Abstract
Vertebral fractures (VFx) are common among older adults. Epidemiological studies report high occurrence of VFx at mid-thoracic and thoracolumbar regions of the spine; however, reasons for this observation remain poorly understood. Prior reports of high ratios of spinal loading to vertebral strength in the thoracolumbar region suggest a possible biomechanical explanation. However, no studies have evaluated load-to-strength ratios (LSRs) throughout the spine for a large number of activities in a sizeable cohort. Thus, we performed a cross-sectional study in a sample of adult men and women from a population-based cohort to: 1) determine which activities cause the largest vertebral LSRs, and 2) examine patterns of LSRs along the spine for these high-load activities. We used subject-specific musculoskeletal models of the trunk to determine vertebral compressive loads for 109 activities in 250 individuals (aged 41 to 90 years, 50% women) from the Framingham Heart Study. Vertebral compressive strengths from T4 to L4 were calculated from computed tomography–based vertebral size and bone density measurements. We determined which activities caused maximum LSRs at each of these spinal levels. We identified nine activities that accounted for >95% of the maximum LSRs overall and at least 89.6% at each spinal level. The activity with the highest LSR varied by spinal level, and three distinct spinal regions could be identified by the activity producing maximum LSRs: lateral bending with a weight in one hand (upper thoracic), holding weights with elbows flexed (lower thoracic), and forward flexion with weight (lumbar). This study highlights the need to consider a range of lifting, holding, and non-symmetric activities when evaluating vertebral LSRs. Moreover, we identified key activities that produce higher loading in multiple regions of the spine. These results provide the first guidance on what activities to consider when evaluating vertebral load-to-strength ratios in future studies, including those examining dynamic motions and the biomechanics of VFx.
Keywords: BIOMECHANICS, BONE QCT, FRACTURE PREVENTION, FRACTURE RISK ASSESSMENT, OSTEOPOROSIS
Introduction
Osteoporotic vertebral fractures (VFx) are associated with high morbidity, increased mortality, and decreased quality of life. VFx are not only the most common fractures in adults older than 50 years but also are second only to hip fractures in terms of length of hospital stay, intensive care unit (ICU) use, and cost.(1,2) The socioeconomic aspect of VFx is exacerbated by an insufficient rate of treatment after diagnosis of osteoporosis.(3) Thus, tools for early detection of increased risk of VFx and improved approaches for VFx prevention are vital to avoid subsequent complications.(3) Areal bone mineral density (BMD), measured by dual-energy X-ray absorptiometry (DXA), is widely used to predict likelihood of fracture;(4) however, many vertebral fractures occur in those who do not have osteoporosis by BMD testing.(5) Moreover, risk assessment by BMD alone may be insufficient because it does not reflect the activity and loading conditions at which fracture may occur (eg, spinal loading).
Several studies have found higher load-to-strength ratios (LSRs) in subjects with prevalent vertebral fractures compared with those without fractures,(6–9) and that higher LSR is a significant predictor of incident vertebral fractures.(10,11) However, the activities used for assessment of LSRs in these prior studies were not selected with any strong rationale. In particular, these studies have evaluated LSRs only for upright standing,(7,8) bending forward,(6–9) bending forward while holding weight,(7–11) and bending and twisting while holding weight.(9) It is unclear whether these activities are likely to cause vertebral fractures, though notably the reported LSRs were mostly less than one, even for fracture cases. Indeed, the activities causing vertebral fractures are not well understood and often unknown.(12–14) Moreover, these studies have only evaluated LSRs at a few levels, T10,(9) L1,(10,11) and L3.(6–9) The one study(9) reporting LSRs at multiple vertebral levels reported LSRs were lower at T10 than at L3, but it is unknown whether different activities might produce relatively more loading at T10. Better understanding of the patterns of loading and LSRs and what activities produce high loading in different parts of the spine would better inform biomechanical studies of vertebral fracture risk.
Vertebral fractures occur most frequently at mid-thoracic and thoracolumbar regions of the spine.(6–9,12,15–17) We previously reported high LSRs in the thoracolumbar region of the spine,(18) suggesting a possible biomechanical explanation for increased incidence of vertebral fractures in this region. However, that study was limited by examining a single representative female musculoskeletal model and vertebral strength. Thus, it is unclear whether this key finding will be upheld across a diverse population. Furthermore, prior studies evaluating the associations of LSRs with vertebral fractures have only examined LSRs for a few activities at a few vertebral levels. Thus, the aims of the current cross-sectional study are to determine which among a large group of activities causes the largest vertebral LSRs for a population-based cohort and to examine patterns of LSRs along the spine for these high-load activities.
Materials and Methods
Subjects
Subjects for a cross-sectional analysis were selected from the Framingham Heart Study Multidetector CT Study cohort(19) in a sex- and age-stratified manner, with 25 men and 25 women included in each of five age groups (40–49, 50–59, 60–69, 70–79, 80+). The 125 men were the same subjects included in our prior study,(20) and the 125 women were selected in a similar manner. Subjects were sampled from cohort participants who had previously collected computed tomography (CT) scans of the chest and abdomen including approximately levels T4 to L4. Scans were collected on an eight-detector helical CT scanner (Lightspeed Plus, General Electric, Milwaukee, WI, USA; scan settings: 120 kVP, 320 mAs, in-plane pixel size 0.68 × 0.68 mm, slice thickness 2.5 mm). Subjects were scanned with a three-chamber hydroxyapatite phantom (Image Analysis, Inc., Lexington, KY, USA). Use of these previously collected, de-identified data was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center.
Prediction of spinal loading using subject-specific musculoskeletal modeling
Similar to our previous studies,(18,20,21) we begin with a generic sex-specific musculoskeletal (MSK) model of the spine and then create subject-specific MSK models by scaling these base models using measured height, weight, trunk muscle morphology, and spinal curvature. In brief, we measure subject-specific trunk muscle morphology and intervertebral joint angles from the CT scans and use these data to adjust the MSK models (Fig. 1). The methods and models for these 250 individuals are available online.(22)
Fig 1.
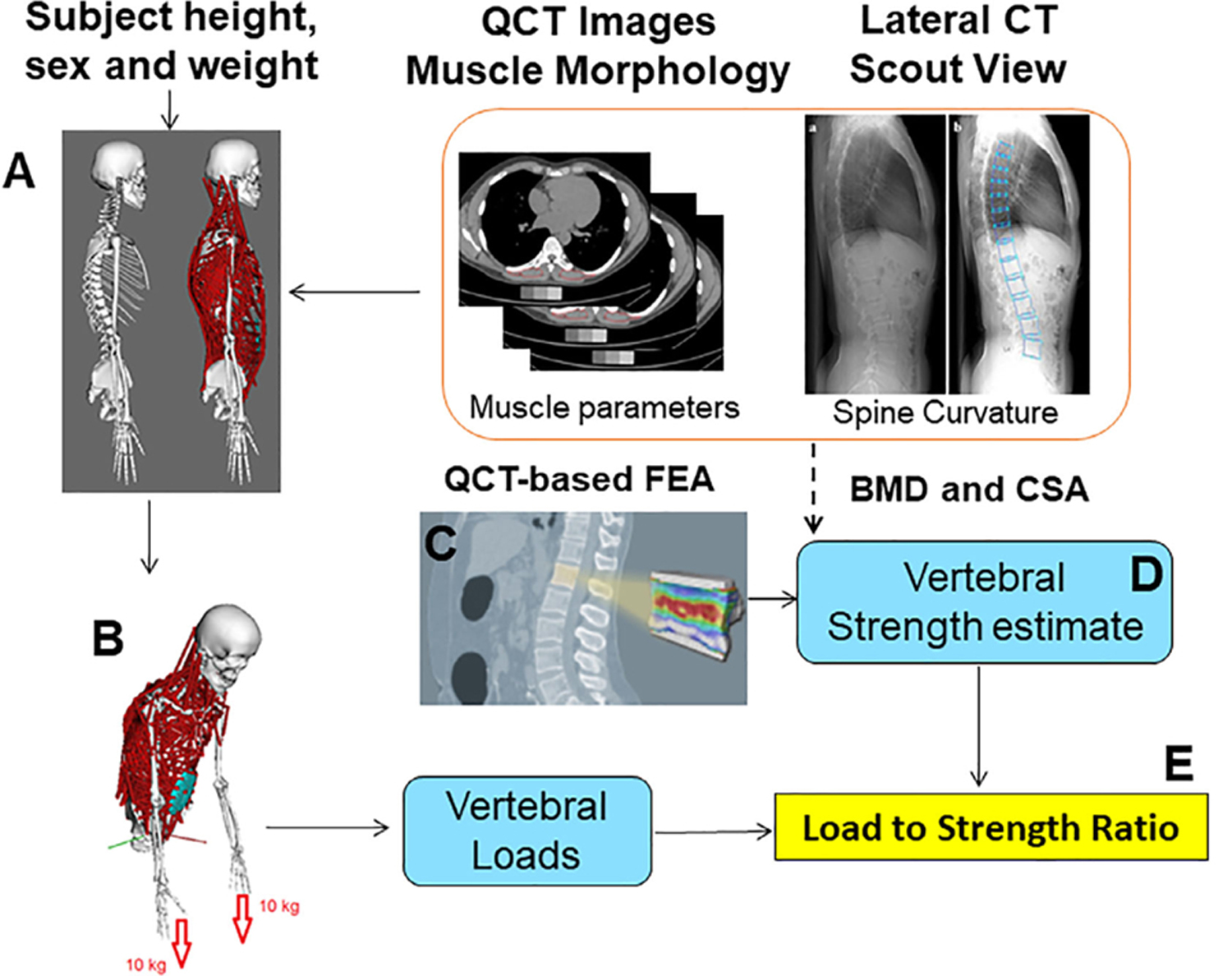
The procedure to develop subject-specific models for everyone in this study. Briefly, a musculoskeletal (MSK) model is adjusted for sex, height/weight, muscle morphology, and spinal curvature (A). Then we applied external loading and postures to simulate an activity (126 activities in this study) (B). Finally, static optimization was used to predict muscle forces, and then a joint analysis tool in OpenSim was used to calculate spinal joint loading.(20) Spinal loading comes from MSK modeling, while vertebral strength comes from a regression equation developed based on QCT-based finite-element analysis (FEA) (C, D) to predict vertebral strength from CSA and vBMD of each vertebra. For those missing levels (where QCT data was missing), we used a multiple imputation approach to fill the missing vertebral strength data along the spine. We then calculated load-to-strength ratio (LSR) at each vertebral level using both vertebral loads and vertebral strength (E).
Using static optimization in OpenSim (version 3.2)(23) to minimize the sum of cubes of muscle activations, we predicted individual muscle forces during each of the activities simulated. We simulated 126 static postures simulating daily activities, similar to previous studies (Supplemental Table S2).(18) Musculoskeletal modeling inputs (kinematics and external loading) for the 126 activities are available online.(22) After prediction of muscle forces, the joint analysis tool in OpenSim was used to calculate intervertebral joint loading along the spine (T4 to L4). In total, we performed 250 × 126 (ie, subjects × activities) = 31,500 MSK modeling simulations.
Prediction of subject-specific vertebral strength along the spine
We compiled data from our prior studies in the Framingham Heart Study CT Study cohort(9,24,25) and identified vertebral levels where we had obtained measurements of integral volumetric BMD, vertebral body cross-sectional area (CSA), and vertebral compressive strength (by CT-based finite element analysis). This data set included 339 vertebral levels (128 T8,4 T9, 75 L2, 129 L3, 3 L4) from 134 individuals. We then created a mixed-effects linear regression with vertebral strength as the dependent variable, BMD × CSA as a fixed-effect independent variable and subject as a random variable. The resulting fixed-effects equation was:
| (Equation1) |
where vertebral strength is in N, CSA is the cross-sectional area of the mid-vertebral body (in cm2), and BMD is in g/cm3. This regression equation fits the finite-element analysis strength data very well (R2 = 0.895) (Fig. 2), and the standard error of the estimate is 920 N.
Fig 2.
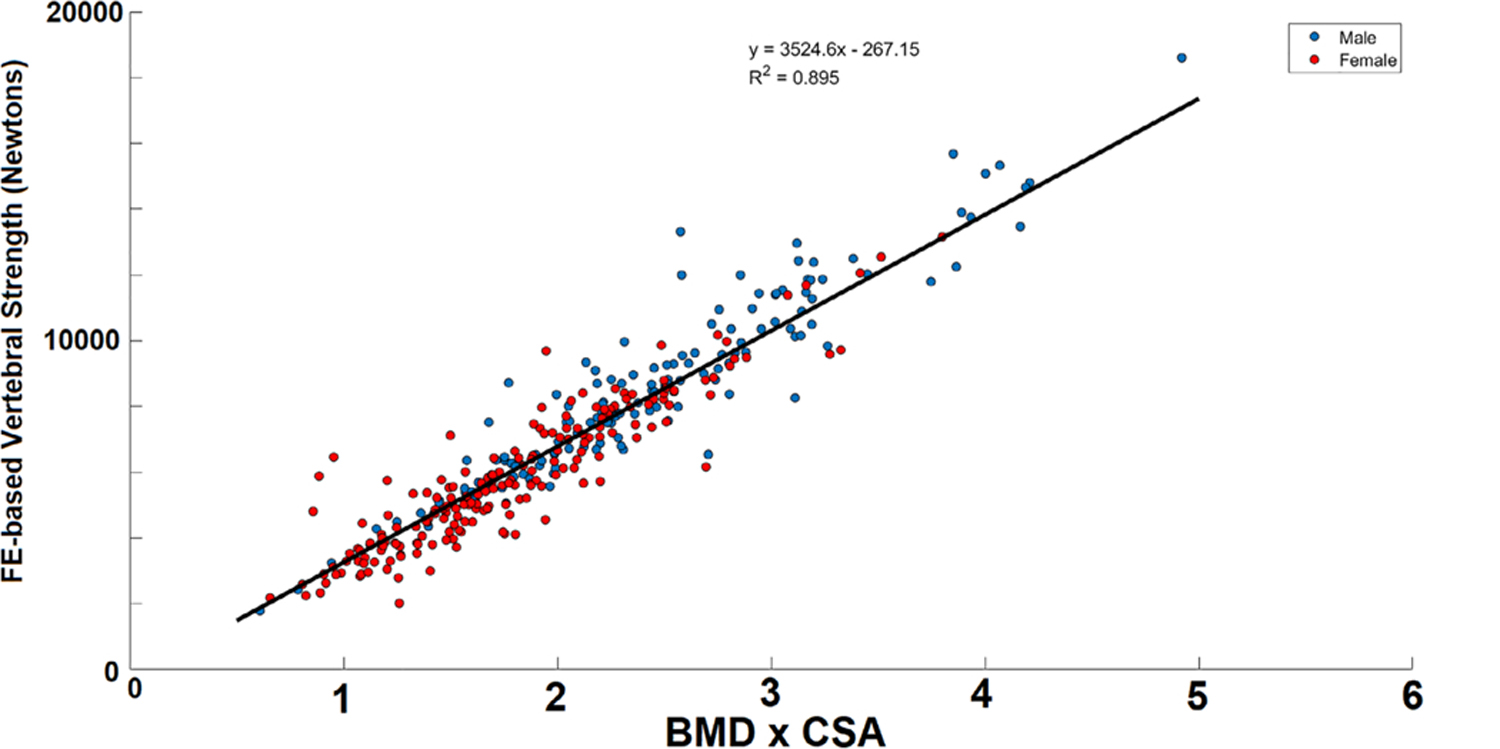
Association between vertebral strength from CT-based finite-element analysis and the product of vertebral cross-sectional area (CSA) and integral volumetric bone mineral density (BMD) (R2 = 0.895).
We then measured BMD and vertebral body CSA and used Eq. 1 to estimate vertebral strength at all available vertebral levels from CT scans, after excluding vertebrae with prevalent vertebral fracture. Of a possible 3250 vertebral levels (250 subjects × 13 vertebral levels per subject), 3086 were available in CT scans. We then excluded 25 vertebrae with prevalent vertebral fractures from measurement. Thus, BMD and CSA were measured at 3061 (94%) vertebral levels. For the 189 levels with missing measurements, we used multiple imputation by chained equations to estimate missing BMD and CSA values (Stata/IC 13.1, StataCorp LP, College Station, TX, USA). We generated 10 imputations of the data, with non-missing values at L3, plus age, sex, height, and weight included as independent variables in the imputations. Each of the 10 imputed data sets was used to generate a corresponding complete strength data set using Eq. 1 and subsequently a complete load-to-strength data set. Further analyses were performed on a final data set created by merging the 10 imputed data sets.
Identify activities that lead to high load-to-strength ratio
After simulation of 126 activities in 250 subject-specific spine models, we calculated spinal load-to-strength ratios at levels T4 to L4. We determined which single activity produced the maximum LSR for each subject at each vertebra examined and for what percentage of examined vertebrae (250 subjects × 13 levels = 3250 vertebrae examined) each activity produced maximum LSRs, or:
| (Equation2) |
Where Xijk indicates for vertebrae i of subject j whether activity k, does (1) or does not (0) produce the maximum LSR among all the activities examined. n is the number of spinal levels T4 to L4 that is n = 13 levels, and N is number of subjects that is N = 250.
Pk is thus the percentage of vertebrae where activity k causes maximum LSR. Activities were then ranked according to the percentage Pk from highest to lowest. Finally, we identified p key activities (in rank order) collectively responsible for maximum LSRs for at least 95% of the examined vertebrae, or:
| (Equation3) |
Where Pr is the percentage of vertebrae where the activity ranked r causes maximum LSR, and p is the minimum number of activities satisfying Eq. 3.
Effect of prevalent vertebral fractures on load-to-strength ratios
As previous studies have noted higher LSRs in individuals with prevalent or incident fractures,(6–11) we also sought to confirm this finding for the key activities identified. Our sample included 23 subjects with one or more prevalent moderate or severe (semiquantitative score of SQ2+) vertebral fractures (28 vertebral fractures total). We compared LSR for these subjects to non-fractured subjects for the key activities identified, using two-way repeated measures ANOVAs for prevalent fracture (subject nested in fracture status) and vertebral level (repeated measure). Subjects younger than 55 years were excluded because vertebral fractures were not routinely assessed below age 55 years. Post hoc comparisons with a Bonferroni correction were performed by level if a significant effect of prevalent fracture or prevalent fracture × level interaction was found. Analysis was performed in Stata/IC 13.1 (StataCorp LP) with significance set at α = 0.05.
Results
Subject characteristics are reported by sex in Table 1. On average, men were taller and heavier than women and had slightly smaller kyphosis and lordosis angles (p < 0.05). Increased age was associated with shorter height and larger kyphosis and lordosis angles (p < 0.05).
Table 1.
Demographics and Characteristics of Study Subjects From the Population-Based Cohort of the Framingham Heart Study
| Men (n = 125) |
Women (n = 125) |
All (n = 250) |
||||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| Age (years) | 64.7 ± 14.0 | 41–88 | 64.3 ± 13.6 | 43–90 | 64.5 ± 13.8 | 41–90 |
| Height (m) | 1.74 ± 0.07 | 1.59–1.92 | 1.60 ± 0.06 | 1.47–1.76 | 1.66 ± 0.10 | 1.59–1.92 |
| Mass (kg) | 85.2 ± 14.2 | 47.2–122.9 | 70.7 ± 14.8 | 43.5–127.0 | 77.9 ± 16.2 | 43.5–127.0 |
| Body mass index (kg/m2) | 28.1 ± 4.2 | 16.0–40.0 | 27.6 ± 5.7 | 16.2–49.5 | 27.9 ± 5.0 | 16.0–49.5 |
| T4/T12 Cobb angle (°) | 33.6 ± 9.0 | 14.4–53.0 | 37.1 ± 10.0 | 14.8–61.4 | 35.3 ± 9.7 | 14.4–61.4 |
| L1/L4 Cobb angle (°) | −15.3 ± 8.0 | −38.2–3.8 | −19.4 ± 10.7 | −46.2–3.8 | −17.4 ± 9.6 | −46.2–3.8 |
Of 126 simulated activities tested, a total of 109 solved successfully in some of the subject-specific models, whereas 17 failed to solve in all models. Of the 109 that solved, we identified 26 activities that produced a maximum LSR in at least one vertebral level. As shown in Table 2 the key activities (top nine ranked) accounted for 95.8% of the maximum LSRs overall, and at least 89.6% at each vertebral level. The most important activity (ie, that which caused the highest percentage of maximum LSRs) varied by vertebral level, as shown in Fig. 3. The two top-ranked activities, holding a weight with 75° or 90° of forward flexion, were the most important from T12 to L4 and contributed to maximum LSRs at every level (Fig. 3; Supplemental Table S1). The next two top-ranked activities, holding weights with elbows flexed, with or without twisting, were the most important from T8 to T11 and also contributed to maximum LSRs at every level. The fifth-ranked activity, lateral bend holding a weight in one hand, was the most important at upper thoracic levels (T4 to T6). The top five activities all contributed from 15% to 25% of maximum LSRs at T7. The remaining activities identified each accounted for <3% of the maximum LSRs overall. Detailed LSR results can be provided upon request and execution of appropriate data-sharing agreements.
Table 2.
Ranking of Activities That Caused Maximum Load-to-Strength Ratios and the Percentage of Subjects for Which the Activity Solved
| Rank | Activity no. | % Subjects solved | Activity description | Percentage of levels at which it causes maximum load-to-strength ratio |
|---|---|---|---|---|
| 1 | 19 | 78% | 75° of trunk flexion with 10 kg weights in each hand | 26.64% |
| 2 | 11 | 29% | 90° trunk flexion with 10 kg weights in each hand | 21.61% |
| 3 | 9 | 100% | Standing, 10 kg weights in each hand, elbows flexed to 90° | 17.92% |
| 4 | 15 | 90% | Standing, 10 kg weight in each hand, elbows flexed to 90°, 30° trunk axial twist | 14.37% |
| 5 | 16 | 86% | 20° trunk lateral bend to the right, with 20 kg in right hand | 8.77% |
| 6 | 61 | 99% | 60° trunk flexion, 5 kg in each hand | 2.39% |
| 7 | 118 | 99% | Sit-up exertion—supine position | 2.03% |
| 8 | 116 | 96% | Upright standing, arms pushing down, shoulders flexed 130° | 1.05% |
| 9 | 73 | 84% | 60° trunk flexion +30° axial twist, 5 kg in each hand | 1.00% |
| 10 | 107 | 94% | Pushing down, arms at side | 0.92% |
| 11 | 114 | 3% | Pushing forward, shoulders flexed 130° | 0.49% |
| 12a | 119 | 100% | Sit-up exertion, 25° flexion at pelvis | 0.49% |
| 13 | 5 | 93% | 90° trunk flexion, arms hanging down | 0.42% |
| 14a | 25 | 93% | 90° of flexion all in lumbar spine (pelvis upright) | 0.42% |
| 15 | 44 | 87% | 60° shoulder flexion, 5 kg in each hand | 0.40% |
| 16 | 32 | 2% | Shoulder flexion 90°, right arm | 0.25% |
| 17 | 14 | 88% | Trunk axial twist and trunk flexion, each at 30° with 10 kg weight | 0.21% |
| 18 | 52 | 49% | 60° shoulder abduction 5 kg in each hand | 0.12% |
| 19 | 8 | 100% | Standing, 20 kg weights in each hand at the side | 0.09% |
| 20a | 20 | 100% | Opening window with both hands (15 N) | 0.09% |
| 21a | 105 | 81% | Pushing forward, arms at side | 0.09% |
| 22 | 17 | 95% | Standing, 20 kg weight in right hand | 0.06% |
| 23a | 113 | 68% | Pushing down, shoulders flexed 130° | 0.06% |
| 24 | 7 | 96% | Standing, 20 kg weight in left hand at the side | 0.03% |
| 25a | 109 | 91% | Pulling back, shoulders flexed 30° | 0.03% |
| 26a | 111 | 98% | Pushing forward, shoulders flexed 130° (49 N) | 0.03% |
Indicates equal importance as previous activity.
Fig 3.
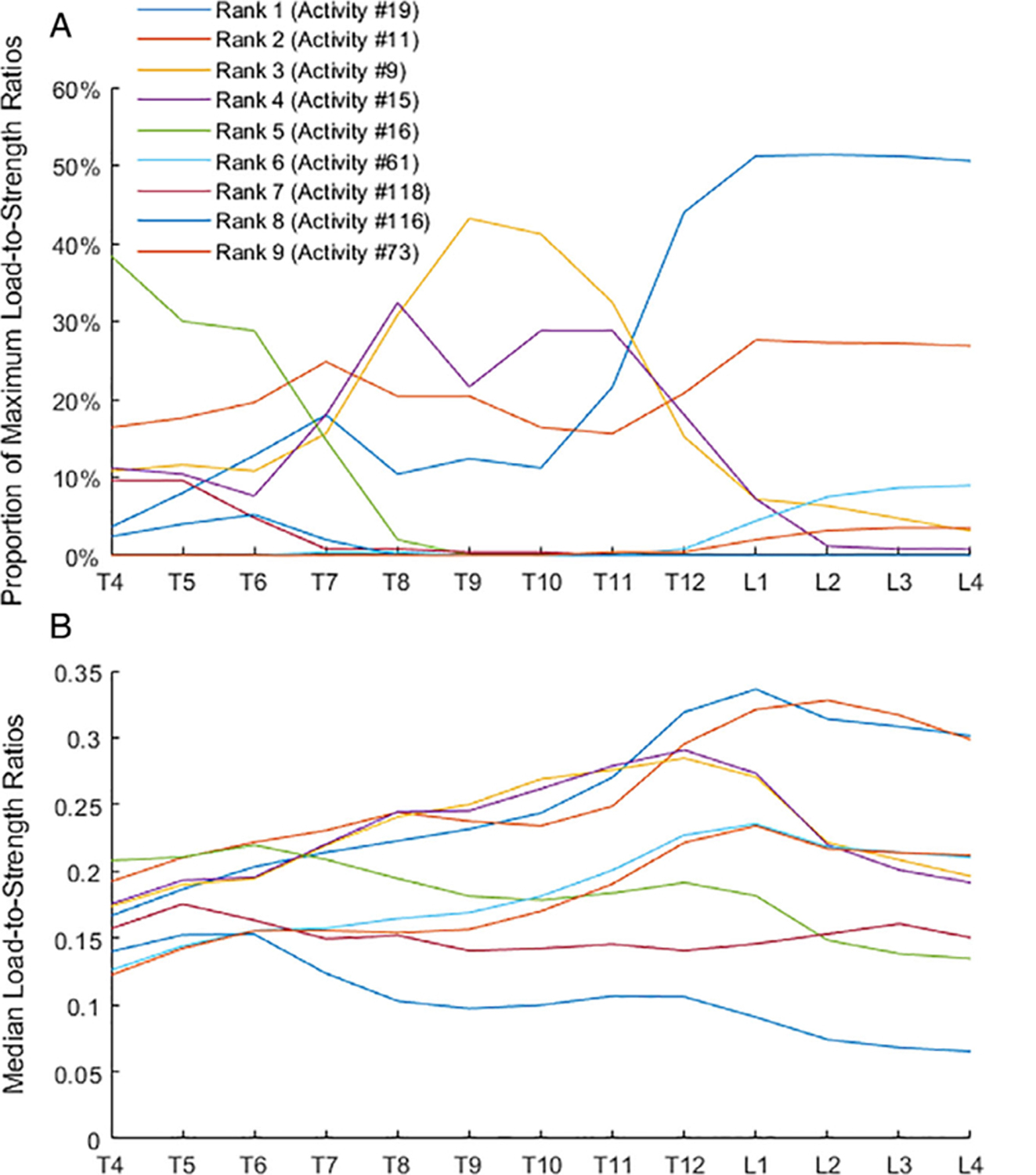
The proportion (%) of maximum load-to-strength ratios at each vertebral level (A) and median values of load-to-strength ratios (B) for nine key activities producing maximum load-to-strength ratios. These nine activities produced 95.8% of the maximum load-to-strength ratios overall among 109 activities examined and at least 89.6% at each vertebral level.
The patterns of LSRs along the spine varied with activity. Specifically, the peak in median LSRs occurred at different spinal locations for different activities (Fig. 3). Activities involving forward flexion with a weight peaked at L1 (activities 19, 61, 73) or L2 (activity 11). Activities involving holding weight with the elbows flexed peaked at T12 (activities 9 and 15). A lateral bend with holding weight on one side peaked at T6 (activity 16). The sit-up and pushing-down activities (activities 118 and 116, respectively) peaked at T5. The value of LSRs for key activities at a level was similar (Fig. 4), whereas the range of LSRs in the population (see 95th percentile error bars in Fig. 4) shows significant overlap. The maximum median value of LSRs for key activities ranged from 0.15 (activity 116 at T6) to 0.34 (activity 19 at L1). For comparison, the median LSRs for neutral standing ranged from 0.04 (L3) to 0.06 (T5).
Fig 4.
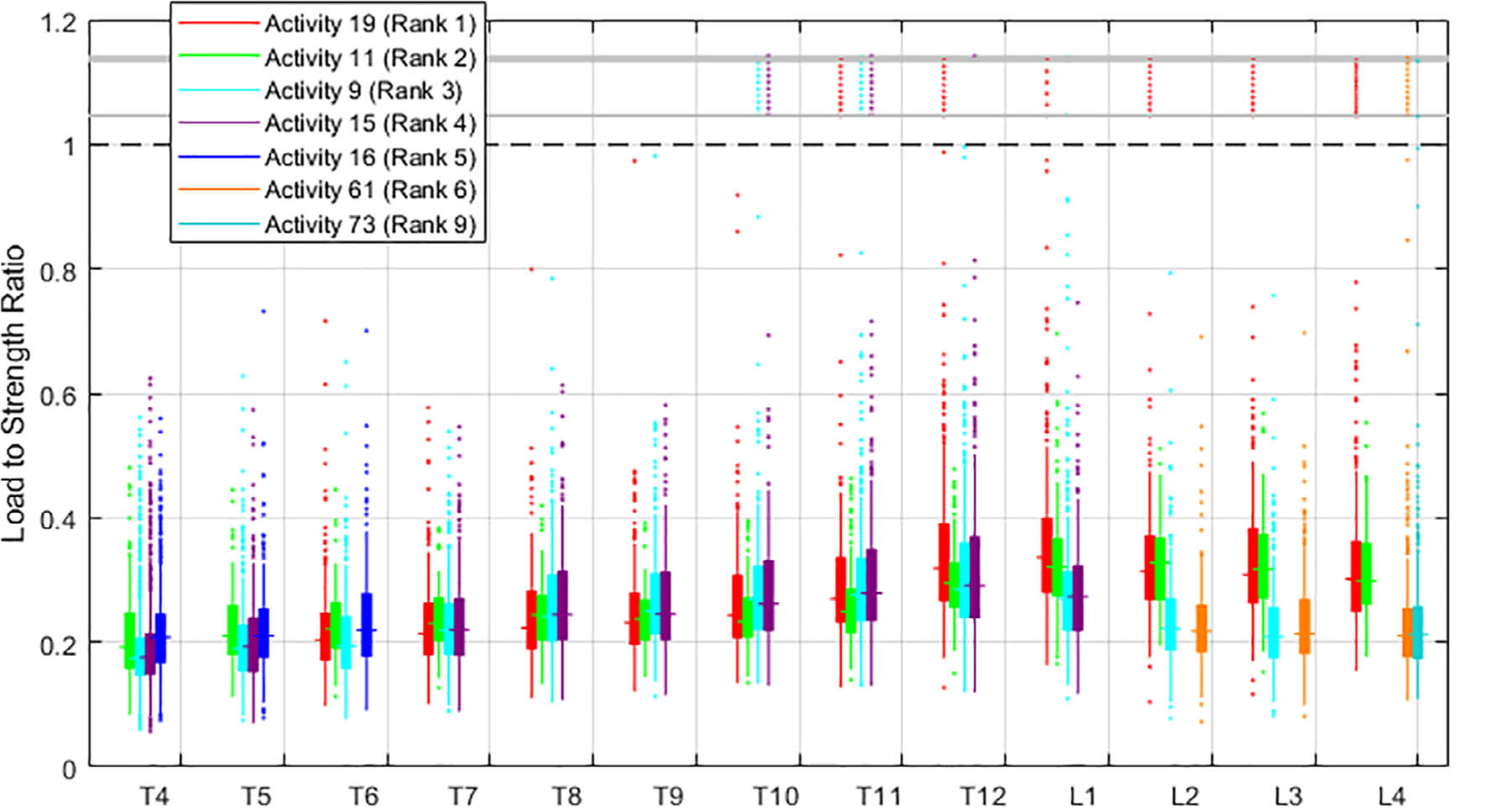
Box plot showing load-to-strength ratios for the top four activities at each level. The four activities shown at each level account for 69.6% to 98.4% of the maximum load-to-strength ratios for that level in the population-based cohort. Three distinct regions of the spine can be found based on the activities contributing to maximum load-to-strength ratios: upper thoracic (T4 to T6), lower thoracic (T8 to T11), lumbar (T12 to L4). For better visibility, outliers of load-to-strength ratio >1 are shown compressed between the gray bars; actual outlier values >1 are found in Supplemental Fig. S1.
Subjects with a prevalent vertebral fracture had significantly higher LSRs than subjects without. Specifically, significant effects (p < 0.05) were found for six of the nine key activities (activities 19, 9, 15, 61, 118, 73), and LSRs were 13% to 32% higher for subjects with prevalent vertebral fractures at levels reporting significant differences in post hoc tests. Detailed results are provided in Supplemental Table S3.
Discussion
In this study, we aimed to identify activities causing large vertebral LSRs for men and women sampled from a population-based cohort and to examine patterns of these LSRs along the spine. By analyzing spinal loading in 250 men and women for 109 postures, representing common activities, we identified nine activities that most frequently caused large LSRs. Some activities only cause large LSRs in certain regions of the spine, and thus we identified three distinct regions of the spine according to the activities producing high LSRs: T4 to T6, T8 to T11, and T12 to L4. Moreover, the activity causing highest LSRs varied among individuals in the population. These findings suggest that studies evaluating LSRs should consider a range of activities and select activities appropriately based on the vertebral levels being examined. The key activities identified here can be used as guidelines when evaluating LSRs in future case-control studies of vertebral fracture and spinal injury.
Patterns of load-to-strength ratios
Based on the LSRs along the spine, we identified key activities of interest for three distinct regions of spine upper thoracic (T4 to T6), lower thoracic (T8 to T11), and lumbar (T12 to L4). It is interesting to note that the boundaries between these regions (T7 to T8 and T12 to L1) correspond to common locations of VFx.(12,15–18) Although LSRs are not notably higher in these locations, there are regional transitions in which activities cause large LSRs. It is possible that these locations are at increased risk of fracture because more types of activities could cause risky loading. Additional work to improve understanding of spine loading, and frequency of loading events, is needed to assess this possibility. Moreover, the current study illustrates the importance of both loading activity and spinal level when evaluating LSRs. For instance, the most common activity simulated in prior studies associating LSRs with vertebral fracture is forward flexion with weight,(7–11) whereas the current results show this type of activity is most relevant in the lumbar spine but less so in the thoracic spine. We found higher LSRs in subjects with prevalent VFx for six key activities, all of which showed significant differences at lumbar vertebral levels. Most activities also showed similar differences at lower thoracic levels but only one (activity 9, holding weight with the elbows flexed) showed higher LSRs in upper thoracic vertebrae (Supplemental Table S3). Finally, our results show that the highest-load activity varies among individuals in the population. This observation aligns with findings using instrumented spinal implants, showing that loading for high-load activities varies significantly among individuals.(26) Thus, future studies need to consider multiple lifting, holding, and non-symmetric activities when evaluating LSRs. Furthermore, the values of LSRs in the current study suggested that these key activities are not likely to cause fracture, as they do not near the theoretical threshold of LSR > 1 for fracture to occur. This is expected, as we simulated common daily activities that are not necessarily expected to lead to vertebral fracture. Moreover, our study included men and women aged 41 to 90 years; thus, the majority would have normal bone strength and low risk of VFx.(27) Nonetheless, the key activities identified represent significant loading on the spine, four to five times greater than the load in neutral standing.
Limitations
We acknowledge several important limitations in our study. Whereas 109 activities is significantly more than the number examined in prior studies, there are near-infinite possibilities for spine loading scenarios. Moreover, 17 activities failed to solve for any subjects, and six more solved in fewer than 10 subjects. These activities are identified in Supplemental Table S2. Some activities, including flexion with weight, likely failed in some subjects because of limitations in spinal muscle strength. However, activities that failed across most or all of the subjects involved large shoulder angles; we believe this is because of limitations in shoulder strength rather than spine strength. Nonetheless, some failed activities would likely produce relatively large spinal loads if successfully solved, and many other loading scenarios could be envisioned to produce large spine loads. Loading in dynamic versions of these activities was not assessed but could produce larger loads than those found in these static tasks.(13,28) Thus, while the activities examined here are varied and instructive, they cannot be deemed fully comprehensive and may be biased toward “moderate” loads due to the nature of the activities attempted and those successfully solved. Our models include a large degree of complexity in terms of subject-specific muscle morphology and spinal curvature; however, we did not include the effect of inter-abdominal pressure (IAP)(29) or non-linearity of material properties of ligaments(30) or intervertebral joints(31) on spinal loading. Although our MSK model has been validated to predict in vivo measurements of spinal loading,(21) future studies could benefit from further advancing the physiological realism of the models. An additional limitation is that only compressive loading was analyzed in this study. We focused on compressive loads because osteoporotic vertebral fractures are predominantly wedge or crush fractures of the vertebral body(12) produced by compressive loading with or without bending. However, activities that produce high loads in other modes of loading such as torsion might not be flagged as activities of interest here. Torsional injuries of the spine may occur due to trauma(32) and typically involve the disc or neural arch(33) rather than the vertebral body. Approaches for estimating torsional loading and strength in vivo are not fully developed or well validated, making this a significant area for future work. A final limitation of note is the potential error in predictions of individual vertebral strengths from the regression model. The average predicted strength of 6870 N in our sample has a 95% confidence interval ± 1.4%, indicating very low uncertainty in the mean prediction of strength, but a 95% prediction interval of ±26%, indicating much larger uncertainty in individual strength predictions. Some individual outliers in LSR were found, and it is likely these are related in part to errors in predictions of vertebral strength (eg, underprediction of strength could produce large LSRs >1). However, our analyses focus on overall trends in the sample, identifying activities that have high LSR in many subjects and examining the median LSR, and these outcomes should not be affected by the uncertainty in vertebral strength estimates. Overall, the limitations noted here are relatively minor alongside the strengths of this study, particularly the large number of subject-specific models and the large number of simulated activities, making this the most comprehensive examination of spinal load-to-strength patterns to date.
In conclusion, this examination of vertebral load-to-strength ratios along the spine in men and women sampled from a population-based cohort is the most comprehensive to date and provides new insights into spinal loading with implications for the biomechanics of vertebral fracture. First, when considering vertebral fracture risk, examining just one activity or posture is unlikely to capture the range of fracture risk that individual patients experience. Rather, a set of activities and postures is needed. Furthermore, the activities that led to the highest load-to-strength ratios varied by vertebral level, highlighting the need to consider a range of lifting, holding, and non-symmetric activities when evaluating the contribution of spinal loading to vertebral fracture risk. Moreover, our findings identified key activities that produce high loading in multiple regions of the spine. Future studies may scrutinize these key activities, particularly in dynamic situations, to better understand the underlying biomechanical mechanisms of vertebral fractures in older adults.
Supplementary Material
Acknowledgments
We acknowledge funding from the National Institutes of Health (R01AR073019, R00AG042458) and National Space Biomedical Research Institute (NCC 9–58). This project includes data from the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This project has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract no. 75N92019D00031.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All authors state that they have no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4222.
References
- 1.Weycker D, Li X, Barron R, Bornheimer R, Chandler D. Hospitalizations for osteoporosis-related fractures: economic costs and clinical outcomes. Bone Rep. 2016;5:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokhtarzadeh H, Anderson DE. The role of trunk musculature in osteoporotic vertebral fractures: implications for prediction, prevention, and management. Curr Osteoporos Rep. 2016;14(3):67–76. [DOI] [PubMed] [Google Scholar]
- 3.Joestl J, Lang N, Bukaty A, Tiefenboeck TM, Platzer P. Osteoporosis associated vertebral fractures—health economic implications. PLoS One. 2017;12(5):e0178209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black DM, Cauley JA, Wagman R, et al. The ability of a single BMD and fracture history assessment to predict fracture over 25 years in postmenopausal women: the study of osteoporotic fractures. J Bone Miner Res. 2018;33(3):389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosman F, Krege JH, Looker AC, et al. Spine fracture prevalence in a nationally representative sample of US women and men aged ≥40 years: results from the National Health and nutrition examination survey (NHANES) 2013–2014. Osteoporos Int. 2017;28(6):1857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan Y, Duboeuf F, Munoz F, Delmas PD, Seeman E. The fracture risk index and bone mineral density as predictors of vertebral structural failure. Osteoporos Int. 2006;17(1):54–60. [DOI] [PubMed] [Google Scholar]
- 7.Melton LJ, Riggs BL, Keaveny TM, et al. Structural determinants of vertebral fracture risk. J Bone Miner Res. 2007;22(12):1885–92. [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ III, Riggs BL, Keaveny TM, et al. Relation of vertebral deformities to bone density, structure, and strength. J Bone Miner Res. 2010;25(9):1922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson DES, Demissie BT, Allaire AG, et al. The associations between QCT-based vertebral bone measurements and prevalent vertebral fractures depend on the spinal locations of both bone measurement and fracture. Osteoporos Int. 2014;25(2):559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29(3):570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Sanyal A, Cawthon PM, et al. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans for the Osteoporotic Fractures in Men (MrOS) research group. J Bone Miner Res. 2012;27(4):808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7(2):221–7. [DOI] [PubMed] [Google Scholar]
- 13.Freitas SS, Barrett-Connor E, Ensrud KE, et al. Rate and circumstances of clinical vertebral fractures in older men. Osteoporos Int. 2008;19(5):615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Li C, Xiang Q, Xiong H, Zhou Y. Epidemiology of spinal fractures among the elderly in Chongqing, China. Injury. 2012;43(12):2109–16. [DOI] [PubMed] [Google Scholar]
- 15.Ismail AA, Cooper C, Felsenberg D, et al. Number and type of vertebral deformities: epidemiological characteristics and relation to back pain and height loss. Osteoporos Int. 1999;9(3):206–13. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ, Lane AW, Cooper C, Eastell R, O’Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3(3):113–9. [DOI] [PubMed] [Google Scholar]
- 17.Siminoski K, Lee KC, Jen H, et al. Anatomical distribution of vertebral fractures: comparison of pediatric and adult spines. Osteoporos Int. 2012;23(7):1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruno AG, Burkhart K, Allaire B, Anderson DE, Bouxsein ML. Spinal loading patterns from biomechanical modeling explain the high incidence of vertebral fractures in the thoracolumbar region. J Bone Miner Res. 2017;32(6):1282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008;102(9):1136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno AG, Mokhtarzadeh H, Allaire BT, et al. Incorporation of CT-based measurements of trunk anatomy into subject-specific musculoskeletal models of the spine influences vertebral loading predictions. J Orthop Res. 2017;35(10):2164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruno AG, Bouxsein ML, Anderson DE. Development and validation of a musculoskeletal model of the fully articulated thoracolumbar spine and rib cage. J Biomech Eng. 2015;137(8):081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson D Mokhtarzadeh H Allaire B Burkhart K, Bouxsein M. Subject-specific spine models for 250 individuals from the Framingham Heart Study [Internet]. Harvard Dataverse. 2020; V 1. Available at: https://dataverse.harvard.edu/dataverse/SpineModeling. [Google Scholar]
- 23.Delp SL, Anderson FC, Arnold AS, et al. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54(11):1940–50. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen BA, Kopperdahl DL, Kiel DP, Keaveny TM, Bouxsein ML. Mechanical contributions of the cortical and trabecular compartments contribute to differences in age-related changes in vertebral body strength in men and women assessed by QCT-based finite element analysis. J Bone Miner Res. 2011;26(5):974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allaire BT, Lu D, Johannesdottir F, et al. Prediction of incident vertebral fracture using CT-based finite element analysis. Osteoporos Int. 2019;30(2):323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohlmann A, Pohl D, Bender A, et al. Activities of everyday life with high spinal loads. PLoS One. 2014;9(5):e98510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johannesdottir F, Allaire B, Anderson DE, Samelson EJ, Kiel DP, Bouxsein ML. Population-based study of age- and sex-related differences in muscle density and size in thoracic and lumbar spine: the Framingham Study. Osteoporos Int. 2018;29(7):1569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGill SM, Norman RW. Dynamically and statically determined low back moments during lifting. J Biomech. 1985;18(12):877–85. [DOI] [PubMed] [Google Scholar]
- 29.Mokhtarzadeh H, Farahmand F, Shirazi-Adl A, Arjmand N, Malekipour F, Parnianpour M. The effects of intra-abdominal pressure on the stability and unloading of the spine. J Mech Med Biol. 2012;12(1):1250014. [Google Scholar]
- 30.Naserkhaki S, Arjmand N, Shirazi-Adl A, Farahmand F, El-Rich M. Effects of eight different ligament property datasets on biomechanics of a lumbar L4-L5 finite element model. J Biomech. 2018;70:33–42. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Mannen EM, Sis HL, et al. Moment-rotation behavior of intervertebral joints in flexion-extension, lateral bending, and axial rotation at all levels of the human spine: a structured review and meta-regression analysis. J Biomech. 2020;100:109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iencean SM. Classification of spinal injuries based on the essential traumatic spinal mechanisms. Spinal Cord. 2003;41(7):385–96. [DOI] [PubMed] [Google Scholar]
- 33.Farfan HF. The torsional injury of the lumbar spine. Spine (Phila. Pa. 1976). 1984;9(1):53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


