Abstract
Background and aim of the study:
The medicalisation of birth pathway may negatively impact on women’s empowerment, enhancing distress even in cases of healthy pregnancies. We have built a program which is comprised of Mindfulness, Yoga, Nutrition, development & Counselling, Coaching, antenatal classes, and Osteopathic treatment (MYNd&CO).
Methods:
This study is a randomized controlled trial involving low-risk pregnant women. They will be randomized to the experimental (MYNd&CO intervention plus standard care) or control group (standard care). The primary (general health and wellbeing, maternal distress) and secondary outcome measures (urinary incontinence, sexual problems, and physical wellbeing) will be assessed via questionnaires at baseline and 6 months after childbirth. The independent-samples t-test and Chi-square will be used to detect changes in the outcomes between intervention and control group.
Discussion:
The trial is expected to increase knowledge about the effectiveness of a holistic approach in low-risk pregnant women, in terms of obstetrical and psychophysiological outcomes.
Keywords: Mindfulness, Yoga, Osteopathy, Prenatal education, Pregnancy
Introduction
In the “Anthropocene” era, pregnancy and childbirth are often characterized by unnecessary medical interventions, even for low-risk women. However, the ‘medicalisation of childbirth’ does not assure a higher level of safety. It can increase the likelihood of false positive cases, resulting in iatrogenic risks due to unnecessary—and sometimes harmful—treatments (1,2). Primarily World Health Organization (WHO) claims for a global woman positive experience assuring the maternal- infant best epigenetics potential during the most critical phase of the first 1000 days (3).
In Italy, medicalisation and over-treatment are deep-rooted phenomena, well-documented by high rates of low-risk pregnancies handled by gynaecologists, hospital births, caesarean sections (CS) and suboptimal breastfeeding (4,5). Women mainly choose gynaecologists for antenatal care, and only a limited percentage opts for public health community services or private midwives (4,5). This ‘epidemiology’ of taking charge contributes to the problem of over-testing and over-diagnosis in pregnancy (4). In fact, against national and international guidelines on low-risk pregnancies, the number of antenatal visits is higher than four in 85.3% of women and more than three ultrasound scans are performed in 74.6% of cases (5). These percentages are not justifiable, in fact the excess of screening tests does not improve the outcomes neither in low- nor in high-risk pregnancies (6).
Regarding birthplaces, the national rate of out-of-hospital births is among the lowest in Europe, estimated at around 0.1% (5,7). In 2010, 38% of Italian infants were born through CS—among the highest rates worldwide (8). The last few years saw some improvement regarding primary and previous CS rates; in 2016, the CS rate was equal to 33.7%, with a significant north-south gradient (5,8). However, this national prevalence must be considered high if compared with the ideal CS rate which, according to the World Health Organization (WHO), should be between 10 and 15% (9).
Similarly, the rates of breastfeeding initiation and continuation show geographical differences, decreasing in areas where education and socio-economic condition levels were low, as in the Southern regions (10). The most recent survey reported that the prevalence of exclusive breastfeeding at two to three months of life was 44.4%, decreasing up to 25.8% at four to five months (11). Consequently, the use of breast milk substitutes is an already widespread practice during the first days of life during the hospital stay (12). Sometimes breast milk substitutes are promoted at discharge, even without an evidence-based clinical reason (12), partly due to healthcare professionals limited training on breastfeeding (13)
Presently, another widespread modern phenomenon is the distress in pregnant women which can continue in the postnatal period, commonly defined as maternal distress. Changes experienced by women during pregnancy can cause anxiety, depression, and distress, even in cases of healthy pregnancies (14,15). This can be associated with several factors, including a variety of coping styles and personal history and circumstances (14).
A study involving 410 Italian primiparous women revealed that 34.9% could be classified as ‘psychologically healthy women’, 47.3% as ‘currently anxious women’ and 17.8% as ‘psychologically distressed’ (16). There were no differences in the three groups in terms of obstetric risks.
The maternal distress level can affect unborn children, exposing them to suboptimal emotional, behavioural, and cognitive development, along with adverse health outcomes (17,18). This occurs not only in severe distress but also in mild or moderate cases if the foetal exposure is recurrent or chronic (19,20).
The medicalisation of natural phenomena such as pregnancy, childbirth and breastfeeding is capable of negatively impacting the empowerment of women and their satisfaction (2,21,22). Women may doubt their ability to cope with motherhood and lose the sense of control over pregnancy, childbirth and newborn care. This can increase the prenatal distress trend.
Therefore, it is important to focus on both evidence-based care for low-risk pregnancies and the psycho-physical well-being of pregnant women. For this reason, we have built a program for low-risk pregnant women, which is comprised of Mindfulness, Yoga, Nutrition, development & Counselling, Coaching, antenatal classes and Osteopathic treatment (MYNd&CO program), according to a holistic or “top-down system” biology approach.
Our hypothesis states that the integration of the standard care planned for low-risk women with the MYNd&CO program will improve the psychological well-being and obstetrical outcomes of pregnant women allocated to the intervention group compared to those assigned to the control group.
Methods
Design and setting
A monocentric randomized controlled trial (RCT) in a two-arm parallel-group design is carried out by January 2019 with repeated measurements at two times (baseline and 6 months after childbirth) at the Clinica Mangiagalli in Milan, Italy.
Pregnant women interested in MYNd&CO program and meeting the inclusion criteria will be assigned to the experimental (MYNd&CO intervention and standard care for pregnancy) or control group (standard care) in a 1:1 ratio, using a randomization software. Ethnicity and Body Mass Index (BMI) will be considered to perform a stratified randomization to ensure their fair allocation in the two arms.
Standard care consists in four obstetrical appointments, screening, and check-ups up until childbirth. MYNd&CO intervention consists in weekly mindfulness and yoga meetups, nutritional screening with further check-ups, if needed, osteopathic screening, five motivational meetings with a coach and attending of a prenatal class. The intervention will last about 28 weeks (from 12 weeks of gestation to the birth) and it will take place in hospital.
The assignment will be communicated to recruited women, who can leave the intervention at any time. The project is offered free of charge.
The study has been approved by the Ethics Committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and registered with the trial code NCT03839004. The steps and features of the study protocol have been described adhering to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist (23).
Participants
The participants will be recruited at Clinica Mangiagalli’s outpatient department. Inclusion criteria for participation in the study are: nulliparous women, singleton pregnancies, spontaneous pregnancies, gestational age ≥12, no maternal medical conditions, maternal age between 18 and 44 years, and no language barrier. Exclusion criteria are: multiple pregnancies, in vitro fertilization pregnancies, maternal or foetal medical conditions, age <18 or >45, and language barrier.
Measures
Primary outcome measure
General health and wellbeing
The Short Form 12 Health Survey (SF12) (24) is one of the main tools used to assess self-reported health-related quality-of-life. This 12-item questionnaire is composed of the Mental Component Summary (MCS) and the Physical Component Summary (PCS) scales which measure 4 health-related domains to evaluate physical status (general health, physical functioning, role limitations due to physical health problems, and body pain) and other 4 to measure psychological condition (vitality, social functioning, role emotional, and mental health) respectively. The SF-12 questionnaire has been validated mainly in case of chronic diseases (25-27), but it has tested also among healthy populations, such as pregnant women (28,29).
Maternal distress
During study timepoints, several instruments will be administered to the participants to assess the degree of maternal distress.
The Psychological Wellbeing (PWB) Scale (30) is a 42-item tool which measures the following six subscales of wellbeing and happiness: autonomy, environmental mastery, personal growth, positive relations, purpose in life, and self-acceptance. Respondents express their level of agreement or disagreement with each item using a 7-point scale (from 1-strongly agree to 7-strongly disagree).
The Edinburgh Depression Scale (EDS) (31,32) is a 10-item self-report measure developed to screen pregnant women or puerperae for emotional distress. The items reflect the woman’s experience of the last 7 days and each answer is given a score of 0 to 3, so the maximum total score is 30 (31). EDS is not a diagnostic tool; therefore psychiatrists/psychologists are needed in case of high scores (>12) for performing a clinical assessment and establishing an appropriate management.
The State-Trait Anxiety Inventory (STAI-Y) (33) includes 40 self-report items in a 4-point Likert scale to detect the level of anxiety. Items 1 to 20 assess state anxiety with the options “not at all”, “somewhat”, “moderately so”, and “very much”, whereas the options “almost never”, “sometimes”, “often”, and “almost always” are possible for items 21 to 40. Participants should respond in accordance with their actual mood.
The Millon Clinical Multiaxial Inventory (MCMI-III) (34) is a 175-item, true-false self-report measure which provides information on personality traits and psychiatric disorders. It contains 24 clinical scales arranged in the following 4 categories: clinical personality patterns, severe personality pathology, clinical syndromes, and severe clinical syndromes.
The Mother to Infant Bonding Scale (MIBS) (35) is composed of 8 items on a 4-point Likert scale (from 0 - “not at all” to 3 - “very much”), having a total score from 0 to 24. High scores indicate disturbances in mothers’ feelings toward their children. This scale will be administered only at the follow-up.
Secondary outcome measures
Urinary incontinence (UI)
The International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI SF) (36) is a 4-item questionnaire used in research and clinical practice in primary and secondary care to screen for UI. It assesses the frequency of UI, amount of leakage, overall impact of UI on quality of life and it includes a self-diagnostic item.
Sexual problems
The Female Sexual Function Index (FSFI) (37) is a 19-item measure on a 5-point Likert scale. It evaluates the sexual functioning in women, taking in account 6 domains (sexual desire, sexual arousal, lubrication, orgasm, satisfaction, and pain). Low scores reveal lower levels of sexual functioning.
Low back pain (LBP) and pelvic girdle pain (PGP)
Assessment of low back and sciatic nerve pain during pregnancy is an indicator of osteo-muscular health and it will perform from the 20th week of gestation to 40 days after delivery. Several tools will be proposed to the recruited women to assess their degree of LBP and PGP.
The Oswestry Disability Index (ODI) (38) is a 10-item tool designed to collect information as to how low back or leg pain affects patient’ ability to manage in everyday life, taking in account these aspects: pain intensity, personal care (washing, dressing, etc), lifting, walking, sitting, standing, sleeping, sex life (if applicable), social life, and travelling. For each section, the maximum score is 5, for a total possible score of 50. The total score, then converted in percentage, classifies a patient’s permanent functional disability as follows: minimal disability (0% to 20%), moderate disability (21%-40%), severe disability (41%-60%), crippled (61%-80%), or bed-bound patient (81%-100%).
The Pelvic Girdle Questionnaire (PGQ) (39) is a tool which comprises 20 activity items and 5 symptom items on a 4-point response scale from 0 to 3, to assess describing the degree of symptoms and activity limitations during pregnancy and postpartum.
The Pregnancy Mobility Index (PMI) (40) is composed of 3 scales (daily mobility, household activities, and mobility outdoors) to assess mobility in relation to back and/or pelvic pain for pregnant women.
The Visual analogue scale (VAS) (41) is the most used pain assessment scale and consists of a horizontal line to collect women’s perceptions of body pain using scores from 0 (no pain) to 10 (extreme pain).
Additional measures
Obstetric and neonatal data
Data on early pregnancy will be collected by the gynaecologists at T0, while information on term pregnancy, delivery, infant health, and post-natal period will be self-reported by participants at T1.
Pregnancy: parity, number of spontaneous and voluntary abortions, last menstrual period (LMP), gestational age at T0, type of conception, type of health professional chosen for pregnancy care, micronutrient supplements, tobacco smoking, and pregnancy health problems/complications.
Delivery: induction, gestational age at birth, type of delivery, and episiotomy/vaginal tears.
Infant health: birth weight, admission to a Neonatal Intensive Care Unit (NICU), and infant feeding practices, including breastfeeding duration, according to WHO/UNICEF definitions.
Demographic data and other information
As part of MYNd&CO Study, demographic data and other information relevant for the research purpose will be collected. These include maternal ethnicity at nationality, age at recruitment, level of education, employment status, marital status, pre-pregnancy body mass index (BMI) and weight gain during pregnancy, diet and nutritional behaviours, physical activity, family/social support, and experience of mindfulness, yoga, coaching, and antenatal classes attendance.
Evaluation of intervention
Questions for evaluation of the program will be addressed to intervention group to assess their satisfaction and perception of efficacy on wellbeing.
Procedure
Every week the pregnant women who have communicated by e-mail their interest in MYNd&CO intervention will be contacted and invited to the first obstetric visit in prenatal outpatient department.
A randomization software will be used to assign the number 1 to the intervention and 0 to the control group. The random numbers will be sequentially paired to women which will show up for the first visit in outpatient department. The allocations will be archived in a document protected by password on group research’s computer. The randomization will guarantee objectivity of the researchers and will avoid bias in the woman’s group allocation. Blinding of researchers of women is not feasible because of the study design.
During the first antenatal visit, two researchers will describe the MYNd&CO project, explaining that specific activities will be proposed to the intervention group, while the control group will receive the standard care.
Women in both the groups will provide written consent for participation in the study. They will have to fill out the questionnaires at T0 and T1.
Women in intervention group will receive the schedule of MYNd&CO activities.
During routine visits, the gynaecologists will check blood glucose curve or fasting glucose (from 24 weeks of pregnancy to the day of delivery) and blood pressure (from the 20th week of gestation to 40 days after delivery) to verify if participants meet inclusion/exclusion criteria related gestational diabetes and hypertension.
An osteopathic treatment will be proposed for the infants of all participants to improve their response rate at T1.
The schedule of recruitment, data collection, intervention and assessments are summarized in Figure 1.
Figure 1.
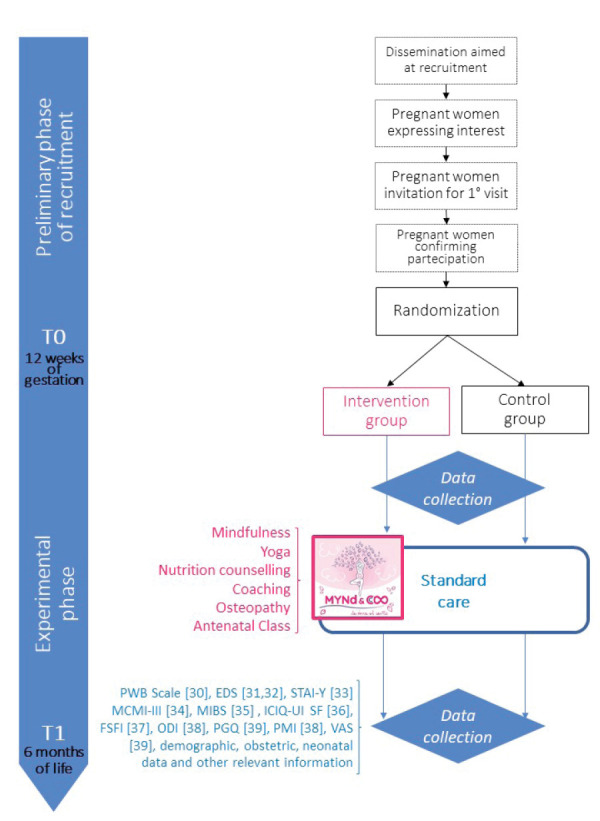
Schedule of the MYND&CO program
Intervention
Intervention includes 6 activities: 1) mindfulness course, 2) yoga classes, 3) nutrition counselling, 4) coaching, 5) osteopathic manipulative treatment, and 6) antenatal classes facilitated by midwives.
The Mindfulness course is inspired by the Mindfulness Based Childbirth and Parenting (MBCP) program. It will include 7 weekly two-hour sessions plus a residential half-day retreat (42).
The Yoga intervention will consist of bi-weekly practice of about 75 minutes. Physical practice of postures (asanas), breathing exercises (pranayama) and meditation (dyana) will be proposed, according to the branch of Hatha Yoga.
Nutrition counselling will focus to promote a healthy, well-balanced, and varied diet, assuring an adequate weight gain to improve the women nutritional status during pregnancy.
The Coaching intervention will provide women the support and tools needed to strengthen their competencies toward childbirth and motherhood. Women will be guided in defining focused goals, supported to meet their expectations related to their first child’s arrival, and helped to find solutions in case of challenges.
The Osteopathic manipulative treatment for low back and pelvic girdle will be preceded by an assessment of lumbar segments, sacrum, pubic symphysis, pelvis, sacroiliac joint, and hip, using the following palpatoric/mobility tests: active straight leg raise test, Gaenslen’s test, long dorsal sacroiliac ligament test, pain provocation of the symphysis by modified Trendelenburg’s test, Patrick’s Faber test, posterior pelvic pain provocation test, symphysis pain palpation test, according to the European Guidelines (43). Moreover, Michaelis test and side bending test pain during and after pregnancy will be performed respectively to test functionality of the thoracolumbar segments and uterus adaptation to the lumbar spine.
The Antenatal Classes will consist of six meetings guided by midwives which will provide information about pregnancy, labour, birth, breastfeeding, and infant care.
These activities will be performed by the team in charge of the MYNd&CO project, made up of gynaecologists, midwives, psychologists, osteopaths, nutritionists, and yoga teachers, who all collaborate on a regular basis with the Clinica Mangiagalli.
Sample size calculation
The primary outcome, psychophysical wellbeing, is calculated as the difference scores between intervention and control group. This change should be greater than or equal to 2 points, according to the instructions of SF-12 user manual. Similarly, the latter indicates that 393 participants are needed to identify difference scores of 2 points (44). For this reason, the MYNd&CO study will include 400 women, divided in 2 groups of 200.
Statistical analysis
The descriptive statistics (mean ± SD, %) will be used for nominal and continuous variables to report the main characteristics of the groups (age; marital status; education; diagnosis; psychosocial variables).
The independent-samples t-test and Chi-square will be used to verify the homogeneity between intervention and control group, comparing baseline characteristics. Any significant differences will be controlled in the subsequent analyses.
T-test will be used to detect changes in the primary outcome (wellbeing) between intervention and control group, in term of differences in the mean values scored in the SF-12.
The level of significance will be set at 0.05 (p < 0.05) with 95% confidence intervals (95% CI). Data will be analysed using SPSS.
Data collection and data management
Two researchers will be responsible for data collection under supervision of the principal investigator. During the trial, a researcher expert in data quality will perform a regular check of collected data to verify their coherence. All data on paper will be archived in a secure area at Mangiagalli Clinica. Digital information will be stored assuring that identifiable data will be separated from anonymized data. Data will be analysed after trial conclusion.
Trial status
The study is currently recruiting participants.
Discussion
We describe a protocol to promote a ‘positive pregnancy’, which supports both the somatic and psychological component of the mother-newborn dyad, and thus organically and proactively prevents the main issues arising during pregnancy and delivery due to unhealthy lifestyles. The intervention is designed according to on a holistic approach to pregnancy which include mindfulness, yoga, nutrition counselling, coaching, osteopathic treatment, and antenatal classes. This innovative model does not aim to substitute the allopathic one which is currently in place (obstetric appointments, ultrasounds, tests, etc.), but to enhance its nature in a more effectively therapeutic manner, increasing the mother-newborn dyad’s potential of health.
The strengths of MYNd&CO project are mainly two: on one hand, to our knowledge, this is the first study focused on a holistic approach in pregnancy. On the other hand, MYNd&CO is a two-arm RCT that will make it possible the generalization of the results and the transfer of this model in other hospitals and settings.
The most significant challenges in this study are the following up of recruited women and quality control. The study purpose will be clearly explicated to raise awareness of women on the importance of the MYNd&CO project. To increase the response rate at follow up, researchers will communicate with participants using various methods as telephone calls, e-mails, and short messages. Moreover, an osteopathic treatment for infants will be proposed. The quality control will be guaranteed by several strategies, regularly checking the process of data entry and analysis. Any dropout will be recorded, indicating the reason. Any relevant protocol modifications decided by the researchers will be communicated to MYND&CO team, participants, and other pertinent parties.
This study has some limitations: first, the self-selection bias due to voluntary participation. However, to reduce this risk, pregnant women will be randomized for their allocation in the intervention or control group. Second, the lack of blinding due to the nature of study may be another source of bias. Moreover, it should be noted that at the end of the study it will be possible to estimate the total effectiveness of MYNd&CO program, but not the effect size of each its component.
If our results will be favourable, the adoption of MYNd&CO will be facilitated, making it affordable and accessible to all women. For the future, it would be desirable to extend the duration of the MYNd&CO program beyond childbirth. Moreover, it would be interesting to include women affected by some of the most common pregestational and gestational pathologies (45,46) such as diabetes to test MYNd&CO program effectiveness also in case of high-risk pregnancies.
Recruitment for this study, began in February 2019, it is currently closed and the follow-up after 6 months of childbirth is ongoingResults will be available in 2021 and published in scientific journals to disseminate the findings internationally.
Conflict of Interest:
Each author declares that he or she has no commercial associations that might pose a conflict of interest in connection with the submitted article
Registration:
This study protocol has been registered on ClinicalTrials.gov. Trial registration number: NCT03839004. Date of registration: 12/02/2019. URL of trial registry record: https://www.clinicaltrials.gov/ct2/show/study/NCT03839004?term=mynd&draw=2&rank=2
References
- Christiaens W, van Teijlingen E. Four meanings of medicalization: childbirth as a case study. Salute e Società. 2009;8(2):123–41. [Google Scholar]
- Shaw JC. The medicalization of birth and midwifery as resistance. Health Care Women Int. 2013;34(6):522–36. doi: 10.1080/07399332.2012.736569. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- Lauria L, Lamberti A, Buoncristiano M, Bonciani M, Andreozzi S, editors. Percorso nascita: promozione e valutazione della qualità di modelli operativi. Le indagini del 2008-2009 e del 2010-2011. Roma: Istituto Superiore di Sanità; 2012. Rapporti ISTISAN 12/39. [Google Scholar]
- Ministero della Salute. Certificato di assistenza al parto (CeDAP). Analisi dell’evento nascita - Anno 2016. Roma: Attività Editoriali Ministero della Salute; 2019. [Google Scholar]
- Miller S, Abalos E, Chamillard M, et al. Beyond too little, too late and too much, too soon: a pathway towards evidence-based, respectful maternity care worldwide. Lancet. 2016;388(10056):2176–92. doi: 10.1016/S0140-6736(16)31472-6. [DOI] [PubMed] [Google Scholar]
- Campiotti M, Campi R, Zanetti M, Olivieri P, Faggianelli A, Bonati M. Low-Risk Planned Out-of-Hospital Births: Characteristics and Perinatal Outcomes in Different Italian Birth Settings. Int J Environ Res Public Health. 2020;17(8):2718. doi: 10.3390/ijerph17082718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colais P, Bontempi K, Pinnarelli L, et al. Vaginal birth after caesarean birth in Italy: variations among areas of residence and hospitals. BMC Pregnancy Childbirth. 2018;18(1):383. doi: 10.1186/s12884-018-2018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. WHO Statement on Caesarean Section Rates. 2015 doi: 10.1016/j.rhm.2015.07.007. Geneva, Switzerland. Retrieved November 11, 2020, from: https://apps.who.int/iris/bitstream/handle/10665/161442/WHO_RHR_15.02_eng.pdf?sequence=1. [DOI] [PubMed] [Google Scholar]
- Cernigliaro A, Palmeri S, Casuccio A, Scondotto S, Restivo V Primis Working Group. Association of the Individual and Context Inequalities on the Breastfeeding: A Study from the Sicily Region. Int J Environ Res Public Health. 2019;16(19):3514. doi: 10.3390/ijerph16193514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauria L, Spinelli A, Buoncristiano M, Bucciarelli M, Pizzi E. Breastfeeding Prevalence at Time of Vaccination: Results of a Pilot Study in 6 Italian Regions. J Hum Lact. 2019;35(4):774–81. doi: 10.1177/0890334418823539. [DOI] [PubMed] [Google Scholar]
- Colaceci S, Chapin EM, Zambri F, et al. Verba volant, scripta manent: breastfeeding information and health messages provided to parents in the neonatal discharge summary in the Lazio Region, Italy. Ann Ist Super Sanita. 2020;56(2):142–149. doi: 10.4415/ANN_20_02_03. [DOI] [PubMed] [Google Scholar]
- Colaceci S, Zambri F, D’Amore C, De Angelis A, Rasi F, Pucciarelli G, Giusti A. Long-Term Effectiveness of an E-Learning Program in Improving Health Care Professionals’ Attitudes and Practices on Breastfeeding: A 1-Year Follow-Up Study. Breastfeed Med. 2020 Apr;15(4):254–260. doi: 10.1089/bfm.2019.0203. [DOI] [PubMed] [Google Scholar]
- Fontein-Kuipers Y, Ausems M, Budé L, Van Limbeek E, De Vries R, Nieuwenhuijze M. Factors influencing maternal distress among Dutch women with a healthy pregnancy. Women Birth. 2015;28(3):e36–e43. doi: 10.1016/j.wombi.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Daglar G, Nur N, Bilgic D, Kadıoglu M. Affective disorder in pregnancy. KASHED. 2015;2(1):27–40. [Google Scholar]
- Molgora S, Fenaroli V, Saita E. Psychological distress profiles in expectant mothers: What is the association with pregnancy-related and relational variables? J Affect Disord. 2020;262:83–9. doi: 10.1016/j.jad.2019.10.045. [DOI] [PubMed] [Google Scholar]
- Monk C, Lugo-Candelas C, Trumpff C. Prenatal developmental origins of future psychopathology: mechanisms and pathways. Annu Rev Clin Psychol. 2019;15:317–44. doi: 10.1146/annurev-clinpsy-050718-095539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aatsinki AK, Keskitalo A, Laitinen V, et al. Maternal prenatal psychological distress and hair cortisol levels associate with infant fecal microbiota composition at 2.5 months of age. Psychoneuroendocrinology. 2020;119:104754. doi: 10.1016/j.psyneuen.2020.104754. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Chrousos GP. Early-life stress: from neuroendocrine mechanisms to stress-related disorders. Horm Res Paediatr. 2018;89:372–9. doi: 10.1159/000488468. [DOI] [PubMed] [Google Scholar]
- Graignic-Philippe R, Dayan J, Chokron S, Jacquet AY, Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci Biobehav Rev. 2014;43:137–62. doi: 10.1016/j.neubiorev.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Colaceci S, Corsi E, Berardi F, Coscarella P, Mariotti M, Ramacciati N. Maternal Satisfaction and Birth: a web-based survey. Prof Inferm. 2020;73(3):181–187. doi: 10.7429/pi.2020.733181. [DOI] [PubMed] [Google Scholar]
- Anderson G, Zega M, D’Agostino F, Rega ML, Colaceci S, Damiani G, Alvaro R, Cocchieri A. Meta-Synthesis of the Needs of Women Cared for by Midwives During Childbirth in Hospitals. J Obstet Gynecol Neonatal Nurs. 2021 Jan;50(1):6–19. doi: 10.1016/j.jogn.2020.10.005. [DOI] [PubMed] [Google Scholar]
- Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Guo X, Tripp C, Huber NL, et al. Patient reported outcomes and quality of life in Chinese patients with implantable cardioverter defibrillators. Heart Lung. 2021;50(1):153–158. doi: 10.1016/j.hrtlng.2020.05.003. [DOI] [PubMed] [Google Scholar]
- Huo T, Guo Y, Shenkman E, Muller K. Assessing the reliability of the short form 12 (SF-12) health survey in adults with mental health conditions: a report from the wellness incentive and navigation (WIN) study. Health Qual Life Outcomes. 2018;16:34. doi: 10.1186/s12955-018-0858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW, Maljanian R, Landes M. Test-retest reliability of short form (SF)-12 component scores of patients with stroke. Int J Rehabil Res. 2004;27:149–50. doi: 10.1097/01.mrr.0000127350.25287.08. [DOI] [PubMed] [Google Scholar]
- Mannocci A, Mipatrini D, D’Egidio V, et al. Health related quality of life and physical activity in prison: a multicenter observational study in Italy. Eur J Public Health. 2018;28(3):570–6. doi: 10.1093/eurpub/ckx183. [DOI] [PubMed] [Google Scholar]
- Hirose M, Tamakoshi K, Takahashi Y, Mizuno T, Yamada A, Kato N. The effects of nausea, vomiting, and social support on health-related quality of life during early pregnancy: A prospective cohort study. J Psychosom Res. 2020;136:110168. doi: 10.1016/j.jpsychores.2020.110168. [DOI] [PubMed] [Google Scholar]
- Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. J Pers Soc Psychol. 1989;57:1069–81. [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Clark G. Discussing emotional health in pregnancy: the Edinburgh Postnatal Depression Scale. Br J Community Nurs. 2000;5(2):91–8. doi: 10.12968/bjcn.2000.5.2.7170. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Millon T, Grossman S, Millon C. MCMI-IV: Millon Clinical Multiaxial Inventory Manual. 1 ed. Bloomington, MN: NCS Pearson, Inc; 2015. [Google Scholar]
- Taylor A, Atkins R, Kumar R, Adams D, Glover V. A new mother-to-infant bonding scale: links with early maternal mood. Arch Womens Ment Health. 2005;8(1):45–51. doi: 10.1007/s00737-005-0074-z. [DOI] [PubMed] [Google Scholar]
- Timmermans L, Falez F, Mélot C, Wespes E. Validation of use of the International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form (ICIQ-UI-SF) for impairment rating: a transversal retrospective study of 120 patients. Neurourol Urodyn. 2013;32(7):974–9. doi: 10.1002/nau.22363. [DOI] [PubMed] [Google Scholar]
- Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25(22):2940–52. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- Stuge B, Garratt A, Krogstad Jenssen H, Grotle M. The pelvic girdle questionnaire: a condition-specific instrument for assessing activity limitations and symptoms in people with pelvic girdle pain. Phys Ther. 2011;91(7):1096–108. doi: 10.2522/ptj.20100357. [DOI] [PubMed] [Google Scholar]
- van de Pol G, de Leeuw JR, van Brummen HJ, Bruinse HW, Heintz AP, van der Vaart CH. The Pregnancy Mobility Index: a mobility scale during and after pregnancy. Acta Obstet Gynecol Scand. 2006;85(7):786–91. doi: 10.1080/00016340500456373. [DOI] [PubMed] [Google Scholar]
- Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB. Measurement Properties of Visual Analogue Scale, Numeric Rating Scale, and Pain Severity Subscale of the Brief Pain Inventory in Patients With Low Back Pain: A Systematic Review. J Pain. 2019;20(3):245–63. doi: 10.1016/j.jpain.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Bardacke N. Mindful birthing: Training the mind, body, and heart for childbirth and beyond. New York: HarperOne; 2012. [Google Scholar]
- Vleeming A, Albert HB, Ostgaard HC, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17(6):794–819. doi: 10.1007/s00586-008-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolone G, Mosconi P, Quattrociocchi L, Gianicolo EAL, Groth N, Ware JE. Questionario sullo stato di salute sf12. Versione italiana 2001. Milano: Guerini e associati; 2001. [Google Scholar]


