Abstract
Background
Ureteral double- J stent is usually inserted by retrograde approach to treating obstructed upper urinary tract. The antegrade approach, can be suitable alternative in certain situations without general or spinal anesthesia. The present study demonstrates the indications, success rate, and complications of this approach in treatmenting malignant obstructive uropathy.
Methods
Data of consecutive patients with malignant obstructive uropathy who underwent antegrade ureteral stenting in the Department of Interventional Radiology at Sahloul hospital from January 2013 to February 2020 was retrieved and retrospectively analyzed.
Result
A total of 188 attempts of antegrade ureteral stent insertion was performed during the study period (left side = 78, right side = 82, bilateral = 14). The mean age was 54 years (range: 9–91 years). The indication of the antegrade stenting was the failure of retrograde approach in 63 patients.The single-stage approach was performed 103 times. A percutaneous nephrostomy was placed for the average duration of 22.4 days (range: 2–60 days) for subsequent attempts. Only four patients required general anesthesia. Ureteral obstruction was caused by bladder cancer (n = 92), uterine cancer (n = 31), prostate cancer (n = 28), colorectal cancer (n = 15) and retroperitoneal tumor (n = 8). A protective nephrostomy was left in situ in 44 cases for 48 h. Clinical success was achieved in 96% of the cases. Two and three patients required hospitalization for perirenal abscess and hematuria, respectively.
Conclusion
This retrospective study shows that antegrade ureteral stent insertion has a high success rate with minimal complications.
Trial registration
ClinicalTrials.gov Identifier: NCT04649970. Registered december 2, 2020- Retrospectively registered,https://clinicaltrials.gov/ct2/show/NCT04649970;
Keywords: Antegrade double-J stent, Malignant obstructive uropathy, Urology
Highlights
-
•
Ureters are easily affected by malignant conditions resulting in the interruption of urinary drainage.
-
•
Double J (JJ) stents placement is the most common method for relieving urinary obstruction in such cases.
-
•
The percutaneous antegrade ureteral stenting (PAUS) technique is a relatively newer technique for ureteral stenting.
-
•
PAUS can be used as an alternative route for relieving ureteral obstruction due to malignancies.
1. Introduction
Ureters have a long and narrow structure; they also have an intimate anatomical relationship with pelvic organs. Malignancies of adjacent organs quickly affectthe ureters and cause interruption of urinary drainage [1]. Double J (JJ) stents placement is the most common method for relieving urinary obstruction in such cases. JJ stents are generally inserted under cystoscopic guidance via retrograde route [2]. However, retrograde placementcan be difficult or even impossible, especially in patients with obstructive malignancies [3]. The percutaneous antegrade ureteral stenting (PAUS) technique is a relatively newer technique for ureteral stenting.PAUS can be used as an alternative route for relieving ureteric obstruction due to malignancies [4].
The purpose of the present study was to evaluate the indications, success rate, and complications of PAUS at our institution since its implementation in 2013. This work has been reported in line with the PROCESS criteria [5].
2. Methods
2.1. Patients
Data of consecutive patients who underwent PAUS for malignant ureteric obstruction at the Department of Interventional Radiology at Sahloul Hospital between January 2013 and February 2020 were retrospectively analyzed using medical records and radiology reports. The study was approved by the Ethics Committee of Sahloul Hospital. Informed consent was obtained from all the patients or their relatives. A total of 174 patients (sex ratio = 0.51, age range 9–91 years; mean age, 54 years) were included in the study.
Ureteral obstruction was caused by bladder cancer (n = 92), uterin cancer (n = 31), prostate cancer (n = 28), colorectal cancer (n = 15) and retroperitoneal tumor (n = 8) (Table 1).
Table 1.
Indications for double J placement.
| Cause of ureteral obstruction | Number |
|---|---|
| Bladder cancer | 92 |
| Uterin cancer | 31 |
| Prostate cancer | 28 |
| Colorectal cancer | 15 |
| Retroperitoneal tumor | 8 |
2.2. Technique of PAUS
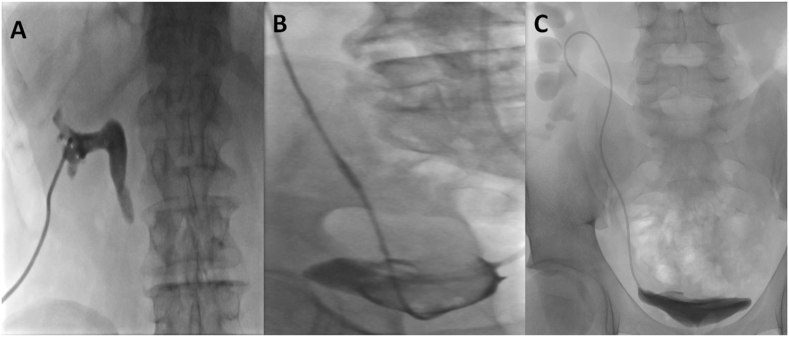
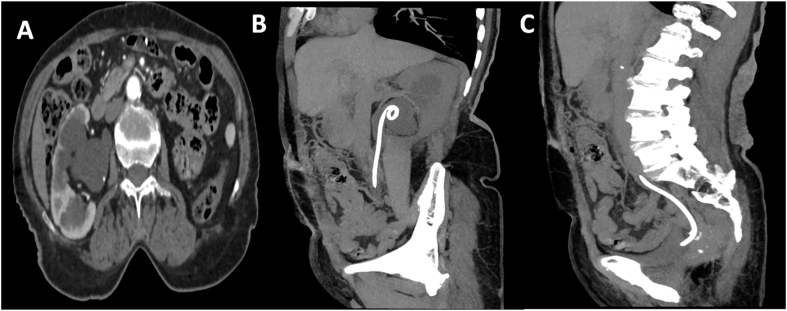
All procedures were performed by an interventional radiologist using ultrasound and fluoroscopy guidance. Local analgesia with conscious sedation was used. General anesthesia was only used exceptionally on the demand of the patient (n = 4). All patients received prophylactic antibiotics preceding the procedure. Percutaneous nephrostomy was carried out with the patient in a 30° prone oblique position. After localization of the collecting system by ultrasound, the lower pole collecting system was punctured with an 18-gauge Chiba needle by a dorsal approach with the Seldinger technique. The collecting system was opacified with nonionic contrast material (Fig. 1).After that, a 0.035-inch guidewire was advanced into the renal pelvis via the needle, and the needle was withdrawn. After tract dilatation, an 8 or 10 French (F) drainage catheter was placed into the renal pelvis for patients with two stage procedure (see Fig. 2).
Fig. 1.
Antegrade insertion of a JJ ureteral stent. (A) A 0.035-inch guidewire is inserted into the renal pelvis. (B) Catheterisation of the ureter into the urinary bladder with a hydrophilic guidewire and a 4 or 5 French catheter. (C) Confirmation of catheter position in the urinary bladder using radiopaque contrast material.
Fig. 2.
(A) abdominal CT scan showing right hydronephrosis secondary to pelvic invasion by a colorectal cancer. (C, D): Antegrade insertion of a JJ ureteral stent.
For patients with single stage approach, antegrade pyelography to visualize all ureteric segments and the ureterovesical junction was performed by contrast material injection. A 5 F multipurpose diagnostic vascular catheter was inserted. Once the pelviureteric junction was crossed, and the ureter was accessed, a straight hydrophilic guidewire and a catheter were inserted. Then, the catheter was advanced into the bladder over the wire. This guidewire was exchanged for an ultra-stiff guidewire, a 7 or 8 F double J ureteral stent was placed over the guidewire, and the safety kit of the stent was removed. A final fluoroscopic image was stored for correcting the stent position.
Technical success of the procedure was defined as maintenance of urinary tract patency and reduction of the severity of hydronephrosis, as determined by imaging (ultrasound or computed tomography). Clinical success was defined as relief of pain and improvement of renal function (reduction in serum creatinine).
2.3. Post-procedure care and follow-up
After the procedure, patients stay in the urology department for 24 h for monitoring. Patients returned to the urology department to assesstent patency, renal function tests (urea and creatinine), a complete blood count, and renal ultrasound.
3. Results
A total of 174 patients (sex ratio = 0.51, age range 9–91 years; mean age, 54 years) were included in the study. Ureteral obstruction was caused by bladder cancer (n = 92), uterine cancer (n = 31), prostate cancer (n = 28), colorectal cancer (n = 15) and retroperitoneal tumor (n = 8) (Table 1).
Totally 188 attempts for JJ stent placement were performed (bilateral JJ stenting in 14 patients).In Sixty three attempts, they underwent previous failed attempts of the retrograde approach(FRA). However, 111 patients (125 attempts) had a directly anterograde approach (DAA), without retrograde attempt Single-stage procedure was performed in 103 attempts, and a percutaneous nephrostomy was placed in multi-stage procedures. The single stage procedure in the DAA group was significantly lower than that of the FRA group (38% vs. 94%, p = 0.03).However Multi-stage procedure in the DAA group was significantly higher than that of the FRA group (62% vs. 3%, p = 0.01)(Table 2).
Table 2.
Outcome and complication rate of directly antegrade approach group and failed retrograde approach group.
| Total (n = 188) | Directly antegrade approach (n = 125) | Failed retrograde approach (n = 63) | P value | |
|---|---|---|---|---|
| Technical success rate | 177 (94%) | 116 (93%) | 61 (97%) | |
| Clinical success rate | 169 (90%) | 108 (86%) | 61 (97%) | |
| Complication rate | 25 (13.3%) | 22 (18%) | 3 (5%) | |
| Single stage procedure | 103 (58%) | 44 (38%) | 59 (94%) | 0,03 |
| Multi-stage procedure | 74 (42%) | 72 (62%) | 2 (3%) – |
0,01 |
The mean duration of percutaneous nephrostomy was 22.4 days (2–60 days) for the other attempts. A protective nephrostomy was left in 44 cases for 48 h.
The mean procedure time was 29 min (range: 15–86 min), clinical success (improvement in serum creatinine and resolution of hydronephrosis) was achieved in 169 attempts (90%), and technical success was noticed in 177 attempts (94%). Eleven patients had unilateral lumbar pain for 24–48 h. Seven patients (3.7%) required postprocedural hospitalization for perirenal abscess (n = 3), hematuria (n = 2) and subcapsular renal hematoma (n = 2) and were considered as major complications according to the guideline of Society of Interventional Radiology(Table 3) [6].The complication rate in the FRA group was lower than that of the DAA group (5% vs 18%). A drainage catheter was inserted for abscess formation and one unit of packed erythrocyte suspension was transfused for hematuria. Subcapsular renal hematomas resolved spontaneously without any blood transfusion or additional medication.
Table 3.
Summary of complications.
| Complication | Number |
|---|---|
| Minor | 11 |
| Lumbar pain | 11 |
| Major | 7 |
| Perirenal abcess | 3 |
| Hematuria | 2 |
| Subcapsular hematoma | 2 |
4. Discussion
For ureteral obstruction due to benign diseases, retrograde stenting has several advantages over PAUS. Using the retrograde approach, it is possible to manage ureteral stones, practice incision of ureteral strictures, or take a ureteral suspicious lesion biopsy [3,7].
A review of literature has shown that the rate of retrograde stenting failure is significantly higher in cases of malignant compression of ureters and that in most cases of or locally advanced prostate cancer or infiltrant bladder cancer, percutaneous nephrostomy is preferable because retrograde stenting would be impossible due to encroachment of tumor into the ureteral orifices [8,9]. In patients presenting with malignant ureteric obstruction, success rates for retrograde ureteral stenting have been reported to be 50%–88% [10,11].
On the other hand, PAUS is more efficient for patients having malignant compression, the technical success rate of 94% of ureteral stenting in the present serie is consistent favorably with other published studies which have reported success rates varying from 85% to 98% [2,[12], [13], [14]] (Table 4).
Table 4.
Comparison of success rates of PAUS.
| Studies | PAUS success |
|---|---|
| Chitale et al.(2) | 39/40 (98%) |
| Uthappa et al.(11) | 24/25 (96%) |
| Harding et al.(12) | 34/37 (92%) |
| Mitty et al.(13) | 67/78 (85%) |
| Kahriman et al.(14) | 639/654 (97%) |
| Our study | 177/188 (94%) |
PAUS should be reserved for patients with fails retrograde insertion and when a patient already has a percutaneous nephrostomy catheter. Because the access to the renal pelvis has already been secured which simplifies PAUS procedure and decrease complications rate [[15], [16], [17]].
In literature, several complications of PAUS have been documented, such as ureteric injury, arterial perforation, and arteriovenous fistula formation caused by vascular injury at the time of stent insertion [18]. In our study, there were only seven significantcomplications (3.7%), all related to percutaneous nephrostomy.
Rutger et al. reported mild hematuria in only 6 cases out of 130 patients after JJ stent insertion. That might be explained by damaged urothelium [19]. Bleeding from the kidney parenchyma is most commonly observed from the site of parenchymal puncture of percutaneous nephrostomy with the reported incidence of 3% of cases after percutaneous nephrostomy catheter placement [20]. In our study, only two patients were hospitalized for hematuria.
Another complication was observed in two patients (1%): subcapsular renal hematoma. In literature: Naeem et al. [21], Jalbani et al. [22], and Romero et al. [23] observed this complication in 4.0%, 5.0%, and 3.5% of their patients, respectively.
The creation of false ureteral tracts is a rare complication. However, it should be kept in mind in case of malfunctioning stent, placement of a percutaneous nephrostomy decrease the pressure of the perforated ureter, usually allowing ureteral cicatrisation [18].
Although this is one of the largest series on PAUS, this study was limited by its retrospective nature where clinical conditions of the patients may be underreported.
5. Conclusion
This retrospective study shows that antegrade, percutaneous insertion of a JJ stent is possible with a high technical success rate (94%) and a low risk of complications. Therefore, antegrade, percutaneous insertion of a JJ stent seems to be a good and safe alternative when retrograde insertion fails, especially for malignant obstruction.
Authors’ contributions
Waad Farhat- data collection, Editing of manuscript.
Dziri sonia– Data collection, Editing of the manuscript.
Sofiene arem – Editing of manuscript, supervision of the manuscript.
Emir akacha- Reviewing the article before submission/Constructing an idea or hypothesis for research/Organising and supervising the course of the article.
Houssem Ammar– Editing of manuscript, literature review, drafting the manuscript.
Azzabi awatef- Reviewing the article before submission not only for spelling and grammar but also for its intellectual content/Taking responsibility in execution of the experiments, patient follow-up.
Rahul Gupta – Editing of manuscript, literature review, drafting the manuscript.
Mehdi jaidane– manuscript correction, supervision of the manuscript.
Azzabi awatef – Supervision of the manuscript, manuscript correction.
Guedri yosra– Supervision of the manuscript, manuscript correction.
Availability of data and materials
Access to the data and the calculation method can be obtained from the authors by email.
Grant support or financial relationship
There is no funding for this study.
Patient consent
Written informed consent was obtained from all patients enrolled in the investigation.
The study protocol conformed to the guidelines of the regional ethical committee of Sahloul Hospital.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was reviewed and approved by the Ethics Committee of Sahloul hospital: approval references: U2352.
Sources of funding
This study has not received any funding.
Author contribution
Study concept or design – GT, HA.
Data collection – SD, WF, RG.
Data interpretation – EA, AA, MJ.
Literature review – SA, SD, RG,AA.
Drafting of the paper – HA, YG, MJ.
Editing of the paper – GT, WF,SD.
Registration of research studies
Name of the registry: ClinicalTrials.gov.
Unique Identifying number or registration ID: NCT04649970.
Hyperlink to the registration (must be publicly accessible): https://clinicaltrials.gov/ct2/show/NCT04649970.
Guarantor
Ghassen tlili.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors gratefully acknowledge all of the investigators for their contributions to the trial.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102726.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dowling R.A., Corriere J.N., Jr., Sandler C.M. Iatrogenic ureteral injury. J. Urol. 1986;135:912–915. doi: 10.1016/s0022-5347(17)45921-0. [DOI] [PubMed] [Google Scholar]
- 2.Chitale S.V., Scott-Barrett S., Ho E.T., Burgess N.A. The management of ureteric obstruction secondary to malignant pelvic disease. Clin. Radiol. 2002;57:1118–1121. doi: 10.1053/crad.2002.1114. [DOI] [PubMed] [Google Scholar]
- 3.Yossepowitch O., Lifshitz D.A., Dekel Y., Gross M., Keidar D.M., Neuman M., Livne P.M., Baniel J. Predicting the success of retrograde stenting for managing ureteral obstruction. J. Urol. 2001;166:1746–1749. [PubMed] [Google Scholar]
- 4.Rutger W., van der Meer, Saskia W. Antegrade ureteral stenting is a good alternative for the retrograde Approach. Curr Urol. 2016;10:87–91. doi: 10.1159/000447157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agha R.A., Sohrabi C., Mathew G., Franchi T., Kerwan A., O'Neill N for the PROCESS Group The PROCESS 2020 guideline: updating consensus preferred reporting of CasE series in surgery (PROCESS) guidelines. Int. J. Surg. 2020;84:231–235. doi: 10.1016/j.ijsu.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Pabon-Ramos W.M., Dariushnia S.R., Walker T.G. Society of interventional radiology standards of practice committee. Quality improvement guidelines for percutaneous nephrostomy. J. Vasc. Intervent. Radiol. 2016;27:410–414. doi: 10.1016/j.jvir.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 7.Song Y., Fei X., Song Y. Percutaneous nephrostomy versus indwelling ureteral stent in the management of gynecological malignancies. Int. J. Gynecol. Canc. 2012;22:697–702. doi: 10.1097/IGC.0b013e318243b475. [DOI] [PubMed] [Google Scholar]
- 8.Kanou T., Fujiyama C., Nishimura K. Management of extrinsic malignant ureteral obstruction with urinary diversion. Int. J. Urol. 2007;14:689–692. doi: 10.1111/j.1442-2042.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 9.Ganatra A.M., Loughlin K.R. The management of malignant ureteral obstruction treated with ureteral stents. J. Urol. 2005;174:2125–2128. doi: 10.1097/01.ju.0000181807.56114.b7. [DOI] [PubMed] [Google Scholar]
- 10.Wang J.Y., Zhang H.L., Zhu Y. Predicting the failure of retrograde ureteral stent insertion for managing malignant ureteral obstruction in outpatients. Oncol Lett. 2016;11:879–883. doi: 10.3892/ol.2015.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uthappa M.C., Cowan N.C. Retrograde or antegrade double-pigtail stent placement for malignant ureteric obstruction? Clin. Radiol. 2005;60:608–612. doi: 10.1016/j.crad.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Harding J.R. Percutaneous antegrade ureteric stent insertion in malignant disease. J. R. Soc. Med. 1993;86:511–513. [PMC free article] [PubMed] [Google Scholar]
- 13.Mitty H.A., Dan S.J., Train J.S. Antegrade ureteral stents: technical and catheter-related problems with polyethylene and polyurethane. Radiology. 1987;165:439–443. doi: 10.1148/radiology.165.2.3659366. [DOI] [PubMed] [Google Scholar]
- 14.Kahriman G., Özcan N., Doğan A. Percutaneous antegrade ureteral stent placement: single center experience. Diagn Interv Radiol. 2019;25:127–133. doi: 10.5152/dir.2019.18252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackethorn J.C., Boren S.R., Dotter C.T. Antegrade internal ureteral stenting: a technical refinement. Radiology. 1985;156:827–828. doi: 10.1148/radiology.156.3.4023252. [DOI] [PubMed] [Google Scholar]
- 16.Lu D.S., Papanicolaou N., Girard M. Percutaneous internal ureteral stent placement: review of technical issues and solutions in 50 consecutive cases. Clin. Radiol. 1994;49:256–261. doi: 10.1016/s0009-9260(05)81852-5. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S.D., Persad R.A., Haq A. A review of antegrade stenting in the management of the obstructed kidney. Br. J. Urol. 1996;78:511–515. doi: 10.1046/j.1464-410x.1996.01673.x. [DOI] [PubMed] [Google Scholar]
- 18.Rao A.R., Alleemudder A., Mukerji G. Extra-anatomical complications of antegrade double-J insertion. Indian J. Urol. 2011;27:19–24. doi: 10.4103/0970-1591.78408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutger W., van der Meer, Saskia W. Antegrade ureteral stenting is a good alternative for the retrograde Approach. Curr Urol. 2016;10:87–91. doi: 10.1159/000447157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wah T.M., Weston M.J., Irving H.C. Percutaneous nephrostomy insertion: outcome data from a prospective multi-operator study at a UK training centre. Clin. Radiol. 2004;59:255–261. doi: 10.1016/j.crad.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Naeem M., Jan M.A., Ullah A. Percutaneous nephrostomy for the relief of upper urinary tract obstruction: an experience with 200 cases. JPMI. 2010;24:147–152. [Google Scholar]
- 22.Jalbani M.H., Deenari R.A., Dholia K.R. Role of percutaneous nephrostomy (PCN) in malignant ureteral obstruction. J. Pakistan Med. Assoc. 2010;60:280–283. [PubMed] [Google Scholar]
- 23.Romero F.R., Broglio M., Pires S.R. Indications for percutaneous nephrostomy in patients with obstructive uropathy due to malignant urogenital neoplasias. Int. Braz J. Urol. 2005;31:117–124. doi: 10.1590/s1677-55382005000200005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to the data and the calculation method can be obtained from the authors by email.