Abstract
Background
Lymphomas usually present with different occurrence patterns across different geographical locations, but their epidemiology in Zambia is yet to be extensively explored.
Objectives
To study the spectrum of lymphoma subtypes prevalent within the Zambian population.
Methods
Histopathological records with suspected lymphoma at the University Teaching Hospital (UTH) in Lusaka from the year 2014 to 2016, diagnosed based on the 2008 World Health Organization (WHO) criteria were reviewed. The analysis was done in terms of type, sex, age, and site of biopsy; and Fisher’s exact test was used for significance testing.
Results
During the study period (2014-2016), there were more B cell neoplasms {222 (92.5%)} than T cell neoplasms {18 (7.5%)}. Non-Hodgkin’s lymphoma (NHL) was seen in 191 (79.6%) whereas classic Hodgkin’s lymphoma (CHL) was seen in 39 (16.3%). Burkitt’s lymphoma (BL) and diffuse large B cell lymphoma (DLBCL) showed equal proportions {17.5% of all lymphoma cases (42/240) each}, as the most prevalent subtypes of NHL whereas marginal zone B cell lymphoma was the rarest subtype with 1.4% (4/240). For CHL, mixed cellularity and lymphocyte rich subtypes (4.6% of all lymphoma cases) were the most common subtypes. There was a statistically significant difference in the occurrences of lymphoma subtypes across different age categories (p = 0.002).
Conclusion
Zambia has a diverse lymphoma subtypes population, affecting a relatively young population. The data from this study will serve as a baseline for improved health care provision and more robust future studies.
Keywords: Spectrum, Lymphoma, Subtypes, Zambia
Background
Lymphoma is a group of blood malignancies that develop from lymphocytes. Also known as lymphoid neoplasms, these blood malignancies comprise a group of closely related yet heterogeneous diseases, with widely varying clinical features, histology, immunophenotypes, and genetic abnormalities [1, 2]. Lymphomas are a fairly common malignancy accounting for approximately half of all newly diagnosed hematological neoplasms, and they comprise the sixth most common group of malignancies worldwide in both men and women, albeit with marked geographic variations and affecting more males than females within the age range of 1 to 85 years but peaking within the second decades of life [3–5]. The highest rates of about 68,000 cases per year are observed in North America while the lowest rates are seen throughout Asia with an incidence rate of around 6.1 [6, 7]. Over the last few decades, the global incidence of lymphoma has recorded an increase of around 3-4% annually [2]. The regions reporting the highest incidence for certain types of lymphomas such as Burkitt’s lymphoma are situated in the developing countries of Africa where poverty levels are still high and the human immunodeficiency virus (HIV) pandemic is still hampering the strides made by health care authorities to reduce disease burdens. The developed countries on the other hand have over the years had a stable increase in the lymphoma incidence [2]. To date, the etiology of most types of lymphoma is not clearly understood [8] but several risk factors have been identified. The most well recognized are acquired immunodeficiency syndrome (AIDS)—infectious agents such as Epstein-Barr virus (EBV), low socio-economic status, advancing age, gender, family history of cancers, dietary, and environmental factors [9–14].
Lymphomas have traditionally been classified as either Hodgkin’s lymphoma (HL) or non-Hodgkin’s lymphoma (NHL) based on the presence or absence of the Reid-Sternberg (RS) cell on histology. HL and NHL are further subtyped into individual entities displaying distinct behavioral, prognostic, and epidemiological characteristics, with varying responses to treatment. NHL subtypes include Burkitt’s lymphoma (BL), diffuse large B cell lymphoma (DLBCL), anaplastic large cell lymphoma, lymphoblastic lymphoma, small lymphocytic lymphoma, and marginal zone B cell lymphoma among others. These make up about 90% of all the lymphoma cases worldwide and have recorded a steady increase in their incidence. By the year 2015, 1 in 78 men and 1 in 110 women at the global level developed NHL between birth and age 79 years. In the same year, NHL was declared the 11th highest cause of cancer deaths [15]. The high prevalence of this cancer in African populations is usually attributed to the high incidence of Burkitt’s lymphoma (BLs) among children in the tropical zone of Africa HL is subtyped into classic Hodgkin’s lymphoma (CHL) and nodular lymphocyte predominance Hodgkin lymphoma (NLPHL). CHL is further subtyped into mixed cellularity, lymphocyte rich, nodular sclerosing, and lymphocyte subtypes [3, 16].
Because of the special geographical location and cultural background in the tropical region of Africa where high levels of poverty, HIV, Epstein-Barr virus, and malaria complicate health care provision, Zambia may have its specific patterns of various cancers. The burden of lymphoma subtypes in Zambia is not clearly known and very few studies have been conducted with pediatric lymphomas being examined for the period 1980 to 1992 [17] and in 2014 [18]. For adult lymphomas, small sample sized studies were carried out [19, 20].
Evidently, all these studies were quite inadequate in scope and this warrants further study. There is need for a holistic investigation of the epidemiology of lymphoma in Zambia, focusing on the subtype, age, sex, and anatomical site. The aim of our study was to determine the overall prevalence of lymphomas and their subtypes at Zambia’s highest referral hospital. The results may establish patterns of lymphoma subtypes currently prevalent in Zambia and will serve as a baseline to carry out more robust studies to design intervention strategies to reduce the burden of lymphomas and other hematological malignancies.
Methods
Aim
To study the spectrum of lymphoma subtypes prevalent within the Zambian population.
Study setting
The study was carried out at the University Teaching Hospital (UTH) in Lusaka, Zambia. UTH is a 2000-bed capacity tertiary hospital and Zambia’s highest referral health institution, and, currently, the final destination of the unsolved medical problems from all over the country [18]. As a highest health institution in the referral system of the country, diseases diagnosed here usually reflect an overall, country wide picture. UTH houses several specialized laboratories including the histopathology laboratory which provide services to most of the country. Also, within the UTH are several training institutions, including the School of Medicine, which is the major provider of medical graduates in the country.
Unfortunately, many medical cases at this institution go undiagnosed only to be discovered at postmortem, mainly due to a lack of prior knowledge about disease burdens, as well as insufficient diagnostic and research infrastructure [21, 22]. Histopathology diagnosis of lymphoma at UTH is based on morphological evaluation of hematoxylin and eosin (H&E)-stained tissues and immunohistochemistry. Flow-cytometry services are insufficient while molecular cytogenetic diagnostic services are not yet available at this institution.
Study design
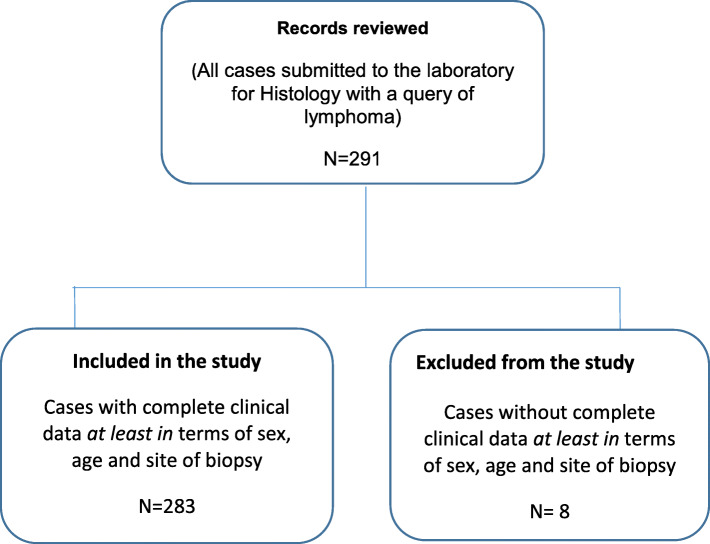
This study reviewed all the histopathological records kept over a 3-year period, from 1st January 2014 to 31st December 2016. These cases were submitted to the laboratory as part of a routine diagnostic schedule, with clinical indications or suspicions of lymphoma. The inclusion criteria were all cases with clinical details comprising of at least of sex, age, site of biopsy, and clinical diagnosis or suspicion. All cases that did not meet these criteria were excluded as shown in Fig. 1.
Fig. 1.
Inclusion and exclusion criteria
Statistical analysis
We analyzed all the cases in terms of sex, age, site of biopsy, and final histological diagnosis. Statistical analysis was performed using Stata Version 14 (Stata Corp. 2015) while Fisher’s exact test was used for statistical significance testing and the level of statistical significance was set at p ≤0.05. The variables considered were sex, age, and site of biopsy. Sex was categorized as either male or female; age was categorized as different age groups which are as a < 15 years, 15 to 24 years, 25 to 34 years, 35 to 44 years, 45 to 54 years, 55 to 64 years, and 64+ years. Site of biopsy was categorized as either nodal (for the biopsies obtained from lymph nodes) or extra nodal (for biopsies obtained from non-lymph node areas of the body). The frequencies of different lymphoma types and sub-types were stratified by sex and age. Lymphoma classification was done based on the 2008 WHO criteria [23].
Results
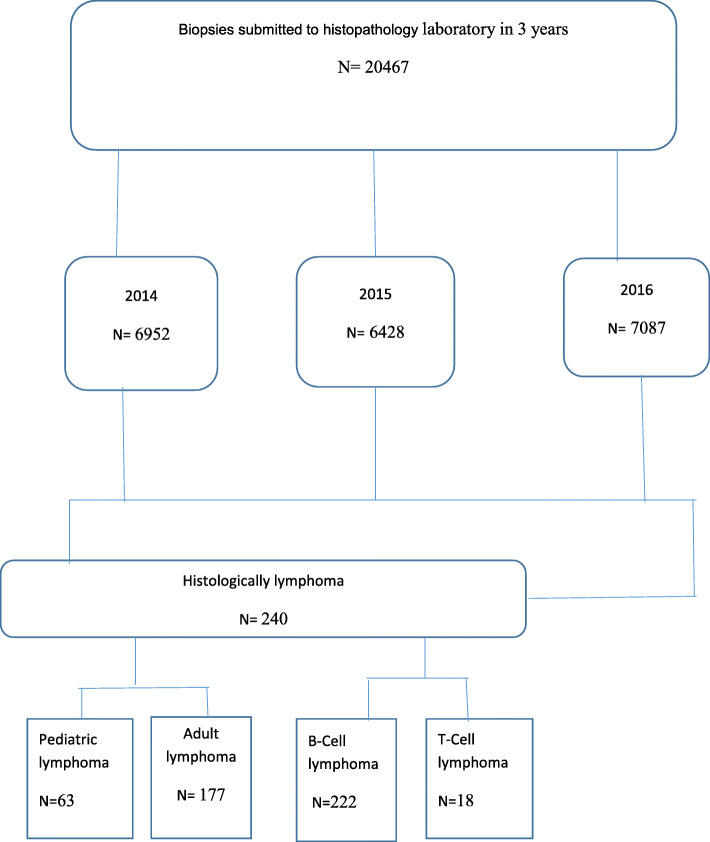
The aim of this study was to find the overall prevalence of lymphoma. During the period considered in this study, a total number of 20,467 biopsies were submitted to UTH histopathology laboratory for routine histological evaluation. The biopsies comprised 6952 for the year 2014, 6428 for the year 2015, and 7087 for the year 2016. Clinical requests querying lymphoma made up 1.4% (291/20467) of the total number of clinical requests to the laboratory in the same period.
Two hundred and ninety-one cases of clinically suspected lymphoma were submitted to the histopathology laboratory during the period under review. Out of these, 8 cases were disqualified for incomplete clinical data. Two hundred and eighty-three cases met the study inclusion criteria. After histological typing, 240 (84.8%) had a laboratory diagnosis of lymphoma, 63 (26.2%) in the pediatric age groups and 177 (73.8%) in the adult age groups. B cell lymphoma made up 222 (92.5%) whereas T cell lymphoma made up 18 (7.5%) as shown in Fig. 2. NHL was diagnosed in 191 (79.6%), whereas 39 (16.3%) were diagnosed as the classic type of HL (CHL) and no case of nodular lymphocyte predominance Hodgkin lymphoma (NLPHL) was detected.
Fig. 2.
General characteristics of reviewed laboratory records
Ten (4.1%) of the cases could not be classified as either NHL or HL. The year 2014 recorded the largest submission of cases of clinically suspected lymphoma (108) followed by 2016 (105) while 2015 recorded the lowest (78), but a laboratory diagnosis of lymphoma was only confirmed in 95 cases for the year 2014, 59 for the year 2015, and 86 for the year 2016 as shown in Table 1.
Table 1.
General demographic characteristics of clinically suspected lymphoma cases at UTH between the year 2014 and 2016 (N = 283)
| Patients’ characteristics | Lymphoma N (%) | Not Lymphoma N (%) | |
|---|---|---|---|
| Total submission to the laboratory | 240 (84.8%) | 43 (15.2%) | |
|
1CHL = 39 (16.3) |
2NHL = 191 (79.6) | ||
| Unclassified = 10 (4.1%) | |||
|
Not subtyped: CHL = 7 NHL = 71 |
|||
| Mean age | 30.6 | ||
| Age groups | |||
| < 15 | 63 (26.2) | 19 (44.2) | |
| 15-24 | 31 (12.9) | 5 (11.6) | |
| 25-34 | 38 (15.8) | 6 (14) | |
| 35-44 | 47 (19.6) | 4 (9.3) | |
| 45-54 | 34 (14.2) | 6 (14) | |
| 55-64 | 16 (6.7) | 2 (4.7) | |
| > 64 | 11 (4.6) | 1 (2.3) | |
| Gender | |||
| Male | 133 (55.4) | 28 (65.1) | |
| Female | 107 (44.6) | 15 (34.9) | |
| Site | |||
| Nodal | 154 (64.2) | 14 (32.6) | |
| Extra-nodal | 86 (35.8) | 29 (67.3) | |
| Year | |||
| 2014 | 95 | ||
| 2015 | 59 | ||
| 2016 | 86 | ||
1Classic Hodgkin’s lymphoma
2Non-Hodgkin’s lymphoma
Of the cases diagnosed as lymphoma, 133 (55.4%) were males while 107 (44.6%) were females, setting the overall male:female ratio at 1.2:1. The age of patients with lymphoma ranged from 1 to 84 years, with a mean age of 30.6 years. The peak occurrence of lymphoma was seen among children under 15 years (26.2% of all lymphoma cases) followed by middle-aged patients in the late third and early fourth decade of life (19.6% of all lymphoma cases). Lymphoma was least prevalent among elderly people in the sixth decade (4.6 %). Nodal involvement (64.2%) was more common than extra nodal involvement (35.8%) (Table 1) and the most commonly involved lymph nodes were cervical (34%) while the least involved were epitrochlear (0.68%). The most common extra nodal site of involvement was abdominal (45.2%). Of the 43 cases without a laboratory diagnosis of lymphoma, 28 (65.1 %) were small round blue cell tumor without definitive diagnosis, 11 (25.6%) were carcinoma, while 4 (9.3%) were Castleman’s disease. There was a statistically significant difference in the occurrences of lymphoma subtypes across different age categories (p = 0.002) (Table 2).
Table 2.
Prevalence of lymphoma subtypes by age group at UTH in Lusaka between the year 2014 and 2016 (N = 152)
| ALCL1 | BL2 | DLBCL3 | LL4 | LD5 | LR6 | MZCL7 | MC8 | NS9 | SLL10 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age groups | |||||||||||
| <15 | 3 | 19 | 3 | 7 | 0 | 3 | 0 | 5 | 2 | 0 | 42 |
| 15-24 | 1 | 6 | 3 | 1 | 0 | 3 | 0 | 1 | 5 | 1 | 21 |
| 25-34 | 4 | 4 | 9 | 0 | 0 | 2 | 0 | 1 | 3 | 0 | 23 |
| 35-44 | 2 | 7 | 12 | 0 | 1 | 2 | 2 | 2 | 0 | 0 | 28 |
| 45-54 | 3 | 4 | 9 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 21 |
| 55-64 | 2 | 1 | 3 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 10 |
| > 65 | 2 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7 |
| Total | 17 | 42 | 42 | 8 | 1 | 11 | 4 | 11 | 10 | 6 | 152 |
p = 0.002
1Anaplastic large cell lymphoma
2Burkitt’s lymphoma
3Diffuse large B cell lymphoma
4Lymphoblastic lymphoma
5Lymphocyte-depleted classic Hodgkin lymphoma
6Lymphocyte rich classic Hodgkin lymphoma
7Marginal zone cell lymphoma
8Mixed cellularity classic Hodgkin lymphoma
9Nodular sclerosing classic Hodgkin lymphoma
10Small lymphocytic lymphoma
Non-Hodgkin’s lymphoma
NHL was diagnosed in 191 cases, making up 79.6% of the total lymphoma patients. BL and DLBCL were seen in equal proportions (42/240) as the most prevalent subtypes of NHL, each of them making up 17.5% of all the lymphoma cases. In both subtypes, the majority (60%) of the cases involved lymph nodes. Both subtypes were seen from early childhood to late adulthood but with stark differences in the peak age categories. BL was mainly a disease of patients under 15 years of age while DLBCL was a disease of individuals in their third, fourth, and fifth decades of life (Table 2). BL affected more males than females with a male:female ratio of 1.6 to 1 while DLBCL showed a completely different picture with a male:female ratio of 0.68 to 1. Anaplastic large cell lymphoma made up 7.1% of all lymphoma cases was seen twice as often in lymph nodes as in extra nodal sites and affected more males than females with a male:female ratio of 1.4:1. It had a preponderance for individuals in their third decade of life. Lymphoblastic lymphomas were mainly seen to be a disease of male patients under 15 years of age and diagnosed in the lymph nodes. Small lymphocytic lymphoma and marginal zone B cell lymphoma were both seen as diseases of the middle aged with 2.5% and 1.4% proportions respectively of the total lymphoma cases. Small lymphocytic lymphoma affected males twice as much as females and was largely nodal in location, whereas marginal zone B cell lymphoma affected males and females equally and was seen more in extra-nodal sites (Tables 2 and 3). Seventy-one cases (29.6% of the lymphoma cases) mostly involving lymph nodes were diagnosed as NHL but could not be subtyped further.
Table 3.
Histological subtype, age range, male to female ratio, frequency, and anatomical location of the lymphoma subtypes diagnosed at the UTH in Lusaka from the year 2014 to 2016 (N = 240)
| Histological type | Age range (years) | M:F ratio | Frequency | Anatomical location | |
|---|---|---|---|---|---|
| (N0) | (%) | (Nodal/extranodal) | |||
| Classic Hodgkin’s lymphoma | |||||
| Mixed cellularity | 6-64 | 2.7:1 | 11 | 4.6 | 10/1 |
| Lymphocyte rich | 9-51 | 1.5:1 | 11 | 4.6 | 11/0 |
| Nodular sclerosing | 12-32 | 4:1 | 10 | 4.2 | 10/0 |
| Lymphocyte depleted | 40 | 1 | 0.4 | 1/0 | |
| Not subtyped | 6-60 | 0.2:1 | 7 | 2.9 | 5/2 |
| Non-Hodgkin’s lymphoma | |||||
| Burkitt’s lymphoma | 1-65 | 1.6:1 | 42 | 17.5 | 24/18 |
| Diffuse large B cell lymphoma | 9-79 | 0.68:1 | 42 | 17.5 | 24/18 |
| Anaplastic large cell lymphoma | 12-71 | 1.4:1 | 17 | 7.1 | 12/5 |
| Lymphoblastic lymphoma | 6-16 | 2.5:1 | 7 | 2.9 | 4/3 |
| Small lymphocytic lymphoma | 19-65 | 2:1 | 6 | 2.5 | 5/1 |
| Marginal zone B cell lymphoma | 41-56 | 1:1 | 4 | 1.4 | 1/3 |
| Not subtyped | 3-78 | 0.9:1 | 73 | 30.4 | 44/29 |
| Others* | 4-84 | 1:1 | 10 | 4.2 | 3/7 |
| Total | 240 | 84.8 | 153/87 | ||
*Lymphoma cases without histological subtypes
Classic Hodgkin’s lymphoma
CHL was diagnosed in 39 (16.3%) of the total number of lymphoma patients. Mixed cellularity and lymphocyte rich subtypes were seen in equal proportions as the most common types of CHL, each making up 4.6% of the total lymphoma cases and both had a preponderance of nodal involvement (91% and 100% respectively). Similar age range and peak age category were equally observed among both subtypes with mixed cellularity occurring between 6 and 64 while lymphocyte rich occurred between 9 and 51 years, but both having a peak occurrence within the first and second decades of life. In both subtypes, the male patients were more affected than their female counterparts with a male:female ratio of 2.7:1 for mixed cellularity and 1.5:1 for lymphocyte rich. Nodular sclerosing subtype was prevalent among patients before 40 years of age but mainly affected patients in the 15 to 24 years age category. It made up 4.2% of the total lymphoma cases with a 100% nodal involvement and a male:female ratio of 4:1. Only one 40-year-old male patient had the lymphocyte-depleted subtype of CHL (Tables 2 and 3). Seven cases (2.9%) of all the lymphoma cases were diagnosed as CHL but could not be subtyped further.
Discussion
Appropriate allocation of resources for cancer prevention, early diagnosis, curative, and palliative care requires detailed knowledge of the local burden of cancer [15]. To the best of our knowledge, our study is the first one to conduct a holistic investigation of the epidemiology of lymphoma in Zambia, focusing on the subtype, age, sex, and anatomical site.
It is a well-established fact that the majority of all lymphoma cases are of B cell lineage [2, 20]. Our study found that the B cell lymphoma accounted for 92.5% of all the lymphoma cases in Zambia, in agreement with most of the findings globally. Our results, however, differ with results obtained from studies done in Kenya, China, Eastern India, and South Korea, which found B cell lymphoma at 80.3%, 76.7%, and 88.8% respectively [1, 24–26]. These variations are not surprising given the geographical trends of lymphoma epidemiology. Even within the same country, ethnic heterogeneity is observed [8, 24, 27]. We found that 79.6% of the lymphoma cases were NHL, and this corroborates the fact that NHL forms a larger proportion of lymphoma diagnosed world over albeit with regional and geographical differences. Our results compare favorably with the Nigerian studies, which found NHL to account for 79.5% and 87.5% of the total lymphoma cases [3, 28]. A representative European study [29] found NHL to be even higher (93.6%) than what is obtaining in sub-Saharan Africa while in Asia, stark variations were observed in the proportions of NHL and HL, but all of the studies agree that NHL comprise over 90% of all lymphoma diagnoses [1, 24, 30]. We contend that variations observed between these studies and ours may be because of varying environmental and genetic factors prevalent in different regions of the world.
In Zambia, only the classical type of HL (CHL) was observed and its subtypes were mainly nodal. It may be deduced that CHL generally affects young people in their first and second decades of life. The absence of nodular lymphocyte predominance Hodgkin lymphoma (NLPHL) in this study corroborates the rarity of this type of HL globally [3, 28, 31–33]. It has been established that the epidemiology of HL exhibits several specific patterns of age-incidence curves, depending on the socioeconomic status of the population [33–35]. Lymphocyte rich and mixed cellularity subtypes of HL were seen more among children under 15 years of age while nodular sclerosing peaked among the young adults, 15 to 24 years old in the Zambian populations. This corroborates the findings that in underdeveloped countries, CHL shows a peak in early childhood with a predominance of mixed cellularity subtype followed by an older adult peak [2]. Epstein-Barr virus (EBV) has traditionally been linked to the etiopathogenesis of HL, especially the mixed cellularity subtype [28, 36]. While the burden of EBV and its involvement in the pathogenesis of lymphoid neoplasms in Zambia has not yet been clearly established, it is noted that the mixed cellularity subtype of CHL is primarily a disease of economically disadvantaged children who are exposed to early childhood viral infection, and older individuals who are immunosuppressed due to old age or HIV infection, while nodular sclerosis is a disease of young adults whose higher social class has delayed their exposure to common childhood infections [34, 37]. This inference is somewhat supported by the findings of one study conducted on herpesviruses among pediatric patients at this site right here in Zambia [38].
Although DLBCL is the most common NHL subtype worldwide, geographical variations exist on a global scale. Most studies in sub-Saharan Africa, including a 1995 pediatric lymphoma study at this same hospital where our study was conducted, found that BLs are the most common subtypes [3, 17, 28, 39]. The situation in sub-Saharan Africa is the opposite of what is obtaining in some regions of Asia, where BL is either present in a small section of the population or non-existent at all [40, 41]. The fact that in our study, BL shares the top notch with DLBCL among the NHL subtypes is not surprising considering our location in the tropical Africa where Malaria is endemic and has been known to exert a cofactor role together with EBV in the etiopathogenesis of BL [9, 42]. However, most studies done at this site found statistics of BL to be very high with some putting it at double the DLCBL proportions. Our study disagrees with these findings and shows equal proportions of these subtypes. The decline in the rates of Burkitt’s lymphoma and the increase in the rates of DLBCL in this region deserve further study. In our opinion, this may be either due to transformation of Lusaka city into a cosmopolitan metropolis or a result of better Malaria control strategies in Zambia that translate into falling incidences of BL. Seven cases of HL and seventy-one cases of NHL could not be subtyped. Failure to subtype these lymphoma cases could be, among other things, a manifestation of insufficient diagnostic infrastructure, which is a common finding in sub-Saharan Africa [3, 43, 44].
The findings of this study should be interpreted in the context of certain limitations. Firstly, while all efforts have been made to present the data as retrieved from the histopathology laboratory records, we feel our findings would have been more robust if we had managed to get more clinical data including the HIV status of the patients. Unfortunately, clinical files were not available at the time of this study. Secondly, while the data presented here is as accurate as when it was used to manage lymphoma patients, misdiagnoses may exist. More robust future research should aim at, among other aspects, establishing the burden of infections among hematological malignancies, as well as locally prevalent genetic and or environmental factors which might be playing a role in the carcinogenesis of hematological malignancies in Zambia.
Conclusion
Lymphomas in Zambia are mostly of B cell lineage, affecting slightly more males than females and mostly involving lymph nodes. The most common HL subtype is mixed cellularity which is very common among children under 15 years of age while DLBCL and BL present in equal proportions as the most common subtypes of NHL. BL is mainly a lymphoma of both male and female children under 15 years of age while DLBCL is mainly a disease affecting more females than males with its peak among individuals in their fourth and fifth decades of life. Besides the establishment of patterns of lymphoma subtypes currently prevalent in Zambia, this study will serve as a baseline to design intervention strategies to reduce the burden of lymphomas and other hematological malignancies.
Acknowledgements
We thank Kenny Mwale for helping with data acquisition from the Laboratory Information System and all the staff at UTH histopathology laboratory for being cooperative during the whole record review process. We also thank the University Teaching Hospital management for granting us permission to review the histopathology records.
Abbreviations
- NHL
Non-Hodgkin’s lymphoma
- HL
Hodgkin’s lymphoma
- WHO
World Health Organization
- BL
Burkitt’s lymphoma
- DLBCL
Diffuse large B cell lymphoma
- AIDS
Acquired immune deficiency syndrome
- HIV
Human immunodeficiency virus
- EBV
Epstein-Barr virus
- SOM
School of Medicine
- CHL
Classic Hodgkin’s lymphoma
- UTH
University Teaching Hospital
Authors’ contributions
PP conceived the research idea. PP, VM, SM, and LZ were involved in designing the study, analyzed the data, participated in interpretation, drafted, and finalized the manuscript. All authors reviewed and approved the final manuscript.
Funding
No funding was obtained from anywhere for this work.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by University of Zambia Biomedical Research Ethics Committee (UNZABREC) (protocol number: 071-06-17). Permission to review histopathology laboratory records was obtained from relevant authorities at UTH. Patient’s confidentiality was upheld by not publishing any patient’s details in the final manuscript and the study adhered to the guidelines of the Helsinki Declaration [45].
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pascal Polepole, Email: pascal.polepole@unza.zm.
Victor C. Mudenda, Email: mudenda2003@yahoo.co.uk
Sody M. Munsaka, Email: s.munsaka@unza.zm
Luwen Zhang, Email: lzhang2@unl.edu.
References
- 1.Sun J, Yang Q, Lu Z, He M, Gao L, Zhu M, Sun L, Wei L, Li M, Liu C, Zheng J, Liu W, Li G, Chen J. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138(3):429–434. doi: 10.1309/AJCP7YLTQPUSDQ5C. [DOI] [PubMed] [Google Scholar]
- 2.Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol. 2012;47(2):92–104. doi: 10.5045/kjh.2012.47.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oluwasola AO, Olaniyi JA, Otegbayo JA, Ogun GO, Akingbola TS, Ukah CO, et al. A fifteen-year review of lymphomas in a Nigerian tertiary healthcare centre. J Health Popul Nutr. 2011;29(4):310. doi: 10.3329/jhpn.v29i4.8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roman E, Smith AG. Epidemiology of lymphomas. Histopathology. 2011;58(1):4–14. doi: 10.1111/j.1365-2559.2010.03696.x. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Parkin D, Whelan S, Ferlay J, Storm H. Cancer incidence in five continents. Vols. I-VIII. Lyon: IARC CancerBase No 7; 2005.
- 7.Forman D, Bray F, Brewster D, Gombe Mbalawa C, Kohler B, Piñeros M, et al. Cancer incidence in five continents, Vol. X (electronic version) Lyon: IARC. 2013. [DOI] [PubMed]
- 8.Müller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin’s lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005;84(1):1–12. doi: 10.1007/s00277-004-0939-7. [DOI] [PubMed] [Google Scholar]
- 9.Young LS, Yap LF, Murray PG. Epstein–Barr virus: more than 50 years old and still providing surprises. Nature Reviews Cancer. 2016;16(12):789–802. doi: 10.1038/nrc.2016.92. [DOI] [PubMed] [Google Scholar]
- 10.Bibas M, Antinori A. EBV and HIV-related lymphoma. Mediterr J Hematol Infect Dis. 2009;1(2):2009-032. [DOI] [PMC free article] [PubMed]
- 11.Morton LM, Slager SL, Cerhan JR, Wang SS, Vajdic CM, Skibola CF, Bracci PM, de Sanjose S, Smedby KE, Chiu BCH, Zhang Y, Mbulaiteye SM, Monnereau A, Turner JJ, Clavel J, Adami HO, Chang ET, Glimelius B, Hjalgrim H, Melbye M, Crosignani P, di Lollo S, Miligi L, Nanni O, Ramazzotti V, Rodella S, Costantini AS, Stagnaro E, Tumino R, Vindigni C, Vineis P, Becker N, Benavente Y, Boffetta P, Brennan P, Cocco P, Foretova L, Maynadie M, Nieters A, Staines A, Colt JS, Cozen W, Davis S, de Roos AJ, Hartge P, Rothman N, Severson RK, Holly EA, Call TG, Feldman AL, Habermann TM, Liebow M, Blair A, Cantor KP, Kane EV, Lightfoot T, Roman E, Smith A, Brooks-Wilson A, Connors JM, Gascoyne RD, Spinelli JJ, Armstrong BK, Kricker A, Holford TR, Lan Q, Zheng T, Orsi L, Dal Maso L, Franceschi S, la Vecchia C, Negri E, Serraino D, Bernstein L, Levine A, Friedberg JW, Kelly JL, Berndt SI, Birmann BM, Clarke CA, Flowers CR, Foran JM, Kadin ME, Paltiel O, Weisenburger DD, Linet MS, Sampson JN. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the interLymph non-Hodgkin lymphoma subtypes project. J Natl Cancer Insti Monog. 2014;2014(48):130–144. doi: 10.1093/jncimonographs/lgu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kricker A, Cocco P, Zheng T, Spinelli JJ, Miligi L, Costantini AS, et al. Occupation and risk of non-Hodgkin lymphoma and its subtypes: a pooled analysis from the interLymph consortium. Environ Health Perspect. 2016;124(4):396-405. [DOI] [PMC free article] [PubMed]
- 13.Morton LM, Hartge P, Holford TR, Holly EA, Chiu BC, Vineis P, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph) Cancer Epidemiol Prev Biomark. 2005;14(4):925–933. doi: 10.1158/1055-9965.EPI-04-0693. [DOI] [PubMed] [Google Scholar]
- 14.Young LS, Yap LF, Murray PG. Epstein–Barr virus: more than 50 years old and still providing surprises. Nature Reviews Cancer. 2016;16(12):789–802. doi: 10.1038/nrc.2016.92. [DOI] [PubMed] [Google Scholar]
- 15.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naresh KN, Raphael M, Ayers L, Hurwitz N, Calbi V, Rogena E, Sayed S, Sherman O, Ibrahim HAH, Lazzi S, Mourmouras V, Rince P, Githanga J, Byakika B, Moshi E, Durosinmi M, Olasode BJ, Oluwasola OA, Akang EE, Akenòva Y, Adde M, Magrath I, Leoncini L. Lymphomas in sub-Saharan Africa - what can we learn and how can we help in improving diagnosis, managing patients and fostering translational research? British Journal of Haematology. 2011;154(6):696–703. doi: 10.1111/j.1365-2141.2011.08772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chintu C, Athale UH, Patil P. Childhood cancers in Zambia before and after the HIV epidemic. Arch Dis Childhood. 1995;73(2):100–105. doi: 10.1136/adc.73.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slone JS, Chunda-Liyoka C, Perez M, Mutalima N, Newton R, Chintu C, Kankasa C, Chipeta J, Heimburger DC, Vermund SH, Friedman DL. Pediatric malignancies, treatment outcomes and abandonment of pediatric cancer treatment in Zambia. PloS one. 2014;9(2):e89102. doi: 10.1371/journal.pone.0089102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zyaambo C, Nzala SH, Babaniyi O, Songolo P, Funkhouser E, Siziya S. Distribution of cancers in Zambia: evidence from the Zambia National Cancer Registry (19902009) J Public Health Epidemiol. 2013;5(2):95–100. [Google Scholar]
- 20.Simakando M, Kaile T, Korolova L, Malyangu E, Shibemba A. B-Cell Non-Hodgkin's lymphoma characterization by Immunohistochemistry in indigenous black Zambians. Journal of Medical Science & Technology. 2015;4(1):46-54.
- 21.Bates M, Mudenda V, Shibemba A, Kaluwaji J, Tembo J, Kabwe M, Chimoga C, Chilukutu L, Chilufya M, Kapata N, Hoelscher M, Maeurer M, Mwaba P, Zumla A. Burden of tuberculosis at post mortem in inpatients at a tertiary referral centre in sub-Saharan Africa: a prospective descriptive autopsy study. The Lancet Infectious diseases. 2015;15(5):544–551. doi: 10.1016/S1473-3099(15)70058-7. [DOI] [PubMed] [Google Scholar]
- 22.Chintu C, Mudenda V, Lucas S, Nunn A, Lishimpi K, Maswahu D, Kasolo F, Mwaba P, Bhat G, Terunuma H, Zumla A. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. The Lancet. 2002;360(9338):985–990. doi: 10.1016/S0140-6736(02)11082-8. [DOI] [PubMed] [Google Scholar]
- 23.Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al. WHO classification of tumors of hematopoietic and lymphoid tissues 4th Ed.(2008). 2008.
- 24.Yoon SO, Suh C, Lee DH, Chi HS, Park CJ, Jang SS, Shin HR, Park BH, Huh J. Distribution of lymphoid neoplasms in the Republic of Korea: analysis of 5318 cases according to the World Health Organization classification. American journal of hematology. 2010;85(10):760–764. doi: 10.1002/ajh.21824. [DOI] [PubMed] [Google Scholar]
- 25.Lazzi S, Ferrari F, Nyongo A, Palummo N, De Milito A, Zazzi M, et al. HIV-associated malignant lymphomas in Kenya (Equatorial Africa) Human Pathol. 1998;29(11):1285–1289. doi: 10.1016/S0046-8177(98)90258-1. [DOI] [PubMed] [Google Scholar]
- 26.Mondal SK, Mandal PK, Roy SD, Chattopadhyay S, Roy S, Biswas PK. Malignant lymphoma in Eastern India: a retrospective analysis of 455 cases according to World Health Organisation classification. Journal of cancer research and therapeutics. 2014;10(2):354–358. doi: 10.4103/0973-1482.136639. [DOI] [PubMed] [Google Scholar]
- 27.Schonfeld SJ, Erdmann F, Wiggill T, Singh E, Kellett P, Babb C, Schüz J. Hematologic malignancies in South Africa 2000–2006: analysis of data reported to the National Cancer Registry. Cancer medicine. 2016;5(4):728–738. doi: 10.1002/cam4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onwubuya IM, Adelusola KA, Durosinmi MA, Sabageh D, Ezike KN. Lymphomas in Ile-Ife, Nigeria: immunohistochemical characterization and detection of Epstein-Barr virus encoded RNA. Journal of clinical and diagnostic research: JCDR. 2015;9(6):EC14–EC19. doi: 10.7860/JCDR/2015/12085.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. British journal of cancer. 2011;105(11):1684–1692. doi: 10.1038/bjc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaqo R, Hughson M, Sulayvani F, Al-Allawi N. Malignant lymphoma in northern Iraq: a retrospective analysis of 270 cases according to the World Health Organization classification. Indian journal of cancer. 2011;48(4):446–451. doi: 10.4103/0019-509X.92276. [DOI] [PubMed] [Google Scholar]
- 31.Pinkus G, Said J. Hodgkin’s disease, lymphocyte predominance type, nodular--a distinct entity? Unique staining profile for L&H variants of Reed-Sternberg cells defined by monoclonal antibodies to leukocyte common antigen, granulocyte-specific antigen, and B-cell-specific antigen. Am J Pathol. 1985;118(1):1. [PMC free article] [PubMed] [Google Scholar]
- 32.Pinkus G, Said J. Hodgkin’s disease, lymphocyte predominance type, nodular--further evidence for a B cell derivation. L & H variants of Reed-Sternberg cells express L26, a pan B cell marker. Am J Pathol. 1988;133(2):211. [PMC free article] [PubMed] [Google Scholar]
- 33.Biasoli I, Stamatoullas A, Meignin V, Delmer A, Reman O, Morschhauser F, Coiffier B, Bosly A, Diviné M, Brice P. Nodular, lymphocyte-predominant Hodgkin lymphoma. Cancer. 2010;116(3):631–639. doi: 10.1002/cncr.24819. [DOI] [PubMed] [Google Scholar]
- 34.Mani H, Jaffe ES. Hodgkin lymphoma: an update on its biology with new insights into classification. Clinical Lymphoma and Myeloma. 2009;9(3):206–216. doi: 10.3816/CLM.2009.n.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaser SL, Jarrett RF. 1 The epidemiology of Hodgkin’s disease. Baillière's clinical haematology. 1996;9(3):401–416. doi: 10.1016/S0950-3536(96)80018-7. [DOI] [PubMed] [Google Scholar]
- 36.Adelusola K, Titiloye N, Rotimi O, Durosinmi M. Epstein Barr virus latent membrane protein-1 in Hodgkin’s lymphoma in Nigerians. Afr Health Sci. 2009;9(3):174-8. [PMC free article] [PubMed]
- 37.Jarrett R. Viruses and Hodgkin’s lymphoma. Ann Oncol. 2002;13(suppl_1):23–29. doi: 10.1093/annonc/13.S1.23. [DOI] [PubMed] [Google Scholar]
- 38.Minhas V, Brayfield BP, Crabtree KL, Kankasa C, Mitchell CD, Wood C. Primary gamma-herpesviral infection in Zambian children. BMC infectious diseases. 2010;10(1):115. doi: 10.1186/1471-2334-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tumwine LK, Orem J, Kerchan P, Byarugaba W, Pileri SA. EBV, HHV8 and HIV in B cell non Hodgkin lymphoma in Kampala, Uganda. Infectious agents and cancer. 2010;5(1):12. doi: 10.1186/1750-9378-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sukpanichnant S. Analysis of 1983 cases of malignant lymphoma in Thailand according to the World Health Organization classification. Human pathology. 2004;35(2):224–230. doi: 10.1016/j.humpath.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Chuang S-S, Chen S-W, Chang S-T, Kuo Y-T. Lymphoma in Taiwan: review of 1347 neoplasms from a single institution according to the 2016 Revision of the World Health Organization Classification. Journal of the Formosan Medical Association. 2017;116(8):620–625. doi: 10.1016/j.jfma.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Moormann AM, Snider CJ, Chelimo K. The company malaria keeps: how co-infection with Epstein-Barr virus leads to endemic Burkitt lymphoma. Current opinion in infectious diseases. 2011;24(5):435–441. doi: 10.1097/QCO.0b013e328349ac4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naresh KN, Raphael M, Ayers L, Hurwitz N, Calbi V, Rogena E, Sayed S, Sherman O, Ibrahim HAH, Lazzi S, Mourmouras V, Rince P, Githanga J, Byakika B, Moshi E, Durosinmi M, Olasode BJ, Oluwasola OA, Akang EE, Akenòva Y, Adde M, Magrath I, Leoncini L. Lymphomas in sub-Saharan Africa–what can we learn and how can we help in improving diagnosis, managing patients and fostering translational research? Br J Haematol. 2011;154(6):696–703. doi: 10.1111/j.1365-2141.2011.08772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gopal S, Krysiak R, Liomba NG, Horner M-J, Shores CG, Alide N, Kamiza S, Kampani C, Chimzimu F, Fedoriw Y, Dittmer DP, Hosseinipour MC, Hoffman IF. Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PloS one. 2013;8(8):e70361. doi: 10.1371/journal.pone.0070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Association GAotWM World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dentists. 2014;81(3):14–18. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.