Abstract
In 2015, the Nobel Committee for Physiology or Medicine, in its only award for treatments of infectious diseases since six decades prior, honoured the discovery of ivermectin (IVM), a multifaceted drug deployed against some of the world’s most devastating tropical diseases. Since March 2020, when IVM was first used against a new global scourge, COVID-19, more than 20 randomized clinical trials (RCTs) have tracked such inpatient and outpatient treatments. Six of seven meta-analyses of IVM treatment RCTs reporting in 2021 found notable reductions in COVID-19 fatalities, with a mean 31% relative risk of mortality vs. controls. During mass IVM treatments in Peru, excess deaths fell by a mean of 74% over 30 days in its ten states with the most extensive treatments. Reductions in deaths correlated with the extent of IVM distributions in all 25 states with p < 0.002. Sharp reductions in morbidity using IVM were also observed in two animal models, of SARS-CoV-2 and a related betacoronavirus. The indicated biological mechanism of IVM, competitive binding with SARS-CoV-2 spike protein, is likely non-epitope specific, possibly yielding full efficacy against emerging viral mutant strains.
Keywords: COVID-19, H. pylori, ivermectin, SARS-CoV-2, spike protein
Introduction
The 2015 Nobel prize for the discovery of ivermectin (IVM) and an antimalarial treatment was the Nobel committee’s first award for treatment agents for infectious diseases since the one in 1952 for streptomycin [1]. A macrocyclic lactone of multifaceted potency [2,3], IVM as deployed worldwide since 1987 has made major inroads against two devastating tropical diseases, onchocerciasis and lymphatic filariasis [4]. During the year since IVM treatment was first applied to COVID-19, another global scourge [5], results from more than 20 randomized clinical trials (RCTs) of IVM treatment of COVID-19 have been reported [2,6,7], with inpatient and outpatient treatments of COVID-19 conducted in 25 countries [2]. A likely biological mechanism has been indicated to be competitive binding with SARS-CoV-2 spike protein sites, as reviewed [8,9].
Recently, Dr Satoshi Omura, the Nobel co-laureate for the discovery of IVM, and colleagues conducted a comprehensive review of IVM clinical activity against COVID-19, concluding that the preponderance of the evidence demonstrated major reductions in mortality and morbidity [2]. Our review of that evidence, updated with consideration of several new studies, supports the same conclusion.
Animal studies for IVM treatment of SARS-CoV-2 and a closely related betacoronavirus
A framework for the examination of clinical IVM treatment results for COVID-19 is provided by related animal studies using IVM at low human-equivalent doses. In golden hamsters that were intranasally inoculated with SARS-CoV-2, causing symptomatic COVID-19 infections, concurrent dosing with IVM significantly reduced the severity of clinical signs (p < 0.001). While viral load was not reduced, these improvements included one-third of the incidence of anosmia and sharp reductions in the Il-6/Il-10 ratio in lung tissue [10]. In another animal model, mice were infected with mouse hepatitis virus MHV-A59 [11], a betacoronavirus strain that does not express hemagglutinin esterase [12], like SARS-CoV-2, SARS-CoV, and MERS [8]. Whereas infected mice had severe histopathological liver damage, IVM-treated mice had half the hepatic viral load and minimal liver damage, not significantly different than that observed in uninfected controls.
RCTs for IVM treatment and prevention of COVID-19
More than 20 RCTs for IVM treatment of COVID-19 have been conducted to date, as cited above. A search of Google Scholar for meta-analyses of IVM treatment studies of COVID-19 that appeared in 2021 [13] yielded seven such studies that drew conclusions from RCTs only [6,[14], [15], [16], [17], [18], [19]]. The relative risk (RR) of mortality with IVM treatment vs. controls as calculated in four of these meta-analyses using Cochrane analysis methodology ranged from 0.25 to 0.37, with a mean of 0.31 [6,14,15,19]. The three other meta-analyses reported odds ratios of 0.16, 0.21 and 0.33, with a mean of 0.23 [[16], [17], [18]]. Six of these seven meta-analyses concluded that there was a significant [6,[14], [15], [16]] or possible [17,18] indication of the efficacy of IVM in reducing COVID-19 mortality. One found no evidence of IVM efficacy in its first version [20], reporting an RR of 1.11 for IVM treatment vs. controls, and stuck with that finding even after changing this RR value to 0.37 and correcting switched treatment and control deaths it had misreported for one study [21] in a revised version [19]. Among the most recent and comprehensive of these seven meta-analyses reported a pooled total of 31 deaths among 1101 subjects in IVM treatment groups and 91 deaths among 1064 controls from 11 RCTs, amounting to a 67% reduction in mortality, with a statistical significance for an overall effect of p = 0.005 [16]. The RCT that used the largest dose of IVM, 400 μg/kg on each of days 1-4 [22], had 2 vs. 24 deaths in the treatment vs. control groups (n = 200 each).
An objection that had been raised earlier in 2021 to the preponderance of clinical evidence for the efficacy of IVM treatment of COVID-19 as summarized above was that none of these RCTs had been published in mainstream peer-reviewed scientific journals [23]. Closing that gap, however, was the publication in 2021 in journals from major scientific publishers of five such RCTs for COVID-19 treatment [[24], [25], [26], [27], [28]], each showing multiple clinical benefits for IVM vs. controls, most of these to statistical significance at p < 0.002. Also published in 2021 were three other RCTs for IVM treatment of COVID-19: one that reported briefer hospital stays for IVM treatment short of statistical significance (p = 0.08) [29], another that compared IVM with two other drug treatment groups but not a placebo group and found no benefit [30], and an additional study conducted in Cali, Columbia with mix-ups between treatment and placebo doses as described below.
Another objection that has been raised to the RCT evidence supporting IVM efficacy was that study populations were too small [31]. Yet, it is well known in clinical trial design that highly effective drugs will establish statistically significant results with smaller sample sizes, with larger study populations required for minimally effective drugs [32,33]. But for a drug with a more modest RR of 75%, for example, the treatment and control arms would need more than 3800 subjects each to yield the same statistical significance [33]. Although large study populations are useful to screen for adverse effects (AEs) of new drugs, IVM has been used safely in 3.7 billion doses worldwide since 1987 [2,3] and is well tolerated even at much greater doses than the standard single dose of 200 μg/kg [34,35]. It has been used in RCTs for COVID-19 treatment at cumulative doses of 1500 μg/kg [36], 1600 μg/kg [22] and 3000 μg/kg [37] over 4 or 5 days with only small percentages of mild or transient adverse effects.
Among these RCTs that established safety for high-dose IVM treatment of COVID-19 was one conducted in Cali, Columbia, with generally mild COVID-19 cases, median age 37, having only one death in the control group [36]. The study found no statistically significant symptom improvements with IVM treatment yet reported a striking anomaly: AEs distinctive for its high IVM dose, described in the study protocol as ‘security parameters’ for its IVM use, occurred at almost identical rates in its IVM and placebo arms. These included transient incidences of blurred vision (11.3%, 11.6%) and dizziness (35.6%, 34.3%). These indications of IVM use in controls occurred as over-the-counter sales of IVM surged in the study region during the study period (Supplementary Table 1). Further questions as to the study’s treatment/control boundaries were raised by the mistaken substitution of IVM for placebo for 38 patients, discovered by the lead pharmacist a month after the fact (study, p. 3; study protocol supplement, p. 43). In addition, blinding was breached by the use of the dextrose-saline solution as the placebo for 64 control patients (IVM tastes distinctively bitter), while the composition of the replacement placebo solution was not specified [38].
Supporting the findings of IVM efficacy in COVID-19 treatment as summarized above were indications of activity against SARS-CoV-2 in prevention studies. Three RCTs evaluated the prophylactic effect of IVM administered to cohorts of 100 [22], 117 [39] and 203 [40] subjects exposed to COVID-19 patients. These studies, all using IVM in doses of at least 150 μg/kg per week, reported statistically significant reductions in COVID-19 incidences, with respective RRs of 20%, 26% and 13% as compared with controls, and greater reductions in incidences of moderate and severe cases. Another RCT for COVID-19 prevention administered just one dose of IVM at 12 mg (about 150 μg/kg) to 617 subjects on day one of a 42-day observation period, while three other preventative regimens were each administered daily over that period [41]. IVM at that single low dose yielded the best results of these four regimens, with highly statistically significant reductions of close to 50% in both symptomatic COVID-19 and acute respiratory symptoms vs. controls.
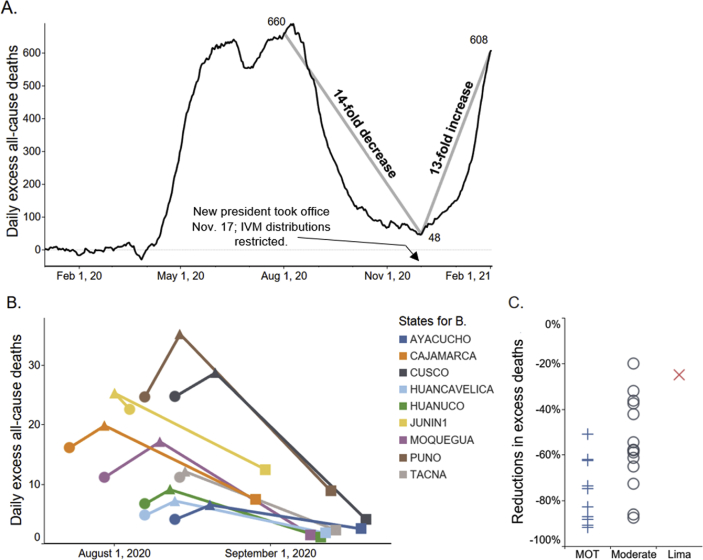
14-fold reductions in excess deaths with IVM use in Peru, then 13-fold increase after IVM restricted
The clinical experience of IVM treatments of COVID-19 in 25 countries extends far beyond the RCT results summarized, yet incomplete tracking and lack of control data exclude most of this for evaluation. The record of nationally authorized such treatments in Peru provides a notable exception [42]. In ten states of Peru, mass IVM treatments of COVID-19 were conducted through a broadside, army-led effort, Mega-Operación Tayta (MOT), that began on different dates in each state. In these MOT states, excess deaths dropped sharply over 30 days from peak deaths by a mean of 74%, in close time conjunction with MOT start date (Fig. 1B). In 14 states of Peru having locally administered IVM distributions, the mean reduction in excess deaths over 30 days from peak deaths was 53%, while in Lima, which had minimal IVM distributions during the first wave of the pandemic due to restrictive government policies there, the corresponding 30-day decrease in excess deaths was 25%.
Fig. 1.
A) Excess all-cause deaths (all ages), the national population of Peru. These decreased 14-fold from 1st August through 1st December 2020; then, after IVM use was restricted, increased 13-fold through 1st February. For A and B, y values are 7-day moving averages; for B and C, ages ≥60. Data are from Peru’s National Death Information System (SINADEF). (B) Drops in excess deaths for all states of operation MOT, an army-led program of mass IVM distributions, but Pasco, which had them on three dates. • MOT start date; ▴ peak deaths; ▪ day of peak deaths +30 days. Junin distributed IVM through local channels 13 days before MOT start. (C) Reductions in excess deaths at +30 days after peak deaths for the 25 states by extent of IVM distributions: maximal-MOT ( ), mean -74%; moderate-local distributions (
), mean -74%; moderate-local distributions ( ), mean -53%; and minimal-Lima (
), mean -53%; and minimal-Lima ( ), -25%. The absolute value of these reductions by state correlated with extent of IVM distributions with Kendall τb = 0.524, p < 0.002 (Spearman rho = 0.619, p < 0.001). All these data are from publicly accessible Peruvian national databases, with associated frozen datasets available from the Dryad data repository [42].
), -25%. The absolute value of these reductions by state correlated with extent of IVM distributions with Kendall τb = 0.524, p < 0.002 (Spearman rho = 0.619, p < 0.001). All these data are from publicly accessible Peruvian national databases, with associated frozen datasets available from the Dryad data repository [42].
Reductions in excess deaths by state (absolute values) correlated with the extent of IVM distribution (maximal-MOT states, moderate-local distributions, and minimal-Lima) with Kendall τb = 0.524, p < 0.002, as shown in Fig. 1C. Nationwide, excess deaths decreased 14-fold over four months through 1st December 2020. After a restrictive IVM treatment policy was enacted under a new Peruvian president who took office on 17th November, however, deaths increased 13-fold over the two months following 1st December through 1st February 2021 (Fig. 1A). Potential confounding factors, including lockdowns and herd immunity, were ruled out using Google community mobility data, seropositivity rates, population densities and geographic distributions of SARS-CoV-2 genetic variations and by restricting all analysis except that for Fig. 1A to ages ≥ 60. Excess deaths were used in all analyses rather than COVID-19 case fatalities as gross underreporting of pandemic deaths by case fatalities was known to the Peruvian Ministry of Health since July 2020 [43]. This disparity has been consistently manifested in the national health database figures for COVID-19 case fatalities vs. all natural-cause deaths since that date [42].
IVM-based combination treatments and other research in progress
Combination treatments using IVM and adjuncts have shown indications of efficacy against COVID-19 in RCTs conducted to date [24,44]. Results using IVM, doxycycline and zinc to treat serious and critical cases having spO2 ≤ 90 prior to treatment, with spO2 changes tracked 24 hours after treatment, will be reported by TJB with Sabine Hazan, MD. Pronounced improvements of serious COVID-19 symptoms within 1–2 days after IVM administration have been observed in several patients treated by the lead author (ADS), and studies to objectively track such short-term clinical benefits of IVM for COVID-19 are underway. Information on other combination treatments using IVM with agents such as fluvoxamine, for which clinical studies also indicate significant benefits [45], is provided by the USA-based FLCCC alliance (https://covid19criticalcare.com).
The curative potential of combination therapy was demonstrated in a medical breakthrough of three decades prior for another disease, peptic ulcers, for which the discovery of its underlying bacterial cause, Helicobacter pylori, was honoured with the Nobel Prize for Medicine in 2005. In 1990, Dr Thomas J. Borody published the original clinical trial of a combination treatment for H. pylori, achieving a 96% cure rate for a triple therapy consisting of three repurposed drugs, bismuth subcitrate and two antibiotics [46]. Between 1990 and 2015, an estimated 18,665 deaths were prevented by the timely application of this triple therapy for peptic ulcer disease in Australia [47]. After the expiration of the patents for two palliative drugs for this condition, Tagamet and Zantac [48], which had each earned billions of dollars, triple therapy became the standard of care for peptic ulcers in the rest of the world by the late 1990s.
Conclusion
We believe that the evidence to date supports the worldwide extension of IVM treatments for COVID-19, complementary to immunizations. The indicated biological mechanism of IVM, competitive binding with SARS-CoV-2 spike protein, is likely non-epitope specific, as reviewed [8], possibly yielding full efficacy against emerging viral mutant strains. IVM has been safely used in 3.7 billion doses since 1987, well tolerated even at much greater than standard doses [34,35] and used without serious AEs in the three high-dose COVID-19 treatment studies noted above [34,36,37]. In the current international emergency of COVID-19, with mutant viral strains, vaccination refusals and potentially waning immunities over months presenting new challenges, IVM can be an effective component of the mix of therapeutics deployed against this pandemic.
Funding
No funding was received for this perspective.
Ethical approval and consent to participate
This is a review and ethical approval was not required.
Transparency declaration
TJB is a principal in Topelia Therapeutics (Ventura, California), which seeks to commercialize cost-effective treatments for COVID-19, including IVM. All other authors report no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2021.100924.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Ergonul O., Yalcin C.E., Erkent M.A., Demirci M., Uysal S.P., Ay N.Z. Who can get the next Nobel Prize in infectious diseases? Int J Infect Dis. 2016;45:88–91. doi: 10.1016/j.ijid.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Yagisawa M., Foster P.J., Hanaki H., Omura S. Global trends in clinical studies of ivermectin in COVID-19. Japanes J Antib. 2021;74(1) [Google Scholar]
- 3.Campbell W.C. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr Pharm Biotechnol. 2012;13(6):853–865. doi: 10.2174/138920112800399095. [DOI] [PubMed] [Google Scholar]
- 4.Crump A., Ōmura S. Ivermectin, 'wonder drug' from Japan: the human use perspective. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(2):13–28. doi: 10.2183/pjab.87.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajter J.C., Sherman M.S., Fatteh N., Vogel F., Sacks J., Rajter J.-J. Use of ivermectin is associated with lower mortality in hospitalized patients with COVID-19 (ICON study) CHEST. 2020 doi: 10.1016/j.chest.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill A., Abdulamir A., Ahmed S., Asghar A., Babalola O.E., Basri R. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. Res Square. 2021 doi: 10.21203/rs.3.rs-148845/v1. [DOI] [Google Scholar]
- 7.Kory P., Gu Meduri, Varon J., Iglesias J., Marik P.E. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. Am J Therap. 2021;28(3):e299–e318. doi: 10.1097/MJT.0000000000001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheim D.E. 2020. From cold to killer: how SARS-CoV-2 evolved without hemagglutinin esterase to agglutinate, then clot blood cells in pulmonary and systemic microvasculature SSRN.http://ssrn.com/abstract=3706347 Available from: [Google Scholar]
- 9.Zaidi A.K., Dehgani-Mobaraki P. The mechanisms of action of Ivermectin against SARS-CoV-2: an evidence-based clinical review article. J Antib. 2021 doi: 10.1038/s41429-021-00430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melo G.D., Lazarini F., Larrous F., Feige L., Kergoat L., Marchio A. Anti-COVID-19 efficacy of ivermectin in the golden hamster. bioRxiv. 2020 doi: 10.1101/2020.11.21.392639. [DOI] [Google Scholar]
- 11.Arévalo A.P., Pagotto R., Pórfido J.L., Daghero H., Segovia M., Yamasaki K. Ivermectin reduces in vivo coronavirus infection in a mouse experimental model. Scient Reports. 2021;11(1):7132. doi: 10.1038/s41598-021-86679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazi L., Lissenberg A., Watson R., de Groot R.J., Weiss S.R. Expression of hemagglutinin esterase protein from recombinant mouse hepatitis virus enhances neurovirulence. J Virol. 2005;79(24):15064–15073. doi: 10.1128/JVI.79.24.15064-15073.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Search for papers having "ivermectin," "meta," and "COVID" OR "SARS" in the title, appearing in 2021. https://scholar.google.com/scholar?as_q=ivermectin+meta+%28COVID+OR+SARS%29&as_epq=&as_oq=&as_eq=&as_occt=title&as_sauthors=&as_publication=&as_ylo=2021&as_yhi=2021&hl=en&as_sdt=0%2C47 Available from:
- 14.Bryant A., Lawrie T.A., Dowswell T., Fordham E.J., Mitchell S., Hill S.R. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Amer J Therap. 2021 doi: 10.1097/MJT.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariyanto T.I., Halim D.A., Rosalind J., Gunawan C., Kurniawan A. Ivermectin and outcomes from Covid-19 pneumonia: a systematic review and meta-analysis of randomized clinical trial studies. Rev Med Virol. 2021 doi: 10.1002/rmv.2265:e2265. [DOI] [Google Scholar]
- 16.Karale S., Bansal V., Makadia J., Tayyeb M., Khan H., Ghanta S.S. A meta-analysis of mortality, need for ICU admission, use of mechanical ventilation and adverse effects with ivermectin use in COVID-19 patients. medRxiv. 2021 doi: 10.1101/2021.04.30.21256415. [DOI] [Google Scholar]
- 17.Kow C.S., Merchant H.A., Mustafa Z.U., Hasan S.S. The association between the use of ivermectin and mortality in patients with COVID-19: a meta-analysis. Pharmacol Reports. 2021 doi: 10.1007/s43440-021-00245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez-Gutiérrez R., Raygoza-Cortez K., Garcia-Leal M., Sáenz-Flores M., Solis R.C., Flores-Rodríguez A. 2021. Ivermectin in the prophylaxis and treatment of patients with SARS-CoV-2: a living systematic review and meta-analysis SSRN.http://ssrn.com/abstract=3802499 Available from: [Google Scholar]
- 19.Roman Y.M., Burela P.A., Pasupuleti V., Piscoya A., Vidal J.E., Hernandez A.V. Ivermectin for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. medRxiv. 2021 doi: 10.1101/2021.05.21.21257595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roman Y.M., Burela P.A., Pasupuleti V., Piscoya A., Vidal J.E., Hernandez A.V. Ivermectin for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials (version 1) medRxiv. 2021 doi: 10.1101/2021.05.21.21257595v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niaee M.S., Gheibi H., Namdar P., Allami A. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients; A randomized multi-center clinical trial. Res Square. 2020 doi: 10.21203/rs.3.rs-109670/v1. [DOI] [Google Scholar]
- 22.Elgazzar A., Hany B., Abo Youssef S., Hany B. Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic. Res Square. 2020 doi: 10.21203/rs.3.rs-100956/v1. [DOI] [Google Scholar]
- 23.Sax P.E. Ivermectin for aaCOVID-19 — breakthrough treatment or hydroxychloroquine redux? NEJM J Watch. January 4, 2021 https://blogs.jwatch.org/hiv-id-observations/index.php/ivermectin-for-covid-19-breakthrough-treatment-or-hydroxychloroquine-redux/2021/01/04/] Available from: [Google Scholar]
- 24.Mahmud R., Rahman M.M., Alam I., Ahmed K.G.U., Kabir A.K.M.H., Sayeed S.K.J.B. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021;49(5) doi: 10.1177/03000605211013550. 03000605211013550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okumuş N., Demirtürk N., Çetinkaya R.A., Güner R., Avcı İ.Y., Orhan S. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21(1):411. doi: 10.1186/s12879-021-06104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samaha A.A., Mouawia H., Fawaz M., Hassan H., Salami A., Bazzal A.A. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in Lebanon. Viruses. 2021;13(6):989. doi: 10.3390/v13060989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Shahbaznejad L., Davoudi A., Eslami G., Markowitz J.S., Navaeifar M.R., Hosseinzadeh F. Effect of ivermectin on COVID-19: a multicenter double-blind randomized controlled clinical trial. Clin Therap. 2021 doi: 10.1016/j.clinthera.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaccour C., Casellas A., Blanco-Di Matteo A., Pineda I., Fernandez-Montero A., Ruiz-Castillo P. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClin Med. 2021 doi: 10.1016/j.eclinm.2020.100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abd-Elsalam S., Noor R.A., Badawi R., Khalaf M., Esmail E.S., Soliman S. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study. J Med Virol. 2021 doi: 10.1002/jmv.27122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galan L.E.B., Santos N.M.D., Asato M.S., Araújo J.V., de Lima Moreira A., Araújo A.M.M. Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection. Pathog Glob Heal. 2021;115(4):235–242. doi: 10.1080/20477724.2021.1890887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.COVID-19 scientific advisory group rapid evidence report: ivermectin in the treatment and prevention of COVID-19: alberta health services. February 2, 2021. https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-sag-ivermectin-in-treatment-and-prevention-rapid-review.pdf Available from: [Google Scholar]
- 32.Sakpal T.V. Sample size estimation in clinical trial. Persp Clin Res. 2010;1(2):67–69. [PMC free article] [PubMed] [Google Scholar]
- 33.Kohn M., Senyak J. April 29, 2021. Sample size calculators: UCSF CTSI.https://www.sample-size.net/] Available from: [Google Scholar]
- 34.Navarro M., Camprubí D., Requena-Méndez A., Buonfrate D., Giorli G., Kamgno J. Safety of high-dose ivermectin: a systematic review and meta-analysis. J Antimicrob Chemother. 2020;75(4):827–834. doi: 10.1093/jac/dkz524. [DOI] [PubMed] [Google Scholar]
- 35.Guzzo C.A., Furtek C.I., Porras A.G., Chen C., Tipping R., Clineschmidt C.M. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42(10):1122–1133. doi: 10.1177/009127002401382731. [DOI] [PubMed] [Google Scholar]
- 36.López-Medina E., López P., Hurtado I.C., Dávalos D.M., Ramirez O., Martínez E. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021 doi: 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krolewiecki A., Lifschitz A., Moragas M., Travacio M., Valentini R., Alonso D.F. 2020. Antiviral effect of high-dose ivermectin in adults with COVID-19: a pilot randomised, controlled, open label, multicentre trial: SSRN.http://ssrn.com/abstract=3714649 Available from: [Google Scholar]
- 38.Scheim D.E., Hibberd J.A., Chamie J.J. 2021. Protocol violations in López-Medina et al.: 38 switched ivermectin (IVM) and placebo doses, failure of blinding, ubiquitous IVM use OTC in Cali, and nearly identical AEs for the IVM and control groups: OSF Preprints. Available from: [DOI] [Google Scholar]
- 39.Chahla R.E., Medina Ruiz L., Ortega E.S., Morales M.F., Barreiro F., George A. A randomized trial - intensive treatment based in ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID-19 in healthcare agents. medRxiv. 2021 doi: 10.1101/2021.03.26.21254398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shouman W., Hegazy A., Nafae R., Ragab M., Samra S., Ibrahim D. Use of ivermectin as a prophylactic option in asymptomatic family close contacts with patients of COVID-19 (NCT number: 04422561) J Clin Diagnos Res. 2021;15(2):OC27. [Google Scholar]
- 41.Seet R.C.S., Quek A.M.L., Ooi D.S.Q., Sengupta S., Lakshminarasappa S.R., Koo C.Y. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial. Int J Infect Dis. 2021;106:314–322. doi: 10.1016/j.ijid.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chamie J.J., Hibberd J.A., Scheim D.E. 2021. Ivermectin for COVID-19 in Peru: 14-fold reduction in nationwide excess deaths, p<0.002 for effect by state, then 13-fold increase after ivermectin use restricted: OSF Preprints. Available from: Access date June 10, 2021. Associated frozen data from the Peruvian SINADEF database used in this analysis is available from the Dryad data repository at https://doi.org/10.5061/dryad.dv41ns1xr. [DOI] [Google Scholar]
- 43.Covid-19: segundo informe para actualizar cifra de fallecidos se conocerá esta semana. Andina. Agencia Peruana de Noticias; 2020. 2020/07/26. [Google Scholar]
- 44.Hashim H.A., Maulood M.F., Rasheed A.M., Fatak D.F., Kabah K.K., Abdulamir A.S. Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv. 2020 doi: 10.1101/2020.10.26.20219345. [DOI] [Google Scholar]
- 45.Sukhatme V.P., Reiersen A.M., Vayttaden S.J., Sukhatme V.V. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharmacol. 2021;12(763) doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.George L.L., Borody T.J., Andrews P., Devine M., Moore-Jones D., Walton M. Cure of duodenal ulcer after eradication of Helicobacter pylori. Med J Aust. 1990;153(3):145–149. doi: 10.5694/j.1326-5377.1990.tb136833.x. [DOI] [PubMed] [Google Scholar]
- 47.Eslick G.D., Tilden D., Arora N., Torres M., Clancy R.L. Clinical and economic impact of "triple therapy" for Helicobacter pylori eradication on peptic ulcer disease in Australia. Helicobacter. 2020;25(6) doi: 10.1111/hel.12751. [DOI] [PubMed] [Google Scholar]
- 48.Berndt E.R., Kyle M., Ling D. In: Scanner data and price indexes. Feenstra R.C., Shapiro M.D., editors. Univeristy of Chicago Press; Chicago: 2003. The long shadow of patent expiration: generic entry and rx-to-OTC switches; pp. 229–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.