Abstract
Background:
We recently reported that operant social choice-induced voluntary abstinence prevents incubation of methamphetamine craving. Here, we determined whether social choice-induced voluntary abstinence would prevent incubation of heroin craving. We also introduce a fully-automatic social reward self-administration model that eliminates the intense workload and rat-human interaction of the original semi-automatic model.
Methods:
In Exp. 1, we trained male and female rats for social self-administration (6 d) and then for heroin self-administration (12 d). Next, we assessed relapse to heroin seeking after 1 and 15 abstinence days. Between tests, the rats underwent either forced or social choice-induced abstinence. In Exp. 2, we developed a fully-automatic social self-administration procedure by introducing a screen between the self-administration chamber and the social-peer chamber; the screen allows physical contact but prevents rats from crossing chambers. Next, we compared incubation of craving in rats with a history of standard (no-screen) or automatic (screen) social self-administration and social choice-induced abstinence.
Results:
The time-dependent increase in heroin seeking after cessation of drug self-administration (incubation of craving) was lower after social choice-induced abstinence than after forced abstinence. There were no differences in social self-administration, social choice-induced abstinence, and incubation of craving in rats trained in the standard semi-automatic procedure versus the novel fully-automatic procedure.
Conclusion:
Our study demonstrates the protective effect of rewarding social interaction on heroin self-administration and incubation of heroin craving and introduces a fully-automatic social self-administration and choice procedure to investigate the role of volitional social interaction in drug addiction and other psychiatric disorders.
Keywords: animal models, voluntary abstinence, social, opioid, addiction, incubation, rats, reward, motivation, operant, self-administration, choice
Introduction
Research on neural substrates of drug reward, withdrawal, and relapse has yet to be translated into an advancement in addiction treatment (1, 2). The reasons for the limited translational success of studies using rodent addiction models are complex and multi-factorial (2–4). This state-of-affairs has led us to change the classic translational approach involved in identifying unique mechanisms of relapse-provoking stimuli (stress, discrete and contextual cues, drug priming) (5, 6) to a different “reverse-translational” approach (7, 8). The goal of the reverse-translational approach is to develop animal models that mimic successful behavioral treatments in humans—contingency management (9) and community reinforcement approach (10)—to improve mechanistic understanding of abstinence and relapse.
Contingency management maintains prolonged abstinence by giving nondrug rewards (monetary vouchers) in exchange for negative drug tests (11–13). However, when contingency management discontinues, former drug users relapse to drug use (14, 15). The community reinforcement approach employs similar learning principles and its goal is to substitute drug use with alternative nondrug social rewards (e.g., family support, employment) contingent, at least in part, on cessation of drug use (10, 16). However, as with contingency management, when the treatment discontinues, former drug users relapse to drug use (13, 15). We recently developed rat models of voluntary abstinence and relapse based on these human behavioral treatments.
In the rat contingency management model, we first trained rats to self-administer palatable food (the alternative nondrug reward) and then to self-administer a drug (heroin or methamphetamine) for several weeks. We then assessed relapse to drug seeking during early and late abstinence days in the absence of the food reward. Between tests, we expose rats to mutually exclusive choice sessions between drug and food (8, 17). Under these “contingency management” conditions, rats voluntarily abstain from drug self-administration when the alternative nondrug reward is available, but relapse when the food reward is removed (7, 18–20).
In the rat community reinforcement model, our goal was to improve the translational utility of the voluntary abstinence model by using social interaction as the alternative nondrug reward (8), because in humans, the rewards that compete with drugs are primarily social (e.g. family, friends, employment) (16, 21–23). We found that the availability of a mutually exclusive operant social reward prevented methamphetamine and heroin self-administration in the escalation model of addiction (24), and methamphetamine self-administration in the DSM-IV-based (25) and intermittent access (26) addiction models. Social choice-induced abstinence also prevented incubation of methamphetamine craving (8), the progressive increase in drug seeking after cessation of drug self-administration (27, 28).
Here, based on studies showing behavioral and mechanistic differences between opiate and psychostimulant drugs (29–33), we determined whether the inhibitory effect of social choice-induced abstinence on incubation of methamphetamine craving generalizes to incubation of heroin craving. Additionally, we developed a fully-automatic social self-administration procedure by introducing a screen between the self-administration chamber and the social-peer chamber; the screen allows physical contact and prevents rats from crossing chambers. Next, we compared incubation of heroin craving in rats with a history of standard (no-screen) or automatic (screen) social self-administration and social choice-induced abstinence. We developed the “screen” model to eliminate limitations of the original model: intense workload and repeated physical interaction between the experimenter and rats, which can introduce experimenter-related confounds and induce rodent-related allergies.
Material and Methods
Subjects
We used male and female Sprague-Dawley rats (Charles River, n=192 [96 “Resident” (48 males/48 females) and 96 “Social partners” (48 males/48 females)], weighing 150–175 g upon arrival. We housed the rats two/cage by sex for 2–3 weeks and then individually starting one week prior to social self-administration. We randomly assigned rats to “Resident (drug user)” and “Social partner (drug naïve)” conditions. We maintained rats on a reverse 12-h light/dark cycle (lights off at 9:30 AM) with free access to laboratory chow and water. The study followed the guidelines outlined in the Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf) and was approved by NIDA-IRP ACUC. We excluded 8 rats (3 males/5 females) because of catheter failure.
Surgery
We anesthetized the rats with isoflurane (5% induction; 2%−3% maintenance) and inserted Silastic catheters into the jugular vein, which passed subcutaneously to the mid-scapular region and attached to a modified 22-gauge cannula cemented to polypropylene mesh (Sefar). We injected ketoprofen (2.5 mg/kg, s.c., Butler Schein) after surgery to relieve pain. We allowed the rats to recover from surgery for 3–4 days. We flushed the catheters daily with sterile saline containing gentamicin (4.25 mg/ml, APP Pharmaceuticals).(8, 19, 34)
Self-administration chambers
Procedures
Social self-administration
We trained rats to self-administer for access to the social partner during daily 40-min (20 trials/session, 60-s, Exp. 1–2) or 120-min sessions (60 trials/session, 60-s, Exp. 1), using a discrete-trial design. We housed Resident rats with their social partners (cage-mate) until 1 week prior to social-interaction self-administration, and each resident rat lever pressed for their previously-paired partner. As previously described (8), the trials started with illumination of the social-paired houselight followed 10-s later with insertion of the social-paired lever; we allowed the resident rat 60 s to press the active lever (fixed-ratio-1[FR1] reinforcement schedule) before lever retraction and houselight turning off. Successful lever-presses caused the retraction of the active lever, a discrete 20-s tone cue and opening of the guillotine-style sliding door. Resident rats were subsequently allowed to interact with the social partners for 60-s until the houselight turned off, at which point, the guillotine door closed. We manually removed the social partner rats in Exp. 1–2, for the ‘standard’ group rats.
Drug self-administration
We trained rats to self-administer heroin (FR1 20-s timeout reinforcement schedule, 0.1 mg/kg/infusion (8, 19, 34)) during six 1-h sessions that were separated by 10-min off periods. We limited the number of infusions to 15/h. We started the self-administration sessions at the onset of the dark cycle and sessions began with the presentation of the red light and 10-s later with the insertion of the drug-paired lever; the red light remained on for the duration of the session and served as a discriminative cue for drug availability. At the end of each 1-h session, the red light was turned off, and the active lever retracted (8, 19).
Discrete choice procedure
We conducted the discrete-choice sessions using the same parameters used during social and heroin self-administration training. We allowed the rats to choose between the social- and drug-paired levers in a discrete-trial choice procedure. We divided each 120-min choice session into 15 discrete trials that were separated by 8 min (8). Each trial began with presentations of the discriminative stimuli for social interaction and heroin, followed 10-s later by insertion of the levers paired with the rewards. Rats could then select one of the two levers. If rats responded within 6 min, they only received the reward corresponding with the selected lever. Each reward delivery was signaled by the social or drug-associated cue, retraction of both levers, and turning off the discriminative cues. If rats failed to respond on either active lever within 6 min, both levers were retracted, and the discriminative stimuli were turned off with no reward delivery. We manually replaced both resident and social partner rats in their appropriate chambers after 60 s of social interaction (Exp. 1–2, ‘standard’ group rats).
Social choice-induced abstinence
After the training phase, we allowed the rats to choose between the drug-paired lever (delivering 1 infusion) or social interaction (60-s) during 15 discrete-choice trials (8 min apart) for 10 sessions over 14 days.
Forced abstinence
After the day-1 relapse test, we returned the rats to their homecage for 14 days, and then assessed relapse to heroin seeking on abstinence day 15. We handled the rats twice/week.
Relapse tests
The 30-min relapse tests were conducted in the presence of the heroin-associated cues. The sessions began with presenting the heroin-paired discriminative cue, followed 10-s later by insertion of the heroin-paired lever; the red light remained on for the session duration. Active lever- presses during testing, the operational measure of drug seeking in incubation of craving and relapse studies (8, 35), caused contingent presentations of the light cue previously paired with heroin infusions, but not heroin.
Specific experiments
Exp. 1: Incubation of heroin craving after social choice-induced abstinence
We previously reported that operant social reward prevented incubation of methamphetamine craving (8). In Exp. 1 we tested whether social choice-induced abstinence would prevent incubation of heroin craving. We used 2 groups of rats (34 males/32 females) in an experimental design that included the between-subjects factors of Abstinence condition (Forced, Voluntary) and Sex (Male, Female), and the within-subjects factor of Abstinence day (1, 15).
Training:
We first trained rats to self-administer social interaction (6 sessions, 20 [23 females/23 males] or 60 [9 females/n=11 males] trials/session) and then trained them to self-administer heroin (12 sessions, 6 h/session). We used the standard (semi-automatic) social self-administration and choice procedure.
Discrete choice tests:
We determined social interaction versus heroin preference for 10 sessions in the voluntary abstinence group.
Relapse tests:
We tested the forced and voluntary abstinence rats for heroin seeking under extinction conditions on abstinence days 1 and 15. The duration of the test session was 30 min to minimize carryover effect of extinction learning, which may decrease drug seeking on day-15 testing.
Exp. 2: Incubation of heroin craving after social choice-induced abstinence using the automatic procedure
In Exp. 2, we compared social self-administration, social choice-induced abstinence, and incubation of heroin craving in rats trained in the semi-automatic standard (no screen) procedure with rats trained in the new automatic (screen) procedure (Fig. 2 and SOM). We used 2 groups of rats (4 males/3 females in the no-screen group; 7 males/8 females in the screen group) in an experimental design that included the between-subjects factors of Voluntary abstinence condition (standard, screen) and the within-subjects factor of Abstinence day (1, 15).
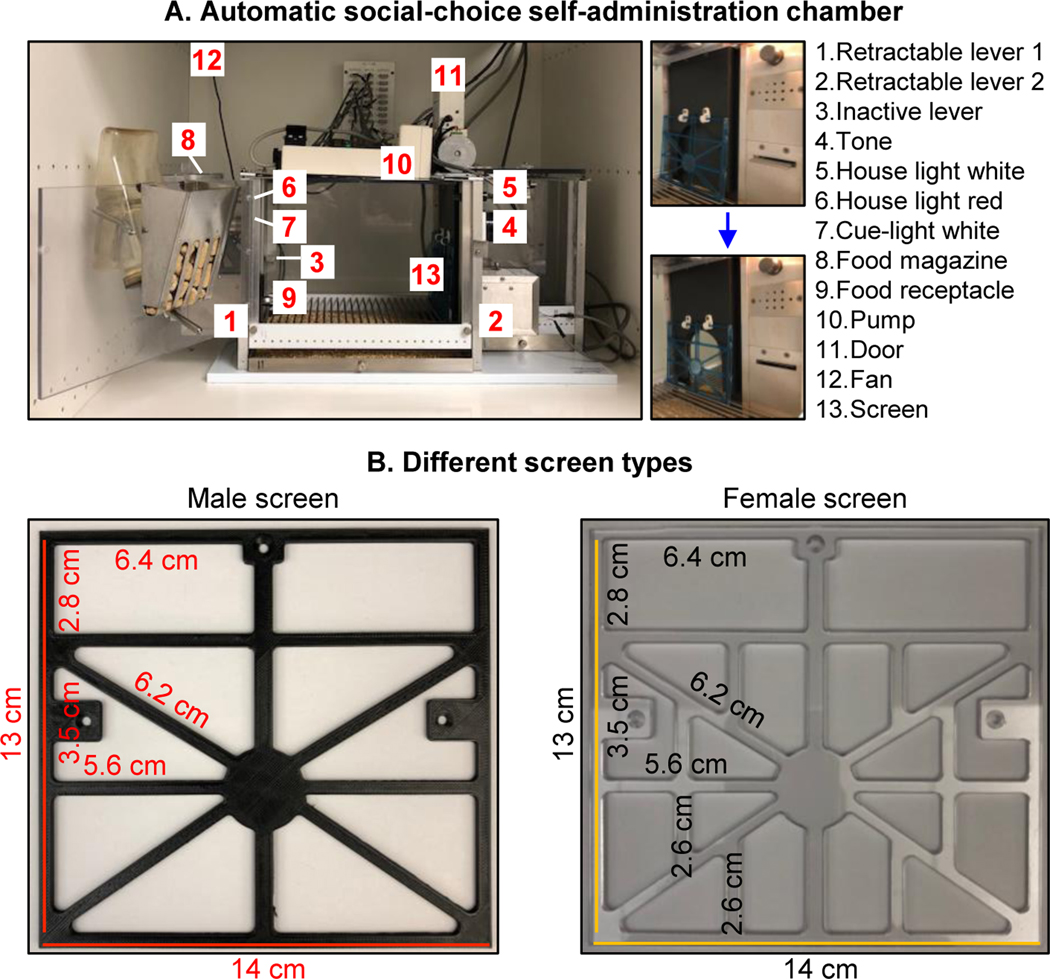
Figure 2. Automatic social-choice self-administration chamber.
(A) Picture of the chamber. The chamber has two active levers (drug-paired and social-paired), one inactive lever, two discriminative cues (red light for drug, white light for social), two discrete cues (white light for drug, tone for social), a food magazine and receptacle, a pump, a fan, and social-peer chamber separated by a sliding door, and a plastic grid barrier (termed ‘screen’) with triangular openings. (B) Different screen types. Left panel: screen for male rats; Right panel: screen for female rats (all measurements are in cm). Chamber dimensions: Length: 58.5 cm; Width: 35.6 cm; Height: 44.5 cm; this chamber fits into the standard sound attenuating Med-Associates chambers.
Training:
We first trained rats to self-administer social interaction (6 sessions, 20 trials/session) and then trained them to self-administer heroin (12 sessions, 6 h/session).
Discrete choice tests:
We determined social interaction versus heroin preference during training, after every three drug self-administration sessions, and for 10 sessions to achieve voluntary abstinence after heroin self-administration training.
Relapse tests:
We tested the screen and no-screen voluntary abstinence groups for heroin seeking under extinction conditions on abstinence days 1 and 15. The session duration was 30 min.
Statistical analysis
We used factorial ANOVAs and t-tests using SPSS (IBM, version 25, GLM procedure). We followed significant main and interaction effects (p<0.05, two-tailed) with post-hoc tests (Fisher PLSD). We only report significant effects critical for data interpretation and indicate results of post-hoc analyses in the figures. For choice data, the statistical analyses were performed on a social preference ratio score (# of social rewards/[# of social reward + # of heroin infusions]). In Exp. 2, we combined the male and female rats in each group for the statistical analysis because we did not observe sex differences in Exp. 1. We indicate p-values for those less than 0.001 as p<0.001 and report exact p-values for values <0.05 and <0.001. In Table S1 we report the full statistical results of the experiments and in Table S2 we provide results of inactive lever presses during the relapse tests.
Results
Incubation of heroin craving after social choice-induced abstinence
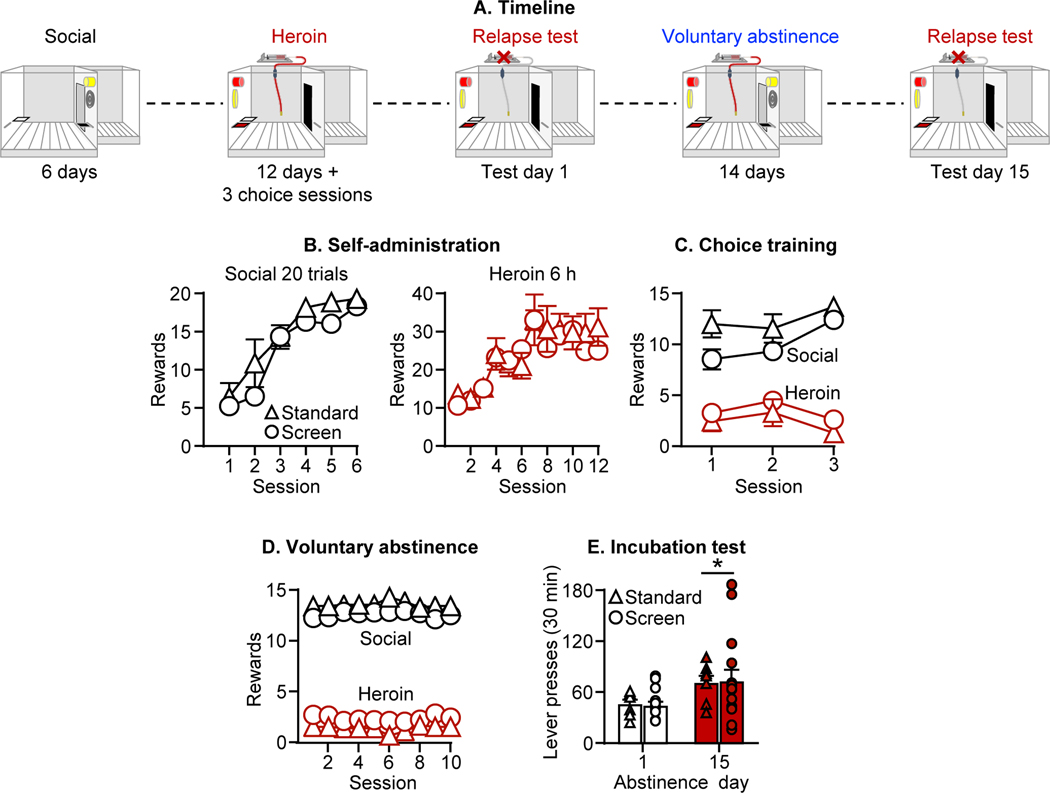
In Exp. 1 we determined whether social choice-induced abstinence would decrease incubation of heroin craving. The experiment consisted of 3 phases (Fig. 1A): self-administration training (3 weeks), relapse tests 1 day after the last self-administration session or after 15 days of either social choice-induced abstinence or homecage forced abstinence.
Figure 1. Social choice-induced voluntary abstinence decreases incubation of heroin craving.
(A) Timeline of the experiments. (B) Self-administration training (rewards: social or heroin infusion). Number of social rewards (60 or 20 trials) or heroin infusions (6 h), in male and female rats. (C) Voluntary Abstinence. Social rewards and heroin infusions earned during 10 discrete-choice sessions (15 trials/session). (D) Incubation (relapse) test. Active lever presses during the 30-min test sessions (including individual data), for both forced (left panel) and social-choice (right panel) groups. During testing, active lever presses led to contingent presentation of the discrete light cue previously paired with heroin infusions during training, but not heroin (extinction conditions). * Different from test day 1, p<0.05. # Different from the social-choice voluntary abstinence group on test day 15, p<0.05. Forced condition: 16/16 females; social choice condition: 18 males/16 females. Data are mean±SEM.
Training:
The male and female rats lever pressed for social interaction and no sex differences were observed (Fig. 1B). The analysis of number of operant social interactions showed a significant main effect of Session (60-trials group: F5,80=68.2, p<0.001; 20-trials group: F5,210=133.2, p<0.001), but not Sex or Session x Sex interaction (p values>0.05). The male and female rats also reliably lever pressed for heroin infusions and no sex differences were observed (Fig. 1B). The analysis of number of infusions showed a significant effect of Session (F11,682=15.4, p<0.001), but not Session x Sex interaction (p values>0.05).
Abstinence phase:
The male and female rats in the voluntary abstinence groups showed strong preference for social interaction over heroin and no sex differences were observed (Fig. 1C). The analysis of the social preference score showed a significant effect of Session (F9,270=3.1, p=0.001), but not Sex or Session X Sex interaction (p values>0.05).
Relapse tests:
Active lever presses during testing were higher after 15 abstinence days than after 1 day (Fig. 1D), demonstrating incubation of heroin craving after either forced abstinence or social choice-induced abstinence. However, the latter condition decreased this incubation effect. There were no sex differences in incubation after either voluntary or forced abstinence. The analysis, which included the between-subjects factors of Sex and Abstinence condition (forced, voluntary), and the within-subjects factor of Abstinence day (1, 15), showed significant effects of Abstinence condition (F1,62=6.2, p=0.02) and Abstinence condition x Abstinence day interaction (F1,62=9.8, p=0.003), but no other interactions (p values>0.05). Inactive lever presses were very low (Table S2) and did not differ between the abstinence days, access conditions, or sexes.
Exp. 1 demonstrates that social choice-induced voluntary abstinence decreased but did not completely prevent incubation of heroin craving.
Incubation of heroin craving after social choice-induced abstinence using the automatic procedure
In Exp. 2 we determined whether the automatic (screen, see Fig. 2) procedure could be used to study social self-administration, social choice-induced abstinence, and incubation of heroin craving after voluntary abstinence. The experiment consisted of 3 phases (Fig. 3A & S1A): self-administration training (3 weeks) that also includes 3 discrete choice sessions, voluntary abstinence (14 days), and relapse tests. [Note: we combined male and female rats in each group (standard and screen) for the statistical analysis because we did not observe sex differences in Exp. 1; also, the n for the no-screen group was too low for meaningful analysis of sex differences; for male versus female comparison of the screen group, see Fig. S1].
Figure 3. Automatic social-choice self-administration procedure to study social choice-induced voluntary abstinence and incubation of heroin craving.
(A) Timeline of the experiments. (B) Self-administration training (rewards: social interaction or heroin). Number of social rewards (20 trials) or heroin infusions (6 h). (C) Choice trials during training. Social rewards and heroin infusions earned during 3 discrete-choice sessions performed after every three days of heroin self-administration training (15 trials/session). (D) Voluntary Abstinence. Social rewards and heroin infusions earned during the 10 discrete-choice sessions (15 trials/session). (E) Incubation (relapse) test. Active lever presses during the 30-min test sessions (including individual data) for both the standard and screen groups. * Different from test day 1, p<0.05. Standard (no screen) condition: n=7 (4 males/3 females); Screen condition: n=15: 7 males/8 females. Data are mean±SEM.
Training:
The rats in the semi-automatic (no-screen) and the automatic (screen) procedure lever pressed for a social peer and there were no group differences. The analysis showed a significant effect of Session (F5,100=72.1, p<0.001), but not Access condition or Session x Access condition (standard, screen) interaction (p values>0.05). During training for heroin self-administration, the rats increased their drug intake over time (Fig. 3B). The analysis showed a main effect of Session (F11,220=25.7, p<0.001) but not Access condition or Session x Access condition interaction (p values>0.05).
Discrete choice sessions during training:
During the three discrete choice sessions, the rats in both access conditions showed a strong preference for social interaction (Fig. 3C). The analysis of the social preference score showed a significant effect of Session (F2,40=5.0, p=0.01), but no effect of Access condition or interaction between the two factors (p values>0.05).
Abstinence phase:
The rats showed strong preference for social interaction, an effect that was independent of the access condition (Fig. 3D). The analysis of the social preference scores showed no significant effects of Access condition, Session, or an interaction between the two factors (p values>0.05).
Relapse tests:
Active lever presses during the tests were higher after 15 abstinence days than after 1 day in both the standard and screen conditions (Fig. 3E), demonstrating incubation of heroin craving after social choice-induced abstinence under both access conditions. The analysis, which included the between-subjects factors of Access condition and the within-subjects factors of Abstinence day, showed a significant effect of Abstinence day (F1,20=5.5, p=0.03), but no significant effects of Abstinence condition or an interaction between the two factors (p values>0.05). Inactive lever presses were very low (Table S2) and did not differ between abstinence days or access conditions.
Exp. 2 confirms that incubation of heroin craving occurs after social choice-induced voluntary abstinence. More importantly, this experiment demonstrates that the automatic social self-administration and choice procedure can replace the original semi-automatic labor-intensive social self-administration procedure.
Discussion
There are two main findings in our study. First, independent of sex, social choice-induced abstinence decreased incubation of heroin craving. Second, there were minimal differences in social self-administration, social choice-induced abstinence, and incubation of heroin craving in rats trained in the semi-automatic procedure (8) and those trained in the fully-automatic screen procedure. The fully automatic procedure overcomes two main limitations of the semi-automatic procedure (8)—intense workload and rat-human interaction—and can facilitate the study of social factors in addiction and other psychiatric disorders.
Incubation of drug craving after voluntary abstinence
As in previous studies using both sexes (19) or male rats (36–39), we report incubation of heroin craving after forced abstinence. More importantly, we found reliable, albeit reduced (compared with forced abstinence), incubation of heroin craving after social choice-induced abstinence. This pattern of results is different from that seen in our recent studies using choice-induced abstinence with social interaction or palatable food. Specifically, social choice-induced abstinence prevented incubation of methamphetamine craving (8) and food choice-induced abstinence prevented incubation of heroin craving (19). Below we discuss these different results across drug classes and voluntary abstinence conditions. We caution that our conclusions/speculations are based on comparisons across studies.
The different effect of social choice on incubation of methamphetamine versus heroin craving may be due to the differential impact of social interaction on dissociable behavioral and brain mechanisms controlling opioid versus psychostimulant reward (29, 30, 32, 33, 40, 41) and relapse (31, 42, 43). Relevant here are data indicating that different mechanisms control incubation of psychostimulant versus opiate craving (44). Specifically, inhibition GDNF signaling in VTA decreases incubation of cocaine but not heroin craving (39, 45). In contrast, chronic delivery of the Toll-like receptor 4 antagonist (+)–naltrexone decreases incubation of heroin but not methamphetamine craving (38). Additionally, reversible inactivation of orbitofrontal cortex decreases incubation of heroin but not methamphetamine craving (37, 46).
A more challenging task is explaining the different effects of social choice-induced abstinence (partial inhibition) versus palatable food choice-induced abstinence (complete inhibition) (19) on incubation of heroin craving. We expected similar effects of the voluntary abstinence conditions, because endogenous opioids are critical for heroin (29, 33, 40, 47) and palatable food (48–51) reward, and also contribute to social reward (52–54). We previously proposed that in the food choice-induced abstinence procedure, the palatable food acts as a substitute for heroin in a manner akin to agonist substitution therapy (55), leading to decreased heroin seeking one day after the removal of the food substitute. A similar mechanism may account for the partial inhibitory effect of social choice-induced abstinence, but to a lesser degree, potentially due to a more prominent role of endogenous opioids in palatable food reward than in social reward, which is critically dependent on other neuromodulators like oxytocin and vasopressin (56, 57).
Finally, a main finding in our study is the lack of sex differences in heroin self-administration, preference for social interaction over heroin, and relapse after social choice-induced abstinence or forced abstinence. These results confirm and extend our previous study on lack of sex differences in heroin self-administration, food choice-induced abstinence, and relapse after food choice-induced abstinence or forced abstinence (19).
Methodological and interpretation considerations
An alternative explanation for the lower incubation in the voluntary abstinence condition in Exp. 1 is that, unlike the forced abstinence condition, during social choice-induced abstinence the rats were exposed to low amounts of heroin (mean of 1.5±0.2 [males] and 1.9±0.2 [females] infusions/d). While we cannot rule out this explanation, we believe it is unlikely because we found no correlation between heroin intake during the 10 sessions of voluntary abstinence and active lever presses during the day 15 relapse test in Exp. 1 (Pearson r=0.3, p=0.1), and a positive (rather than negative) correlation between the two measures in Exp. 2 (Pearson r=0.55, p=0.01).
Another issue to consider is that the rats in the homecage forced abstinence condition were not exposed to the self-administration chambers between day 1 and day 15 relapse test. However, it is unlikely that exposing them to the self-administration chambers without access to their social partners would decrease incubation of drug craving, because in previous studies we found that incubation of heroin or cocaine craving reliably occurs after forced abstinence in either the self-administration chambers or the homecage (27, 36, 39, 58). However, we cannot rule out the possibility that forced abstinence plus operant social interaction in the self-administration chambers without choice will decrease incubation of drug craving. In this regard, previous studies have shown that homecage environmental enrichment, which includes a social interaction component, decreases incubation of cocaine and sucrose craving (59–61).
From the perspective of the translation of results from studies on incubation of craving in rat models (which primarily have used forced abstinence (28, 44, 62, 63)) to the human condition, it should be noted that this phenomenon is less robust and more variable in human than in rat studies (64–66). There are many reasons for this state-of-affairs, but based on our present and previous study (8) on the inhibitory effect of volitional social interaction on incubation of drug craving, one reason might be different degrees of positive (and negative) social interaction of the subjects in the human studies.
Automatic social-choice self-administration and choice procedure
In our original social self-administration and choice procedure, we manually removed the social- partner rats after each social interaction (8). The intense experimenter workload is a limiting factor for timely data collection and a potential experimental confound due to extensive human-rat physical interaction. These two limitations decrease the likelihood that other researchers will use our operant social interaction procedure (8). Here, we introduced an automatic social self-administration and choice procedure by adding a custom-made screen to separate the self-administration chambers from the social-partner chambers. This modification was partially inspired by an early study showing that rhesus monkeys prefer to open a window for visual access to a room containing another monkey over food (67). In pilot studies, we tested different screen designs with different shapes and hole sizes (circle versus rectangles). We excluded the circle design because rats did not reliably perform the operant task with small holes that prevented extensive physical social interaction or became stuck in larger holes that allowed social interaction but not chamber crossing. We chose the current rectangle design with smaller holes for females because they were able to cross to the other chambers with the “male-size” screen (Fig. 2). The new automatic screen procedure allows the rats to physically interact with their peers, which is a critical component of social reward in rodents (53) and eliminates procedural limitations of the semi-automatic procedure (8).
Concluding remarks
We showed that the protective effect of social interaction on drug relapse (8) generalizes to male and female rats with a history of heroin self-administration. More broadly, the results from our study and our previous choice-induced voluntary abstinence studies with palatable food and social rewards (68) highlight the notion that incorporating choice procedures and social factors into rodent models is critical for a more complete behavioral and mechanistic understanding of drug addiction (2, 4, 69–72). From a clinical perspective and within the context of the current opioid crisis (73, 74), our findings highlight the importance of combining social-based behavioral treatments (16, 23) with opioid agonist maintenance treatments (1). Finally, beyond addiction, the automatic social self-administration and choice procedure provides an ideal tool to study the mechanisms of social reward, and its disruption in animal models of autism, depression, PTSD, and schizophrenia.
Supplementary Material
Acknowledgment and Financial Disclosure:
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the text of the paper. The research was supported by the Intramural Research Program of NIDA, a fellowship from the NIH Center on Compulsive Behaviors (MV), and NARSAD Distinguished Investigator Grant Award (YS).
Footnotes
Data availability and resource sharing: Materials, datasets, protocols and chamber details are available upon request to Marco Venniro (venniro.marco@nih.gov) or Yavin Shaham (yshaham@intra.nida.nih.gov).
References
- 1.Epstein DH, Heilig M, Shaham Y (2018): Science-based actions can help address the opioid crisis. Trends Pharmacol Sci. 39:911–916. [DOI] [PubMed] [Google Scholar]
- 2.Heilig M, Epstein DH, Nader MA, Shaham Y (2016): Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci. 17:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit H, Epstein DH, Preston KL (2018): Does human language limit translatability of clinical and preclinical addiction research? Neuropsychopharmacology. 43:1985–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed SH (2010): Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 35:172–184. [DOI] [PubMed] [Google Scholar]
- 5.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003): The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 168:3–20. [DOI] [PubMed] [Google Scholar]
- 6.Kalivas PW, McFarland K (2003): Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 168:44–56. [DOI] [PubMed] [Google Scholar]
- 7.Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. (2015): Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 78:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, et al. (2018): Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 21:1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, et al. (1991): A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 148:1218–1224. [DOI] [PubMed] [Google Scholar]
- 10.Hunt GM, Azrin NH (1973): A community-reinforcement approach to alcoholism. Behav Res Ther. 11:91–104. [DOI] [PubMed] [Google Scholar]
- 11.Higgins ST, Heil SH, Lussier JP (2004): Clinical implications of reinforcement as a determinant of substance use disorders. Annu Rev Psychol. 55:431–461. [DOI] [PubMed] [Google Scholar]
- 12.Preston KL, Umbricht A, Epstein DH (2002): Abstinence reinforcement maintenance contingency and one-year follow-up. Drug Alcohol Depend. 67:125–137. [DOI] [PubMed] [Google Scholar]
- 13.Stitzer ML, Jones HE, Tuten M, Wong C (2011): Community reinforcement approach and contingency management interventions for substance abuse Handbook of Motivational Counseling: Goal-Based Approaches to Assessment and Intervention with Addiction and Other Problems (eds Cox WM and Klinger E), John Wiley & Sons, Ltd, Chichester, UK [Google Scholar]
- 14.Roll JM (2007): Contingency management: an evidence-based component of methamphetamine use disorder treatments. Addiction. 102 Suppl 1:114–120. [DOI] [PubMed] [Google Scholar]
- 15.Silverman K, DeFulio A, Sigurdsson SO (2012): Maintenance of reinforcement to address the chronic nature of drug addiction. Prev Med. 55 Suppl:S46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azrin NH (1976): Improvements in the community-reinforcement approach to alcoholism. Behav Res Ther. 14:339–348. [DOI] [PubMed] [Google Scholar]
- 17.Caprioli D, Zeric T, Thorndike EB, Venniro M (2015): Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addict Biol. 20:913–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, et al. (2017): Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci. 37:1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venniro M, Zhang M, Shaham Y, Caprioli D (2017): Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacology. 42:1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, et al. (2017): The anterior insular cortex-->central amygdala glutamatergic pathway Is critical to relapse after contingency management. Neuron. 96:414–427 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn K, DeFulio A, Everly JJ, Donlin WD, Aklin WM, Nuzzo PA, et al. (2015): Employment-based reinforcement of adherence to oral naltrexone in unemployed injection drug users: 12-month outcomes. Psychol Addict Behav. 29:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristjansson AL, Sigfusdottir ID, Thorlindsson T, Mann MJ, Sigfusson J, Allegrante JP (2016): Population trends in smoking, alcohol use and primary prevention variables among adolescents in Iceland, 1997–2014. Addiction. 111:645–652. [DOI] [PubMed] [Google Scholar]
- 23.Aklin WM, Wong CJ, Hampton J, Svikis DS, Stitzer ML, Bigelow GE, et al. (2014): A therapeutic workplace for the long-term treatment of drug addiction and unemployment: eight-year outcomes of a social business intervention. J Subst Abuse Treat. 47:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed SH, Koob GF (1998): Transition from moderate to excessive drug intake: change in hedonic set point. Science. 282:298–300. [DOI] [PubMed] [Google Scholar]
- 25.Deroche-Gamonet V, Belin D, Piazza PV (2004): Evidence for addiction-like behavior in the rat. Science. 305:1014–1017. [DOI] [PubMed] [Google Scholar]
- 26.Zimmer BA, Oleson EB, Roberts DC (2012): The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology. 37:1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimm J, Hope B, Wise R, Shaham Y (2001): Neuroadaptation - Incubation of cocaine craving after withdrawal. Nature. 412:141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venniro M, Caprioli D, Shaham Y (2016): Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 224:25–52. [DOI] [PubMed] [Google Scholar]
- 29.Badiani A, Belin D, Epstein D, Calu D, Shaham Y (2011): Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 12:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caprioli D, Celentano M, Dubla A, Lucantonio F, Nencini P, Badiani A (2009): Ambience and drug choice: cocaine- and heroin-taking as a function of environmental context in humans and rats. Biol Psychiatry. 65:893–899. [DOI] [PubMed] [Google Scholar]
- 31.De Luca MT, Montanari C, Meringolo M, Contu L, Celentano M, Badiani A (2019): Heroin versus cocaine: opposite choice as a function of context but not of drug history in the rat. Psychopharmacology (Berl). 236:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Pirro S, Galati G, Pizzamiglio L, Badiani A (2018): The affective and neural correlates of heroin versus cocaine use in addiction are influenced by environmental setting but in opposite directions. J Neurosci. 38:5182–5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ettenberg A, Pettit HO, Bloom FE, Koob GF (1982): Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology (Berl). 78:204–209. [DOI] [PubMed] [Google Scholar]
- 34.Bossert JM, Adhikary S, St Laurent R, Marchant NJ, Wang HL, Morales M, et al. (2016): Role of projections from ventral subiculum to nucleus accumbens shell in context-induced reinstatement of heroin seeking in rats. Psychopharmacology. 233:1991–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shalev U, Grimm J, Shaham Y (2002): Neurobiology of relapse to heroin and cocaine seeking: A review. Pharmacol Rev. 54:1–42. [DOI] [PubMed] [Google Scholar]
- 36.Shalev U, Morales M, Hope B, Yap J, Shaham Y (2001): Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 156:98–107. [DOI] [PubMed] [Google Scholar]
- 37.Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT (2012): Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci. 32:11600–11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, et al. (2013): Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry. 73:729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Airavaara M, Pickens CL, Stern AL, Wihbey KA, Harvey BK, Bossert JM, et al. (2011): Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addiction Biol. 16:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mello NK, Negus SS (1996): Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 14:375–424. [DOI] [PubMed] [Google Scholar]
- 41.Badiani A (2013): Substance-specific environmental influences on drug use and drug preference in animals and humans. Curr Opin Neurobiol. 23:588–596. [DOI] [PubMed] [Google Scholar]
- 42.Bossert J, Ghitza U, Lu L, Epstein D, Shaham Y (2005): Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur J Pharmacol. 526:36–50. [DOI] [PubMed] [Google Scholar]
- 43.Bossert JM, Marchant NJ, Calu DJ, Shaham Y (2013): The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 229:453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011): Neurobiology of the incubation of drug craving. Trends Neurosci. 34:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, et al. (2009): Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 66:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y (2015): The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology. 40:1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Ree JM, Gerrits MA, Vanderschuren LJ (1999): Opioids, reward and addiction: An encounter of biology, psychology, and medicine. Pharmacol Rev. 51:341–396. [PubMed] [Google Scholar]
- 48.Levine AS, Morley JE, Gosnell BA, Billington CJ, Bartness TJ (1985): Opioids and consummatory behavior. Brain Res Bull. 14:663–672. [DOI] [PubMed] [Google Scholar]
- 49.Kelley AE, Berridge KC (2002): The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 22:3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldo BA (2016): Prefrontal Cortical Opioids and Dysregulated Motivation: A Network Hypothesis. Trends Neurosci. 39:366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pecina S, Berridge KC (2005): Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 25:11777–11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG (1980): Endogenous opioids and social behavior. Neurosci Biobehav Rev. 4:473–487. [DOI] [PubMed] [Google Scholar]
- 53.Vanderschuren LJ, Achterberg EJ, Trezza V (2016): The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev. 70:86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ (2011): Nucleus accumbens mu-opioid receptors mediate social reward. J Neurosci. 31:6362–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dole VP, Nyswander M (1965): A medical treatment for diacetylmorphine (heroin) addiction. A clinical trial with methadone hydrochloride. JAMA. 193:646–650. [DOI] [PubMed] [Google Scholar]
- 56.Johnson ZV, Young LJ (2017): Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev. 76:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caldwell HK, Albers HE (2016): Oxytocin, Vasopressin, and the Motivational Forces that Drive Social Behaviors. Curr Top Behav Neurosci. 27:51–103. [DOI] [PubMed] [Google Scholar]
- 58.Lu L, Grimm J, Dempsey J, Shaham Y (2004): Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 176:101–108. [DOI] [PubMed] [Google Scholar]
- 59.Chauvet C, Goldberg SR, Jaber M, Solinas M (2012): Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology. 63:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thiel KJ, Painter MR, Pentkowski NS, Mitroi D, Crawford CA, Neisewander JL (2012): Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict Biol. 17:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grimm JW, Barnes JL, Koerber J, Glueck E, Ginder D, Hyde J, et al. (2016): Effects of acute or chronic environmental enrichment on regional Fos protein expression following sucrose cue-reactivity testing in rats. Brain Struct Funct. 221:2817–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong Y, Taylor JR, Wolf ME, Shaham Y (2017): Circuit and synaptic plasticity mechanisms of drug relapse. J Neurosci. 37:10867–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolf ME (2016): Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 17:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Venniro M, Shaham Y (2016): Translational Research on Incubation of Cocaine Craving. JAMA Psychiatry. 73:1115–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, et al. (2011): Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 69:708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parvaz MA, Moeller SJ, Goldstein RZ (2016): Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry. 73:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corwin RL, Schuster CR (1993): Anorectic specificity as measured in a choice paradigm in rhesus monkeys. Pharmacol Biochem Behav. 45:131–141. [DOI] [PubMed] [Google Scholar]
- 68.Venniro M, Caprioli D, Shaham Y (2019): Novel models of drug relapse and craving after voluntary abstinence. Neuropsychopharmacology. 44:234–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banks ML, Negus SS (2017): Insights from preclinical choice models on treating drug addiction. Trends Pharmacol Sci. 38:181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nader MA, Banks ML (2014): Environmental modulation of drug taking: Nonhuman primate models of cocaine abuse and PET neuroimaging. Neuropharmacology. 76 Pt B:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed SH (2018): Trying to make sense of rodents’ drug choice behavior. Prog Neuropsychopharmacol Biol Psychiatry. 87:3–10. [DOI] [PubMed] [Google Scholar]
- 72.Bardo MT, Neisewander JL, Kelly TH (2013): Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 65:255–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soelberg CD, Brown RE Jr., Du Vivier D, Meyer JE, Ramachandran BK (2017): The US Opioid Crisis: Current Federal and State Legal Issues. Anesth Analg. 125:1675–1681. [DOI] [PubMed] [Google Scholar]
- 74.Hedegaard H, Warner M, Minino AM (2017): Drug Overdose Deaths in the United States, 1999–2016. NCHS Data Brief.1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.