Abstract
Background In Western developed countries, food-based dietary patterns have been associated with the risk of cardiometabolic diseases, but little is known about such associations in less developed ethnic minority regions (LEMRs), where the cardiometabolic disease burden is growing rapidly and food patterns differ substantially.
Methods Between May 2018 and September 2019, we recruited 99556 participants aged 30-79 years from the China Multi-Ethnic Cohort (CMEC) Study. We measured habitual dietary intake with validated food frequency questionnaire (FFQ) and then calculated dietary pattern scores for two of the most studied a priori dietary patterns, i.e., Dietary Approaches to Stop Hypertension (DASH) and alternative Mediterranean (aMED) style diets, and three a posteriori dietary patterns. Four cardiometabolic risks, including hypertension, diabetes, dyslipidaemia and metabolic syndrome (MetS), were newly diagnosed by medical examination and blood tests. We estimated adjusted odds ratios (OR) relating various dietary pattern scores to cardiometabolic risks using marginal structural models under the guidance of directed acyclic graphs. For the above associations, we further calculated the proportion mediated by overweight (PM) using regression-based mediation analysis for better public health implications.
Findings The final study sample consisted of 68834 participants. Among them, we newly diagnosed 12803 hypertension, 3527 diabetes, 16342 hyperlipidaemia, and 8198 MetS cases. Overall, all 5 dietary patterns showed considerable associations with risks of hypertension and MetS. Comparing the highest with the lowest quintiles, the DASH score showed the strongest inverse associations with risks of hypertension (OR=0.74, 95% CI:0.70-0.79; PM=10%) and MetS (OR=0.79, 95% CI:0.74-0.85; PM=35%); conversely, scores of the localized a posteriori Yunnan-Guizhou plateau dietary pattern in LEMRs showed the strongest positive associations with risks of hypertension (OR=1.44, 95% CI:1.35-1.52; PM=10%) and MetS (OR=1.35, 95% CI:1.26-1.46; PM=33%), with all P values for trend <0.001. These associations were consistent in various subgroups defined by sex, age, smoking and physical activity, but with magnitudes that differed substantially across different ethnic regions and urbanicity. By investigating the single-component effects of dietary patterns, the dairy intake component contributed a major proportion to the beneficial effects of DASH (41.9% for hypertension and 100.5% for MetS).
Interpretation Substantial socioeconomic status and ethnic disparities in diet quality and related cardiometabolic risks were seen in LEMRs, with hypertension being the top diet-related cardiometabolic risk. Our findings support that DASH provides superior dietary guidance compared to aMED for reducing cardiometabolic risks in LEMRs. In particular, the dairy intake encouraged by DASH may produce considerable beneficial effects.
Funding This study was funded by the National Key R&D Program of China; full funding sources listed in the acknowledgements.
Keyword: Dietary pattern, Cardiometabolic risks, socioeconomic status disparity, ethnic disparity, epidemiological study
Introduction
Cardiometabolic diseases are the top-ranked causes of morbidity and mortality worldwide. In 2019, it was estimated that over 25% of the global disability-adjusted life-years (DALYs) in the elderly population are attributable to three major cardiometabolic diseases: ischaemic heart disease, stroke and diabetes.1 Over past decades, cardiometabolic disease mortality has experienced a steady decline in high-income countries (HICs)[2], [3], [4] but has increased rapidly in low- and middle-income countries (LMICs),[5], [6], [7], [8] as well as in populations of low socioeconomic status (SES) and racial/ethnic minorities.[8], [9], [10], [11], [12] Reducing SES and racial/ethnic disparities in cardiometabolic diseases has therefore become a rising public health concern globally and a top priority to achieve United Nations Sustainable Development Goals (SDGs) target 3.4.13,14
An unhealthy diet is the leading modifiable risk factor for cardiometabolic diseases.15,16 In particular, both randomized trials and long-term cohort studies suggest that healthful food-based dietary patterns produce significant benefits on cardiometabolic diseases[17], [18], [19] but that little benefit was identified from controlling single isolated nutrients.[20], [21], [22] Dietary patterns also have the advantage of public health implications because they can facilitate dietary guidance and lessen industry manipulation.15,23 For such reasons, healthful food-based dietary patterns such as Dietary Approaches to Stop Hypertension (DASH) and Mediterranean (MED)-style diets have been recommended by the U.S. Department of Agriculture and American Heart Association and used worldwide to reduce the risk of cardiometabolic diseases.[24], [25], [26] Nevertheless, almost all well-known dietary pattern guidance has been developed based on Western-like diets from developed countries. An emerging question then is whether Western dietary guidance can be extrapolated to other populations, particularly racial/ethnic minority groups in less-developed regions, for whom the cardiometabolic disease burden is growing rapidly and food patterns differ substantially.[27], [28], [29] Besides, as the above populations have been rarely studied before, another crucial question is what insights we can gain from their disparate food patterns, which may also help for cardiometabolic disease prevention. However, reliable evidence from large-scale epidemiological studies on this topic is scarce.
The China Multi-Ethnic Cohort (CMEC) Study is a large-scale epidemiological study undertaken in Southwest China (an area of 2.3 million square kilometres), covering the Qinghai-Tibet Plateau, Yunnan-Guizhou Plateau and Sichuan Basin, with great diversity in SES, ethnicity, cardiometabolic disease burden, habitual diet, living environment, etc.30 Nearly 0.1 million participants from seven ethnic groups with comprehensive information were enrolled in the CMEC study. Overall, CMEC presents an ideal and unique opportunity to finely characterize the relationship between dietary patterns and cardiometabolic disease risks in the setting of diverse less-developed ethnic minority regions (LEMRs).
In this cross-sectional analysis of CMEC baseline data, we aimed to assess associations of two of the most studied a priori dietary patterns from Western developed countries, i.e., DASH and alternative MED diets, as well as three a posteriori dietary patterns derived from CMEC dietary data with newly diagnosed cardiometabolic risks (hypertension, diabetes, dyslipidaemia and metabolic syndrome). Given that increasing evidence suggests that associations between diet and cardiometabolic risks might be partly mediated by overweight,[31], [32], [33] we further aimed to examine how such associations between dietary patterns and cardiometabolic risks might be mediated by overweight to obtain better public health implications.
Methods
Study population
The CMEC study is an ongoing community-based prospective cohort study undertaken in Southwest China, where is the major ethnic area in China and home to 56 ethnic groups. Ethnic characteristics, SES, population size and disease patterns were given special consideration when selecting the study population and survey sites. A detailed description of the study design, sampling strategy and baseline characteristics has been published elsewhere.30 In brief, a total of 99556 participants from six ethnic minority groups (Tibetan, Yi, Miao, Bai, Bouyei and Dong) as well as the majority Han group were recruited between May 2018 and September 2019. The baseline survey consisted of a tablet-based electronic questionnaire via face-to-face interviews, anthropometric measurements, thorough medical examinations, and blood and urine tests. All the participants provided written informed consent prior to the data collection, and the study was approved by the Sichuan University Medical Ethical Review Board and local ethics committee at each participating site.
In the present study, we focused on adults aged 30-79 years with complete and plausible diet- and outcome-related data, total energy intakes and body mass index (BMI). We excluded 22618 participants who self-reported physicians diagnosed hypertension, diabetes, hyperlipidaemia, coronary heart disease, or stroke at the baseline survey to eliminate potential reverse causality. The final sample consisted of 68834 participants. More details are provided in the Appendix text 1s.
Assessment of dietary intakes
For the baseline survey, habitual diets were assessed using quantitative food frequency questionnaire (FFQ) (see the full text in the Appendix text 2s). For each food group, participants were required to report the quantity (average grams per meal according to standard serving size moulds) and frequency (four frequency categories ranged from how many times per day to year) that they consumed during the past 12 months. We also asked information about alcohol, tea, Sugar Sweetened Beverages (SSBs), cooking oil and salt in separate sections. In particular, daily alcohol consumption was calculated as grams of pure alcohol according to alcohol type, amount drunk and frequency.34 From the above FFQ, we estimated the total daily energy intake according to the China food exchange lists and the 2018 China food composition tables (see more details in the Appendix text 3s).35,36
We conducted the repeated FFQ and 24-hour dietary recall (24 HDRs) to assess the reproducibility and validity of the baseline FFQ. Regarding reproducibility, intraclass correlation coefficients (ICC) for food groups ranged from 0.15 for fresh vegetables to 0.67 for alcohol. Regarding validity, de-attenuated Spearman rank correlation coefficients for food groups ranged from 0.10 for soybean products to 0.66 for rice. More details are provided in the Appendix text 4s.
Assessment of dietary patterns
To better capture the dietary features of our study population, we scored all participants according to their adherence to specific dietary patterns, including the two of the most studied a priori dietary patterns (i.e., DASH- and MED-style diets) relating to cardiometabolic diseases, as well as three a posteriori dietary patterns derived from CMEC data.
To represent adherence to a DASH-style diet, we used a modified DASH score with nonfat and low-fat dairy replaced by full-fat dairy products,37 given that the consumption of nonfat and low-fat dairy was extremely low in our study population. Compared to the original DASH diet,17 the modified DASH diet was shown by a randomized controlled trial to be more effective at reducing cardiometabolic risks.37 In addition, we excluded the food group component of Sugar Sweetened Beverages (SSBs) because regular consumption of SSBs in our study population was only 7.2%. To represent adherence to a MED-style diet, we used an alternative Mediterranean diet (aMED) score, which is an adapted version of the traditional MED for the non-Greek population.[38], [39], [40] We also eliminated the food group component of nuts due to the lack of a separate food group for nut consumption. For each of food group components of DASH or aMED, we categorized the food group consumption into quintiles and scored all participants from 1 to 5 according to their intake ranking,41 and then obtained the total score by summing up the component scores. More details are provided in the Appendix text 5s.
To determine the major a posteriori dietary patterns, we used principal component factor analysis (PCFA) with varimax rotation based on the same food group data transformed to z scores.23,42 Three major dietary patterns were identified via comprehensive considerations of eigenvalues, variance explained, scree plot and interpretability (see more details in the Appendix text 6s). For each dietary pattern, factor scores were calculated for all participants by summing up the standardized intakes of food groups weighted by their factor loadings. The robustness of the characteristics of three identified dietary patterns were assessed by a split-sample validation (see more details in the Appendix text 7s).
Assessment of outcomes
The outcomes in this study were newly diagnosed cardiometabolic risks, including hypertension, diabetes, dyslipidaemia and metabolic syndrome (MetS). We identified all cardiometabolic risks based on objective indicators from medical examinations or blood tests at the baseline survey. More detailed information about assessment of cardiometabolic outcomes is provided in the Appendix text 8s.
Assessment of covariates
We obtained covariate information from the baseline questionnaire. To guide the selection of potential confounders, we constructed directed acyclic graphs (DAGs) under the protocol of “Evidence Synthesis for Constructing Directed Acyclic Graphs” (ESC-DAGs), which combined evidence synthesis strategies and causal inference principles.43 We further performed independent tests to continuously modify the proposed DAGs until the implied conditional independences were satisfied. See more details in the Appendix text 9s and e-table. On the basis of these causal diagrams and backdoor criteria,44 we adjusted the final models for sex, age, urbanicity, ethnicity, marital status, highest education attained, annual household income, occupation, regular smoking, physical activity in hours of metabolic equivalent tasks per day (METs-h/day), total energy intake (kcal per day), regular intake of sweeten beverage, regular intake of dietary supplements, regular intake of spicy food, regular intake of pepper food, insomnia symptoms, depressive symptom, anxiety symptom, menopause status for women, and family history of cardiometabolic diseases.
Statistical analysis
To assess ethnic and regional variations in dietary patterns, we compared the median (25th, 75th percentiles) of five dietary pattern scores across ethnic regions. We categorized all dietary pattern scores into quintiles for the entire study population. Baseline characteristics are described as the median (25th, 75th percentiles) for continuous variables and percentage for categorical variables according to quintiles of dietary pattern scores.
We employed marginal structural models which combined logistic regression with the inverse probability of exposure weighting (IPEW) to estimate associations between the five dietary pattern scores (quintiles of dietary pattern scores were modelled as categorical variable with five levels) and four cardiometabolic risks separately,45,46 with the lowest fifth of the dietary pattern score as the reference group. To determine the preferable weighting method, we adopted six weighting methods in our primary analysis,47 with the quintiles of dietary pattern scores as the dependent variable and confounders decided by the ESC-DAGs as the independent variables, and then assessed their balances of confounders among different exposure groups.48 In our final model, we used the entropy balancing weighting method due to the optimal balance of confounders.49 See the detailed results in the Appendix text 10s. To evaluate the linear trend across quintiles, we assigned a median value to each quintile of dietary scores and then modelled the median score as a continuous variable. To facilitate interpretations of the effects of overall dietary patterns, we carried out single-component and single group analyses. For DASH and aMED, we evaluated the association of each of the dietary components with cardiometabolic risks by eliminating one component at a time from the overall score separately.50,51 We also assessed the association of each of the single food groups in our FFQ with cardiometabolic risks. To examine the mediation effects of overweight (BMI <24 OR ≥24 kg/m2), regression-based mediation analyses were used to decompose the total effects of dietary patterns on cardiometabolic risks into natural direct and indirect effects through overweight and to calculate the proportion of mediation accordingly.52,53 Due to the built-in quality control in the tablet-based questionnaire and stringent data audit, the missing proportion in this study was very low (<5% except for oil and salt consumption). Most missing values were generated from unverifiable outliers after audio review. For missing values of food groups, we performed multiple imputation (with 5 imputations) by the chained equations method.54 To simultaneously account for the uncertainty of estimations of both weighting and exposure-outcome associations, a bootstrap-based method with 1000 replicates was used to obtain 95% confidence intervals (CIs).55,56
To examine potential effect modifiers, we conducted stratification analysis among predefined subgroups, including sex, age, regular smoking, physical activity, urbanicity and ethnic regions. Heterogeneity among different strata was assessed using the chi square (χ2) test. To assess the robustness of our findings, we also performed several sensitivity analyses. First, we used a more stringent exclusion criterions by excluding self-reported physicians diagnosed hepatic and gastrointestinal diseases. Second, we used all identified cardiometabolic risks (self-reported plus newly identified) as outcomes to examine the magnitude of potential reverse causality. Third, we adopted the conventional covariate adjustment approach to estimate the associations. Fourth, we alternatively adjusted for BMI as a confounder rather than a mediator. Fifth, we ran a minimally adjusted analysis, without justification for spicy food, pepper food, insomnia, depression, and anxiety. Sixth, we alternatively calculated the DASH score with the inclusion of SSBs. Last, we ran a complete case analysis instead of the multiple imputation approach.
All analyses were performed with R Project for Statistical Computing version 4.0.2 (Vienna, Austria).57 The maps displayed in the results were produced by ArcGIS Desktop version 10.2.1 (authorization number: EFL734321752).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. XX, JY, and XZ had access to all data and had final responsibility for the decision to submit for publication.
Results
Characteristics of dietary patterns
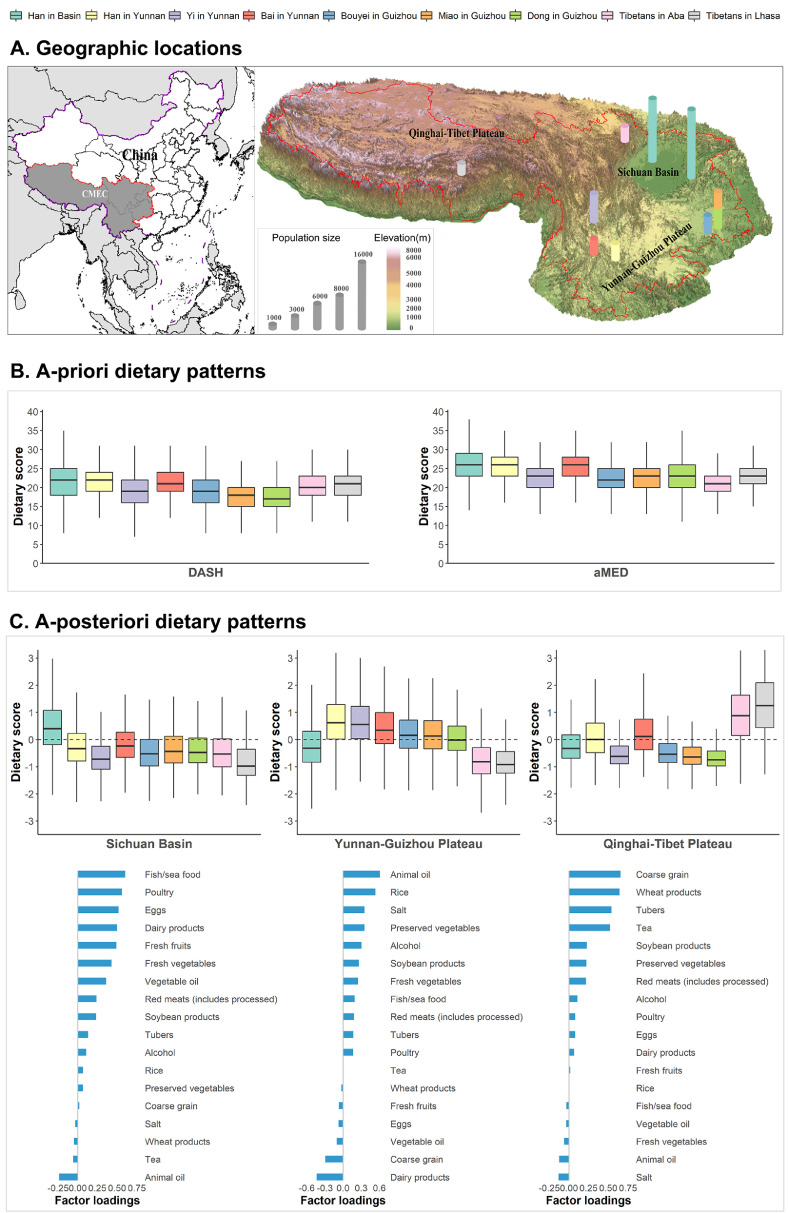
Figure 1 shows the geographic and ethnic variations of various dietary pattern scores. Regarding Western-like diet-based a priori dietary patterns, no substantial difference in median scores was found across different regions and ethnic groups, with DASH scores ranging from 17 (Dong in Guizhou) to 22 (Han in Basin) and aMED scores ranging from 21 (Tibetans in Aba) to 26 (Han in Basin) (figure 1B). In contrast, a posteriori dietary patterns showed a better ability to discriminate different ethnic groups, with various ethnic groups clustered into three geography-related a posteriori dietary patterns. The first one predominated by the Han majority population in the more developed Sichuan Basin (with two mega-cities located in this region) was characterized by relatively high intakes of fish/sea food, poultry, eggs, dairy products, fresh fruits, fresh vegetables and vegetable oil, implying a modern and balanced dietary pattern: the Sichuan Basin dietary pattern. The second one predominated by various ethnic minority populations in the less developed Yunnan-Guizhou Plateau was characterized by relatively high intakes of animal oil, rice, salt, preserved vegetables, alcohol and soybean products and low intakes of dairy products and coarse grain, which suggested a poor and agricultural dietary pattern: the Yunnan-Guizhou Plateau dietary pattern. The third one predominated by the Tibetan population in the less developed Qinghai-Tibet Plateau was characterized by relatively high intakes of coarse grain, wheat products, tubers and tea, indicating a featured high-altitude Tibetan dietary pattern: the Qinghai-Tibet Plateau dietary pattern (figure 1A and 1C). More detailed information in table format on the comparisons of dietary scores across ethnic regions is provided in the Appendix text 11s.
Figure 1.
Geographic and ethnic variations of various dietary patterns among various ethnic groups. (A) Geographic locations and terrains of study sites. The administrative boundary data with scale of 1:4 million was obtained from national fundamental geoinformation database. The digital elevation data with 30 meters resolution used in terrain map was obtained from the advanced spaceborne thermal emission and reflection radiometer global digital elevation Model. (B) Comparisons of a priori dietary pattern scores (includes DASH and aMED) among various ethnic groups. (C) Characteristics of three a posteriori dietary patterns and related comparisons among various ethnic groups. Boxplot based on median (25th, 75th percentiles) was used to visually compare the distribution of dietary scores across ethnic regions.
Baseline characteristics according to the lowest and highest quintiles of the various dietary scores are reported in Table 1. Among the 68834 participants included in this study, the median age was 49.5 (42, 56) years, 62% of the participants were women, 35% of the participants were urban residents, and 53% of the participants were ethnic minority populations. Participants with higher adherence to DASH, aMED and Sichuan Basin dietary patterns shared similar characteristics. They tended to have higher education and SES levels as well as healthier lifestyles, and they were less likely to report mental disorders but more likely to report a family history of cardiometabolic diseases. In contrast, participants with higher adherence to the Yunnan-Guizhou Plateau or Qinghai-Tibet Plateau dietary patterns generally tended to have lower education and SES levels as well as unhealthier lifestyles.
Table 1.
Baseline characteristics in the CMEC Study, according to quintiles of various dietary pattern scores. a
| Characteristic | No. of participants b | Overall |
DASH |
aMED |
Sichuan Basin |
Yunnan-Guizhou Plateau |
Qinghai-Tibet Plateau |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | |||
| Dietary score | 68834 | - | 15 (13, 16) |
26 (24, 27) |
19 (17, 20) |
30 (28, 31) |
-1.1 (-1.3, -0.9) |
1.4 (1.1, 1.9) |
-1.2 (-1.4, -1.0) |
1.3 (1.0, 1.8) |
-1.0 (-1.1, -0.9) |
1.2 (0.8, 1.8) |
| Age (yr) | 68834 | 49 (42, 56) |
50 (44, 59) |
47 (39, 55) |
50 (43, 60) |
48 (41, 55) |
51 (44, 59) |
47 (39, 54) |
46 (38, 55) |
51 (45, 58) |
49 (42, 56) |
49 (42, 57) |
| Female sex (%) | 68834 | 62 | 54 | 70 | 53 | 69 | 66 | 57 | 75 | 47 | 75 | 50 |
| Urban residence (%) | 68834 | 35 | 26 | 49 | 22 | 49 | 13 | 60 | 52 | 21 | 31 | 29 |
| Ethnic group (%) c | ||||||||||||
| Sichuan Basin | 32116 | 47 | 36 | 62 | 29 | 64 | 15 | 79 | 62 | 31 | 42 | 35 |
| Yunnan-Guizhou Plateau | 29924 | 43 | 57 | 30 | 51 | 34 | 63 | 18 | 11 | 68 | 56 | 32 |
| Qinghai-Tibet Plateau | 6794 | 10 | 7 | 8 | 19 | 2 | 23 | 3 | 27 | 2 | 1 | 34 |
| Marital status (%) | 68833 | 90 | 88 | 91 | 89 | 91 | 88 | 90 | 88 | 90 | 88 | 91 |
| Highest education (%) | ||||||||||||
| No formal school | 17140 | 25 | 37 | 14 | 41 | 13 | 45 | 9 | 23 | 27 | 30 | 32 |
| Primary school | 17101 | 25 | 28 | 19 | 28 | 22 | 31 | 17 | 16 | 35 | 24 | 28 |
| Middle and high school | 26340 | 38 | 29 | 47 | 25 | 49 | 21 | 53 | 39 | 35 | 35 | 32 |
| College or university | 8252 | 12 | 6 | 20 | 6 | 16 | 3 | 21 | 22 | 4 | 11 | 9 |
| Annual household income (%) | ||||||||||||
| <¥12000 | 11095 | 16 | 27 | 9 | 26 | 9 | 28 | 8 | 10 | 22 | 21 | 15 |
| ¥12000-19999 | 12475 | 18 | 21 | 15 | 23 | 14 | 24 | 11 | 16 | 21 | 19 | 21 |
| ¥20000-59999 | 25420 | 37 | 35 | 36 | 34 | 38 | 36 | 35 | 35 | 40 | 35 | 39 |
| ¥60000-99999 | 10148 | 15 | 10 | 19 | 9 | 19 | 8 | 21 | 19 | 10 | 13 | 13 |
| ¥100000-199999 | 7677 | 11 | 6 | 16 | 6 | 15 | 4 | 19 | 16 | 6 | 10 | 9 |
| >¥200000 | 1932 | 3 | 1 | 5 | 1 | 4 | 1 | 6 | 5 | 1 | 2 | 3 |
| Occupation (%) d | ||||||||||||
| Primary industry | 24065 | 35 | 46 | 23 | 50 | 24 | 54 | 15 | 18 | 56 | 39 | 38 |
| Secondary industry | 5464 | 8 | 9 | 7 | 7 | 8 | 5 | 10 | 6 | 9 | 8 | 6 |
| Tertiary industry | 26388 | 38 | 30 | 46 | 28 | 45 | 29 | 48 | 51 | 24 | 38 | 35 |
| Unemployed | 12860 | 19 | 15 | 24 | 15 | 23 | 11 | 27 | 25 | 12 | 15 | 21 |
|
Physical activity (METs-h/day) e |
68556 | 23.7 (13.4, 38.7) |
26.7 (13.9, 42.0) |
21.0 (13.0, 34.6) |
25.8 (12.6, 41.7) |
22.2 (13.5, 36.1) |
27.2 (13.0, 43.0) |
20.6 (13.0, 33.6) |
17.8 (10.3, 29.2) |
33.0 (18.2, 47.2) |
25.5 (14.0, 39.9) |
22.2 (12.1, 38.3) |
| BMI (kg/m2) | 68834 | 23.6 (21.4, 25.9) |
23.8 (21.5, 26.2) |
23.4 (21.4, 25.5) |
23.7 (21.4, 26.2) |
23.6 (21.5, 25.8) |
23.5 (21.2, 25.9) |
23.7 (21.7, 25.9) |
23.7 (21.6, 25.9) |
23.3 (21.1, 25.6) |
23.4 (21.3, 25.6) |
24.1 (21.8, 26.3) |
| Total energy intake (kcal/day) | 68834 | 1751.8 (1382.3, 2205.6) |
1665.8 (1292.7, 2140.2) |
1796.9 (1463.5, 2228.3) |
1691.1 (1282.5, 2183.4) |
1857.5 (1516.7, 2273.5) |
1407.7 (1084.1, 1802.1) |
2123.5 (1739.8, 2597.1) |
1569.2 (1244.6, 1945.7) |
2235.7 (1840.7, 2683.0) |
1427.5 (1139.0, 1805.6) |
2225.1 (1809.9, 2703.6) |
| Regular smoking (%) | ||||||||||||
| Never | 52482 | 76 | 70 | 82 | 72 | 79 | 80 | 72 | 87 | 62 | 85 | 68 |
| Previous | 2789 | 4 | 4 | 4 | 4 | 4 | 3 | 5 | 3 | 5 | 2 | 6 |
| Current | 13563 | 20 | 26 | 14 | 23 | 17 | 17 | 22 | 10 | 33 | 12 | 26 |
| Dietary supplement (%) | 68832 | 15 | 9 | 23 | 8 | 21 | 10 | 21 | 19 | 13 | 14 | 13 |
| Regular sweeten beverage intake (%) | ||||||||||||
| Never | 63506 | 92 | 94 | 94 | 87 | 96 | 89 | 94 | 87 | 95 | 96 | 82 |
| Previous | 264 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Current | 5064 | 7 | 6 | 6 | 12 | 4 | 10 | 6 | 13 | 5 | 3 | 17 |
| Regular spicy food intake (%) | 68834 | 79 | 78 | 81 | 71 | 85 | 71 | 85 | 70 | 84 | 79 | 73 |
| Regular pepper food intake (%) | 68834 | 68 | 57 | 75 | 54 | 78 | 53 | 79 | 64 | 73 | 58 | 68 |
| Insomnia symptom (%) | 68772 | 42 | 47 | 38 | 45 | 40 | 45 | 40 | 40 | 42 | 46 | 40 |
| Depressive symptom (%) | 68774 | 4 | 7 | 3 | 6 | 3 | 6 | 3 | 3 | 5 | 7 | 3 |
| Anxiety symptom (%) | 68772 | 5 | 9 | 3 | 7 | 4 | 8 | 3 | 3 | 7 | 8 | 3 |
| Menopausal status in women (%) f | ||||||||||||
| Premenopause | 22439 | 52 | 44 | 58 | 45 | 56 | 43 | 61 | 60 | 47 | 51 | 53 |
| Perimenopause | 3078 | 7 | 7 | 7 | 7 | 8 | 7 | 7 | 7 | 7 | 7 | 7 |
| Postmenopause | 17351 | 40 | 50 | 35 | 48 | 36 | 50 | 32 | 34 | 46 | 42 | 40 |
| Family history (%) g | 68834 | 32 | 25 | 39 | 21 | 39 | 21 | 40 | 36 | 27 | 29 | 29 |
Abbreviations: Q1 and Q5: the lowest and highest quintiles of dietary pattern scores; METs-h/day: hours of metabolic equivalent tasks per day; BMI: body mass index;
a. Data are presented as median (25th, 75th percentiles) for continuous variables and percentages (%) for categorical variables. We only display the results of Q1 and Q5 of dietary pattern scores for simplicity, with the corresponding median (25th, 75th percentiles) displayed in the following bracket.
b. Missing values are observed for marital status, highest education, annual household income, occupation, physical activity, dietary supplement, insomnia symptom, depressive symptom and anxiety symptom. The number of missing values per variable equal to the total number of participants (68834) minus the number presented in this column.
c. We aggregate various ethnic groups into three geographic regions due to their high similarity in dietary pattern and baseline characteristics.
d. Primary industry refers to occupations that involves getting raw materials, such as agriculture, forestry, fishing, and mining. Secondary industry refers to occupations that involves the transformation of the raw material into manufactured goods, such as factory worker. Tertiary industry refers to occupations that involves the giving away direct services to its consumers, such as administrator, teacher, sales, etc.
e. Physical activity considers participants’ occupational, traffic, chores, and leisure time activities. We then calculated the hours of metabolic equivalent tasks per day (MET-h) for each participant.
f. Data are only available for women.
g. Family history refers to the self-reported hypertension, diabetes or cardiovascular disease from at least one first-degree relative (biological parents, sibling) in the baseline survey.
Associations of dietary patterns with cardiometabolic risks
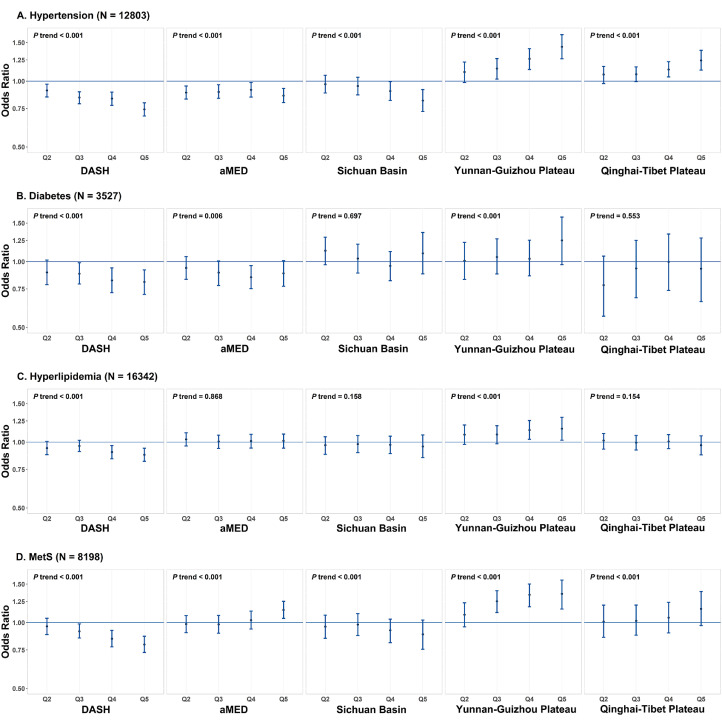
In the baseline survey of the CMEC study, we newly identified cases of 12803 hypertension, 3527 of diabetes, 16342 of hyperlipidaemia and 8198 of MetS. Figure 2 displays the estimated associations between dietary patterns and cardiometabolic risks after adjusting for potential confounders. Overall, dietary patterns showed considerable associations with risks of hypertension and MetS but only borderline associations with risks of diabetes and hyperlipidaemia. Comparing the highest with lowest quintiles, the DASH score showed the strongest inverse associations with risks of hypertension (OR=0.74, 95% CI:0.69-0.80) and MetS (OR=0.79, 95% CI:0.73-0.87), whereas the score of a posteriori Yunnan-Guizhou plateau dietary pattern showed the strongest positive associations with risks of hypertension (OR=1.44, 95% CI:1.27-1.63) and MetS (OR=1.35, 95% CI:1.15-1.57), with all P values for trend <0.001. For other dietary patterns, the Sichuan Basin showed similar but weaker associations with various cardiometabolic risks as DASH; a similar situation was also found between the Qinghai-Tibet and Yunnan-Guizhou Plateau dietary patterns. See the data presented in Figure 2 in table format in the Appendix text 12s.
Figure 2.
Estimated associations between various dietary patterns and cardiometabolic risks according to quintiles of dietary pattern scores, with the lowest quintile as reference group. Analyses were adjusted for sex, age, urbanicity, ethnicity, marital status, highest education attained, household income, profession, regular smoking, physical activity, total energy intake, regular intake of sweeten beverage, regular intake of dietary supplements, regular intake of spicy food, regular intake of pepper food, insomnia symptoms, depressive symptom, anxiety symptom, menopause status for women, and family history of cardiometabolic diseases using the inverse probability of exposure weighting. N in the brackets represent the number of newly diagnosed cardiometabolic risks. Q2-Q5 represent the second to fifth quintiles of dietary pattern scores. The filled blue dots represent adjusted odds ratios and the vertical blue lines represent 95% confidence intervals.
Although DASH and aMED shared some common food group components, aMED had notably different impacts on cardiometabolic risks. By comparing the highest with the lowest quintiles, aMED was associated with a 14% lower risk of hypertension (OR=0.86, 95% CI:0.80-0.93) but a 14% higher risk of MetS (OR=1.14, 95% CI:1.05-1.25) (figure 2). The discrepant results between DASH and aMED can be well confirmed by single-component analyses (Appendix text 13s: tables 19-22s). Among the discrepant food group components, the dairy product components included in DASH (but not in aMED) contributed a majority of the beneficial effects of DASH on hypertension (41.9%) and MetS (100.5%). In contrast, the monounsaturated fatty acids: saturated fatty acids (MUFA: SFA) ratio included in aMED (but not in DASH) had harmful effects, contributing a major proportion to the positive associations of aMED with hypertension (62.7%) and MetS (83.1%). Single food group analyses also showed similar results, especially for the strongest inverse association between dairy products and cardiometabolic risks (Appendix text 13s: tables 23s).
For simplicity, only associations of DASH and Yunnan-Guizhou plateau dietary patterns with hypertension and MetS are reported in the following results. In stratification analysis, the inverse associations of DASH and the positive associations of the Yunnan-Guizhou plateau dietary pattern with hypertension and MetS were consistent across various subgroups, with the only exceptions being ethnic region and urbanicity (figure 3). Both dietary patterns showed stronger associations with hypertension among the Han majority in Sichuan Basin compared to the ethnic minorities in other regions. For MetS, dietary patterns were prone to exhibit a risker association among the ethnic minorities on the Qinghai-Tibet Plateau compared to others, probably due to the extraordinarily high prevalence of central obesity in the Tibetan population.30,58 The stratified results of urbanicity highly agreed with those of ethnic regions because nearly all of the ethnic minority populations lived in rural areas. By decomposing the total associations into natural direct and indirect effects, these proportions mediated (PM) by overweight differed substantially between hypertension and MetS but remained relatively stable for hypertension or MetS, regardless of the type of dietary patterns (table 2). For associations with hypertension, the PM were 10% for DASH and 10% for the Yunnan-Guizhou plateau dietary pattern. Regarding associations with MetS, these PM increased to 35% for DASH and 33% for the Yunnan-Guizhou plateau dietary pattern.
Figure 3.
Stratified analysis of estimated associations between various dietary patterns and cardiometabolic risks according to predefined characteristics, by comparing the highest with the lowest quintiles. Analyses were adjusted for sex, age, urbanicity, ethnicity, marital status, highest education attained, household income, profession, regular smoking, physical activity, total energy intake, regular intake of sweeten beverage, regular intake of dietary supplements, regular intake of spicy food, regular intake of pepper food, insomnia symptoms, depressive symptom, anxiety symptom, menopause status for women, and family history of cardiometabolic diseases using the inverse probability of exposure weighting, with exclusion of the stratified variable as appropriate. Chi-square tests (χ2) were performed to examine heterogeneity among different subgroups. The filled blue dots represent adjusted odds ratios and the vertical blue lines represent 95% confidence intervals.
Table 2.
Mediation analysis of the associations between dietary patterns and cardiometabolic risks mediated by overweight, by comparing the highest with the lowest quintiles. a
| Dietary patterns | Cardiometabolic risks |
No. of overweight |
No. of cardiometabolic risks |
ORNIE | ORNDE | ORTE b | PM (%) c |
| DASH | Hypertension | 31130 | 12803 | 0.97 (0.96, 0.98) | 0.73 (0.68, 0.8) | 0.71 (0.65, 0.77) | 10 |
| Yunnan-Guizhou Plateau | Hypertension | 31130 | 12803 | 1.04 (1.02, 1.06) | 1.44 (1.26, 1.64) | 1.50 (1.31, 1.70) | 10 |
| DASH | MetS | 31130 | 8198 | 0.92 (0.90, 0.95) | 0.86 (0.79, 0.94) | 0.80 (0.72, 0.88) | 35 |
| Yunnan-Guizhou Plateau | MetS | 31130 | 8198 | 1.10 (1.05, 1.16) | 1.22 (1.05, 1.42) | 1.35 (1.15, 1.59) | 33 |
Abbreviations: ORNIE: odds ratio for the natural indirect effects; ORNDE: odds ratio for the natural direct effects; ORTE: odds ratio for the total effects; PM: proportion of mediation.
a. Analyses were adjusted for sex, age, urbanicity, ethnicity, marital status, highest education attained, household income, profession, regular smoking, physical activity, total energy intake, regular intake of sweeten beverage, regular intake of dietary supplements, regular intake of spicy food, regular intake of pepper food, insomnia symptoms, depressive symptom, anxiety symptom, menopause status for women, and family history of cardiometabolic diseases using the inverse probability of exposure weighting.
b. The estimated total effects of mediation analysis in this table were slightly different from that estimated in figure 2 because the calculation of natural direct effects needs to be conditional on the level of the covariates. In this analysis, continuous covariates were fixed at their median and categorical covariates were set at levels observed most frequently in the study population.
c. Proportion of mediation = natural indirect effect/(natural direct effect + natural indirect effect) × 100.
Our conclusions were robust with regard to all types of sensitivity analyses (Appendix text 14s). As expected, the inverse associations of DASH and positive associations of the Yunnan-Guizhou plateau dietary pattern with cardiometabolic risks (hypertension and MetS) were slightly attenuated but remained statistically significant when we further included self-reported cardiometabolic outcomes or adjusted for BMI as a confounder, which could be attributable to the potential inverse causality and mediation effects of BMI, respectively.
Discussion
Summary of main results
In this CMEC study covering diverse less-developed ethnic regions, greater adherence to DASH but not aMED was consistently associated with lower cardiometabolic risks, especially for hypertension and MetS. In contrast, two localized a posteriori dietary patterns in LEMRs were associated with increased cardiometabolic risks. Those associations were consistent in various subgroups defined by sex, age, smoking and physical activity, but with the magnitudes that differed substantially across different ethnic regions and urbanicity. If those associations were mainly causal, DASH-like diets would be a superior dietary recommendation to reduce cardiometabolic risks in settings similar to the LEMRs in CMEC.
Substantial SES and ethnic disparities
In recent decades, diet quality has experienced global trends similar to those of cardiometabolic disease mortality.59 Accordingly, suboptimal diets have now become one of the major driving factors for the rise of cardiometabolic diseases in LMICs.6 Although the Chinese government has done remarkable achievements in improving the living standards in LEMRs, our findings still found the huge SES and ethnic disparity in diet quality and related cardiometabolic risks. Compared to the Han majority in the more developed Sichuan Basin, ethnic minorities in less developed plateau areas generally adhered to much worse dietary patterns, which contributed to a substantial increase in cardiometabolic risks. A recent systematic analysis of the global burden of disease reported that high sodium intake and low whole-grain and fruit intake were the leading three dietary risk factors for death and DALYs worldwide.60 In China, national nutrition surveys have shown that high consumption of sodium and low consumption of fruits are associated with the greatest number of cardiometabolic deaths.61 Unfortunately, we can see the suboptimal intakes of sodium, whole-grain and fruit simultaneously in LEMRs compared to the more developed region in our study (i.e. Sichuan basin). Given that China already has the highest rates of diet-related cardiometabolic disease deaths worldwide,60 there is an urgent need for dietary actions to prevent the ongoing epidemics of cardiometabolic diseases in LEMRs.
Hypertension is the top priority
It is well acknowledged that suboptimal diets are associated with a myriad of cardiometabolic risks.15,16 However, in this study, hypertension manifested a substantially stronger association with dietary patterns than the other three included cardiometabolic risks, suggesting that hypertension is the predominant diet-related cardiometabolic risk in LEMRs. Although MetS (a combined indicator of multiple cardiometabolic risks) was also considerably associated with dietary patterns, the majority of its association can be attributable to hypertension as well, given that only weak associations were observed between dietary patterns and other cardiometabolic risks, such as diabetes and dyslipidaemia. These results were not surprising because hypertension in China have been a major concern for a long time.62 Our study indicates that hypertension control should be the top priority target among various diet-related cardiometabolic risks in LEMRs.
Weight loss may be not an ideal control target
In the development of cardiometabolic diseases, it is well recognized that obesity plays a crucial role and is highly associated with multiple cardiometabolic risks.63,64 Taking hypertension as an example, previous population studies suggest that overweight can directly account for 65% to 75% of primary hypertension.65 A growing number of studies also indicate that overweight may be an important mediator of the association between diet and cardiometabolic risks.[31], [32], [33] In this CMEC study with a relatively lean population, we found that overweight only mediated 10% of the associations between dietary patterns and hypertension but that the overwhelming majority of the risk of hypertension was attributable to the direct effects of dietary patterns. Due to the high correlation between central obesity (prerequisite for MetS) and overweight, the proportions of mediations were expected to be relatively high for MetS, but the major part of the risk of MetS (approximately 65%) was still attributable to the direct effects of dietary patterns. Given that the total energy content of a diet is the main determinant of an overweight status, our results imply that the principal risk of diet on cardiometabolic health in the CEMC study may derive from dietary components directly rather than energy imbalance. Therefore, weight loss or solely energy limit may not be an ideal control target for diet-related cardiometabolic risks in LEMRs.
DASH offers superior dietary guidance compared to aMED
DASH and Mediterranean style diets are the most well-studied healthy dietary patterns for reducing cardiometabolic risks.15 In our study, DASH was consistently and inversely associated with various cardiometabolic risks, in line with multiple previous systematic reviews.[66], [67], [68], [69], [70] It is also worth noting that dairy products contribute a predominate proportion to the beneficial effects of DASH. To date, evidence from both systematic reviews and Mendelian randomization studies in HICs have only suggested weak inverse associations between dairy products and cardiometabolic risk,15,71,72 but few of those studies were from LMICs, especially in those LMICs where dairy consumption is considerably low, such as China, Southeast Asia and Africa. Our findings indicate that dairy products may produce strong protective effects on cardiometabolic risks in LEMRs with low levels of dairy consumption, which coincides with the findings from the Prospective Urban Rural Epidemiology (PURE) study and another cohort study in China.73,74 According to previous systematic reviews, the beneficial effects of Mediterranean-style diets compared to DASH on cardiometabolic risks were controversial,[75], [76], [77], [78] especially for non-Mediterranean populations and racial/ethnic minority groups.79 In our study, we only observed weak inverse associations of aMED with hypertension and diabetes and even a positive association between aMED and MetS. As the major features of Mediterranean-style diets, the component of MUFA: SFA ratio did not show the expected beneficial effects on cardiometabolic risks based on single-component analyses. As another ethnic minority study suggested, the MUFA: SFA ratio may represent types of oils other than the intended healthy olive oil in non-Mediterranean populations.80 Similarly, we found that the MUFA: SFA ratio can only reflect a high consumption of vegetable oils (Appendix table 6s) due to a serious lack of high-quality sources of MUFA (such as olive oil or marine fish) in LEMRs. Besides, the stir-frying cooking manner with high temperature in LEMRs could be another reason why we did not observe the beneficial effects of the MUFA: SFA ratio in this study. In summary, our results indicate that DASH provides superior dietary guidance compared to aMED regarding reducing cardiometabolic risks in LEMRs.
Remaining issues related to translating dietary guidance into practices
Owing to the poor availability and affordability of cardiometabolic medicines in LMICs,81 dietary intervention is still an effective and feasible way to prevent ongoing cardiometabolic epidemics.82,83 However, adherence to healthy dietary guidance heavily relies on access to high-quality food.84 For the highly geography-related dietary patterns in our study, the food choices in LEMRs were confined by the relatively infertile and inconvenient plateau environment. The crux of translating healthy dietary guidance into practices is still the availability and affordability of high-quality food. This may explain why stronger inverse associations of DASH with cardiometabolic risks were found among the Han majority in more developed regions than ethnic minorities in less developed regions. Deep-rooted culinary culture is another challenge to reduce the diet-related cardiometabolic risks in LEMRs. Although a global shift in dietary patterns has been seen worldwide,59 most ethnic minorities still live distinct lifestyles with unique culinary cultures that can hardly be altered. Thus, healthy dietary guidance is far from sufficient, and multi-sectional cooperation in refining food quality and acceptable dietary guidance that are suitable for local culture are remaining challenges for improving diet-related cardiometabolic health in LEMRs.
Strengths and limitations
To our knowledge, this is the first large-scale epidemiological study to comprehensively examine associations between dietary patterns and cardiometabolic risks in LEMRs. Our study provides a unique opportunity to understand the diet quality and related cardiometabolic risks in LEMRs, which has rarely been performed. We used standardized and validated methods to measure diet and objective indicators to record cardiometabolic outcomes. In addition, we performed analyses under the framework of causal inference, including the application of ESC-DAGs and marginal structure models, which provides transparent and evidence-based approaches to guide the selection of potential confounders and estimation methods. Nonetheless, limitations are worth noting. First, for the sake of feasibility, the FFQ used in our study only includes thirteen crude food groups rather than specific food items. Although it should not have a significant impact on the assessment of dietary patterns, crude food groups may undermine the accuracy of the calculation of particular dietary components (i.e., the MUFA: SFA ratio in aMED) and total energy intake. Our assessment tool is imperfect, but this simple tool might be the only feasible way to collect dietary information in LEMRs. For those participants from LEMRs, many of them are illiterate (they cannot read or fill the food questionnaire by themselves), speak different local languages (interviews need help from the local translator) and consume distinct foods (many foods are not included in any existing food database). Given the huge number of participants recruited and comprehensive other information collected, a food group-based and simplified food questionnaire may be the only option that can ensure the efficiency of communication, the cooperation of participants, and the comparability among various regions. By analysing subsample data for 24 HDRs, differences between the calculation of total energy intakes based on crude food groups and that based on specific food items were small and symmetrically distributed around zero (Appendix text 15s). Second, we modified the dietary pattern scores by omitting SSBs from the DASH score and nuts from the MED score, which may attenuate the associations between dietary scores and cardiometabolic risks. For omitting the consumption of nuts, it may also lead to the underestimation of MUFA: SFA ratio. Nevertheless, we believe that this defect should not have a marked impact on our conclusion given that the consumption of both SSBs and healthy nuts in LEMRs was very low. Third, we used questions on household use as a proxy for individual-level intake to estimation both sodium and oil intake. In China, people always consumed mixed dishes and shared dishes with each other, and the standard recipes with amounts of condiments specified precisely is also rarely used, which make it infeasible to recall and estimate salt and oil intake by questionnaire. Fourth, we did not perform measurement error correction, as the overwhelming majority of current statistical approaches are only suitable for a single food item or nutrient.85,86 By conducting a preliminary simulation study, we found that the dietary patterns were robust to the measurement errors and that the impact of measurement errors on the diet-disease association was still the attenuation effect (Appendix text 16s). Fifth, dietary pattern analysis can capture only a portion of the variation in food consumption87 and therefore may not fully represent diet quality. Sixth, residual confounding is still possible, even though we carefully adjusted for potential confounders under the framework of causal inference. Last, the intrinsic nature of the cross-sectional study design also limits the reliable inference of causality, but we did exclude self-reported cases to eliminate potential reverse causality.
Conclusion
In conclusion, using the recently collected CMEC data, we observed substantial SES and ethnic disparities in diet quality and related cardiometabolic risks in LEMRs. Among the four included cardiometabolic risks, hypertension showed the strongest association with dietary patterns and should be the top priority of control target for ensuing dietary actions. Our study also suggests that DASH offers superior dietary guidance to aMED for reducing cardiometabolic risks in LEMRs. In particular, the dairy intake encouraged by DASH may produce considerable beneficial effects for lowering cardiometabolic risks. Although the spectrum of cardiometabolic diseases and dietary patterns may vary among different LEMRs, our study reaffirms the important role of diet in the prevention of cardiometabolic diseases and DASH may provide a solution of dietary guidance with availability and affordability in the setting of LEMRs.
Contributors
XX, JY, and XZ contributed to the design of the present study. XZ was the principal investigator and JY was the co- principal investigator of the CMEC study. XX and ZQ wrote the analysis plan, and the first and final draft of the paper. JY and XZ reviewed and commented on the data analysis, all drafts and the final paper. All other authors were involved in conduct of the study, analysis of data, interpretation of results, and provided critical comments on all drafts of the report.
Research in context
Evidence before this study
We searched for PubMed for relevant literature between Jan 1, 1980, and Feb 1, 2021, using the search terms “dietary pattern” or “eating pattern” or “dietary index” or “dietary guidance” or “diet quality” or “dietary approaches to stop hypertension” or “Mediterranean” and “cardiovascular” or “metabolic” or “cardiometabolic” or “heart” or “atherosclerosis” or “stroke” or “hypertension” or “diabetes” or “hyperlipidaemia”. Few studies on the associations between dietary patterns and cardiometabolic risks have been conducted in less-developed ethnic minority regions (LEMRs).
Added value of this study
To our knowledge, this is the first large-scale epidemiological study to comprehensively examine associations between dietary patterns and cardiometabolic risks in LEMRs. Our study provides a unique opportunity to understand diet quality and related cardiometabolic risks in LEMRs, which has rarely been conducted previously.
Implications of all the available evidence
Given the substantial socioeconomic status and ethnic disparities in diet quality and related cardiometabolic risks shown in this study, there is an urgent need for dietary actions to prevent the ongoing epidemics of cardiometabolic diseases in LEMRs, especially hypertension. Our findings also suggest that Dietary Approaches to Stop Hypertension (DASH) offers superior dietary guidance compared to alternative Mediterranean (aMED)-style diets for reducing cardiometabolic risks in LEMRs.
Declaration of interests
All authors declared no competing interest.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgements
The CMEC study was funded by the National Key R&D Program of China (Grant No. 2017YFC0907302, 2017YFC0907300). XX was supported by the National Natural Science Foundation of China (Grant No. 81903415), the Key R&D Project of Sichuan Province Science and Technology Support Program (Grant No. 2020YFS0215) and CNS-ZD Tizhi and Health Fund (Grant No. CNS-ZD2020-149). JY was supported by the National Natural Science Foundation of China (Grant No. 81860597). XZ was supported by the National Natural Science Foundation of China (Grant No. 81973151). We thank all the team members and participants involved in the China Multi-Ethnic Cohort (CMEC). We also thank Drs Junmin Zhou and Huan Song for their critical reading of the manuscript, and MScs Jiaojiao Lu, Yuan Zhang, Xinyu Wu, Xingren Zhu for their significant contributions on the research plan and data analysis. We are grateful to Prof. Xiaosong Li at Sichuan University for his leadership and fundamental contribution to the establishment of the CMEC. Prof. Li was the former principal investigator of the CMEC study who passed away in 2019.
Data sharing
Study data are available on request to the authors.
Editor note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100252.
Contributor Information
Jianzhong Yin, Email: yinjianzhong2005@sina.com.
Xing Zhao, Email: xingzhao@scu.edu.cn.
Appendix. Supplementary materials
References
- 1.Vos T, Lim SS, Abbafati C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ezzati M, Obermeyer Z, Tzoulaki I, Mayosi BM, Elliott P, DA Leon. Contributions of risk factors and medical care to cardiovascular mortality trends. Nat Rev Cardiol. 2015;12(9):508–530. doi: 10.1038/nrcardio.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mensah George A, Wei Gina S, Sorlie Paul D. Decline in Cardiovascular Mortality. Circ Res. 2017;120(2):366–380. doi: 10.1161/CIRCRESAHA.116.309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES, Capewell S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: public health versus clinical care. Annu Rev Publ Health. 2011;32:5–22. doi: 10.1146/annurev-publhealth-031210-101211. [DOI] [PubMed] [Google Scholar]
- 5.Ezzati M, Pearson-Stuttard J, Bennett JE, Mathers CD. Acting on non-communicable diseases in low- and middle-income tropical countries. Nature. 2018;559(7715):507–516. doi: 10.1038/s41586-018-0306-9. [DOI] [PubMed] [Google Scholar]
- 6.Miranda JJ, Barrientos-Gutiérrez T, Corvalan C. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat Med. 2019;25(11):1667–1679. doi: 10.1038/s41591-019-0644-7. [DOI] [PubMed] [Google Scholar]
- 7.Lijing LY, Shangzhi X, Hongsheng L, Enying G, Nicholas P, Shenglan T. World Health Organization. Regional Office for South-East Asia; New Delhi: 2019. Strengthening primary health care for the prevention and management of cardiometabolic disease in low- and middle-income countries. [Google Scholar]
- 8.Templin T, Cravo Oliveira Hashiguchi T, Thomson B, Dieleman J, Bendavid E. The overweight and obesity transition from the wealthy to the poor in low- and middle-income countries: A survey of household data from 103 countries. Plos Med. 2019;16(11) doi: 10.1371/journal.pmed.1002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong B, Arnold LW, Peng Y, Wang Z. Ethnic differences in cardiometabolic risk among adolescents across the waist–height ratio spectrum: National Health and Nutrition Examination Surveys (NHANES) Int J Cardiol. 2016;222:622–628. doi: 10.1016/j.ijcard.2016.07.169. [DOI] [PubMed] [Google Scholar]
- 10.Agbim U, Carr RM, Pickett-Blakely O, Dagogo-Jack S. Ethnic Disparities in Adiposity: Focus on Non-alcoholic Fatty Liver Disease, Visceral, and Generalized Obesity. Curr Obes Rep. 2019;8(3):243–254. doi: 10.1007/s13679-019-00349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Cesare M, Khang YH, Asaria P. Inequalities in non-communicable diseases and effective responses. The Lancet. 2013;381(9866):585–597. doi: 10.1016/S0140-6736(12)61851-0. [DOI] [PubMed] [Google Scholar]
- 12.Niessen LW, Mohan D, Akuoku JK. Tackling socioeconomic inequalities and non-communicable diseases in low-income and middle-income countries under the Sustainable Development agenda. The Lancet. 2018;391(10134):2036–2046. doi: 10.1016/S0140-6736(18)30482-3. [DOI] [PubMed] [Google Scholar]
- 13.Ralston J, Nugent R. Toward a broader response to cardiometabolic disease. Nat Med. 2019;25(11):1644–1646. doi: 10.1038/s41591-019-0642-9. [DOI] [PubMed] [Google Scholar]
- 14.Bennett JE, Stevens GA, Mathers CD. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. The Lancet. 2018;392(10152):1072–1088. doi: 10.1016/S0140-6736(18)31992-5. [DOI] [PubMed] [Google Scholar]
- 15.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozaffarian D. Dietary and policy priorities to reduce the global crises of obesity and diabetes. Nature Food. 2020;1(1):38–50. [Google Scholar]
- 17.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 18.Estruch R, Ros E, Salas-Salvadó J. Primary prevention of cardiovascular disease with a Mediterranean diet. New Engl J Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 19.Schulze MB, Hoffmann K, Manson JE. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82(3) doi: 10.1093/ajcn.82.3.675. 675-84; quiz 714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tinker LF, Bonds DE, Margolis KL. Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women's Health Initiative randomized controlled dietary modification trial. Arch Intern Med. 2008;168(14):1500–1511. doi: 10.1001/archinte.168.14.1500. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury R, Warnakula S, Kunutsor S. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160(6):398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 22.Howard BV, Van Horn L, Hsia J. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 23.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services and U.S . Washington, D.C.: U.S. Department of Agriculture. 8th Edition. 2015. Department of Agriculture. 2015 –2020 Dietary Guidelines for Americans. [Google Scholar]
- 25.Dietary Guidelines Advisory Committee . U.S. Department of Agriculture; Washington, DC: 2020. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. In: U.S. Department of Agriculture, U.S. Department of Health and Human Services. editors. [Google Scholar]
- 26.Sacks FM, Lichtenstein AH, Wu JHY. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation. 2017;136(3):e1–e23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 27.Davis NJ, Schechter CB, Ortega F, Rosen R, Wylie-Rosett J, Walker EA. Dietary patterns in blacks and Hispanics with diagnosed diabetes in New York City's South Bronx. Am J Clin Nutr. 2013;97(4):878–885. doi: 10.3945/ajcn.112.051185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nettleton JA, Schulze MB, Jiang R, Jenny NS, Burke GL, Jacobs DR. Jr. A priori-defined dietary patterns and markers of cardiovascular disease risk in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2008;88(1):185–194. doi: 10.1093/ajcn/88.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker KL. Dietary patterns, approaches, and multicultural perspective. Appl Physiol Nutr Me. 2010;35(2):211–218. doi: 10.1139/H10-010. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X, Hong F, Yin J. Cohort profile: the China Multi-Ethnic cohort (CMEC) study. Int J Epidemiol. 2020 doi: 10.1093/ije/dyaa185. dyaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.André P, Proctor G, Driollet B. The role of overweight in the association between the Mediterranean diet and the risk of type 2 diabetes mellitus: a mediation analysis among 21 585 UK biobank participants. Int J Epidemiol. 2020;49(5):1582–1590. doi: 10.1093/ije/dyaa103. [DOI] [PubMed] [Google Scholar]
- 32.Joris PJ, Plat J, Kusters YH. Diet-induced weight loss improves not only cardiometabolic risk markers but also markers of vascular function: a randomized controlled trial in abdominally obese men. Am J Clin Nutr. 2017;105(1):23–31. doi: 10.3945/ajcn.116.143552. [DOI] [PubMed] [Google Scholar]
- 33.Schoenaker DA, Soedamah-Muthu SS, Mishra GD. Quantifying the mediating effect of body mass index on the relation between a Mediterranean diet and development of maternal pregnancy complications: the Australian Longitudinal Study on Women's Health. Am J Clin Nutr. 2016;104(3):638–645. doi: 10.3945/ajcn.116.133884. [DOI] [PubMed] [Google Scholar]
- 34.Millwood IY, Walters RG, Mei XW. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. The Lancet. 2019;393(10183):1831–1842. doi: 10.1016/S0140-6736(18)31772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institute for Nurtition and Health. China food composition tables. 6 ed. Beijing: Peking university medical press; 2018.
- 36.National Health Commission of the People's Republic of China. Dietary guide for adult diabetes patients (WS/T 429-2013). 2013.
- 37.Chiu S, Bergeron N, Williams PT, Bray GA, Sutherland B, Krauss RM. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: a randomized controlled trial. Am J Clin Nutr. 2016;103(2):341–347. doi: 10.3945/ajcn.115.123281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. New Engl J Med. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 39.Fung TT, McCullough ML, Newby PK. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82(1):163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 40.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119(8):1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shan Z, Li Y, Baden MY. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern Med. 2020;180(8):1090–1100. doi: 10.1001/jamainternmed.2020.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edefonti V, Randi G, La Vecchia C, Ferraroni M, Decarli A. Dietary patterns and breast cancer: a review with focus on methodological issues. Nutr Rev. 2009;67(6):297–314. doi: 10.1111/j.1753-4887.2009.00203.x. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson KD, McCann M, Katikireddi SV. Evidence synthesis for constructing directed acyclic graphs (ESC-DAGs): a novel and systematic method for building directed acyclic graphs. Int J Epidemiol. 2020;49(1):322–329. doi: 10.1093/ije/dyz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearl J, Glymour M, Jewell NP. Wiley; United Kingdom: 2016. Causal Inference in Statistics: A Primer. West Sussex. [Google Scholar]
- 45.Ukoumunne OC, Williamson E, Forbes AB, Gulliford MC, Carlin JB. Confounder-adjusted estimates of the risk difference using propensity score-based weighting. Stat Med. 2010;29(30):3126–3136. doi: 10.1002/sim.3935. [DOI] [PubMed] [Google Scholar]
- 46.Elze MC, Gregson J, Baber U. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J Am Coll Cardiol. 2017;69(3):345–357. doi: 10.1016/j.jacc.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 47.Greifer N. WeightIt: Weighting for Covariate Balance in Observational Studies. R package version 0.10.2. ed; 2020.
- 48.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hainmueller J. Entropy Balancing for Causal Effects: A Multivariate Reweighting Method to Produce Balanced Samples in Observational Studies. Political Analysis. 2012;20(1):25–46. [Google Scholar]
- 50.Trichopoulou A, Bamia C, Trichopoulos D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ. 2009;338:b2337. doi: 10.1136/bmj.b2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.InterAct C, Romaguera D, Guevara M. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: the InterAct project. Diabetes care. 2011;34(9):1913–1918. doi: 10.2337/dc11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VanderWeele T. Oxford University Press; New York: 2015. Explanation in Causal Inference: Methods for Mediation and Interaction. [Google Scholar]
- 53.Gaynor SM, Schwartz J, Lin X. Mediation analysis for common binary outcomes. Stat Med. 2019;38(4):512–529. doi: 10.1002/sim.7945. [DOI] [PubMed] [Google Scholar]
- 54.van Buuren S. Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 55.Bradley Efron, Tibshirani RJ . Chapman and Hall/CRC; New York: 1994. An Introduction to the Bootstrap. [Google Scholar]
- 56.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–5655. doi: 10.1002/sim.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Team RC. R Foundation for Statistical Computing. Vienna; Austria: 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 58.Zhang X, Zhang M, Zhao Z. Geographic Variation in Prevalence of Adult Obesity in China: Results From the 2013-2014 National Chronic Disease and Risk Factor Surveillance. Ann Intern Med. 2020;172(4):291–293. doi: 10.7326/M19-0477. [DOI] [PubMed] [Google Scholar]
- 59.Imamura F, Micha R, Khatibzadeh S. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. The Lancet Glob Health. 2015;3(3):e132–ee42. doi: 10.1016/S2214-109X(14)70381-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Afshin A, Sur PJ, Fay KA. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2019;393(10184):1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He Y, Li Y, Yang X. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982–2012: a cross-sectional population-based study. The Lancet Diabetes Endo. 2019;7(7):540–548. doi: 10.1016/S2213-8587(19)30152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Chen Z, Zhang L. Status of Hypertension in China: Results From the China Hypertension Survey, 2012–2015. Circulation. 2018;137(22):2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 63.Poirier P, Giles TD, Bray GA. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 64.Krauss RM, Winston M, Fletcher BJ. Grundy SM. Obesity. Circulation. 1998;98(14):1472–1476. [PubMed] [Google Scholar]
- 65.Hall JE, JMd Carmo, AAd Silva, Wang Z, Hall ME. Obesity-Induced Hypertension. Circ Res. 2015;116(6):991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shirani F, Salehi-Abargouei A, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition. 2013;29(7-8):939–947. doi: 10.1016/j.nut.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 67.Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Brit J Nutr. 2015;113(1):1–15. doi: 10.1017/S0007114514003341. [DOI] [PubMed] [Google Scholar]
- 68.Filippou CD, Tsioufis CP, Thomopoulos CG. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Nutr. 2020;11(5):1150–1160. doi: 10.1093/advances/nmaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Nutr. 2016;7(1):76–89. doi: 10.3945/an.115.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovas. 2014;24(12):1253–1261. doi: 10.1016/j.numecd.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 71.Ding M, Huang T, Bergholdt HK, Nordestgaard BG, Ellervik C, Qi L. Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study. BMJ. 2017;356:j1000. doi: 10.1136/bmj.j1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anand SS, Hawkes C, de Souza RJ. Food Consumption and its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System: A Report From the Workshop Convened by the World Heart Federation. J Am Coll Cardiol. 2015;66(14):1590–1614. doi: 10.1016/j.jacc.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dehghan M, Mente A, Rangarajan S. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. The Lancet. 2018;392(10161):2288–2297. doi: 10.1016/S0140-6736(18)31812-9. [DOI] [PubMed] [Google Scholar]
- 74.Zong G, Sun Q, Yu D. Dairy Consumption, Type 2 Diabetes, and Changes in Cardiometabolic Traits: A Prospective Cohort Study of Middle-Aged and Older Chinese in Beijing and Shanghai. Diabetes Care. 2014;37(1):56–63. doi: 10.2337/dc13-0975. [DOI] [PubMed] [Google Scholar]
- 75.Bendall CL, Mayr HL, Opie RS, Bes-Rastrollo M, Itsiopoulos C, Thomas CJ. Central obesity and the Mediterranean diet: A systematic review of intervention trials. Crit Rev Food Sci Nutr. 2018;58(18):3070–3084. doi: 10.1080/10408398.2017.1351917. [DOI] [PubMed] [Google Scholar]
- 76.Liyanage T, Ninomiya T, Wang A. Effects of the Mediterranean Diet on Cardiovascular Outcomes-A Systematic Review and Meta-Analysis. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0159252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nissensohn M, Román-Viñas B, Sánchez-Villegas A, Piscopo S, Serra-Majem L. The Effect of the Mediterranean Diet on Hypertension: A Systematic Review and Meta-Analysis. J Nutr Educ Behav. 2016;48(1):42–53. doi: 10.1016/j.jneb.2015.08.023. e1. [DOI] [PubMed] [Google Scholar]
- 78.Rees K, Takeda A, Martin N. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Db Syst Rev. 2019;3(3) doi: 10.1002/14651858.CD009825.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sotos-Prieto M, Mattei J. Mediterranean diet and cardiometabolic diseases in racial/ethnic minority populations in the United States. Nutrients. 2018;10(3):352. doi: 10.3390/nu10030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gardener H, Wright CB, Gu Y. Mediterranean-style diet and risk of ischemic stroke, myocardial infarction, and vascular death: the Northern Manhattan Study. Am J Clin Nutr. 2011;94(6):1458–1464. doi: 10.3945/ajcn.111.012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khatib R, McKee M, Shannon H. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. The Lancet. 2016;387(10013):61–69. doi: 10.1016/S0140-6736(15)00469-9. [DOI] [PubMed] [Google Scholar]
- 82.Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of Different Dietary Interventions on Blood Pressure: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Hypertension. 2016;67(4):733–739. doi: 10.1161/HYPERTENSIONAHA.115.06853. [DOI] [PubMed] [Google Scholar]
- 83.Papamichou D, Panagiotakos DB, Itsiopoulos C. Dietary patterns and management of type 2 diabetes: A systematic review of randomised clinical trials. Nutr Metab Cardiovas. 2019;29(6):531–543. doi: 10.1016/j.numecd.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Baker EA, Schootman M, Barnidge E, Kelly C. The role of race and poverty in access to foods that enable individuals to adhere to dietary guidelines. Prev Chronic Dis. 2006;3(3):A76. [PMC free article] [PubMed] [Google Scholar]
- 85.Bennett DA, Landry D, Little J, Minelli C. Systematic review of statistical approaches to quantify, or correct for, measurement error in a continuous exposure in nutritional epidemiology. BMC Med Res Methodol. 2017;17(1):146. doi: 10.1186/s12874-017-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Looman M, Boshuizen HC, Feskens EJ, Geelen A. Using enhanced regression calibration to combine dietary intake estimates from 24 h recall and FFQ reduces bias in diet-disease associations. Public Health Nutr. 2019;22(15):2738–2746. doi: 10.1017/S1368980019001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schulze MB, Martinez-Gonzalez MA, Fung TT, Lichtenstein AH, Forouhi NG. Food based dietary patterns and chronic disease prevention. BMJ. 2018;361:k2396. doi: 10.1136/bmj.k2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.