Highlights
-
•
Long-term gefitinib induction could increase the level of oxidative stress in lung adenocarcinoma cells and reduce the antioxidant capacity.
-
•
Acquired resistance to gefitinib in lung adenocarcinoma was closely related to the high expression of HIF-1 and ALDH1 and the enrichment of CSCs.
-
•
The inhibitory effect of aerobic exercise on oxidative stress can effectively reduce the expression of HIF-1 and ALDH1 and inhibit the enrichment of CSCs, which can enhance the response of drug-resistant cells to gefitinib.
Keywords: Aerobic exercise, Lung adenocarcinoma, Gefitinib, Acquired drug resistance, CSCs
Abstract
Lung adenocarcinoma patients with epidermal growth factor receptor (EGFR)-activating mutations respond well to tyrosine kinase inhibitors but typically develop resistance. Current therapies mainly target differentiated cells, not cancer stem cells (CSCs), but CSCs affect the occurrence, invasion, metastasis and treatment sensitivity of malignant tumours. Recently, aerobic exercise has emerged as adjuvant therapy for cancer. Aerobic exercise can accelerate blood circulation, improve tissue oxygen supply, reduce the stress level of patients, improve the antioxidant capacity of the body, and facilitate the degradation of hypoxia-inducible factor-1 (HIF-1) in tumour tissues, thus weakening its maintenance effect on CSCs. In this study, we successfully established lung adenocarcinoma cell lines with gefitinib resistance. Long-term gefitinib induction could increase the level of oxidative stress in lung adenocarcinoma cells and reduce the antioxidant capacity, resulting in the high expression of HIF-1 and ALDH1 and leading to the enrichment of CSCs, and a decreased response to gefitinib. This may be one of the important reasons for gefitinib-acquired resistance in lung adenocarcinoma. In the case of drug resistance, effective aerobic exercise could reduce ROS, activate SOD, inhibit HIF-1 and ALDH1, and cause a reduction in CSCs to sensitise cells to gefitinib again and ultimately inhibit the malignant proliferation of tumours. Therefore, in the treatment of lung adenocarcinoma, the inhibitory effect of aerobic exercise on oxidative stress can enhance the response of drug-resistant cells to gefitinib and can be used as an effective adjunct measure in the treatment of lung adenocarcinoma.
Introduction
The incidence and mortality of lung cancer are extremely high. Approximately 80% of cases are non-small-cell lung cancer (NSCLC), and lung adenocarcinoma is one of the main pathological types of NSCLC [1,2]. In the early stage, lung adenocarcinoma usually features the general symptoms of respiratory diseases, which are easily overlooked. Diagnosis usually occurs in the advanced stage, and the prognosis is very poor [3,4]. With advances in medical research, targeted therapy has gradually become a new method of treatment. Activating mutations in EGFR have been shown to be key drivers of lung adenocarcinoma. Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI)-based molecular targeted therapy has provided hope for patients with advanced lung adenocarcinoma [5]. However, the development of drug resistance makes long-term treatment impossible. Therefore, exploring the mechanism of acquired resistance and identifying ways to delay the occurrence of resistance are reasonable goals for reducing the mortality rate of lung adenocarcinoma. Many teams have published research on the mechanism underlying EGFR-TKI resistance. Within these published studies, research related to cancer stem cells (CSCs) has garnered increasing attention, and CSCs have been successfully isolated and enriched in lung cancer [6,7].
CSCs are a small subset of cells with a high potential for self-renewal and abnormal differentiation in tumours [8]. Although conventional radiotherapy and chemotherapy have certain therapeutic effects on tumours, clinical data show that CSCs are resistant to various treatments. CSCs have been considered the root cause of drug resistance, recurrence and metastasis of tumours [9]. Acquired resistance to lung adenocarcinoma may be caused by the enrichment of CSCs by TKIs. Studies have confirmed that acetaldehyde dehydrogenase 1 (ALDH1) can be used as a CSC marker for lung adenocarcinoma [10,11].
In recent years, aerobic exercise has been used as a novel adjuvant therapy for cancer. Effective aerobic exercise after treatment for breast cancer, colorectal cancer and prostate cancer can significantly improve quality of life and prolong survival [12,13]. However, the recurrence and metastasis rates of malignant tumour patients with obesity and metabolic syndrome and those who do not exercise are significantly increased [14]. It is suggested that aerobic exercise may be an effective adjunct measure for tumour treatment. Clinical data show that aerobic exercise can accelerate blood circulation, improve tissue oxygen supply, reduce the production of reactive oxygen species (ROS), activate endogenous antioxidant enzymes, and facilitate the degradation of hypoxia-inducible factor-1 (HIF-1) in tumour tissues [15,16], weakening its role in maintaining CSCs [17].
In this study, we established lung adenocarcinoma cell lines with gefitinib resistance. The mechanism underlying acquired resistance to gefitinib in lung adenocarcinoma was preliminarily explored by detecting ROS-related indicators, HIF-1 and ALDH1 proteins and the populations of CSCs in drug-resistant cell lines and parental cell lines. In addition, aerobic exercise combined with gefitinib was used as an intervention in a subcutaneous tumour-bearing nude mouse model. Here, we studied the effect of aerobic exercise on acquired gefitinib resistance in lung adenocarcinoma, providing new ideas and effective strategies for the clinical treatment of lung adenocarcinoma.
Materials and methods
Establishment of cell lines with gefitinib resistance
The human lung adenocarcinoma cell lines PC-9 and HCC827 were kindly donated by Dr. Shimada at Kyoto University (Kyoto, Japan). Cell lines were maintained at a density of 5 × 105 cells/mL in RPMI-1640 medium (Gibco) supplemented with 10% foetal bovine serum (Gibco) in an incubator (37 °C, 5% CO2). To help prevent or control contamination with Mycoplasma, anti-Mycoplasma reagent (InvivoGen) was used prophylactically at a working concentration of 2.5 mg/mL. Cell lines with acquired gefitinib resistance were established by a stepwise induction method. The concentration of gefitinib (Selleck Chemicals) was increased gradually from 5 nmol/L to 2 μmol/L, and the culture was continued for 6 months. The successfully induced drug-resistant cell lines were named PC-9-GR and HCC827-GR [18].
Determination of gefitinib IC50
A 100 μl cell suspension was prepared in a 96-well plate with 1000 cells per well and cultured in an incubator (37 °C, 5% CO2). Different concentrations of gefitinib were added to the cells, which were incubated for specific times [18]. CCK-8 (Dojindo) solution (10 μL) was added to each well, and the samples were incubated in an incubator for 1 h. The absorbence at 450 nm was measured with an enzyme plate analyser, and the IC50 values of different groups were compared. The experiment was repeated 3 times.
Determination of ROS-related indicators
ROS kits (Nanjing Jiancheng Bioengineering Institute) were used to detect the ROS levels in cells and tumours of each group. The fluorescence value of the control group was 1, and the relative ROS content of the other groups was compared with that of the control group. For detailed steps, refer to the instructions and literature [18]. The supernatant of cell culture or serum of nude mice was taken from each group. MDA and SOD kits (Nanjing Jiancheng Bioengineering Institute) were used to detect MDA content and SOD activity in cells and nude mice of each group. For detailed steps, refer to the instructions. The experiment was repeated 3 times.
Western blotting
RIPA buffer (Solarbio) was used to extract fresh cell and tissue proteins. Protein concentration was determined and quantified using the BCA Protein Quantitative Kit (Solarbio). A total of 30 μg of total protein were loaded per lane. The proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) with a mass fraction of 10% and transferred to PVDF membranes (Millipore). The samples were sealed with TBST containing 5% skim milk for 1 h at room temperature. HIF-1, ALDH1 and GAPDH antibodies (1:1000, Abcam) were added and incubated overnight at 4 °C. Goat anti-rabbit IgG (1:2000, Abcam) was added and incubated at room temperature for 2 h. The protein bands were detected with an enhanced chemiluminescence (ECL) luminescence developer (Invitrogen). A gel imaging system (BIO-RAD) was used to capture images. Image Lab software was used to measure the grey value of the protein strips. The ratio of the greyscale value of the target protein to the internal reference protein band was used as the relative expression level of the final protein [19,20]. Each experiment was repeated 3 times.
Determination of the ALDH1+ CSCs populations
The concentration of cells in each group was adjusted to 1 × 106 cells/mL with Aldefluor™ detection buffer (STEMCELL Technologies). Each sample to be tested was provided with a "test" tube (DEAB-) and a "control" tube (DEAB+). The cell suspension (1 mL) was extracted from each group and placed into each test tube, and 5 μL DEAB reagent was added to each control tube. Activated Aldefluor™ reagent (5 μL) was added to the cell suspension in each test tube, and 0.5 mL of the mixture was immediately extracted and placed into the control tube. Each sample was incubated at 37 °C for 30 to 60 min. The cell precipitate was resuspended in 0.5 mL Aldefluor™ detection buffer, and the populations of ALDH1+ CSCs were measured by flow cytometry (Beckman Coulter, Inc.) [18]. The experiment was repeated 3 times.
Lentiviral vector packaging
293T cells were maintained in our laboratory at a density of 70% in DMEM (Gibco) supplemented with 10% foetal bovine serum in an incubator (37 °C, 5% CO2). The overexpressed or knockdown plasmids of the packaged lentivirus (Cyagen) were mixed with the packaged lentivirus plasmids (Cyagen) and then mixed with the transfection reagent (GeneCopoeia) to form a DNA-Endofectin mixture. After sitting for 30 min at room temperature, the mixture was added to 293T cells. After 12 h of conventional culture, the medium was replaced with fresh medium. The virus supernatant was collected after 48 h of conventional culture and filtered through a 0.45 μm filter to remove cell debris. When the confluency of target cells reached 50–60%, the virus supernatant was added for transfection. The cells were screened and cultured with puromycin (Gibco) after 48 h of infection. After stable cell lines were obtained, the knockdown and overexpression efficiency of HIF-1 and ALDH1 was determined by Western blotting. The stable cell lines with knockdown and overexpression of HIF-1 and ALDH1 were constructed according to the above steps.
In vivo studies
Forty-eight healthy male SPF-grade BALB/C nude mice aged 4–6 weeks and weighing approximately 20 g were obtained from China Changzhou Cavens Experimental Animal Co., Ltd. (animal licence no.: SCXK (SU) 2016-0010). The temperature of the animal room was 25 ± 2 °C, the humidity was 40–60%, and natural day and night lighting was used. The feed and drinking water were SPF grade, and the nude mice were allowed to freely eat and drink. After being adaptively fed for 1 week, the mice were randomly divided into 8 groups: control, gefitinib monotherapy, aerobic exercise, aerobic exercise + gefitinib, HIF-1 knockdown, HIF-1 knockdown + gefitinib, ALDH1 knockdown, and ALDH1 knockdown + gefitinib. Six mice in each group were maintained under SPF conditions. PC-9-GR cells, PC-9-GR (HIF-1 knockdown) cells, and PC-9-GR (ALDH1 knockdown) cells were fluorescently labelled with PKH67 (Sigma-Aldrich), and all cells were detected to carry fluorescence. After skin disinfection of the nude mice, 5 ×€106 labelled cells were inoculated in the right armpit of each nude mouse according to the group. After tumour formation, the nude mice were orally administered gefitinib at 75 mg/kg every day until the end of the experiment [18]. The nude mice in the aerobic exercise group and aerobic exercise + gefitinib group were given exercise training [21]. After the adaptive platform (China Jiangsu Cylon Biotechnology Co., Ltd.) training for 5 days (the speed was 1 m/min, and the slope was 0°), the platform speed was 17 m/min, and the slope was 0° (84% VO2max). Calibration was repeated every 15 days. Before each training, warm-up exercises were performed for 10 min (the speed was 1 m/min, and the slope was 0°). The nude mice were exercised once a day for 60 min each time (they were exercised 6 days a week and rested 1 day). The experiment lasted for 8 weeks (56 days). The other six groups did not exercise.
During the experiment, the tumour size was measured by a small animal in vivo imager (PerkinElmer). After the experiment, the nude mice were sacrificed. Their serum was used to measure MDA content and SOD activity. Their tumours were peeled and ground. The ROS content in each tumour was detected by a kit. The protein expression of HIF-1 and ALDH1 in the tumours of nude mice in each group was detected by Western blotting. The populations of ALDH1+ CSCs in each group of nude mice were determined by flow cytometry using an Aldefluor® stem cell assay kit. The mean value of nude mice in each group was taken as the final value in the above detection methods. The animal study (including the mouse euthanasia procedure) was carried out in compliance with the regulations and the Henan University of Science and Technology Institutional Animal Care Guidelines (Ethical code: 2021-03-B027).
Statistical analysis
SPSS 26.0 software was used for statistical processing of the data. All measurement data are expressed as the mean ± standard deviation (± s). The difference between the two groups was compared by t-test. Differences between three or more groups were compared by one-way ANOVA. P < 0.05 was considered to indicate statistically significant differences.
Results
ROS content increased and SOD activity decreased in gefitinib-resistant lung adenocarcinoma cells
After 6 months of induction of gefitinib resistance, inverted optical microscopy (400 × , Nikon) showed that the drug-resistant PC-9-GR and HCC827-GR cells showed significantly different morphological structures (unclear boundaries, large nuclei and increased pseudopods) compared with the parental PC-9 and HCC827 cells (Fig. 1A). The CCK8 method was used to detect the response of each cell to gefitinib. The results showed that the gefitinib IC50 of the drug-resistant cells was significantly higher than that of the parental cells (Fig. 1B-C, P < 0.05). PC-9-GR and HCC827-GR drug-resistant cell lines induced by gefitinib were established successfully. The content of ROS and MDA and the activity of SOD in each cell were detected by commercial kits. The results showed that compared with the parental cells, the contents of ROS and MDA in the drug-resistant cells were significantly increased, while the activity of SOD was significantly decreased (Fig. 1D-I, P < 0.05). These results suggest that long-term gefitinib induction can enhance the oxidative stress level of lung adenocarcinoma cells and weaken the antioxidant capacity.
Fig. 1.
ROS content increased and SOD activity decreased in gefitinib-resistant lung adenocarcinoma cells. A: Gefitinib induced morphological changes in lung adenocarcinoma cells (400 × ). B/C: Dose-response curves for PC-9 (B) and HCC827 (C) cells after long-term treatment with gefitinib. D/G: A t-test was used to analyse the difference in ROS content between parental cells and drug-resistant cells. E/H: A t-test was used to analyse the difference in MDA content between parental cells and drug-resistant cells. F/I: A t-test was used to analyse the difference in SOD activity between parental cells and drug-resistant cells. (1) Compared with parental cells, P < 0.05.
Long-term gefitinib induction induces therapeutic resistance to lung adenocarcinoma through overexpression of HIF-1 and ALDH1
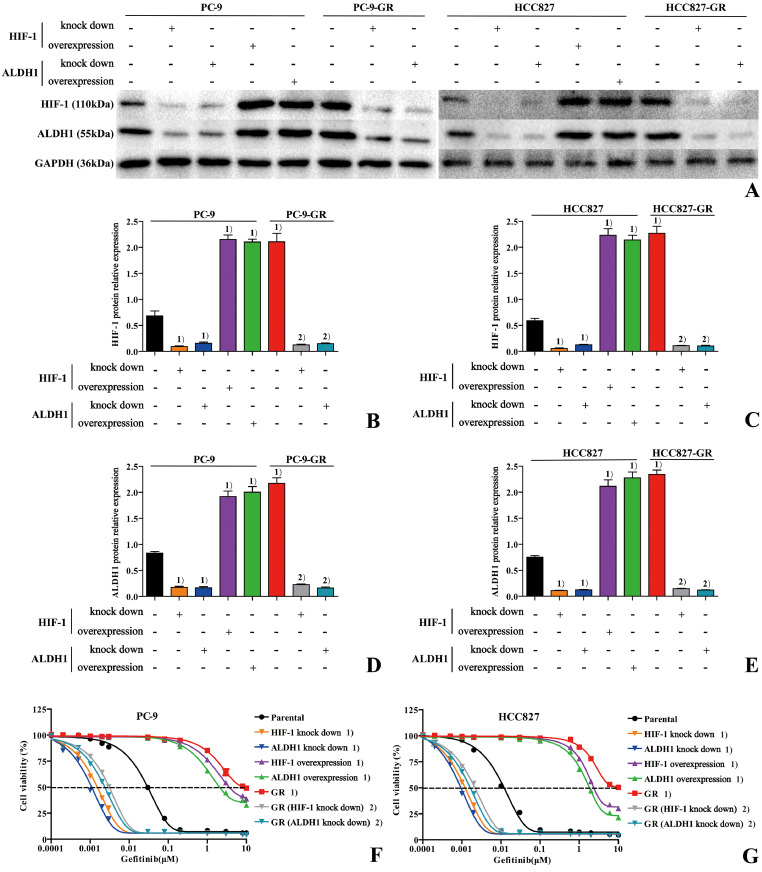
Western blotting was used to detect the protein expression of HIF-1 and ALDH1 in the parental and drug-resistant cells (Fig. 2A), and the results showed that the expression of HIF-1 and ALDH1 in the drug-resistant cells was significantly increased compared with that in the parental cells (Fig. 2B–E, P < 0.05). To detect the role of HIF-1 and ALDH1 in resistance to gefitinib treatment in lung adenocarcinoma, knockdown and overexpression of HIF-1 and ALDH1 were performed in parental cell lines, and knockdown was performed in drug-resistant cell lines with high expression of both. The results showed that knockdown of HIF-1 or ALDH1 in parental cells decreased the expression of both (Fig. 2B–E, P < 0.05) and increased the sensitivity to gefitinib (Fig. 2F and G, P < 0.05). However, the overexpression of both was completely opposite, consistent with drug-resistant cells. Similarly, knockdown of HIF-1 or ALDH1 in drug-resistant cells also resulted in decreased expression of both (Fig. 2B–E, P < 0.05) and increased sensitivity to gefitinib (Fig. 2F and G, P < 0.05). These results suggest that long-term gefitinib induction may induce therapeutic resistance to lung adenocarcinoma through overexpression of HIF-1 and ALDH1. However, knockdown of HIF-1 or ALDH1 can enhance the sensitivity of parental cells to gefitinib and enhance the efficacy of the gefitinib response in drug-resistant cells.
Fig. 2.
Long-term gefitinib induction induces therapeutic resistance to lung adenocarcinoma through overexpression of HIF-1 and ALDH1. A: The stable cell lines with knockdown and overexpression of HIF-1 and ALDH1 were constructed by lentiviral vectors. The protein expression of HIF-1 and ALDH1 in each group was detected by Western blotting. B/C: The differences in HIF-1 protein expression amongst groups were compared by one-way ANOVA. D/E: The differences in ALDH1 protein expression amongst groups were compared by one-way ANOVA. F/G: Dose-response curves for each group after treatment with gefitinib. (1) Compared with parental cells, P < 0.05. (2) Compared with drug-resistant cells, P < 0.05.
ALDH1+ CSCs were enriched in drug-resistant lung adenocarcinoma cells
The populations of ALDH1+ CSCs in each group of cells were determined by flow cytometry using the Aldefluor® Stem Cell Detection Kit (Fig. 3A,B). The results showed that the populations of ALDH1+ CSCs in the drug-resistant cells were significantly higher than those in the parental cells, while the populations of CSCs in both cell lines decreased when HIF-1 or ALDH1 was knocked down (Fig. 3C,D, P < 0.05). In addition, the overexpression of HIF-1 or ALDH1 in parental cells resulted in an increase in the populations of CSCs (Fig. 3C,D, P < 0.05), which was consistent with drug-resistant cells. These results suggest that the long-term induction of gefitinib can induce the enrichment of ALDH1+ CSCs by activating HIF-1 and ALDH1, while the knockdown of HIF-1 or ALDH1 can inhibit the enrichment of CSCs.
Fig. 3.
ALDH1+ CSCs were enriched in drug-resistant lung adenocarcinoma cells. A/B: The populations of ALDH1+ CSCs in each group were determined by flow cytometry. C/D: The differences in the populations of ALDH1+ CSCs amongst groups were compared by one-way ANOVA. (1) Compared with parental cells, P < 0.05. (2) Compared with drug-resistant cells, P < 0.05.
Effect of aerobic exercise on acquired drug resistance in lung adenocarcinoma cells
The effect of aerobic exercise on the acquired drug resistance of lung adenocarcinoma cells was tested by subcutaneous tumour-bearing tests in nude mice. In vivo imaging (Fig. 4A,B) and tumour weight (Fig. 4C) showed that compared with the control group, the gefitinib monotherapy group showed no significant difference in tumour formation ability, while the tumour formation ability of the aerobic exercise group and the HIF-1 or ALDH1 knockdown groups were significantly decreased (P < 0.05). It is suggested that drug-resistant lung adenocarcinoma cells were no longer sensitive to gefitinib, and aerobic exercise, HIF-1 or ALDH1 knockdown could effectively inhibit the malignant proliferation of drug-resistant lung adenocarcinoma cells. Compared with the gefitinib monotherapy group, the aerobic exercise + gefitinib group and the HIF-1 or ALDH1 knockdown + gefitinib groups were significantly decreased (P < 0.05). It is suggested that, in the case of gefitinib resistance, effective aerobic exercise or inhibition of HIF-1 or ALDH1 can enhance the response efficacy of gefitinib in drug-resistant cells. Compared with the aerobic exercise group and the HIF-1 or ALDH1 knockdown groups, the three groups treated with gefitinib showed significantly decreased tumour formation ability (P < 0.05). It is suggested that aerobic exercise, HIF-1 or ALDH1 knockdown combined with gefitinib therapy can more effectively inhibit the malignant proliferation of tumours.
Fig. 4.
Effect of aerobic exercise on acquired drug resistance in lung adenocarcinoma cells. A: Tumour size and fluorescence intensity were detected by in vivo imaging in each group. B: The differences in tumour size and fluorescence intensity amongst groups were compared by one-way ANOVA. C: The differences in tumour weight amongst groups were compared by one-way ANOVA. (1) Compared with the control group, P < 0.05. (2) Compared with the gefitinib monotherapy group, P < 0.05. (3) Compared with the aerobic exercise group, P < 0.05. (4) Compared with the HIF-1 knockdown group, P < 0.05. (5) compared with the ALDH1 knockdown group, P < 0.05.
Effects of aerobic exercise on ROS-related indicators, HIF-1, ALDH1 and ALDH1+ CSCs
ROS-related indicators (Fig. 5A–C), HIF-1 and ALDH1 proteins (Fig. 5D–F), and the populations of CSCs (Fig. 5G,H) were detected in nude mice of each group. The results showed that compared with the control group, the gefitinib monotherapy group showed no difference, while the ROS and MDA contents in the aerobic exercise group and the HIF-1 or ALDH1 knockdown groups had significantly decreased, and the SOD activity was increased (P < 0.05). Meanwhile, the expression of HIF-1 and ALDH1 and the populations of CSCs were decreased in the three groups (P < 0.05). Compared with the gefitinib monotherapy group, the ROS and MDA contents in the aerobic exercise + gefitinib group and the HIF-1 or ALDH1 knockdown + gefitinib groups were decreased (P < 0.05), and the SOD activity was increased (P < 0.05). In addition, the expression of HIF-1 and ALDH1 and the populations of CSCs were decreased in the three groups (P < 0.05). Compared with the aerobic exercise group and the HIF-1 or ALDH1 knockdown groups, the three groups combined with gefitinib showed no difference.
Fig. 5.
Effects of aerobic exercise on ROS-related indicators, HIF-1, ALDH1 and ALDH1+ CSCs. A: The differences in ROS content in tumours amongst groups were compared by one-way ANOVA. B: The differences in MDA content in serum amongst groups were compared by one-way ANOVA. C: The differences in SOD activity in serum amongst groups were compared by one-way ANOVA. D: The protein expression of HIF-1 and ALDH1 in tumours amongst groups was detected by Western blotting. E: The differences in HIF-1 protein expression in tumours amongst groups were compared by one-way ANOVA. F: The differences in ALDH1 protein expression in tumours amongst groups were compared by one-way ANOVA. G: The populations of ALDH1+ CSCs in tumours amongst groups were determined by flow cytometry. H: The differences in the populations of ALDH1+ CSCs in tumours amongst groups were compared by one-way ANOVA. (1) Compared with the control group, P < 0.05. (2) Compared with the gefitinib monotherapy group, P < 0.05.
Discussion
Lung adenocarcinoma patients with EGFR-activating mutations respond well to TKIs but are prone to developing drug resistance [5]. Studies have shown that the current treatment methods (radiotherapy and chemotherapy) mainly target differentiated cells rather than CSCs. However, these CSCs, which are present in small numbers, affect the occurrence, invasion, metastasis and treatment sensitivity of malignant tumours [8,9]. The detection and sorting of CSCs have become key goals of cancer treatment. ALDH1 is an NAD(P)+-dependant enzyme that oxidises aldehydes into carboxylic acids and has high oxidative activity, participating in gene expression, tissue differentiation and the maintenance of internal environment stability [22]. In 2007, Ginestier and his colleagues [23] found that ALDH1 is a marker of breast CSCs, marking the start of ALDH1 research in the field of CSCs. Since then, successive studies have confirmed that ALDH1 is a CSC marker in many cancers, including lung adenocarcinoma [[24], [25], [26]]. Its expression is closely related to the clinical prognosis of patients. To date, our team has used the fluorescent dye Aldefluor to detect and classify CSCs according to the different fluorescence intensities of ALDH1 in cells [18].
The potential for abnormal proliferation and differentiation of CSCs is closely related to their microenvironment. Hypoxia caused by the rapid growth of tumour cells and the relatively insufficient blood supply of tumour tissues is one of the characteristics of the tumour microenvironment. When tumour cells are in a hypoxic environment, the metabolic pathway of oxidative phosphorylation is blocked, and the transmission of the electron respiratory chain in the mitochondrial intima is abnormal, leading to an increase in ROS, which cannot be cleared in time, resulting in oxidative stress. As a lipid peroxidation product, MDA can reflect the severity of ROS attack on cells. As a key antioxidant enzyme, the activity of SOD reflects the ability of cells to eliminate oxygen free radicals [15]. To adapt to anoxic environments and meet the energy requirements of rapid growth, the metabolic process of tumour cells is reshaped. Excessive ROS production can inhibit the activity of proline hydroxylase and block its degradation of HIF-1, leading to the abnormal activation of HIF-1, thus regulating the related target genes and various cell signalling pathways to tolerate hypoxia and eventually resulting in the enhancement of CSCs and resistance to radiotherapy and chemotherapy [17]. In this study, it was found that compared with the parental cells, the ROS and MDA contents in the drug-resistant PC-9-GR and HCC827-GR cells were increased, and the SOD activity was decreased (P < 0.05). In addition, the expression of HIF-1 and ALDH1 and the populations of CSCs were decreased in the drug-resistant cells. It is suggested that long-term administration of gefitinib may enhance the oxidative stress level of lung adenocarcinoma cells, weaken the antioxidant capacity, activate HIF-1, cause the high expression of ALDH1 protein, and lead to the enrichment of ALDH1+ CSCs, which may be an important mechanism leading to the formation of acquired resistance to gefitinib in lung adenocarcinoma.
Aerobic exercise has been successfully used to treat different types of cancer. It has shown significant effects on reducing cancer incidence, controlling and improving uncomfortable symptoms, improving patients’ physiological functions, improving patients’ negative emotions and cultivating patients’ confidence in overcoming cancer [12–14]. Data have shown that aerobic exercise can reduce the production of reactive oxygen species (ROS) and the stress level of patients. It can also upregulate endogenous antioxidant enzymes such as glutathione peroxidase and mitochondrial superoxide dismutase, accelerate HIF-1 degradation, and improve the antioxidant capacity of the body. In addition, aerobic exercise can inhibit the production of HIF-responsive genes [16,27], regulate multiple signalling pathways, and inhibit CSCs, which is consistent with the results of this study.
The subcutaneous tumour-bearing nude mouse model used in this study showed that compared with the control group, the gefitinib monotherapy group showed no significant differences in tumour formation ability, ROS-related indicators, HIF-1, ALDH1 and CSCs. These results suggest that due to the enrichment of ROS, the level of oxidative stress was enhanced, the antioxidant capacity was weakened, HIF-1 was highly activated, and ALDH1 was overexpressed, leading to the enrichment of CSCs in PC-9-GR cells, which are no longer sensitive to gefitinib. However, the tumour formation ability and ROS and MDA contents of the aerobic exercise group and the HIF-1 or ALDH1 knockdown groups were significantly decreased, and SOD activity was increased. Meanwhile, the expression of HIF-1 and ALDH1 and the populations of CSCs were decreased in the three groups. The results suggest that the inhibition of oxidative stress by aerobic exercise could inhibit the expression of HIF-1 and ALDH1 proteins and inhibit the enrichment of CSCs, ultimately leading to the inhibition of tumour proliferation. Compared with the gefitinib monotherapy group, the tumour formation ability and ROS and MDA contents of the aerobic exercise + gefitinib group and the HIF-1 or ALDH1 knockdown + gefitinib groups were decreased, and SOD activity was increased. In addition, the expression of HIF-1 and ALDH1 and the populations of CSCs were decreased in the three groups. It is suggested that effective aerobic exercise in patients with gefitinib resistance can reduce the level of oxidative stress, enhance antioxidant capacity, cause the reduction of HIF-1 and ALDH1, and inhibit the enrichment of CSCs to re-sensitise tumour cells to gefitinib and ultimately inhibit malignant proliferation. Compared with the aerobic exercise group and the HIF-1 or ALDH1 knockdown groups, the three groups combined with gefitinib showed no significant differences in ROS-related indicators, HIF-1, ALDH1 and CSCs, but the tumour formation ability was significantly decreased. It is suggested that other tumour cells of non-CSCs also have certain tumour formation ability, and this group of cells is highly sensitive to gefitinib, indicating that inhibition of CSCs combined with gefitinib therapy could effectively inhibit the malignant proliferation of tumours.
In conclusion, the inhibitory effect of aerobic exercise on oxidative stress can effectively reduce the expression of HIF-1 and ALDH1 and inhibit the enrichment of CSCs, which can enhance the response of drug-resistant cells to gefitinib and can be used as an effective additional strategy in the treatment of lung adenocarcinoma. Due to the diversity and complexity of the disease, the regulatory mechanism of aerobic exercise in lung adenocarcinoma remains to be further explored, but reasonable and effective aerobic exercise is of great significance for the clinical treatment of patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kong J.Y., Xu F.X., He M. The incidence of lung cancer by histological type: a population based study in tianjin, China during 1981-2005. Respirology. 2014;19(8):1222–1228. doi: 10.1111/resp.12373. [DOI] [PubMed] [Google Scholar]
- 2.Kong J.Y., Xu F.X., Qu J.L. Genetic polymorphisms in the vitamin D pathway in relation to lung cancer risk and survival. Oncotarget. 2015;6(4):2573–2582. doi: 10.18632/oncotarget.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong J.Y., Chen X.J., Wang J. Genetic polymorphisms in the vitamin D pathway and non-small cell lung cancer survival. Pathol. Oncol. Res. 2020;26(3):1709–1715. doi: 10.1007/s12253-019-00702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y.W., Yuan X., Chen K.S. Clinical significance and prognostic value of Porphyromonas gingivalis infection in lung cancer. Transl. Oncol. 2021;14(1) doi: 10.1016/j.tranon.2020.100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu S.G., Shih J.Y. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol. Cancer. 2018;17(1):38–51. doi: 10.1186/s12943-018-0777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Codony-Servat J., Codony-Servat C., Cardona A.F. Cancer stem cell biomarkers in EGFR-mutation-positive non-small-cell lung cancer. Clin. Lung Cancer. 2019;20(3):167–177. doi: 10.1016/j.cllc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Testa U., Castelli G., Pelosi E. Lung cancers: molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers. 2018;10(8):248–328. doi: 10.3390/cancers10080248. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atashzar M.R., Baharlou R., Karami J. Cancer stem cells: a review from origin to therapeutic implications. J. Cell Physiol. 2020;235(2):790–803. doi: 10.1002/jcp.29044. [DOI] [PubMed] [Google Scholar]
- 9.Smith A.G., Macleod K.F. Autophagy, cancer stem cells and drug resistance. J. Pathol. 2019;247(5):708–718. doi: 10.1002/path.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voronkova M.A., Rojanasakul L.W., Kiratipaiboon C. The SOX9-aldehyde dehydrogenase axis determines resistance to chemotherapy in non-small-cell lung cancer. Mol. Cell Biol. 2020;40(2):e00307–e00319. doi: 10.1128/MCB.00307-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonagh L., Santiago R.M., Gray S.G. Exploitation of the vitamin A/retinoic acid axis depletes ALDH1-positive cancer stem cells and re-sensitises resistant non-small cell lung cancer cells to cisplatin. Transl. Oncol. 2021;14(4) doi: 10.1016/j.tranon.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadimitriou N., Dimou N., Tsilidis K.K. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat. Commun. 2020;11(1):597–606. doi: 10.1038/s41467-020-14389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos C., Sotomayor P., Jerez D. Exercise and prostate cancer: from basic science to clinical applications. Prostate. 2018;78(9):639–645. doi: 10.1002/pros.23502. [DOI] [PubMed] [Google Scholar]
- 14.Dieli-Conwright C.M., Courneya K.S., Demark-Wahnefried W. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. 2018;20(1):124–134. doi: 10.1186/s13058-018-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simioni C., Zauli G., Martelli A.M. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9(24):17181–17198. doi: 10.18632/oncotarget.24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiggins J.M., Opoku-Acheampong A.B., Baumfalk D.R. Exercise and the tumor microenvironment: potential therapeutic implications. Exerc. Sport Sci. Rev. 2018;46(1):56–64. doi: 10.1249/JES.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 17.Tong W.W., Tong G.H., Liu Y. Cancer stem cells and hypoxia-inducible factors (Review) Int. J. Oncol. 2018;53(2):469–476. doi: 10.3892/ijo.2018.4417. [DOI] [PubMed] [Google Scholar]
- 18.Sun J.T., Li G.F., Liu Y.W. Targeting histone deacetylase SIRT1 selectively eradicates EGFR TKI-resistantcancer stem cells via regulation of mitochondrial oxidative phosphorylation in lung adenocarcinoma. Neoplasia. 2020;22(1):33–46. doi: 10.1016/j.neo.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao S., Liu Y., Duan X. Porphyromonas gingivalis infection exacerbates oesophageal cancer and promotes resistance to neoadjuvant chemotherapy. Br. J. Cancer. 2021 doi: 10.1038/s41416-021-01419-5. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi Y.J., Jiao Y.L., Chen P. Porphyromonas gingivalis promotes progression of esophageal squamous cell cancer via TGFβ-dependent Smad/YAP/TAZ signaling. PLoS Biol. 2020;18(9) doi: 10.1371/journal.pbio.3000825. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Denou E., Marcinko K., Surette M.G. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2016;310(11):E982–E993. doi: 10.1152/ajpendo.00537.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Ma H., Zhang J. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci. Rep. 2018;8(1):4276–4290. doi: 10.1038/s41598-018-22220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginestier C., Hur M.H., Charafe-Jauffret E. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed S.Y., Kaf R.M., Ahmed M.M. The prognostic value of cancer stem cell markers (Notch1, ALDH1, and CD44) in primary colorectal carcinoma. J. Gastrointest. Cancer. 2019;50(4):824–837. doi: 10.1007/s12029-018-0156-6. [DOI] [PubMed] [Google Scholar]
- 25.Vieira V., Campos L.H., Jesus L.H. Overexpression of ALDH1 and EMT marker profile are linked with unfavorable outcome in head and neck cancer. Med. Oral Patol. Oral Cir. Bucal. 2020;25(6):e752–e761. doi: 10.4317/medoral.23777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J., Alharbi A., Shan H. TAZ induces lung cancer stem cell properties and tumorigenesis by up-regulating ALDH1A1. Oncotarget. 2017;8(24):38426–38443. doi: 10.18632/oncotarget.16430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hojman P., Gehl J., Christensen J.F. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27(1):10–21. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]