Abstract
Mechanotransduction, a conversion of mechanical forces into biochemical signals, is essential for human development and physiology. It is observable at all levels ranging from the whole body, organs, tissues, organelles down to molecules. Dysregulation results in various diseases such as muscular dystrophies, hypertension-induced vascular and cardiac hypertrophy, altered bone repair and cell deaths. Since mechanotransduction occurs at nanoscale, nanosciences and applied nanotechnology are powerful for studying molecular mechanisms and pathways of mechanotransduction. Atomic force microscopy, magnetic and optical tweezers are commonly used for force measurement and manipulation at the single molecular level. Force is also used to control cells, topographically and mechanically by specific types of nano materials for tissue engineering. Mechanotransduction research will become increasingly important as a sub-discipline under nanomedicine. Here we review nanotechnology approaches using force measurements and manipulations at the molecular and cellular levels during mechanotransduction, which has been increasingly play important role in the advancement of nanomedicine.
Keywords: nanomedicine, mechanotransduction, mechanical force, differentiation, tissue engineering
Introduction
Mechanotransduction is the process by which organisms perceive physical forces by producing biochemical signals in response. Such signals have been shown to control cell proliferation, migration, differentiation, and death[1–8]. The significant influence of mechanotransduction on cells suggests potential clinical importance in modulating, altering, and controlling the process.
The recent discovery of the underlying molecular mechanisms of mechanotransduction advances the study closer to the point of clinical importance. Although mechanotransduction has been discovered for a long time and extensively studied at the cellular and tissue levels[9], it is just recently that its mechanism has been revealed at the single molecular level[4]. Although studies at cellular and tissue levels are still necessary to understand mechanotransduction, research on the process will increasingly apply nanotechnology. Clinical research into the applications of nanotechnology in mechanotransduction is becoming a sub-set of nanomedicine.
One of nanomedicine uses nanoparticles (NPs) for site-specific delivery of drugs and diagnostic agents. Some NPs show promise in helping to reveal the workings of mechanotransduction for tissue engineering, drug delivery, diagnostics, and other medical advancements.
Mechanotransduction
Internal and external mechanical forces to the human body such as shear forces in blood vessels, stretching, actomyosin contraction, gravity, acoustic vibration, and pressure can regulate cellular development and various processes[4,10–15] (Fig. 1). For example, exposure to the microgravity environment of space travel reduces bone and muscle formation, and changes immune response and metabolism[25]. Mechanical niches of stem and cancer cells also regulate their fates such as differentiation and proliferation[26–27]. During aging, rigidity of human epithelial cells markedly increases, which also affects differentiation of their progenitor cells[28–29]. In these biological processes, DNA sequencing identified many expressed genes induced by the signals produced via mechanotransduction in response to mechanical inputs[30–31]. However, what is missing in mechanotransduction research is a complete understanding of how forces are sensed, transmitted, and transduced into gene expression. Fig. 2 illustrates possible mechanotransduction pathways that have been partially revealed as described below. The major obstacle to reveal the molecular mechanism of mechanotransduction and its translation into gene expression is the difficulty in reconstitution of the reaction in vitro, where mechanical force is a difficult parameter to reproduce and disappears once biological samples are lysed. Despite the challenges, methods have been developed to unravel the molecular mechanisms of mechanotransduction.
Figure 1.
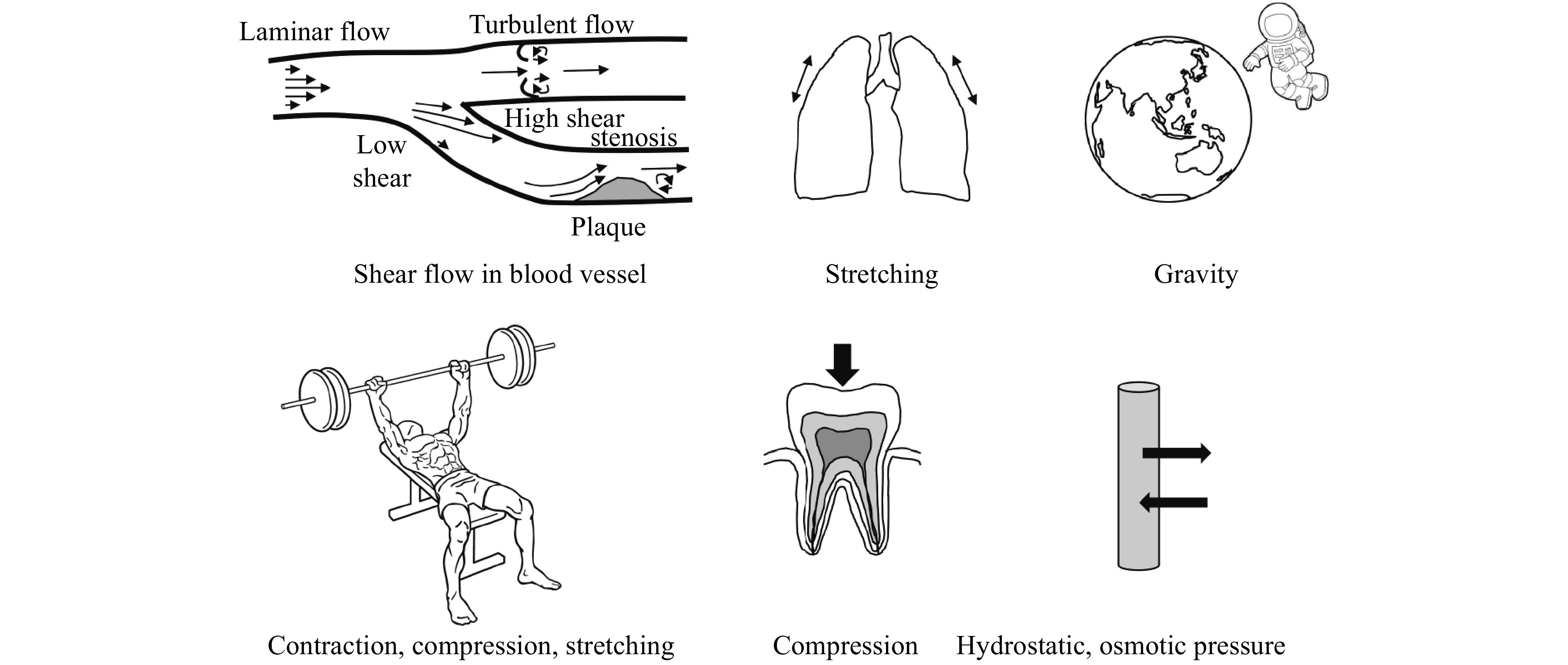
Mechanical forces influence human physiology and pathophysiology.
Shear flow in blood vessel influences physiology and pathophysiology of endothelial cells and blood cells (adapted from Nakamura et al[4]). Stretching of lung tissue regulates the synthesis of extracellular matrix[16–17]. Exposure to microgravity is associated with atrophy in heart, muscle, and bone, which is also frequented in aging[18–19]. Exercise-induced mechanical stimulus regulates gene expression for muscle fiber hypertrophy[20–21]. Application of mechanical stress on periodontal ligament fibroblasts induces gene expression to regulate the development, differentiation, and maintenance of periodontal tissues[22]. Hydrostatic or osmotic pressure promotes chondrogenesis of mesenchymal stem cells and the transition and differentiation of notochordal cells into nucleus pulposus cells in the intervertebral disc[23–24].
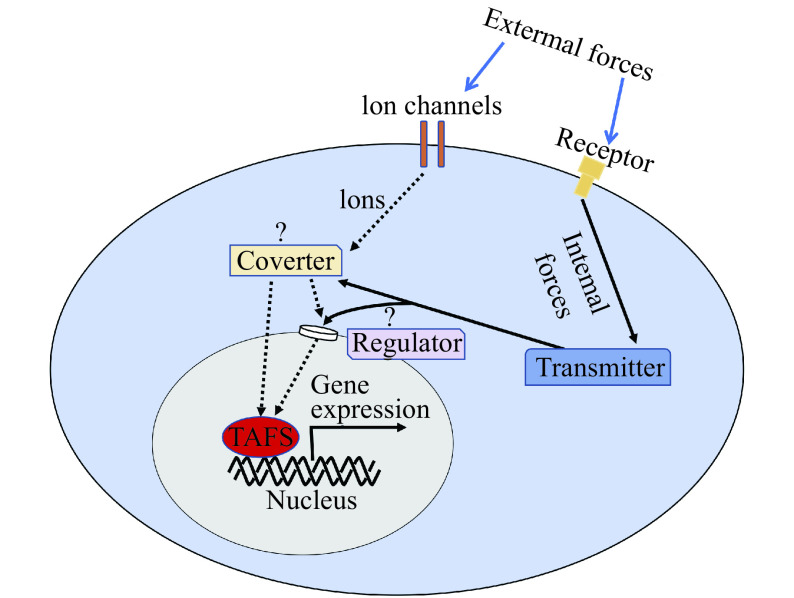
Figure 2.
A schematic overview of how mechanical forces are converted to gene expression in a cell.
Some illustrated pathways were experimentally demonstrated. For example, external forces such as touching and stretching are sensed by mechanosensitive ion channels (e.g., Piezo channels)[32]. Internal forces such as actomyosin contraction trigger mechanotransduction as well. Actin cytoskeleton mediates sensing and transmission of forces to regulate nuclear pore size, which controls localization of a trans-acting factor (red) such as Yes-associated protein 1 and megakaryoblastic leukemia 1[33–34]. Note that this diagram only depicts mechanotransduction pathways to gene expression. Mechanotransduction is also known to induce apoptosis, cell migration, and shape change[35].
Methods for studying mechanotransduction
To understand a biological system, scientists apply "input" into the system and read "output" as a standard approach. For mechanotransduction research, input is the mechanical forces applied to a cell or tissue (Fig. 1). Applying a controlled input of force can be achieved in several ways. For example, tissue culture cells can be repeatedly stretched on an elastic substrate in various directions and frequencies to mimic muscle, blood vessels, and lungs[36–38]. Hydrostatic pressure and membrane-stretching can activate channel proteins[39–40]. Cells can be compressed using a dynamic compressive bioreactor to engineer articular cartilage tissue and to mimic periodontal cells[41–43]. Shear stress can be generated by a pump to mimic blood flow[37,44]. Even microgravity can be tested on satellites and space stations, or in a rotating wall vessel bioreactor on the Earth[12,45–47]. Infrasound (0–20 Hz) and low-frequency noise (20–500 Hz) are also mechanical stimuli that influence physiology of cells and can be applied in research settings[48]. In addition to these external forces, internal forces can be controlled by inhibiting myosin. Stiffness of the substrate also affects the forces on adhesion molecules that link to the cytoskeleton and the nucleus[49–51].
"Output" in the experimental methods above includes gene expression, morphological changes, translocation of protein, and post-translational modification as these methods are well-established. However, as previously mentioned, these methods do not address how forces are sensed, transmitted, and transduced into gene expression, which have remained challenging questions in the field of mechanotransduction research.
How mechanical forces are detected
Accumulated evidence demonstrates that mechanical forces trigger conformational changes of proteins to activate them. For example, piezo cation channels use a lever-like mechanogating mechanism to function as a mechanotransduction channel[52]. Filamin A, an actin cross-linking protein, exposes a cryptic binding site for integrins and other binding partners when actomyosin contraction dissociates the domain-domain pair of filamin A[53–57]. Talin unfolding occurs in the R8 domain upon force application to activate downstream signaling[58]. Blood shear force can induce unfolding of the A2 domain of von Willebrand factor to expose the binding site for the glycoprotein Iα receptor in the A1 domain, cryptic A disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13) binding sites, and the cleavage site in the A2 domain[59–60]. Deflection of stereocilia of hair cells by acoustic forces pulls open a calcium channel and activates the current through the channel[61].
All of these demonstrations rely on detailed structural information as no robust method is available to identify a mechanosensing molecule. A nanotechnology-based method that specifically recognizes mechanosensitive changes without recognizing non-mechanosensitive changes which could take place at the same time as mechanosensitive changes, is necessary to advance our knowledge of force sensing mechanisms. These changes could include not only conformational changes of protein but also other biological molecules such as membrane lipids and sugars.
How mechanical forces are transmitted
In theory, force transmission can be mediated by cell-matrix interaction, cell-to-cell interaction, cytoskeleton, pressure, and fluidic flow and vibration (Fig. 2)[62–64]. The cytoskeleton-mediated transmission is fast even across long distances compared to molecular diffusion in cells, presumably due to the direct connection of the sensor to a target[65]. However, in theory, pressure change, stretching, and vibration can also transmit faster than molecular diffusion. The cytoskeleton is directly connected to the linker of nucleoskeleton and cytoskeleton (LINC) complex to regulate gene expression[51,66–67]. In addition, osmotic shocks stretch the nucleus and nuclear pores to facilitate active transport of Yes-associated protein 1 (YAP), a transcriptional co-factor, into the nucleus[68].
The only means to identify a force transmitter is the perturbation of a candidate. For example, cytoskeleton can be perturbed by depolymerization agents such as latrunculin and nocodazole[69–70]. The LINC complex can be disrupted by targeting the component of the complex using siRNA and genome editing[68]. Another sophisticated method is necessary to identify and monitor force transmission at micro or nanoscales. For example, CRISPR screening may facilitate discovery of key mechanotransmitter and rationally designed fluorescent probe may monitor force transmission in real time[71].
How mechanical forces are converted to biochemical signals
The mechanotransduction channels can directly convert mechanical forces into biochemical signals by incorporating ions into cytosol[52,61] (Fig. 2). Upon mechanical activation, filamin A can connect to integrin, smoothelin, and fimbacin, and dissociate FilGAP, a Rac-specific GTPase-activating protein[54–57]. The filamin A-integrin interaction regulates cell adhesion and migration, but functions of force-dependent interactions with smoothelin and fimbacin are not known[72]. Binding of FilGAP to filamin A targets FilGAP to sites of membrane protrusion, where it antagonizes Rac to control actin remodeling[73]. Mechanical forces also trigger proteolysis by exposing a cleavage site[59–60]. Enlargement of nuclear pore size regulated by mechanical forces such as substrate stiffness, indentation of plasma membrane by atomic force microscopy (AFM), and osmotic shocks enables the transport of YAP from cytosol to the nucleus to regulate gene expression[68,74]. Other means to convert mechanical forces into biochemical signals use actin polymerization that is triggered by mechanical forces by unknown mechanisms[75–79]. Polymerization of actin dissociates myocardin-related transcription factor A from unpolymerized actin in cytosol facilitates nuclear localization of mitochondrial transcription factor A to associate with several serum response factor target promoters[80–82].
Although several other molecules such as cadherin, catenin, and merlin are shown to be involved in mechanotransduction pathways, how mechanical forces are exactly converted to biochemical signals through these molecules are not known[83–86]. The major obstacle is the lack of nanoscale structural information before and after activation by mechanical forces.
Measurement of mechanical forces
Measurement of forces applied to tissue, cells, and eventually a single molecule is the basis of mechanotransduction research and application (Fig. 3A and B). However, reported values of cell stiffness and viscosity vary substantially depending on methods even when different groups use the same instruments (e.g., elastic and viscous moduli of MCF-7 breast cancer cells can vary 1000-fold and 100-fold, respectively)[87]. Therefore, scientists need to be aware of the limitations of force measurement and confounding factors such as heat introduced by probes. Unfolding forces of protein domains such as fibronectin type Ⅲ and immunoglobulin domains also vary depending on methods due to different loading forces, loading rate, and other factors[88–91]. AFM measures the forces required to unfold individual domains of titin, ranging from 150 to 300 pN, whereas magnetic tweezers detected the critical force (~5.4 pN) at which unfolding and folding have equal probability[90,92]. This is the case for unfolding of filamin immunoglobulin domains too. The unfolding force ranged from 50 to 220 pN by AFM, whereas magnetic tweezers revealed two different modes of unfolding at <10 pN and >20 pN [88,93]. The difference between AFM and the magnetic tweezers is that the loading rate of the magnetic tweezers is much lower than that used in the AFM experiments[93–94]. Although the magnetic tweezers is a choice to measure physical property of biological molecule in physiological condition, AFM can be used to characterize molecule under high-force pulling and to determine molecular-molecular interaction.
Figure 3.
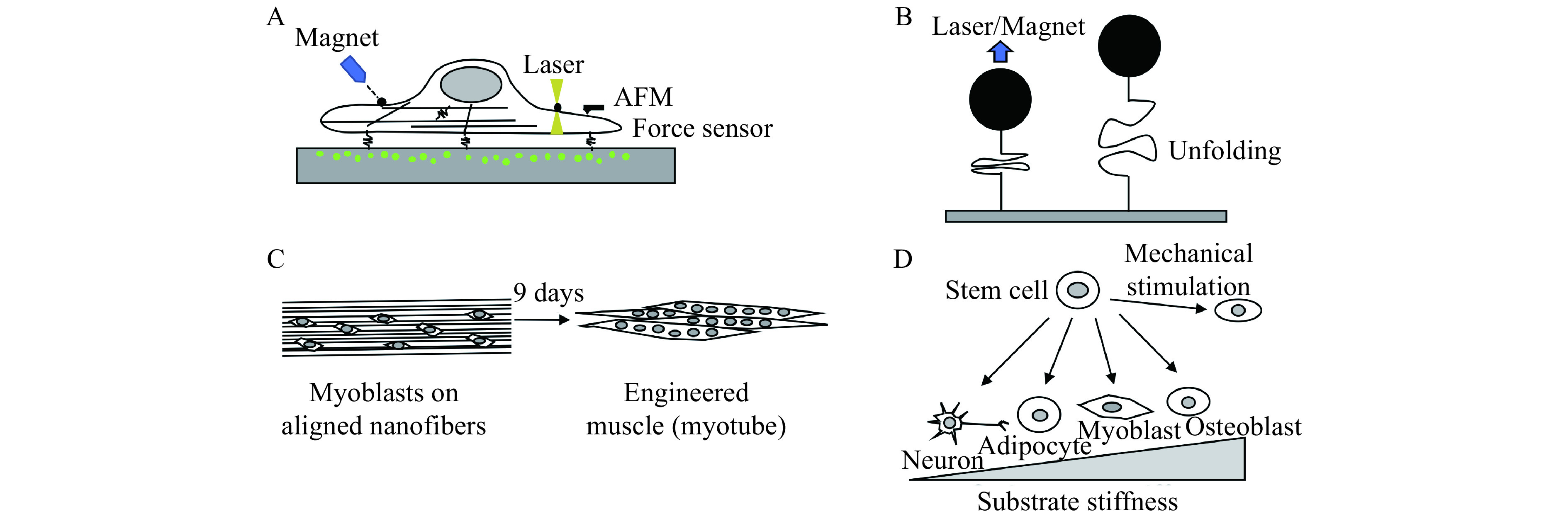
Application of nanotechnology on mechanotransduction research.
A: Measurement of mechanics and mechanical forces of aliving cell. Magnetic tweezers and optical tweezers measure mechanics of a cell using a magnetic bead and microscopic objects coated with specific ligand that attaches to cell surface receptor. Atomic forcemicroscopy (AFM) measures cell mechanics by directly touching a cell. Force sensor fused into adhesion molecule or attached on substrate measure traction force of a cell. Traction force microscopy measuresdisplacement of microbeads embedded in the substrate to determine traction force. Other methods, not shown in this figure, includemicropillar array to detect traction force and fluorescent resonance energy transfer (FRET)-based probe to map stress within a cell. B:Measurement of mechanical properties of a single molecule using magnetic and optical tweezers. AFM can be used for a single molecular analysis but loading rate is much higher than that used in the magnetic and optical tweezers. Fusing an internal control molecule whose mechanical property is known to a test molecule can be used to warrant single molecular analysis. C: Pattern of nanoscale scaffold regulates cell behaviors. D: Stiffness of nanoscale scaffold and mechanical stimulation regulate cell differentiation. For example, nanoparticles can be mechanically manipulated to activate a specific signaling pathway to induce differentiation, growth, and death.
The optical tweezers demonstrated that application of 2–5 pN to filamin domain increases the affinity to its binding partners[95]. The computational calculation of the critical force required to denature an immunoglobulin domain is calculated to be 3.5–5 pN using the energy difference between the folded and unraveled domain[96]. Since the forces generated by single myosin or kinesin molecules are 2- to 7-pN force, single and multiple motor molecules are capable of unfolding an immunoglobulin domain[97–98].
Nanoscale molecular sensors can also measure mechanical forces loaded on a single molecule in living cells. Fluorescent resonance energy transfer (FRET)-based probes can be genetically constructed and expressed in living cells. Since FRET changes correlate with forces, force can be measured in the cells. For example, a vinculin probe demonstrated that tension across vinculin in stable focal adhesion is about 2.5 pN and changes as cells migrate[99]. In talin, the rod domain experiences a force gradient (in the single piconewton regime) upon integrin-mediated cell adhesion[100], which is consistent with single molecular analysis[101]. At cell-cell junctions, it was estimated that cadherin-catenin complex is subjected to a tension of ~5 pN under resting conditions rising to ~50 pN in stressed conditions consistent with experimental measurement[102]. FRET-based tension sensors can be used to determine traction forces at the cell surface that attaches to substrate as well[103]. Recently developed nanofiber optic force transducers have the potential to measure intracellular forces with sub-piconewton force sensitivity and a nanoscale footprint[104].
Manipulation of mechanical forces
Nanotechnology is powerful to mechanically manipulate conformational changes of a molecule. For example, magnetic NP is a promising technique for activating a specific signaling pathway, controlling stem-cell differentiation, inducing cancer-cell death, and treating nervous system diseases[105–107]. The NPs can be made in customized sizes and surface characteristics with a high surface to volume ratio, and can be ingested by cells. Magnetic micropillar substrate can also be used for mechanotransduction research and application[108]. Optical and magnetic tweezers that control from nano- to 2–3 micron-particles can be attached to specific position of a molecule through linkers and internal control such as antibody and green fluorescent protein[109–111]. AFM allows a single molecule to be imaged but also to be manipulated using a cantilever tip[112–113]. Furthermore, high-speed AFM has recently been developed to record dynamic action of biological molecules (currently at 10–16 frames/s)[114]. These techniques can be applied to mechanically stimulate cells[68,115–116].
Application of mechanotransduction research in nanomedicine
Although mechanotransduction is essential during development and repair of tissues, the difficulty of mimicking the natural properties of tissues is one of the bottlenecks in applying mechanotransduction research to tissue engineering. Nanotechnology through customized nanomaterials has the potential to solve this problem. For example, stem cells can be differentiated into different types of cells by manipulating substrate rigidity and topography[117–119] (Fig. 3C and D). More specifically, a defined substrate topography induces chondrogenesis and osteogenesis from human mesenchymal stem cells[120–123]. Culturing myoblasts on aligned nanofibers engineer muscle (myotube) that can be used for skeletal muscle repair and generation[124]. Moreover, reduced graphene oxide, a low-weight 3D aerogel-like material with pore diameter in the range of approximately 5–10 μm, induces neuronal differentiation of human neuroblastoma cells by modulating RhoA/Hippo pathway[125]. Doping NPs into hydrogel improves the scaffold mechanics for tissue engineering such as treatment of myocardial infarction and skin scar, bone regeneration, proliferation and differentiation of bone marrow and periodontal stem cells[126–130]. These results suggest that nanotechnology can be used to manipulate matrix and tissue mechanics to control cell fate to repair and generate tissues. In this aspect, an organoid, a simplified and miniaturized version of an organ produced in vitro in 3D, can be a good model to study application of mechanotransduction regulated by nanotechnology[131–132].
Although NP-based diagnosis and therapy are the major topics of nanomedicine, it was recently demonstrated that cellular uptake of NPs are cell mechanics dependent[133]. Since mechanical properties of normal and diseased cells are significantly different[134], these results suggest the application of mechanotargeting of NPs in nanomedicine[135]. Moreover, the success of a shear-activated drug-delivery system inspired by platelet activation suggests that such a system can be applicable to perturb a specific mechanotransduction pathway[136].
Magnetic NP is a promising tool to remotely activate mechanotransduction. For example, mechanical activation of force-sensitive TWIK related potassium (TREK1 K+) channel and integrin using magnetic NPs promotes mineralization of bones[137]. The magnetic NPs can also remotely control brain circuits[138].
Other nanomaterials possessing particular physical and chemical properties, such as carbon nanotubes and nanofibers can be used for tissue engineering. These materials are biocompatible, stable, easy to fabricate and functionalize, and have a potential effect on neurogenesis, osteogenesis, and stem cell differentiation due to their mechanical properties related to mechanotransduction[123].
Although application of nanotechnology in mechanotransduction research and tissue engineering is already beginning to happen and show promising results, safety of nanomaterial should be vigorously tested before medical application[139–140].
Conclusion and future perspectives
Mechanical forces have a profound effect on human physiology and pathophysiology. Although research on tissue and cellular level is still necessary to understand mechanotransduction, its molecular mechanism at nano level should eventually be revealed for the potential of clinically significant findings. Nanotechnology provides a new set of tools for studying mechanotransduction and for its application in nanomedicine. Such nanomedicine includes force-induced therapeutics, diagnosis, and tissue engineering, which will offer unprecedented opportunities for innovative medicine. However, an understanding of mechanotransduction at the molecular level is still nascent, retaining its application in nanomedicine. Of critical importance is the need to identify a full-set of mechanosensing molecules and reveal how forces are sensed, transmitted, and converted to biochemical signals and gene expression. Such understanding should lead to the development of more selective drugs and treatment.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31771551 to F.N.).
Footnotes
CLC number:R318.01, Document code: A
The authors reported no conflict of interests.
References
- 1.Urner S, Kelly-Goss M, Peirce SM, et al Mechanotransduction in blood and lymphatic vascular development and disease. Adv Pharmacol. 2018;81:155–208. doi: 10.1016/bs.apha.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Malakou LS, Gargalionis AN, Piperi C, et al Molecular mechanisms of mechanotransduction in psoriasis. Ann Transl Med. 2018;6(12):245. doi: 10.21037/atm.2018.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broders-Bondon F, Nguyen Ho-Bouldoires TH, Fernandez-Sanchez ME, et al Mechanotransduction in tumor progression: the dark side of the force. J Cell Biol. 2018;217(5):1571–1587. doi: 10.1083/jcb.201701039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura F Mechanotransduction in blood cells. Asia-Pacific J Blood Types Genes. 2017;1(1):1–9. [Google Scholar]
- 5.Maycas M, Esbrit P, Gortázar AR Molecular mechanisms in bone mechanotransduction. Histol Histopathol. 2017;32(8):751–760. doi: 10.14670/HH-11-858. [DOI] [PubMed] [Google Scholar]
- 6.Lyon RC, Zanella F, Omens JH, et al Mechanotransduction in cardiac hypertrophy and failure. Circ Res. 2015;116(8):1462–1476. doi: 10.1161/CIRCRESAHA.116.304937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent TL Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr Opin Pharmacol. 2013;13(3):449–454. doi: 10.1016/j.coph.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Cheng CM, Tang MJ Review series - mechanotransduction from physiology to disease states. J Cell Mol Med. 2013;17(2):223–224. doi: 10.1111/jcmm.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French AS Mechanotransduction. Annu Rev Physiol. 1992;54:135–152. doi: 10.1146/annurev.ph.54.030192.001031. [DOI] [PubMed] [Google Scholar]
- 10.Petridou NI, Spiró Z, Heisenberg CP Multiscale force sensing in development. Nat Cell Biol. 2017;19(6):581–588. doi: 10.1038/ncb3524. [DOI] [PubMed] [Google Scholar]
- 11.Vogel V, Sheetz M Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7(4):265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 12.Najrana T, Sanchez-Esteban J Mechanotransduction as an adaptation to gravity. Front Pediatr. 2016;4:140. doi: 10.3389/fped.2016.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphrey JD, Dufresne ER, Schwartz MA Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wozniak MA, Chen CS Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10(1):34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uroz M, Wistorf S, Serra-Picamal X, et al Regulation of cell cycle progression by cell-cell and cell-matrix forces. Nat Cell Biol. 2018;20(6):646–654. doi: 10.1038/s41556-018-0107-2. [DOI] [PubMed] [Google Scholar]
- 16.Young SM, Liu S, Joshi R, et al Localization and stretch-dependence of lung elastase activity in development and compensatory growth. J Appl Physiol (1985) 2015;118(7):921–931. doi: 10.1152/japplphysiol.00954.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Yu W, Liu Y, et al Mechanical stretching stimulates collagen synthesis via down-regulating SO2/AAT1 pathway . Sci Rep. 2016;6(1):21112. doi: 10.1038/srep21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang E Age-dependent atrophy and microgravity travel: what do they have in common? FASEB J. 1999;13(9001):S167–S174. doi: 10.1096/fasebj.13.9001.s167. [DOI] [PubMed] [Google Scholar]
- 19.Blaber E, Marçal H, Burns BP Bioastronautics: the influence of microgravity on astronaut health. Astrobiology. 2010;10(5):463–473. doi: 10.1089/ast.2009.0415. [DOI] [PubMed] [Google Scholar]
- 20.Bamman MM, Roberts BM, Adams GR Molecular regulation of exercise-induced muscle fiber hypertrophy. Cold Spring Harb Perspect Med. 2018;8(6):a029751. doi: 10.1101/cshperspect.a029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen LA, Nicoll JX, Fry AC The skeletal muscle fiber: a mechanically sensitive cell. Eur J Appl Physiol. 2019;119(2):333–349. doi: 10.1007/s00421-018-04061-x. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Choi YS, Jeong MJ, et al Expression of UNCL during development of periodontal tissue and response of periodontal ligament fibroblasts to mechanical stress in vivo and in vitro . Cell Tissue Res. 2007;327(1):25–31. doi: 10.1007/s00441-006-0304-3. [DOI] [PubMed] [Google Scholar]
- 23.O'Conor CJ, Case N, Guilak F Mechanical regulation of chondrogenesis. Stem Cell Res Ther. 2013;4(4):61. doi: 10.1186/scrt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fearing BV, Hernandez PA, Setton LA, et al Mechanotransduction and cell biomechanics of the intervertebral disc. JOR Spine. 2018;1(3):e1026. doi: 10.1002/jsp2.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradamante S, Barenghi L, Maier JAM Stem cells toward the future: the space challenge. Life (Basel) 2014;4(2):267–280. doi: 10.3390/life4020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanovska IL, Shin JW, Swift J, et al Stem cell mechanobiology: diverse lessons from bone marrow. Trends Cell Biol. 2015;25(9):523–532. doi: 10.1016/j.tcb.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei SC, Yang J Forcing through tumor metastasis: the interplay between tissue rigidity and epithelial-mesenchymal transition. Trends Cell Biol. 2016;26(2):111–120. doi: 10.1016/j.tcb.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokolov I, Iyer S, Woodworth CD Recovery of elasticity of aged human epithelial cells in vitro . Nanomedicine. 2006;2(1):31–36. doi: 10.1016/j.nano.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Pelissier FA, Garbe JC, Ananthanarayanan B, et al Age-related dysfunction in mechanotransduction impairs differentiation of human mammary epithelial progenitors. Cell Rep. 2014;7(6):1926–1939. doi: 10.1016/j.celrep.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casey T, Patel OV, Plaut K Transcriptomes reveal alterations in gravity impact circadian clocks and activate mechanotransduction pathways with adaptation through epigenetic change. Physiol Genomics. 2015;47(4):113–128. doi: 10.1152/physiolgenomics.00117.2014. [DOI] [PubMed] [Google Scholar]
- 31.Uhler C, Shivashankar GV Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat Rev Mol Cell Biol. 2017;18(12):717–727. doi: 10.1038/nrm.2017.101. [DOI] [PubMed] [Google Scholar]
- 32.Barzegari A, Omidi Y, Ostadrahimi A, et al The role of Piezo proteins and cellular mechanosensing in tuning the fate of transplanted stem cells. Cell Tissue Res. 2020;381(1):1–12. doi: 10.1007/s00441-020-03191-z. [DOI] [PubMed] [Google Scholar]
- 33.Dupont S, Morsut L, Aragona M, et al Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 34.Huang XW, Yang NH, Fiore VF, et al Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47(3):340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaalouk DE, Lammerding J Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asano S, Ito S, Morosawa M, et al Cyclic stretch enhances reorientation and differentiation of 3-D culture model of human airway smooth muscle. Biochem Biophys Rep. 2018;16:32–38. doi: 10.1016/j.bbrep.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto K, Ando J Vascular endothelial cell membranes differentiate between stretch and shear stress through transitions in their lipid phases. Am J Physiol Heart Circ Physiol. 2015;309(7):H1178–H185. doi: 10.1152/ajpheart.00241.2015. [DOI] [PubMed] [Google Scholar]
- 38.Takahara N, Ito S, Furuya K, et al Real-time imaging of ATP release induced by mechanical stretch in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2014;51(6):772–782. doi: 10.1165/rcmb.2014-0008OC. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, Montell C Forcing open TRP channels: Mechanical gating as a unifying activation mechanism. Biochem Biophys Res Commun. 2015;460(1):22–25. doi: 10.1016/j.bbrc.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitzthum C, Clauss WG, Fronius M Mechanosensitive activation of CFTR by increased cell volume and hydrostatic pressure but not shear stress. Biochim Biophys Acta. 2015;1848:2942–2951. doi: 10.1016/j.bbamem.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Yang L, Yang Y, Wang S, et al In vitro mechanical loading models for periodontal ligament cells: from two-dimensional to three-dimensional models . Arch Oral Biol. 2015;60(3):416–424. doi: 10.1016/j.archoralbio.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Chukkapalli SS, Lele TP Periodontal cell mechanotransduction. Open Biol. 2018;8(9):180053. doi: 10.1098/rsob.180053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson DE, Johnstone B Dynamic mechanical compression of chondrocytes for tissue engineering: a critical review. Front Bioeng Biotechnol. 2017;5:76. doi: 10.3389/fbioe.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gongol B, Marin T, Zhang J, et al Shear stress regulation of miR-93 and miR-484 maturation through nucleolin. Proc Natl Acad Sci U S A. 2019;116(26):12974–12979. doi: 10.1073/pnas.1902844116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torday JS Parathyroid hormone-related protein is a gravisensor in lung and bone cell biology. Adv Space Res. 2003;32(8):1569–1576. doi: 10.1016/S0273-1177(03)90397-8. [DOI] [PubMed] [Google Scholar]
- 46.Baio J, Martinez AF, Silva I, et al Cardiovascular progenitor cells cultured aboard the International Space Station exhibit altered developmental and functional properties. npj Microgravity. 2018;4:13. doi: 10.1038/s41526-018-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu DY, Sun SJ, Zhang F, et al Microgravity-induced hepatogenic differentiation of rBMSCs on board the SJ-10 satellite. FASEB J. 2019;33(3):4273–4286. doi: 10.1096/fj.201802075R. [DOI] [PubMed] [Google Scholar]
- 48.Lim K, Kim J, Seonwoo H, et al In vitro effects of low-intensity pulsed ultrasound stimulation on the osteogenic differentiation of human alveolar bone-derived mesenchymal stem cells for tooth tissue engineering . Biomed Res Int. 2013;2013:269724. doi: 10.1155/2013/269724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang L, Sun ZL, Chen XF, et al Cells sensing mechanical cues: stiffness influences the lifetime of cell-extracellular matrix interactions by affecting the loading rate. ACS Nano. 2016;10(1):207–217. doi: 10.1021/acsnano.5b03157. [DOI] [PubMed] [Google Scholar]
- 50.Gong Z, Szczesny SE, Caliari SR, et al Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc Natl Acad Sci U S A. 2018;115(12):E2686–E2695. doi: 10.1073/pnas.1716620115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hieda M Signal transduction across the nuclear envelope: role of the LINC complex in bidirectional signaling. Cells. 2019;8(2):124. doi: 10.3390/cells8020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao QC, Zhou H, Li XM, et al The mechanosensitive Piezo1 channel: a three-bladed propeller-like structure and a lever-like mechanogating mechanism. FEBS J. 2019;286(13):2461–2470. doi: 10.1111/febs.14711. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura F, Stossel TP, Hartwig JH The filamins: organizers of cell structure and function. Cell Adh Migr. 2011;5(2):160–169. doi: 10.4161/cam.5.2.14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehrlicher AJ, Nakamura F, Hartwig JH, et al Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature. 2011;478(7368):260–263. doi: 10.1038/nature10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Razinia Z, Mäkelä T, Ylänne J, et al Filamins in mechanosensing and signaling. Annu Rev Biophys. 2012;41:227–246. doi: 10.1146/annurev-biophys-050511-102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Nakamura F Identification of Filamin A mechanobinding partner I: smoothelin specifically interacts with the filamin A Mechanosensitive domain 21. Biochemistry. 2019;58(47):4726–4736. doi: 10.1021/acs.biochem.9b00100. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Nakamura F Identification of Filamin A mechanobinding partner Ⅱ: fimbacin is a novel actin cross-linking and Filamin A binding protein. Biochemistry. 2019;58(47):4737–4743. doi: 10.1021/acs.biochem.9b00101. [DOI] [PubMed] [Google Scholar]
- 58.Haining AWM, Rahikainen R, Cortes E, et al Mechanotransduction in talin through the interaction of the R8 domain with DLC1. PLoS Biol. 2018;16(7):e2005599. doi: 10.1371/journal.pbio.2005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang XH, Halvorsen K, Zhang CZ, et al Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science. 2009;324(5932):1330–1334. doi: 10.1126/science.1170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crawley JTB, de Groot R, Xiang YZ, et al Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. 2011;118(12):3212–3221. doi: 10.1182/blood-2011-02-306597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goutman JD, Elgoyhen AB, Gómez-Casati ME Cochlear hair cells: the sound-sensing machines. FEBS Lett. 2015;589(22):3354–3361. doi: 10.1016/j.febslet.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen CH, Lin YH, Chen CH, et al Transforming growth factor beta 1 mediates the low-frequency vertical vibration enhanced production of tenomodulin and type I collagen in rat Achilles tendon. PLoS One. 2018;13(10):e0205258. doi: 10.1371/journal.pone.0205258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turner DC, Edmiston AM, Zohner YE, et al Transient intraocular pressure fluctuations: source, magnitude, frequency, and associated mechanical energy. Invest Ophthalmol Vis Sci. 2019;60(7):2572–2582. doi: 10.1167/iovs.19-26600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathieu S, Manneville JB Intracellular mechanics: connecting rheology and mechanotransduction. Curr Opin Cell Biol. 2019;56:34–44. doi: 10.1016/j.ceb.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Wang N, Tytell JD, Ingber DE Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 66.Belaadi N, Millon-Frémillon A, Aureille J, et al. Analyzing mechanotransduction through the LINC complex in isolated nuclei[M]//Gundersen GG, Worman HJ. The LINC Complex. New York: Humana Press, 2018: 73–80.
- 67.Wang SS, Stoops E, Cp U, et al Mechanotransduction via the LINC complex regulates DNA replication in myonuclei. J Cell Biol. 2018;217(6):2005–2018. doi: 10.1083/jcb.201708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elosegui-Artola A, Andreu I, Beedle AEM, et al Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171(6):1397–1410.e14. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Shim JW, Wise DA, Elder SH Effect of cytoskeletal disruption on Mechanotransduction of hydrostatic pressure by C3H10T1/2 murine fibroblasts. Open Orthop J. 2008;2:155–162. doi: 10.2174/1874325000802010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomasy SM, Morgan JT, Wood JA, et al Substratum stiffness and latrunculin B modulate the gene expression of the mechanotransducers YAP and TAZ in human trabecular meshwork cells. Exp Eye Res. 2013;113:66–73. doi: 10.1016/j.exer.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang B, McJunkin K CRISPR screening strategies for microRNA target identification. FEBS J. 2020 doi: 10.1111/febs.15218. [DOI] [PubMed] [Google Scholar]
- 72.Calderwood DA, Huttenlocher A, Kiosses WB, et al Increased filamin binding to β-integrin cytoplasmic domains inhibits cell migration. Nat Cell Biol. 2001;3(12):1060–1068. doi: 10.1038/ncb1201-1060. [DOI] [PubMed] [Google Scholar]
- 73.Ohta Y, Hartwig JH, Stossel TP FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8(8):803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 74.Fal K, Asnacios A, Chabouté ME, et al Nuclear envelope: a new frontier in plant mechanosensing? Biophys Rev. 2017;9(4):389–403. doi: 10.1007/s12551-017-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hirata H, Tatsumi H, Sokabe M Mechanical forces facilitate actin polymerization at focal adhesions in a Zyxin-dependent manner. J Cell Sci. 2008;121(17):2795–2804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- 76.Moreno-Vicente R, Pavon DM, Martin-Padura I, et al Caveolin-1 Modulates Mechanotransduction responses to substrate stiffness through actin-dependent control of YAP. Cell Rep. 2018;25(6):1622–1635.e6. doi: 10.1016/j.celrep.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang L, Azzolin L, Di Biagio D, et al The SWI/SNF complex is a mechanoregulated inhibitor of YAP and TAZ. Nature. 2018;563(7730):265–269. doi: 10.1038/s41586-018-0658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halder G, Dupont S, Piccolo S Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13(9):591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 79.Zhang XQ, Hu XY, Lei HZ, et al Mechanical force-induced polymerization and depolymerization of F-actin at water/solid interfaces. Nanoscale. 2016;8(11):6008–6013. doi: 10.1039/C5NR08713A. [DOI] [PubMed] [Google Scholar]
- 80.Finch-Edmondson M, Sudol M Framework to function: mechanosensitive regulators of gene transcription. Cell Mol Biol Lett. 2016;21(1):28. doi: 10.1186/s11658-016-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miralles F, Posern G, Zaromytidou AI, et al Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113(3):329–342. doi: 10.1016/S0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 82.Hu X, Liu ZZ, Chen XY, et al MKL1-actin pathway restricts chromatin accessibility and prevents mature pluripotency activation. Nat Commun. 2019;10(1):1695. doi: 10.1038/s41467-019-09636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dorland YL, Huveneers S Cell-cell junctional mechanotransduction in endothelial remodeling. Cell Mol Life Sci. 2017;74(2):279–292. doi: 10.1007/s00018-016-2325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen TC, Saw TB, Mège RM, et al Mechanical forces in cell monolayers. J Cell Sci. 2018;131(24):jcs218156. doi: 10.1242/jcs.218156. [DOI] [PubMed] [Google Scholar]
- 85.Das T, Safferling K, Rausch S, et al A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol. 2015;17(3):276–287. doi: 10.1038/ncb3115. [DOI] [PubMed] [Google Scholar]
- 86.Chakraborty S, Njah K, Pobbati AV, et al Agrin as a Mechanotransduction signal regulating YAP through the hippo pathway. Cell Rep. 2017;18(10):2464–2479. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 87.Wu PH, Aroush DRB, Asnacios A, et al A comparison of methods to assess cell mechanical properties. Nat Methods. 2018;15(7):491–498. doi: 10.1038/s41592-018-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Furuike S, Ito T, Yamazaki M Mechanical unfolding of single filamin A (ABP-280) molecules detected by atomic force microscopy. FEBS Lett. 2001;498(1):72–75. doi: 10.1016/S0014-5793(01)02497-8. [DOI] [PubMed] [Google Scholar]
- 89.Ferrer JM, Lee H, Chen J, et al Measuring molecular rupture forces between single actin filaments and actin-binding proteins. Proc Natl Acad Sci U S A. 2008;105(27):9221–9226. doi: 10.1073/pnas.0706124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen H, Yuan GH, Winardhi RS, et al Dynamics of equilibrium folding and unfolding transitions of Titin immunoglobulin domain under constant forces. J Am Chem Soc. 2015;137(10):3540–3546. doi: 10.1021/ja5119368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cossio P, Hummer G, Szabo A On artifacts in single-molecule force spectroscopy. Proc Natl Acad Sci U S A. 2015;112(46):14248–14253. doi: 10.1073/pnas.1519633112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rief M, Gautel M, Oesterhelt F, et al Reversible unfolding of individual Titin immunoglobulin domains by AFM. Science. 1997;276(5315):1109–1112. doi: 10.1126/science.276.5315.1109. [DOI] [PubMed] [Google Scholar]
- 93.Chen H, Zhu XY, Cong PW, et al Differential mechanical stability of filamin A rod segments. Biophys J. 2011;101(5):1231–1237. doi: 10.1016/j.bpj.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neuman KC, Nagy A Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods. 2008;5(6):491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rognoni L, Stigler J, Pelz B, et al Dynamic force sensing of filamin revealed in single-molecule experiments. Proc Natl Acad Sci U S A. 2012;109(48):19679–19684. doi: 10.1073/pnas.1211274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Erickson HP Reversible unfolding of fibronectin type Ⅲ and immunoglobulin domains provides the structural basis for stretch and elasticity of Titin and fibronectin. Proc Natl Acad Sci U S A. 1994;91(21):10114–10118. doi: 10.1073/pnas.91.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finer JT, Simmons RM, Spudich JA Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 98.Hunt AJ, Gittes F, Howard J The force exerted by a single kinesin molecule against a viscous load. Biophys J. 1994;67(2):766–781. doi: 10.1016/S0006-3495(94)80537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grashoff C, Hoffman BD, Brenner MD, et al Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466(7303):263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ringer P, Weißl A, Cost AL, et al Multiplexing molecular tension sensors reveals piconewton force gradient across talin-1. Nat Methods. 2017;14(11):1090–1096. doi: 10.1038/nmeth.4431. [DOI] [PubMed] [Google Scholar]
- 101.del Rio A, Perez-Jimenez R, Liu RC, et al Stretching single talin rod molecules activates vinculin binding. Science. 2009;323(5914):638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Charras G, Yap AS Tensile forces and Mechanotransduction at cell-cell junctions. Curr Biol. 2018;28(8):R445–R457. doi: 10.1016/j.cub.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 103.Ma VPY, Salaita K DNA nanotechnology as an emerging tool to study Mechanotransduction in living systems. Small. 2019;15(26):1900961. doi: 10.1002/smll.201900961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang Q, Lee J, Arce FT, et al Nanofibre optic force transducers with sub-piconewton resolution via near-field plasmon-dielectric interactions. Nat Photonics. 2017;11(6):352–355. doi: 10.1038/nphoton.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seo D, Southard KM, Kim JW, et al A Mechanogenetic toolkit for interrogating cell signaling in space and time. Cell. 2016;165(6):1507–1518. doi: 10.1016/j.cell.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen YJ, Cheng Y, Uyeda TQP, et al Cell Mechanosensors and the possibilities of using magnetic nanoparticles to study them and to modify cell fate. Ann Biomed Eng. 2017;45(10):2475–2486. doi: 10.1007/s10439-017-1884-7. [DOI] [PubMed] [Google Scholar]
- 107.Gonçalves AI, Miranda MS, Rodrigues MT, et al Magnetic responsive cell-based strategies for diagnostics and therapeutics. Biomed Mater. 2018;13(5):054001. doi: 10.1088/1748-605X/aac78b. [DOI] [PubMed] [Google Scholar]
- 108.Nagayama K, Inoue T, Hamada Y, et al Direct application of mechanical stimulation to cell adhesion sites using a novel magnetic-driven micropillar substrate. Biomed Microdevices. 2018;20(4):85. doi: 10.1007/s10544-018-0328-y. [DOI] [PubMed] [Google Scholar]
- 109.Norregaard K, Metzler R, Ritter CM, et al Manipulation and motion of organelles and single molecules in living cells. Chem Rev. 2017;117(5):4342–4375. doi: 10.1021/acs.chemrev.6b00638. [DOI] [PubMed] [Google Scholar]
- 110.Zhao DY, Liu SY, Gao Y Single-molecule manipulation and detection. Acta Biochim Biophys Sin (Shanghai) 2018;50(3):231–237. doi: 10.1093/abbs/gmx146. [DOI] [PubMed] [Google Scholar]
- 111.Cordova JC, Das DK, Manning HW, et al Combining single-molecule manipulation and single-molecule detection. Curr Opin Struct Biol. 2014;28:142–148. doi: 10.1016/j.sbi.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 112.Fisher TE, Marszalek PE, Fernandez JM Stretching single molecules into novel conformations using the atomic force microscope. Nat Struct Biol. 2000;7(9):719–724. doi: 10.1038/78936. [DOI] [PubMed] [Google Scholar]
- 113.Fotiadis D, Scheuring S, Muller SA, et al Imaging and manipulation of biological structures with the AFM. Micron. 2002;33(4):385–397. doi: 10.1016/S0968-4328(01)00026-9. [DOI] [PubMed] [Google Scholar]
- 114.Ando T Directly watching biomolecules in action by high-speed atomic force microscopy. Biophys Rev. 2017;9(4):421–429. doi: 10.1007/s12551-017-0281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Charras GT, Lehenkari PP, Horton MA Atomic force microscopy can be used to mechanically stimulate osteoblasts and evaluate cellular strain distributions. Ultramicroscopy. 2001;86(1-2):85–95. doi: 10.1016/S0304-3991(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 116.Targosz-Korecka M, Malek-Zietek KE, Brzezinka GD, et al Morphological and nanomechanical changes in mechanosensitive endothelial cells induced by colloidal AFM probes. Scanning. 2016;38(6):654–664. doi: 10.1002/sca.21313. [DOI] [PubMed] [Google Scholar]
- 117.Discher D, Dong C, Fredberg JJ, et al Biomechanics: cell research and applications for the next decade. Ann Biomed Eng. 2009;37(5):847–859. doi: 10.1007/s10439-009-9661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pemberton GD, Childs P, Reid S, et al Nanoscale stimulation of osteoblastogenesis from mesenchymal stem cells: nanotopography and nanokicking. Nanomedicine (Lond) 2015;10(4):547–560. doi: 10.2217/nnm.14.134. [DOI] [PubMed] [Google Scholar]
- 119.Teo BK, Wong ST, Lim CK, et al Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano. 2013;7(6):4785–4798. doi: 10.1021/nn304966z. [DOI] [PubMed] [Google Scholar]
- 120.Wu YN, Law JBK, He AY, et al Substrate topography determines the fate of chondrogenesis from human mesenchymal stem cells resulting in specific cartilage phenotype formation. Nanomedicine. 2014;10(7):1507–1516. doi: 10.1016/j.nano.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 121.Panadero JA, Lanceros-Mendez S, Ribelles JLG Differentiation of mesenchymal stem cells for cartilage tissue engineering: Individual and synergetic effects of three-dimensional environment and mechanical loading. Acta Biomater. 2016;33:1–12. doi: 10.1016/j.actbio.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 122.Dobbenga S, Fratila-Apachitei LE, Zadpoor AA Nanopattern-induced osteogenic differentiation of stem cells - A systematic review. Acta Biomater. 2016;46:3–14. doi: 10.1016/j.actbio.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 123.Tay CY, Koh CG, Tan NS, et al Mechanoregulation of stem cell fate via micro-/nano-scale manipulation for regenerative medicine. Nanomedicine (Lond) 2013;8(4):623–638. doi: 10.2217/nnm.13.31. [DOI] [PubMed] [Google Scholar]
- 124.Nakayama KH, Quarta M, Paine P, et al Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration. Commun Biol. 2019;2(1):170. doi: 10.1038/s42003-019-0416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Catanesi M, Panella G, Benedetti E, et al YAP/TAZ mechano-transduction as the underlying mechanism of neuronal differentiation induced by reduced graphene oxide. Nanomedicine (Lond) 2018;13(24):3091–3106. doi: 10.2217/nnm-2018-0269. [DOI] [PubMed] [Google Scholar]
- 126.Jawad H, Boccaccini AR, Ali NN, et al Assessment of cellular toxicity of TiO2 nanoparticles for cardiac tissue engineering applications . Nanotoxicology. 2011;5(3):372–380. doi: 10.3109/17435390.2010.516844. [DOI] [PubMed] [Google Scholar]
- 127.Li N, Fan XL, Tang KY, et al Nanocomposite scaffold with enhanced stability by hydrogen bonds between collagen, polyvinyl pyrrolidone and titanium dioxide. Colloids Surf B Biointerfaces. 2016;140:287–296. doi: 10.1016/j.colsurfb.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 128.Pan LL, Pei XB, He R, et al Multiwall carbon nanotubes/polycaprolactone composites for bone tissue engineering application. Colloids Surf B Biointerfaces. 2012;93:226–234. doi: 10.1016/j.colsurfb.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 129.Rodriguez-Lozano FJ, García-Bernal D, Aznar-Cervantes S, et al Potential of graphene for tissue engineering applications. Transl Res. 2015;166(4):399–400. doi: 10.1016/j.trsl.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 130.Chen GY, Pang DWP, Hwang SM, et al A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials. 2012;33(2):418–427. doi: 10.1016/j.biomaterials.2011.09.071. [DOI] [PubMed] [Google Scholar]
- 131.Bayir E, Sendemir A, Missirlis YF Mechanobiology of cells and cell systems, such as organoids. Biophys Rev. 2019;11(5):721–728. doi: 10.1007/s12551-019-00590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abdel Fattah AR, Ranga A Nanoparticles as versatile tools for Mechanotransduction in tissues and organoids. Front Bioeng Biotechnol. 2020;8:240. doi: 10.3389/fbioe.2020.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei Q, Huang CJ, Zhang Y, et al Mechanotargeting: mechanics-dependent cellular uptake of nanoparticles. Adv Mater. 2018;30(27):1707464. doi: 10.1002/adma.201707464. [DOI] [PubMed] [Google Scholar]
- 134.Butcher DT, Alliston T, Weaver VM A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9(2):108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mpekris F, Angeli S, Pirentis AP, et al Stress-mediated progression of solid tumors: effect of mechanical stress on tissue oxygenation, cancer cell proliferation, and drug delivery. Biomech Model Mechanobiol. 2015;14(6):1391–1402. doi: 10.1007/s10237-015-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Korin N, Kanapathipillai M, Matthews BD, et al Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337(6095):738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- 137.Henstock JR, Rotherham M, Rashidi H, et al Remotely activated mechanotransduction via magnetic nanoparticles promotes mineralization synergistically with bone morphogenetic protein 2: applications for injectable cell therapy. Stem Cells Transl Med. 2014;3(11):1363–1374. doi: 10.5966/sctm.2014-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tay A, Di Carlo D Remote neural stimulation using magnetic nanoparticles. Curr Med Chem. 2017;24(5):537–548. doi: 10.2174/0929867323666160814000442. [DOI] [PubMed] [Google Scholar]
- 139.Jiang ZY, Shan KZ, Song J, et al Toxic effects of magnetic nanoparticles on normal cells and organs. Life Sci. 2019;220:156–161. doi: 10.1016/j.lfs.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 140.Rezvani E, Rafferty A, McGuinness C, et al Adverse effects of nanosilver on human health and the environment. Acta Biomater. 2019;94:145–159. doi: 10.1016/j.actbio.2019.05.042. [DOI] [PubMed] [Google Scholar]