AT. Psychotic states associated with disorders of thyroid function. Int J Psychiatry Med. 1989;19(1):47–56. [DOI] [PubMed] [Google Scholar]
- 92.Evans TC, Hodges RE. Myxedema: a study of 400 cases. Arch Intern Med. 1965;116(2):183–190. doi: 10.1001/archinte.1965.03870020023008 [DOI] [PubMed] [Google Scholar]
- 93.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331(19):1249–1252. doi: 10.1056/NEJM199411103311901 [DOI] [PubMed] [Google Scholar]
- 95.Biondi B, Fazio S, Carella C, et al. Cardiac effects of long term thyrotropin-suppressive therapy with levothyroxine. J Clin Endocrinol Metab. 1993;77(2):334–338. doi: 10.1210/jcem.77.2.8345037 [DOI] [PubMed] [Google Scholar]
- 96.Alex C, Kumar R. An interesting case of myxedema mania-a case report. RGUHS J Med Sci. 2015;5:27–29 [Google Scholar]
- 97.Levitte SS. Coexistent hypomania and severe hypothyroidism. Psychosomatics. 1993;34(1):96–97. doi: 10.1016/S0033-3182(93)71934-9 [DOI] [PubMed] [Google Scholar]
- 98.Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol. 2003;60(2):164–171. doi: 10.1001/archneur.60.2.164 [DOI] [PubMed] [Google Scholar]
- 99.Menon V, Subramanian K, Thamizh JS. Psychiatric presentations heralding Hashimoto’s encephalopathy: a systematic review and analysis of cases reported in literature. J Neurosci Rural Pract. 2017;8(2):261–267. doi: 10.4103/jnrp.jnrp_440_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Holanda NCP, de Lima DD, Cavalcanti TB, Lucena CS, Bandeira F. Hashimoto’s encephalopathy: systematic review of the literature and an additional case. J Neuropsychiatry Clin Neurosci. 2011;23(4):384–390. doi: 10.1176/jnp.23.4.jnp384 [DOI] [PubMed] [Google Scholar]
- 101.Brain L. Hashimoto’s disease and encephalopathy. Lancet. 1966;288(7462):512–514. doi: 10.1016/S0140-6736(66)92876-5 [DOI] [PubMed] [Google Scholar]