Abstract
Purpose
We analysed incidence, treatment, survival, occurrence of ductal carcinoma in situ (DCIS) and invasive breast cancer (IBC) after lobular carcinoma in situ (LCIS) in the Netherlands.
Methods
All women diagnosed with classic LCIS between 1989 and 2017 were identified from the Netherlands Cancer Registry. We calculated overall (OS), relative survival (RS) and cumulative incidence functions (CIF, accounting for competing risks) of mortality, DCIS and IBC. For IBC, standardised incidence ratios (SIR) of IBC were calculated. Analyses were stratified for surgical treatment.
Results
We included 1890 patients. Median age was 51 years. Median follow-up was 8.5 years. In 1989–2017, LCIS incidence increased from 41 to 124, surgical treatment decreased from 100% to 41.1 % – mostly BCS. 10-year OS and 20-year RS exceeded 90 % in all subgroups. Overall, 48 (2.5 %) and 270 (14.3 %) patients were diagnosed with DCIS and IBC. IBCs were mostly early-stage. After mastectomy, 13 of 14 IBCs presented contralaterally. In the other groups, 64.8–70.9 % of IBCs presented ipsilaterally, 34.5–53.9 % of these were lobular. The SIR of ipsilateral IBC was highest after no surgery (6.9, 95%CI:4.9–9.4), lowest after mastectomy (0.2, 95%CI:0.4–0.8).
Conclusion
LCIS incidence increased, surgical treatment decreased. The low mortality risks support consideration of active surveillance. However, the increased IBC incidence suggests careful monitoring.
Keywords: Lobular carcinoma in situ, Invasive breast cancer, Survival, Cumulative incidence function, Standardised incidence ratio, Population-based study
Highlights
-
•
The incidence of LCIS increased, while surgical treatment decreased over time.
-
•
10- and 20-year relative survival rates exceeded 90 %, irrespective of surgery.
-
•
The risk of breast cancer was highest after no surgery, lowest after mastectomy.
-
•
Subsequent breast cancers were generally of low stage.
-
•
Active surveillance for LCIS is advised, with the current follow-up five years.
1. Introduction
In 1941, lobular carcinoma in situ (LCIS) was described as breast cancer [1]. In 1983, it was included in the AJCC staging system [2]. Due to its high disease-specific specific survival [[3], [4], [5]], it was removed from the 8th edition. Subsequently, LCIS was considered as lobular neoplasia. LCIS often remains undetected due to lack of specific clinical and radiological features. Consequently, the diagnosis is mostly based on an accidental finding or mass lesions/calcifications on screening mammography [[6], [7], [8]]. LCIS is histologically classified into classic, pleomorphic and florid LCIS [9]. Since pleiomorphic and florid LCIS are described to behave similar to high grade ductal carcinoma in situ (DCIS) [10], those patients are recommended to be treated as such. Regarding classic LCIS, the optimal treatment is subjected to debate [11]. Based on its high relative risk of subsequent invasive breast cancer (IBC) [9,11,12], LCIS is often recommended to be treated with surgery [[13], [14], [15]]. However, other studies describe a more conservative approach as the absolute risk is considered to be low [16,17]. The latter was adopted in the Dutch breast cancer guideline in which active surveillance for classic LCIS is recommended [18]. Analysing the effect of surgical treatment is difficult, as patients not treated with surgery can have an invasive component which remains undetected. It is therefore crucial to analyse survival and the risk of IBC separately for surgically and non-surgically treated patients.
Here, we aimed to describe trends in incidence and treatment of LCIS over time, as well as long-term survival and incidence of DCIS and IBC, according to surgical treatment.
2. Methods
2.1. Design and patients
We used the Netherlands Cancer Registry (NCR), containing patient-, tumour and treatment-related characteristics of all newly diagnosed malignancies covering the entire Dutch population since 1989. We selected all LCIS patients, identified through a histologically confirmed diagnosis between 1989 and 2017. We obtained additional data on expected mortality risks and size of the general population from Statistics Netherlands. Vital status was obtained from the Municipal Personal Records Database (complete until January 31, 2019). We excluded men, women with a history of invasive breast cancer or DCIS and patients registered as having had pleomorphic LCIS.
2.2. Outcomes and definitions
First, we investigated age-standardised trends in incidence and surgical treatment over time, using European Standardised Rates (ESR), in which we corrected for the European population structure (ESP, 1976). Second, we analysed long-term overall (OS), relative survival (RS) and standardised incidence ratios (SIR) of IBC and DCIS following LCIS in the Netherlands. All outcomes were analysed according to (type of) surgery. This grouping is crucial, as patients not treated with surgery could have had concurrent IBC, which is unknown due to lack of surgery. Opposingly, patients treated with surgery in whom – next to LCIS – an invasive component was found, were registered as if they had IBC (without coding concurrent LCIS). These patients are therefore not included in our selection and not traceable. This information is crucial in interpreting the results.
To obtain better insights in IBC risks, this outcome was further analysed according to its laterality. Ipsilateral and contralateral IBC were defined as IBC diagnosed in the same and opposite breast as LCIS. Follow-up was calculated from date of LCIS diagnosis to date of event (death or IBC) or last observation.
2.3. Statistical analysis
Numerical variables and categorical data were summarized using the median (interquartile range, IQR) and frequency (percentage), respectively. For OS, differences between treatment groups were visualized by Kaplan-Meier curves and tested by the log-rank test. RS (observed survival divided by expected survival of general population, matched by age, sex and calendar year) was estimated with 95 % confidence intervals (CIs) using the Ederer II method.
The incidences of subsequent DCIS and IBC as first event were assessed by the cumulative incidence function (CIF). By considering the other events as competing risks, it estimates the crude risk of an event accounting for the possibility to experience another event [19].
For subsequent IBC, SIRs were calculated by dividing the observed number of IBC cases by the number of cases in the general population (expected). Statistical analyses were performed in Stata (v16.1).
3. Results
From the NCR, 2448 LCIS patients diagnosed between 1989 and 2017 were identified. We excluded males (n = 2,0.1 %), patients with pleiomorphic LCIS (n = 3,0.1 %) and prior (breast) malignancies (n = 443,22.6 %), leading to 1890 included patients (77 % of total). Of these, 505 (26.7 %) received no surgery, 954 (50.5 %) breast-conserving surgery (BCS), 193 (10.2 %) mastectomy and 238 (12.6 %) an unknown type of surgery (mainly in earlier years). Patients treated with mastectomy were the oldest, patients with an unknown type of surgery were the youngest (54 versus 49 years). Since 2011, detection through screening was regularly registered in the NCR, therefore for many patients the method of detection was unknown. However, in patients not treated with surgery, most tumours were detected by the national screening program (49.5 %). From 2011 on, approximately 70 % of all eligible patients (50–74 years) was diagnosed through screening (data not shown). After unknown surgery, 23.5 % of the tumours were incidentally detected during a surgical breast procedure for a benign indication. There were 61 patients treated with radiotherapy (56 received BCS). Three patients received endocrine therapy, all treated with surgery (Table 1).
Table 1.
Baseline characteristics of women diagnosed with classic LCIS in 1989–2017 according to surgical management.
| Characteristics | No surgery (n = 505) | Breast-conserving surgery (n = 954) | Mastectomy (n = 193) | Surgery of unknown type (n = 238) |
|---|---|---|---|---|
| Age in years (median, IQR) | 52 (50–58) | 51 (48–59) | 54 (48–62) | 49 (44–54) |
| Age group | ||||
| <50 years | 119 (23.6) | 324 (34.0) | 65 (33.7) | 127 (53.4) |
| 50–64 years | 309 (61.2) | 492 (51.6) | 91 (47.2) | 95 (39.9) |
| ≥65 years | 77 (15.3) | 138 (14.5) | 37 (19.2) | 16 (6.7) |
| Socioeconomic status | ||||
| Low | 111 (22.0) | 269 (28.2) | 53 (27.5) | 61 (25.6) |
| Medium | 189 (37.4) | 371 (38.9) | 79 (40.9) | 98 (41.2) |
| High | 205 (40.6) | 314 (32.9) | 61 (31.6) | 79 (33.2) |
| Period of diagnosis | ||||
| 1989–1998 | 12 (2.4) | 206 (21.6) | 72 (37.3) | 148 (62.2) |
| 1999–2008 | 41 (8.1) | 370 (38.8) | 60 (31.1) | 52 (21.9) |
| 2009–2017 | 452 (89.5) | 378 (39.6) | 61 (31.6) | 38 (16.0) |
| Detection through screening∗ | ||||
| No | 127 (25.2) | 102 (10.7) | 25 (13.0) | 26 (10.9) |
| Yes | 250 (49.5) | 171 (17.9) | 17 (8.8) | 1 (0.42) |
| Unknown | 128 (25.4) | 681 (71.4) | 151 (78.2) | 211 (88.7) |
| Incidental finding during surgical breast procedure | ||||
| No | 505 (100) | 922 (96.7) | 179 (92.8) | 182 (76.5) |
| Yes | 0 (0.0) | 32 (3.4) | 14 (7.3) | 56 (23.5) |
| Tumour lateralisation | ||||
| Left | 268 (53.1) | 507 (53.1) | 103 (53.4) | 111 (46.6) |
| Right | 236 (46.7) | 446 (46.8) | 90 (46.6) | 126 (52.9) |
| Unknown | 1 (0.2) | 1 (0.1) | 0 (0.0) | 1 (0.42) |
| Sublocalisation | ||||
| Outer quadrants | 254 (50.3) | 469 (49.2) | 85 (44.0) | 70 (29.4) |
| Inner quadrants | 58 (11.5) | 130 (13.6) | 12 (6.2) | 28 (11.8) |
| Central parts | 62 (12.3) | 95 (10.0) | 8 (4.2) | 18 (7.6) |
| Overlapping lesions | 89 (17.6) | 181 (19.0) | 55 (28.5) | 49 (20.6) |
| Unknown | 42 (8.3) | 79 (8.3) | 33 (17.1) | 73 (30.7) |
| Multifocality | ||||
| No | 403 (79.8) | 504 (52.8) | 74 (38.3) | 75 (31.5) |
| Yes | 14 (2.8) | 28 (2.9) | 15 (7.8) | 9 (3.8) |
| Unknown | 6 (1.2) | 1 (0.1) | 1 (0.5) | 0 (0.0) |
| Palpable mass | ||||
| No | 309 (61.2) | 220 (23.1) | 32 (16.6) | 17 (7.1) |
| Yes | 48 (9.5) | 44 (4.6) | 7 (3.6) | 2 (0.8) |
| Unknown | 148 (29.3) | 690 (72.3) | 154 (79.8) | 219 (92.0) |
| Tumour grade | ||||
| Well differentiated (I) | 37 (7.3) | 49 (5.1) | 11 (5.7) | 3 (1.3) |
| Intermediately differentiated (II) | 4 (0.8) | 34 (3.6) | 14 (7.3) | 0 (0.0) |
| Poorly differentiated (III) | 2 (0.4) | 29 (3.0) | 9 (4.7) | 1 (0.4) |
| Unknown | 462 (91.5) | 842 (88.3) | 159 (82.4) | 234 (98.3) |
| Radiotherapy | ||||
| No | 504 (99.8) | 898 (94.1) | 193 (100) | 234 (98.3) |
| Yes | 1 (0.2 %) | 56 (5.9) | 0 (0) | 4 (1.7 %) |
| Endocrine therapy | ||||
| No | 0 (0.0) | 1 (0.1) | 1 (0.5) | 1 (0.4) |
| Yes | 505 (100.0) | 953 (99.9) | 192 (99.5) | 237 (99.6) |
Numbers are n(%) unless otherwise specified. ∗Detection through screening was only registered from 2011 on. Abbreviations: n, number; cm, centimetre.
The incidence of LCIS increase over time, from 41 diagnoses in 1989 to 124 diagnoses in 2017. In 1989, all patients received surgery, while in 2017 this was 41.1 %. ESRs as well as the distribution of surgical treatments are shown in Fig. 1.
Fig. 1.
European standardised incidence rates and treatment patterns of classic lobular carcinoma in situ in the Netherlands (1989–2017). Abbreviations: ESR, European Standardised Rate; BCS, Breast-conserving surgery.
3.1. Overall and relative survival
Median follow-up to death or last observation was 8.5 years (IQR:4.2–16.8 years). Ultimately, 245 patients died (13.0 %). These percentages were 3.6 %, 14.2 %, 26.4 % and 17.2 % after no surgery, BCS, mastectomy and unknown surgery. Overall 10-year OS and RS were 91.5 % (95%CI:89.7–92.8 %) and 98.5 % (95%CI:96.7–100 %), respectively. Twenty-year OS and RS were 76.0 % (95%CI:72.7–79.0 %) and 95.2 %, 95%CI:91.0–98.9 %), respectively. Both outcomes were highest after unknown surgery and lowest after mastectomy. RS rates exceeded 90 % in all groups (Table 2,Supplementary Figure 1).
Table 2.
Survival outcomes and cumulative incidence functions of mortality following a diagnosis of classic lobular carcinoma in situ.
| Characteristics | Overall survival (95 % CI) |
Relative survival (Ederer II) (95 % CI) |
Cumulative incidence function of mortality (95 % CI) |
|||
|---|---|---|---|---|---|---|
| 10 years | 20 years | 10 years | 20 years | 10 years | 20 years | |
| Entire cohort | 91.5 % (89.7–97.7 %) | 76.0 % (72.7–79.0 %) | 98.5 % (96.7–100.0 %) | 95.2 % (91.0–98.9 %) | 7.5 % (6.0–9.1 %) | 16.7 % (14.0–19.5 %) |
| Management | ||||||
| No surgery | 90.6 % (82.8–94.9 %) | 88.2 % (78.5–93.7 %) | 97.2 % (88.4–100.0 %) | 101.0 % (93.9–114.0 %) | 12.1 % (5.5–21.5 %) | 12.1 % (5.5–21.5 %) |
| BCS | 92.2 % (89.9–93.9 %) | 74.0 % (69.2–78.2 %) | 99.1 % (96.7–101 %) | 92.4 % (86.5–97.6 %) | 6.4 % (4.7–8.4 %) | 15.5 % (11.9–19.6 %) |
| Mastectomy | 84.5 % (77.9–89.3 %) | 67.5 % (58.4–75.02 %) | 92.7 % (85.4–97.9 %) | 90.7 % (78.6–100.0 %) | 13.7 % (8.8–19.7 %) | 29.2 % (21.2–37.7 %) |
| Unknown | 94.3 % (89.9–96.8 %) | 84.6 % (78.1–89.3 %) | 101.0 % (96.3–103.7 %) | 103.0 % (95.1–108.8 %) | 5.5 % (2.8–9.4 %) | 13.1 % (8.4–18.9 %) |
AbbreviationsCI, confidence interval; BCS, Breast-conserving surgery.
3.2. Cumulative mortality incidence
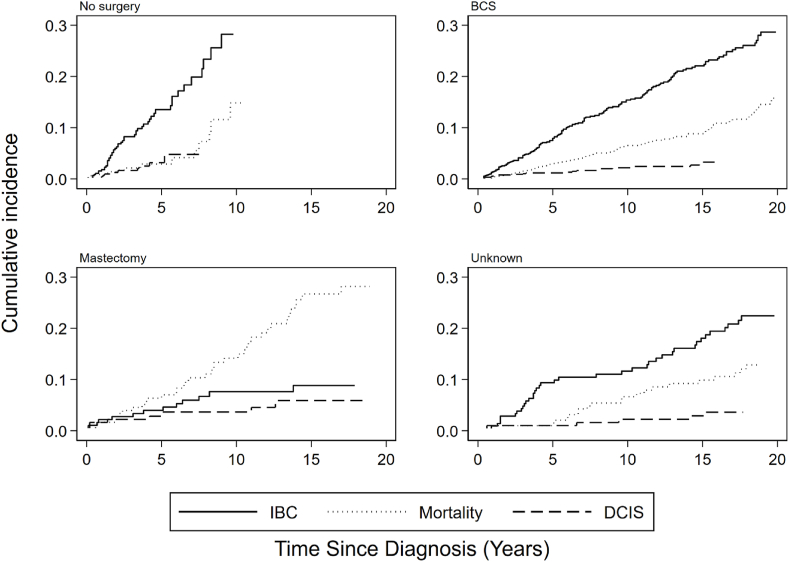
CIFs for all outcomes, corrected for competing risks, are shown in Fig. 2.
Fig. 2.
Cumulative incidence functions of invasive breast cancer, mortality and DCIS following a LCIS diagnosis in the Netherlands, according to type of surgical management and corrected for competing risks. Abbreviations: IBC, invasive breast cancer; DCIS, ductal carcinoma in situ.
Because in the first half period of our cohort all patients received surgery, the follow-up of patients not treated with surgery was limited. Taking into account IBC as competing risk, the overall CIFs for 10- and 20-year mortality were 7.5 % (95%CI:6.0–9.1 %) and 16.7 % (95%CI:14.0–19.5 %), respectively. Patients treated with unknown surgery had the lowest CIFs, followed by BCS, while patients treated with mastectomy had the highest CIFs(Table 2).
3.3. DCIS and IBC
After a median follow-up to first event of 6.0 years (IQR:2.6–13.8 years), subsequent DCIS and IBC were diagnosed in 48 (2.5 %) and 270 (14.2 %) of all patients, respectively. Patients not treated with surgery had the shortest follow-up (3.0 years, IQR:1.4–5.1 years), and patients treated with unknown surgery had the longest follow-up (12.9 years, IQR:4.1–21.1 years), corresponding with the developments in surgical treatment over time (Fig. 1). Of patients diagnosed with DCIS, most patients (n = 21,43.8 %) received BCS for primary LCIS. For IBC this was 58.9 %. As subsequent DCIS in this population was rare – and IBC is more decisive for prognosis – we focused on IBC only in further analyses.
IBC was mostly diagnosed in patients treated with unknown surgery (n = 42,17.6 %), followed by BCS (n = 159,16.7 %), no surgery (n = 55,10.9 %) and mastectomy (n = 14,7.3 %). IBCs were mostly stage I-II, hormonal receptor positive, HER2 negative. Among subsequent IBCs in patients not treated with surgery, 39 (70.9) presented ipsilaterally. Opposingly, only one IBC (7.1 %) in patients treated with mastectomy for LCIS presented in the ipsilateral breast, which was invasive ductal cancer (IDC). Of all ipsilateral IBCs in patients not treated with surgery for LCIS, 53.9 % was invasive lobular cancer (ILC), compared to 12.5 % of all contralateral IBCs in this group. Similar results were found for patients treated with BCS and unknown surgery (Supplementary Table 1).
3.4. Cumulative IBC incidence
Taking into account death as competing risk, overall 10- and 20-year CIFs of IBC were 14.9 % (95%CI:13.0–17.0 %) and 25.9 % (95%CI:22.9–29.0 %), respectively. CIFs were lowest after mastectomy and highest after no surgery. Overall CIFs for 10- and 20-year ipsilateral IBC were 9.5 % (95%CI:7.9–11.2) and 18.0 % (95%CI:15.2–20.8). For contralateral IBC, CIFs were 6.0 % (95%CI:5–8%) and 9.8 % (95%CI:7.8–12.1 %). For ipsilateral IBCs, 10- and 20-year CIFs for no surgery were the highest (21.3 % (95%CI:12.7–31.3 %)), and for mastectomy they were the lowest (both 0.7 % (95%CI:0.1–3.5 %)) (Table 3).
Table 3.
Cumulative incidence functions for subsequent invasive breast cancer after diagnosis of lobular carcinoma in situ.
| Characteristics | Overall CIF (95 % CI) |
CIF for ipsilateral IBC (95 % CI) |
CIF for contralateral IBC (95 % CI) |
|||
|---|---|---|---|---|---|---|
| 10 years | 20 years | 10 years | 20 years | 10 years | 20 years | |
|
Entire cohort(Median follow-up: 6.0 (IQR 0–29) years) |
14.9 % (13.0–17.0 %) | 25.9 % (22.9–29.0 %) | 9.5 % (7.9–11.2 %) | 18.0 % (15.2–20.8 %) | 6.0 % (4.8–7.5 %) | 9.8 % (7.8–12.1 %) |
| Type of management | ||||||
| No surgery (Median follow-up: 3.0 (IQR 1.4-5.1) years) |
29.1 % (19.5–39.4 %) | 29.1 % (19.5–39.4 %) | 21.3 % (12.7–31.3 %) | 21.3 % (12.7–31.3 %) | 10.0 % (4.4–18.4 %) | 10.0 % (4.4–18.4 %) |
| BCS (Median follow-up: 8.3 (IQR 3.7-15.3 years) |
15.2 % (12.6–18.0 %) | 29.3 % (24.9–33.8 %) | 9.5 % (7.4–11.9 %) | 20.7 % (16.8–25.0 %) | 6.3 % (4.6–8.4 %) | 10.9 % (7.9–14.5 %) |
| Mastectomy (Median follow-up: 9.7 (IQR 4.9-18.1 years) |
6.9 % (3.6–11.5 %) | 9.1 % (5.0–14.8 %) | 0.7 % (0.1–3.5 %) | 0.7 % (0.1–3.5 %) | 6.2 % (3.2–10.7 %) | 8.5 % (4.5–14.1 %) |
| Unknown surgery (Median follow-up: 12.9 (IQR 4.1-21.1 years) | 11.1 % (7.2–16.0 %) | 22.8 % (16.7–29.6 %) | 7.8 % (4.6–12.2 %) | 16.7 % (11.3–23.1 %) | 3.5 % (1.6–6.8 %) | 7.4 % (3.9–12.2 %) |
Median follow-up was calculated from diagnosis of LCIS to IBC event, DCIS, death or last observation. Abbreviations: CIF, cumulative incidence function; CI, confidence interval; IBC, invasive breast cancer; BCS, Breast-conserving surgery; IQR, interquartile range.
3.5. Standardised IBC incidence
Fig. 3 shows the SIRs (compared to the general population) of IBC according to laterality and surgical treatment. The SIR for ipsilateral IBC was highest for patients not treated with surgery for LCIS (6.9, 95%CI:4.9–9.4) and lowest for patients treated with mastectomy (0.2, 95%CI 0.4–0.8). The SIR for contralateral IBC was highest for patients not treated with surgery (2.9, 95%CI:1.6–4.7) and lowest for patients treated with unknown type of surgery (1.5, 95%CI:0.8–2.5). Further stratification for age (<50, 50–64 and ≥ 65 years) revealed no significant differences in IBC risk among age groups (Supplementary Figure 2). Patients not treated with surgery in 1999–2008 and 2009–2017 had higher IBC risks than patients treated in 1989–1998 (Supplementary Figure 3).
Fig. 3.
Standardised incidence ratios of subsequent invasive breast cancer following a LCIS diagnosis in the Netherlands, according to type of surgical management. Error bars represent 95 % confidence intervals. Abbreviations: BCS, breast-conserving surgery.
3.6. Sensitivity analysis
Overall, 41 patients (2.2 %) were diagnosed with poorly differentiated LCIS(Table 1). We hypothesized that these patients actually had pleiomorphic LCIS, therefore we analysed the this group separately. These patients were diagnosed in the entire range of incidence years. At the end of the follow-up four patients (9.8 %) died and six patients (14.6 %) developed IBC. These percentages are similar to the entire cohort, so we expect our results not to be affected.
4. Discussion
This population-based study showed that LCIS incidence increased over time, while surgical treatment decreased. Survival was high (RS exceeding 90 % at 20 years) over the entire period, irrespective of surgical treatment. Survival was highest in patients treated with unknown surgery and lowest in patients treated with mastectomy. However, LCIS was associated with an increased SIR of subsequent IBC which was highest in patients not treated with surgery and lowest in patients treated with mastectomy.
We analysed outcomes for each type of surgical treatment separately due to the possible presence of invasive components in patients not treated with surgery, and the developments in surgical treatment over time. Both factors are intertwined with our results. It is therefore not the intention to directly compare outcomes between treatment groups, but our results give insights in treatment of LCIS and outcomes in daily practice, which was the aim of our study.
The increased incidence over time may partly be related to improved detection methods, and the growing and ageing population. The clear increase starting from 2004 likely relates to the introduction of full-field digital mammography in the Netherlands [20], which is confirmed by our finding that most of the eligible patients (50–74 years) were diagnosed through screening. A similar increase was observed for DCIS [21]. Regarding surgery, BCS was mostly performed, followed by no surgery, mastectomy and unknown surgery. Unknown surgery was mostly registered in the earlier years, where less specific coding rules were valid. However, it was also partly related to incidental findings for benign indications and slightly declined over time, probably related to the large detection rates within the screening program (thereby lowering the chances of detecting LCIS during other procedures). There was an increasing trend (>50 %) towards no surgery over the last decade, which is consistent with the recommendation for active surveillance in the Dutch guidelines, but contrary to LCIS treatment in the United States, where it followed the general trend of more mastectomies [22].
The favourable survival outcomes are confirmed by others [[3], [4], [5]], and contribute to increasing evidence that LCIS barely affects mortality. After mastectomy, survival rates were the lowest (although they were still high), which can be attributed to the slightly older median age, alongside a possible higher frequency of comorbidities as compared to the other groups. Although survival rates were high, IBC risks were significantly higher than the general population for all patients not treated with mastectomy, with the highest SIR after no surgery. In patients not treated with surgery the SIR of IBC slightly increased over time, which – although not significant – may feel counterintuitive as patients diagnosed more recently have shorter follow-up time in which they could have developed IBC. This most likely reflects improved methods of detection [20], as in the most recent time periods of our study population no surgery became generally accepted as treatment option, mostly as part of active surveillance. This reasoning is underlined by our data, in which time intervals between the LCIS and IBC diagnoses were the shortest in the most recent period, and the longest in the earliest period.
The finding that LCIS is related to IBC is supported by previous registry-based studies [5,23,24] and molecular studies [[25], [26], [27]]. Although most IBCs presented ipsilaterally, the contralateral IBC risk was still higher than the breast cancer risk in the general population. A previous Dutch study showed that ILC occurred more often bilaterally than IDC [28]. As we showed that LCIS leads 3–5 times as often to ILC than the general breast cancer population [29], this might additionally explain our result that LCIS is associated with contralateral IBC. However, this needs cautious interpretation, as contralateral IBC was less often ILC than ipsilateral IBC. Importantly, developing contralateral IBC could be related to any other breast cancer risk factor. Notwithstanding, patients with subsequent IBC presented mostly with early-stage disease, which corresponds with the high survival rates.
4.1. Strengths and limitations
This is the first nationwide population-based study combining trends in incidence and treatment of LCIS with long-term mortality and IBC risks, compared to the general population, specified for surgical treatment. The NCR is reliable and complete, ensuring robust analyses. However, as pleiomorphic LCIS is not included in the ICDO-3, and it was only since mid-2018 that it was included in the NCR coding manual, we assume that many of the pleiomorphic LCIS have been registered as classic LCIS. We identified 41 potential pleiomorphic LCIS (2.2 % of total), but we expect this number to be larger. In 2019, 5.7 % of all LCIS diagnoses in the NCR was pleiomorphic. Members of our group, analysing biopsies, showed that up to 12 % of all LCIS diagnoses was pleiomorphic (unpublished results). We therefore expect that 5.7–12 % of the patients in our study actually had pleiomorphic LCIS. These patients most likely were treated with surgery, as pleiomorphic LCIS is recommended to be treated as DCIS (in the studied period DCIS was usually surgically treated). Furthermore, we did not identify any florid LCIS cases, also because it is not taken up in the ICD-O3. Florid LCIS is rare, and similar to pleiomorphic LCIS, described to warrant surgical excision [30]. Although we expect its influence on our results to be minimal based on our sensitivity analysis, the IBC risk in the surgically treated groups may have been overestimated.
4.2. Clinical implications
IBC risks are highest in patients not treated with surgery, which is probably largely due to the fact that active surveillance was introduced more recently and therefore is associated with improved methods of detection. Since survival is very high and most patients will not develop subsequent IBC – and if so they generally have a good prognosis – our results support the recommendation for active surveillance. We showed that the median follow-up time between LCIS and IBC diagnosis in patients not treated with surgery was three years (IQR:1.4–5.1 years) supporting the current 5-year clinical follow-up period.
5. Conclusion
LCIS incidence has increased over time, while surgical treatment decreased. Survival rates are very high, thereby supporting active surveillance. LCIS is associated with increased IBC risk, especially ipsilateral ILC, which contributes to the evidence that LCIS is a non-obligate precursor of IBC. Future research should focus on the identification of patients with high IBC risk, who – in contrast to most LCIS patients – may benefit from surgical treatment and/or more intensive follow-up.
Declaration of competing interest
None.
Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.07.020.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data statement
The data underlying this article cannot be shared publicly due to privacy regulations of the Netherlands Cancer Registry. The data will be shared on reasonable request to the corresponding author (data request study number K19.057, www.iknl.nl).
Ethics approval
This study was approved by the local privacy committee of the Netherlands Cancer Registry (study number K19.057).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Foote F.W., Stewart F.W. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol. 1941;17(4):491–496. doi: 10.3322/canjclin.32.4.234. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beahrs O.H., Myer M.H. second ed. J.B. Lippincot company; Philadelphia: 1983. American Joint Committee on Cancer: manual for staging of cancer. [Google Scholar]
- 3.Wong S.M., King T., Boileau J.F., Barry W.T., Golshan M. Population-based analysis of breast cancer incidence and survival outcomes in women diagnosed with lobular carcinoma in situ. Ann Surg Oncol. 2017;24(9):2509–2517. doi: 10.1245/s10434-017-5867-6. [DOI] [PubMed] [Google Scholar]
- 4.Chuba P.J., Hamre M.R., Yap J., Severson R.K., Lucas D., Shamsa F. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol. 2005;23(24):5534–5541. doi: 10.1200/JCO.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 5.King T.A., Pilewskie M., Muhsen S., Patil S., Mautner S.K., Park A. Lobular carcinoma in situ: a 29-year longitudinal experience evaluating clinicopathologic features and breast cancer risk. J Clin Oncol. 2015;33(33):3945–3952. doi: 10.1200/JCO.2015.61.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu A.J., Cho N., Park I.A., Cho S.W. Features of pure lobular carcinoma in situ on magnetic resonance imaging associated with immediate Re-excision after lumpectomy. J Breast Cancer. 2016;19(2):199–205. doi: 10.4048/jbc.2016.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amos B., Chetlen A., Williams N. Atypical lobular hyperplasia and lobular carcinoma in situ at core needle biopsy of the breast: an incidental finding or are there characteristic imaging findings? Breast Dis. 2016;36(1):5–14. doi: 10.3233/BD-150194. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell A.J., Clements K., Dodwell D.J., Evans A.J., Francis A., Hussain M. The radiological features, diagnosis and management of screen-detected lobular neoplasia of the breast: findings from the Sloane Project. Breast. 2016;27:109–115. doi: 10.1016/j.breast.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Wen H.Y., Brogi E. Lobular carcinoma in situ. Surg Pathol Clin. 2018;11(1):123–145. doi: 10.1016/j.path.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginter P.S., D'Alfonso T.M. Current concepts in diagnosis, molecular features, and management of lobular carcinoma in situ of the breast with a discussion of morphologic variants. Arch Pathol Lab Med. 2017;141(12):1668–1678. doi: 10.5858/arpa.2016-0421-RA. [DOI] [PubMed] [Google Scholar]
- 11.Hussain M., Cunnick G.H. Management of lobular carcinoma in-situ and atypical lobular hyperplasia of the breast--a review. Eur J Surg Oncol. 2011;37(4):279–289. doi: 10.1016/j.ejso.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Sen L.Q., Berg W.A., Hooley R.J., Carter G.J., Desouki M.M., Sumkin J.H. Core breast biopsies showing lobular carcinoma in situ should Be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol. 2016;207(5):1132–1145. doi: 10.2214/AJR.15.15425. [DOI] [PubMed] [Google Scholar]
- 13.Arpino G., Allred D.C., Mohsin S.K., Weiss H.L., Conrow D., Elledge R.M. Lobular neoplasia on core-needle biopsy--clinical significance. Cancer. 2004;101(2):242–250. doi: 10.1002/cncr.20318. [DOI] [PubMed] [Google Scholar]
- 14.Foster M.C., Helvie M.A., Gregory N.E., Rebner M., Nees A.V., Paramagul C. Lobular carcinoma in situ or atypical lobular hyperplasia at core-needle biopsy: is excisional biopsy necessary? Radiology. 2004;231(3):813–819. doi: 10.1148/radiol.2313030874. [DOI] [PubMed] [Google Scholar]
- 15.Londero V., Zuiani C., Linda A., Vianello E., Furlan A., Bazzocchi M. Lobular neoplasia: core needle breast biopsy underestimation of malignancy in relation to radiologic and pathologic features. Breast. 2008;17(6):623–630. doi: 10.1016/j.breast.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Kopans D.B. Lobular neoplasia on core-needle biopsy--clinical significance. Cancer. 2004;101(12):2902–2903. doi: 10.1002/cncr.20682. [DOI] [PubMed] [Google Scholar]
- 17.Renshaw A.A., Cartagena N., Derhagopian R.P., Gould E.W. Lobular neoplasia in breast core needle biopsy specimens is not associated with an increased risk of ductal carcinoma in situ or invasive carcinoma. Am J Clin Pathol. 2002;117(5):797–799. doi: 10.1309/T4XF-C61J-C95Y-VR4Q. [DOI] [PubMed] [Google Scholar]
- 18.Integraal Kankercentrum Nederland . 2020. Richtlijn mammacarcinoom.https://richtlijnendatabase.nl/richtlijn/borstkanker/algemeen.html Accessed. [Google Scholar]
- 19.Putter H., Fiocco M., Geskus R.B. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 20.Sankatsing V.D.V., Fracheboud J., de Munck L., Broeders M.J.M., van Ravesteyn N.T., Heijnsdijk E.A.M. Detection and interval cancer rates during the transition from screen-film to digital mammography in population-based screening. BMC Canc. 2018;18(1):256. doi: 10.1186/s12885-018-4122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannu G.S., Wang Z., Broggio J., Charman J., Cheung S., Kearins O. Invasive breast cancer and breast cancer mortality after ductal carcinoma in situ in women attending for breast screening in England, 1988-2014: population based observational cohort study. BMJ. 2020;369:m1570. doi: 10.1136/bmj.m1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portschy P.R., Marmor S., Nzara R., Virnig B.A., Tuttle T.M. Trends in incidence and management of lobular carcinoma in situ: a population-based analysis. Ann Surg Oncol. 2013;20(10):3240–3246. doi: 10.1245/s10434-013-3121-4. [DOI] [PubMed] [Google Scholar]
- 23.McEvoy M.P., Coopey S.B., Mazzola E., Buckley J., Belli A., Polubriaginof F. Breast cancer risk and follow-up recommendations for young women diagnosed with atypical hyperplasia and lobular carcinoma in situ (LCIS) Ann Surg Oncol. 2015;22(10):3346–3349. doi: 10.1245/s10434-015-4747-1. [DOI] [PubMed] [Google Scholar]
- 24.To T., Wall C., Baines C.J., Miller A.B. Is carcinoma in situ a precursor lesion of invasive breast cancer? Int J Canc. 2014;135(7):1646–1652. doi: 10.1002/ijc.28803. [DOI] [PubMed] [Google Scholar]
- 25.Andrade V.P., Ostrovnaya I., Seshan V.E., Morrogh M., Giri D., Olvera N. Clonal relatedness between lobular carcinoma in situ and synchronous malignant lesions. Breast Cancer Res. 2012;14(4):R103. doi: 10.1186/bcr3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aulmann S., Penzel R., Longerich T., Funke B., Schirmacher P., Sinn H.P. Clonality of lobular carcinoma in situ (LCIS) and metachronous invasive breast cancer. Breast Canc Res Treat. 2008;107(3):331–335. doi: 10.1007/s10549-007-9557-0. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.Y., Schizas M., Geyer F.C., Selenica P., Piscuoglio S., Sakr R.A. Lobular carcinomas in situ display intralesion genetic heterogeneity and clonal evolution in the progression to invasive lobular carcinoma. Clin Canc Res. 2019;25(2):674–686. doi: 10.1158/1078-0432.CCR-18-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setz-Pels W., Duijm L.E., Groenewoud J.H., Louwman M.W., Jansen F.H., van Beek M. Patient and tumor characteristics of bilateral breast cancer at screening mammography in The Netherlands, a population-based study. Breast Canc Res Treat. 2011;129(3):955–961. doi: 10.1007/s10549-011-1545-8. [DOI] [PubMed] [Google Scholar]
- 29.Lips E.H., Mukhtar R.A., Yau C., de Ronde J.J., Livasy C., Carey L.A. Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast Canc Res Treat. 2012;136(1):35–43. doi: 10.1007/s10549-012-2233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaaban A.M. Why is LCIS important – pathological review. Curr Breast Canc Rep. 2021 doi: 10.1007/s12609-021-00415-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.