Abstract
Objective
Proopiomelanocortin (POMC) neurons of the hypothalamic arcuate nucleus are essential regulators of energy balance. Selective loss of POMC production in these cells results in extreme obesity and metabolic comorbidities. Neurogenesis occurs in the adult hypothalamus, but it remains uncertain whether functional POMC neurons emerge in physiologically significant numbers during adulthood. Here, we tested whether Rax-expressing precursors generate POMC neurons in adult mice and rescue the metabolic phenotype caused by congenital hypothalamic POMC deficiency.
Methods
Initially, we identified hypothalamic Rax-expressing cell types using wild-type and Rax-CreERT2:Ai34D mice. Then we generated compound Rax-CreERT2:ArcPomcloxTB/loxTB mice in which endogenous hypothalamic Pomc expression is silenced, but can be restored by tamoxifen administration selectively in neurons derived from Rax+ progenitors. The number of POMC neurons generated by Rax+ progenitors in adult mice and their axonal projections was determined. The metabolic effects of these neurons were assessed by measuring food intake, bodyweight, and body composition, along with glucose and insulin levels.
Results
We found that Rax is expressed by tanycytes and a previously unrecognized cell type in the hypothalamic parenchyma of adult mice. Rax+ progenitors generated ~10% of the normal adult hypothalamic POMC neuron population within two weeks of tamoxifen treatment. The same rate and steady state of POMC neurogenesis persisted from young adult to aged mice. These new POMC neurons established terminal projections to brain regions that were involved in energy homeostasis. Mice with Rax+ progenitor-derived POMC neurons had reduced body fat mass, improved glucose tolerance, increased insulin sensitivity, and decreased bodyweight in proportion to the number of new POMC neurons.
Conclusions
These data demonstrate that Rax+ progenitors generate POMC neurons in sufficient numbers during adulthood to mitigate the metabolic abnormalities of hypothalamic POMC-deficient mice. The findings suggest that adult hypothalamic neurogenesis is a robust phenomenon in mice that can significantly impact energy homeostasis.
Keywords: POMC, Rax, Adult neurogenesis, Tanycyte, Stem cell, Arcuate nucleus
Highlights
-
•
Rax+ precursors generate 10% of the hypothalamic POMC neurons during adulthood.
-
•
The same rate of POMC neurogenesis persists from young adult to aged mice.
-
•
Adult-born POMC neurons develop normal efferent projections.
-
•
Adult-born POMC neurons improve metabolism in hypothalamic POMC-deficient mice.
Abbreviations
- α-MSH
α-melanocyte-stimulating hormone
- Arc
arcuate nucleus
- ANOVA
analysis of variance
- AUC
area under the curve
- BNST
bed nucleus of the stria terminalis
- BrdU
bromodeoxyuridine
- DMH
dorsomedial hypothalamic nucleus
- FISH
fluorescence in situ hybridization
- GFAP
glial fibrillary acidic protein
- GTT
glucose tolerance test
- IF
immunofluorescence
- loxTB
loxP-flanked transcription blocker sequence
- MCT8
monocarboxylate transporter 8
- NTS
nucleus of the solitary tract
- POA
preoptic area
- POMC
proopiomelanocortin
- PVH
paraventricular hypothalamic nucleus
- TAM
tamoxifen
- tdTom
tdTomato
- VMH
ventromedial hypothalamic nucleus
- WT
wild-type
1. Introduction
Obesity and type 2 diabetes have emerged as a global health crisis in recent decades. Bodyweight and energy balance are regulated by multiple interacting neural circuits in the brain [1], an essential component of which is the melanocortin system in both rodents and humans [2]. Proopiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus (Arc) inhibit food intake and stimulate energy expenditure by releasing α-melanocyte-stimulating hormone (α-MSH), which further stimulates downstream satiety neurons expressing melanocortin receptors [3]. Mice with a selective deletion of Pomc expression in Arc neurons develop a severely obese phenotype characterized by hyperphagia, hyperinsulinemia, and reduced energy expenditure [4,5]. Using a reversible genetic mouse model of Arc Pomc deficiency, we previously demonstrated that the restoration of eutopic Pomc expression in Arc neurons of weanling mice resulted in the complete rescue of the obese phenotype [5]. Reactivation of Arc Pomc expression in adult mice with increased levels of overweight – while not completely normalizing bodyweight or leptin sensitivity – markedly reduced the level of obesity and associated comorbidities [5]. However, chronic food restriction to normalize bodyweight before the reactivation of Arc Pomc expression restored leptin sensitivity and resulted in the indefinite maintenance of normal bodyweight and body composition [6].
Considering the efficacy of Pomc expression rescue, and recent evidence of adult hypothalamic neurogenesis in mammals [7], promoting Pomc neurogenesis postnatally could lead to a new treatment for obesity and associated metabolic comorbidities in humans. However, it remains uncertain whether functional Pomc neurons emerge normally during adulthood and reach a critical mass that can favorably impact energy homeostasis. Studies that applied brain delivery of the cell proliferation marker, bromodeoxyuridine (BrdU), detected only a small number of Arc Pomc neurons that derived from new cell divisions in adult mice [8,9]. Somewhat more, albeit still a low number of adult-born Pomc neurons, were identified by fate mapping of Sox2-expressing neural progenitors of the mediobasal hypothalamic parenchyma in adult mice [8]. However, an indirect approach based on tracking the survival rate of prenatally generated POMC neurons into adulthood suggested that a stunning 50% of Arc POMC neurons might be replaced by ongoing neurogenesis between 4 and 12 weeks of age in mice [10].
Besides parenchymal progenitors, another population of adult hypothalamic stem/progenitor cells is tanycytes – radial glia-like ependymal cells lining the third ventricle – that can differentiate into glia and neurons [[11], [12], [13], [14], [15]]. While tanycytes were recently shown to give rise to some POMC neurons in the early postnatal period (postnatal day 8) [16], the capacity of tanycytes to generate functionally relevant POMC neurons during adulthood is unknown. The present study was designed to systematically test whether tanycytes can differentiate into melanocortin-secreting POMC neurons that integrate into the normal anatomical projection pathways, and rescue the obesity phenotype caused by the loss of Pomc expression in Arc Pomc-deficient mice. We generated an inducible compound genetic mouse model by crossing ArcPomcloxTB/loxTB mice with reversible Pomc silencing in the Arc [5] with Rax-CreERT2 knock-in mice [17] expressing tamoxifen (TAM)-inducible CreERT2 under the endogenous Rax promoter/enhancers, the most selective marker known for tanycytes [[17], [18], [19], [20]]. TAM treatment of these mice induces Cre recombinase activity that matches the endogenous Rax expression pattern and relieves the transcriptional silencing of Pomc in Rax-positive cells—thus allowing Pomc transcriptional activity only in neurons derived from Rax+ progenitors. Using this model, we aimed to dissect the role of Rax+ progenitors in the generation of hypothalamic POMC neurons in young adult to aged mice, and to decipher the capacity of these new neurons to restore the metabolic abnormalities observed in Arc Pomc-deficient mice.
2. Materials and methods
2.1. Animal care
All animal procedures were approved by the Institutional Animal Care and Use Committees at the University of Michigan and Tufts Medical Center, and followed the National Research Council's “Guide for the Care and Use of Laboratory Animals”. Mice were group-housed (3–5 per cage) on cellulose bedding in ventilated cages under controlled temperature (25 ± 4 °C) and photoperiod (12-h light–dark cycle, lights on from 6:00 am to 6:00 pm), with filtered tap water and standard laboratory chow (5LOD; LabDiet, St. Louis, MO) that contained 28.5 kcal% protein, 13.5 kcal% fat, and 58.0 kcal% carbohydrate available ad libitum. All breeders were fed ad libitum with breeder chow (5008; LabDiet) that contained 26.53 kcal% protein, 16.97 kcal% fat, and 56.5 kcal% carbohydrate. For fasting-refeeding and metabolic experiments, mice were individually housed and either fed ad libitum or preweighed amount of food, or were fasted overnight by removing the chow diet from the cages depending on the purpose of the experiments and according to the approved experimental protocol.
2.2. Generation and breeding of mice
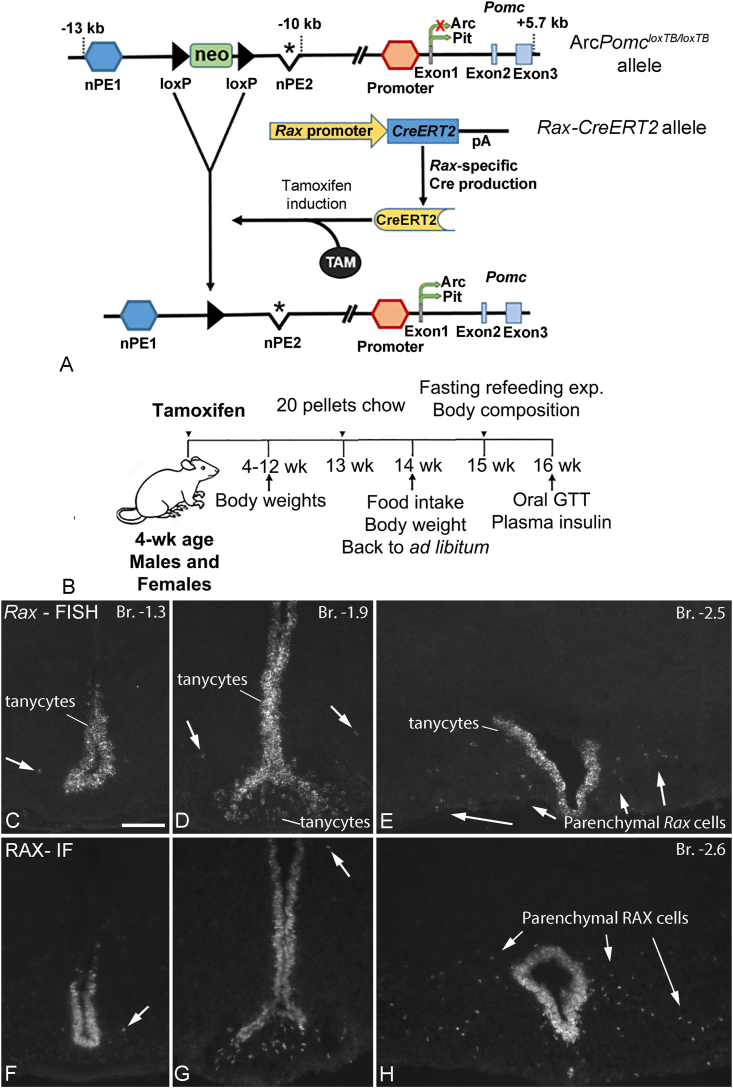
ArcPomcloxTB/loxTB mice were generated on the C57BL/6J background as described with the alternate name ArcPomc−/− [5,21]. ArcPomcloxTB/loxTB mice carry a loxP-flanked transcription blocker sequence (loxTB; a pGK-neo cassette), inserted between the first upstream neuronal enhancer (nPE1) and the deleted second neuronal enhancer (nPE2) of the Pomc gene (Figure 1A). The presence of loxTB in the neuronal enhancer region selectively blocks Pomc gene expression in neurons of the hypothalamic arcuate nucleus, while expression in the pituitary and nucleus of the solitary tract (NTS) remains intact [21] (Figure 1A). ArcPomcloxTB/loxTB mice are obese and hyperphagic due to the lack of Pomc gene expression in the Arc, which can be rescued by TAM-induced CreERT2-mediated removal of loxTB [5].
Figure 1.
Generation of Rax-CreERT2/+:ArcPomcloxTB/loxTBmice, experimental design, and cellular distribution of Rax mRNA/RAX protein in the mouse hypothalamus. (A) ArcPomcloxTB/loxTB mice carry a floxed transcription blocker (neo cassette) in the neuronal enhancer region of the Pomc gene that prevents Pomc transcription in Arc neurons, but not the pituitary. When Rax-specific CreERT2 knock-in mice are crossed to ArcPomcloxTB/loxTB mice, the transcription blocker is excised in Rax+ cells in response to TAM induction, allowing Pomc gene expression in Arc neurons derived from Rax+ precursors. (B) Timeline and experimental design of the metabolic study. Male and female mice were injected with TAM at age 4–5 weeks, followed by food intake and bodyweight measurements for an additional 16 weeks. Body composition measurements were taken at age 15 weeks and an oral glucose tolerance test (GTT) with insulin measurements were performed at age 16 weeks. (C–E) FISH shows the distribution of Rax mRNA-expressing cells at different rostrocaudal levels (Bregma coordinates are indicated). Rax is expressed in tanycytes lining the 3rd ventricle, in the median eminence, and in parenchymal cells (arrows) that constitute a small but significant cell population in the caudal Arc. (F–H) Immunofluorescence (IF) for RAX (transcription factor protein encoded by Rax), labels cell nuclei in the same distribution pattern as Rax mRNA. Scale bar: 100 μm (C, for C–H).
ArcPomcloxTB/loxTB mice were crossed with Rax-CreERT2 knock-in mice (Raxtm1.1(cre/ERT2)Sbls/J; The Jackson Laboratory) to obtain compound Rax-CreERT2/+:ArcPomcloxTB/loxTB mice (Figure 1A). Rax-CreERT2/+ transgenic mice were generated on the C57BL/6J background and express TAM-inducible Cre recombinase activity under the direction of the Rax promoter and enhancers [17].
The inducible triple compound Rax-CreERT2/+:ArcPomcloxTB/loxTB:Ai34(RCL-Syp/tdT)-D genetic model was generated by crossing Rax-CreERT2/+:ArcPomcloxTB/loxTB mice with Ai34(RCL-Syp/tdT)-D mice (B6; 129S-Gt(ROSA)26Sortm34.1(CAG-Syp/tdTomato)Hze/J; The Jackson Laboratory). Ai34D mice carry a knock-in mutation at the Gt(ROSA)26Sor locus with a loxP-flanked STOP cassette that prevents the transcription of a CAG (cytomegalovirus early enhancer element; the promoter, the first exon and the first intron of the chicken beta-Actin gene; and the splice acceptor of the rabbit beta-Globin) promoter-driven synaptophysin-tdTomato fusion gene. Cre-mediated removal of the STOP cassette results in synaptophysin-tdTomato expression in neuronal soma, projections, and synaptic terminals in brain regions.
2.3. Genotyping
Mouse genotyping was performed by extracting the DNA from tail tips; it was collected carefully with fresh razor blades to avoid cross-contamination between the samples. Tails were incubated at 55 °C overnight in a lysis solution that contained proteinase K (0.1 μg/μl). DNA extraction was performed as described previously [22].
After the compound Rax-CreERT2/+:ArcPomcloxTB/loxTB:Ai34(RCL-Syp/tdT)-D transgenic line was established, the presence of the Rax-CreERT2 allele was determined by polymerase chain reaction (PCR) using primers amplifying the Cre recombinase: forward 5′-GGACATGTTCAGGGATCGCCAGGCG-3′ and reverse 5′-GCATAACCAGTGAAACAGCATTGCTG-3′, which produced a 268 bp PCR product. Genotyping for ArcPomcloxTB/loxTB was performed using primers: forward 5′-GGGTGGGCTACTGTGCTAATA-3′ and reverse 5′-AAGCCAAGAGCACACTGAAGGAGA -3′, which produced a 178 bp band for the loxTB knock-in allele and 346 bp band for the wild-type (WT) allele. Ai34(RCL-Syp/tdT)-D mice were genotyped by using the forward primer 5′-GGAGTGTGCCAACAAGACGGAGA-3′ and reverse primer 5′-CCAGCCTGTCTCCTTGAACACGA-3′, which produced a 523 bp band for the transgenic allele and 297 bp band for the WT allele. The PCR protocol used was as follows: 94 °C for 4 min, followed by 35 cycles of 94 °C for 1 min, 67 °C for 1 min, 72 °C for 1 min, 72 °C for 5 min, and 4 °C on hold.
2.4. General experimental design
Experiment 1
Rax mRNA and RAX protein expression study in WT mice. Juvenile male and female (n = 4 each) C57BL/6NTac mice were purchased from Taconic Farms (Germantown, NY) and euthanized on postnatal day 33.
Experiment 2
Metabolic study in early adult mice. Ten cohorts of mice each containing multiple genotypes of mice were generated by using the above breeding strategies. After the genotypes were known, males (WT, n = 9; Rax-CreERT2/+, n = 6; ArcPomcloxTB/loxTB, n = 6; Rax-CreERT2/+:ArcPomcloxTB/loxTB, n = 8) and females (WT, n = 3; Rax-CreERT2/+, n = 6; ArcPomcloxTB/loxTB, n = 8; Rax-CreERT2/+:ArcPomcloxTB/loxTB, n = 6) at age 4–5 weeks were injected intraperitoneally with TAM (100 mg/kg dose; T5648, Sigma–Aldrich, dissolved in sesame oil, S3547, Sigma–Aldrich) for five consecutive days [23]. Some of these mice also carried the Ai34D allele; refer Results section 3.7. Following TAM treatment, mice were monitored for serial bodyweight, food intake, whole body composition (fat mass, lean mass, and fluid mass), oral glucose tolerance test (GTT), and insulin measurements for 16 additional weeks (Figure 1B) [24].
Experiment 3
Age cohort study. Eight cohorts of mice, from 15 to 50 weeks old, were injected with TAM as described and monitored weekly for bodyweight for 12–16 weeks, and then, euthanized. In total, this experiment included 4 male and 6 female Rax-CreERT2/+; 5 male and 3 female ArcPomcloxTB/loxTB; and 14 male and 12 female Rax-CreERT2/+:ArcPomcloxTB/loxTB mice. The exact ages of the mice are described in Results section 3.5 and Figure 7.
Experiment 4
Short-term survival study. Mice were injected with TAM and euthanized 7 days or 16 days after the first of five daily TAM injections. For the histologic analysis, the 7-day group included 3 male and 5 female Rax-CreERT2/+:ArcPomcloxTB/loxTB mice (some carrying the Ai34D allele, refer Results section 3.7), 1 male and 1 female ArcPomcloxTB/loxTB mice, and 1 male and 2 female Rax-CreERT2/+:Ai34D mice. The 16-day group included 3 male and 1 female Rax-CreERT2/+:ArcPomcloxTB/loxTB mice and 3 male ArcPomcloxTB/loxTB mice. The age of the mice is described in Figure 8 and Supplementary Fig. 5.
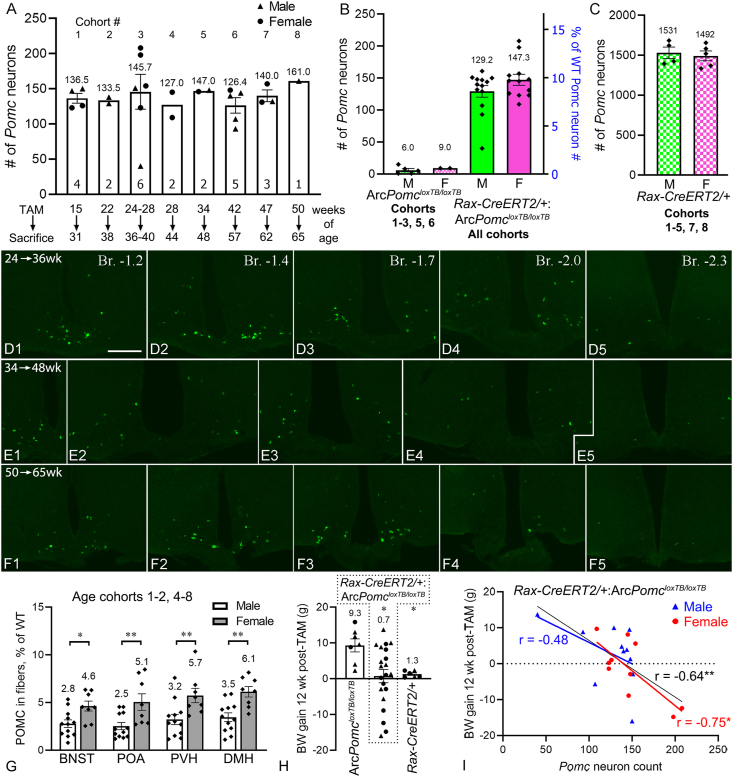
Figure 7.
Generation of hypothalamic Pomc neurons by Rax+progenitors in mature adult and middle aged mice. (A)Pomc neuron counts in 8 different age cohorts of Rax-CreERT2/+:ArcPomcloxTB/loxTB mice that were perfused at either 12 week (cohort 3) or 14–16 weeks (all other cohorts) after TAM treatment. Sample sizes are shown inside the columns. (B)Pomc neuron counts in male (n = 13) and female (n = 12) Rax-CreERT2/+:ArcPomcloxTB/loxTB mice from all cohorts combined (male vs female not significantly different, p = 04777, Mann–Whitney test); and male (n = 5) and female (n = 2) ArcPomcloxTB/loxTB mice (negative controls) from 5 cohorts (n = 1–2 each). (C)Pomc neuron counts in male (n = 4) and female (n = 5) Rax-CreERT2/+mice (positive controls) from 7 cohorts (n = 1–2 each); their combined mean was used as 100% for scaling the right Y axis in B. (D1-F5) Distribution of Pomc neurons (FISH) in Rax-CreERT2/+:ArcPomcloxTB/loxTB brains with the highest Pomc neuron counts from cohort 3 (D1-5), cohort 5 (E1-5), and cohort 8 (F1-5). Five rostrocaudal levels are shown for each cohort. Images show all Pomc neurons in the sections. Note that several Pomc neurons are in very lateral positions in E1-5; the most lateral is ~600 μm away from the ventricle in E4. (G) Semiquantitative analysis of POMC fiber staining in male (n = 12) and female (n = 8) Rax-CreERT2/+:ArcPomcloxTB/loxTB mice from all age cohorts except cohort 3. Values (area% covered by POMC fibers) are expressed as % of WT. ∗p < 0.05, ∗∗p < 0.01; BNST: p = 0.0108, DMH: p = 0.0016, POA: p = 0.0077, unpaired t-test; PVH: p = 0.0077, Mann–Whitney test. (H) Bodyweight gains at 12 weeks after TAM injection in cohorts 1–6; ∗significantly different from ArcPomcloxTB/loxTB. W(2.00,13.18) = 8.885, p = 0.0036, Welch's ANOVA; Rax-CreERT2/+:ArcPomcloxTB/loxTB (n = 21) vs ArcPomcloxTB/loxTB (n = 7): p = 0.0114; Rax-CreERT2/+(n = 6) vs ArcPomcloxTB/loxTB: p = 0.0139, Dunnett's T3 multiple comparisons. The mean values for each genotype are shown above their respective columns. (I) Bodyweight gain in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice (cohorts 1–6) showed significant inverse correlation with the number of observed Pomc neurons in females (red line; r = −0.75, p = 0.0123, two-tailed, n = 10) but not in males (blue line; r = −0.48, p = 0.1352, two-tailed, n = 11). The correlation was also significant when the sexes were combined (black line; r = −0.64; p = 0.0019, two-tailed, n = 21). Scale bar: 200 μm (D1, for D1-F5). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
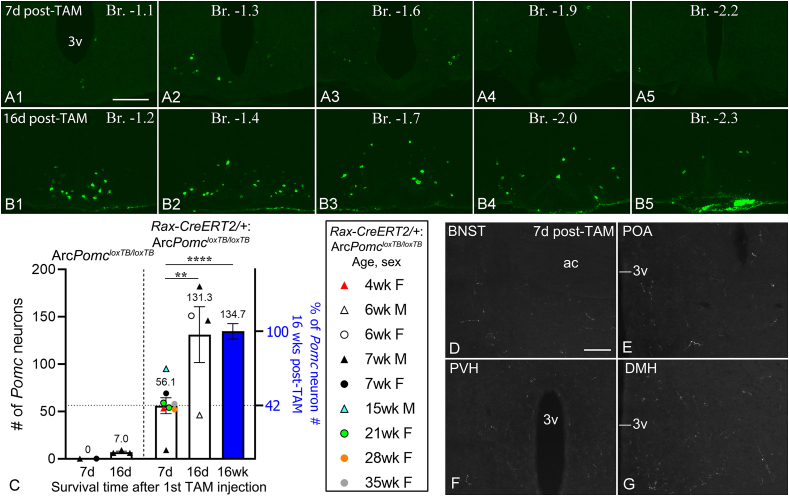
Figure 8.
Rapid generation of Pomc neurons from Rax+progenitors. (A1-B5) FISH detection of hypothalamic Pomc neurons in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice that were euthanized 7 days (A1-5) or 16 days (B1-5) after the first TAM injection. The two brains shown here are that of the 35 weeks and 6 weeks old females (refer graph in C). Brains from the 7 day-group and 16 week-group were PFA-perfused, while brains from the 16 day-group were collected fresh-frozen. (C)Pomc neuron counts in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice from the 7 day-group (n = 8) and 16 day-group (n = 4). Sex and age (when TAM treatment began) are shown for each mouse in the boxed panel. The blue column represents the Pomc neuron count 16 weeks after TAM treatment, combined from both sexes from Figure 3E. F(2,23) = 14.57, p < 0.0001, one-way ANOVA; ∗∗∗∗p < 0.0001, ∗∗p = 0.004, Tukey's multiple comparisons. ArcPomcloxTB/loxTB mice (negative control) were 7-week-old males (triangles) and 13 weeks old females (circle). (D–G) POMC fibers (IF) in a Rax-CreERT2/+:ArcPomcloxTB/loxTB mouse (7 weeks female) perfused 7 days after TAM treatment began. BNST, bed nucleus of the stria terminalis; POA, preoptic area; PVH, hypothalamic paraventricular nucleus; DMH, hypothalamic dorsomedial nucleus; 3v, third ventricle; ac, anterior commissure. Scale bars: 200 μm (A1, for A1-B5), 100 μm (D, for D-G). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.5. Bodyweight and food intake measurements
After TAM treatment, both male and female mice were subjected to bodyweight measurements that started at the age of 4–5 weeks. At 12 weeks' age, mice were acclimated to single housing with standard laboratory chow and filtered tap water ad libitum for one week. After one-week acclimation, at 13 weeks' age, mice were weighed to ensure the recovery of their bodyweights after changes in the housing conditions. Mice were then transferred to clean cages and provided with preweighed food (20 chow pellets = ~80–100 g per mouse). After one week, at 14 weeks' age, the mice were weighed and the remaining food was measured to determine average daily food consumption. Mice were then given ad libitum food for the remaining week. At 15 weeks' age, the body composition measurements were obtained by nuclear magnetic resonance (NMR), followed by a fasting-refeeding experiment that started at 4 pm on the same day. In this experiment, mice were given preweighed food (6 chow pellets = ~20–25 g per mouse) and food intake was measured daily. After 48 h, mice were weighed and fasted for 18 h. After an 18 h fast, they were again weighed and provided with 3 chow pellets of food. The food intake was measured at 1, 2, 4, and 24 h after the food was given. Mice were weighed after refeeding and fed ad libitum over the weekend. At 16 weeks’ age, an oral GTT was performed after a 5 h fast. After two days, mice were euthanized by transcardial perfusion as described below.
2.6. Body composition measurements
Body composition measurements were performed by the University of Michigan Animal Phenotyping Core. Body fat, lean mass, and free fluids were measured in live mice using an NMR-based analyzer (Minispec LF90II, Bruker Optics). The noninvasive individual measurements took less than 2 min during which conscious mice were placed into the measuring tube with minimal restraint. Before use, the machine performance was checked daily using a reference sample (canola seeds), as recommended by the manufacturer.
2.7. Oral GTT and insulin measurements
Oral GTT with insulin measurements were performed by the University of Michigan Animal Phenotyping Core. Mice were fasted for 5 h (8 am–1 pm) before the oral administration of 2 g/kg bodyweight glucose (50% glucose in 1X PBS) using 18-gauge oral gavage needles (FNS-18-2; Kent Scientific Corporation). Blood samples were collected through tail vein blood before and after the glucose gavage at 0, 15, 30, 60, and 120 min and were assayed for blood glucose using a glucometer (Acucheck, Roche) and plasma insulin levels using an ELISA kit (#90080, Crystal Chem Ultra-Sensitive Mouse Insulin ELISA Kit, IL, USA). Animals were restrained repeatedly for less than a minute to collect blood samples. The total area under the curve (AUC) for glucose and insulin was calculated using the trapezoidal rule [25]. The glucose-insulin index, an indicator of insulin resistance, was calculated as the product of the AUC for glucose and insulin [26]. HOMA-IR index, as a homeostatic model assessment of β-cell function and insulin resistance, was calculated as the product of fasting glucose and insulin [27].
2.8. Tissue collection
Mice from Experiment 1 and the 16-day group in Experiment 4 were deeply anesthetized with ketamine/xylazine or isoflurane (Piramal Enterprises Ltd.), respectively, and decapitated. The brains were removed from the skulls and either rapidly immersed in dry ice-chilled isopentane or snap-frozen in powdered dry ice, respectively. Mice from Experiments 2, 3, and 4 (7-day group) were deeply anesthetized with isoflurane and transcardially perfused with 50 ml 0.1 M RNase-free PBS (pH 7.4), followed by 50 ml 4% paraformaldehyde (PFA) in PBS. Brains were dissected out, postfixed overnight in 4% PFA, and then cryoprotected in 30% sucrose (Fisher Scientific) in RNase-free PBS at 4 °C, till the brain tissues sank. All dissections were carried out at mid-day.
Coronal sections were cut through the Arc (fresh-frozen brains) or the entire forebrain (perfused brains) on a CM3050 S cryostat (Leica Microsystems, Nussloch GmbH, Germany). Sections from fresh-frozen brains were thaw-mounted on Superfrost Plus glass slides (Fisher Scientific Co., Pittsburgh, PA) and stored at −80 °C. Sections from perfused brains were collected free-floating in cryoprotectant (30% ethylene-glycol; 25% glycerol; 0.05 M PB), and stored at −20 °C. One-in-seven series of 16-μm thick sections were collected from the brains in Experiment 1 and one-in-five series of 20-μm thick sections were collected in Experiments 2–4.
2.9. Fluorescence in situ hybridization (FISH)
Pomc FISH. Free-floating sections containing the Arc (15–16 sections per brain) were transferred from cryoprotectant to PBS, mounted on Superfrost Plus slides, and desiccated overnight at 42 °C. The sections were hybridized with a digoxigenin-labeled antisense riboprobe corresponding to the full-length mouse Pomc mRNA (NCBI GenBank accession numbers NM_001278584.1), as described previously for PFA-perfused brains [28]. After stringent washes, 0.5% Triton X-100/0.5% H2O2 treatment and a 10 min incubation in 1% blocking reagent for nucleic acid hybridization (Roche Applied Sciences), the sections were incubated overnight in peroxidase-conjugated antidigoxigenin Fab fragments (Millipore Sigma, Burlington, MA; diluted 1:100 in 1% blocking reagent). Signal amplification was performed for 30 min using the TSA Biotin Tyramide system (PerkinElmer, Waltham, MA), and detected with Alexa Fluor 488-conjugated streptavidin (diluted 1:500 in 1% blocking reagent, Thermo Fisher). Fresh-frozen sections from the 16-day group in Experiment 4 were hybridized as already described for fresh-frozen brains [28], using reduced (15 min) tyramide amplification time.
Rax FISH. Sections were hybridized as per protocol for fresh-frozen brains [28], using a digoxigenin-labeled 1030 base long antisense probe corresponding to the entire coding region of mouse Rax mRNA (276–1304 bases of NM_013833.2). The template DNA was synthesized and cloned into pBluescript KS(−) plasmid by GenScript (Piscataway, NJ). Signal amplification was applied for 30 min, using the TSA Plus Biotin kit (PerkinElmer) with the TSA Plus biotin reagent diluted 1:400 in 0.05 M Tris and 0.01% H2O2. The biotin deposits were detected with Alexa Fluor 488-conjugated streptavidin.
2.10. Post-FISH immunofluorescence
Following Pomc FISH, brains with the Synaptophysin-tdTomato (Ai34D) allele were processed for immunofluorescence, with overnight incubation in the cocktail of a rabbit red fluorescent protein (RFP) antibody (1:400 dilution; Rockland Cat# 600-401-379, RRID:AB_2209751) that recognizes the tdTomato protein, and either a mouse monoclonal HuC/D antibody (2 μg/ml concentration; ThermoFisher Cat# A21271, RRID:AB_221448) or a Guinea pig NeuN antibody (1:2K dilution; Millipore Cat# ABN90, RRID:AB_11205592), to confirm neuronal identity. The primary antibodies were detected with Cy3-conjugated anti-rabbit and Alexa Fluor 647-conjugated anti-mouse or anti-Guinea pig IgGs (1:200 dilution each; Jackson Immunoresearch, West Grove, PA). Sections were coverslipped with SlowFade™ Diamond Antifade Mountant with DAPI (Thermo Fisher).
2.11. Immunofluorescence (IF)
Single IF for RAX. Fresh-frozen sections on slides (Experiment 1) were fixed with 4% PFA in 0.1 M PB (pH 7.4) for 15 min, then permeabilized with 0.5% Triton-X-100 for 20 min, and blocked with antibody diluent (2% normal horse serum, 0.2% Kodak Photo-Flo, 0.2% sodium-azide in PBS) for 20 min. Sections were incubated overnight in Guinea pig anti-RAX antibody (1:5 K dilution; RIKEN Institute for Developmental Biology Cat# MS8407-3, RRID:AB_2783560; gift from Dr. Hidetaka Suga, Nagoya University Graduate School of Medicine, Japan), followed by incubation in Cy3-conjugated anti-Guinea pig IgG (1:200) for 2 h. In preliminary experiments, this antibody yielded the same immunostaining pattern as a commercial Guinea pig RAX antibody (Cat# M229, Takara Bio Inc., Shiga, Japan; RRID: AB_2783559), but with slightly higher sensitivity.
Dual IF for RAX/MCT8 and RAX/HuC. Free-floating sections of Rax-CreERT2/+:Ai34D mice from Experiment 4 (7-day group; 1 male, 2 female) were treated with 0.5% Triton-X-100 for 30 min, rinsed in PBS (3 × 10 min), and blocked with antibody diluent for 20 min. The sections were incubated for 2 days in a cocktail of the Guinea pig RAX antibody (1:1K) and either a rabbit monocarboxylate transporter 8 (MCT8) antiserum (1:2K dilution; Visser TJ lab Erasmus University Medical Center, The Netherlands, Cat# 1306; RRID:AB_2661880; a gift from Dr. Theo J. Visser) or a biotinylated anti-HuC/D antibody (5 μg/ml concentration; Thermo Fisher Scientific, Cat# A-21272, RRID:AB_2535822). The unconjugated primary antibodies were detected with Alexa Fluor 647 anti-Guinea pig and Alexa Fluor 488 anti-rabbit (1:200 each) for 2 h. The biotinylated HuC/D antibody was detected with avidin-biotin-peroxidase complex (1:500 dilution for 2 h; Elite ABC-HRP Kit, Vector, Burlingame, CA), followed by 15 min biotin-tyramide amplification and fluorescein (DTAF) streptavidin (1:300 dilution, Jackson Immunoresearch).
Dual IF for POMC/α-MSH. Sections from Experiments 2 and 4 were treated with 0.5% Triton X-100 and 0.5% H2O2 for 15 min, blocked with antibody diluent for 20 min, and incubated in the cocktail of rabbit anti-POMC (1:15K dilution; Phoenix Pharmaceuticals, Cat# H-029-30, RRID:AB_2307442) and sheep anti-α-MSH (1:20K dilution; Millipore, Cat# AB5087, RRID:AB_91683; gift of Jeffrey B. Tatro, Tufts Medical Center, Boston MA) antisera for 22 h at room temperature. The primary antibodies were detected with Alexa Fluor 647 anti-rabbit and Alexa 488 anti-sheep IgGs (1:200 each) for 2 h. In Experiment 3, to eliminate high lipofuscin autofluorescence, sections were incubated in 0.3% Sudan Black B (Sigma–Aldrich) in 70% ethanol for 10 min, followed by a vigorous wash in 70% ethanol for 5 min, before the antibody diluent step. Sections from Experiment 3 were incubated for 24 h in the cocktail of 1:10K POMC and 1:20K anti-α-MSH antisera, and subsequently, in Cy3-conjugated anti-rabbit and Alexa 488 anti-sheep IgGs (1:200 each) for 2 h. Sections were mounted and coverslipped with SlowFade™ Diamond Mountant with DAPI.
2.12. Image acquisition and analysis
All images were captured with a Zeiss Axioplan 2 epifluorescent microscope (Carl Zeiss, Göttingen, Germany) equipped with an RT SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI). Cell counts (RAX IF, Pomc FISH, POMC IF, including dual-labeling with tdTomato), were carried out manually on every 5th coronal 20 μm thick section covering the entire rostrocaudal length of tanycyte and POMC neuron distribution (retrochiasmatic area and Arc, 15–16 sections per brain; the same number also for fresh-frozen collected sections in the 16-day group in Experiment 4). Because of partial tissue loss of the Arc, one male Rax-CreERT2/+:ArcPomcloxTB/loxTB brain in Experiment 3 was excluded from the Pomc cell count, but was included in the POMC IF fiber analysis. Semiquantitative analysis of POMC fibers was performed using ImageJ software (public domain at http://rsb.info.nih.gov/ij). Images were acquired from each region bilaterally in two consecutive sections (in a one-in-five section series) using a 10× objective. POMC-labeled fibers were separated from the background using the same threshold value for all images and the %Area covered by POMC fibers was measured. Nonspecific signals such as artifacts or labeling of blood vessels in some brains were removed manually. %Area values were expressed as a percentage of the %Area measured in WT and Rax-CreERT2/+brains in Experiments 2 (n = 2 males, 2 females) and 3 (n = 2 males, 3 females).
2.13. Statistics
GraphPad Prism 8 software was used to calculate statistics and plot graphs. All data are presented as mean ± SEM. Datasets were tested for normality by using the Shapiro–Wilk (when n < 8) or D'Agostino–Pearson normality tests. Comparisons between Pomc cell counts or POMC fiber densities were made using a two-tailed unpaired t-test or one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test, or Mann–Whitney test for non-Gaussian data distributions. In the metabolic study, the differences in bodyweights and other parameters were tested for significance using one-way ANOVA followed by Tukey's multiple comparisons test. In the age cohort experiment, bodyweight gains were compared using Welch's ANOVA followed by Dunnett's T3 multiple comparisons test. To correlate bodyweight gains with Pomc cell counts, the Pearson correlation (r) was calculated with a two-tailed p-value. Differences were considered significant for p < 0.05. The number of mice (n) per group, statistical tests used, and p values are described in the Results or figure legends.
3. Results
3.1. Distribution of Rax/RAX+ cells in the mediobasal hypothalamus
Rax has been regarded as a tanycyte-specific marker in the postnatal and adult hypothalamus [[17], [18], [19], [20]]. To identify all potential Rax+ progenitors in the hypothalamus, we examined the distribution of Rax+ cells using FISH for Rax mRNA, and IF for the transcription factor it encodes, retinal homeobox protein Rx (RAX) that is localized in cell nuclei. In agreement with previous studies [18,29], the vast majority of Rax-expressing cells corresponded to the distribution of tanycytes in the third ventricular wall and the median eminence (Figure 1C–E). A minor but conspicuous population of Rax+ cells was also observed in the parenchyma of the caudal Arc (caudal of Bregma −2.30 mm; Figure 1E), but only a few scattered Rax+ cells were found in the parenchyma alongside the rostral and mid portions of the tanycyte region (Figure 1C,D). RAX immunostaining labeled cell nuclei in the same distribution pattern as Rax mRNA, including parenchymal cells (Figure 1F–H).
3.2. RAX is expressed in tanycytes, tanycyte-like cells, and a distinct glial cell type in the hypothalamic parenchyma
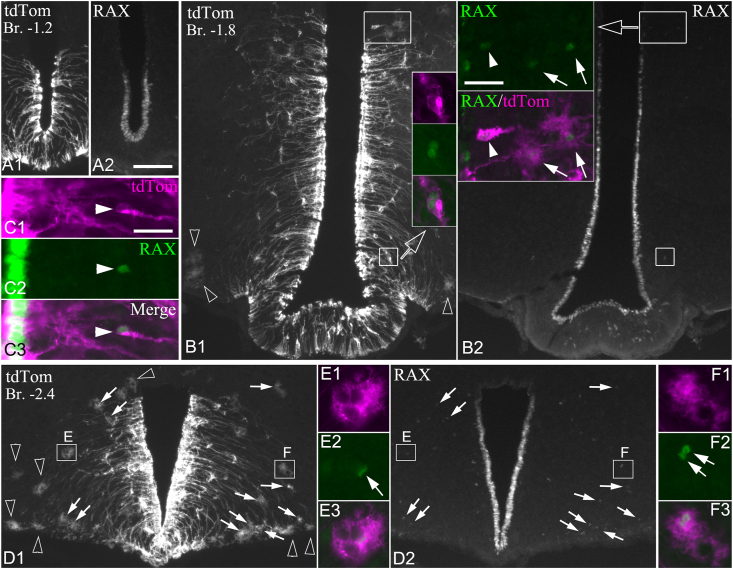
To examine the morphology of parenchymal RAX+ cells, we performed RAX immunolabeling in hypothalamic sections from TAM-treated Rax-CreERT2/+:Ai34D mice. In these mice, TAM-induced CreERT2 activates the floxed Synaptophysin-tdTomato fusion protein reporter allele under transcriptional control of the constitutively active CAG promoter, resulting in synaptophysin-tdTom expression in the entire cytoplasm of RAX+ cells and their progeny. Seven days after the first TAM injection, synaptophysin-tdTom labeled cell bodies and processes of tanycytes, corresponding to the distribution of RAX, were observed (Figure 2). Of the RAX+ cells counted in the parenchyma (122 ± 21 cells total, n = 3 mice; 4.2 ± 0.6 and 18.1 ± 2.6 cells per section rostral and caudal from Bregma −2.30 mm, respectively), about half (49.2 ± 2.8%) did not express any detectable synaptophysin-tdTom. The other half that expressed synaptophysin-tdTom in their cytoplasm could be roughly classified into two morphological types: one with size and shape similar or virtually identical to tanycytes (16.4 ± 6.0% of all parenchymal RAX+ cells) (Figure 2B1-2, C1-3); and another with a different, nonelongated shape characterized by the extension of fine processes, often making the cells appear as large patches (34.4% ± 3.4%) (Figure 2B1-2, D-F). The processes of these cells, which will be referred to as “frizzy cells”, often encircled the cell bodies of adjacent neurons (Supplementary Fig 1B1-4 and Figure 2E1-E3). Morphologically, frizzy cells were most reminiscent of protoplasmic astrocytes observed in the brainstem [30]; however, they were negative for GFAP immunostaining and the tanycyte markers, vimentin and MCT8 (Supplementary Fig. 2). The vast majority of frizzy RAX+ cells were found in the caudal Arc (Figure 2D), while few were observed rostral to Bregma −2.30 mm, in the dorsomedial (DMH) and ventromedial nuclei (VMH) of the hypothalamus, and the lateral Arc. We often observed doublets of RAX-positive nuclei that belonged to frizzy cells (Figure 2F2, Supplementary Fig 1B2), suggesting recent cell divisions. We also observed numerous frizzy synaptophysin-tdTom+ cells of the same or very similar morphology that lacked RAX staining, indicating the downregulation of Rax expression after the induction of synaptophysin-tdTomato reporter expression. These cells were more broadly distributed—not only in the caudal Arc (Figure 2D1), but also more rostrally in the DMH, VMH, and lateral Arc (Figure 2B1) and spreading out laterally as far as the lateral hypothalamus, often adjacent to the meninges (Supplementary Fig. 1A). While the mean lateral distance of RAX+ frizzy cells from the third ventricle was 209 ± 9 μm (n = 109 cells, from 3 mice) and the maximum was 433 μm, RAX-negative frizzy cells had a mean distance of 407 ± 14 μm (n = 298 cells, from 3 mice) with 1120 μm as maximum distance, and 31% were farther than 500 μm from the third ventricle. Altogether, RAX-negative, frizzy tdTom+ cells were approximately three times more numerous than RAX-positive frizzy tdTom+ cells. Several RAX-negative frizzy tdTom+ cells were conspicuous in the hypothalamic paraventricular nucleus (PVH); they were occasionally observed as far rostral as the preoptic area (POA) and the bed nucleus of the stria terminalis (BNST). Importantly, RAX immunostaining was never observed in neurons identified by HuC immunofluorescence (Supplementary Fig 1B1-4).
Figure 2.
Morphology of parenchymal RAX cells as revealed by the synaptophysin-tdTomato reporter in Rax-CreERT2/+:Ai34D mice. (A1-2, B1-2, D1-2) Low magnification grayscale images show native tdTomato fluorescence (A1, B1, and D1) and RAX IF (A2, B2, and D2) of the same fields from rostral, mid, and caudal levels of the tanycyte region. In the wall of the 3rd ventricle, tdTomato-labeled tanycytes are distributed in the same pattern as RAX. In the parenchyma, arrows indicate tdTomato-labeled frizzy cells that are RAX positive, open arrowheads that are RAX negative. (Insets, C1-3, E1-3, F1-3) Higher magnification images show dual-labeled, tdTomato (magenta) and RAX (green) positive parenchymal cells. B1 inset shows two closely adjacent RAX cells in the Arc parenchyma with morphology similar to tanycytes. B2 inset shows a tanycyte translocated into the parenchyma (white arrowhead) and two frizzy cells (arrows) in the dorsomedial nucleus. C1-3 shows a RAX positive cell well inside the Arc parenchyma (~Bregma −2.40 mm) with morphology identical to tanycytes. E1-3 and F1-3 show frizzy cells inside the boxed areas from D1-2. The double arrows in F2 point to two adjacent RAX nuclei. Scale bars: 100 μm (A2, for all low magnification images); 25 μm (B2 inset); 25 μm (C1-3, for B1 inset, E1-3, F1-3). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
It is important to note the different distribution of tdTomato+ tanycyte-like cells and frizzy cells, particularly at rostral and mid-levels of the Arc. Tanycyte-like cells were observed only in the medial portion of the Arc or VMH, a maximum of 200 μm away from the ventricle wall, in agreement with previous studies [31,32]. Frizzy cells were distributed more laterally, as they appeared to be largely excluded from the area dominated by the ventrolaterally arching tanycyte processes (Supplementary Fig. 1A and E), except in the caudal Arc (caudal of Bregma −2.30 mm). Frizzy cells could be adjacent to the ventricle in the VMH (Supplementary Fig. 1C), but their distance from the ventricle increased to a minimum of ~120 μm at the dorsal border of the Arc (Supplementary Fig. 1D) and a minimum of ~170–180 μm in the Arc (Supplementary Fig. 1D and E). Frizzy cells were frequent in the lateral portion of the Arc, >200 μm from the ventricle wall (Supplementary Fig. 1E).
3.3. Rax+ progenitors give rise to Pomc neurons that develop normal efferent projections in young adult mice
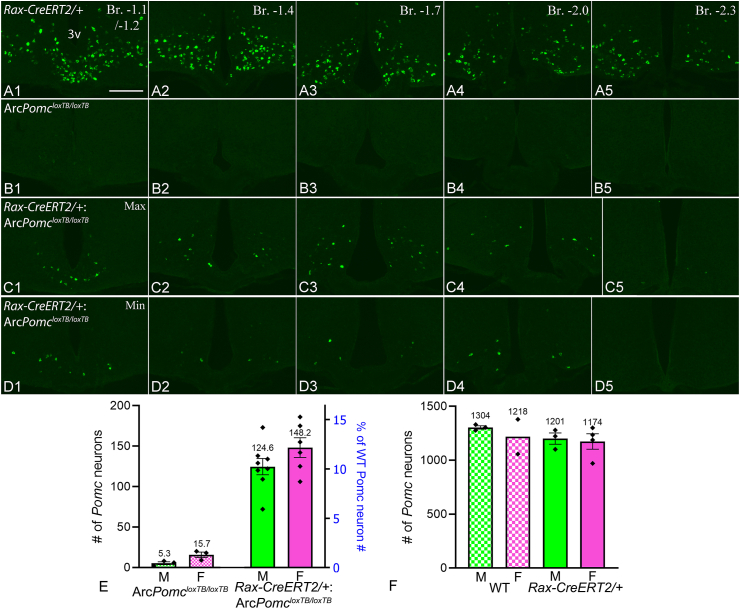
The generation of new Arc Pomc neurons by Rax+ progenitors during early adulthood was assessed by Pomc FISH in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice that were injected with TAM at 4–5 weeks of age and euthanized 16 weeks later (Figure 1A,B). TAM-treated ArcPomcloxTB/loxTB littermates were used as negative controls, while WT and Rax-CreERT2/+littermates were used as positive controls. In ArcPomcloxTB/loxTB mice, very few Arc neurons had sufficient FISH signal to be counted as Pomc positive (Figure 3B1-5, E). The same was observed in the Rax-CreERT2/+:ArcPomcloxTB/loxTB genotype without TAM treatment (data not shown). In all TAM-treated Rax-CreERT2/+:ArcPomcloxTB/loxTB mice, a prominent population of Pomc neurons was observed in the Arc. The distribution of Pomc neurons in two Rax-CreERT2/+:ArcPomcloxTB/loxTB brains that had the maximum and minimum Pomc cell counts (186 and 72, respectively; counted on every 5th 20 μm thick Arc section) is illustrated in Figure 3C1-D5. Mean Pomc neuron counts were 125 ± 10 for males and 148 ± 12 for females (not significantly different, t(12) = 1.49, p = 0.161, unpaired t-test), which amounted to approximately 10–12% of the normal Pomc neuron population observed in WT or Rax-CreERT2/+ mice (Figure 3A1-5, F). The distribution of Pomc neurons was very similar in all Rax-CreERT2/+:ArcPomcloxTB/loxTB mice: the majority were located in the medial Arc, <200 μm from the ventricle, and a smaller subset was located more laterally (Figure 3C1-D5). Few Pomc neurons were caudal of Bregma level −2.30 mm (Figure 3C5, D5).
Figure 3.
Distribution and quantification of hypothalamic Pomc neurons generated by Rax+progenitors in early adult mice. (A1-5) FISH shows normal Pomc expression at 5 rostrocaudal levels of the Arc in a Rax-CreERT2/+mouse (positive control). (B1-5)Pomc neurons were barely detected by FISH in TAM-treated ArcPomcloxTB/loxTB mice (negative control). (C1-5, D1-5) Distribution of Pomc neurons in two Rax-CreERT2/+:ArcPomcloxTB/loxTB mice that were TAM-treated at 4–5 weeks of age and sacrificed 16 weeks later; C1-5 had the highest, D1-5 the lowest Pomc neuron count. Rax-CreERT2/+:ArcPomcloxTB/loxTB images show all Pomc neurons in the sections; Rax-CreERT2/+images were cropped and do not show Pomc neurons in more lateral positions. (E)Pomc neuron counts in ArcPomcloxTB/loxTB (n = 3 per sex) and Rax-CreERT2/+:ArcPomcloxTB/loxTB mice (n = 8 males, n = 6 females). (F)Pomc neuron counts in male and female WT and Rax-CreERT2/+mice (n = 2–4); their combined average was used as 100% for scaling the right Y axis in E. Cell counts were made on every 5th, 20 μm thick coronal Arc section. 3v, third ventricle. Scale bar: 200 μm (A1, for A1-D5).
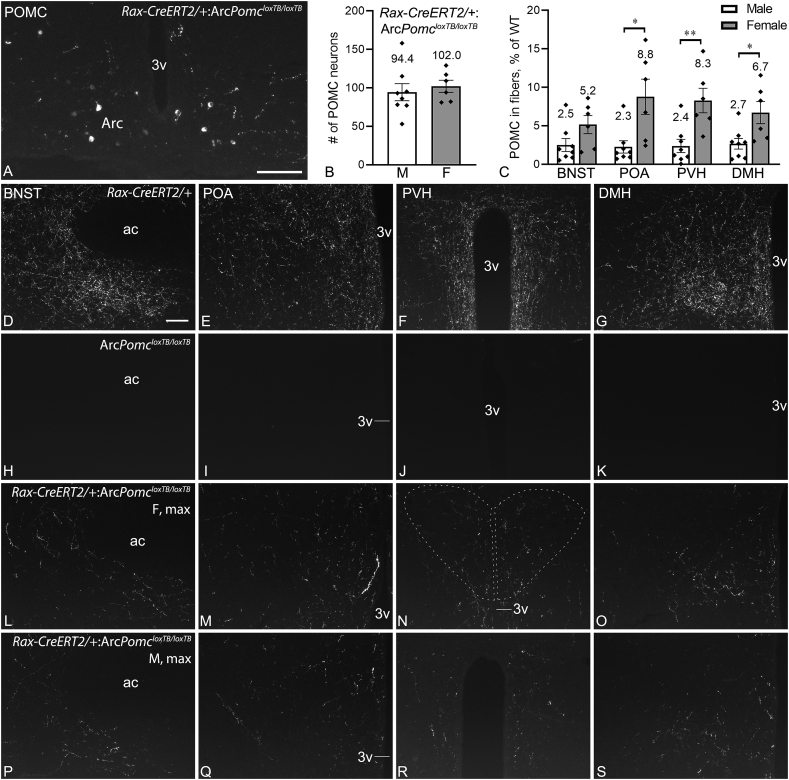
The number of POMC neurons detected by IF in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice was ~70–75% of the numbers detected by FISH, but in the same distribution patterns (Figure 4A,B). POMC fibers in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice were observed in all major target regions of Arc POMC neurons, including the BNST, POA, PVH, DMH (Figure 4L–S, compared to normal fiber density in Figure 4D–G), lateral hypothalamus, medial amygdaloid nucleus, and thalamic paraventricular nucleus. POMC fiber densities in these regions varied greatly among Rax-CreERT2/+:ArcPomcloxTB/loxTB brains, ranging from a moderate fiber density expected to arise from ~10% of the POMC neuron population to much lower densities or only sparse fibers. Brains with more Pomc neurons tended to have higher POMC fiber densities, but exceptions to this tendency were also observed. In general, females had more POMC fibers, and semiquantitative analysis revealed significantly higher POMC fiber density in females than males in the POA, PVH, and DMH (Figure 4C; BNST: p = 0.0872, POA: p = 0.0080, PVH: p = 0.0080, Mann–Whitney tests; DMH: t(12) = 2.749, p = 0.0176 unpaired t-test). Similar to WT mice, practically all POMC fibers in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice were also immunopositive for α-MSH (data not shown), which indicate normal proteolytic processing of POMC in these neurons. POMC fibers were virtually absent from the forebrain in ArcPomcloxTB/loxTB mice (Figure 4H–K), as described previously [33].
Figure 4.
Immunofluorescent detection of Rax+progenitor-derived POMC neurons and their efferents in young adult mice. (A) Cell bodies of POMC neurons in the rostral Arc of a Rax-CreERT2/+:ArcPomcloxTB/loxTB mouse. (B) POMC neuron counts in male (n = 8) and female (n = 6) Rax-CreERT2/+:ArcPomcloxTB/loxTB mice. (C) Quantification of POMC-immunoreactive fibers in the bed nucleus of the stria terminalis (BNST), preoptic area (POA), hypothalamic paraventricular nucleus (PVH), and dorsomedial nucleus (DMH) in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice. Values (area% covered by POMC fibers) are expressed as % of WT; n = 8 males, n = 6 females; ∗p < 0.05, ∗∗p < 0.01; BNST: p = 0.0872, POA: p = 0.0080, PVH: p = 0.0080, Mann–Whitney tests; DMH: p = 0.0176, unpaired t-test. (D–S) POMC fibers shown in Rax-CreERT2/+(positive control; D-G), ArcPomcloxTB/loxTB (negative control; H–K), and female (L–O) and male (P–S)Rax-CreERT2/+:ArcPomcloxTB/loxTB mice with the highest POMC fiber densities in the quantified regions; BNST: (D, H, L, P), POA: (E, I, M, Q), PVH: (F, J, N, R), DMH: (G, K, O, S). 3v, third ventricle; ac, anterior commissure. Scale bars: 100 μm (A) and 100 μm D, for D-S).
3.4. Rax-CreERT2/+:ArcPomcloxTB/loxTB mice have reduced fat mass, improved glucose tolerance, and insulin sensitivity than ArcPomcloxTB/loxTB mice
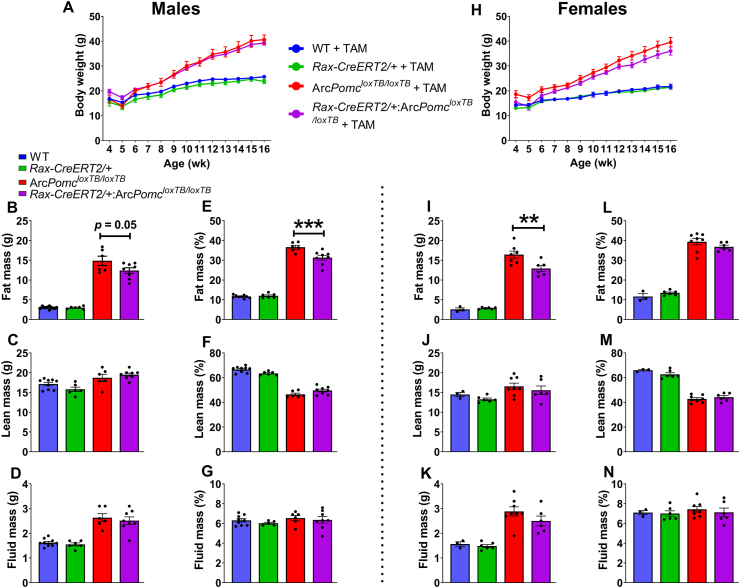
In an attempt to understand the capacity of these newly generated POMC+ neurons in rescuing the obesity phenotype caused by the loss of Arc Pomc expression in ArcPomcloxTB/loxTB mice, we performed the functional metabolic study outlined in Figure 1B. There was no significant difference in bodyweight for either sex between ArcPomcloxTB/loxTB and Rax-CreERT2/+:ArcPomcloxTB/loxTB mice over 16 weeks (Figure 5A,H). However, there were significant differences in body composition (Figure 5); both male and female Rax-CreERT2/+:ArcPomcloxTB/loxTB mice showed significantly reduced fat mass compared to ArcPomcloxTB/loxTB mice (Figure 5B,E, I; male fat mass: F(3, 25) = 97.93, p < 0.0001, one-way ANOVA; p = 0.05, Tukey's multiple comparisons; male fat mass%: F(3, 25) = 240.8, p < 0.0001, one-way ANOVA; p = 0.0009, Tukey's multiple comparisons; female fat mass: F(3, 18) = 94.73, p < 0.0001, one-way ANOVA; p = 0.007, Tukey's multiple comparisons). There were no significant differences in lean mass and fluid mass for either sex between ArcPomcloxTB/loxTB and Rax-CreERT2/+:ArcPomcloxTB/loxTB mice (Figure 5C,D, F, G, J, K, M, N).
Figure 5.
Rax-CreERT2:ArcPomcloxTB/loxTBmice show significantly reduced fat mass compared to ArcPomcloxTB/loxTBmice. (A, H) Bodyweight growth curves of males and females following TAM treatment. There was no significant change in bodyweight between ArcPomcloxTB/loxTB and Rax-CreERT2:ArcPomcloxTB/loxTB mice over 16 weeks (p > 0.05, one-way ANOVA; Tukey's multiple comparisons). Body composition - Fat mass (B, I); lean mass (C, J); and fluid mass (D, K) of male (B–D) and female (I–K) mice analyzed by NMR. (E-G, L-N) Body composition mass measurements are shown as a percentage of total body mass. Rax-CreERT2/+:ArcPomcloxTB/loxTB mice show reduced fat mass (males: p = 0.05, females: ∗∗p = 0.007; Tukey's multiple comparisons), and reduced fat mass% in males (∗∗∗p = 0.0009; Tukey's multiple comparisons) compared to ArcPomcloxTB/loxTB mice. Data are shown as mean ± SEM (males, n = 6–9/group; females, n = 3–8/group). Individual mouse values are represented by filled circles. The data were analyzed using one-way ANOVA followed by Tukey's multiple comparisons test.
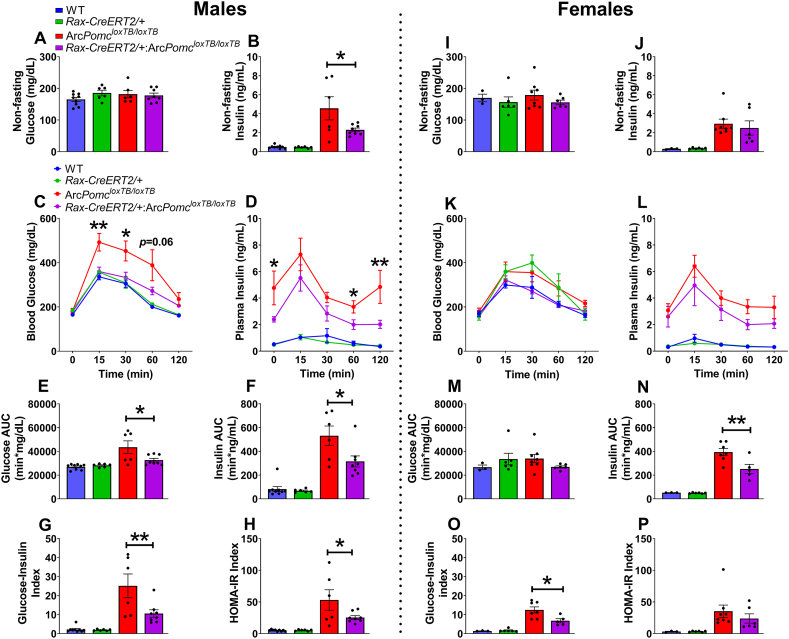
To examine glucose tolerance and insulin sensitivity, mice were subjected to oral glucose challenge after 5 h fasting. Before fasting, the blood glucose and plasma insulin levels were measured. Rax-CreERT2/+:ArcPomcloxTB/loxTB male mice had significantly lower nonfasting plasma insulin levels compared with ArcPomcloxTB/loxTB mice (Figure 6B; F(3, 25) = 13.17, p < 0.0001, one-way ANOVA; p = 0.024, Tukey's multiple comparisons); with no differences in male and female nonfasting blood glucose (Figure 6A,I) and female nonfasting plasma insulin levels (Figure 6J). Following oral glucose challenge, male Rax-CreERT2/+:ArcPomcloxTB/loxTB mice showed significantly reduced blood glucose levels at 15 (F(3, 25) = 9.89, p = 0.0002, one-way ANOVA; p = 0.001, Tukey's multiple comparisons) and 30 min (F(3, 25) = 6.03, p = 0.003, one-way ANOVA; p = 0.023, Tukey's multiple comparisons), and significantly reduced plasma insulin levels at 0 (F(3, 25) = 13.16, p < 0.0001, one-way ANOVA; p = 0.024, Tukey's multiple comparisons), 60 (F(3, 25) = 18.65, p < 0.0001, one-way ANOVA; p = 0.023, Tukey's multiple comparisons), and 120 min (F(3, 25) = 13.98, p < 0.0001, one-way ANOVA; p = 0.006, Tukey's multiple comparisons) compared with ArcPomcloxTB/loxTB mice (Figure 6C,D). In addition, male Rax-CreERT2/+:ArcPomcloxTB/loxTB mice showed significantly reduced calculated glucose AUC (F(3, 25) = 8.78, p = 0.0004, one-way ANOVA; p = 0.023, Tukey's multiple comparisons), calculated insulin AUC (F(3, 25) = 22.28, p < 0.0001, one-way ANOVA; p = 0.012, Tukey's multiple comparisons), glucose-insulin index (F(3, 25) = 13.69, p < 0.0001, one-way ANOVA; p = 0.007, Tukey's multiple comparisons), and HOMA-IR index (measure of insulin resistance; F(3, 25) = 10.01, p = 0.0002, one-way ANOVA; p = 0.047, Tukey's multiple comparisons) compared with ArcPomcloxTB/loxTB mice (Figure 6E–H). Similarly, female Rax-CreERT2/+:ArcPomcloxTB/loxTB mice showed significantly reduced calculated insulin AUC (F(3, 17) = 38.53, p < 0.0001, one-way ANOVA; p = 0.006, Tukey's multiple comparisons) and glucose-insulin index (F(3, 17) = 19.65, p < 0.0001, one-way ANOVA; p = 0.014, Tukey's multiple comparisons) compared with ArcPomcloxTB/loxTB mice (Figure 6N, O). There were no differences in blood glucose and plasma insulin levels over time, calculated glucose AUC, and HOMA-IR index in females (Figure 6K-M, P).
Figure 6.
Rax-CreERT2:ArcPomcloxTB/loxTBmice show significantly improved glucose tolerance and insulin sensitivity compared to ArcPomcloxTB/loxTBmice in response to an oral glucose tolerance test. (A, I) Blood glucose levels before fasting in male and female mice, measured using a glucometer. There was no significant difference in nonfasting blood glucose levels between the groups for either sex. (B, J) Plasma insulin levels before fasting in male and female mice, measured by ELISA. Rax-CreERT2/+:ArcPomcloxTB/loxTB male mice show lower nonfasting plasma insulin levels (∗p = 0.024; Tukey's multiple comparisons) compared to ArcPomcloxTB/loxTB mice. (C, K)Rax-CreERT2:ArcPomcloxTB/loxTB male, but not female, mice show significantly reduced blood glucose levels at 15 (∗∗p = 0.001, Tukey's multiple comparisons) and 30 min (∗p = 0.023, Tukey's multiple comparisons) after oral glucose challenge, compared to ArcPomcloxTB/loxTB mice. (D, L)Rax-CreERT2:ArcPomcloxTB/loxTB male, but not female, mice show significantly reduced plasma insulin levels at 0 (∗p = 0.024, Tukey's multiple comparisons), 60 (∗p = 0.023, Tukey's multiple comparisons), and 120 min (∗∗p = 0.006, Tukey's multiple comparisons) after oral glucose challenge, compared to ArcPomcloxTB/loxTB mice. (E, M)Rax-CreERT2:ArcPomcloxTB/loxTB male, but not female mice, show significantly reduced glucose areas under the curve (AUC; ∗p = 0.023, Tukey's multiple comparisons) compared to ArcPomcloxTB/loxTB mice. (F, N)Rax-CreERT2:ArcPomcloxTB/loxTB male and female mice show significantly reduced insulin areas under the curve (AUC; male: ∗p = 0.012, female: ∗∗p = 0.006; Tukey's multiple comparisons) compared to ArcPomcloxTB/loxTB mice. (G, O)Rax-CreERT2:ArcPomcloxTB/loxTB male and female mice show significantly reduced glucose-insulin indexes (male: ∗∗p = 0.007, female: ∗p = 0.014; Tukey's multiple comparisons). (H, P)Rax-CreERT2:ArcPomcloxTB/loxTB male, but not female, mice show significantly reduced HOMA-IR indexes (∗p = 0.047, Tukey's multiple comparisons) compared to ArcPomcloxTB/loxTB mice. Data are shown as mean ± SEM (males, n = 6–9/group; females, n = 3–8/group). Individual mouse values are represented by filled circles. The data were analyzed using one-way ANOVAs followed by Tukey's multiple comparisons test.
The average daily food intake measured at age 14 and 15 weeks did not differ between genotypes (Supplementary Fig. 3A and C). Furthermore, a fasting-refeeding experiment performed at age 15 weeks showed the normal compensatory hyperphagic response to an 18-h fast and return of bodyweight to baseline without any genotype or sex differences (Supplementary Fig. 3).
3.5. Pomc neurons continuously arise from Rax+ progenitors at least until senescence
To determine whether Rax+ progenitors continue to generate new Pomc neurons after early adulthood, we performed Pomc FISH in different age groups (8 cohorts) of Rax-CreERT2:ArcPomcloxTB/loxTB mice that received TAM treatment between ages 15–50 weeks, and were euthanized either 12 weeks (cohort 3) or 14–16 weeks (all other cohorts) later (Figure 7A). The two oldest cohorts (cohorts 7 and 8) were euthanized at 14–15 months of age, which is considered the end of middle age in mice [34]. TAM-treated ArcPomcloxTB/loxTB littermates were used as negative controls and Rax-CreERT2/+littermates were used as positive controls. The number of Pomc neurons across these age groups was very similar to that observed in early adult Rax-CreERT2/+:ArcPomcloxTB/loxTB mice (males: 129 ± 9; females: 147 ± 9) (Figure 7A,B). This amounted to ~9–10% of the normal Pomc neuron number counted in Rax-CreERT2/+ littermates, which was somewhat higher than in early adult Rax-CreERT2/+ or WT mice (Figure 7C; compare to Figure 3F). There was no difference in the number of Pomc neurons between the 12 and 16 weeks survival time after TAM treatment (Figure 7A). Brains with the highest Pomc neuron counts from cohorts 3, 5, and 8 are shown in Figure 7D1-F5.
POMC/α-MSH IF was performed in all Rax-CreERT2/+:ArcPomcloxTB/loxTB mice (except cohort 3) to study the distribution of POMC fibers. POMC fibers were observed in all major projection regions, including BNST, POA, PVH, and DMH (Supplementary Fig. 4E-L, compared to normal fiber density in A-D). POMC fiber densities in these regions were similar to that observed in early adult Rax-CreERT2/+:ArcPomcloxTB/loxTB mice, although the highest fiber densities remained lower compared with early adults. Female mice had significantly higher densities of POMC fibers than males in all measured regions (Figure 7G; BNST: t(18) = 2.84, p = 0.0108, DMH: t(18) = 3.72, p = 0.0016, POA: t(18) = 3.00, p = 0.0077, unpaired t-tests; PVH: p = 0.0077, Mann–Whitney test).
Bodyweight gain at 12 weeks after TAM treatment, combined from cohorts 1–6, are shown in Figure 7H (cohorts 7 and 8 were excluded from this analysis due to higher likelihood of age-related bodyweight loss, independent of genotype). The initial pre-TAM bodyweights of Rax-CreERT2/+:ArcPomcloxTB/loxTB and ArcPomcloxTB/loxTB mice were characteristic of obesity in all age coorts (from 40.2 ± 1.6 g in the younger cohort 1 to 65.8 ± 2.5 g in the 27-week-old cohort 6). Rax-CreERT2/+:ArcPomcloxTB/loxTB mice gained significantly less weight than ArcPomcloxTB/loxTB mice (0.7 ± 1.8 g vs 9.3 ± 1.8 g; W(2.00, 13.18) = 8.885, p = 0.0036, Welch's ANOVA; p = 0.0114, Dunnett's T3 multiple comparisons); several Rax-CreERT2/+:ArcPomcloxTB/loxTB mice lost weight up to 16 g (Figure 7H). Bodyweight gain in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice negatively correlated with the number of Pomc neurons observed in these mice (Figure 7I; Pearson r = −0.64, p = 0.0019, n = 21). When analyzed separately by sex, the negative correlation was statistically significant in females (r = −0.75, p = 0.0123, n = 10), but not in males (r = −0.48, p = 0.1352, n = 11) (Figure 7I).
3.6. New Pomc neurons are generated rapidly from Rax+ progenitors
The previous results that showed no difference in Pomc neuron counts in mice sacrificed 12 or 16 weeks after TAM treatment suggested that Pomc neurons derived from Rax+ progenitors may not accumulate linearly with time. To study the dynamics of Pomc neuron generation by Rax+ progenitors during shorter intervals, we performed Pomc FISH on Rax-CreERT2/+:ArcPomcloxTB/loxTB mice sacrificed at 7 (7-day group) or 16 (16-day group) days following the first TAM injection (Figure 8A1-B5). At 7 days, we counted 56 ± 8 Pomc neurons (n = 8; 3 males, 5 females), which is equivalent to ~42% of the Pomc neurons observed in mice sacrificed 16 weeks after TAM induction in the early adult experiment (Figure 8C). At 16 days, 131 ± 30 Pomc neurons were counted (n = 4; 3 males, 1 female), which is identical to the number observed 16 weeks after TAM treatment (Figure 8C). These results indicate a linear increase in Pomc neuron number from the first TAM injection until around 16 days, with no further increase.
To assess how rapidly new Pomc neurons develop efferent projections, we examined POMC fiber distribution in four Rax-CreERT2/+:ArcPomcloxTB/loxTB mice from the 7-day group. We observed sparse to modest fiber densities in the BNST, POA, PVH, DMH (Figure 8D–G), along with the thalamic paraventricular and medial amygdaloid nuclei (data not shown), comparable to the low-tier POMC fiber densities observed 16 weeks after TAM treatment, suggesting the rapid development of fibers from new Pomc neurons.
3.7. Congenital Arc Pomc deficiency does not significantly affect the rate of Pomc neurogenesis from Rax+ progenitors
Finally, we aimed to determine whether the observed number of newly generated Pomc neurons in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice represents normal levels of neurogenesis that also occurs in WT mice, or rather the result of congenital Arc Pomc deficiency itself and/or its metabolic effects. Therefore, we performed lineage tracing using the Ai34D reporter allele, synaptophysin-tdTomato, to compare Pomc neurogenesis in ArcPomcloxTB/loxTB mice with mice expressing the WT Pomc alleles. Compound Rax-CreERT2/+:ArcPomcloxTB/loxTB:Ai34D and Rax-CreERT2/+:Ai34D mice were TAM-treated and euthanized either 16 weeks later as part of the metabolic experiment in early adult mice, or at 7 days as part of the short-term survival experiment. As described above, TAM treatment of these mice results in synaptophysin-tdTomato expression in Rax+ cells and their progeny. Following Pomc FISH, sections were processed for tdTomato IF, and Pomc neurons containing the synaptophysin-tdTomato lineage trace were counted (Supplementary Fig 5A1-C3). The numbers of dual-labeled tdTom/Pomc neurons were very similar in the two genotypes (Supplementary Fig. 5D), suggesting that Arc Pomc deficiency does not significantly affect the rate of Pomc neurogenesis from Rax+ progenitors.
Surprisingly, not all Pomc neurons in Rax-CreERT2/+:ArcPomcloxTB/loxTB:Ai34D mice contained synaptophysin-tdTomato (Supplementary Fig 5A1-A2) – the theoretically expected result because both the rescue of Pomc transcription and activation of synaptophysin-tdTomato expression are CreERT2-dependent. Only a small fraction of Pomc neurons, approximately 15–20% was tdTomato positive, in both the 7-day and 16-week groups (Supplementary Fig. 5E). Using an alternative detection method in the 16-week group, native tdTomato fluorescence was detected in similarly low percentages of immunofluorescently labeled POMC neurons (Supplementary Fig. 5E) and corresponding proportions of POMC fibers. Possible explanations for this discrepancy are presented in the Discussion. Notably, the two mice homozygous for the synaptophysin-tdTomato allele had both the highest number and percentage of tdTom/Pomc neurons (red triangles in Supplementary Fig. 5D and E). It should also be noted that synaptophysin-tdTomato expression itself did not affect Pomc neurogenesis from Rax+ progenitors, as Pomc neuron numbers were similar in Rax-CreERT2/+:ArcPomcloxTB/loxTB:Ai34D and Rax-CreERT2/+:ArcPomcloxTB/loxTB mice (Supplementary Fig. 5F).
4. Discussion
In this study, we demonstrated that Rax+ progenitor cells generate a functionally significant population of Pomc neurons in adult mice that are capable of mitigating the metabolic abnormalities resulting from congenital Arc Pomc-deficiency.
4.1. Validity of the findings
The reliability of genetic fate mapping based on Cre-mediated recombination depends on the specific expression of Cre in the targeted stem/progenitor cell population. In Rax-CreERT2/+ mice, CreERT2 was inserted into the Rax locus and is expressed under the endogenous Rax promoter, thus minimizing, if not eliminating, the possibility of ectopic expression [17]. We did not observe Rax expression in hypothalamic neurons by FISH or IF, and Rax transcript was not detected in Pomc neurons of adult mice in a single-cell RNA-Seq study [35]. Moreover, in a developmental single-cell RNA-Seq analysis of hypothalamic POMC neurons from the Low lab (submitted, [36]), we found that not more than 2% of 3,533 Pomc-positive neurons at postnatal age 12 days had any detectable UMIs for Rax transcripts (mostly 1–2) compared to typical Pomc UMI counts of 50–300 per neuron. As recombination efficiency depends on CreERT2 expression levels [37] and because incomplete recombination occurs even when the CreERT2 driver gene is easily detectable [38,39], these data suggest that it is unlikely for TAM treatment to reactivate Pomc expression in already existing Arc neurons. Consequently, the vast majority of Arc Pomc neurons observed in Rax-CreERT2/+:ArcPomcloxTB/loxTB mice were generated from Rax-expressing precursors after TAM induction. Considering that Pomc expression in the pituitary and NTS remain intact in ArcPomcloxTB/loxTB mice [21], and there is no evidence of Rax expression in the brainstem [17] where the only other POMC neuron population is located [22], the observed metabolic changes can only be attributed to the newly generated Arc Pomc neurons.
4.2. Adult-born POMC neurons may derive from two different Rax+ precursors
Although previous studies identified Rax as a tanycyte marker [[18], [19], [20]], we found that Rax is also expressed in a small population of parenchymal cells. While a subset of these cells had tanycyte-like morphology and probably represents tanycytes that migrated into the parenchyma before differentiation [31,40], the majority represented a different cell type that we termed “frizzy cells”, which were located primarily in the caudal Arc. Rax-negative frizzy cells expressing tdTomato were more broadly distributed, suggesting that frizzy cells move over long distances and downregulate Rax while migrating. Frizzy cells were also observed by other studies, such as by Yoo and colleagues [16] who used the same Rax-CreERT2/+ line, and showed a reporter-positive cell with glial cell-like morphology that is identical to a frizzy cell. Another tanycyte lineage-tracing study using Fgf10-CreERT2/+ mice described reporter-positive glial-like cells [40] that are morphologically identical to frizzy cells. A third study using GLAST::CreERT2 mice to fate map α-tanycytes noted that a large portion of parenchymal reporter-expressing cells was morphologically distinct, unidentified cells, negative for glial markers such as GFAP, nestin, and NG2 [15]. This description resembles frizzy cells, although based on the photographs we could not unambiguously identify them as such. The above studies referred to these cells as having glial morphology or morphologically distinct, confirming that it is difficult to categorize frizzy cells into any known hypothalamic cell type based on morphology. However, frizzy cells may represent a subtype of protoplasmic astrocytes with low GFAP expression/immunoreactivity [30,41], and potentially with neural progenitor characteristics akin to a subtype of hippocampal astrocytes [42]. In a recent RNA-Seq study that categorized cells of the Rax lineage, frizzy cells were probably classified as astrocytes and/or oligodendrocyte precursor cells, and it was noted that astrocytes appear to arise directly from α1 and α2 tanycytes [16]. Interestingly, we observed that dorsal α1 tanycytes translocated into the parenchyma with nearby RAX+ frizzy cells (Figure 2B2 inset), which may offer a snapshot into the process of tanycytes entering the parenchyma and differentiating into frizzy cells. Overall, frizzy cells appear to be tanycyte-derived, undifferentiated cells. Further studies are required to investigate whether they are the direct precursors of Rax+ progenitor-derived Pomc neurons in the lateral Arc; considering their anatomical distribution and the absence of tanycyte-like cells >200 μm away from the third ventricle.
4.3. Dynamics of adult POMC neurogenesis
We found that the number of Rax+ progenitor-derived Pomc neurons increased linearly up to 16 days after TAM treatment, with no further accumulation. This suggests that Pomc neurons are constantly born from Rax+ progenitors, have an average lifespan of about 16 days, and undergo turnover. Of note, the population of tdTomato-labeled neurons in Ai34D mice increased in a similarly moderate degree between 7 days and 4 months after TAM treatment, further suggesting that Rax+ progenitor-derived neurons do not permanently integrate into the brain. Previous studies have suggested a high neuronal turnover rate among both prenatally generated and adult-born hypothalamic neurons, including Pomc neurons [9,10]. Pomc neurogenesis from Rax+ progenitors remained constant up to 15 months of age, in agreement with a previous study on adult hypothalamic neurogenesis [43].
Our observation that Rax+ progenitors generated ~10% of the Arc Pomc population within 16 days indicates a much more robust Pomc neurogenesis than we suspected, based on studies that used BrdU to identify Pomc neurons derived from recent cell divisions [8,9]. While this can be partly explained by the superior sensitivity of genetic lineage tracing compared to BrdU labeling, it raises the possibility that the majority of Rax+ progenitors might exit the cell cycle several days or weeks before differentiating into Pomc neurons. In support, neurogenesis in the Arc from postmitotic precursors without cell division has been reported in adult mice following a monosodium-glutamate–induced lesion [44]. This proposed mechanism raises the question of whether tanycyte proliferation is sufficient to replace the number of postmitotic precursors that exit the ventricular wall and differentiate into neurons. Although basal tanycyte proliferation in mice is modest [15], this question will require careful analysis. In rats, a proliferative zone exists within the α1 tanycyte region [45,46], where proliferation appears to occur in bursts [32]. In addition, tanycyte movement from the β1 to the α domain (ventral to dorsal) was observed in mice [40]. Proliferative activity even among posterior pituitary pituicytes – which also expresses Rax [17] and have similar characteristics to tanycytes [47] – could supply new hypothalamic tanycytes by cell migration through the infundibulum. However, proliferation might be insufficient to maintain tanycyte numbers, as age-related attrition in the number of α-tanycytes was described [43].
Using a fluorescent lineage marker, we demonstrated that similar numbers of tdTom/Pomc neurons were generated in mice with or without ArcPomcloxTB/loxTB silencing. However, this method dramatically underestimated the number of Rax+ progenitor-derived Pomc neurons. A possible explanation is that Rax+ tanycytes (and frizzy cells) that remained tdTomato-negative in Rax-CreERT2:ArcPomcloxTB/loxTB:Ai34D mice (Supplementary Fig 6A1-B) generated a significant number of Pomc neurons. Cre recombination efficiency depends on the genomic location of the loxP sites [37,48], and although we could not determine recombination in the Pomc neuronal enhancer at the single-cell level, the loxP sites in the Pomc gene may be more easily recombined than in the synaptophysin-tdTomato allele in the Rosa26 locus. Nevertheless, as tdTomato-negative Rax+ cells represented a small minority of Rax+ cells, they may not fully explain why the majority of Pomc neurons were tdTomato-negative, unless they overrepresented highly active progenitors. Another possibility is that the synaptophysin-tdTomato allele may be silenced in differentiated neurons (refer [49,50] for transgene downregulation following differentiation), as we often observed less intense tdTomato signal in neurons than in tanycytes (examples in Supplementary Fig 5A1-C3). While the exact cause remains to be determined, this provides an important caveat for future lineage tracing studies that rely on similar transgenic alleles.
4.4. Adult-born Pomc neurons become functionally integrated
Our results showed that Pomc neurons born from Rax+ progenitors develop rapidly and form long-range projections within a week, suggesting fast neuronal maturation, in agreement with findings that adult-born tanycyte-derived neurons become leptin-responsive within 8 days [14]. A recent study also demonstrated that tanycyte-derived neurons are capable of firing action potentials within 12 days, receive synaptic inputs, and thus integrate into the hypothalamic neurocircuitry [16]. Newly generated Pomc neurons successfully incorporated into functional neural circuits—as indicated by improved glucose tolerance in males, improved insulin sensitivity, and reduced fat mass that were observed in both sexes, but more pronounced in males. However, food intake and bodyweight did not differ significantly. Interestingly, the opposite effects were observed in studies that interfered with hypothalamic neurogenesis in adult mice. The ablation of tanycytes resulted in modest, but significantly increased fat mass and reduced insulin sensitivity in males only [51]; whereas the inhibition of hypothalamic neurogenesis from Sox2-expressing progenitor cells (that also include tanycytes [17]) was accompanied by a ~10% decrease in POMC neuron number, and resulted in glucose intolerance and hyperinsulinemia, but also increased food intake and obesity [8].
It is tempting to speculate that Rax+ progenitor-derived Pomc neurons primarily regulate glucose homeostasis and energy expenditure, rather than food intake. Recent evidence indicates functional heterogeneity among POMC neurons in the regulation of feeding and energy expenditure [38,52]. For example, rescuing Pomc expression in a subset of GABAergic POMC neurons normalized food intake in Arc Pomc-deficient mice, while a similar, but nonspecific rescue of Pomc expression failed to decrease food intake, though it reduced bodyweight [38]. Alternatively, Rax+ progenitor-derived POMC neurons may be involved in food intake regulation, but their number does not reach the threshold to exert a significant effect [38]. Intriguingly, mature adult and middle-aged Rax-CreERT2:ArcPomcloxTB/loxTB mice gained significantly less bodyweight than ArcPomcloxTB/loxTB mice following TAM treatment. The weight gain correlated inversely with the number of Pomc neurons in females, but was not statistically significant in males.
The different metabolic responses observed in males vs females occurred despite similar numbers of newly generated Pomc neurons in the sexes. Of note, a previous study also found similar levels of neurogenesis in the Arc between males and females [53]. However, female Rax-CreERT2:ArcPomcloxTB/loxTB mice consistently exhibited greater densities of POMC fibers than males, which may translate into different metabolic outcomes between the sexes. In addition, distinct POMC neuron subpopulations are known to have disparate effects in males and females. For example, the restoration of Pomc expression in ArcPomcloxTB/loxTB mice mediated by a Htr2c-Cre knock-in mouse strain normalized the obesity and metabolic phenotype in male offspring [54]. However, females continued to exhibit hypolocomotor activity, decreased energy expenditure, and obesity, despite normalized feeding behavior and insulin levels. Furthermore, chemogenetic activation of leptin-receptor-expressing and glucagon-like peptide 1 receptor-expressing POMC neurons suppresses feeding in male mice, but not in female mice [55].
5. Conclusions
In conclusion, adult-born Pomc neurons generated by Rax+ progenitors make up ~10% of the total Pomc neuron population and are sufficient to mitigate the metabolic abnormalities of congenital Arc Pomc-deficiency, revealing a remarkable novel regulatory capacity of Pomc neurons. However, further experiments are required to determine the underlying mechanisms involved in the generation of Pomc neurons from Rax+ progenitors. Because adult Pomc neurogenesis has the potential to slow the progression of advanced obesity caused by Arc Pomc deficiency in mice, we propose that developing interventions to increase the number of adult-born Pomc neurons in humans may lead to a novel clinical approach in the treatment of diabetes and obesity.
Author contributions
Study concept: RML. Overall research plan and study supervision: MJL and RML. Generation of experimental mice, metabolic experiments, and data analysis: S. Histology and data analysis: GW. Article writing: S and GW. Critical revision of the article: MJL and RML.
Acknowledgments
This study was funded by NIH grants AG059004 and AG050663 (to R.M.L.), DK068400 (to M.J.L.), and used core services provided by the Michigan Mouse Metabolic Phenotyping Center's Animal Phenotyping Core (U2CDK110768 to M.J.L.). The authors thank Graham Jones and Talisha Sutton for generating preliminary data for this project, Dr. Hidetaka Suga for providing the RAX antiserum, and Dr. Hui Yu for providing an analysis of Rax and Pomc co-expression by scRNA-seq in the arcuate nucleus of postnatal day-12 mice.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2021.101312.
Contributor Information
Surbhi, Email: sgahlot@umich.edu.
Gábor Wittmann, Email: gabor.wittmann11@gmail.com.
Malcolm J. Low, Email: mjlow@umich.edu.
Ronald M. Lechan, Email: rlechan@tuftsmedicalcenter.org.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Berthoud H.R., Munzberg H., Morrison C.D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology. 2017;152(7):1728–1738. doi: 10.1053/j.gastro.2016.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krashes M.J., Lowell B.B., Garfield A.S. Melanocortin-4 receptor-regulated energy homeostasis. Nature Neuroscience. 2016;19(2):206–219. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson E.J., Cakir I., Carrington S.J., Cone R.D., Ghamari-Langroudi M., Gillyard T. 60 years OF POMC: regulation of feeding and energy homeostasis by alpha-MSH. Journal of Molecular Endocrinology. 2016;56(4):T157–T174. doi: 10.1530/JME-16-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smart J.L., Tolle V., Low M.J. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. Journal of Clinical Investigation. 2006;116(2):495–505. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bumaschny V.F., Yamashita M., Casas-Cordero R., Otero-Corchon V., de Souza F.S., Rubinstein M. Obesity-programmed mice are rescued by early genetic intervention. Journal of Clinical Investigation. 2012;122(11):4203–4212. doi: 10.1172/JCI62543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhabra K.H., Adams J.M., Jones G.L., Yamashita M., Schlapschy M., Skerra A. Reprogramming the bodyweight set point by a reciprocal interaction of hypothalamic leptin sensitivity and Pomc gene expression reverts extreme obesity. Molecular Metabolism. 2016;5(10):869–881. doi: 10.1016/j.molmet.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo S., Blackshaw S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Progress in Neurobiology. 2018;170:53–66. doi: 10.1016/j.pneurobio.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Tang Y., Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nature Cell Biology. 2012;14(10):999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouaze A., Brenachot X., Rigault C., Krezymon A., Rauch C., Nedelec E. Cerebral cell renewal in adult mice controls the onset of obesity. PloS One. 2013;8(8) doi: 10.1371/journal.pone.0072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNay D.E., Briancon N., Kokoeva M.V., Maratos-Flier E., Flier J.S. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. Journal of Clinical Investigation. 2012;122(1):142–152. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez E.M., Blazquez J.L., Pastor F.E., Pelaez B., Pena P., Peruzzo B. Hypothalamic tanycytes: a key component of brain-endocrine interaction. International Review of Cytology. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y., Tamamaki N., Noda T., Kimura K., Itokazu Y., Matsumoto N. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Experimental Neurology. 2005;192(2):251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Lee D.A., Bedont J.L., Pak T., Wang H., Song J., Miranda-Angulo A. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nature Neuroscience. 2012;15(5):700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haan N., Goodman T., Najdi-Samiei A., Stratford C.M., Rice R., El Agha E. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. Journal of Neuroscience. 2013;33(14):6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robins S.C., Stewart I., McNay D.E., Taylor V., Giachino C., Goetz M. alpha-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nature Communications. 2013;4:2049. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- 16.Yoo S., Kim J., Lyu P., Hoang T.V., Ma A., Trinh V. Control of neurogenic competence in mammalian hypothalamic tanycytes. 2021. bioRxiv. [DOI] [PMC free article] [PubMed]
- 17.Pak T., Yoo S., Miranda-Angulo A.L., Wang H., Blackshaw S. Rax-CreERT2 knock-in mice: a tool for selective and conditional gene deletion in progenitor cells and radial glia of the retina and hypothalamus. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranda-Angulo A.L., Byerly M.S., Mesa J., Wang H., Blackshaw S. Rax regulates hypothalamic tanycyte differentiation and barrier function in mice. Journal of Comparative Neurology. 2014;522(4):876–899. doi: 10.1002/cne.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell J.N., Macosko E.Z., Fenselau H., Pers T.H., Lyubetskaya A., Tenen D. A molecular census of arcuate hypothalamus and median eminence cell types. Nature Neuroscience. 2017;20(3):484–496. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R., Wu X., Jiang L., Zhang Y. Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Reports. 2017;18(13):3227–3241. doi: 10.1016/j.celrep.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam D.D., de Souza F.S., Nasif S., Yamashita M., Lopez-Leal R., Otero-Corchon V. Partially redundant enhancers cooperatively maintain Mammalian pomc expression above a critical functional threshold. PLoS Genetics. 2015;11(2) doi: 10.1371/journal.pgen.1004935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young J.I., Otero V., Cerdan M.G., Falzone T.L., Chan E.C., Low M.J. Authentic cell-specific and developmentally regulated expression of pro-opiomelanocortin genomic fragments in hypothalamic and hindbrain neurons of transgenic mice. Journal of Neuroscience. 1998;18(17):6631–6640. doi: 10.1523/JNEUROSCI.18-17-06631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahn H.M., Kasakow C.V., Helfer A., Michely J., Verkhratsky A., Maurer H.H. Refined protocols of tamoxifen injection for inducible DNA recombination in mouse astroglia. Scientific Reports. 2018;8(1):5913. doi: 10.1038/s41598-018-24085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvela T.S., Surbhi, Shakya M., Bachor T., White A., Low M.J. Reduced stability and pH-dependent activity of a common obesity-linked PCSK1 polymorphism, N221D. Endocrinology. 2019;160(11):2630–2645. doi: 10.1210/en.2019-00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allison D.B., Paultre F., Maggio C., Mezzitis N., Pi-Sunyer F.X. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 26.Henriksen E.J., Jacob S., Fogt D.L., Dietze G.J. Effect of chronic bradykinin administration on insulin action in an animal model of insulin resistance. American Journal of Physiology. 1998;275(1):R40–R45. doi: 10.1152/ajpregu.1998.275.1.R40. [DOI] [PubMed] [Google Scholar]
- 27.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Wittmann G., Hrabovszky E., Lechan R.M. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. Journal of Comparative Neurology. 2013;521(14):3287–3302. doi: 10.1002/cne.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvatierra J., Lee D.A., Zibetti C., Duran-Moreno M., Yoo S., Newman E.A. The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation. Journal of Neuroscience. 2014;34(50):16809–16820. doi: 10.1523/JNEUROSCI.1711-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardona S.M., Dunphy J.M., Das A.S., Lynch C.R., Lynch W.P. Astrocyte infection is required for retrovirus-induced spongiform neurodegeneration despite suppressed viral protein expression. Frontiers in Neuroscience. 2019;13:1166. doi: 10.3389/fnins.2019.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jourdon A., Gresset A., Spassky N., Charnay P., Topilko P., Santos R. Prss56, a novel marker of adult neurogenesis in the mouse brain. Brain Structure and Function. 2016;221(9):4411–4427. doi: 10.1007/s00429-015-1171-z. [DOI] [PubMed] [Google Scholar]
- 32.Wittmann G., Lechan R.M. Prss56 expression in the rodent hypothalamus: inverse correlation with pro-opiomelanocortin suggests oscillatory gene expression in adult rat tanycytes. Journal of Comparative Neurology. 2018;526(15):2444–2461. doi: 10.1002/cne.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam D.D., Attard C.A., Mercer A.J., Myers M.G., Jr., Rubinstein M., Low M.J. Conditional expression of Pomc in the Lepr-positive subpopulation of POMC neurons is sufficient for normal energy homeostasis and metabolism. Endocrinology. 2015;156(4):1292–1302. doi: 10.1210/en.2014-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flurkey K., Currer J., Harrison D. In: The mouse in biomedical research. 2nd ed. Fox J., editor. Academic Press; 2007. The mouse in aging research; pp. 637–672. [Google Scholar]
- 35.Lam B.Y.H., Cimino I., Polex-Wolf J., Nicole Kohnke S., Rimmington D., Iyemere V. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Molecular Metabolism. 2017;6(5):383–392. doi: 10.1016/j.molmet.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H., Rubinstein M., Low M.J. bioRxiv; 2021. Dual origin and multiple neuropeptidergic trajectories of hypothalamic POMC progenitors revealed by developmental single-cell transcriptomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Chacon M., Casquero-Garcia V., Luo W., Francesca Lunella F., Ferreira Rocha S., Del Olmo-Cabrera S. iSuRe-Cre is a genetic tool to reliably induce and report Cre-dependent genetic modifications. Nature Communications. 2019;10(1):2262. doi: 10.1038/s41467-019-10239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trotta M., Bello E.P., Alsina R., Tavella M.B., Ferran J.L., Rubinstein M. Hypothalamic Pomc expression restricted to GABAergic neurons suppresses Npy overexpression and restores food intake in obese mice. Molecular Metabolism. 2020;37:100985. doi: 10.1016/j.molmet.2020.100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarvie B.C., Hentges S.T. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. Journal of Comparative Neurology. 2012;520(17):3863–3876. doi: 10.1002/cne.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman T., Nayar S.G., Clare S., Mikolajczak M., Rice R., Mansour S. Fibroblast growth factor 10 is a negative regulator of postnatal neurogenesis in the mouse hypothalamus. Development. 2020;147(13) doi: 10.1242/dev.180950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeSilva T.M., Borenstein N.S., Volpe J.J., Kinney H.C., Rosenberg P.A. Expression of EAAT2 in neurons and protoplasmic astrocytes during human cortical development. Journal of Comparative Neurology. 2012;520(17):3912–3932. doi: 10.1002/cne.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batiuk M.Y., Martirosyan A., Wahis J., de Vin F., Marneffe C., Kusserow C. Identification of region-specific astrocyte subtypes at single cell resolution. Nature Communications. 2020;11(1):1220. doi: 10.1038/s41467-019-14198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaker Z., George C., Petrovska M., Caron J.B., Lacube P., Caille I. Hypothalamic neurogenesis persists in the aging brain and is controlled by energy-sensing IGF-I pathway. Neurobiology of Aging. 2016;41:64–72. doi: 10.1016/j.neurobiolaging.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Yulyaningsih E., Rudenko I.A., Valdearcos M., Dahlen E., Vagena E., Chan A. Acute lesioning and rapid repair of hypothalamic neurons outside the blood-brain barrier. Cell Reports. 2017;19(11):2257–2271. doi: 10.1016/j.celrep.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-Martin M., Cifuentes M., Grondona J.M., Lopez-Avalos M.D., Gomez-Pinedo U., Garcia-Verdugo J.M. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. European Journal of Neuroscience. 2010;31(9):1533–1548. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- 46.Recabal A., Fernandez P., Lopez S., Barahona M.J., Ordenes P., Palma A. The FGF2-induced tanycyte proliferation involves a connexin 43 hemichannel/purinergic-dependent pathway. Journal of Neurochemistry. 2020 doi: 10.1111/jnc.15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez E., Guerra M., Peruzzo B., Blazquez J.L. Tanycytes: a rich morphological history to underpin future molecular and physiological investigations. Journal of Neuroendocrinology. 2019;31(3) doi: 10.1111/jne.12690. [DOI] [PubMed] [Google Scholar]
- 48.Vooijs M., Jonkers J., Berns A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Reports. 2001;2(4):292–297. doi: 10.1093/embo-reports/kve064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jakobsson J., Rosenqvist N., Thompson L., Barraud P., Lundberg C. Dynamics of transgene expression in a neural stem cell line transduced with lentiviral vectors incorporating the cHS4 insulator. Experimental Cell Research. 2004;298(2):611–623. doi: 10.1016/j.yexcr.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 50.Macarthur C.C., Xue H., Van Hoof D., Lieu P.T., Dudas M., Fontes A. Chromatin insulator elements block transgene silencing in engineered human embryonic stem cell lines at a defined chromosome 13 locus. Stem Cells and Development. 2012;21(2):191–205. doi: 10.1089/scd.2011.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoo S., Cha D., Kim S., Jiang L., Cooke P., Adebesin M. Tanycyte ablation in the arcuate nucleus and median eminence increases obesity susceptibility by increasing body fat content in male mice. Glia. 2020;68(10):1987–2000. doi: 10.1002/glia.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saucisse N., Mazier W., Simon V., Binder E., Catania C., Bellocchio L. bioRxiv; 2020. POMC neurons functional heterogeneity relies on mTORC1 signaling. [DOI] [PubMed] [Google Scholar]
- 53.Lee D.A., Yoo S., Pak T., Salvatierra J., Velarde E., Aja S. Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice. Frontiers in Neuroscience. 2014;8:157. doi: 10.3389/fnins.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burke L.K., Doslikova B., D'Agostino G., Greenwald-Yarnell M., Georgescu T., Chianese R. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Molecular Metabolism. 2016;5(3):245–252. doi: 10.1016/j.molmet.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biglari N., Gaziano I., Schumacher J., Radermacher J., Paeger L., Klemm P. Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nature Neuroscience. 2021 doi: 10.1038/s41593-021-00854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.