Abstract
Mutant isocitrate dehydrogenase 1/2 (mIDH1/2) gain a novel function for the conversion of α-ketoglutarate (α-KG) to oncometabolite R-2-hydroxyglutarate (R-2-HG). Two molecular entities namely enasidenib (AG-221) and ivosidenib (AG-120) targeting mIDH2 and mIDH1 respectively, have already been approved by FDA for the treatment of relapsed/refractory acute myeloid leukemia (R/R AML). However, the low responses, drug-related adverse effects, and most significantly, the clinically-acquired resistance of AG-221 and AG-120 has shown great influence on their clinical application. Therefore, searching for novel therapeutic strategies to enhance tumor sensitivity, reduce drug-related side effects, and overcome drug resistance have opened a new research field for defeating IDH-mutated cancers. As the effective methods, synthetic lethal interactions and synergetic therapies are extensively investigated in recent years for the cure of different cancers. In this review, the molecules displaying synergetic effects with mIDH1/2 inhibitors, as well as the targets showing relevant synthetic lethal interactions with mIDH1/2 are described emphatically. On these foundations, we discuss the opportunities and challenges for translating these strategies into clinic to combat the defects of existing IDH inhibitors.
Keywords: Mutant IDH, Inhibitor, Drug resistance, Synthetic lethality, Synergetic therapy
Background
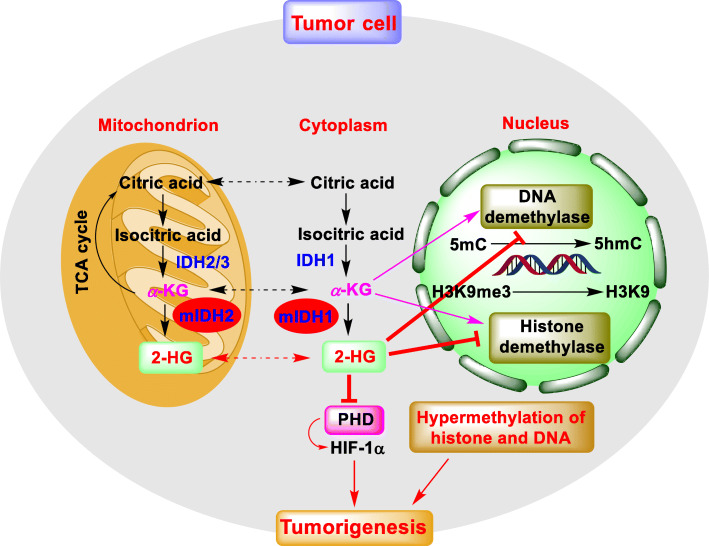
IDH1/2 are metabolic enzymes that are responsible for catalyzing the conversion of isocitrate to α-KG in tricarboxylic acid cycle (TCA) [1]. Somatic mutations, such as R132H in IDH1 and R140Q or R172H in IDH2 have been found in various types of human cancers, including AML, glioma, chondrosarcoma and so on [2, 3]. Moreover, R132H accounts for more than 93% of IDH1 variants while R140Q and R172K mutants are predominant among all IDH2 variants in AML [4]. All these IDH mutants gain a neomorphic function for converting α-KG to R-2-HG. The accumulating R-2-HG acts as an α-KG analog to competitively inhibit α-KG dependent dioxygenases such as histone and DNA demethylases, resulting in DNA and histone hypermethylation (Fig. 1), which are responsible for the blockade of cell differentiation, and hence cancer development [5]. Also, R-2-HG can inhibit the activity of prolyl hydroxylase (PHD), which is an α-KG dependent dioxygenase focusing on the degradation of hypoxia-inducible factor 1α (HIF-1α), finally facilitate oncogenesis and tumor progression [5]. Interestingly, the stability of HIF-1α can also be regulated by α-KG. It was reported that the increased expression of HIF-1α is often detected in tumor cells with low levels of α-KG [6, 7].
Fig. 1.
The presentation model for R-2-HG in tumorigenesis. R-2-HG produced by mIDH1/2 inhibits the α-KG dependent dioxygenases include DNA demethylase, histone demethylase, and PHD, leading to the hypermethylation of DNA and histone, and the increase of HIF-1α, respectively. These epigenetic alterations result in the blockade of cell differentiation and promote the initial engraftment and proliferation of tumor cells
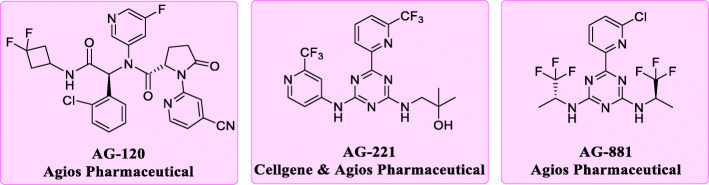
Currently, several inhibitors targeting IDH1/2 mutants have been reported [8, 9]. Among them, AG-221 and AG-120 are both first-in-class inhibitors approved by FDA in 2017 and 2018 respectively [10, 11]. Also, they are prescribed to treat adults suffering from R/R AML with IDH1/2 mutations. Vorasidenib (AG-881), a potent, oral, brain-penetrant dual inhibitor of both IDH1 and IDH2 mutants, is now undergoing several clinical tests (Fig. 2) [12].
Fig. 2.
The representative structures of mIDH1/2 inhibitors. The phenyl glycine based AG-120 is an orally available mIDH1 inhibitor (IC50 [IDH1R132H] = 12 nM). The triazines based AG-221 is an orally available mIDH2 inhibitor (IC50 [IDH2R140Q] = 100 nM versus IC50 [IDH2wild-type] = 1.8 μM). AG-881 is a pan-IDH inhibitor against both mIDH1 and mIDH2 (IC50 [IDH1R132H] = 6 nM versus IC50 [IDH2R140Q] = 12 nM)
Despite encouraging results were obtained from enasidenib and ivosidenib, their clinical applications remain limited because of several key factors. Firstly, the majority of AML patients treated with enasidenib have low responses (38.5% overall response rate, 20.2% complete remission) [13]. Worse results are identified in IDH-mutated glioma and cholangiocarcinoma, whose objective response rate in clinical trials are only 2.9 and 2%, respectively [12, 14]. These studies strongly suggest that precise clinical classification of patients is needed before treatment. Indeed, IDH mutations only drive the early stage of gliomagenesis [15], and are always accompanied with the secondary genetic abnormalities including tumor protein 53 (TP53) mutations, ATP-dependent helicase alpha-thalassemia syndrome protein (ATRX) loss, (RAS)-signaling pathway activation, chromosomal region 1p/19q co-deletion and so on [16, 17]. These alterations often become the leading causes of gliomagenesis in the late stage, and therefore should be considered as the foundations for the histological classification to improve the therapeutic response. Also, these secondary variations are the theoretical basis for novel therapeutics, including synthetic lethality and drug combination. Secondly, some nonnegligible drug-related adverse effects such as differentiation syndrome, leukocytosis and nausea also weaken the clinical value of these two drugs [11–13, 18]. Last, and most important of all, acquired clinical resistance has been reported in AML patients treated with enasidenib and ivosidenib due to the secondary mutations in the allosteric pocket of mIDH1/2 [19, 20]. Therefore, there is an urgent need for discovering novel therapeutic methods to overcome the problems above in IDH-mutated cancers [21, 22].
Drug combination is an available method to produce synergetic effect for treating disease with complicated pathogenesis and several druggable targets [23]. This synergism modulates these targets simultaneously, enhancing the susceptibility of pathogen to multi-drug therapy and overcoming the drug resistance originated from the single-target treatment [24]. In addition, the concept of synthetic lethality has been paid extensive attention due to its successfully application in oncotherapy, such as the poly (ADPribose) polymerase (PARP) inhibitor Olaparib, which is the most famous example of synthetic lethality between BRCA1/2 and PARP in the DNA repair pathway. It has been approved by FDA for the treatment of ovarian cancer and breast cancer carrying BRCA mutation [25]. It is desirable that more and more synthetic lethal interactions are being or waiting to be discovered by using genome-wide RNA interference (RNAi) screening or pharmacology methods [26, 27].
Besides the epigenetic reprogramming, R-2-HG has also been found to be involved in the abnormal regulation of other physiological processes, such as cellular metabolism, cellular apoptosis, redox homeostasis, cell cycle, DNA damage response, immune response and so on. These correlations are basic to the synthetic lethal interactions and synergetic therapies associated with mIDH. In this review, we first enumerate the targets exhibiting relevant synthetic lethal interactions with mIDH1/2. In detail, the inhibitors of these targets could suppress the growth or induced the redifferentiation of tumor cells expressing mIDH1/2. The mechanisms of these synthetic lethal interactions are also well elucidated. Then the compounds which displayed synergetic effect with mIDH1/2 inhibitors are also listed, especially the molecules combined with enasidenib or ivosidenib. We proposed that in the future these promising strategies would be applied in the clinic for the treatment of tumor harbor IDH mutations to combat the defects of existing mIDH1/2 inhibitors.
Synthetic lethality in IDH-mutated tumors
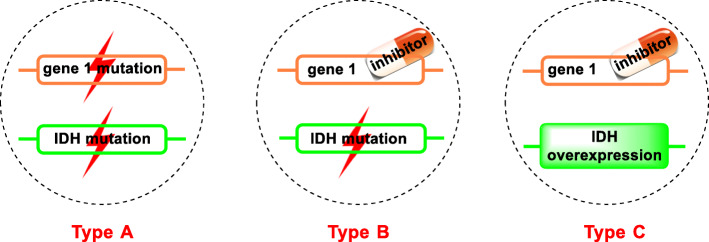
Synthetic lethality describes a phenomenon that the concurrent defects of two genes result in cell death, whereas a defect in either of them has little or no effect on cell viability [28]. Base on different principles, synthetic lethality of IDH can be classified into three types (Fig. 3) [26], the mutations in both genes (Type A), IDH mutation plus another gene’s inhibition (Type B), and IDH overexpression plus another gene’s inhibition (Type C).
Fig. 3.
Three types of synthetic lethality associated with IDH. The orange and green frames represent two genes. The shape of lightning means gene mutation. Inhibitor here indicates the low expression of one gene
In recent years, increasing studies have uncovered that IDH-mutated tumors are sensitive to the inhibition of several other targets. For example, the physiological process involved in DNA repair machinery was known to display synthetic lethal interaction with mIDH, making the tumor cells sensitive to radiation or chemotherapy [29]. It was reported that the glioma cells transduced with IDH1R132H or IDH2R172K exhibited increased cytotoxic actions to radiation [30]. Further, tumor cells expressing IDH1R132H showed more susceptibility to chemotherapeutic agents such as temozolomide (TMZ) and cis-diamminedichloroplatinum (CDDP) in a time- and dose-dependent manner [31]. Collectively, the potential therapeutic efficacy will spur scientists to identify more synthetic lethal partner genes for IDH.
IDH and glutaminase
Mitochondrial glutaminase (GLS) is often in charge of translating glutamine into glutamate and serving as a key role in cell metabolism, growth, and proliferation [32]. It has been widely accepted that cancer cells exhibited “glutamine addiction”, with the overexpression of GLS and rapid dying after glutamine depletion [33]. Given its broad role in energetic and biomass requirements for rapidly proliferating cells, GLS has become an attractive target for cancer therapeutics. Telaglenastat (CB-839), the first-in-class, selective, and orally active GLS inhibitor developed by Calithera Biosciences, is now undergoing clinical trials for the treatment of advanced solid tumors and hematological malignancies [34].
It was reported that the tumor cells expressing mutations in IDH1/2 often appear distinct metabolic characteristics, especially the strong dependency on glutamine as the main cellular source of α-KG. In the meantime, the generated α-KG would be reduced to R-2-HG with NADPH and Mg2+ as the cofactors [35]. Additionally, the metabolic stress produced in diffuse glioma cells expressing IDH1R132H can be alleviated by lactate and glutamate [36]. Therefore, glutamine is crucial for the survival of IDH-mutated tumor cells, and interfering with the glutamine metabolism by GLS inhibitors would result in the deficiency of α-KG, and hence the decreased level of R-2-HG. This would be a novel therapeutic strategy for cancers carrying IDH mutations. It is worth noting that GLS inhibitor BPTES, a lead compound of CB-839, could suppress the growth of five primary AML cells expressing mIDH1/2, but lacking of effectiveness in wild-type cells [37]. Apart from AML, glioma cells with IDH1R312H also showed this metabolic synthetic lethality, exhibiting the preferential sensibility to glutaminase inhibitor. Moreover, inhibiting glutaminase by siRNA or BPTES markedly slowed the growth of IDH1-mutated glioblastoma cells when compared to those expressing wild-type IDH1 [38]. All these studies open a way for novel approaches to target a specific subtype of AML or glioma metabolically with IDH mutations and a unique reprogramming of intermediary metabolism that culminates in glutamine dependency of cancer cells for survival.
IDH and DOT1L
Disruptor of telomeric silencing 1-like (DOT1L) is a histone lysine methyltransferase (HMT) that specifically catalyzes the mono-, di-, and tri-methylation of the histone H3-lysine79 (H3K79) [39]. DOT1L-mediated H3K79 methylations play an important role in transcriptional elongation, cell cycle regulation, and DNA damage response [40]. The aberrant methylation of H3K79 by DOT1L would enhance the expression of leukemogenic genes homeobox A9 (HOXA9) and Meis Homeobox 1 (MEIS1), driving the pathogenic of mixed lineage leukemia (MLL) rearranged leukemia [41]. At this point, DOT1L has been found to be an important drug target for the therapy of acute leukemia with MLL gene translocations, and several kinds of DOT1L inhibitors were discovered in the past decade [42].
It was found that the global levels of H3K79 dimethylation (H3K79me2) were abnormally increased in IDH1/2-mutated tumor cells due to the overexpression of DOT1L [43]. Ley group from Washington University rendered DOT1L inhibitors to primary AML cells carrying IDH1/2 mutations to investigate whether these molecules could also be active in AML cases that lacking MLL rearrangements [44]. To its surprise, the selective DOT1L inhibitor EPZ004777 induced redifferentiation associated with apparent morphologic changes of these AML cells after 10 days’ incubation, which indicated the synthetic lethal-like effect resulted from mIDH and DOT1L. Nevertheless, this effect didn’t lead to the death of AML cells. It appears that treating IDH mutated tumor with DOT1L inhibitor may also be a feasible strategy for AML.
IDH1 and Bcl-2/Bcl-xL
Since the initial discovery in the context of B-cell lymphoma (Bcl) in the 1980s, the Bcl-2 family has long been identified as the apoptosis-suppressing oncogene. Based on different structural and functional features, the Bcl-2 family is divided into three subfamilies, including anti-apoptotic proteins, pro-apoptotic (BH3-only) proteins, and pore-forming or ‘executioner’ (pro-apoptotic) proteins [45]. The anti-apoptotic subfamily contains several members such as Bcl-2, Bcl-xL, BCL-w, BCL-B, MCL-1, and Bfl1/A-1 [46]. Among them, Bcl-2 is the first identified member due to its important role in B-cell lymphoma [47]. Bcl-2, as well as Bcl-xL, were found overexpressed in various tumors [48]. As a druggable target, the first Bcl-2 inhibitor venetoclax was approved by FDA in 2016 for the treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic leukemia (SLL) in adults [49].
In 2015, Chan and his co-workers identified Bcl-2 as a target that can form synthetic lethal interaction with IDH1R132H using a large-scale RNAi screening [50]. The results showed that primary human AML cells with IDH1/2 mutation were more sensitive to venetoclax in both ex vivo and in vivo models than the wild-type cells. Also, the oncometabolite R-2-HG suppressed the activity of cytochrome c oxidase (COX) in the mitochondrial electron transport chain (ETC), subsequently lowered the mitochondrial threshold to trigger apoptosis upon Bcl-2 inhibition, which accounts for the enhanced sensitivity of AML cells to venetoclax. This synthetic lethal interaction constructs the rational basis for combining agents with venetoclax to target resistant cancer cells and maximize the clinical utility of this promising drug.
Similarly, researchers from Columbia University Medical Center have identified Bcl-xL as another target that held synthetic lethal interaction with IDH1R132H [51]. They found U87MG and T98G glioblastoma cells with IDH1R132H mutation displayed significantly higher sensitivity to the Bcl-xL inhibitor ABT263 than the wild-type counterparts. Moreover, the glioblastoma cells treated with ABT263 showed a markedly enhanced cleavage of caspases 9, 3, and PARP, which well explained the mechanism responsible for the enhanced anti-proliferative response toward the Bcl-xL inhibitor. These results support more therapy choices for IDH1-mutated gliomas.
IDH and PARP
As the important DNA damage sensors and signal transducers, PARP enzymes are essential for the repair of single-strand DNA breaks (SSBs) and other types of DNA lesion via the base excision repair pathway [52]. The activated PARP triggers DNA damage response (DDR) that stall the cell cycle and mediate DNA repair, thus maintaining the integrity and stability of the genome in healthy cells. Besides that, BRCA1/2 plays a critical role in the cellular response to DNA double-strand breaks (DSBs) by the homologous recombination (HR) pathway [53]. Mutations in BRCA1/2 would lead to the impairment of the HR pathway and therefore unable to repair the DSBs [54]. Further, inhibition of PARP causes an increase of SSBs, and subsequently the rise of DSBs because of the replication fork collapse, showing synthetic lethal effect as well as inducing the cell death in BRCA1/2-mutated tumors [55].
Sulkowski et al. discovered a markedly reduced capacity to repair DSBs in several genetically diverse cell lines with IDH1 mutation [56]. It also suggests that mutations in IDH1/2 always induce HR defect that renders tumor cells exquisitely sensitive to PARP inhibition. The R-2-HG mediated direct inhibition of αKG dependent dioxygenases (especially KDM4A and KDM4B) are the main reasons for HR suppression. In total, this “BRCAness” phenotype was originated from synthetic lethal interaction between mIDH1/2 and the inhibition of PARP.
IDH1 and mTORC1
The mammalian target of rapamycin (mTOR) is a large serine/theronine protein kinase which is activated by the PI3K/Akt signaling pathway [57]. It is involved in several physiological processes, such as regulating cellular metabolism, cellular growth, protein synthesis, proliferation and survival, whose overactivation is closely associated with the initial and progression of cancer [58]. Thus, mTOR has been regarded as an effective target for cancer therapy [59]. Actually, mTOR resides in two distinct multiprotein complexes: the rapamycin- sensitive complex (mTORC1) and the rapamycin-resistant complex (mTORC2). The mTORC1 is responsible for modulating the translation, ribosome and mitochondrial biogenesis, autophagy, lipid biosynthesis via integrating signals from cell surface receptors, energy status, stress, and availability of oxygen and amino acids [60]. As an inhibitor of mTORC1, rapamycin exhibits potential therapeutic effects on age-related diseases, such as cancer, neurodegenerative disease, and type 2 diabetes [61].
Hujber and co-workers found the IDH1-mutated tumor cells displayed remarkable sensitivity to mTORC1 inhibitor [62]. It was reported that rapamycin could reduce the production of lactate and R-2-HG in fibrosarcoma cells via inhibiting LDH-A and glutaminase, respectively. Additionally, rapamycin also elicited robust inhibitory effects in the proliferation and migration of IDH1-mutated HT-1080 cells, as well as altered their metabolic activity. These results strongly suggest that targeting mTOR activity could be an effective and novel strategy to eliminate tumor with IDH1 mutations.
IDH and SRC
The Src is a group of non-receptor tyrosine kinase families associated with several cellular processes, such as proliferation, migration, differentiation and survival. This family consists of 11 members, among which Src is a representative one, which is ubiquitously expressed in mammals [63]. Src has been found directly involved in the transformation and oncogenesis along with its overexpression and high activation in a wide variety of human cancers [64]. Thus, it has been intensively investigated as a target for anti-tumor and several Src inhibitors have exerted fine therapeutic efficacy in the suppression of tumor growth and angiogenesis [65].
Dasatinib is the first Src/Abl dual inhibitor approved by the FDA for the treatment of chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia [66]. Saha et al. revealed that the IDH-mutated intrahepatic cholangiocarcinoma (ICC) cells exhibited sensitivity to dasatinib [67]. The inhibition of Src signaling was defined as the key factor for this sensitivity. What’s more, dasatinib could also cause rapid and widespread cell death in patient-derived xenografts with IDH mutation. Thus, this novel and dramatic synthetic lethal effect conferred by mIDH and SRC will be a potential treatment protocol for relevant cancers.
IDH1 and GDH
Glutamate dehydrogenase (GDH) is in charge of catalyzing the reversible inter-conversion of L-glutamate to α-KG using ammonia using NAD(P)+ as a coenzyme [68]. This metabolic enzyme can be found in all living organisms, while high levels of GDH activity are always detected in mammalian liver, kidney, brain and pancreas. Emerging evidence indicated that GDH was significantly overexpressed across many cancer subtypes [69].
The metabolic reprogramming in IDH-mutated cells always induced high metabolic stress [70]. In order to overcome this abnormal stress, the mutated cell often adopts the rescue mechanisms, such as upregulating glutaminase and glutamate processing [36]. At this point, the inhibition of glutamate processing with GDH inhibitor epigallocatechin-3-gallate (EGCG) would decrease the synthesis of NAD(P)H and α-KG in IDH-mutated cancers, resulting in increased oxidative stress and metabolic stress, and subsequently improved sensitivity to radiotherapy [71]. Studies have validated that EGCG could significantly decrease the proliferation rates of HCT-116 colorectal cells with IDH1R132H knock-in under ionizing radiation. However, this synthetic lethal effect was diminished when treating with mIDH1 inhibitor AGI-5198. It therefore appears that the synthetic lethal activity derived from mIDH1 and GDH may be an effective and promising option for IDH-mutated cancers.
IDH and DLL3
Delta-like ligand 3 (DLL3) is an inhibitory Notch ligand that is minimally expressed in normal tissues but always specially and highly expressed on the surface of small cell lung cancer (SCLC) cells and other high-grade neuroendocrine tumor cells [72, 73]. Researches have indicated that Notch could be inhibited by overexpressed DLL3, which would exert an important role in the onset and progression of cancer [74]. At this point, DLL3 has been defined as a potential therapeutic target in SCLC or neuroendocrine tumors [75]. Rovalpituzumad is a first-in-class antibody-drug conjugate directed against DLL3 for the treatment of SCLC [76]. Spino et al. reported that IDH mutated glioma cells showed significantly higher expression of DLL3 than wild-type cells. What’s more, the patient-derived IDH-mutated glioma displayed robustly sensitivity to rovalpituzumad in an antigen-dependent manner [77]. These considerations highlight that DLL3 inhibition may be a favorable therapeutic strategy for the cure of glioma with IDH mutation.
IDH1 and Nrf2
Glutathione (GSH) is responsible for defending against oxidative stress in all mammalian cells. As the key determinant of redox signaling, GSH is vital in detoxification of xenobiotics and modulating cell proliferation, apoptosis, immune function, and fibrogenesis [78]. The abnormal levels of GSH are known to contribute to the initial and development of many pathological conditions, such as diabetes mellitus, pulmonary and liver fibrosis, alcoholic liver disease, drug-resistant tumor and so on. Glutamate cysteine ligase (GCL) and GSH synthetase (GS) are two important enzymes in GSH synthesis, whose activity is always regulated at multiple levels in a coordinated manner [79]. Among them, NF-E2 related factor 2 (Nrf2) is the key transcription factor that regulate the expression of these genes via the antioxidant response element (ARE) [80].
Xu group found reactive oxygen species (ROS) and the oxidative damage were remarkable accumulation in IDH1-mutated cells [81]. Consistently, the cells harboring IDH1 mutations exhibited enhanced transcriptional activity of Nrf2 along with the elevation of oxidative stress, which would raise the expression of GLC subsequently. Thus, blockading Nrf2 activation by genetic or pharmacologic methods could not only disrupt ROS homeostasis but also enhance oxidative DNA damage and decrease the proliferation of IDH1-mutated cells [82–84]. Triptolide, a potent Nrf2 inhibitor, has been shown to effectively suppress patient-derived IDH1-mutated glioma cells and exhibited selective cytotoxicity to preclinical glioma xenograft with IDH1 mutations [81]. In general, this synthetic lethality derived from IDH1 mutants and Nrf2 displayed the therapeutic potential in refractory malignancies.
IDH1 and H3K27M
As the mark characteristic for facultative heterochromatin, H3K27 trimethylation (H3K27me3) is associated with transcriptionally repressed regions [85]. Polycomb repressive complex 2 (PRC2) is responsible for depositing, recognizing and propagating H3K27 methylation to maintain the silent state of the genes during development and cell differentiation [86]. Previous studies have identified the H3K27 mutation (H3K27M) in more than 80% diffuse intrinsic pontine gliomas (DIPGs) [87], which is fatal and lacks effective treatments in childhood brain tumors [88]. H3K27M showed a strong affinity for methyltransferase EZH2, the catalytic subunit of the PRC2, repressing H3K27me3 and thereby inhibiting PRC2’s enzymatic activity. Among histone H3, H3.3 is a major variant, and the K27M mutation in histone H3.3 (H3.3K27M) has been identified as a driver mutation for DIPGs [89]. The mutant cells showed decreased levels and altered distribution of H3K27 trimethylation (H3.3K27me3) via multiple mechanisms, including aberrant PRC2 interactions and hampered H3.3K27me3 spreading.
The cell harbor H3.3K27M mutation showed a distinct metabolic pattern, such as enhanced glycolysis, glutaminolysis, and TCA metabolism with high α-KG production [90]. Nevertheless, α-KG often displays contrary level of quantity with H3K27me3 in H3.3K27M cells [91]. The low level of α-KG production by inhibiting the key enzymes in glycolysis or glutaminolysis showed survival benefit in animal models. It was noteworthy that mIDH glioma cells consumed excessive α-KG and subsequently resulted in the increased level of H3K27me3. Moreover, Chung and co-workers found that H3.3K27M and mIDH1 adopt the opposite ways to hold a critical and conserved metabolic pathway and these two mutants displayed mutually exclusive and experimental synthetic lethality [91]. To inhibit IDH1 by shRNA or pharmacological methods in H3.3K27M cells could increase H3K27me3 levels and suppress cell proliferation duo to the altered chromatin accessibility at gene loci related to neuroglial differentiation. Ultimately, the therapeutic potential in preclinical DIPG animal models was presented when combined inhibition of α-KG-producing enzymes in glucose and glutamine metabolism pathways in DIPG animal models.
Synergetic therapies in IDH-mutated tumors
mIDH inhibitors and chemotherapeutic agents
Azacitidine (AZA) is a hypomethylating agent that prevents methylation by covalently binding DNA methyltransferases [92]. The mIDH1 inhibitor ivosidenib has the similar mechanism of action, which inhibits the hypermethylation of DNA and histone. Therefore, the combination of them has been considered to obtain more clinical benefits than monotherapy [93]. Indeed, preclinical work has shown that combination of ivosidenib and AZA could enhance mIDH1 inhibition-related redifferentiation or apoptosis in IDH1-transformed leukemic cell line [94]. On this foundation, an open-label, multicenter, phase Ib trial (NCT02677922) to evaluate safety and efficacy of combining ivosidenib with AZA in patients with newly diagnosed IDH1-mutated AML ineligible for intensive induction chemotherapy was performed [95]. This combination was proved well tolerated, with an expected safety profile consistent to monotherapy. Preliminary efficacy data were promising in difficult-to-treat patients, with the deep and durable remissions and the clearance of IDH1 mutation in the majority of responders.
Apart from this combination above, ivosidenib is also discovered in the clinical trials of combinatorial therapy with PD-1 inhibitor nivolumab (NCT04056910), the dual-drug liposomal encapsulation of cytarabine and daunorubic in CPX-351 (NCT04493164), decitabine/cedazuridine and BCL-2 inhibitor venetoclax (NCT04774393, NCT03471260), the chemotherapeutics such as cytarabine or fludarabine (NCT04250051), and cisplatin (NCT04088188). Also, enasidenib is investigated to combine with AZA (NCT03683433, NCT03383575), JAK inhibitor ruxolitinib (NCT04281498), venetoclax (NCT04092179), CPX-351 (NCT03825796), decitabine/cedazuridine and venetoclax (NCT04774393). Unfortunately, these clinical trials are under recruiting or not yet recruiting thereby no available data are disclosed (Table 1). However, these combined pharmacotherapies would represent an important tendency for the treatment AML or other tumors.
Table 1.
Overview of selected clinical trials of combined pharmacotherapy between mIDH1/2 inhibitors and other molecules
| Drug combination | Phase | Number Enrolled | Inclusion criteria | Trail registration | Status | |
|---|---|---|---|---|---|---|
| Ivosidenib | AZA | Ib/II | 131 | Newly diagnosed AML | NCT02677922 | Active, not recruiting |
| venetoclax | II | 48 | AML | NCT03471260 | Recruiting | |
| FLAG Chemotherapy | I | 25 | R/R AML | NCT04250051 | Not yet recruiting | |
| CPX-351 | II | 30 | AML/MDS | NCT04493164 | Recruiting | |
| gemcitabine + cisplatin | I | 40 | Cholangiocarcinoma | NCT04088188 | Recruiting | |
| Decitabine/ ASTX727 + venetoclax | Ib/II | 84 | R/R AML | NCT04774393 | Not yet recruiting | |
| Enasidenib | AZA | II | 50 | R/R AML | NCT03683433 | Recruiting |
| AZA | II | 105 | MDS | NCT03383575 | Recruiting | |
| Ruxolitinib | II | 32 | Myeloproliferative neoplasm | NCT04281498 | Not yet recruiting | |
| CPX-351 | II | 18 | Relapsed AML | NCT03825796 | Recruiting | |
| venetoclax | Ib/II | 48 | AML | NCT04092179 | Recruiting | |
| Decitabine/ ASTX727 + venetoclax | Ib/II | 84 | R/R AML | NCT04774393 | Not yet recruiting | |
mIDH and radiotherapy/chemotherapy strategies
Like normal cells, tumor cells constantly suffer DNA damages every day and insufficient repairs will drive them towards apoptosis. Therefore, a complete DNA repair system is needed to prevent the cytotoxic or mutagenic effects derived from various types of DNA lesions. As the major enzyme for repairing the methylated lesions on DNA and RNA, the function of α-KG -dependent dioxygenase AlkB superfamily is known to remove N-methyl groups preferentially from 1-methyladenine or 3-methylcytosine in single-stranded DNA [96]. Human cells consist nine distinct ALKB homologs (ALKBH), namely ALKBH1–8 and fat mass and obesity-associated protein (FTO) respectively [97]. Notably, ALKBH2 and ALKBH3 have been shown to catalyze nucleic acid N-demethylation, repairing DNA alkylation lesion preferentially [98].
As a Fe2+ and α-KG-dependent dioxygenase, the activity of AlkB was reported to be inhibited by R-2-HG [99]. It therefore appears that tumor cells with IDH1 mutation display more sensitive to alkylating agents than wild-type cells due to the reduced repair kinetics and increased DNA damages. On the other hand, R-2-HG could elicit the downregulation of DNA damage sensor ataxia telangiectasia mutated (ATM) and subsequently increase the impairment of DNA repair, finally enhance the sensitivity of tumor cells to alkylating agents [100]. Typically, AML cells or glioma cells harbor IDH mutations showed increased sensitivity to radiotherapy and chemotherapy [101, 102]. It is interesting that the deletion of mIDH1 allele or overexpression of ALKBH2 or AKLBH3 could reverse this synergetic effect. These results strongly suggest that the mIDH1/2 derived impairment of DNA repair might contribute to tumorigenesis and classical alkylating agents may offer a way forward the “targeted” therapy for patients with IDH-mutated cancers.
mIDH1 inhibitor and TET inducer
As a hallmark of cancer, the disruption of epigenetic landscapes such as the changes of DNA methylation patterns, are closely associated with the pathogenesis of tumor [103, 104]. TET is known to catalyze the demethylation of 5-methylcytosine (5mC) [105]. It contains three subunits, TET1, TET2, and TET3, that catalyze the successive oxidation of 5-methylcytosine to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) [106]. Several studies have recognized TET enzymes as the important tumor suppressors and the mutants in all three TET genes were uncovered [107]. They showed reduced expression and impaired activity in a wide range of different cancer types [108].
TET is an α-KG dependent dioxygenase and its activity could be inhibited by mIDH1/2 derived R-2-HG, which leads to epigenetic alteration [109]. Gerecke et al. found that Vitamin C (VC), the inducer of TET enzymes, could obviously reduce R-2-HG to the levels comparable to those in wild-type HCT116 cell line when combinatorial treating with mIDH1 inhibitor ML309 [110]. Additionally, this reduction of R-2-HG was accompanied by an enhanced global DNA hydroxymethylation and an increased gene expression of certain tumor suppressors. Most important of all, IDH1-mutated tumor showed an increased percentage of apoptotic cells after combining use ML309 and VC. It is also critical to note that high doses of VC can exert its anticancer effect via multiple mechanism of actions, including modulating infiltration of the tumor microenvironment by cells in immune system to delay cancer growth [111], accumulating ROS and inhibiting glyceraldehyde 3-phosphate dehydrogenase (GAPDH) to kill the colorectal cancer cells harboring KRAS or BRAF mutations [112], targeting redox homeostasis in pheochromocytomas and paragangliomas (PCPG) cells with low levels of succinate dehydrogenase B subunit (SDHB) to suppress metastatic lesion and prolong overall survival [113], and et al. Therefore, this synergetic therapy needs to be further investigated. Collectively, these results strongly illustrate that combinatorial therapy is of great interest to rescue TET activity and treat IDH1/2-mutated cancers.
mIDH inhibitors and immune checkpoint inhibitors
Indeed, R-2-HG is not only the epigenetic modifier involved in neoplasia, but also an immunosuppressive metabolite. It has been reported that R-2-HG always accumulate in tumor environment and can be taken up by CD8+ T cells, hence impairing their capacity of anticancer immune responses [114]. Additionally, R-2-HG was also explored to inhibit the tumor-infiltrating lymphocytes and natural killer cells, causing enhanced immune suppression in tumor microenvironments [115, 116]. On the basis of these considerations, the inhibitors of mIDH actually play dual antitumor effect by causing the epigenetic reprogramming of malignant cells and relieving R-2-HG-mediated immunosuppression. From this it appears that combining cancer metabolism therapy with immunotherapy will be a prospective strategy for overcoming cancer [117], and in particularly, mIDH inhibitors should be paired with the immune checkpoint inhibitors to improve the clinical response of patients with IDH-mutated tumors [118].
It is worth noting that IDH1R132H inhibitor BAY1436032 collocated with PD-1 inhibitor would result in an increase of overall survival in C57BL/6 J mice bearing IDH1-mutated glioma [119]. To our knowledge, the phase II study involved in ivosidenib in combination with immune checkpoint inhibitor nivolumab for patients with IDH1-mutated R/R AML and high risk myelodysplastic syndrome (MDS) [120], advanced solid tumors or enhancing gliomas (NCT04056910) is carrying out. Unfortunately, there is no available data uncovered at present.
Conclusions
As the druggable targets, mIDH1/2 have been paid much attentions due to their therapeutic potential as well as the swiftly successful in drug discovery. Nevertheless, the insuperable weaknesses of two approved inhibitors render researchers to look for alternative methods. With the successful application of PARP inhibitors, several screening programs using siRNA or pharmacological methods have been conducted to identify the targets that can generate synthetic lethal interaction with mIDH1/2 and the molecules can supply synergetic therapy with mIDH1/2 inhibitors. Indeed, these novel therapeutic strategies have exhibited significant effectiveness with the enhanced sensitivity to diverse tumor cells, especially AML and glioma with IDH mutations.
Despite strenuous efforts have been paid, there are still several challenges for transforming these synthetic lethal interactions and synergetic therapies into clinical benefits. The magnitude of a given synthetic lethal effect is the one of the major challenges. Some of the synthetic lethal interactions showed statistically significant and even biologically meaningful, but are unable to translate into clinic due to their small efficacy in the trails. Consider olaparib as a reference, whose synthetic lethal effect with mutant BRCA1/2 is about 100-fold difference in sensitivity when compared to those in wild-type cells. The second challenge is the heterogeneity of cancer [121], with many synthetic lethal targets only existed in a small number of cancer subtypes. In addition, this heterogeneity also makes a portion of cells response to synthetic lethal effect, while it is ineffective to other portion of cells. Actually, the biological targets of R-2-HG are more than 60 members in cells [122], which are involved in profoundly diverse molecular pathways. Thus, it remains not clear for the real oncogenic mechanisms of glioma and it will be a great challenge for applying the synthetic lethal effect to glioma. Other challenges for synthetic lethal effect include how to obtain cell lines stably expressing mutant IDH and avoid the false positive screening by using the novel techniques. Last, favorable blood-brain barrier (BBB) penetration of mutant IDH inhibitors and other drugs is the foundation of synthetic lethal interaction and synergetic therapies to induce anti-tumor effects in CNS tumors, especially for gliomas. In summary, we trust that induction of these therapeutic strategies into IDH-mutated neoplasms is feasible and potentially highly efficacious in future clinical treatment.
Acknowledgements
Not applicable.
Abbreviations
- mIDH1/2
Mutant isocitrate dehydrogenase 1/2
- α-KG
α-ketoglutarate
- R-2-HG
R-2-hydroxyglutarate
- TCA
Tricarboxylic acid cycle
- AML
Acute myeloid leukemia
- PARP
Poly ADPribose polymerase
- RNAi
RNA interference
- TMZ
Temozolomide
- CDDP
Cis-diamminedichloroplatinum
- GLS
Glutaminase
- DOT1L
Disruptor of telomeric silencing 1-like
- HMT
Methyltransferase
- H3K79
Histone H3-lysine79
- MLL
Mixed lineage leukemia
- CLL
Chronic lymphocytic leukemia
- SLL
Small lymphocytic leukemia
- COX
Cytochrome c oxidase
- ETC
Electron transport chain
- SSBs
Single-strand DNA breaks
- DDR
DNA damage response
- DSBs
Double strand breaks
- HR
Homologous recombination
- mTOR
mammalian target of rapamycin
- GDH
Glutamate dehydrogenase
- 5mC
5-methylcytosine
- PHD
Prolyl hydroxylase
- EGCG
Epigallocatechin-3-gallate
- DLL3
Delta-like ligand 3
- SCLC
Small cell lung cancer
- GSH
Glutathione
- GCL
Glutamate cysteine ligase
- GS
GSH synthetase
- Nrf2
NF-E2 related factor 2
- ARE
Antioxidant response element
- ROS
Reactive oxygen species
- H3K27me3
H3K27 trimethylation
- PRC2
Polycomb repressive complex 2
- DIPGs
Diffuse intrinsic pontine gliomas
- AZA
Azacitidine
- PI
Proteasome inhibitors
- MM
Multiple myeloma
- MCL
Mantle cell lymphoma
- R/R AML
Relapsed/refractory acute myeloid leukemia
- TP53
Tumor protein 53
- ATRX
Alpha-thalassaemia syndrome protein
- HOXA9
Homeobox A9
- MEIS1
Meis Homeobox 1
- Bcl
B-cell lymphoma
- ICC
Intrahepatic cholangiocarcinoma
- ATM
Ataxia telangiectasia mutated
- MDS
Myelodysplastic syndrome
Authors’ contributions
KY and HL conceived the idea and wrote the manuscript. JY helped to search for related papers and collect useful information. HT and JY (corresponding author) revised and edited the manuscript. The author(s) read and approved the final manuscript.
Funding
This work was supported by Natural Science Foundation of Suzhou (SYSD2017154) and Fund for development of Science and Technology of Nanjing Medical University (NMUB2020295).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kun Yao and Hua Liu contributed equally to this work.
Contributor Information
Jianmin Yuan, Email: 13358118723@163.com.
Hong Tao, Email: tjzthxlf@163.com.
References
- 1.Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 2010;102(13):932–941. doi: 10.1093/jnci/djq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark O, Yen K, Mellinghoff IK. Molecular pathways: isocitrate dehydrogenase mutations in cancer. Clin Cancer Res. 2016;22(8):1837–1842. doi: 10.1158/1078-0432.CCR-13-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pusch S, Sahm F, Meyer J, Mittelbronn M, Hartmann C, von Deimling A. Glioma IDH1 mutation patterns off the beaten track. Neuropathol Appl Neurobiol. 2011;37(4):428–430. doi: 10.1111/j.1365-2990.2010.01127.x. [DOI] [PubMed] [Google Scholar]
- 4.Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Krönke J, Bullinger L. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28(22):3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 5.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324(5924):261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumor growth and progression. Nat Rev Cancer. 2011;12(1):9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma T, Zou F, Pusch S, Xu Y, Deimling A, Zha X. Inhibitors of mutant isocitrate dehydrogenases 1 and 2 (mIDH1/2): an update and perspective. J Med Chem. 2018;61(20):8981–9003. doi: 10.1021/acs.jmedchem.8b00159. [DOI] [PubMed] [Google Scholar]
- 9.Dang L, Yen K, Attar EC. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol. 2016;27(4):599–608. doi: 10.1093/annonc/mdw013. [DOI] [PubMed] [Google Scholar]
- 10.Molenaar RJ, Maciejewski JP, Wilmink JW, Noorden CJF. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene. 2018;37(15):1949–1960. doi: 10.1038/s41388-017-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SL, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomized, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiNardo CD, Stein EM, Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–2398. doi: 10.1056/NEJMoa1716984. [DOI] [PubMed] [Google Scholar]
- 14.Mellinghoff IK, Ellingson BM, Touat M, Maher E, De La Fuente MI, Holdhoff M, et al. Ivosidenib in isocitrate dehydrogenase 1-mutated advanced glioma. J Clin Oncol. 2020;38(29):3398–3406. doi: 10.1200/JCO.19.03327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johannessen T, Mukherjee J, Viswanath P, Ohba S, Ronen SM, Bjerkvig R, et al. Rapid conversion of mutant IDH1 from driver to passenger in a model of human gliomagenesis. Mol Cancer Res. 2016;14(10):976–983. doi: 10.1158/1541-7786.MCR-16-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Gerges N, Korshunov A, Sabha N, Khuong-Quang D, Fontebasso AM, et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124(5):615–625. doi: 10.1007/s00401-012-1031-3. [DOI] [PubMed] [Google Scholar]
- 17.Amatangelo MD, Quek L, Shih A, Stein EM, Roshal M, David MD, et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood. 2017;130(6):732–741. doi: 10.1182/blood-2017-04-779447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norsworthy KJ, Mulkey F, Scott EC, Ward AF, Przepiorka D, Charlab R, et al. Differentition syndrome with ivosidenib and enasidenib treatment in patients with relapsed or refractory IDH-mutated AML: a U.S. food and drug administration systematic analysis. Clin Cancer Res. 2020;26(16):4280–4288. doi: 10.1158/1078-0432.CCR-20-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Intlekofer AM, Shih AH, Wang B, Nazir A, Rustenburg AS, Albanese SK, et al. Acquired resistance to IDH inhibition through trans or cis dimer-interface mutations. Nature. 2018;559(7721):125–129. doi: 10.1038/s41586-018-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choe S, Wang H, DiNardo CD, Stein EM, Botton S, Roboz GJ, et al. Molecular mechanisms mediating relapse following ivosidenib monotherapy in IDH1-mutant relapsed or refractory AML. Blood Adv. 2020;4(9):1894–1905. doi: 10.1182/bloodadvances.2020001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waitkus MS, Diplas BH, Yan H. Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell. 2018;34(2):186–195. doi: 10.1016/j.ccell.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han S, Liu Y, Cai SJ, Qian M, Ding J, Larion M, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580–1589. doi: 10.1038/s41416-020-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kummar S, Chen HX, Wright J, Holbeck S, Millin MD, Tomaszewski J, et al. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements. Nat Rev Drug Discov. 2010;9(11):843–856. doi: 10.1038/nrd3216. [DOI] [PubMed] [Google Scholar]
- 24.Cheng F, Kovács IA, Barabási AL. Network-based prediction of drug combinations. Nat Commun. 2019;10(1):1197. doi: 10.1038/s41467-019-09186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griguolo G, Dieci MV, Guarneri V, Conte P. Olaparib for the treatment of breast cancer. Expert Rev Anticancer Ther. 2018;18(6):519–530. doi: 10.1080/14737140.2018.1458613. [DOI] [PubMed] [Google Scholar]
- 26.Ashworth A, Lord CJ. Synthetic lethal therapies for cancer: what's next after PARP inhibitors? Nat Rev Clin Oncol. 2018;15(9):564–576. doi: 10.1038/s41571-018-0055-6. [DOI] [PubMed] [Google Scholar]
- 27.McDonald ER, Weck A, Schlabach MR, Billy E, Mavrakis KJ, Hoffman GR, et al. Project DRIVE: a compendium of cancer dependencies and synthetic lethal relationships uncovered by largescale, deep RNAi screening. Cell. 2017;170(3):577–592. doi: 10.1016/j.cell.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Mullard A. Synthetic lethality screens point the way to new cancer drug targets. Nat Rev Drug Discov. 2017;16(9):589–591. doi: 10.1038/nrd.2017.165. [DOI] [PubMed] [Google Scholar]
- 29.Karpel-Massler G, Nguyen TT, Shang E, Siegelin MD. Novel IDH1-targeted glioma therapies. CNS Drugs. 2019;33(12):1155–1166. doi: 10.1007/s40263-019-00684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Chou AP, Chen W, Chen R, Deng Y, Phillips HS, et al. Overexpression of isocitrate dehydrogenase mutant proteins renders glioma cells more sensitive to radiation. Neuro-Oncology. 2013;15(1):57–68. doi: 10.1093/neuonc/nos261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J, Sun B, Shi W, Zuo H, Cui D, Ni L, et al. Decreasing GSH and increasing ROS in chemosensitivity gliomas with IDH1 mutation. Tumour Biol. 2015;36(2):655–662. doi: 10.1007/s13277-014-2644-z. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Meng Y, Li L, Xu P, Wang J, Li Z, et al. Overview of the development of glutaminase inhibitors: achievements and future directions. J Med Chem. 2019;62(3):1096–1115. doi: 10.1021/acs.jmedchem.8b00961. [DOI] [PubMed] [Google Scholar]
- 33.Xiang Y, Stine ZE, Xia J, Lu Y, O’Connor RS, Altman BJ, et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest. 2015;125(6):2293–2306. doi: 10.1172/JCI75836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song M, Kim SH, Im CY, Hwang HJ. Recent development of small molecule glutaminase inhibitors. Curr Top Med Chem. 2018;18(6):432–443. doi: 10.2174/1568026618666180525100830. [DOI] [PubMed] [Google Scholar]
- 35.Salamanca-Cardona L, Shah H, Poot AJ, Correa FM, Gialleonardo VD, Lui H, et al. In vivo imaging of glutamine metabolism to the oncometabolite 2-hydroxyglutarate in IDH1/2 mutant tumors. Cell Metab. 2017;26(6):830–841. doi: 10.1016/j.cmet.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenting K, Khurshed M, Peeters TH, Heuvel CN, Lith SA, Bitter T, et al. Isocitrate dehydrogenase 1-mutated human gliomas depend on lactate and glutamate to alleviate metabolic stress. FASEB J. 2019;33(1):557–571. doi: 10.1096/fj.201800907RR. [DOI] [PubMed] [Google Scholar]
- 37.Emadi A, Jun SA, Tsukamoto T, Fathi AT, Minden MD, Dang CV. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Exp Hematol. 2014;42(4):247–251. doi: 10.1016/j.exphem.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70(22):8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min J, Feng Q, Li Z, Zhang Y, Xu R. Structure of the catalytic domain of human DOT1L, a non-SET domain nucleosomal histone methyltransferase. Cell. 2003;112(5):711–723. doi: 10.1016/S0092-8674(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 40.Kim W, Choi M, Kim JE. The histone methyltransferase Dot1/DOT1L as a critical regulator of the cell cycle. Cell Cycle. 2014;13(5):726–738. doi: 10.4161/cc.28104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, et al. MLL rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20(1):66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anglin JL, Song Y. A medicinal chemistry perspective for targeting histone H3 lysine-79 methyltransferase DOT1L. J Med Chem. 2013;56(22):8972–8983. doi: 10.1021/jm4007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarkaria SM, Christopher MJ, Klco JM, Ley TJ. Primary acute myeloid leukemia cells with IDH1 or IDH2 mutations respond to a DOT1L inhibitor in vitro. Leukemia. 2014;28(12):2403–2406. doi: 10.1038/leu.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruckheimer EM, Cho SH, Sarkiss M, Herrmann J, McDonnell TJ, et al. The Bcl-2 gene family and apoptosis. Adv Biochem Eng Biotechnol. 1998;62:75–105. doi: 10.1007/BFb0102306. [DOI] [PubMed] [Google Scholar]
- 46.Warren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10(3):177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, et al. Solution structure of the antiapoptotic protein bcl-2. Proc Natl Acad Sci U S A. 2001;98(6):3012–3017. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garner TP, Lopez A, Reyna DE, Spitz AZ, Gavathiotis E. Progress in targeting the BCL-2 family of proteins. Curr Opin Chem Biol. 2017;39:133–142. doi: 10.1016/j.cbpa.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong W, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21(2):178–184. doi: 10.1038/nm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karpel-Massler G, Ishida CT, Bianchetti E, Zhang Y, Shu C, Tsujiuchi T, et al. Induction of synthetic lethality in IDH1-mutated gliomas through inhibition of Bcl-xL. Nat Commun. 2017;8(1):1067. doi: 10.1038/s41467-017-00984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lord CJ, Ashworth A. PARP inhibitors: the first synthetic lethal targeted therapy. Science. 2017;355(6330):1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vyas S, Chesarone-Cataldo M, Todorova T, Huang Y, Chang P. A systematic analysis of the PARP protein family identifies new functions critical for cell physiology. Nat Commun. 2013;4:2240. doi: 10.1038/ncomms3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lord CJ, Ashworth A. Targeted therapy for cancer using PARP inhibitors. Curr Opin Pharmacol. 2008;8(4):363–369. doi: 10.1016/j.coph.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Saffhill R, Ockey CH. Strand breaks arising from the repair of the 5-bromodeoxyuridine-substituted template and methyl methanesulphonate-induced lesions can explain the formation of sister chromatid exchanges. Chromosoma. 1985;92(3):218–224. doi: 10.1007/BF00348697. [DOI] [PubMed] [Google Scholar]
- 56.Sulkowski PL, Corso CD, Robinson ND, Scanlon SE, Purshouse KR, Bai H, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med. 2017;9(375):eaal2463. doi: 10.1126/scitranslmed.aal2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15(3):155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 58.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169(2):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12(1):71. doi: 10.1186/s13045-019-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015;25(9):545–555. doi: 10.1016/j.tcb.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schreiber KH, Apelo SIA, Yu D, Brinkman JA, Velarde MC, Syed FA, et al. A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat Commun. 2019;10(1):3194. doi: 10.1038/s41467-019-11174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hujber Z, Petővári G, Szoboszlai N, Dankó T, Nagy N, Kriston C, et al. Rapamycin (mTORC1 inhibitor) reduces the production of lactate and 2-hydroxyglutarate oncometabolites in IDH1 mutant fibrosarcoma cells. J Exp Clin Cancer Res. 2017;36(1):74. doi: 10.1186/s13046-017-0544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bagnato G, Leopizzi M, Urciuoli E, Peruzzi B. Nuclear functions of the tyrosine kinase Src. Int J Mol Sci. 2020;21(8):2675. doi: 10.3390/ijms21082675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19(49):5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 65.Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012;33(3):122–128. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gnoni A, Marech I, Silvestris N, Vacca A, Lorusso V. Dasatinib: an anti-tumour agent via Src inhibition. Curr Drug Targets. 2011;12(4):563–578. doi: 10.2174/138945011794751591. [DOI] [PubMed] [Google Scholar]
- 67.Saha SK, Gordan JD, Kleinstiver BP, Vu P, Najem MS, Yeo JC, et al. Isocitrate dehydrogenase mutations confer dasatinib hypersensitivity and SRC dependence in intrahepatic cholangiocarcinoma. Cancer Discov. 2016;6(7):727–739. doi: 10.1158/2159-8290.CD-15-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spanaki C, Plaitakis A. The role of glutamate dehydrogenase in mammalian ammonia metabolism. Neurotox Res. 2012;21(1):117–127. doi: 10.1007/s12640-011-9285-4. [DOI] [PubMed] [Google Scholar]
- 69.Spinelli JB, Yoon H, Ringel AE, Jeanfavre S, Clish CB, Haigis MC. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science. 2017;358(6365):941–946. doi: 10.1126/science.aam9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Pusch S, Innes J, Sidlauskas K, Ellis M, Lau J, et al. Mutant IDH sensitizes gliomas to endoplasmic reticulum stress and triggers apoptosis via miR-183-mediated inhibition of semaphoring 3E. Cancer Res. 2019;79(19):4994–5007. doi: 10.1158/0008-5472.CAN-19-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peeters TH, Lenting K, Breukels V, Lith SA, Heuvel CN, Molenaar R, et al. Isocitrate dehydrogenase 1-mutated cancers are sensitive to the green tea polyphenol epigallocatechin-3-gallate. Cancer Metab. 2019;7:4. doi: 10.1186/s40170-019-0198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol. 2017;14(9):549–561. doi: 10.1038/nrclinonc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015;7(302):302ra136. doi: 10.1126/scitranslmed.aac9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12(5):535–542. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 75.Owen DH, Giffin MJ, Bailis JM, Smit MD, Carbobe DP, He K. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol. 2019;12(1):61. doi: 10.1186/s13045-019-0745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgenszterm D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18(1):42–51. doi: 10.1016/S1470-2045(16)30565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spino M, Kurz SC, Chiriboga L, Serrano J, Zeck B, Sen N, et al. Cell surface notch ligand DLL3 is a therapeutic target in Isocitrate dehydrogenase-mutant Glioma. Clin Cancer Res. 2019;25(4):1261–1271. doi: 10.1158/1078-0432.CCR-18-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calabrese G, Morgan B, Riemer J. Mitochondrial glutathione: regulation and functions. Antioxid Redox Signal. 2017;27(15):1162–1177. doi: 10.1089/ars.2017.7121. [DOI] [PubMed] [Google Scholar]
- 79.Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu H, Jia Z, Misra BR, Zhang L, Cao Z, Yamamoto M, et al. Nuclear factor E2-related factor 2-dependent myocardiac cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol. 2008;8(2):71–85. doi: 10.1007/s12012-008-9016-0. [DOI] [PubMed] [Google Scholar]
- 81.Yu D, Liu Y, Zhou Y, Ruiz-Rodado V, Larion M, Xu G, et al. Triptolide suppresses IDH1-mutated malignancy via Nrf2-driven glutathione metabolism. Proc Natl Acad Sci U S A. 2020;117(18):9964–9972. doi: 10.1073/pnas.1913633117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi J, Zuo H, Ni L, Xia L, Zhao L, Gong M, et al. An IDH1 mutation inhibits growth of glioma cells via GSH depletion and ROS generation. Neurol Sci. 2014;35(6):839–845. doi: 10.1007/s10072-013-1607-2. [DOI] [PubMed] [Google Scholar]
- 83.Tang X, Fu X, Liu Y, Yu D, Cai SJ, Yang C. Blockade of glutathione metabolism in IDH1-mutated glioma. Mol Cancer Ther. 2020;19(1):221–230. doi: 10.1158/1535-7163.MCT-19-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y, Lu Y, Celiku O, Li A, Wu Q, Zhou Y, et al. Targeting IDH1-mutated malignancies with NRF2 blockade. J Natl Cancer Inst. 2019;111(10):1033–1041. doi: 10.1093/jnci/djy230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kouzarides T. Chromatin modifications and their function. Cell. 2017;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science. 2002;298(5595):1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 87.Khuong-Quang DA, Buczkowicz P, Rakopoulos P, Liu X, Fontebasso AM, Bouffet E, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis PW, Müller MM, Koletsky MS, Cordero F, Lin S, Banaszynski LA, et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340(6134):857–861. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Delaney K, Strobino M, Wenda JM, Pankowski A, Steiner FA. H3.3K27M-induced chromatin changes drive ectopic replication through misregulation of the JNK pathway in C. elegans. Nat Commun. 2019;10(1):2529. doi: 10.1038/s41467-019-10404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venneti S, Garimella MT, Sullivan LM, Martinez D, Huse JT, Heguy A, et al. Evaluation of histone 3 lysine 27 trimethylation (H3K27me3) and enhancer of zest 2 (EZH2) in pediatric glial and glioneuronal tumors shows decreased H3K27me3 in H3F3A K27M mutant glioblastomas. Brain Pathol. 2013;23(5):558–564. doi: 10.1111/bpa.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chung C, Sweha SR, Pratt D, Tamrazi B, Panwalkar P, Banda A, et al. Integrated metabolic and Epigenomic reprograming by H3K27M mutations in diffuse intrinsic Pontine Gliomas. Cancer Cell. 2020;38(3):334–349. doi: 10.1016/j.ccell.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cogle CR, Scott BL, Boyd T, Garcia-Manero G. Oral azacitidine (CC-486) for the treatment of myelodysplastic syndromes and acute myeloid leukemia. Oncologist. 2015;20(12):1404–1412. doi: 10.1634/theoncologist.2015-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bewersdorf JP, Shallis R, Stahl M, Zeidan AM. Epigenetic therapy combinations in acute myeloid leukemia: what are the options? Ther Adv Hematol. 2019;10:1–19. doi: 10.1177/2040620718816698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yen K, Chopra VS, Tobin E, Avanzino B, Mavrommatis K, DiMartino J, et al. Abstract 4956: Functional characterization of the ivosidenib (AG-120) and azacitidine combination in a mutant IDH1 AML cell model. Cancer Res. 2018;78(13 Suppl):4956. [Google Scholar]
- 95.DiNardo CD, Stein AS, Stein EM, Fathi AT, Frankfurt O, Schuh AC, et al. Mutant isocitrate dehydrogenase 1 inhibitor ivosidenib in combination with azacitidine for newly diagnosed acute myeloid leukemia. J Clin Oncol. 2021;39(1):57–65. doi: 10.1200/JCO.20.01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Oxidative demethylation by E. coli alkB directly reverts DNA base damage. Nature. 2002;419(6903):174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 97.Sedgwick B, Bates PA, Paik J, Jacobs SC, Lindahl T. Repair of alkylated DNA: recent advances. DNA Repair (Amst) 2007;6(4):429–442. doi: 10.1016/j.dnarep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Tsujikawa K, Koike K, Kitae K, Shinkawa A, Arima H, Suzuki T, et al. Expression and sub-cellular localization of human ABH family molecules. J Cell Mol Med. 2007;11(5):1105–1116. doi: 10.1111/j.1582-4934.2007.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang P, Wu J, Ma S, Zhang L, Yao J, Hoaddley KA, et al. Oncometabolite D-2-Hydroxyglutarate inhibits ALKBH DNA repair enzymes and sensitizes IDH mutant cells to alkylating agents. Cell Rep. 2015;13(11):2353–2361. doi: 10.1016/j.celrep.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inoue S, Li WY, Tseng A, Beerman I, Elia AJ, Bendall SC, et al. Mutant IDH1 downregulates ATM and alters DNA repair and sensitivity to DNA damage independent of TET2. Cancer Cell. 2016;30(2):337–348. doi: 10.1016/j.ccell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Molenaar RJ, Radivoyevitch T, Nagata Y, Khurshed M, Przychodzen B, Makishima H, et al. IDH1/2 mutations sensitize acute myeloid leukemia to PARP inhibition and this is reversed by IDH1/2-mutant inhibitors. Clin Cancer Res. 2018;24(7):1705–1715. doi: 10.1158/1078-0432.CCR-17-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Y, Kwintkiewicz J, Liu Y, Tech K, Frady LN, Su YT, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Caner Res. 2017;77(7):1709–1718. doi: 10.1158/0008-5472.CAN-16-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu M, Hazelton WD, Luebeck GE, Grady WM. Epigenetic aging: more than just a clock when it comes to cancer. Cancer Res. 2020;80(3):367–374. doi: 10.1158/0008-5472.CAN-19-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 105.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333(6047):1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lio CJ, Yuita H, Rao A. Dysregulation of the TET family of epigenetic regulators in lymphoid and myeloid malignancies. Blood. 2019;134(18):1487–1497. doi: 10.1182/blood.2019791475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rasmussen KD, Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30(7):733–750. doi: 10.1101/gad.276568.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Losman JA, Kaelin WG. What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev. 2013;27(8):836–852. doi: 10.1101/gad.217406.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gerecke C, Schumacher F, Berndzen A, Homann T, Kleuser B. Vitamin C in combination with inhibition of mutant IDH1 synergistically activates TET enzymes and epigenetically modulates gene silencing in colon cancer cells. Epigenetics. 2020;15(3):307–322. doi: 10.1080/15592294.2019.1666652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Magrì A, Germano G, Lorenzato A, Lamba S, Chilà R, Montone M, et al. High-dose vitamin C enhances cancer immunotherapy. Sci Transl Med. 2020;12(532):eaay8707. doi: 10.1126/scitranslmed.aay8707. [DOI] [PubMed] [Google Scholar]
- 112.Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Y, Pang Y, Zhu B, Uher O, Caisova V, Huynh T, et al. Therapeutic targeting of SDHB-mutated pheochromocytoma/paraganglioma with pharmacologic ascorbic acid. Clin Cancer Res. 2020;26(14):3868–3880. doi: 10.1158/1078-0432.CCR-19-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Galluzzi L, Kroemer G. Potent immunosuppressive effects of the oncometabolite R-2-hydroxyglutarate. Oncoimmunology. 2018;7(12):e1528815. doi: 10.1080/2162402X.2018.1528815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berghoff AS, Kiesel B, Widhalm G, Wilhelm D, Rajky O, Kurscheid S, et al. Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro-Oncology. 2017;19(11):1460–1468. doi: 10.1093/neuonc/nox054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X, Rao A, Sette P, Deibert C, Pomerantz A, Kim WJ, et al. IDH mutant gliomas escape natural killer cell immune surveillance by downregulation of NKG2D ligand expression. Neuro-Oncology. 2016;18(10):1402–1412. doi: 10.1093/neuonc/now061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mullard A. Cancer metabolism pipeline breaks new ground. Nat Rev Drug Discov. 2016;15(11):735–737. doi: 10.1038/nrd.2016.223. [DOI] [PubMed] [Google Scholar]
- 118.Kohanbash G, Carrera DA, Shrivastav S, Ahn BJ, Jahan N, Mazor T, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest. 2017;127(4):1425–1437. doi: 10.1172/JCI90644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018;24(8):1192–1203. doi: 10.1038/s41591-018-0095-6. [DOI] [PubMed] [Google Scholar]
- 120.Chandhok NS, Wei W, Halene S, Prebet T. Ivo-Nivo: A phase II study of the IDH1 inhibitor ivosidenib (AG-120) in combination with the checkpoint blockade inhibitor nivolumab for patients with IDH1 mutated relapsed/refractory AML and high risk MDS. Blood. 2019;134(Supplement_1):1374. doi: 10.1182/blood-2019-130033. [DOI] [Google Scholar]
- 121.Hofree M, Shen JP, Carter H, Gross A, Ideker T. Network-based stratification of tumor mutations. Nat Methods. 2013;10(11):1108–1115. doi: 10.1038/nmeth.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7(9):715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.