Key Points
Question
Is there an association between the timing of exposure to and severity of COVID-19 disease in close contacts of index patients with COVID-19?
Findings
In this cohort study of 730 index patients with a COVID-19 diagnosis and 8852 close contacts, transmission potential was greatest in the first 2 days before and 3 days after onset of symptoms in the index patient. When contacts received a diagnosis of COVID-19 infection, they were more likely to present asymptomatically if they had been exposed to an asymptomatic patient.
Meaning
These results suggest that the quantity of exposure to a patient with COVID-19 may be associated with clinical presentation among close contacts who develop COVID-19.
Abstract
Importance
Much remains unknown about the transmission dynamics of COVID-19. How the severity of the index case and timing of exposure is associated with disease in close contacts of index patients with COVID-19 and clinical presentation in those developing disease is not well elucidated.
Objectives
To investigate the association between the timing of exposure and development of disease among close contacts of index patients with COVID-19 and to evaluate whether the severity of the index case is associated with clinical presentation in close contacts who develop COVID-19.
Design, Setting, and Participants
This study used a large, population-based cohort of 730 individuals (index patients) who received a diagnosis of COVID-19 in Zhejiang Province, China, from January 8 to July 30, 2020, along with a contact tracing surveillance program. Field workers visited 8852 close contacts of the index patients and evaluated them for COVID-19 through August 2020. A timeline was constructed to characterize different exposure periods between index patients and their contacts.
Main Outcomes and Measures
The primary outcome was the attack rate of COVID-19, defined as the total number of new COVID-19 cases diagnosed among contacts of index patients divided by the total number of exposed contacts. A secondary outcome was asymptomatic clinical presentation among infected contacts. Relative risks were calculated to investigate risk factors for COVID-19 among contacts and asymptomatic clinical presentation among infected contacts.
Results
Among 8852 close contacts (4679 male contacts [52.9%]; median age, 41 years [interquartile range, 28-54 years]) of 730 index patients (374 male patients [51.2%]; median age, 46 years [interquartile range, 36-56 years]), contacts were at highest risk of COVID-19 if they were exposed between 2 days before and 3 days after the index patient’s symptom onset, peaking at day 0 (adjusted relative risk [ARR], 1.3; 95% CI, 1.2-1.5). Compared with being exposed to an asymptomatic index patient, the risk of COVID-19 among contacts was higher when they were exposed to index patients with mild (ARR, 4.0; 95% CI, 1.8-9.1) and moderate (ARR, 4.3; 95% CI, 1.9-9.7) cases of COVID-19. As index case severity increased, infected contacts were less likely to be asymptomatic (exposed to patient with mild COVID-19: ARR, 0.3; 95% CI, 0.1-0.9; exposed to patient with moderate COVID-19: ARR, 0.3; 95% CI, 0.1-0.8).
Conclusions and Relevance
This cohort study found that individuals with COVID-19 were most infectious a few days before and after symptom onset. Infected contacts of asymptomatic index patients were less likely to present with COVID-19 symptoms, suggesting that quantity of exposure may be associated with clinical presentation in close contacts.
This cohort study evaluates the association between the timing of exposure to and severity of COVID-19 disease in close contacts of index patients with COVID-19.
Introduction
SARS-CoV-2 is a novel beta coronavirus originating in late 2019.1,2 By the end of 2020, hundreds of millions of individuals had acquired COVID-19 infection, leading to the disability and death of millions3 and a substantial indirect influence on other diseases.4 COVID-19 is characterized by high transmission rates, at times leading to large outbreaks, mostly in indoor congregate settings.5,6,7 However, many aspects of COVID-19 transmission remain to be fully understood,8,9 and there is uncertainty regarding the effectiveness of public health strategies that attempt to prevent COVID-19 transmission.8,9
Prompt diagnosis and quarantine is largely recommended by national and global health organizations to limit COVID-19 transmission. However, when individuals with COVID-19 are most infectious during their disease process requires further elucidation.8 The duration of viable SARS-CoV-2 RNA virus is short, as viral shedding often decreases quickly shortly after symptom onset.10,11,12 Few studies have reported COVID-19 rates among contacts based on the serial interval of exposure to the index patient. A recent study suggested that individuals with COVID-19 are most infectious a few days after symptom onset; however, this study had few total cases and low statistical power.13 Additional epidemiologic data regarding the transmission potential of COVID-19 in association with the timing of symptoms and diagnosis are needed.
To address knowledge gaps concerning the transmission dynamics of SARS-CoV-2, we performed a population-based, case-contact study to analyze the association between timing of exposure of individuals with COVID-19 to their social network and the subsequent development of COVID-19 among contacts. We also examined the association of exposure to asymptomatic COVID-19 infection with the attack rate of COVID-19 and the clinical presentation of infection in contacts.
Methods
Study Design and Participants
This was a cohort study of contacts of COVID-19 index patients. In brief, we identified newly diagnosed patients with COVID-19 from the provincial Center for Disease Control and Prevention in Zhejiang Province, China (Zhejiang CDC), from January through August 2020. Index patients were defined as the first eligible patient with a diagnosed case of COVID-19 with 1 or more contacts. All index patients’ cases of COVID-19 were microbiologically confirmed through positive reverse transcriptase–polymerase chain reaction (RT-PCR) test results. The research protocol was approved by the institutional review board at the Zhejiang Provincial Center for Disease Control and Prevention, which waived the need for consent because all data were deidentified. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The first wave of the COVID-19 epidemic in Zhejiang Province began in early January 2020 and continued until late February 2020, after which sporadic cases were observed. We conducted contact tracing of all index patients with microbiologically confirmed symptomatic and asymptomatic cases of COVID-19 and successfully traced most contacts whose symptom onset dates were between January 8 and July 30, 2020. Most index patients were able to recall their recent contacts to Zhejiang CDC officials, after which those individuals were contacted. Contacts were quarantined for at least 14 days and received clinical and epidemiologic investigation. Individual-level data were collected from both contacts and index patients (eAppendix 1 and eAppendix 2 in the Supplement).
Procedures and Definitions
We analyzed confirmed COVID-19 cases identified by the Zhejiang CDC between January and August 2020, and close contacts of index patients prior to August 22, 2020. Surveys were administered to both contacts and index patients regarding demographic, clinical, and exposure-related characteristics by county-level Zhejiang CDC health officials. Information on employment and the workplace was also collected. A timeline was constructed for symptom onset and exposure times between index patients and their contacts.
COVID-19 cases were defined according to China’s guidelines for diagnosis and management of COVID-19 (eAppendix 1 in the Supplement). Six editions of these guidelines were released during the study period. Patients with laboratory-confirmed COVID-19 were individuals with positive detection of SARS-CoV-2 nucleic acid by real-time RT-PCR using respiratory specimens. Because of mass screening and contact tracing, this definition also encompassed asymptomatic COVID-19 infection. COVID-19 cases were classified as either asymptomatic, mild, moderate, severe, or critically ill (eAppendix 2 in the Supplement). All patients with confirmed cases of COVID-19 and close contacts were isolated or quarantined after being identified through contact tracing. Trained health professionals investigated each confirmed case with a predefined questionnaire through which basic health and demographic information was collected. We defined timing of exposure by the first day and duration of the social contact between index patients and their contacts.
During the isolation and quarantine period, index patients and their contacts received regular testing and daily symptom screening for fever, cough, and shortness of breath. If an index patient or contact had a positive test result but had no symptoms, they would be temporarily classified as asymptomatic or presymptomatic. All patients were followed up for at least 90 days after their initial positive test result to distinguish between asymptomatic and presymptomatic illness. Among these patients, those who developed symptoms later received a final classification as symptomatic. Others who never developed symptoms between their initial positive test result and first subsequent negative RT-PCR test result were classified as asymptomatic.
A household contact was defined as an individual in the same household or an individual who dined together with the index patient. A close nonhousehold contact was defined as an individual exposed (within 1 meter) to an index patient with microbiologically confirmed COVID-19 and included coworkers, hospital settings, or shared vehicle transportation. Index patients were assigned by the contact tracing investigation, but the classification of index and contact was further examined by comparing the date with the earliest symptom onset from symptomatic disease. The nature and setting of index patient–contact exposure included conversation, dining together, being in an enclosed space without direct contact, a health care setting, living together, or shared transportation.
Statistical Analysis
Close contacts were included in this analysis if they had at least 1 positive or negative RT-PCR test result. We summarized continuous variables as median values with interquartile ranges (IQRs) and categorical variables using proportions.
There were 2 outcomes in our analysis. Our primary outcome was the attack rate of COVID-19, defined as the total number of new COVID-19 cases diagnosed among contacts of index patients divided by the total number of exposed contacts.14 The attack rate was estimated among all contacts and then estimated separately for each included close contact, index patient, and exposure-related characteristic using standard 2 × 2 contingency tables. A secondary outcome of our analysis was asymptomatic clinical presentation among infected contacts.
We used a modified mixed-effects Poisson regression with robust SE variance to conduct all analyses. This model has a logarithmic link function, can take into account clustering of contacts, and allows for direct estimation of relative risks (RRs) in observational studies.15,16 P values and 95% CIs were used to assess statistical significance in all models. All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Analytical Plan for Outcome 1: Development of COVID-19
We presented various multivariable models assessing the association between exposure time and index symptom onset with the development of COVID-19 after adjustment for potential confounding variables. We reported estimations of the timing of exposure through distributed lag nonlinear models.17,18 Distributed lag nonlinear models allow for both exposure-response functions and lag-response functions, and are widely used in environmental studies to compare the risk of exposures prior to the outcome.19,20
We restricted the study population to contacts exposed to symptomatic patients, as the start of index patient–contact exposure to asymptomatic index patients is unclear. We constructed the timing of index patient–contact exposure using index patient symptom onset and the reported exposure period (confirmed by both index patients and contacts). We included only contacts whose exposure occurred between 14 days prior to and 10 days after the index patient’s symptom onset date21,22,23 and compared the risk of COVID-19 at different exposure days. The last day of exposure time was assigned as the last reported day of exposure by the index patient and contact. We compared timing of exposure between index patients and their contacts by single-day bins. The risk of COVID-19 was estimated for each single-day bin (from 14 days before index patient symptom onset to 10 days after index patient symptom onset). The RR was estimated by the exposure-lag-response association in the distributed lag nonlinear models. It was defined on a grid of values of the binary variable of exposure and a continuous variable of lag time (days).17 For example, if a contact was first exposed on the day of symptom onset of their index patient (day 0), the RR is derived by comparing the estimated risk of COVID-19 without exposure at the same day but with exposure at different time points. We restricted our analysis to 10 days after symptom onset owing to low statistical power after more than 10 days. We also compared COVID-19 in contacts based on when the first exposure occurred compared with symptom onset as well as the duration of index patient–contact exposure. We used a constrained distributed lag function of index patients’ exposure to estimate the lag day–specific RR of index patient contact from 14 days before and 10 days after index patient symptom onset date.17,18 To explore the lag day association of index patient exposure, we used a natural cubic B spline on the lag scale and used the Akaike information criterion to guide the selection of knots. More details are provided in eAppendix 3 in the Supplement. We conducted a sensitivity analysis stratifying on exposure time and index symptom onset by the exposure setting (household and nonhousehold contacts) between close contacts and index patients.
Analytical Plan for Outcome 2: Asymptomatic Clinical Presentation Among Infected Contacts
A secondary outcome was asymptomatic clinical presentation among infected contacts. This outcome was restricted only to contacts infected with COVID-19. Other clinical presentations among these contacts were grouped together. We compared the risk of asymptomatic clinical presentation in infected contacts stratified by severity of the index patient’s COVID-19 case. We then evaluated whether asymptomatic clinical presentation was associated with clinical index patient severity in a multivariable model adjusting for potential confounders.
All analyses were performed using Stata, version 14.1 (StataCorp) and R, version 4.0.3 (R Foundation for Statistical Computing). The R packages dlnm and lme4 were used for distributed lag nonlinear models and mixed-effects modeling.
Results
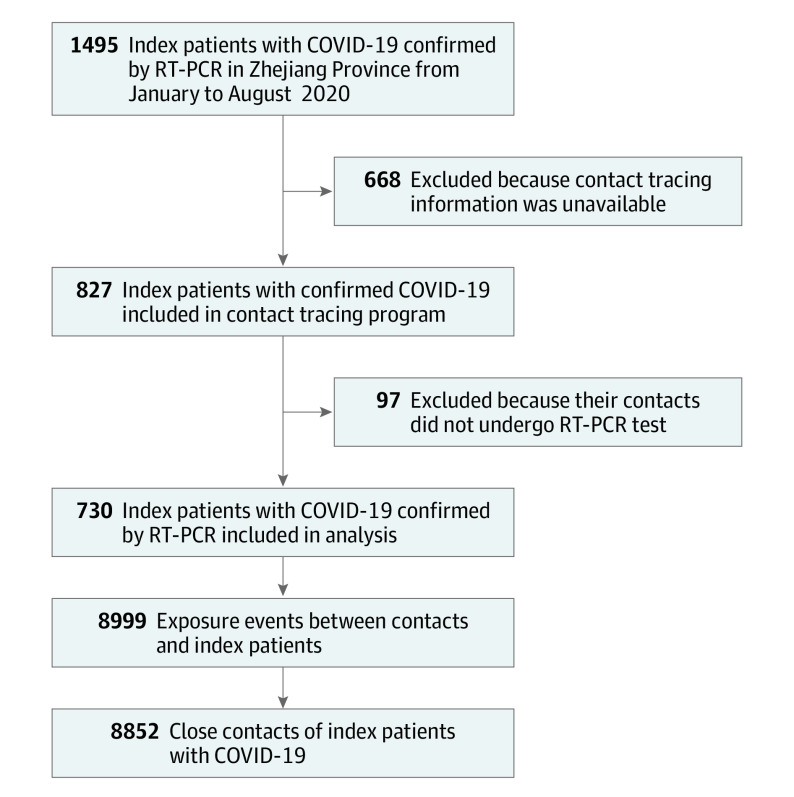
In total, 1495 patients were diagnosed with COVID-19 in Zhejiang province during the study period, of whom 827 patients had complete contact tracing information. Of these, 730 index patients had at least 1 traced close contact with a confirmed RT-PCR test performed, including 8999 contact events and 8852 close contacts (Figure 1). Index patients included 356 women (48.8%) and 374 men (51.2%), with a median age of 46 years (IQR, 36-56 years). Close contacts included 4173 women (47.1%) and 4679 men (52.9%) with a median age of 41 years (IQR, 28-54 years). The characteristics of excluded and included contacts were largely similar; however, excluded participants were more likely to be older (median age, 43 years [IQR, 30-54 years] vs 41 years [IQR, 28-54 years]; P < .001) and be exposed in a household setting (compared with nonhousehold, 1846 of 6004 [30.7%] vs 2484 of 8852 [28.1%]; P < .001) (eTable 3 and eTable 4 in the Supplement). The median duration from index symptom onset to isolation was 5 days (IQR, 2-8 days), while the median duration from first to last exposure between index patients and contacts was 3 days (IQR, 0-7 days) (eFigure 1 in the Supplement).
Figure 1. Study Flow Diagram of Eligibility and Inclusion of COVID-19 Index Patients and Their Social Network in Zhejiang Province, China.
The sample population and restriction of inclusion of participants into the study population are described in further detail in eTable 1 and eTable 2 in the Supplement. The number of exposure events does not equal the number of close contacts because some contacts may have been exposed to multiple index patients. RT-PCR indicates reverse transcriptase–polymerase chain reaction.
The most common contact types were conversational (2687 of 8999 [29.9%]), living in the same household (1499 of 8999 [16.7%]), and enclosed space without direct contact (1408 of 8999 [15.6%]) (Table 1). Most COVID-19 cases among index patients were either mild (336 [46.0%]) or moderate (313 [42.9%]), while only 81 index patients (11.1%) were asymptomatic.
Table 1. Demographic Characteristics of Index Patients and Close Contacts.
| Characteristics | No. (%) | |
|---|---|---|
| Index patients (n = 730) | Close contacts (n = 8852)a | |
| Age, median (IQR), y | 46 (36-56) | 41 (28-54) |
| Age group, y | ||
| <20 | 22 (3.0) | 1101 (12.4) |
| 20-29 | 67 (9.2) | 1337 (15.1) |
| 30-39 | 153 (21.0) | 1800 (20.3) |
| 40-49 | 174 (23.8) | 1718 (19.4) |
| 50-59 | 176 (24.1) | 1445 (16.3) |
| 60-69 | 90 (12.3) | 851 (9.6) |
| ≥70 | 48 (6.6) | 600 (6.8) |
| Sex | ||
| Female | 356 (48.8) | 4173 (47.1) |
| Male | 374 (51.2) | 4679 (52.9) |
| Severity of COVID-19 in index patient | ||
| Asymptomatic | 81 (11.1) | 1098 (12.2) |
| Mild | 336 (46.0) | 4867 (54.1) |
| Moderate | 313 (42.9) | 3034 (33.7) |
| Primary contact type | ||
| Conversation | NA | 2687 (29.9) |
| Dine together | NA | 1066 (11.8) |
| Enclosed space without direct contact | NA | 1408 (15.6) |
| Health care setting | NA | 459 (5.1) |
| Live together | NA | 1499 (16.7) |
| Multiple | NA | 44 (0.5) |
| Shared transportation | NA | 1055 (11.7) |
| Others | NA | 781 (8.7) |
| Source of index patient | ||
| Importationb | 42 (5.8) | 439 (4.9) |
| Local | 688 (94.2) | 8560 (95.1) |
Abbreviation: NA, not applicable.
For contact characteristics, the statistics in this column are assigned to contacts. For index case characteristics (ie, severity of COVID-19 in index patient, primary contact type, source of index patient), the statistics in this column represent the number of contacts exposed to that type of index patient. For example, 1098 contacts were exposed to an asymptomatic index patient. Also, 8560 contacts were exposed to a local index patient. For these characteristics there were 8999 contact events among 8852 total contacts, as some contacts were exposed to multiple index patients. Therefore, for index patient characteristics (eg, severity of COVID-19 in index patient) assigned to contacts there is a sum of 8999 rather than 8852, which is the sum for contact characteristics in this column (eg, sex).
Not infected in Zhejiang Province.
Of 8852 screened close contacts, 327 (3.6%; 95% CI, 3.3%-4.0%) received a diagnosis of COVID-19. Most index patients’ close contacts had no cases of COVID-19. The median number of COVID-19 cases among contacts per index was 0.4 (IQR, 0-1). Of these, 31 cases (9.5%) were critical, while most cases manifested as either mild (98 [30.0%]) or moderate (137 [41.9%]). A total of 61 contacts with a diagnosis of COVID-19 (18.7%) were asymptomatic.
Contacts exposed to index patients had a higher COVID-19 attack rate if their exposure time was a few days before or after symptom onset in the index patient (Figure 2). In a mixed-effects model adjusted for age, sex, duration of exposure, and the index patient–contact exposure setting, contacts were at higher risk of COVID-19 if they were exposed between days −2 and 3 (Figure 2) and peaked at day 0 (adjusted relative risk [ARR], 1.3; 95% CI, 1.2-1.5). A lower risk was seen among contacts exposed on days −6 (ARR, 0.8; 95% CI, 0.6-1.0) and −5 (ARR, 0.8; 95% CI, 0.6-1.0) (Figure 2 and eTable 5 in the Supplement). The risk of COVID-19 was nonstatistically higher among contacts exposed on other days.
Figure 2. Adjusted Relative Risk of Development of COVID-19 in Close Contacts of Index Patients by the Timing of Exposure and the Distribution of Contact Events.
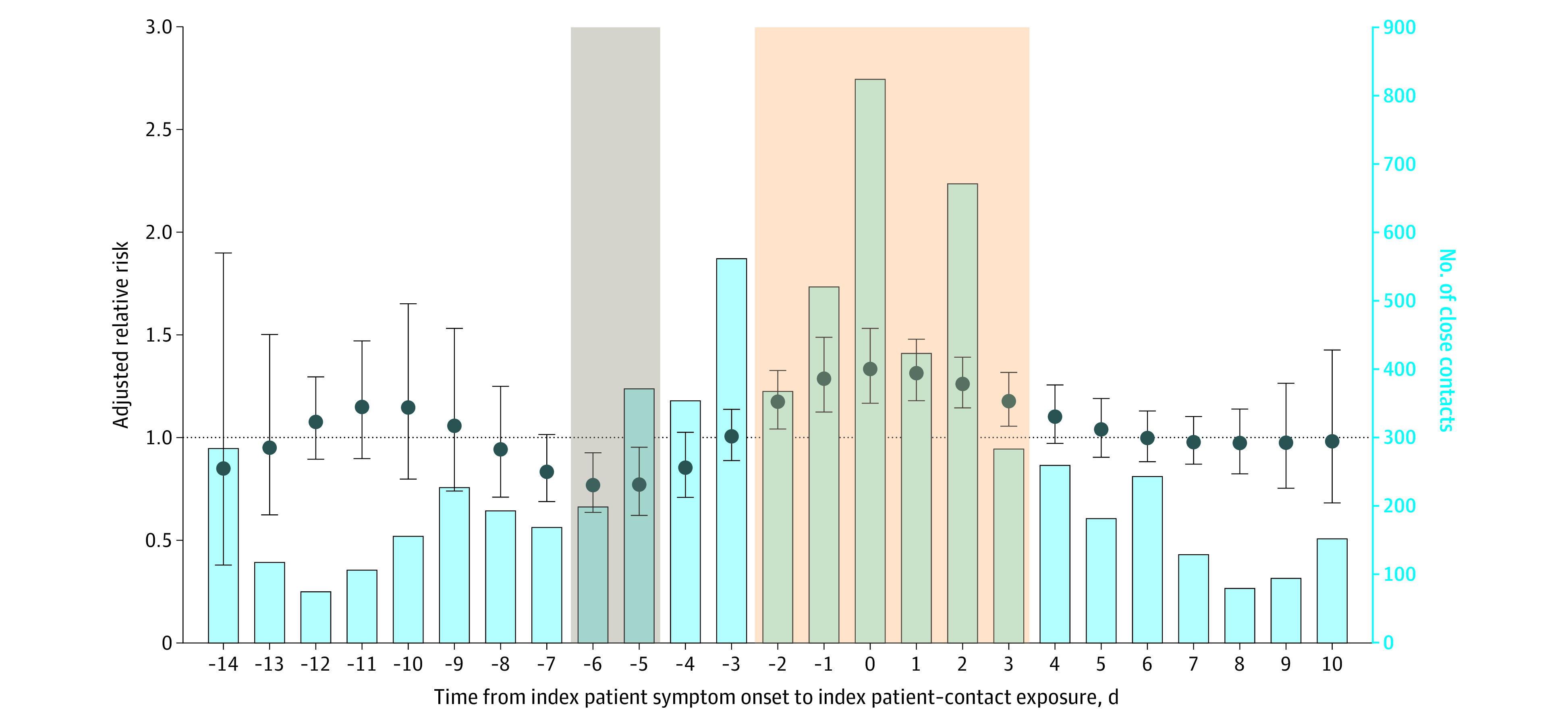
The x-axis represents the length of time between index patient–contact exposure and the index patient’s symptom onset time. For example, 0 signifies that the index patient’s symptom onset was the first day of index patient–contact exposure. Negative and positive days represent the days before and after exposure occurred relative to the date of index symptom onset. The left y-axis and dots represent the adjusted relative risk of COVID-19 transmission in contacts. The error bars indicate 95% CIs. The horizontal dotted line at 1.0 is a reference for the left axis. Adjusted relative risks for each day were estimated through a distributed lag nonlinear, multivariable mixed-effects model with adjustment for age and sex of the contact and index patient, the exposure setting, and contact exposure duration. A random intercept was included for overlapping index patients. Further explanation of this model is described in the Methods section. The adjusted relative risks were estimated by comparing COVID-19 infection risk at different exposure points. The right y-axis and bars represent the number of close contacts exposed on each day. Contacts exposed in the tan highlighted area had significantly higher relative risk, while those exposed in the gray highlighted area had significantly lower relative risks (6 days before to 5 days before; the risk may still be higher compared with those with no exposure to patients with COVID-19). The reference group and model approach were distinct from eFigures 3, 4, and 5 in the Supplement; therefore, relative risks from this figure may not be directly comparable to those in eFigures 3, 4, and 5.
We then grouped contacts into multiday bins (before −3 days; days −3 to −1; days 0 to 2; days 3 and 5; and days 6 and 10) to assess whether certain time periods were associated with higher risk. In a multivariable, mixed-effect model adjusted for age, sex, duration of contact, and setting of the contact, contacts were at higher risk of COVID-19 if they were exposed between day −3 and −1 from their index patient’s symptom onset (ARR, 3.4; 95% CI, 1.9-5.8) or day 0 and 2 days after their index patient’s symptom onset (ARR, 2.8; 95% CI, 1.5-5.0) (eFigure 3 in the Supplement). The risk of COVID-19 was nonstatistically higher among contacts exposed between days 3 and 5 (ARR, 2.0; 95% CI, 0.8-4.7) or days 6 and 10 (ARR, 1.8; 95% CI, 0.7-4.7) after their index patient’s symptom onset. When we stratified this analysis by whether the index patient–contact exposure was from household or nonhousehold settings, we found that the increased risk of COVID-19 among contacts exposed between days −3 and 2 from index symptom onset was consistent in both contact groups but clearer in household contacts. Among nonhousehold contacts there was a higher risk in this time range but low statistical power.
Contacts were at increased risk of disease if they had a longer duration of exposure (eFigure 6 in the Supplement). Relative risks were elevated when exposure was longer; this elevation was accentuated when exposure occurred between −2 and 3 days from the index patient’s symptom onset. For example, if a contact event occurred at day −2 and exposure lasted for 13 days, the ARR of COVID-19 among contacts was 4.7 (95% CI, 1.9-11.4) (eFigure 6 in the Supplement). At days −6 and −5 from index symptom onset, the duration of exposure was associated with risk of COVID-19; at short exposure duration, the risk was statistically lower (ARRs between 0.4 and 0.8), but individuals exposed for long durations had an increased risk of COVID-19 (eFigure 6 in the Supplement).
COVID-19 transmission was consistently associated with the severity of the index patient’s case of COVID-19 (Table 2). Attack rates were highest among household members of index patients (260 of 2565 [10.1%; 95% CI, 9.0%-11.4%]) and contacts exposed in multiple settings to the same index patient (3 of 44 [6.8%; 95% CI, 1.4%-18.7%]). In a multivariable model, household members had an ARR of 8.1 (95% CI, 5.9-11.4), and contacts exposed in multiple settings to the same index patient had an ARR of 6.0 (95% CI, 1.7-21.0) for risk of COVID-19 compared with an “Other” category that included shared transportation, enclosed space without direct contact, and conversation. Contacts exposed in a health care setting had a lower, but not statistically significant, risk of COVID-19 (ARR, 0.4; 95% CI, 0.1-1.7). Compared with contacts exposed to 1 index patient with COVID-19, contacts were at higher risk if exposed to 2 (ARR, 1.8; 95% CI, 1.1-3.2) and 3 (ARR, 10.2; 95% CI, 1.4-72.6) index patients with COVID-19.
Table 2. Index Patient, Contact, and Exposure-Related Risk Factors for COVID-19 Among Close Contacts of Index Patients With COVID-19.
| Variable | No. contacts who developed COVID-19/No. total contacts | Attack rate (95% CI)a | Adjusted risk ratio (95% CI) | P value |
|---|---|---|---|---|
| Contact age, per 10-y | NA | NA | 1.1 (1.1-1.2) | <.001 |
| Contact sex | ||||
| Female | 160/4254 | 3.5 (3.0-4.1) | 1 [Reference] | NA |
| Male | 167/4747 | 3.8 (3.2-4.3) | 1.0 (0.8-1.2) | .77 |
| Index patient sex | ||||
| Female | 154/4398 | 3.5 (2.8-3.9) | 1 [Reference] | NA |
| Male | 173/4609 | 3.8 (3.2-4.3) | 1.0 (0.7-1.5) | .88 |
| Severity of COVID-19 in index patient | ||||
| Asymptomatic | 11/1098 | 1.0 (0.5-1.8) | 1 [Reference] | NA |
| Mild | 180/4867 | 3.7 (3.2-4.3) | 4.0 (1.8-9.1) | .001 |
| Moderate | 136/3034 | 4.5 (3.8-5.3) | 4.3 (1.9-9.7) | <.001 |
| Primary contact typeb | ||||
| Other | 62/5931 | 1.0 (0.8-1.3) | 1 [Reference] | NA |
| Health care setting | 2/457 | 0.4 (0.1-1.6) | 0.4 (0.1-1.7) | .22 |
| Household member | 260/2565 | 10.1 (9.0-11.4) | 8.1 (5.9-11.4) | <.001 |
| Multiple settings | 3/44 | 6.8 (1.4-18.7) | 6.0 (1.7-21.0) | .005 |
Abbreviation: NA, not applicable.
We calculated 95% binomial exact CIs around these estimates.
We grouped exposure settings into an “Other” category that includes shared transportation, enclosed space without direct contact, conversation, and Others from Table 1.
Transmission from asymptomatic index patients was associated with both transmission and clinical presentation among infected contacts (Figure 3). Compared with moderate and mild index cases, asymptomatic index patients were the least likely to transmit to contacts (attack rate, 1.0% [11 of 1098]; 95% CI, 0.5%-1.8%) (Table 2). In a multivariable model adjusting for age and sex of case and contact, as well as exposure setting, the risk of COVID-19 in contacts was much higher when exposed to index patients with mild (ARR, 4.0; 95% CI, 1.8-9.1) and moderate (ARR, 4.3; 95% CI, 1.9-9.7) cases. However, infected contacts were most likely to manifest without symptoms when exposed to an asymptomatic index patient. Compared with exposure to an asymptomatic index patient, the risk of asymptomatic infection in contacts was lower among contacts exposed to mild (ARR, 0.3; 95% CI, 0.1-0.9) and moderate (ARR, 0.3; 95% CI, 0.1-0.8) index cases.
Figure 3. Adjusted Relative Risk of COVID-19 in Close Contacts and Asymptomatic Clinical Presentation in Infected Contacts, Stratified by the Severity of the Index Case.
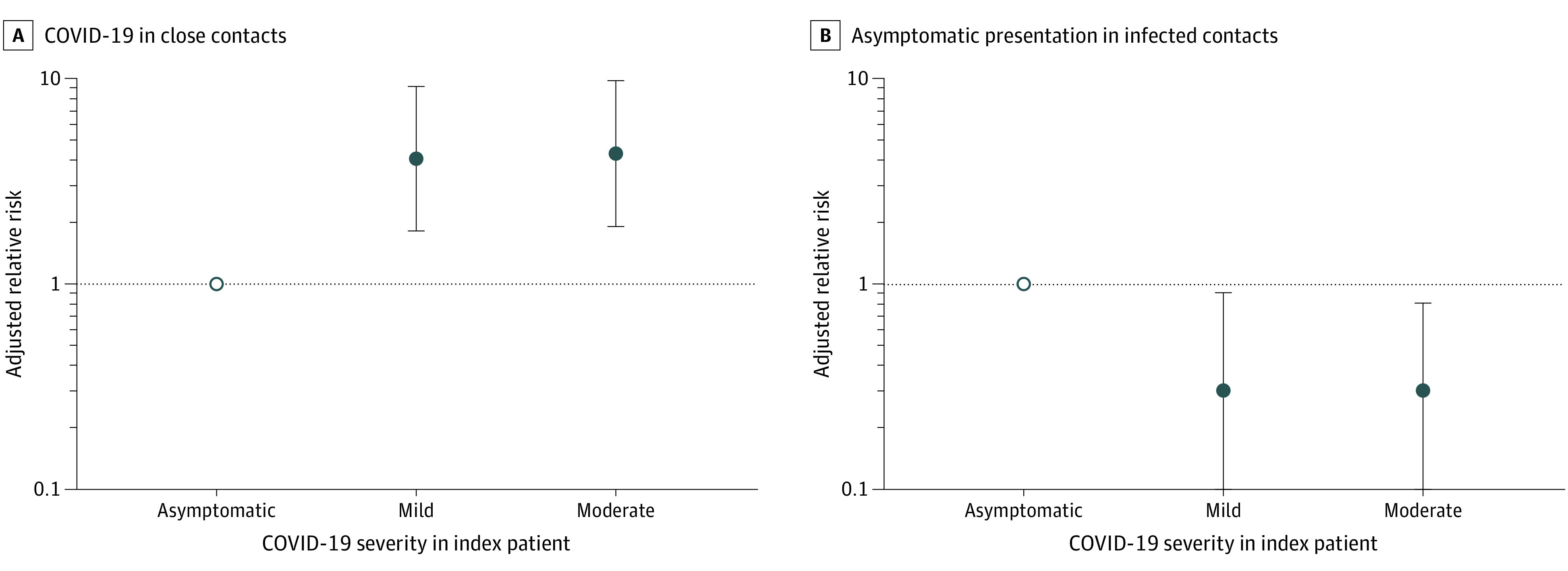
A, COVID-19 in close contacts. B, Asymptomatic clinical presentation in infected contacts. Two distinct multivariable models were built. In the first model of the outcome of COVID-19 in close contacts, all 8852 contacts were included; in the second model of the outcome of asymptomatic clinical presentation in contacts with disease, only the 327 contacts who developed COVID-19 were included. The outcome was asymptomatic disease. Other clinical presentations were grouped together. Both models were adjusted for age and sex of index patients and contacts and for exposure setting. COVID-19 cases (both in index patients and contacts who were diagnosed) were classified as either asymptomatic, mild, moderate, severe, or critically ill. The empty circles are reference groups (corresponding to the dotted lines). The dots and error bars indicate relative risks and 95% CIs. The y-axis is on a log scale. Criteria for COVID-19 case definitions and disease severity are provided in eAppendix 2 in the Supplement.
Discussion
In this population-based, index patient–contact cohort study, we found that the risk of COVID-19 transmission to close contacts was higher if exposure occurred between −2 and 3 days from symptom onset in index patients. Furthermore, the COVID-19 attack rate increased based on the presence or absence of symptoms in the index patient. Among contacts developing COVID-19, asymptomatic infection was more common if they were exposed to an asymptomatic index patient, suggesting that the severity of the COVID-19 case in the index patient may be associated with the clinical presentation of disease.
Our study suggests that transmission of COVID-19 is most likely if contacts are exposed shortly before and after symptom onset in the index patient. This association was consistent regardless of adjustment for additional transmission risk factors, such as the duration of exposure, age and sex of the contact, and exposure setting. We used 2 distinct models to identify high-risk COVID-19 time windows. Although the reference group and approaches were different in each model, both identified similar results supporting the robustness of our conclusions. A study in Taiwan found high COVID-19 attack rates among contacts exposed less than 3 days after symptom onset in index patients and suggested that the period close to symptom onset in index patients is important for transmission.13 However, that study had limited statistical power and included only 22 total cases of COVID-19 among contacts, limiting their ability to comprehensively investigate the association of timing of exposure with COVID-19 risk to a close contact. Our large population-based sample allowed us to examine timing of exposure at each individual day while also examining presymptomatic periods.
These results have important implications for understanding transmission dynamics of COVID-19 and are consistent with recent results suggesting that viral load may peak at 2 days before symptom onset12,13 and decline quickly after 1 week of symptoms.10,11 Compared with contacts exposed −14 to −4 days from symptom onset in index patients, the risk of COVID-19 was nonsignificantly higher among contacts exposed 4 to 10 days after symptom onset in index patients. Our results may be biased if contacts with more intense exposure, such as household contacts, are more likely to be exposed to their index patient around the time of symptom onset. However, when conducting a sensitivity analysis restricting our study population to household and nonhousehold contacts, we found roughly consistent results. We also found a relatively reduced risk time window around days −5 and −6. The reasoning for this reduced risk is not clear, and future studies, including contact investigations and viral shedding studies, are needed to further understand this period before the onset of COVID-19 symptoms among persons who develop COVID-19.
We found that contacts exposed to asymptomatic index patients were less likely to develop COVID-19, and, given infection, were more likely to be asymptomatic. This result suggests that there may be a dose-response association between severity of the index patient’s case of COVID-19 and clinical presentation among contacts. If confirmed in other studies, this result may suggest additional secondary benefits associated with reducing case severity of individuals with COVID-19 through vaccination or prompt diagnosis and treatment.24 Recently, the hypothesis that mask use may reduce disease severity by decreasing the magnitude of exposure has been postulated25; although our study results cannot be extended to understand the implications of mask use (because we did not have detailed information on mask or personal protective equipment use), these results suggest this should be further investigated empirically. We found that health care workers were at a nonstatistically lower risk of COVID-19, which may be owing to mask use or policy regarding testing.26 Unfortunately, we were unable to further stratify by other measurements of exposure because of low statistical power. Alternatively, strain congruencies between close contacts and their index patients may explain our findings if particular COVID-19 strains are more likely to cause severe disease than others. Further studies are needed to explore underlying mechanisms for this association.
Limitations
There are limitations to our analysis. First, reporting bias may be present if contacts and/or index patients did not accurately recall their symptom onset. However, results were verified by both index patients and contacts. Family-related contact events may be more reliable to recall because these contacts had a closer level of exposure and the relationships are more likely to be permanent. However, in the sensitivity analysis stratifying the model by whether exposure occurred inside or outside the household, the conclusion of a high-risk time window was consistent (eFigure 4 and eFigure 5 in the Supplement). Second, directionality of transmission was identified by index patients’ recall and then determined based on the chronological order of symptom onset time. Although this method is widely used,7,13,27,28,29 there is potential for misclassification if an index patient had an unusually long incubation period compared with contacts. Third, not all contacts were traced and screened with RT-PCR testing (eFigure 2 in the Supplement). Because of this, contacts with asymptomatic disease may have been missed. However, our study covers a large proportion of all index patients diagnosed in Zhejiang province, and characteristics of included and excluded contacts were largely similar, indicating minimal selection bias. Our study may not accurately represent transmission dynamics in communities exposed to novel SARS-CoV-2 variants. Fourth, nonpharmaceutical interventions were widely conducted in Zhejiang Province early in 2020, reducing transmission risk. Therefore, caution is needed when applying our results to other settings with limited health interventions.
Conclusions
Among 8852 close contacts and 730 index patients from Zhejiang Province, China, we found that COVID-19 transmission was especially common among close contacts exposed 2 to 3 days before or after onset of symptoms among the index patients. Infected contacts exposed to an individual with asymptomatic COVID-19 were more likely to also clinically present without symptoms.
eAppendix 1. Additional Methodological Information
eAppendix 2. Definition of COVID-19 Severity
eAppendix 3. Distributed Lag Non-Linear Models (DLNMs)
eReferences.
eTable 1. Sample Size of Each Flow Step for the Larger Sample Analysis of Risk Factor Analyses of Asymptomatic Disease and Intensity of Exposure
eTable 2. Sample Size of Each Flow Step for the Analysis of Exposure Time
eTable 3. Comparison of Basic Demographics Between Included and Excluded Index Cases
eTable 4. Comparison of Basic Demographics Between Included and Excluded Contacts
eTable 5. Adjusted Relative Risks Shown in Figure 2
eFigure 1. Contact Events Description (PCR Positive Counts/Total Number of Contacts)
eFigure 2. Proportion of Contacts Received PCR Tested for COVID-19
eFigure 3. Relative Risk of Covid-19 Infection Among Contacts Between Different Exposure Time
eFigure 4. Relative Risk of Covid-19 Infection Among Household Contacts Between Different Exposure Time
eFigure 5. Relative Risk of Covid-19 Infection Among Non-Household Contacts Between Different Exposure Time
eFigure 6. The Risk of Covid-19 in Close Contacts of Index Cases by the Duration and Timing of Exposure
References
- 1.Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Weekly epidemiological update—5 January 2021. Accessed July 1, 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update---5-january-2021
- 4.Liu Q, Lu P, Shen Y, et al. Collateral impact of the coronavirus disease 2019 (COVID-19) pandemic on tuberculosis control in Jiangsu Province, China. Clin Infect Dis. Published online August 28, 2020. doi: 10.1093/cid/ciaa1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawks L, Woolhandler S, McCormick D. COVID-19 in prisons and jails in the United States. JAMA Intern Med. 2020;180(8):1041-1042. doi: 10.1001/jamainternmed.2020.1856 [DOI] [PubMed] [Google Scholar]
- 6.Shen Y, Li C, Dong H, et al. Community outbreak investigation of SARS-CoV-2 transmission among bus riders in eastern China. JAMA Intern Med. 2020;180(12):1665-1671. doi: 10.1001/jamainternmed.2020.5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung HF, Martinez L, Alarid-Escudero F, et al. The household secondary attack rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a rapid review. Clini Infect Dis. Published online October 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:m3862. doi: 10.1136/bmj.m3862 [DOI] [PubMed] [Google Scholar]
- 9.Cevik M, Marcus JL, Buckee C, Smith TC. SARS-CoV-2 transmission dynamics should inform policy. Clin Infect Dis. 2020;ciaa1442. doi: 10.1093/cid/ciaa1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13-e22. doi: 10.1016/S2666-5247(20)30172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672-675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 12.To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565-574. doi: 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng HY, Jian SW, et al; Taiwan COVID-19 Outbreak Investigation Team . Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180(9):1156-1163. doi: 10.1001/jamainternmed.2020.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dicker RC, Coronado F, Koo D, Parrish RG. Principles of Epidemiology in Public Health Practice; an Introduction to Applied Epidemiology and Biostatistics. Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 15.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 16.Zocchetti C, Consonni D, Bertazzi PA. Relationship between prevalence rate ratios and odds ratios in cross-sectional studies. Int J Epidemiol. 1997;26(1):220-223. doi: 10.1093/ije/26.1.220 [DOI] [PubMed] [Google Scholar]
- 17.Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw. 2011;43(8):1-20. doi: 10.18637/jss.v043.i08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Stat Med. 2010;29(21):2224-2234. doi: 10.1002/sim.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasparrini A, Guo Y, Hashizume M, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015;386(9991):369-375. doi: 10.1016/S0140-6736(14)62114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasparrini A, Guo Y, Sera F, et al. Projections of temperature-related excess mortality under climate change scenarios. Lancet Planet Health. 2017;1(9):e360-e367. doi: 10.1016/S2542-5196(17)30156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577-582. doi: 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Zhang X, Zhang X, et al. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: a prospective contact-tracing study. J Infect. 2020;80(6):e1-e13. doi: 10.1016/j.jinf.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan W-J, Ni Z-Y, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi M, Rutherford GW. Facial masking for COVID-19—potential for “variolation” as we await a vaccine. N Engl J Med. 2020;383(18):e101. doi: 10.1056/NEJMp2026913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin ET, Huynh BQ, Chapman LAC, Murrill M, Basu S, Lo NC. Frequency of routine testing for coronavirus disease 2019 (COVID-19) in high-risk healthcare environments to reduce outbreaks. Clin Infect Dis. 2020;ciaa1383. doi: 10.1093/cid/ciaa1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911-919. doi: 10.1016/S1473-3099(20)30287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke RM, Midgley CM, Dratch A, et al. Active monitoring of persons exposed to patients with confirmed COVID-19—United States, January-February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(9):245-246. doi: 10.15585/mmwr.mm6909e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun K, Wang W, Gao L, et al. Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science. 2021;371(6526):eabe2424. doi: 10.1126/science.abe2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Additional Methodological Information
eAppendix 2. Definition of COVID-19 Severity
eAppendix 3. Distributed Lag Non-Linear Models (DLNMs)
eReferences.
eTable 1. Sample Size of Each Flow Step for the Larger Sample Analysis of Risk Factor Analyses of Asymptomatic Disease and Intensity of Exposure
eTable 2. Sample Size of Each Flow Step for the Analysis of Exposure Time
eTable 3. Comparison of Basic Demographics Between Included and Excluded Index Cases
eTable 4. Comparison of Basic Demographics Between Included and Excluded Contacts
eTable 5. Adjusted Relative Risks Shown in Figure 2
eFigure 1. Contact Events Description (PCR Positive Counts/Total Number of Contacts)
eFigure 2. Proportion of Contacts Received PCR Tested for COVID-19
eFigure 3. Relative Risk of Covid-19 Infection Among Contacts Between Different Exposure Time
eFigure 4. Relative Risk of Covid-19 Infection Among Household Contacts Between Different Exposure Time
eFigure 5. Relative Risk of Covid-19 Infection Among Non-Household Contacts Between Different Exposure Time
eFigure 6. The Risk of Covid-19 in Close Contacts of Index Cases by the Duration and Timing of Exposure