Summary
Whereas the role of calcium ions (Ca2+) in plant signaling is well studied, the physiological significance of pH-changes remains largely undefined.
Here we developed CapHensor, an optimized dual-reporter for simultaneous Ca2+ and pH ratio-imaging and studied signaling events in pollen tubes (PTs), guard cells (GCs), and mesophyll cells (MCs). Monitoring spatio-temporal relationships between membrane voltage, Ca2+- and pH-dynamics revealed interconnections previously not described.
In tobacco PTs, we demonstrated Ca2+-dynamics lag behind pH-dynamics during oscillatory growth, and pH correlates more with growth than Ca2+. In GCs, we demonstrated abscisic acid (ABA) to initiate stomatal closure via rapid cytosolic alkalization followed by Ca2+ elevation. Preventing the alkalization blocked GC ABA-responses and even opened stomata in the presence of ABA, disclosing an important pH-dependent GC signaling node. In MCs, a flg22-induced membrane depolarization preceded Ca2+-increases and cytosolic acidification by c. 2 min, suggesting a Ca2+/pH-independent early pathogen signaling step. Imaging Ca2+ and pH resolved similar cytosol and nuclear signals and demonstrated flg22, but not ABA and hydrogen peroxide to initiate rapid membrane voltage-, Ca2+- and pH-responses.
We propose close interrelation in Ca2+- and pH-signaling that is cell type- and stimulus-specific and the pH having crucial roles in regulating PT growth and stomata movement.
Keywords: abscisic acid (ABA), calcium, flg22, guard cells, imaging, ion signaling, pH, pollen tube
Introduction
Plant growth, development and adaptation are influenced by biotic and abiotic stimuli that must be perceived and transduced to elicit physiological responses. The role of calcium ions (Ca2+) as a second messenger has been studied intensively, but the role of protons (H+/pH) as a signaling mechanism has proved more recalcitrant to understand. Ca2+ and H+ can function as second messengers in many stress responses, such as pathogen- and drought-stress (Gao et al., 2004; Kudla et al., 2010; Reddy et al., 2011; Romeis & Herde, 2014; Wilkins et al., 2016). Changes in free cytosolic Ca2+-concentration ([Ca2+]cyt) and free cytosolic H+-concentration ([H+]cyt) and membrane voltage (Vm) are associated with early electric signaling (Felle et al., 2005; Mousavi et al., 2013; Choi et al., 2017; Vincent et al., 2017; Devireddy et al., 2018; Kumari et al., 2019). However, the hierarchy and possible interdependence of these three signaling components remain an intriguing fundamental question.
Guard cells (GCs) and pollen tubes (PTs) are the best studied plant cell types in terms of Ca2+-, pH- and ion-signaling (Murata et al., 2015; Jezek & Blatt, 2017; Michard et al., 2017; Konrad et al., 2018). The mechanisms by which the phytohormone abscisic acid (ABA) and pathogen attack lead to stomatal closure has been studied intensively during the last decades (Kollist et al., 2014; McLachlan et al., 2014; Murata et al., 2015; Chen et al., 2020). Perception of the bacterial flagella epitope flg22 (Melotto et al., 2006) and ABA seem to share common [Ca2+]cyt downstream signaling networks (McLachlan et al., 2014; Güzel Deger et al., 2015; Zheng et al., 2018). A mechanism similarly to GCs is thought to operate also in mesophyll cells (MCs) (Montillet et al., 2013; Güzel Deger et al., 2015) albeit it can be triggered by other stimuli (Elzenga et al., 1995; Elzenga & Volkenburgh, 1997; Stoelzle et al., 2003; Roelfsema et al., 2012). Genetic evidence support the concept that Ca2+-dependent responses branched from the ABA pathway in GCs, yet in mesophyll pathogen-induced signaling, the Ca2+-dependent protein kinases (CDPKs) were likely co-opted to activate Ca2+-channels through reactive oxygen species (ROS) generated by NADPH-oxidases (Mori et al., 2006; Boudsocq & Sheen, 2013; Merilo et al., 2013; Brandt et al., 2015; Yuan et al., 2017; Tian et al., 2019). CDPKs and ROS-signaling were shown to be involved in immunity and ABA signaling in both GCs and MCs (Boudsocq & Sheen, 2013; Suzuki et al., 2013; Kadota et al., 2014; Sierla et al., 2016) but are not associated with early electric response and the initiation of stomatal closure (Güzel Deger et al., 2015). It should be noted that GCs need to integrate various signals from antagonistic stimuli, many of which use Ca2+ as a second messenger. Stomatal opening was reported to be associated with an increase in [Ca2+]cyt (Irving et al., 1992; Cousson & Vavasseur, 1998; Young et al., 2006; Harada & Shimazaki, 2008) along with a cytosolic acidification in GCs, whereas ABA-induced stomatal closure was associated with a similar [Ca2+]cyt rise but cytosolic alkalization (Irving et al., 1992). This raises questions about the specificity of [Ca2+]cyt-changes in stoma behavior and implies [H+]cyt-changes as an important signal transmitter. Changes in extracellular H+-fluxes as well as [H+]cyt-dynamics are coupled to pathogen and ABA-triggered stomatal closure in GCs (Irving et al., 1992; Yan et al., 2015) and are associated with early defense responses in the mesophyll (Jeworutzki et al., 2010). However, the physiological role of pH-changes in all these responses is still unclear and reports on temporal aspects of pH signaling are rare. A cytosolic alkalization in GCs begins c. 2 min after ABA exposure (Irving et al., 1992; Blatt & Armstrong, 1993) and precedes ROS production during stomatal closure (Suhita et al., 2004; Ma et al., 2013). Early responses in MCs upon perception of flg22 include an apoplastic alkalization (Kimura et al., 2017) as well as ROS-production and Ca2+-influx via NADPH oxidases and cyclic nucleotide-gated channels (CNGCs) or glutamate receptor-like channels (GLRs), respectively (McLachlan et al., 2014; Moeder et al., 2019; Tian et al., 2019).
An interweaving, but still not totally understood [Ca2+]cyt and [H+]cyt signaling network exists in PTs, too (Konrad et al., 2011; Michard et al., 2017). PTs exhibit steep [Ca2+]cyt- and [H+]cyt-gradients, with a focused peak of concentration at the tip (Pierson et al., 1994; Feijó et al., 1999). Whereas some mechanistic links of [Ca2+]cyt-regulation in PT growth are established, the role of pH-signaling is however unclear. Yet, the often occurring oscillatory PT growth behavior has been explored to characterize links between growth, [Ca2+]cyt and extracellular H+ fluxes (Damineli et al., 2017). More recently, the demonstration that disruption of the pH gradient abrogates PT growth is suggestive of its critical role (Hoffmann et al., 2020).
Live-cell imaging became an essential tool to visualize [Ca2+]cyt and [H+]cyt dynamics, monitoring signaling mechanisms and reporting on real-time ion transport (Uslu & Grossmann, 2016; Behera et al., 2018; Grossmann et al., 2018; Hilleary et al., 2018; Walia et al., 2018). The genetically encoded Ca2+-sensors called GECOs (Zhao et al., 2011), yield a fluorescence signal with a superior dynamic range compared to the ratiometric Ca2+-sensor Yellow Cameleon 3.6 (YC3.6) (Keinath et al., 2015). However, the drawback of the single-wavelength GECO-probes is that they are not ratiometric. Thus, spatial variations in protein abundance may reflect [Ca2+]cyt dynamics inappropriately. This difficulty can be overcome by co-expression of a reference fluorescent protein for normalization (Waadt et al., 2017). With exceptions (Ast et al., 2017; Demes et al., 2020; Waadt et al., 2020), many of these methods do not warrant an equilibrated amount of the two proteins to allow stable ratiometry free from unwanted FRET (Förster Resonance Energy Transfer). Close proximity of a reference and sensor fluorescent protein within a translational fusion protein carries the risk of FRET-effects to occur that may influence reporter readout, especially when the reference fluorescence protein is sensitive to cellular changes, too. This risk is particularly acute in the case of multiparametric monitoring. While recent advantages in Ca2+-imaging highlight an interconnection of Ca2+- and electric-signaling (Nguyen et al., 2018) as well as Ca2+- and pH-signaling (Behera et al., 2018; Waadt et al., 2020), the role for cytosolic pH-changes in plant physiology lags largely behind.
Here we set up a novel imaging technique and established an optimized biosensor we named ‘CapHensor’ enabling simultaneous ratiometric imaging of Ca2+ and pH, using two well-known genetically encoded probes arranged in a multicistronic vector harboring a P2A self-cleavage site. This arrangement allows spatial separation and equilibrated expression of the sensors from one messenger RNA (mRNA). We quantified coherency between cytosolic Ca2+-, H+- and electric-signals and describe new interactions in ion signaling networks involved in the control of PT growth, stomata movement and leaf defense mechanisms.
Materials and Methods
Plant material, growth conditions and media
Growth of Nicotiana tabacum plants and pollen collection were performed as described (Gutermuth et al., 2013). Nicotiana benthamiana was grown on soil under a 14 h : 10h, light : dark regime, at 26°C : 22°C in a glasshouse with c. 60% humidity.
The PT growth medium for experiments of Figs 1–3 was composed of: 1 mM 2-(N-morpholino)ethanesulfonic acid (MES), 0.2 mM calcium chloride (CaCl2), 9.6 or 19.6 mM hydrochloric acid (HCl), and 1.6 mM boric acid (H3BO3). The pH of all solutions was adjusted to 5.8 with tris-(hydroxymethyl)-aminomethan (Tris) unless otherwise stated and osmolality adjusted to 420 mosmol kg−1 with d(+)-sucrose. Nicotiana tabacum GCs and N. benthamiana leaves were perfused with a standard solution for leaves (1 mM CaCl2, 1 mM potassium chloride (KCl) and 10 mM MES) adjusted to pH 5.8 with bistris propane (BTP). Either ABA (in 100% ethanol; Sigma-Aldrich, St Louis, MO, USA), hydrogen peroxide (H2O2) (Sigma-Aldrich), acetic acid (HAc; Applichem, Darmstadt, Germany), caffeine (Sigma-Aldrich), butyric acid (BTA) (Sigma-Aldrich) or flg22 (in water; Genscript, Piscataway, NJ, USA) was added to the solutions in the appropriate amounts and in case of pH changes, these were corrected with BTP.
Fig. 1.
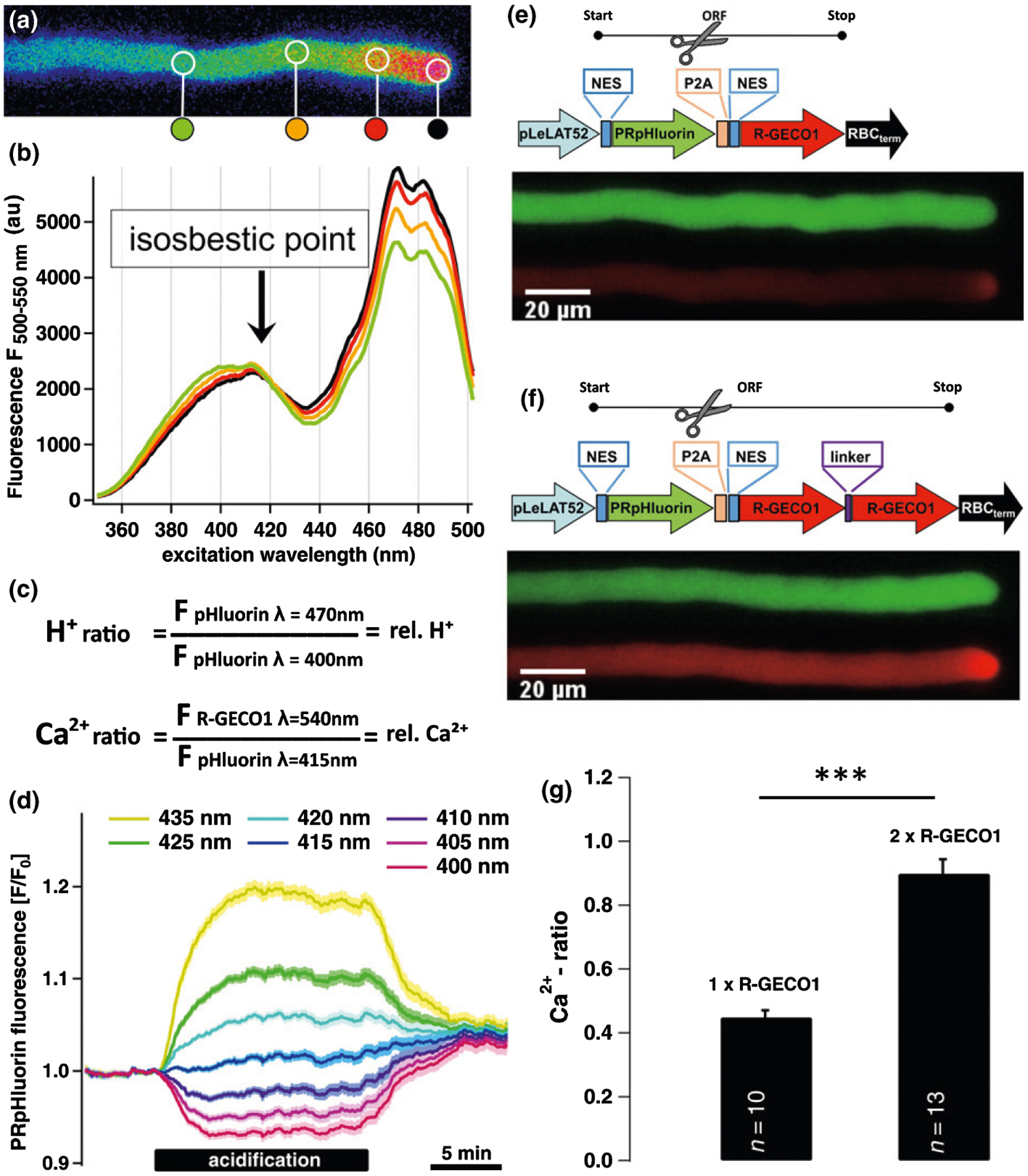
Design, verification and functioning of CapHensor. The structure of the multicistronic CapHensor vectors and expression in PTs to verify its function. (a) False colored [H+]cyt-ratio image of PRpHluorin transiently expressed in Nicotiana tabacum PTs. False color ranges from red, corresponding to slightly acidic lower pH (higher H+ concentration) in the tip, to gradually dissipating green-blue values in the shank indicating moderate alkalization. (b) The marked circular regions-of-interest in (a) reflect the areas of extracting the PRpHluorin excitation spectra which are displayed with the same color. The intersection point of the excitation spectra (415 nm) represents the isosbestic point (marked by arrow), the point where there is no variation of emission at 525 ± 12.5 nm in response to pH. (c) Formulas to generate [Ca2+]cyt and [H+]cyt ratios to represent relative Ca2+ and H+ concentrations, respectively. (d) Mean PRpHluorin fluorescence in mesophyll cells (n = 67) at the designated excitation wavelength after cellular acidification with 5 mM acetate (HAc). Despite progressive acidification, the fluorescence emission from the cytosol remains unchanged when excited with 415 nm. (e, f) Vector design and fluorescence intensity of PRpHluorin (green emission) and R-GECO1 (red emission) with the single or double R-GECO1 arrangement in stable transformed N. tabacum PTs. Spatial separation of the two fluorophores is achieved by the self-cleavage P2A sequence in the ORF indicated by the scissors. (g) Mean Ca2+-ratio values from shank regions of PT expressing vector constructs shown in (e) and (f). The doubling of the ratio reflects the increase in the R-GECO1 emission when ratioed with the same emission generated by the PRpHluorin. Error bars = SE. The t-test was used in (g). ***, P < 0.001.
Fig. 3.
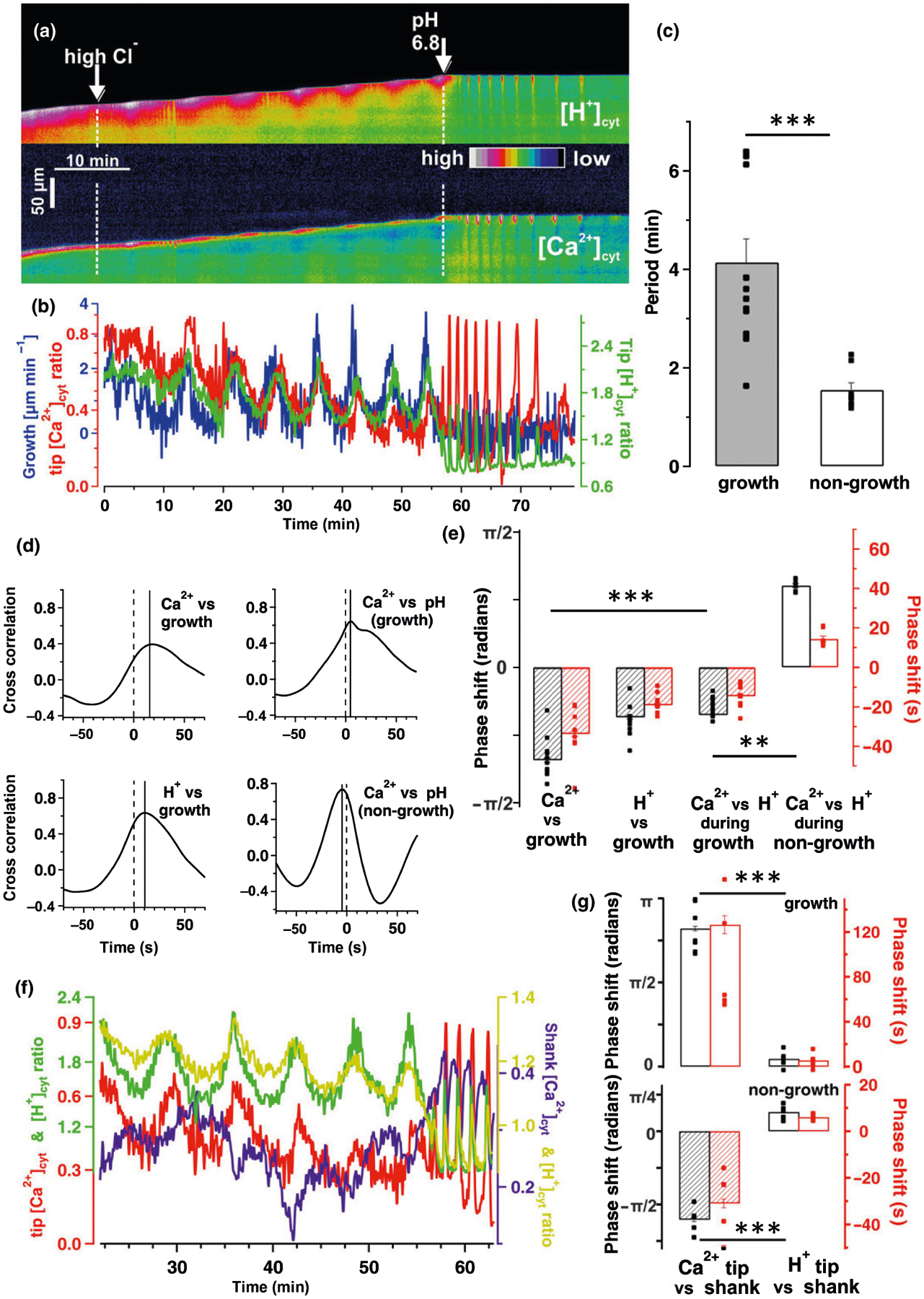
Phase-relation analyses of [Ca2+]cyt, [H+]cyt and growth in oscillating pollen tubes (PTs). Live-cell CapHensor imaging of oscillating Nicotiana tabacum PTs to quantify spatio-temporal interrelation of ion signaling and growth. (a) False colored [H+]cyt-ratio (top) and [Ca2+]cyt-ratio (bottom) kymographs from a representative PT upon external challenging with 20 mM chloride (Cl−) and later challenged with both 20 mM Cl− and pH 6.8 medium. (b) Quantification of PT tip [Ca2+]cyt (red line), [H+]cyt (green line) and growth rate (blue line) of the experiment in (a). (c) Mean period of [Ca2+]cyt- and [H+]cyt-ratio oscillations in growing (grey, n = 13) and non-growing (white, n = 8) PTs in high Cl−-solution. (d, e) Quantification of phase relationships via (d) cross-correlation and (e) cross-wavelet analyses from growing (n = 10) and non-growing (n = 8) PTs in high Cl−-solution as in (b). Time between the solid and dotted vertical lines corresponds to the lagging (shift to right side) or leading (shift to left side) signals in (d). Phase relationship of the signals is presented in radians (black) and also depicted as delay in seconds (red) in (e). (f) [Ca2+]cyt- and [H+]cyt dynamics at the tip (left axis) and shank (right axis) from the cell in (a). (g) Quantification of phase relationships via cross-wavelet analyses between tip (1–5 μm from tip) and shank (35–40 μm from tip) from growing (upper bar diagram) and non-growing (lower bar diagram) PTs as exemplified in (f). It is important to note that delay times depicted in the correlation analyses in (d) and the wavelet analyses (e) are coherent despite being inverse. Error bars = SE. The t-test was used in (c) and (g) while one-way ANOVA was used in (e). **, P < 0.01; ***, P < 0.001.
Molecular biology, cloning and transformation
Cloning was performed with the USER cloning technique (Nour-Eldin et al., 2006) and primers used are listed in Supporting Information Table S1. For stable transformation of N. tabacum plants or transient transformation of N. benthamiana leaves the Agrobacterium tumefaciens strain GV3101 was used. The LeLAT52 promotor (Twell et al., 1991) and RBC-terminator or 35S promotor/terminator were used for PT or ubiquitous expression, respectively. Transient expression of PRpHluorin in PTs was performed as described (Gutermuth et al., 2013).
The construct for cytosolic CapHensor targeting (pCambia3300 NES PRpHluorin NES P2A NES 2xR-GECO1) contained nuclear export sequences (NESs) at the N- and C-terminus of PRpHluorin as well as the N-terminus of the R-GECO1 tandem. The NES targeting sequences at the N- or C-terminus of PRpHluorin were from heat stable inhibitor (PKI) (Wen et al., 1995; Matsushita et al., 2003) and Xenopus MAPKK (Fukuda et al., 1996), respectively. The amino acid sequence of the linker between the two R-GECO1s was GLNLSGG, the ‘self-cleaving’ peptide P2A separating the coding sequence of PRpHluorin and R-GECO1 is as described (Kim et al., 2011). Cytosolic expression of PRpHluorin in tobacco PT was possible with the N-terminal NES only. Nucleus localization was achieved by translational fusion of two nuclear localization sequences (NLSs) (pCambia3300 NLS NLS PRpHluorin P2A R-GECO1 NLS-linker R-GECO1) to the N-terminus of PRpHluorin and a NLS-linker between the two R-GECO1s originating from the simian virus 40 (SV40) T-antigen (Wen et al., 1995; Krylova et al., 2013).
Expression in Escherichia coli and protein purification
R-GECO1 and 2×R-GECO1 in pET24 vector with an additional N-terminal StrepII-tag and YC3.6 in pET30a vector with an additional C-terminal StrepII-tag were introduced in E. coli BL21 (Stratagene, La Jolla, CA, USA). Proteins were expressed and purified as described (Guerra et al., 2020). Great efforts were undertaken to remove Ca2+-contaminations, as it is the main source of variations in quantifying half maximal effective concentration (EC50) values from in vitro titration curves (Patton et al., 2004). Briefly, 500 μl eluate was dialyzed using micro dialysis capsule QuixSep (Roth, Karlsruhe, Germany; 6000–8000-Da cutoff) against two buffer exchanges of 30 mM MES/Tris, 30 mM sodium chloride (NaCl), 5 mM EGTA (ethylene glycolbis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid) and against four buffer exchanges of 30 mM MES/Tris, 30 mM NaCl in ultra-quality water. To remove contaminating Ca2+, plastic bottles, pipette tips, etc. were soaked in 5 mM EGTA and rinsed with ultra-quality water. All chemicals used were of the highest quality (> 99%; Roth, Karlsruhe, Germany).
In vitro biochemical characterization of Ca2+-sensors
For the analyses of Ca2+-sensor conformational changes high-Ca2+-buffer and zero-Ca2+-buffer (30 mM MES/Tris pH 7.35, 30 mM NaCl, 20 mM EGTA, ± 20 mM CaCl2) were mixed. Free Ca2+-concentrations were calculated with the WEBMXC extended website: http://tinyurl.com/y48t33xq based on Patton et al. (2004). To be able to compare the half maximal inhibitory concentration (IC50) values of the three Ca2+-sensor constructs directly with each other, measurements were carried out with the same Ca2+ buffer solutions. Measurements were performed in a Tecan Spark with the following excitation (bandwidth 10 nm) and emission spectra (2 nm steps): R-GECO1 and 2×R-GECO1 (550 nm/584–660 nm), YC3.6 (435 nm/470–600 nm). For analyses of R-GECO1 and 2×R-GECO1 emissionMax from 586 to 600 nm was used, for YC3.6 we calculated the fluorescence ratio using maximal emission values of yellow fluorescent protein (YFP) (522–530 nm) over cyan fluorescent protein (CFP) (476–488 nm). Data was analyzed using Graphpad PRISM 5 (GraphPad Software Inc., La Jolla, CA, USA) and are described by a four parameter logistic equation. The best fit-value obtained for bottom and top ratio are used as Fmin and Fmax values to calculate the normalized fluorescence Fnorm = (F − Fmin)/(Fmax − Fmin).
Live-cell- and confocal-imaging
Live-cell-imaging experiments were carried out with pollen or leaves of N. tabacum plants stably expressing the CapHensor versions. The microscope setup and imaging software is described in Gutermuth et al. (2013). For simultaneous Ca2+ and pH imaging in PTs, R-GECO1 and PRpHluorin were excited sequentially with the following order at 540, 400, 415 and 470 nm every 3 s. Intervals for CapHensor excitation in GCs and MCs were 5 and 3 s, respectively. A dual-band mirror (ET, Chroma 59001bs) was combined with a high-speed filter wheel equipped with bandpass filters for R-GECO (ET 605/26 nm) and PRpHluorin (ET 525/25 nm). To determine the isosbestic point, excitation wavelength was reflected by a LP 500 nm dichroic mirror combined with an ET 525/25 nm filter.
The procedure for pollen grain embedding, hardware and software for imaging is described in Gutermuth et al. (2013). For GC imaging epidermal strips were peeled from leaves of 5 to 6 weeks old tobacco plants and glued (with Medical Adhesive B, Ulrich Swiss, St Gallen, Switzerland) adaxial side down to cover slips mounted in custom made chambers. Epidermal peels recovered in standard solution for leaves for 3 to 6 h under a white light lamp (c. 20–25 μmol m−2 s−1) at room temperature before the experiments. All experiments were carried out under permanent perfusion (700 μl min−1). Stomatal movement was determined by means of quantifying the area within the stomatal pore in Fiji/ImageJ v.1.50 with a custom-made macro. For mesophyll imaging of N. benthamiana the abaxial epidermis of leaf discs was removed, while the adaxial side was glued to the cover slip with Medical Adhesive B followed by incubation in standard solution for leaves in darkness at room temperature for at least 12 h.
Confocal imaging was performed with a Leica TCS SP5 II equipped with a HCX IRAPO 25×/0.95 objective. PRpHluorin and R-GECO1 were excitation at 476 nm and 561 nm and fluorescence was captured at 530/30 nm and 617/26 nm, respectively. Chlorophyll fluorescence was captured at 680/37 nm. Image processing was performed with Fiji/ImageJ v.1.50. Data were processed and plotted with IGOR PRO 5.02 (Wavemetrics Inc., Portland, OR, USA) and R.
Quantification of Ca2+, pH, growth, membrane potential followed by coherence- and phase-relationship analyses
Guard cells (GCs) and mesophyll cells (MCs)
Fluorescence intensity over time was extracted using Fiji/ImageJ followed by H+-ratio (PRpHluorin470nm/PRpHluorin400nm) and Ca2+ ratio (R-GECO1/PRpHluorin415nm) calculations using the ‘image calculator’ tool. Subsequent phase analyses, correlation and wavelet transforms were performed with R v.3.6 (see later).
Pollen tubes (PTs)
Quantification of growth, Ca2+- and H+-ratio were done as follows. Kymographs were generated using IMAGEJ (multiple kymograph plugin) and quantification of H+-ratio (PRpHluorin470nm/PRpHluorin400nm), Ca2+-ratio (R-GECO1/PRpHluorin415nm), Ca2+ raw (R-GECO1) at the tip (1–5 μm behind the tip) and in the shank (35–40 μm behind the tip) was performed with R-scripts based on the CHUKNORRIS algorithm (Damineli et al., 2017). Subsequent coherence analyses were performed.
Cross-wavelet and cross-correlation methodology for periodic phenomena in time series experiments was used to investigate correlation and coherence. Signals for Ca2+, H+, Vm and growth velocity were used to quantify their phase relationship with R-scripts called experiments_auto_analysis, phase_analysis, and CrossWaveGraph_pollen. A script called leaves_analysis was used to detect the time and value of the onset and peak response of Ca2+, H+ and stomata aperture area or Vm in GCs or MCs, respectively. All R-scripts, the CHUKNORRIS algorithm, and a set of example measurements including an instruction are compiled in Notes S1.
The wavelet transform was selected as the preferred method of analysis using the package WaveletComp (Roesch & Schmidbauer, 2018) to estimate each signal as well as the coherent spectrum of each pair of signals (Ca2+-H+, Ca2+-growth, H+-growth, etc.). The dominant frequency of each signal over time was assessed which allows to define a phase relationship among them. The phase relationships reported are found on the ridges of the wavelet transformed and within the regions of statistical significance of the transformation as indicated by areas bordered by a white line in the wavelet spectra. Additionally, a running window correlation analysis of the signals (window size 50) was done allowing one to verify the relationship of the signals during the recordings.
Electrophysiology
Membrane voltage recordings with N. benthamiana leaves were carried out as described (Gutermuth et al., 2013), but current-clamp protocols were applied via WINEDR software (University of Strathclyde, Glasgow, UK). Electrode resistance was 60–120 MΩ.
Statistical analyses
Statistical analyses were carried out using GraphPad software (GraphPad Software Inc.) and OriginPro software (OriginLab, Northampton, MA, USA). Traces are demonstrated by means ± standard errors (SEs). An unpaired t-test or one-way ANOVA was used to compare pairs of experimental conditions for statistically significant differences between groups (*, P < 0.05, **, P < 0.01; ***, P < 0.001).
Results
CapHensor design and functional validation
To monitor Ca2+ and pH in parallel we designed muticistronic vectors harboring two spectral distinct genetically encoded biosensors: PRpHluorin, based on the pH-dependent green fluorescent ratiometric pHluorin (Miesenböck et al., 1998) which was optimized for plant usage (Shen et al., 2013); and the red fluorescent Ca2+-sensor R-GECO1 (Zhao et al., 2011). These two well-characterized sensors were arranged within one original reading frame (ORF) harboring a self-cleavage P2A sequence (Kim et al., 2011) resulting in posttranscriptional cleavage and a stoichiometric amount of both sensors. This dual-sensing approach was tested and verified in growing N. tabacum PTs, which was ideal because they sustain tip-focused [Ca2+]cyt and [H+]cyt gradients. The pH-dependent change in the excitation spectrum of PRpHluorin allows ratiometric imaging of the [H+]cyt gradient in PTs (Fig. 1a) by sequential dual-excitation at 400 nm and 470 nm (Fig. 1b,c). In order to perform simultaneous ratiometric Ca2+-imaging with the single-wavelength R-GECO1 probe (excitation at 540 nm), we ratioed its fluorescence using the excitation isosbestic point of PRpHluorin, where fluorescence is essentially pH- and Ca2+-independent. The isosbestic point of PRpHluorin in planta was screened in growing PTs by recording its excitation spectrum, and determined to be around 410–420 nm (Fig. 1b). To resolve the isosbestic point exactly and validate its pH-insensitivity in planta, we monitored PRpHluorin fluorescence at seven distinct excitation wavelengths around to the isosbestic point while imposing [H+]cyt changes by acetate (HAc) perfusion (Fig. 1d). In N. tabacum PTs and GCs (Fig. S1) as well as in N. benthamiana MCs (Fig. 1d), excitation at 415 nm (isosbestic point) produced no acetate-induced changes in PRpHuorin fluorescence. However, PRpHluorin fluorescence reacted to acidification when excited at wavelengths deviating from 415 nm (Fig. 1b). We concluded that PRpHluorin fluorescence when excited at 415 nm is pH-independent, allowing the use of this wavelength to ratio R-GECO1 fluorescence and allow Ca2+-ratio quantitative measures (Fig. 1c). We further assumed that ratiometric measures using different proteins should be optimized using this strategy given that expression of PRpHluorin and R-GECO1 from the same promoter should result in quasi-equimolar production of both proteins after P2A self-cleavage (Fig. 1e,f). This design should also prevent possible FRET to occur between the two fluorophores, optimizing fluorescence analysis and interpretation on the basis of ratiometry. Yet, a limitation arose from the relative low quantum yield of R-GECO1 when compared to green fluorescent protein (GFP) or YFP (Cranfill et al., 2016). We solved that limitation by introducing two R-GECO1 ORFs in tandem. PT comparison from stable tobacco lines revealed the tandem sensor version to be much more effective (Fig. 1e,f): roughly twice the fluorescence from the R-GECO1, and the same amount of fluorescence emanating from the isosbestic point of PRpHluorin resulted in doubling of the R-GECO1 ratio value (Fig. 1e–g). In vitro biochemical characterization revealed comparable Ca2+ affinities for R-GECO1 and 2×R-GECO1 with a EC50 for Ca2+ of ≈ 350 nM (Fig. S2) consistent with reported values (Zhao et al., 2011; Akerboom et al., 2013; Inoue et al., 2015). The EC50 values were c. 2×as high with YC3.6 (Fig. S2), demonstrating the ability of high sensitivity and high resolution Ca2+-ratio measurements with the 2×R-GECO1 approach. We named the best performing tandem R-GECO1 design CapHensor. The characterization of the CapHensor in PTs could demonstrate its functionality and that of the dual ratiometric imaging method.
The tip focused pH-gradient represents a major determinant for pollen tube growth control
Next, we challenged growing PTs with different treatments to quantify interconnections in [Ca2+]cyt, [H+]cyt and growth. In vitro growing PTs exhibit high [Ca2+]cyt and [H+]cyt at the tip (see Fig. 1a,f) (Holdaway-Clarke et al., 1997; Feijó et al., 1999; Gutermuth et al., 2013; Hoffmann et al., 2020) and extracellular pH changes and hypoosmotic shock were used here to perturb these gradients (Fig. 2a). The dynamics of [Ca2+]cyt, [H+]cyt and growth rate was extracted from PT time-lapse series (Video S1), plotted in false-colored kymographs (Fig. 2a,b), and quantitatively analyzed using the CHUKNORRIS suite of statistical tools for image and time-series analysis (Damineli et al., 2017). Acidification of the optimal growth medium from pH 5.8 to pH 5.0 increased growth rate, tip [H+]cyt and tip [Ca2+]cyt (Fig. 2a,b; Video S1). Inversely, alkalinizing the extracellular medium to pH 6.8 stopped PT growth, dissipated the [H+]cyt constitutive gradient and lead to pronounced tip-focused high-amplitude [Ca2+]cyt and [H+]cyt oscillations (Fig. 2a,b; Video S1). Oscillations ceased and growth resumed after moving back to optimal pH 5.8 growth medium (Fig. 2a,b). Hypoosmotic shock resulted in transiently accentuated [Ca2+]cyt and [H+]cyt gradient at the tip (Fig. 2a,b; Video S1). Accentuating or dissipating the tip-focused [H+]cyt gradient by experimental manipulations was associated to low or high growth rates, respectively (Fig. 2a,b). To test whether elevated growth rates were due to extracellular, or otherwise caused by cytosolic acidification in the apex, we made use of a medium supplemented with 2 mM acetate (HAc) adjusted to pH 5.8. The protonated form of HAc, a weak acid, is able to permeate membranes, releasing H+ inside and thereby acidifying the cytosol (Brummer et al., 1984). Therefore, using HAc with the control medium adjusted to pH 5.8 only the cytosol acidifies whereas using low pH medium (pH 5.0), the cell wall and cytosol become acidified, due to the higher H+ influx in the PT tip (Fig. 2a–c). The exclusive acidification of the cytosol by HAc increased growth rates (Fig. 2c) which is suggestive of an important role of tip [H+]cyt to function as a key signal in PT growth control. Cytosolic acidification induced by extracellular pH 5, and HAc was both accompanied by a slight increase in [Ca2+]cyt (Fig. 2b,c). Employing 3 mM caffeine to diminish the [Ca2+]cyt gradient (Holdaway-Clarke et al., 1997; Diao et al., 2018), we recognized this treatment to dissipate both, the apical [Ca2+]cyt as well as the [H+]cyt gradient and to abrogate growth (n = 8, Fig. 2d,e; Video S2). Correlation coefficients were calculated to evaluate the coherence of growth with tip [Ca2+]cyt or [H+]cyt in selected time windows marked by symbols in Fig. 2(b–e). It was evident, that during re-establishment of growth upon caffeine washout (Fig. 2e, triangle t = 20–30 min), but also during recovery after the forced growth stop (Fig. 2b, diamond t = 55–75 min) or during growth spurt caused by pH changes (Fig. 2b, square t = 15–25 min and Fig. 2c, closed circle t = 15–25 min, open circle t = 50–60 min), growth rate correlated significantly better with tip [H+]cyt rather than tip [Ca2+]cyt (Fig. 3f). Coherence between growth and tip [H+]cyt is consistent with other studies (Winship et al., 2017; Hoffmann et al., 2020) showing a prominent role of the [H+]cyt gradient for PT growth.
Fig. 2.
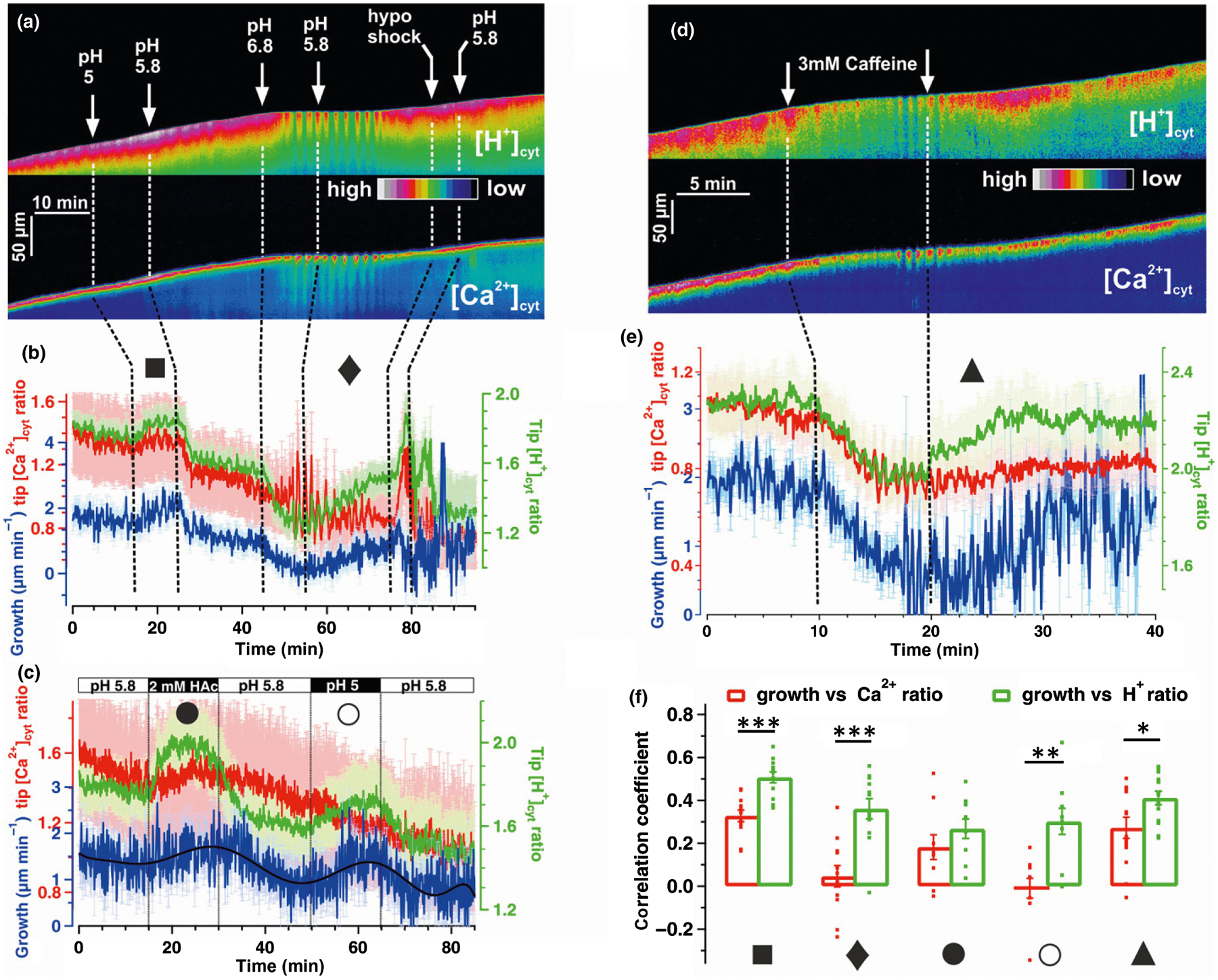
Interrelation of [Ca2+]cyt, [H+]cyt and growth in pollen tubes (PTs). Live-cell CapHensor imaging of Nicotiana tabacum PTs to quantify spatio-temporal interrelation of tip [Ca2+]cyt, [H+]cyt and growth under different conditions. (a) False colored [H+]cyt-ratio (top) and [Ca2+]cyt-ratio (bottom) kymographs from a representative CapHensor PT imaging time-course experiment with sequential extracellular medium perfusions of different pH or low osmolarity (hypo shock). (b) Quantification of mean PT tip [Ca2+]cyt (red line), [H+]cyt (green line) and growth rate (blue line) from experiments (n = 12) with the same sequence of treatment as in (a). (c) Quantification of mean PT tip [Ca2+]cyt (red line), [H+]cyt (green line) and growth rate (blue line) from experiments (n = 8) upon 2 mM acetate (HAc) treatment or extracellular acidification (pH 5) in order to increase [H+]cyt. (d) False colored [H+]cyt-ratio (top) and [Ca2+]cyt-ratio (bottom) kymographs from a representative CapHensor PT imaging time-course experiment with sequential extracellular medium perfusion of 3 mM caffeine. (e) Quantification of mean PT tip [Ca2+]cyt (red line), [H+]cyt (green line) and growth rate (blue line) from experiments (n = 15) with the same sequence of treatment as in (d). (f) Comparison of correlation coefficients of growth vs tip [Ca2+]cyt-ratio (red) and growth vs tip [H+]cyt-ratio (green) from experiments in (b, ■ 15–25 min; ◆ 55–75 min), (c, ● 15–25 min; ○ 50–60 min) and (e, ▲ 20–30 min). Error bars = SE. Dots in (f) indicate individual measurements and t-test was used. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To analyze coherence between [Ca2+]cyt, [H+]cyt and growth with a good temporal resolution, we explored ion dynamics behavior in oscillating PTs. We used wavelet transformation (Torrence & Compo, 1998) as it is advantageous over cross-correlation analysis because dynamics in period length and power can be resolved in time, whereas cross-correlation analysis lack temporal information (Damineli et al., 2017). Growth rate oscillations were induced by increasing Cl−-concentration in the medium according to Gutermuth et al. (2013) (Video S3). Kymographs and corresponding quantifications of the PTs growth rate, tip [Ca2+]cyt and [H+]cyt over time demonstrated the three parameters to be clearly linked (Fig. 3a,b). Phase relationships between oscillations of growth rate, tip [Ca2+]cyt and [H+]cyt were quantified via wavelet and cross-wavelet analyses for representative sequences (Fig. 3a,b) and displayed in terms of dynamics of oscillatory power and period in the [Ca2+]cyt and [H+]cyt wavelet spectra (Fig. S3). Interestingly, the period of [Ca2+]cyt and [H+]cyt oscillations was reduced to roughly one-third upon alkalization induced growth arrests (pH 6.8) (Fig. 3c). This experimental sequence (increase in chloride followed by alkalization) was further explored to characterize phase delay changes between growth, [Ca2+]cyt and [H+]cyt oscillations. To that purpose, phase shifts in the [Ca2+]cyt and [H+]cyt oscillations were also converted into radians (−π; π) because it normalizes changes in period length allowing for better comparison of phase relations between measurements, and even between species (Holdaway-Clarke & Hepler, 2003). Quantification of the phase shifts between oscillations in growing PTs with cross-correlation analyses (n = 10) (Fig. 3d) and cross-wavelet analyses (n = 10) (Fig. 3e) revealed similar phase-shifts with mean [Ca2+]cyt periods lagging behind [H+]cyt periods by 14.55 ± 0.60 s, while growth lead [H+]cyt periods by 17.87 ± 0.46 s (Fig. 3e). Note that lagging and leading terms are used here not to imply causation, but simply to denominate the smallest interval of time, or the best cross-correlation between the two variables within the whole period length. It should be noted, however, that there is a growth spurt first, followed by the change in the [H+]cyt gradient and then the change in the [Ca2+]cyt gradient (Fig. 3d,e). Remarkably, phase relationships between [Ca2+]cyt and [H+]cyt inverted when PT growth was arrested (Fig. 3d,e). Non-growing oscillating PTs exhibited [Ca2+]cyt-leading [H+]cyt dynamics by 14.36 ± 1.42 s when growth arrest was induced by alkalinizing the medium pH to 6.8 (Fig. 3e), but also displayed this phase relation in other media as long as growth was compromised (Fig. S4). Another interesting observation was that oscillations in [Ca2+]cyt at the tip (red trace; 1–5 μm behind tip) and the shank (purple trace; 35–40 μm behind tip) were almost in antiphase (with absolute phase shift in radians values higher than half π) to one-another albeit the amplitude of shank Ca2+-oscillations is smaller (Fig. 3f,g). The delay of [Ca2+]cyt between the tip and shank was 126.48 ± 7.96 s in growing cells and 30.96 ± 1.86 s in non-growing cells, which was consistent with the period difference between growth and non-growth conditions (Figs 3g, S3). By contrast, [H+]cyt alterations at the tip (green trace) were in phase (with absolute phase shift in radians values lower than half π) with the ones in the shank (light green; Fig. 3f, g) and unaltered by the PT growth state.
Distinct [Ca2+]cyt and [H+]cyt regimes triggered by different stimuli have particular control over stomatal movement
Another classic model system for ion signaling are stomata. Biotic and abiotic stimuli may induce second messenger signals but the relationship between [Ca2+]cyt and [H+]cyt have not yet been decoded. Initially, we set up an approach for synchronized imaging and stomatal movement in N. tabacum epidermal peels to investigate possible interrelation in GC motion, [Ca2+]cyt and [H+]cyt dynamics. Unexpectedly, stable expression of the CapHensor with a NES at the N-terminus of PRpHluorin and 2×R-GECO1 (Fig. 1f) resulted in additional fluorescence of PRpHluorin in the nucleus, while R-GECO1 located to the cytosol as expected (Fig. S5), so we designed different constructs to correct for that in stable transgenic tobacco. Of special note is, that none of the transgenic tobacco lines expressing various CapHensor versions had any negative effect on plant growth or reproduction (Fig. S6). Ideal for expression in leaves was a construct design harboring a second NES inserted at the C-terminus of PRpHluorin, resulting in exclusive cytosolic localization (Fig. S5) and fulfilling our criteria to optimize ratiometric imaging of [Ca2+]cyt and [H+]cyt (Fig. 4a).
Fig. 4.
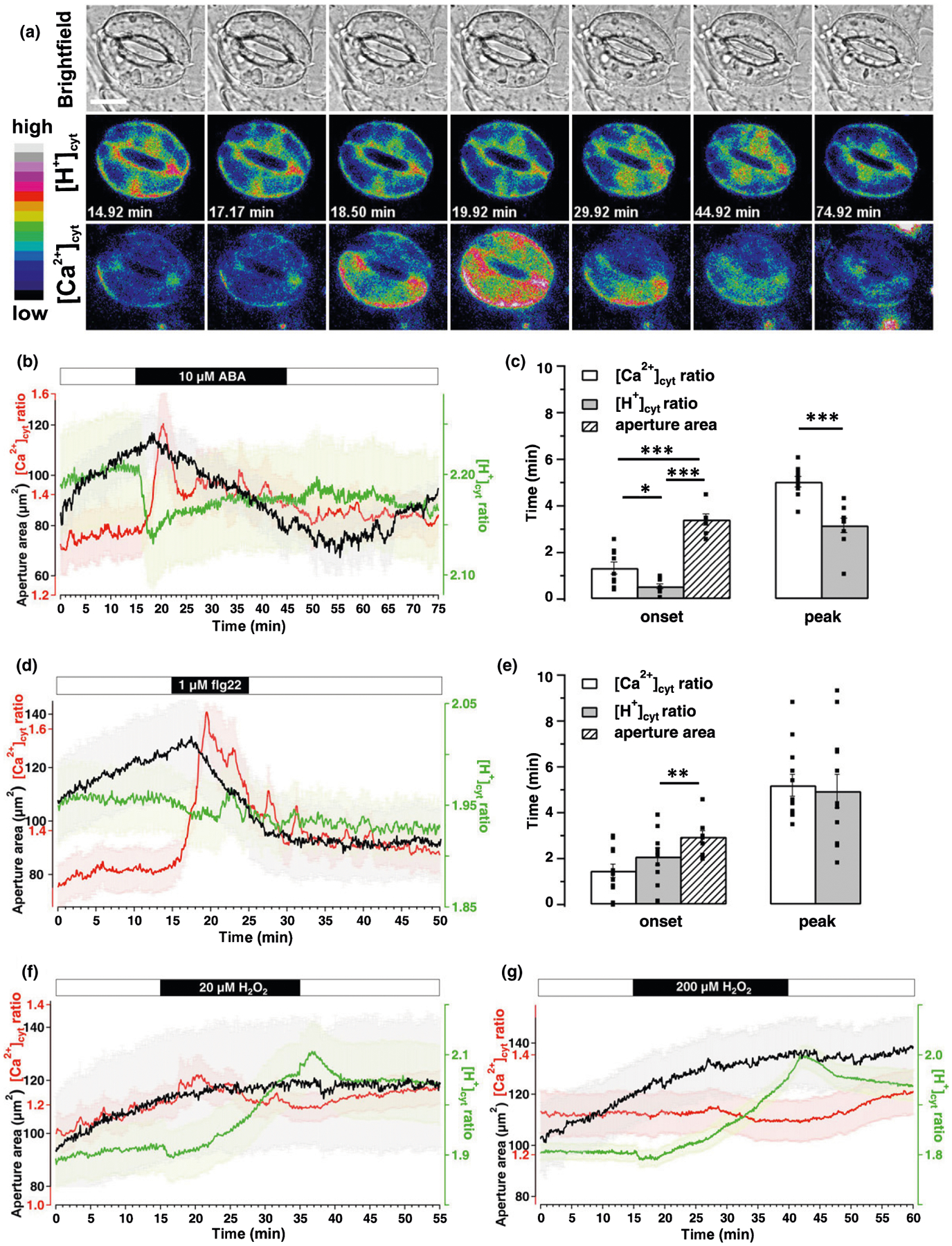
Different stimuli trigger distinct [Ca2+]cyt and [H+]cyt signatures in guard cells (GCs) during stomata movement. Time-lapse CapHensor imaging together with stomata aperture monitoring in GCs of Nicotiana tabacum epidermal strips. (a) Brightfield (top) as well as false colored [H+]cyt (middle) and [Ca2+]cyt (bottom) ratio images of a representative time-lapse CapHensor imaging series upon the application of 10 μM ABA. Bar, 20 μm. (b, d, f, g) Mean [Ca2+]cyt ratio (red), [H+]cyt ratio (green) and stomatal aperture area (black) over time upon application of (b) 10 μM ABA (n = 10), (d) 1 μM flg22 (n = 12), (f) 20 μM H2O2 (n = 10) and (g) 200 μM H2O2 (n = 22). The bars above the mean traces indicate the time of treatments. (c, e) Bar diagram displays the average time of the onset and to the peak-response after 10 μM ABA and 1 μM flg22 treatment from data shown in (b) and (d), respectively. Dots in (c) and (e) indicate individual measurements. Error bars = SE. The t-test was used for peak time analyses while one-way ANOVA was used for onset time analyses in (c) and (e). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In average, the application of 10 μM ABA ((±)-cis,trans ABA) to the pre-opened stomata induced a rapid [H+]cyt decrease (alkalization) within 32.78 ± 6.13 s and an increase in [Ca2+]cyt 80 ± 15.70 s after treatment, followed by stomatal closure (Fig. 4a,b; Video S4). Peak alkalization occurred at 190 ± 18.63 s and [Ca2+]cyt peaked at 302.22 ± 13.47 s after ABA application, demonstrating the pH-response to occur before the [Ca2+]cyt-change (Fig. 4c). We then investigated pathogen-induced stomatal closure induced by flg22, which despite inducing a similar stomatal closure as ABA, induced no significant change in [H+]cyt. Surprisingly though, [Ca2+]cyt response was more pronounced and lasted longer (n = 12, Fig. 4d,e; Video S5). During stomatal closure induced by ABA and flg22 repetitive and sometimes oscillating spikes of [Ca2+]cyt-increases occurred as previously described (Staxen et al., 1999; Thor & Peiter, 2014) side-by-side with [H+]cyt-oscillations (Fig. S7). Besides Ca2+, ROS are widely considered important second messengers in ABA- and pathogen-signaling of GCs (Song et al., 2014; Sierla et al., 2016). For example, H2O2 at concentrations of 0.1–1 mM affect [Ca2+]cyt-dynamics and in turn stomatal aperture (Pei et al., 2000; Zhang et al., 2001; Ma et al., 2013; Martí et al., 2013; Wu et al., 2020). Surprisingly, application of 1 mM H2O2 resulted in loss of GC integrity in parallel with [Ca2+]cyt- and [H+]cyt increases (Fig. S8; Video S6), but 20 and 200 μM H2O2 resulted in strong cytosol acidification within 10–20 min, while [Ca2+]cyt and stomatal aperture were unaltered (Fig. 4f,g; Video S6).
All these results are suggestive of different signaling pathways to affect differently [Ca2+]cyt and [H+]cyt, and thus we examined in more detail their possible correlation. Stomatal closure can be prevented by pH-clamping with the weak acid butyrate (Islam et al., 2010; Ma et al., 2013). Indeed, we could inhibit ABA-induced stomatal closure by perfusion with 10 μM ABA together with 3 mM BTA and as expected, BTA induced a moderate acidification (Fig. 5a) (c. 0.4 pH units according to the Henderson–Hasselbalch equation (Colcombet et al., 2005)). In all cases, washing BTA while upholding 10 μM ABA caused an immediate drop in [H+]cyt (alkalization), an increase in [Ca2+]cyt and stomatal closure (Fig. 5a) (n = 22). Intriguingly, ABA pre-incubated closed stomata opened immediately when an ABA-medium containing 3 mM BTA was applied (Fig. 5b). Similar to the BTA-inhibition of ABA-responses, 3 mM BTA inhibited flg22 induced stomata closure, however, pronounced [Ca2+]cyt-increases occurred upon flg22-treatment in the presence of BTA (n = 24, Fig. 5c). This suggests that [Ca2+]cyt cannot be solely responsible for stomatal closure per se, and (as can be seen in Fig. 4b,c) supports our findings that ABA and flg22 trigger partially different ion signaling pathways but share pH-dependent mechanisms to trigger stomatal closure. To understand the interconnection between [Ca2+]cyt and [H+]cyt signals we increased extracellular Ca2+ known to close stomata (Gilroy et al., 1991). This led to GC deflating together with transient oscillatory cytosolic alkalizations and [Ca2+]cyt increases (n = 22, Figs 5d, S7) which is suggestive for control of stomata closure by alkalization and the existence of functional links between [Ca2+]cyt and [H+]cyt.
Fig. 5.
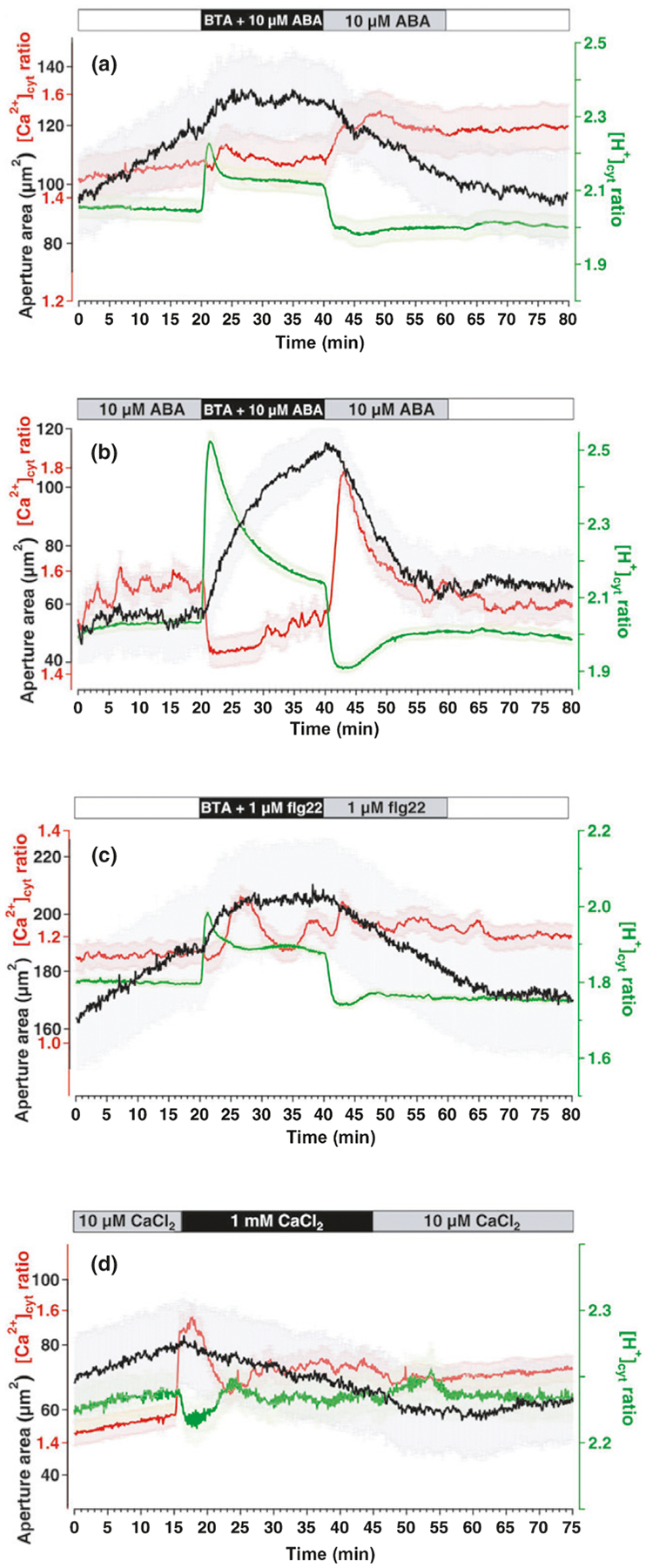
The cytosolic pH in guard cells (GCs) – important factor for stoma movement. Time-lapse CapHensor imaging in GCs of Nicotiana tabacum epidermal strips together with stomata aperture monitoring. Mean [Ca2+]cyt ratio (red), [H+]cyt ratio (green) and stomata aperture area (black) over time. (a) Simultaneous application of 3 mM BTA and 10 μM ABA followed by exclusive ABA treatment (n = 22). (b) 10 μM ABA-pretreated GCs were challenged with 3 mM BTA plus 10 μM ABA followed by treatment with 10 μM ABA only (n = 22). (c) Simultaneous application of 3 mM BTA together with 1 μM flg22 followed by exclusive flg22 treatment (n = 24). (d) Increase of the medium Ca2+-concentration via perfusion from 10 μM CaCl2 to 1 mM CaCl2 (n = 22) and back. Error bars = SE.
Cytosolic and nuclear Ca2+- and pH-changes are linked to electric signals in the mesophyll
Leaf mesophyll react to various stress stimuli with changes in [Ca2+]cyt, [H+]cyt and Vm, and functional links between them have been suggested (Gilroy et al., 2016). Hence, we carried out Vm recordings via sharp microelectrodes together with [Ca2+]cyt/[H+]cyt imaging in transiently transformed N. benthamiana leaves. Great care was taken to ensure that electrode insertion was close (next or above) to the imaging section. In contrast to the flg22 response in GCs (Fig. 4d), CapHensor expression in the cytosol of MCs (Fig. 6a) revealed pronounced [H+]cyt- and [Ca2+]cyt increase upon 100 nM flg22 (Fig. 6b; Video S7). Interestingly, in all cases (n = 9), flg22-induced a depolarization 125.72 ± 15.68 s after the given stimulus in the mesophyll (Fig. 6k, down 54 mV from a resting Vm of −114 ± 6.84 mV) that significantly preceded the [Ca2+]cyt and [H+]cyt changes by 121.61 ± 22.04 and 145.12 ± 20.13 s, respectively (Fig. 6b,i). However, the application of ABA and 200 μM H2O2 to MCs had no obvious chemo-electric response (Fig. 6c,d) and was comparable to control measurements (Fig. S9). It is well-known that pathogen attack (flg22) and drought stress (ABA) are transduced by H2O2, leading to genetic reprogramming (Boudsocq et al., 2010; Tsuda & Somssich, 2015; Couto & Zipfel, 2016; Li et al., 2016; Birkenbihl et al., 2017). Increases in nuclear Ca2+ concentrations ([Ca2+]nuc) play key roles in stress signaling pathways and induce changes in gene expression via CDPK-mediated and CaM-binding transcription factors (Reddy et al., 2011; Dubiella et al., 2013; Gao et al., 2013). Based on the correlations between [Ca2+]cyt and [H+]cyt in PTs, GCs and MCs, we investigated their existence in the nucleus by generating a nuclear CapHensor version (Figs 6e, S9) to report [Ca2+]nuc and nuclear H+ concentrations ([H+]nuc). Similar to the chemo-electric response in the cytosol, [Ca2+]nuc and [H+]nuc transiently increased upon 100 nM flg22 (Fig. 6f,j; Video S7). The mean [Ca2+]nuc and [H+]nuc changes lagged behind the onset of the voltage changes of about 141.01 ± 22.09 and 117.51 ± 17.15 s, respectively, while the onset of [Ca2+]nuc and [H+]nuc approximately coincide at 226.29 ± 16.94 and 202.79 ± 12.59 s after treatment (Fig. 6j). This indicates that changes in concentration of both Ca2+ and H+ in the cytosol induce similar changes in nuclear Ca2+ and H+. By contrast, application of ABA and 200 μM H2O2 to MCs revealed no obvious responses in Vm, [Ca2+]nuc and [H+]nuc (Fig. 6g,h).
Fig. 6.
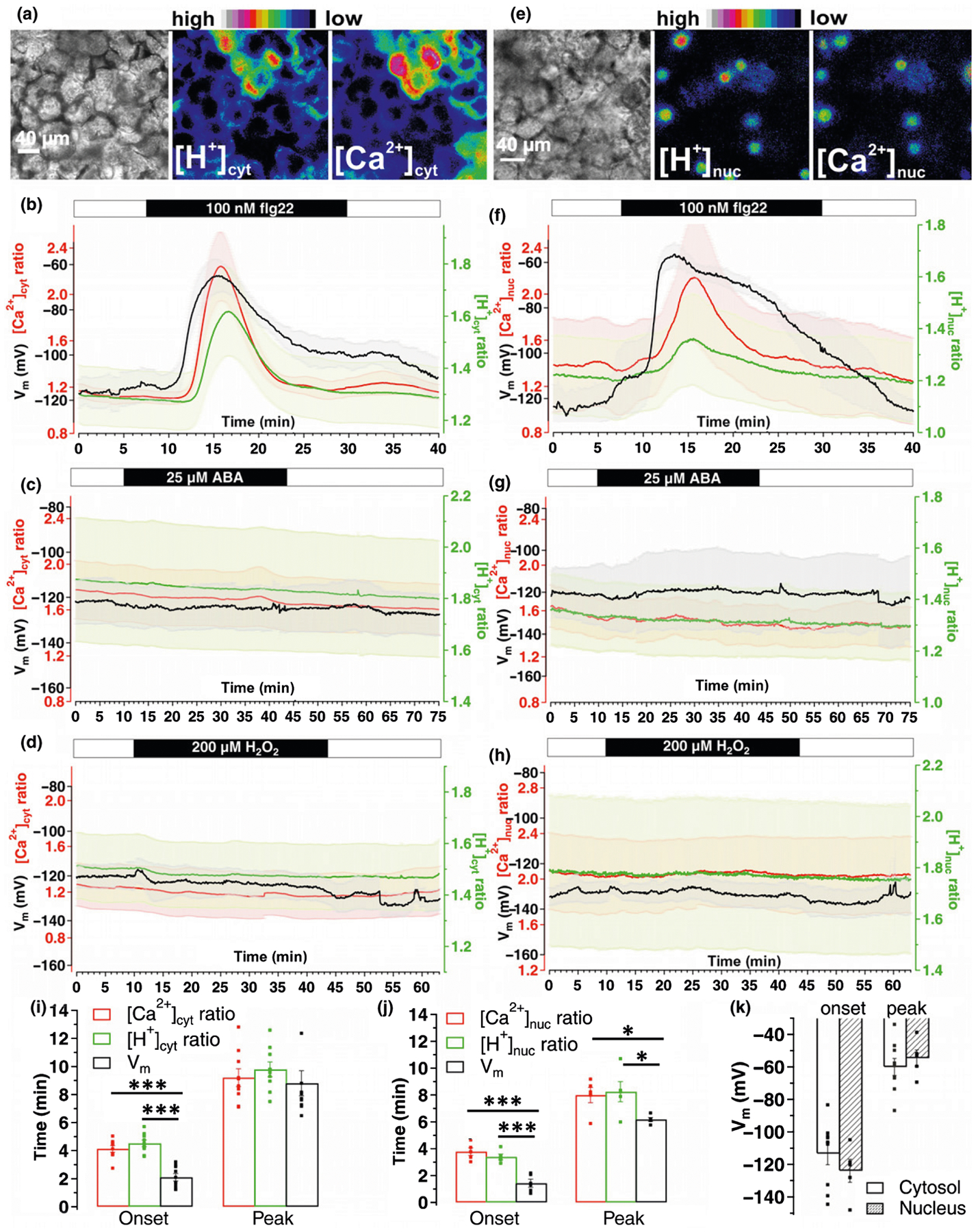
Distinct cytosolic and nuclear Ca2+- and H+-signatures in mesophyll cells upon different stimuli. Simultaneous Ca2+, H+ and Vm recordings in mesophyll cells of Nicotiana benthamiana leaves transiently expressing a cytosolic or nuclear version of the CapHensor. (a, e) Representative brightfield (left) as well as false colored [H+]- (middle) and [Ca2+] (right) ratio images of a leave mesophyll section expressing the (a) cytosolic- or (e) nuclear CapHensor version. (b–d) Mean [Ca2+]cyt ratio (red), [H+]cyt ratio (green) and membrane potential (Vm, black) dynamics over time upon application of (b) 100 nM flg22 (n = 9), (c) 25 μM ABA (n = 6) and (d) 200 μM H2O2 (n = 5). (f–h) Mean [Ca2+]nuc ratio (red), [H+]nuc ratio (green) and Vm (black) dynamics over time upon application of (f) 100 nM flg22 (n = 6), (g) 25 lM ABA (n = 4) and (h) 200 μM H2O2 (n = 5). (i, j) Bar diagram of mean onset and peak times of Ca2+ ratio (red), H+ ratio (green) and Vm after 100 nM flg22 with the cytosolic (i) or nuclear (j) expressed CapHensor. (k) Bar diagram of mean membrane potential (Vm) in mesophyll cells at the onset (left) and peak (right) response when the cytosol (blank, n = 9) or nucleus (shadow, n = 6) expressed version for CapHensor was used. Error bars = SE. Dots in (i–k) indicate individual measurements. One-way ANOVA was used in (i) and (j). *, P < 0.05; ***, P < 0.001.
Discussion
CapHensor advantages for high-resolution spatio-temporal imaging of Ca2+ and pH
Here we developed CapHensor a sophisticated dual reporter for simultaneous ratiometric imaging of Ca2+ and pH. Our design is based on multicistronic vectors for co-expression of an optimized pH-dependent GFP for plants and a red-fluorescent Ca2+-sensor with a high dynamic range (Fig. 1). Our design circumvented, with advantage, other commonly observed problems with methods tailored for the same objectives (e.g. Waadt et al., 2020), namely in terms of: (1) overcoming the low quantum yield of red fluorophores (Cranfill et al., 2016); (2) increasing Ca2+-sensitivity; (3) using the isosbestic point of PRpHluorin for Ca2+-ratioing; (4) using of a self-cleavage P2A sequence to separate the two fluorophores; (5) allowing quantitative analysis free of variations in expression level and unwanted FRET events; (6) optimized subcellular targeting (Figs 1, 6, S5) avoiding problems with mixed signals from more than one compartment, a problem especially acute when considering different cytosolic and nuclear Ca2+ dynamics (Hanson & Köhler, 2001; Bootman et al., 2009; Mazars et al., 2011; Huang et al., 2017; Charpentier, 2018; Kelner et al., 2018).
The pH-sensitivity of R-GECO1 in planta has been reported (Keinath et al., 2015) and affects R-GECO1 fluorescence positively with alkalization (Zhao et al., 2011). In our experiments no such pH-effects on R-GECO1 fluorescence were observed upon triggering acidification in live PTs (Fig. 2c) or GCs (Fig. 5a–c). Moreover, no such visible pH effect on R-GECO1 fluorescence was observed during pronounced pH oscillations in PTs, GCs and MCs with different [Ca2+]cyt- and [H+]cyt phase relations (Figs 3e,g, 5a–d, 6b,f).
It should be noted that many conventional confocal laser microscopes lack a 415 nm laser line, the wavelength to excite PRpHluorin at its isosbestic point (Fig. 1). This does not allow Ca2+-ratio measurements, that we achieved using a polychromator, or which is possible with a light-emitting diode-based system for illumination, however, CapHensor enables simultaneous monitoring of Ca2+ intensiometrically (ΔF/F0) and pH ratiometrically (405 nm laser diode and 476 nm argon laser line) with all advantages of its design.
Importance of the tip [H+]cyt gradient in the signaling network of pollen tubes
Recently H+-ATPases were genetically established as the basis for the formation of the [H+]cyt-gradient in PTs, with consequences on growth rates and plant fertility (Hoffmann et al., 2020). In accordance, we generated here more evidence supporting an important role of the tip [H+]cyt-gradient in PT growth regulation. CapHensor enabled visualizing decreased or increased growth rates when low or high tip [H+]cyt was triggered on demand, respectively (Fig. 2a–f). This important relationship fits to the correlation coefficients between growth and [H+]cyt, which were significantly higher than those between growth and [Ca2+]cyt (Fig. 2f). Detailed quantifications of phase relationships in oscillatory growing tobacco PTs showed the tip [H+]cyt transients to precede the ones of tip [Ca2+]cyt by c. 15 s, albeit [H+]cyt transients still lagged growth by c. 18 s (Fig. 3b,e). In lily PTs a similar sequence of events was observed by Winship et al. (2017). The large phase shift between [Ca2+]cyt and growth by c. 34 s, together with a lack of coherence between them (Fig. 2f) makes it difficult to argue for a causal relationship of Ca2+ inducing growth, though the involvement of Ca2+ channels in PT growth is well established. Our present data asks for a re-analysis of what the Ca2+ dependency of the mechanism behind PT growth might be.
Recently, it has been postulated, based on measurements on roots and leaves of Arabidopsis, that [Ca2+]cyt increases lead to acidification of the cytosol (Behera et al., 2018). PT oscillations however displayed phase relationships of [Ca2+]cyt lagging [H+]cyt, that could be swapped during growth arrests, pointing to a more dynamic interrelation of [Ca2+]cyt and [H+]cyt than previously thought. This has profound consequences for the understanding of a possible interdependence of these signaling ions and regulation of the corresponding transport proteins.
Another novel aspect revealed by CapHensor is the coupling of shank and tip oscillations, observed to be in antiphase for [Ca2+]cyt while in phase for [H+]cyt (Fig. 3f,g). Such delays may find an explanation on the physical properties of these ions and their interactions with organic molecules in vivo. On the one hand, the cytosol is presumed to have a high buffer capacity for Ca2+ and H+, but on the other hand, protons are predicted to have much higher diffusion rates (Vanýsek, 1993). Delays in shank vs tip could be viewed in the context of distinct subcellular locations and Ca2+-affinities of CDPKs (Boudsocq et al., 2012; Konrad et al., 2018). Thus, it is conceivable that Ca2+-regulated processes determining cell polarity like cytoskeleton dynamics (Cardenas et al., 2008; Wang et al., 2008; Hepler, 2016), organelle movement (Lovy-Wheeler et al., 2007), and ion homeostasis (Tavares et al., 2011; Zhao et al., 2013; Gutermuth et al., 2018) could be synchronized according to different temporal windows generated by the dynamics of these two ions.
Ca2+ and pH control stomata movement in concert – cytosolic pH affects ABA and pathogen signaling
Stomatal closure usually happens within 15 min after ABA application (McAinsh et al., 1992; Staxen et al., 1999; Tanaka et al., 2005; Hubbard et al., 2012). We posit that precise quantification of [Ca2+]cyt and [H+]cyt together with aperture monitoring, as we now describe, constitutes a new and relevant routine assay to ensure proper understanding of stomata signaling. In consonance, we demonstrate that [Ca2+]cyt and [H+]cyt are associated with different behaviors in GCs during ABA, flg22 and H2O2 responses. Stomatal closure by ABA depended on a rapid cytosolic alkalization, followed by [Ca2+]cyt elevation (Figs 4, S7), preventing it blocked both stomatal closure and [Ca2+]cyt elevations (Fig. 5a,b). The fact that closed stomata could be opened without delay by reversing the cytosolic alkalization even in the presence of ABA (Fig. 5a,b) is suggestive that the core ABA-signaling pathway is under pH-control. Such is the case of a key regulator in ABA-signaling, ABI1, where pH changes like those evoked by ABA were shown to modulate its activity (Leube et al., 1998). ABI1 is one of the central regulators in ABA-signaling as it interacts with ABA receptors, SnRKs, CBL-CIPK and anion channels. This example could make the case for the presence of pH-sensing effector candidate proteins to exist and act in sensing pH signatures. The impact of such mechanisms could be far-reaching given that relevant roles for pH signaling, such as protons acting as bona fide neuro-transmitters (Beg et al., 2008) have been proposed before. The fact that [H+]cyt changes in GCs occur earlier than [Ca2+]cyt changes and that the relationship between them is not fixed (Figs 4, 5, S7, S8) indicates a more complex interrelation between [Ca2+]cyt and [H+]cyt than previously thought (Behera et al., 2018).
In contrast to the pH-dependent ABA-signaling pathway, the flg22-induced stomatal response mostly relies on a pronounced [Ca2+]cyt-increase as the [H+]cyt dynamics was basically unchanged (Fig. 4d). This is in line with forward genetic screens that identified CDPKs and ROS production to be involved in flg22-dependent defense mechanisms (Boudsocq et al., 2010). However, a quadruple loss-of-function mutant (CPK3/5/6/11) containing those CDPKs found in the screen was recently shown to have normal stomata behavior to ABA and flg22 (Güzel Deger et al., 2015). This is suggestive of other Ca2+-sensing or Ca2+-independent mechanisms to be in place, as we could also block flg22-induced stomata closure by cytosolic acidification (Fig. 5c).
ABA- and flg22-induced stomata closure are believed to affect the production of ROS (Chinchilla et al., 2007; Song et al., 2014; Kimura et al., 2017; Qi et al., 2017), which were proposed to trigger yet unknown Ca2+-channels (Mori & Schroeder, 2004; Song et al., 2014). NADPH oxidases (RboHD/F) produce the major bulk of ROS required for flg22-induced stomatal closure, but not essential for ABA-induced stomatal closure (Toum et al., 2016). In contrast to other studies in Arabidopsis (Allen et al., 2000; Kwak et al., 2003; Suhita et al., 2004; Zou et al., 2015; Wu et al., 2020), ≤ 200 μM H2O2 treatment hardly closed tobacco stomata and increased [Ca2+]cyt modestly, if at all (Fig. 4f,g). Slow stomata closure and a [Ca2+]cyt rise was only observed with very high H2O2 concentrations (1 mM) where cell integrity was lost during a time-course of c. 15–30 min (Figs 4f,g, S8). It should be noted that H2O2 is known to be unspecific in terms of mimicking ABA-responses in terms of gene-expression (Trouverie et al., 2008) and GC ion channel adjustment (Köhler et al., 2003). However, we found physiological H2O2 concentrations to provoke a concentration-dependent, reversible and pronounced cytosolic acidification (Fig. 4f,g). Compatible with our results of a H2O2-induced [H+]cyt increase and a block of the ABA- and flg22-induced stomatal closure response by high [H+]cyt in tobacco (Figs 4f,g, 5a–c), physiological concentrations of H2O2 were recently reported to open stomata in Arabidopsis (Li et al., 2020).
Interconnection of Ca2+-, pH- and electric signals in the mesophyll
Compared to GCs, MC physiology and its regulation by chemical and electrical signals is less well characterized. The leaf mesophyll is known to react quickly to wounding, pathogen infection or drought stress, sometimes generating rapid chemoelectric and transcriptional changes (Boudsocq et al., 2010; Jeworutzki et al., 2010; Krol et al., 2010; Mousavi et al., 2013; Keinath et al., 2015; Toyota et al., 2018; Berens et al., 2019). Here, we combined the CapHensor approach with electrophysiology in mesophyll and revealed a steep [Ca2+]cyt increase 4 min after flg22 treatment, that preceded a [H+]cyt increase (acidification) by c. 24 s (Fig. 6b,i). The [H+]cyt increase is consistent with an alkalization of the apoplast (Gust et al., 2007; Jeworutzki et al., 2010) suggested to play a role in long-distance stress signaling (Felle et al., 2005; Zimmermann et al., 2009; Geilfus, 2017). Cytosolic acidification was proposed to be involved in triggering defense gene expression in tobacco (Mathieu et al., 1996; Lapous et al., 1998). Indeed, we observed [Ca2+]nuc and [H+]nuc changes with lower amplitude but similar timing in the nucleus upon flg22 treatment (Fig. 6f,j). Permeation of Ca2+ through nuclear pores is contentious, but the existence of Ca2+-activated Ca2+-channels in the nuclear envelope has been described (Kim et al., 2019). Combining the improved features of CapHensor with electric recordings revealed novel aspects of the spatio-temporal relationships between Vm, [Ca2+]cyt and [H+]cyt. Placing the electrode with the best possible precision was pivotal to monitor Vm and CapHensor fluorescence in the same area. It allowed the observation that large depolarizations start c. 2 min after flg22 application and precede [Ca2+]cyt and [H+]cyt increases by c. 2 min (Fig. 6b,f,i–k). This phenomenon was also recognized by others upon wounding (Zimmermann et al., 2009) where Vm signals clearly travel faster than the accompanying [Ca2+]cyt-waves (Nguyen et al., 2018). Faster propagation of the depolarization signal when compared to the [Ca2+]cyt-signal cannot be accounted for by a high cytosolic buffering capacity because diffusion of Ca2+ in cellular dimensions is accomplished in the millisecond to second range (Donahue & Abercrombie, 1987) and R-GECO1 is very sensitive to [Ca2+]cyt-changes even close to the cells resting Ca2+-level (Fig. S2). This questions the well-described mechanism of Ca2+-dependent anion channel activation via CDPKs (Geiger et al., 2011) to induce fast Vm changes seen here. The mesophyll depolarization was considered to be associated with anion efflux (Jeworutzki et al., 2010), but post-translational modification of the H+-ATPase or its relocation into lipid domains may also contribute (Nühse et al., 2007; Jeworutzki et al., 2010; Keinath et al., 2010). We thus have to conclude that the fast flg22-induced depolarization is neither mediated by Ca2+ nor H+ or their signaling downstream effectors.
Analyses of Vm, Ca2+ and H+ changes upon application of ABA and H2O2 resulted in no significant effects (Fig. 6c,d,g,h), showing that these do not induce fast cytosolic and/or nuclear signals in MCs. Hence, other Ca2+ and pH-independent mechanisms must exist. MCs and GCs thus seem to share signaling mechanisms for flg22, but not for ABA and H2O2. Likewise, no responses to ABA and H2O2 on [Ca2+]cyt and [H+]cyt were recently reported in roots (Waadt et al., 2020). We can therefore assume that GCs, PTs and MCs show different [Ca2+]cyt/[H+]cyt interrelationships, which is consistent with the concept of distinct responses of different cell types (Harada & Shimazaki, 2008; Ranf et al., 2011; Martí et al., 2013). The relationship of Ca2+-induced acidification in leaves and roots described recently (Behera et al., 2018) is not apparent in every cell type as we presented for PTs and GCs here. In fact, Ca2+/pH interrelations monitored via CapHensor displayed remarkable variability (Figs 3, 4, S7, S8). We conclude that when studying plant cellular signaling networks it is not possible to overgeneralize and draw conclusions from the response of one cell type to another.
Supplementary Material
Fig. S1 Isosbestic point of PRpHluorin in pollen tubes and guard cells.
Fig. S2 Ca2+ binding to YC3.6 and R-GECO1 in vitro.
Fig. S3 [Ca2+]cyt and [H+]cyt oscillations period in pollen tube under high Cl− solution.
Fig. S4 Pollen tube growth arrest is associated with tip [Ca2+]cyt oscillations preceding [H+]cyt oscillations, which is independent on the pH-gradient across the plasma membrane.
Fig. S5 Subcellular localization of CapHensor versions in guard cells.
Fig. S6 Transgenic Nicotiana tabacum CapHensor lines grow normal.
Fig. S7 [Ca2+]cyt and [H+]cyt oscillations in individual guard cells upon ABA, flg22 and high Ca2+ treatment.
Fig. S8 1 mM H2O2 results in loss of guard cell integrity.
Fig. S9 Ca2+, H+ and Vm regime in the mesophyll under control conditions.
Notes S1 R-scripts for quantitative analysis of data from pollen tubes, guard cells and mesophyll cells.
Table S1 Primers for cloning and sequencing of CapHensor constructs.
Video S7 Two time-lapse imaging series of [H+]- and [Ca2+]-ratio images as well as brightfield images from Nicotiana benthamiana mesophyll tissue responding to 0.1 μM flg22 when expressing the cytosolic or nuclear version of the CapHensor, corresponding to data presented in Fig. 6(b,f).
Video S6 Two time-lapse CapHensor imaging series of representative Nicotiana tabacum GCs in epidermal strips upon 200 μM H2O2 or 1 mM H2O2 treatment corresponding to data presented in Figs 4(g) and S8 showing false colored [H+]cyt and [Ca2+]cyt ratios, as well as widefield images.
Video S5 Time-lapse CapHensor imaging of a representative Nicotiana tabacum GC in epidermal strip presented in Fig. 4(d) with false colored [H+]cyt and [Ca2+]cyt ratios, and widefield image.
Video S4 Time-lapse CapHensor imaging of a representative Nicotiana tabacum GC in epidermal strip presented in Fig. 4(a) with false colored [H+]cyt and [Ca2+]cyt ratios, and widefield image.
Video S3 Time-lapse CapHensor imaging of a representative Nicotiana tabacum PT from data presented in Fig. 3(a,b) with false colored [H+]cyt and [Ca2+]cyt ratios, and widefield image.
Video S2 Time-lapse CapHensor imaging of a representative Nicotiana tabacum PT from Fig. 2(d,e) with false colored [H+]cyt and [Ca2+]cyt ratios, and widefield image upon 3 mM caffeine perfusion.
Video S1 Time-lapse CapHensor imaging of a representative Nicotiana tabacum PT from Fig. 2(a,b) with false colored [H+]cyt and [Ca2+]cyt ratios, and widefield image (bottom) upon sequential extracellular medium perfusions of different pH or low osmolarity (hypo shock).
Acknowledgements
The authors thank Robert Campbell and Gero Miesenböck for their permission to use R-GECO1 and pHluorin, respectively. The plant ratiometric pHluorin was kindly provided by Liwen Jiang. Early work on legacy versions of the CapHensor by Tilman Güthoff is appreciated. The authors thank Bernadette Eichstädt for cloning of YC3.6 in the E. coli expression vector. For advice and discussion the authors are grateful to Dirk Becker. Financial support by the Deutsche Forschungsgemeinschaft (DFG KO3657/2-3) and Chinese Scholarship Council to Kai R. Konrad and Kunkun Li, respectively, is greatly acknowledged. The laboratory of JAF was supported by the National Institutes of Health (NIH, R01 GM131043) and the National Science Foundation (NSF, MCB1616437, MCB1637673 and MCB1930165). DSCD is funded by the grant 19/23343-7 from the São Paulo Research Foundation (FAPESP). For the use of the High Performance Computing Cluster the authors thank the ‘Rechenzentrum’ University of Wuerzburg. The authors would like to thank Marcel Dunkel (Visitron Systems) for providing the macro to measure excitation spectra with the VisiView software.
Footnotes
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Supporting Information
Additional Supporting Information may be found online in the Supporting Information section at the end of the article.
References
- Akerboom J, Carreras Calderón N, Tian L, Wabnig S, Prigge M, Tolö J, Gordus A, Orger M, Severi K, Macklin J et al. 2013. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Frontiers in Molecular Neuroscience 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF et al. 2000. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342. [DOI] [PubMed] [Google Scholar]
- Ast C, Foret J, Oltrogge LM, De Michele R, Kleist TJ, Ho C-H, Frommer WB. 2017. Ratiometric Matryoshka biosensors from a nested cassette of green- and orange-emitting fluorescent proteins. Nature Communications 8: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Ernstrom GG, Nix P, Davis MW, Jorgensen EM. 2008. Protons act as a transmitter for muscle contraction in C. elegans. Cell 132: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera S, Xu Z, Luoni L, Bonza MC, Doccula FG, De Michelis MI, Morris RJ, Schwarzländer M, Costa A. 2018. Cellular Ca2+ signals generate defined pH signatures in plants. The Plant Cell 30: 2704–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens ML, Wolinska KW, Spaepen S, Ziegler J, Nobori T, Nair A, Krüler V, Winkelmüller TM, Wang Y, Mine A et al. 2019. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proceedings of the National Academy of Sciences, USA 116: 2364–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenbihl RP, Kracher B, Roccaro M, Somssich IE. 2017. Induced genome-wide binding of three Arabidopsis WRKY transcription factors during early MAMP-triggered immunity. The Plant Cell 29: 20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR, Armstrong F. 1993. K+ channels of stomatal guard cells – abscisic acid evoked control of the outward rectifier mediated by cytoplasmic pH. Planta 191: 330–341. [Google Scholar]
- Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. 2009. An update on nuclear calcium signalling. Journal of Cell Science 122: 2337–2350. [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Droillard M-J, Regad L, Lauriére C. 2012. Characterization of Arabidopsis calcium-dependent protein kinases: activated or not by calcium? Biochemical Journal 447: 291–299. [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Sheen J. 2013. CDPKs in immune and stress signaling. Trends in Plant Science 18: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng S-H, Sheen J. 2010. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B, Munemasa S, Wang C, Nguyen D, Yong T, Yang PG, Poretsky E,Belknap TF, Waadt R, Aleman F et al. 2015. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 4: e03599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer B, Felle H, Parish RW. 1984. Evidence that acid solutions induce plant cell elongation by acidifying the cytosol and stimulating the proton pump. FEBS Letters 174: 223–227. [Google Scholar]
- Cardenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK. 2008. Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiology 146: 1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier M 2018. Calcium signals in the plant nucleus: origin and function.Journal of Experimental Botany 69: 4165–4173. [DOI] [PubMed] [Google Scholar]
- Chen K, Li G-J, Bressan RA, Song C-P, Zhu J-K, Zhao Y. 2020. Abscisic acid dynamics, signaling, and functions in plants. Journal of Integrative Plant Biology 62: 25–54. [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, Felix G, Boller T. 2007. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500. [DOI] [PubMed] [Google Scholar]
- Choi W-G, Miller G, Wallace I, Harper J, Mittler R, Gilroy S. 2017.Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. The Plant Journal 90: 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombet J, Lelievre F, Thomine S, Barbier-Brygoo H, Frachisse JM. 2005. Distinct pH regulation of slow and rapid anion channels at the plasma membrane of Arabidopsis thaliana hypocotyl cells. Journal of Experimental Botany 56: 1897–1903. [DOI] [PubMed] [Google Scholar]
- Cousson A, Vavasseur A. 1998. Putative involvement of cytosolic Ca2+ and GTP-binding proteins in cyclic GMP-mediated induction of stomatal opening by auxin in Commelina communis L. Planta 206: 308–314. [Google Scholar]
- Couto D, Zipfel C. 2016. Regulation of pattern recognition receptor signalling in plants. Nature Reviews Immunology 16: 537–552. [DOI] [PubMed] [Google Scholar]
- Cranfill PJ, Sell BR, Baird MA, Allen JR, Lavagnino Z, de Gruiter HM,Kremers G-J, Davidson MW, Ustione A, Piston DW. 2016. Quantitative assessment of fluorescent proteins. Nature Methods 13: 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damineli DSC, Portes MT, Feijó JA. 2017. Oscillatory signatures underlie growth regimes in Arabidopsis pollen tubes: computational methods to estimate tip location, periodicity, and synchronization in growing cells. Journal of Experimental Botany 68: 3267–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demes E, Besse L, Cubero-Font P, Satiat-Jeunemaitre B, Thomine S, De Angeli A. 2020. Dynamic measurement of cytosolic pH and [NO3−] uncovers the role of the vacuolar transporter AtCLCa in cytosolic pH homeostasis. Proceedings of the National Academy of Sciences, USA 117: 15343–15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy AR, Zandalinas SI, Gómez-Cadenas A, Blumwald E, Mittler R. 2018. Coordinating the overall stomatal response of plants: rapid leaf-to-leaf communication during light stress. Science Signaling 11: eaam9514. [DOI] [PubMed] [Google Scholar]
- Diao M, Qu X, Huang S. 2018. Calcium imaging in Arabidopsis pollen cells using G-CaMP5. Journal of Integrative Plant Biology 60: 897–906. [DOI] [PubMed] [Google Scholar]
- Donahue BS, Abercrombie RF. 1987. Free diffusion coefficient of ionic calcium in cytoplasm. Cell Calcium 8: 437–448. [DOI] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte C-P, Schulze WX, Romeis T. 2013. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proceedings of the National Academy of Sciences, USA 110: 8744–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzenga JTM, Prins HBA, Van Volkenburgh E. 1995. Light-induced membrane potential changes of epidermal and mesophyll cells in growing leaves of Pisum sativum. Planta 197: 127–134. [Google Scholar]
- Elzenga JTM, Volkenburgh EV. 1997. Kinetics of Ca2+- and ATP-dependent, voltage-controlled anion conductance in the plasma membrane of mesophyll cells of Pisum sativum. Planta 201: 415–423. [DOI] [PubMed] [Google Scholar]
- Feijó JA, Sainhas J, Hackett GR, Kunkel JG, Hepler PK. 1999. Growing pollen tubes possess a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. Journal of Cell Biology 144: 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH, Herrmann A, Huckelhoven R, Kogel KH. 2005. Root-to-shoot signalling: apoplastic alkalinization, a general stress response and defence factor in barley (Hordeum vulgare). Protoplasma 227: 17–24. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh I, Gotoh Y, Nishida E. 1996. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. Journal of Biological Chemistry 271: 20024–20028. [DOI] [PubMed] [Google Scholar]
- Gao D, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C. 2004. Self-reporting arabidopsis expressing pH and Ca2+ indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiology 134: 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen X, Lin W, Chen S, Lu D, Niu Y, Li L, Cheng C, McCormack M,Sheen J et al. 2013. Bifurcation of Arabidopsis NLR immune signaling via Ca2+-dependent protein kinases. PLoS Pathogens 9: e1003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, AL-Rasheid KAS, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E et al. 2011. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Science Signaling 4: ra32. [DOI] [PubMed] [Google Scholar]
- Geilfus C-M. 2017. The pH of the Apoplast: dynamic factor with functional impact under stress. Molecular Plant 10: 1371–1386. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R. 2016. ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiology 171: 1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Fricker MD, Read ND, Trewavas AJ. 1991. Role of calcium in signal transduction of Commelina guard cells. The Plant Cell 3: 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann G, Krebs M, Maizel A, Stahl Y, Vermeer JEM, Ott T. 2018. Green light for quantitative live-cell imaging in plants. Journal of Cell Science 131: jcs209270. [DOI] [PubMed] [Google Scholar]
- Guerra T, Schilling S, Hake K, Gorzolka K, Sylvester F-P, Conrads B,Westermann B, Romeis T. 2020. Calcium-dependent protein kinase 5 links calcium signaling with N-hydroxy-l-pipecolic acid- and SARD1-dependent immune memory in systemic acquired resistance. New Phytologist 225: 310–325. [DOI] [PubMed] [Google Scholar]
- Gust AA, Biswas R, Lenz HD, Rauhut T, Ranf S, Kemmerling B, Götz F, Glawischnig E, Lee J, Felix G et al. 2007. Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. Journal of Biological Chemistry 282: 32338–32348. [DOI] [PubMed] [Google Scholar]
- Gutermuth T, Herbell S, Lassig R, Brosche M, Romeis T, Feijo JA, Hedrich R, Konrad KR. 2018. Tip-localized Ca2+-permeable channels control pollen tube growth via kinase-dependent R- and S-type anion channel regulation. New Phytologist 218: 1089–1105. [DOI] [PubMed] [Google Scholar]
- Gutermuth T, Lassig R, Portes M-T, Maierhofer T, Romeis T, Borst J-W, Hedrich R, Feijó JA, Konrad KR. 2013. Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20. The Plant Cell Online 25: 4525–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güzel Deger A, Scherzer S, Nuhkat M, Kedzierska J, Kollist H, Brosché M, Unyayar S, Boudsocq M, Hedrich R, Roelfsema MRG. 2015. Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytologist 208: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MR, Köhler RH. 2001. GFP imaging: methodology and application to investigate cellular compartmentation in plants. Journal of Experimental Botany 52: 529–539. [PubMed] [Google Scholar]
- Harada A, Shimazaki K-i. 2008. Measurement of changes in Cytosolic Ca2+ in Arabidopsis guard cells and mesophyll cells in response to blue light. Plant and Cell Physiology 50: 360–373. [DOI] [PubMed] [Google Scholar]
- Hepler PK. 2016. The cytoskeleton and its regulation by calcium and protons.Plant Physiology 170: 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleary R, Choi W-G, Kim S-H, Lim SD, Gilroy S. 2018. Sense and sensibility: the use of fluorescent protein-based genetically encoded biosensors in plants. Current Opinion in Plant Biology 46: 32–38. [DOI] [PubMed] [Google Scholar]
- Hoffmann RD, Portes MT, Olsen LI, Damineli DSC, Hayashi M, Nunes CO, Pedersen JT, Lima PT, Campos C, Feijó JA et al. 2020. Plasma membrane H+-ATPases sustain pollen tube growth and fertilization. Nature Communications 11: 2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Feijó JA, Hackett GR, Kunkel JG, Hepler PK. 1997. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. The Plant Cell 9: 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Hepler PK. 2003. Control of pollen tube growth: role of ion gradients and fluxes. New Phytologist 159: 539–563. [DOI] [PubMed] [Google Scholar]
- Huang F, Luo J, Ning T, Cao W, Jin X, Zhao H, Wang Y, Han S. 2017. Cytosolic and nucleosolic calcium signaling in response to osmotic and salt stresses are independent of each other in roots of Arabidopsis seedlings. Frontiers in Plant Science 8: 1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard KE, Siegel RS, Valerio G, Brandt B, Schroeder JI. 2012. Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Annals of Botany 109: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Takeuchi A, Horigane S-i, Ohkura M, Gengyo-Ando K, Fujii H,Kamijo S, Takemoto-Kimura S, Kano M, Nakai J et al. 2015. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nature Methods 12: 64–70. [DOI] [PubMed] [Google Scholar]
- Irving HR, Gehring CA, Parish RW. 1992. Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proceedings of the National Academy of Sciences, USA 89: 1790–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Hossain MA, Jannat R, Munemasa S, Nakamura Y, Mori IC,Murata Y. 2010. Cytosolic alkalization and cytosolic calcium oscillation in Arabidopsis guard cells response to ABA and MeJA. Plant and Cell Physiology 51: 1721–1730. [DOI] [PubMed] [Google Scholar]
- Jeworutzki E, Roelfsema MRG, Anschütz U, Krol E, Elzenga JTM, Felix G, Boller T, Hedrich R, Becker D. 2010. Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca2+-associated opening of plasma membrane anion channels. The Plant Journal 62: 367–378. [DOI] [PubMed] [Google Scholar]
- Jezek M, Blatt MR. 2017. The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiology 174: 487–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones Jonathan D, Shirasu K, Menke F, Jones A et al. 2014. Direct regulation of the NADPH OXIDASE RBOHD by the PRR-associated kinase BIK1 during plant immunity. Molecular Cell 54: 43–55. [DOI] [PubMed] [Google Scholar]
- Keinath NF, Kierszniowska S, Lorek J, Bourdais G, Kessler SA, Shimosato-Asano H, Grossniklaus U, Schulze WX, Robatzek S, Panstruga R. 2010. PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. The Journal of Biological Chemistry 285: 39140–39149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath NF, Waadt R, Brugman R, Schroeder JI, Grossmann G, Schumacher K, Krebs M. 2015. Live cell imaging with R-GECO1 sheds light on flg22- and chitin-induced transient [Ca2+]cyt patterns in Arabidopsis. Molecular Plant 8: 1188–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner A, Leitao N, Chabaud M, Charpentier M, de Carvalho-Niebel F. 2018. Dual color sensors for simultaneous analysis of calcium signal dynamics in the nuclear and cytoplasmic compartments of plant cells. Frontiers in Plant Science 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee S-R, Li L-H, Park H-J, Park J-H, Lee KY, Kim M-K, Shin BA, Choi S-Y. 2011. High cleavage efficiency of a 2A peptide derived from Porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE 6: e18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Zeng W, Bernard S, Liao J, Venkateshwaran M, Ane J-M, Jiang Y. 2019. Ca2+-regulated Ca2+ channels with an RCK gating ring control plant symbiotic associations. Nature Communications 10: 3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Waszczak C, Hunter K, Wrzaczek M. 2017. Bound by fate: the role of reactive oxygen species in receptor-like kinase signaling. The Plant Cell 29: 638–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler B, Hills A, Blatt MR. 2003. Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiology 131: 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MRG. 2014. Closing gaps: linking elements that control stomatal movement. New Phytologist 203: 44–62. [DOI] [PubMed] [Google Scholar]
- Konrad KR, Maierhofer T, Hedrich R. 2018. Spatio-temporal aspects of Ca2+ signalling: lessons from guard cells and pollen tubes. Journal of Experimental Botany 69: 4195–4214. [DOI] [PubMed] [Google Scholar]
- Konrad KR, Wudick MM, Feijó JA. 2011. Calcium regulation of tip growth: new genes for old mechanisms. Current Opinion in Plant Biology 14: 721–730. [DOI] [PubMed] [Google Scholar]
- Krol E, Mentzel T, Chinchilla D, Boller T, Felix G, Kemmerling B, Postel S, Arents M, Jeworutzki E, Al-Rasheid KA et al. 2010. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. Journal of Biological Chemistry 285: 13471–13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylova I, Kumar RR, Kofoed EM, Schaufele F. 2013. A versatile, bar-coded nuclear marker/reporter for live cell fluorescent and multiplexed high content imaging. PLoS ONE 8: e63286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Batistic O, Hashimoto K. 2010. Calcium signals: the lead currency of plant information processing. The Plant Cell 22: 541–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Chételat A, Nguyen CT, Farmer EE. 2019. Arabidopsis H+-ATPase AHA1 controls slow wave potential duration and wound-response jasmonate pathway activation. Proceedings of the National Academy of Sciences, USA 116: 201907379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE,Bodde S, Jones JD, Schroeder JI. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO Journal 22: 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapous D, Mathieu Y, Guern J, Laurière C. 1998. Increase of defense gene transcripts by cytoplasmic acidification in tobacco cell suspensions. Planta 205: 452–458. [Google Scholar]
- Leube MP, Grill E, Amrhein N. 1998. ABI1 of Arabidopsis is a protein serine/threonine phosphatase highly regulated by the proton and magnesium ion concentration. FEBS Letters 424: 100–104. [DOI] [PubMed] [Google Scholar]
- Li B, Meng X, Shan L, He P. 2016. Transcriptional regulation of pattern-triggered immunity in plants. Cell Host & Microbe 19: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-G, Fan M, Hua W, Tian Y, Chen L-G, Sun Y, Bai M-Y. 2020. Brassinosteroid and hydrogen peroxide interdependently induce stomatal opening by promoting guard cell starch degradation. The Plant Cell 32: 984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Cardenas L, Kunkel JG, Hepler PK. 2007. Differential organelle movement on the actin cytoskeleton in lily pollen tubes. Cell Motility and the Cytoskeleton 64: 217–232. [DOI] [PubMed] [Google Scholar]
- Ma Y, She X, Yang S. 2013. Cytosolic alkalization-mediated H2O2 and NO production are involved in darkness-induced stomatal closure in Vicia faba. Canadian Journal of Plant Science 93: 119–130, 112. [Google Scholar]
- Martí MC, Stancombe MA, Webb AAR. 2013. Cell- and stimulus type-specific intracellular free Ca2+ signals in Arabidopsis. Plant Physiology 163: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu Y, Lapous D, Thomine S, Laurière C, Guern J. 1996. Cytoplasmic acidification as an early phosphorylation-dependent response of tobacco cells to elicitors. Planta 199: 416–424. [Google Scholar]
- Matsushita T, Mochizuki N, Nagatani A. 2003. Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature 424: 571–574. [DOI] [PubMed] [Google Scholar]
- Mazars C, Brière C, Bourque S, Thuleau P. 2011. Nuclear calcium signaling: an emerging topic in plants. Biochimie 93: 2068–2074. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. 1992. Visualizing changes in cytosolic free Ca2+ during the response of stomatal guard cells to abscisic acid. The Plant Cell 4: 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan DH, Kopischke M, Robatzek S. 2014. Gate control: guard cell regulation by microbial stress. New Phytologist 203: 1049–1063. [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. 2006. Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980. [DOI] [PubMed] [Google Scholar]
- Merilo E, Laanemets K, Hu H, Xue S, Jakobson L, Tulva I, Gonzalez-Guzman M, Rodriguez PL, Schroeder JI, Broschè M et al. 2013. PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiology 162: 1652–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E, Simon AA, Tavares B, Wudick MM, Feijó JA. 2017. Signaling with ions: the keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiology 173: 91–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G, De Angelis DA, Rothman JE. 1998. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394: 192–195. [DOI] [PubMed] [Google Scholar]
- Moeder W, Phan V, Yoshioka K. 2019. Ca2+ to the rescue – Ca2+ channels and signaling in plant immunity. Plant Science 279: 19–26. [DOI] [PubMed] [Google Scholar]
- Montillet J-L, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, Boudsocq M, Garcia AV, Douki T, Bigeard J, Laurière C et al. 2013. An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biology 11: e1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang YZ, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR et al. 2006. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biology 4: 1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Schroeder JI. 2004. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiology 135: 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. 2013.Glutamate receptor-like genes mediate leaf-to-leaf wound signalling. Nature 500: 422–426. [DOI] [PubMed] [Google Scholar]
- Murata Y, Mori IC, Munemasa S. 2015. Diverse stomatal signaling and the signal integration mechanism. Annual Review of Plant Biology 66: 369–392. [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Kurenda A, Stolz S, Chételat A, Farmer EE. 2018. Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proceedings of the National Academy of Sciences, USA 115: 10178–10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour-Eldin HH, Hansen BG, Norholm MH, Jensen JK, Halkier BA. 2006. Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Research 34: e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Bottrill AR, Jones AME, Peck SC. 2007. Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. The Plant Journal: For Cell and Molecular Biology 51: 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton C, Thompson S, Epel D. 2004. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium 35: 427–431. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E,Schroeder JI. 2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734. [DOI] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, Shipley AM, Rivers BA, Cresti M,Hepler PK. 1994. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: effect of BAPTA-type buffers and hypertonic media. The Plant Cell Online 6: 1815–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Wang J, Gong Z, Zhou J-M. 2017. Apoplastic ROS signaling in plant immunity. Current Opinion in Plant Biology 38: 92–100. [DOI] [PubMed] [Google Scholar]
- Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D. 2011. Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. The Plant Journal 68: 100–113. [DOI] [PubMed] [Google Scholar]
- Reddy ASN, Ali GS, Celesnik H, Day IS. 2011. Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. The Plant Cell 23: 2010–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R, Geiger D. 2012. Anion channels: master switches of stress responses. Trends in Plant Science 17: 221–229. [DOI] [PubMed] [Google Scholar]
- Roesch A, Schmidbauer H. 2018. WaveletComp: computational wavelet analysis. R package v. [Google Scholar]
- 1.1. [WWW document] URL https://CRAN.R-project.org/package=WaveletComp.
- Romeis T, Herde M. 2014. From local to global: CDPKs in systemic defense signaling upon microbial and herbivore attack. Current Opinion in Plant Biology 20: 1–10. [DOI] [PubMed] [Google Scholar]
- Shen J, Zeng Y, Zhuang X, Sun L, Yao X, Pimpl P, Jiang L. 2013. Organelle pH in the Arabidopsis endomembrane system. Molecular Plant 6: 1419–1437. [DOI] [PubMed] [Google Scholar]
- Sierla M, Waszczak C, Vahisalu T, Kangasjärvi J. 2016. Reactive oxygen species in the regulation of stomatal movements. Plant Physiology 171: 1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Miao Y, Song C-P. 2014. Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytologist 201: 1121–1140. [DOI] [PubMed] [Google Scholar]
- Staxen II, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR. 1999. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proceedings of the National Academy of Sciences, USA 96: 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoelzle S, Kagawa T, Wada M, Hedrich R, Dietrich P. 2003. Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proceedings of the National Academy of Sciences, USA 100: 1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. 2004. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology 134: 1536–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K et al. 2013. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. The Plant Cell 25: 3553–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S. 2005. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiology 138: 2337–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares B, Dias PN, Domingos P, Moura TF, Feijó JA, Bicho A. 2011. Calcium-regulated anion channels in the plasma membrane of Lilium longiflorum pollen protoplasts. New Phytologist 192: 45–60. [DOI] [PubMed] [Google Scholar]
- Thor K, Peiter E. 2014. Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytologist 204: 873–881. [DOI] [PubMed] [Google Scholar]
- Tian W, Hou C, Ren Z, Wang C, Zhao F, Dahlbeck D, Hu S, Zhang L, Niu Q, Li L et al. 2019. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 572: 131–135. [DOI] [PubMed] [Google Scholar]
- Torrence C, Compo GP. 1998. A practical guide to wavelet analysis. Bulletin of the American Meteorological Society 79: 61–78. [Google Scholar]
- Toum L, Torres PS, Gallego SM, Benavídes MP, Vojnov AA, Gudesblat GE. 2016. Coronatine inhibits stomatal closure through guard cell-specific inhibition of NADPH oxidase-dependent ROS production. Frontiers in Plant Science 7: 1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S. 2018. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361: 1112–1115. [DOI] [PubMed] [Google Scholar]
- Trouverie J, Vidal G, Zhang Z, Sirichandra C, Madiona K, Amiar Z, Prioul J-L, Jeannette E, Rona J-P, Brault M. 2008. Anion channel activation and proton pumping inhibition involved in the plasma membrane depolarization induced by ABA in Arabidopsis thaliana suspension cells are both ROS dependent. Plant and Cell Physiology 49: 1495–1507. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Somssich IE. 2015. Transcriptional networks in plant immunity. New Phytologist 206: 932–947. [DOI] [PubMed] [Google Scholar]
- Twell D, Yamaguchi J, Wing RA, Ushiba J, McCormick S. 1991. Promoter analysis of genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Genes & Development 5: 496–507. [DOI] [PubMed] [Google Scholar]
- Uslu VV, Grossmann G. 2016. The biosensor toolbox for plant developmental biology. Current Opinion in Plant Biology 29: 138–147. [DOI] [PubMed] [Google Scholar]
- Vanýsek P 1993. Ionic conductivity and diffusion at infinite dilution. In: Linde DR, ed. CRC hand book of chemistry and physics, 74th edn.Boca Raton, FL, USA: CRC Press, 5–90–5–92. [Google Scholar]
- Vincent TR, Avramova M, Canham J, Higgins P, Bilkey N, Mugford ST,Pitino M, Toyota M, Gilroy S, Miller AJ et al. 2017. Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. The Plant Cell 29: 1460–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Koster P, Andres Z, Waadt C, Bradamante G, Lampou K, Kudla J, Schumacher K. 2020. Dual-reporting transcriptionally linked genetically encoded fluorescent indicators resolve the spatiotemporal coordination of cytosolic abscisic acid and second messenger dynamics in Arabidopsis. The Plant Cell 32: 2582–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Krebs M, Kudla J, Schumacher K. 2017. Multiparameter imaging of calcium and abscisic acid and high-resolution quantitative calcium measurements using R-GECO1-mTurquoise in Arabidopsis. New Phytologist 216: 303–320. [DOI] [PubMed] [Google Scholar]
- Walia A, Waadt R, Jones AM. 2018. Genetically encoded biosensors in plants: pathways to discovery. Annual Review of Plant Biology 69: 497–524. [DOI] [PubMed] [Google Scholar]
- Wang H-J, Wan A-R, Jauh G-Y. 2008. An actin-binding protein, LlLIM1, mediates calcium and hydrogen regulation of actin dynamics in pollen tubes. Plant Physiology 147: 1619–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Meinkotht JL, Tsien RY, Taylor SS. 1995. Identification of a signal for rapid export of proteins from the nucleus. Cell 82: 463–473. [DOI] [PubMed] [Google Scholar]
- Wilkins KA, Matthus E, Swarbreck SM, Davies JM. 2016. Calcium-mediated abiotic stress signaling in roots. Frontiers in Plant Science 7: 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship LJ, Rounds C, Hepler PK. 2017. Perturbation analysis of calcium, alkalinity and secretion during growth of lily pollen tubes. Plants 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Chi Y, Jiang Z, Xu Y, Xie L, Huang F, Wan D, Ni J, Yuan F, Wu X et al. 2020. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 578: 577–581. [DOI] [PubMed] [Google Scholar]
- Yan S, McLamore ES, Dong S, Gao H, Taguchi M, Wang N, Zhang T, Su X,Shen Y. 2015. The role of plasma membrane H+-ATPase in jasmonate-induced ion fluxes and stomatal closure in Arabidopsis thaliana. The Plant Journal 83: 638–649. [DOI] [PubMed] [Google Scholar]
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. 2006. CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proceedings of the National Academy of Sciences, USA 103: 7506–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Jauregui E, Du L, Tanaka K, Poovaiah BW. 2017. Calcium signatures and signaling events orchestrate plant–microbe interactions. Current Opinion in Plant Biology 38: 173–183. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong FC, Gao JF, Song CP. 2001. Hydrogen peroxide-induced changes in intracellular pH of guard cells precede stomatal closure. Cell Research 11: 37–43. [DOI] [PubMed] [Google Scholar]
- Zhao L-N, Shen L-K, Zhang W-Z, Zhang W, Wang Y, Wu W-H. 2013. Ca2+-dependent protein Kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. The Plant Cell Online 25: 649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Araki S, Wu J, Teramoto T, Chang Y-F, Nakano M, Abdelfattah AS,Fujiwara M, Ishihara T, Nagai T et al. 2011. An expanded palette of genetically encoded Ca2+ indicators. Science 333: 1888–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Kang S, Jing Y, Ren Z, Li L, Zhou J-M, Berkowitz G, Shi J, Fu A, Lan W et al. 2018. Danger-associated peptides close stomata by OST1-independent activation of anion channels in guard cells. The Plant Cell 30: 1132–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MR, Maischak H, Mithöfer A, Boland W, Felle HH. 2009. System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiology 149: 1593–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J-J, Li X-D, Ratnasekera D, Wang C, Liu W-X, Song L-F, Zhang W-Z, Wu W-H. 2015. Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. The Plant Cell 27: 1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Isosbestic point of PRpHluorin in pollen tubes and guard cells.
Fig. S2 Ca2+ binding to YC3.6 and R-GECO1 in vitro.
Fig. S3 [Ca2+]cyt and [H+]cyt oscillations period in pollen tube under high Cl− solution.
Fig. S4 Pollen tube growth arrest is associated with tip [Ca2+]cyt oscillations preceding [H+]cyt oscillations, which is independent on the pH-gradient across the plasma membrane.
Fig. S5 Subcellular localization of CapHensor versions in guard cells.
Fig. S6 Transgenic Nicotiana tabacum CapHensor lines grow normal.
Fig. S7 [Ca2+]cyt and [H+]cyt oscillations in individual guard cells upon ABA, flg22 and high Ca2+ treatment.
Fig. S8 1 mM H2O2 results in loss of guard cell integrity.
Fig. S9 Ca2+, H+ and Vm regime in the mesophyll under control conditions.
Notes S1 R-scripts for quantitative analysis of data from pollen tubes, guard cells and mesophyll cells.
Table S1 Primers for cloning and sequencing of CapHensor constructs.
Video S7 Two time-lapse imaging series of [H+]- and [Ca2+]-ratio images as well as brightfield images from Nicotiana benthamiana mesophyll tissue responding to 0.1 μM flg22 when expressing the cytosolic or nuclear version of the CapHensor, corresponding to data presented in Fig. 6(b,f).
Video S6 Two time-lapse CapHensor imaging series of representative Nicotiana tabacum GCs in epidermal strips upon 200 μM H2O2 or 1 mM H2O2 treatment corresponding to data presented in Figs 4(g) and S8 showing false colored [H+]cyt and [Ca2+]cyt ratios, as well as widefield images.
Video S5 Time-lapse CapHensor imaging of a representative Nicotiana tabacum GC in epidermal strip presented in Fig. 4(d) with false colored [H+]cyt and [Ca2+]cyt ratios, and widefield image.
Video S4 Time-lapse CapHensor imaging of a representative Nicotiana tabacum GC in epidermal strip presented in Fig. 4(a) with false colored [H+]cyt and [Ca2+]cyt ratios, and widefield image.
Video S3 Time-lapse CapHensor imaging of a representative Nicotiana tabacum PT from data presented in Fig. 3(a,b) with false colored [H+]cyt and [Ca2+]cyt ratios, and widefield image.
Video S2 Time-lapse CapHensor imaging of a representative Nicotiana tabacum PT from Fig. 2(d,e) with false colored [H+]cyt and [Ca2+]cyt ratios, and widefield image upon 3 mM caffeine perfusion.
Video S1 Time-lapse CapHensor imaging of a representative Nicotiana tabacum PT from Fig. 2(a,b) with false colored [H+]cyt and [Ca2+]cyt ratios, and widefield image (bottom) upon sequential extracellular medium perfusions of different pH or low osmolarity (hypo shock).


