Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurological disorder characterized by progressive paralysis caused by the degeneration of motor neurons throughout the central nervous system. Mutations of the free radical scavenging enzyme Cu/Zn superoxide dismutase 1 (SOD1) are a cause of familial ALS. In the present study, we demonstrated an age-dependent increase in taurine transporter (TauT) immunoreactivity in spinal cord motor neurons of ALS transgenic mice (mutant SOD1 (G93A)) and a similar increase in TauT in spinal motor neurons of patients with ALS. Chromatin immunoprecipitation analysis verified that heat shock factor 1 (HSF1) preferentially occupies the HSF1 binding element in the promoter of TauT under oxidative stress conditions. Knockdown of HSF1 by small interfering RNA reduced the transcriptional activity of TauT. Using [3H] taurine, we confirmed that an elevated expression of TauT directly contributes to increased taurine uptake in ALS motor neurons. In addition, we showed that taurine plays an antioxidant role and may prevent motor neuron loss due to oxidative stress in ALS. Our findings suggest that HSF1-induced TauT expression partially protects motor neurons by compensating for constitutive oxidative stress, which is thought to be a key mechanism contributing to the pathogenesis of ALS. Taken together, our results suggest that TauT is a novel pathological marker for stressed motor neurons in ALS and that modulation of TauT and taurine may slow neuronal degeneration in ALS.
Keywords: Taurine transporter, Heat shock factor 1, Transcription, Taurineuptake, Amyotrophic lateral sclerosis
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder characterized by a loss of upper and lower motor neurons resulting in progressive muscle wasting and paralysis [1]. The incidence of ALS is 1–2/100,000/year and may be rising. The vast majority of ALS cases are sporadic, but about 5–10 % of cases is familial. Missense mutations of Cu/Zn superoxide dismutase 1 (SOD1) cause about 25 % of familial ALS (FALS) cases [2], whose clinical and pathological features are indistinguishable from those in sporadic ALS (SALS). Transgenic mice expressing the G93A or G37R human SOD1 mutations with elevated levels of SOD1 activity develop progressive hind limb weakness, muscle wasting, and neuropathological sequelae similar to those observed in both SALS and FALS patients [3–6]. Despite beneficial therapeutic results having been generated in transgenic ALS mice, unfortunately, human clinical trials have had minimal success [7–10]. A better understanding of the molecular events leading to motor neuron death in ALS may lead to new therapies targeting these pathways. It has well been established that glutamate-mediated toxicity is associated with neuronal death, particularly in ALS. Several converging lines of inquiry have also shown the abnormalities of glutamate regulation in ALS [11–13], suggesting that excessive synaptic glutamate may initiate or propagate motor neuron loss in ALS [14]. Some therapeutic strategies based on those mechanisms have been applied in ALS treatment. Despite this fact, however, no cure or effective therapy is currently available.
Deregulation of amino acid metabolism has been linked to ALS [15–17]. For example, strikingly, arginine concentrations are decreased in the plasma of ALS patients. Interestingly, we have previously found that arginine administration prevents motor neuronal loss and improves neuropathology and survival of ALS transgenic mtSOD1 (G93A) mice. In addition, three decades ago, levels of the sulfur amino acid taurine (2-aminoethanesulphonic acid) were often found to be increased in the motor cortex and the spinal cord of ALS patients, suggesting that the metabolism of sulfur amino acids may be affected as the disease progresses [17–19]. Since taurine is not a protein-composing amino acid, it is not likely that elevated levels are a result of protein breakdown as is the case with other branched amino acids. In general, intracellular levels of taurine in mammalian tissues are as high as millimolar concentrations compared to micromolar concentrations in plasma [20]. Taurine uptake is directly mediated by a sodium-dependent mechanism via the taurine transporter (TauT) [21]. TauT plays a pivotal role in the maintenance of the high concentration of taurine in tissues and TauT expression is increased in hyperosmotic conditions [22, 23]. Taurine is released from glia through an osmoregulatory mechanism and may have neuroprotective properties via its antioxidant effects [24]. Given that taurine levels are preserved in ALS [17, 18], it is important to determine whether taurine uptake is regulated through a TauT-dependent mechanism in spinal motor neurons.
Diverse physiological signals, including oxidative stress, are thought to activate heat shock factor 1 (HSF1) which binds to the sequence-specific heat shock element (HSE) contained in the promoters of many genes, such as heat shock proteins (HSPs) [25]. Two HSFs (HSF1 and HSF2, encoding proteins of 75 and 72 kDa, respectively) have been identified in the mouse. Interestingly, both HSF1 andHSF2 are not heat inducible, but HSF1 is hyperphosphorylated in a Ras-dependent manner by mitogen-activated protein kinase families under physiological stresses. During unstressed conditions, both DNA binding activity and transcriptional activity of HSF1 are tightly regulated. In general, transcriptional activation of the HSF1 pathway does not require new protein synthesis because the preexisting HSF1 is inactive in the unstressed state and becomes active in response to stresses. The stresses induce HSF1 monomers to oligomerize as homotrimers, which then bind to an upstream sequence-specific motif (HSE) in the promoter of stress-inducible HSP genes. However, it remains unknown what other important genomes are modulated by HSF1 in the level of transcription under neurodegenerative conditions, such as ALS.
In this study, we demonstrated the essential requirement of the HSF1 pathway in the regulation of TauT gene expression in ALS. We further examined TauT activity and studied the neuroprotective antioxidant effect of taurine in the mtSOD1 (G93A) transgenic cell line model of ALS.
Results
TauT Immunoreactivity is Increased in the Spinal Cords of ALS (G93A) Mice
Spinal cord sections were prepared from wild-type (WT) and G93A animals at 50, 90, and 120 days of age to characterize TauT immunoreactivity. TauT immunoreactivity was increased in G93A mice at 90 and 120 days of age compared to control mice (Fig. 1a) and intense TauT staining was also seen in the peripheral cytoplasm of spinal motor neurons in human ALS (Fig. 1b). The density of TauT was significantly increased in ALS patients compared with controls (p<0.0001) (Fig. 1b). To determine which cell types express high levels of TauT immunoreactivity, double immunofluorescence staining was performed. As seen by confocal microscopy, TauT immunoreactivity was colocalized in ChAT-positive motor neurons in G93A mice compared to WT littermate controls (Fig. 1c). TauT immunoreactivity was not seen in GFAP-positive astrocytes in G93A mice (Fig. 1d). Deconvoluted and reconstructed to 3-D confocal images of TauT staining demonstrated tubular TauT-positive structures in ventral horn motor neurons in G93A mice compared to WT (Fig. 1e).
Fig. 1.

The immunoreactivity of the TauT is increased in ALS. a The immunoreactivity of TauT was increased in the ventral horn of lumbar spinal cord sections in G93A mice (upper panel). Scale bars=60 μm (left) and 30 μm (right). The densitometry analysis showed a significant increase of TauT level in G93A mice in comparison to WT mice (lower panel). b The immunoreactivity of TauT was increased in the motor neurons of human ALS (upper panel). Scale bars=30 μm (left) and 10 μm (right). The densitometry analysis showed a significant increase of TauT level in ALS (lower panel). c Increased TauT immunoreactivity was found in ChAT-positive motor neurons in the lumbar spinal cord of ALS mice. Scale bars (white)=20 μm. d The increased immunoreactivity of TauT was not found in GFAP-positive astrocytes in ALS mice. Scale bars (white)=20 μm. e Isosurface images illustrate clear patterns for the expression of TauT within motor neurons. Upper panel deconvolved images showed intense staining of TauT in G93A mice compared to WT. Lower panel the tubular structure of TauT was increased in motor neurons of the ventral horn in G93A mice compared with WT. Scale bar=40 μm. **p<0.0001, significantly different
TauT Messenger RNA and Protein Levels are Increased in an ALS (G93A) Cell Line Model and in ALS Mice
Since we found that TauT immunoreactivity was elevated in G93A mice, we further examined whether TauT gene or protein expression was affected in motor neuron-like (NSC-34) cell lines and G93A mice. TauT messenger RNA (mRNA) and protein levels in NSC-34 cell lines and G93A mice were determined by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot analysis. Increased levels of TauT mRNA were observed in both NSC-34/hSOD1G93A cells and G93A mouse spinal cord compared to control NSC-34 cells and WT mice (Fig. 2a). In addition, TauT protein was increased twofold in NSC-34/hSOD1G93A cells compared to control NSC-34 cells. In contrast, levels of HSF1, a stress-inducible transcription factor, were only slightly increased in the NSC-34/hSOD1G93A cells (Fig. 2b). Since WT SOD1 is being regarded as a viable target for SALS and is involved in cytotoxicity [26], it seems likely that WT hSOD1 could modulate stress signals, such as HSF1 activation, in order to improve the condition of cells against stresses (Fig. 2b). Western blot and densitometric analysis showed elevated TauT protein levels in the spinal cord of G93A mice at both 50 and 90 days of age compared to WT mice (Fig. 2c).
Fig. 2.

The message and protein levels of TauT are increased in ALS. a TauT mRNA was increased in cellular and animal models of ALS. b Levels of TauT protein were increased in NSC-34/hSOD1G93A cells. c The level of TauT protein was increased in the lumbar spinal cord of G93A mice. *p<0.05, **p<0.005, significantly different
HSF1 Regulates TauT Transcription
In order to determine how the TauT gene is activated at the transcriptional level, we ran the TFSEARCH software (Kyoto University, Japan) using the mouse TauT gene promoter sequence and found DNA binding sites (cis-elements) for transcription factors, such as HSF1 and c-Ets-1 (Fig. 3a). Since several potential HSF1 binding sites emerged, we constructed a series of deletion constructs (TauT, −272/+33; TauT, −132/+33; TauT, −502/−131) to determine which site functions as an important cis-element for the basal activity of the TauT promoter (Fig. 3b). Among three deletion constructs, the TauT promoter (−502/−131) with a deletion of the proximal HSF1 binding site showed significantly decreased luciferase activity compared with the other TauT promoters (−272/+33 and −132/+33) (Fig. 3c). This promoter assay data indicate that the proximal site is an efficient cis-element of HSF1. To confirm whether HSF1-DNA occupancy is identified within the TauT promoter region, we performed chromatin immunoprecipitation (ChIP) assays and found that HSF1-DNA occupancy in the TauT promoter was increased approximately twofold in NSC-34/hSOD1G93A cells compared to NSC-34/hSOD1wt cells (Fig. 3d). Because TauT transcription was regulated by HSF1, we performed Western blot analysis to define how TauT protein levels relate to HSF1. We found that HSF1 overexpression increased TauT levels and that knockdown of HSF1 by small interfering RNA (siRNA) downregulated TauT levels (Fig. 3e, f). These results suggest that TauT levels are directly regulated by HSF1. To clarify whether the transcription of TauT is regulated by HSF1, we cotransfected HSF1 and siRNA of HSF1 with TauT promoter plasmid (−502/+33) in control NSC-34 cells and NSC-34/hSOD1G93A cells and measured the luciferase activity. HSF1 increased TauT transcriptional activity in a dose-dependent manner, while HSF1 knockdown significantly decreased TauT transcription (Fig. 3g, h). Interestingly, the activation of TauT transcription by HSF1 was higher in NSC-34/hSOD1G93A cells than in control NSC-34 cells.
Fig. 3.

HSF1 regulates the transcription of TauT. a The DNA sequence of mouse TauT promoter showed putative transcription factor binding elements. b The deletion constructs of mouse TauT 5′ untranslated region promoter. c TauT promoter activity was determined using a series of deletion reporter constructs (TauT, −502/+33; TauT, −272/+33; TauT, −132/+33; TauT, −502/−131). d The HSF1-DNA occupancy within the TauT promoter region was increased in NSC-34/hSOD1G93A cells. e The level of TauT protein was increased by HSF1 in a dose-dependent manner. f Knockdown of HSF1 with siRNA reduced the level of HSF1 and TauT in comparison to siRNA control. g The transcriptional activity of HSF1 on the TauT promoter was elevated in NSC-34/hSOD1G93A cells in comparison to control NSC-34 cells. h Knockdown of HSF1 with siRNA decreased TauT (−502/+33) and (−132/+33) promoter activity. *p<0.05, **p<0.005, significantly different
Oxidative Stress Induces HSF1 Activation and TauT Expression
Because HSF1 is a transcription factor induced in response to various stresses [25], we hypothesized that the activation of HSF1 could be modulated by oxidative stress, one of the major pathological factors thought to contribute to ALS pathogenesis. In this context, we exposed cells to 100 μM hydrogen peroxide (H2O2) for variable amounts of time and measured HSF1 protein levels. HSF1 protein was increased at 3 h and its elevation persisted for 12 h after treatment with 100 μM H2O2. As we expected, TauT protein levels were increased by oxidative stress in control NSC-34 cells (Fig. 4a). In parallel, TauT expression was induced 12 h after H2O2 treatment. In order to check the stability of TauT protein, we performed a cycloheximide (CHX) chase study in the presence or absence of H2O2 in control NSC-34 cells followed by Western analysis. When CHX (10 μg/ml) was chased for the indicated period of time without oxidative stress, TauT protein stability persisted for more than 24 h. Interestingly, the stability of TauT protein was not affected by oxidative stress over 24 h. It seems likely that oxidative stress does not reduce TauT protein levels (Fig. 4b).
Fig. 4.
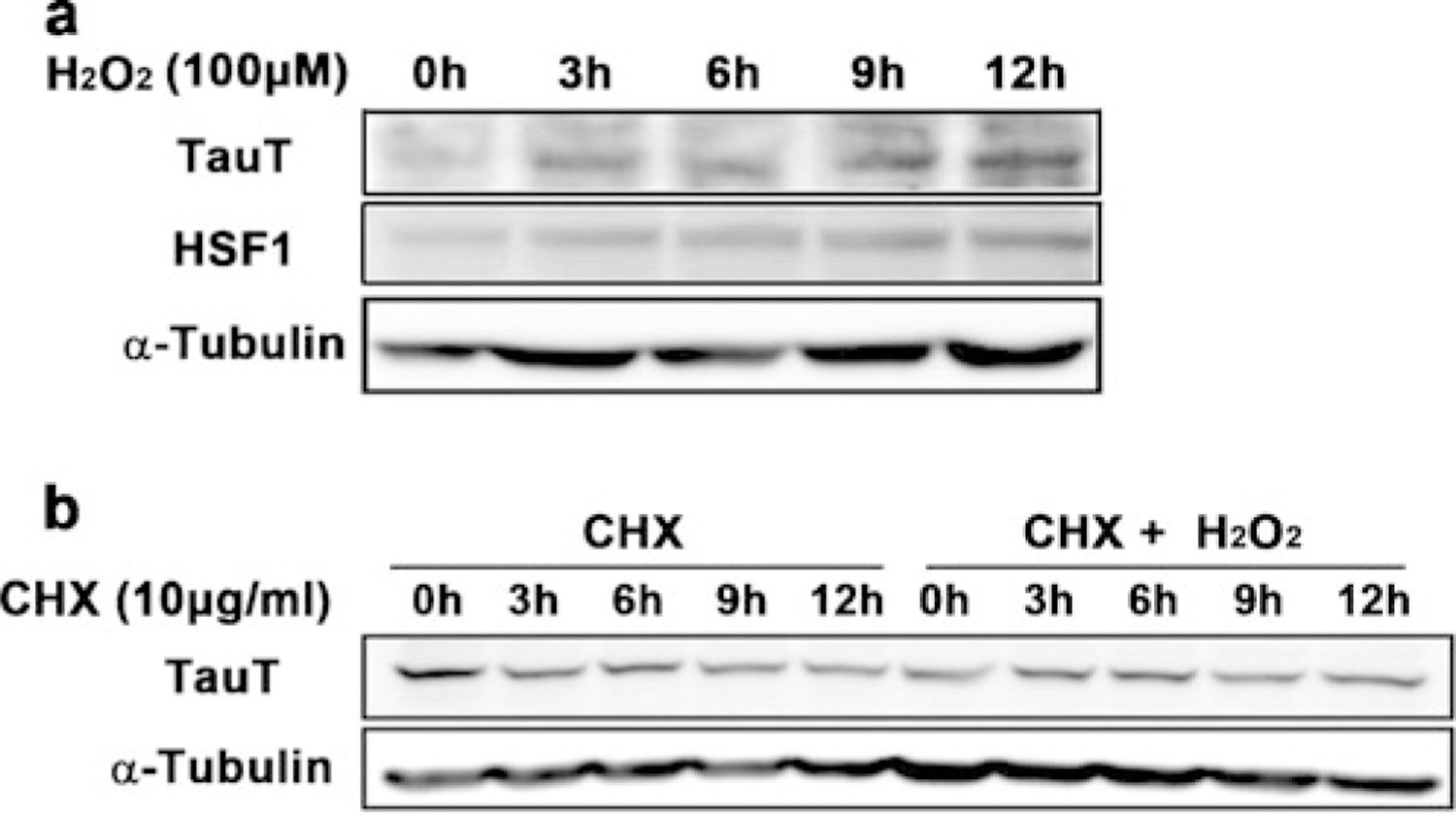
Oxidative stress induces HSF1 activation and TauT expression. a Oxidative stress induced the level of TauT protein level in a time-dependent manner. b Oxidative stress increased the stability of TauT protein
Taurine Levels are Increased in G93A mice
To determine in vivo taurine levels, we performed high-performance liquid chromatography (HPLC) on extracts of lumbar spinal cords from WT and G93A mice. Taurine and several other amino acids were significantly increased in the lumbar spinal cords of G93A mice compared to WT controls (Fig. 5b; Supplementary Table S2). Consistent with the HPLC result, we found that taurine immunoreactivity was elevated in the lumbar spinal cord of G93A mice at 90 and 120 days of age (Fig. 5a). Because both TauT and taurine were increased in ALS motor neurons, we hypothesized that increased TauT activity may be directly involved in extracellular taurine uptake by motor neurons. In order to test our hypothesis, we measured the activity of TauT in NSC-34 cell lines (NSC-34, NSC-34/hSOD1wt, and NSC-34/hSOD1G93A) using radiolabeled [3H] taurine. The ratio of [3H] taurine uptake was studied in a time-dependent manner in NSC-34 cell lines. As expected, [3H] taurine uptake was significantly higher in NSC-34/hSOD1G93A cells than in control NSC-34 and NSC-34/hSOD1wt cells (Fig. 5c). We further tested whether taurine uptake is directly mediated by TauT by competitively inhibiting taurine uptake with β-alanine [27]. When cells were exposed to unlabeled taurine and β-alanine, [3H] taurine uptake was significantly decreased in NSC-34 cell lines, indicating that taurine uptake directly depends on TauT activity (Fig. 5d).
Fig. 5.
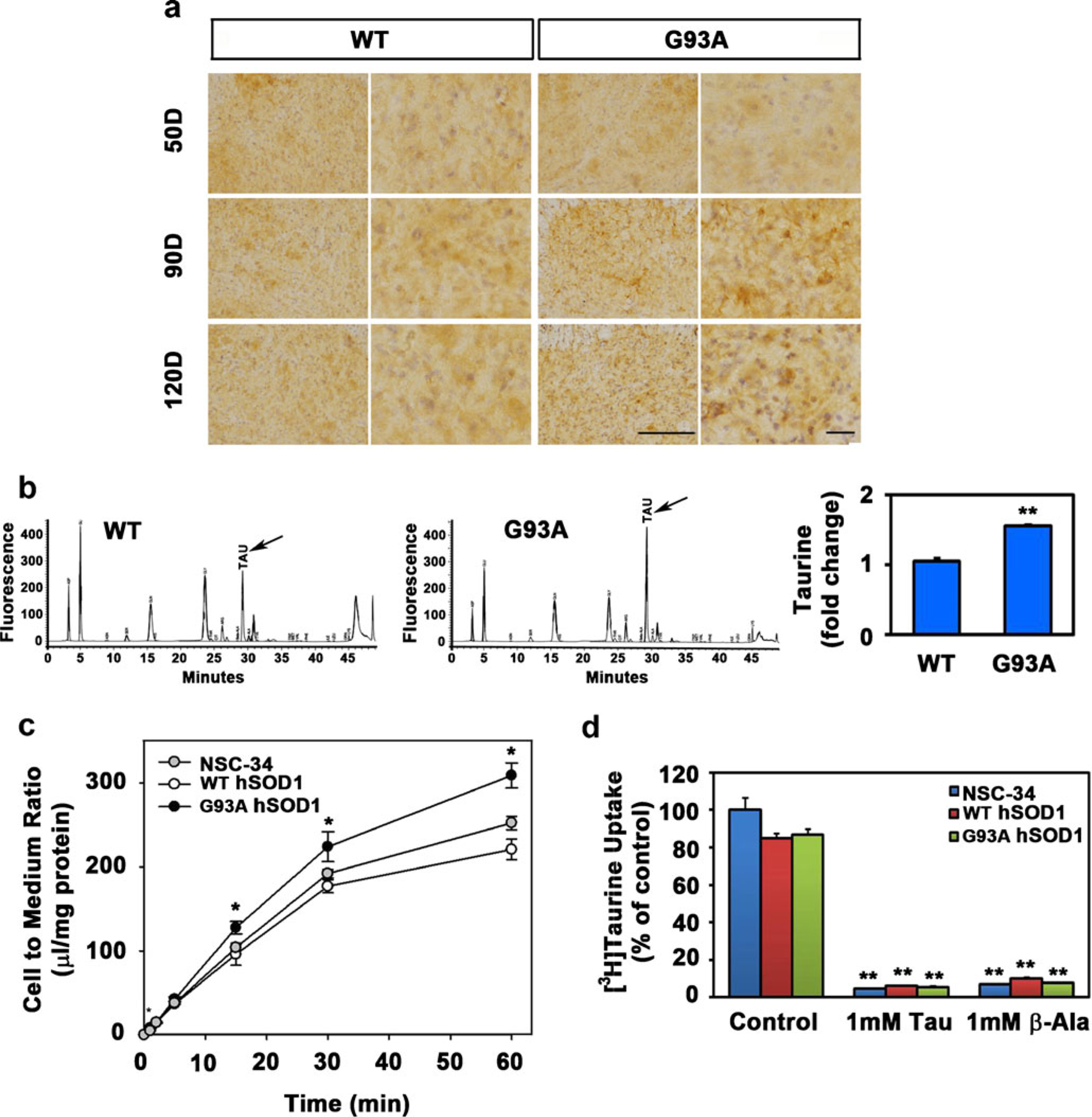
The level and influx of taurine is elevated in ALS. a The immunoreactivity of taurine was increased in motor neurons of G93A mice. Scale bars030 μm (left) and 15 μm (right). b In vivo taurine levels within WT and G93A mice were measured by HPLC. **p<0.005, significantly different. c Time course of [3H] taurine uptake by NSC-34 cell lines (NSC-34, NSC-34/hSOD1wt, and NSC-34/hSOD1G93A). [3H] taurine uptake was performed at 37 °C. Each point. represents the mean±SEM (n04). The error bar is smaller than the size of the symbol in some cases. *p<0.05, significantly different. d Effect of taurine and β-alanine on the uptake of [3H] taurine by NSC-34 cell lines. Taurine uptake was measured after 5 min incubation with [3H] taurine. Each value represents the mean±SEM (n=4). **p<0.001, significantly different
Motor Neurons Viability is Modulated by TauT Activity and Taurine
Based on the results previously described, we hypothesized that oxidative stress-induced TauT expression may drive taurine uptake into motor neuron-like cells and thereby enhance their viability. To determine whether increased taurine uptake can prevent oxidative stress-induced cell death, we performed lactate dehydrogenase (LDH) assays in control NSC-34 cells and we measured motor neuronal cytotoxicity after TauT knockdown with or without 100 μM H2O2, sodium arsenite (NaAsO2), and thapsigargin (Tg) (Fig. 6c). As we expected, cellular stresses induced by H2O2 significantly increased motor neuronal cell death when TauT levels were reduced using siRNA. In addition, knockdown of HSF1 downregulated TauT expression and enhanced motor neuronal cytotoxicity in response to 100 μM H2O2 and NaAsO2 (Fig. 6d). To further test the protective effects of taurine under oxidative stress conditions, we examined cytotoxicity in the absence or presence of H2O2 after preincubation with taurine. Preincubation with taurine prevented motor neuronal cell death produced by H2O2-induced oxidative stress (Fig. 6e, f).
Fig. 6.

Inhibition of taurine uptake by siRNA and β-alanine exacerbates cellular stress-induced motor neuronal cell death. a Knockdown of TauT with siRNA reduced TauT levels compared to siRNA control. b Knockdown of HSF1 with siRNA reduced HSF1 levels compared to siRNA control. c, d Cells were exposed to H2O2, NaAsO2, and Tg. Knockdown of TauT and HSF1 exacerbated cellular stress-induced motor neuronal cell death. The data represent the mean±SEM of three separate experiments. e, f Treatment with taurine prevented the motor neuronal cell death under oxidative stress. g The induction of TauT by HSF1 may play a protective response to compensate oxidative stress in motor neurons under the ALS condition. *p<0.05, **p<0.005, significantly different
Taken together, out results show that HSF1 is activated in response to oxidative stress and modulates TauT expression. Increased TauT and taurine expression in motor neurons contributes to the protection of the motor neurons from oxidative stress (Fig. 6g).
Discussion
Mechanisms of TauT Expression in ALS Motor Neurons
Several pathological factors, such as endoplasmic reticulum (ER) stress, glutamate cytotoxicity, and oxidative stress, are closely linked to motor neuron damage in ALS, but the molecular mechanisms underlying these processes are still poorly understood. In the 1970s, diverse alterations of free amino acids and abnormal metabolism of sulfur amino acids were found in the motor cortex and the spinal cord of ALS patients [17]. The finding of taurine elevation in the precentral white matter in the absence of gliosis suggested that taurine might be increased in neurons [17]. Alternatively, increased taurine could arise from a derangement of sulfur amino acid metabolism because starvation and protein deficiency do not lead to increased taurine. In any case, the cause of taurine accumulation and its role in ALS pathogenesis remain to be elucidated.
In the current study, surprisingly, we found that the levels of TauT expression and taurine are elevated in motor neurons of ALS transgenic (G93A) mice. Although it has been reported that osmotic stress increases levels of TauT mRNA in non-neuronal cells [28], the effects of oxidative stress on TauT expression and the role of TauT in ALS have not been examined prior to our study.
Interestingly, we found that HSF1 is elevated in motor neurons of G93A mice compared to control mice. Overexpression of HSF1 upregulated the level of TauT, whereas knockdown of HSF1 by siRNA downregulated the level of TauT. Our data indicated that the proximal site of the TauT promoter harbors an efficient cis-element for HSF1 binding. ChIP experiments further confirmed that HSF1 constitutively occupies the TauT promoter region and that HSF1-DNA occupancy in the TauT promoter was increased approximately twofold in ALS. These results identified that the expression of TauT is directly regulated by HSF1 under the ALS condition.
The Function of Taurine and TauT in ALS
We hypothesize that the increased expression and activity of TauT in ALS caused by HSF1 may facilitate the ability of motor neurons to cope with oxidative stress and survive. In this paradigm, a likely intermediate to defend the neuron from oxidative stress would be taurine. Our current data confirm that increased TauT levels correlate with enhanced TauT activity and increased taurine uptake. Consequently, elevated intracellular taurine levels reduce motor neuronal death in the context of cellular stress. Thus, the activation of the TauT pathway in response to oxidative stress may be one of the protective responses that prevent motor neuron death. Our CHX chase study shows that the rate of TauT protein turnover (decay) is considerably slower in NSC-34 cells under both physiological condition and oxidative stress. The sustained stability of TauT under highly oxidative conditions may be crucial to maintain taurine uptake into the intracellular space and coping with residual oxidative stress in motor neurons. Not only has taurine been known as a neurotransmitter [29], a regulator of calcium fluxes [30], and an osmoregulator [31] but it also plays a fundamental role as an antioxidant and a cytoprotectant in mammalian cells. A recent study showed that a treatment of PC12 cells with taurine is associated with resistance to oxidative stress, strongly supporting our findings [32]. Our results show that β-alanine-induced inhibition of TauT and subsequent depletion of intracellular taurine exacerbate motor neuronal death in control NSC-34 and NSC-34/hSOD1wt cells compared to NSC-34/hSOD1G93A cells in response to Tg-induced ER stress and H2O2-induced oxidative stress. These results show that altered expression and activity of TauT can contribute to cell-autonomous regulation of motor neuronal survival. Although our data suggest that TauT mediates prosurvival responses in NSC-34/hSOD1G93A cells, these observations imply that the effect of TauT induction may depend on the cell type, the progress/status of the disease, and/or the death stimulus.
In summary, we demonstrate that HSF1 induces TauT in ALS by occupying the promoter region of the TauT gene. We correlate the protective effects of TauT with its ability to increase the uptake of taurine and thereby prevent motor neuronal cell death caused by several stresses. These observations suggest that the induction of TauT by HSF1 is a protective response to compensate for oxidative stress in motor neurons. Activation of TauT and the taurine pathway could contribute to the prevention of motor neuronal damage and lead to improved treatments for neurodegenerative diseases, including ALS.
Materials and Methods
Animals
Male transgenic ALS mice of the mtSOD1 (G93A) H1 high expressor strain (Jackson Laboratories, Bar Harbor, ME, USA) are bred with females with a similar background (B6/SJLF1). These experiments were carried out in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by both the Veterans Administration and Boston University Animal Care Committees.
Cell Culture and DNA Damage
Motor neuron-like cells (NSC-34) transfected with pCl-neo expression vector containing human WT (control NSC-34), hSOD1 WT (NSC-34/hSOD1wt cells), and mutant hSOD1 G93A (NSC34/hSOD1G93A cells) were established previously [33–36]. Cell lines were maintained in Dulbecco’s modified Eagle’s medium (Hyclone, Salt Lake City, UT, USA) supplemented with 10 % (v/v) fetal bovine serum (Hyclone, Salt Lake City, UT, USA), 100 U penicillin/ml, and 0.1 mg streptomycin/ml. Cells were kept in a humidified incubator at 37 °C under 5 % CO2. Cells were subcultured in six-well plates at a density of 5×105 cells per well. After 80 % confluence, cells were treated with 100 μM H2O2 in a time-dependent manner.
Confocal Microscopy
Immunofluorescence staining and confocal microscopy was used to determine the TauT (Santa Cruz Biotech, Santa Cruz, CA, USA) and HSF1 (Santa Cruz Biotech, Santa Cruz, CA, USA). The specimens were incubated for 1 h with DyLight 594 donkey antimouse IgG antibody and DyLight 488 donkey antigoat IgG antibody (Jackson ImmunoResearch, Baltimore Pike, PA, USA; 1:400) after incubation of the primary antibody. Images were analyzed using an Olympus FluoView FV10i confocal microscope (Olympus, Tokyo, Japan). Deconvolution and three-dimensional construction of the confocal image were performed by AQI-COMBO-CWF program (Media Cybernetics Inc., Bethesda, MD, USA). Control experiments were performed in the absence of primary antibody or in the presence of blocking peptide. Immunohisto-chemistry by 3,3′-diaminobenzidine staining was performed as previously described [16]. Immunoreactivity was determined by Multi Gauge V3.0 (Fujifilm, Tokyo, Japan).
Constructs
To generate the TauT promoter region in pGL4.14 (Promega, Fitchburg, WI, USA) with the KpnI and XhoI (New England Biolabs, Ipswich, MA, USA) restriction enzyme sites, the TauT promoter was amplified by PCR (forward, 5′-CGGGGTACCGCCTGTTTTCCCAGTACTGT-3′; reverse, 5′-CCGCTCGAGTTGCAGCTTCTCCTTCGTGG-3′) from genomic NSC-34 cells and sequenced. The TauT deletion constructs of pGL4.14/TauT (−272/+33), TauT (−132/+33), and TauT (−502/−131) were generated by PCR. The sequence of the primers was as follows: pGL4.14/TauT (−272/+33) forward, 5′-GGCCAGTTAGGCCAGAGAAA-3′; TauT (−272/+33) reverse, 5′-CAGGGTACACAGGCTGGTGT-3′; TauT (−132/+33) forward, 5′-AGCTGGCTTCTTTGATG-GAG-3′; TauT (−502/−131) forward, 5′-AGATCCCAAG-GATTTCAATA-3′; TauT (−502/−131) reverse, 5′-GATATCAAGATCTGGCCTCG-3′.
Chromatin Immunoprecipitation
ChIP for HSF1 binding to DNA was performed using a ChIP assay kit (Santa Cruz Biotech, Santa Cruz, CA, USA) as previously described [37]. The cells were cross-linked with 1 % formaldehyde for 10 min at room temperature. The lysates were sonicated ten times with each time for 30 s using Bioruptor (Diagenode, Denville, NJ, USA). After centrifugation, the supernatant was diluted in ChIP dilution buffer and then incubated overnight at 4 °C with the primary antibody. The eluted DNA was quantified by qPCR and normalized to IgG control.
HPLC Analysis of Amino Acids in Spinal Cord
The levels of taurine in the spinal cord were determined using HPLC methods as previously described [38, 39]. Spinal cord tissue was lysed with 0.2 ml of 1.5 mol/L HClO4, and then 0.1 ml of 2 mol/L K2CO3 was added. The neutralized tissue lysates were centrifuged at 10,000×g for 1 min, and an aliquot (0.2 ml) of the supernatant was used for sample cleanup and determination of taurine. An aliquot of the derivatized mixture (25 μl) was injected into a Supelco C18 column (150×4.6 mm I.D.). Amino acids were separated using a solvent gradient consisting of solution A (0.1 M sodium acetate, 2 mM sodium dodecyl sulfate, 0.5 % tetrahydrofuran, and 9 % methanol, pH 7.2) and solution B (100 % methanol and 2 mM sodium dodecyl sulfate). Amino acids in samples were quantified on the basis of authentic standards, using Millenium 32 Software (Waters, Milford, MA, USA).
LDH Assay
Cells were seed to a 48-well plate. Cells were transiently transfected with siTauT and siHSF1 using Lipofectamine 2000 transfection reagent (Invitrogen Life Tech, Carlsbad, CA, USA) for 48 h then exposed to 100 μM H2O2 for 12 h. After this treatment period, cell cytotoxicity was determined by CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit (Promega, Fitchburg, WI, USA).
Promoter Activity Assay
We used the mouse TauT promoter-driven luciferase (Luc) reporter plasmid pGL4.14. Cells (NSC-34, NSC-34/hSOD1wt, and NSC-34/hSOD1G93A) were subcultured in 48-well plates at a density of 5×104 cells per well, and the next day, cells were transfected with the TauT promoter in a dose-dependent manner of HSF1. The luciferase activity was measured 24 h after the transfection.
Quantitative Real-Time PCR
Total RNA was extracted from the motor neuron cell line and spinal cord from ALS mice by TRIzol reagent (MRC, Cincinnati, OH, USA). RNA was measured in a spectrophotometer at 260 nm absorbance. RNA analysis was conducted as follows. Fifty nanograms of RNA was used as a template for qRT-PCR amplification, using SYBR Green Real-Time PCR Master Mix (Toyobo, Osaka, Japan). Primers were standardized in the linear range of the cycle before the onset of the plateau. The sequence of the primers was as follows: GAPDH forward, 5′-TGTGTCCGTCGTGGATCTGA-3′; GAPDH reverse, 5′-CCTGCTTCACCACCTTCTTGA-3′; TauT forward, 5′-ATGACCTCACTGGGAAGCTA-3′; TauT reverse, 5′-GGATGTGATCTGTCCTTCGA-3′. GAPDH was used as an internal control. Two-step PCR thermal cycling for DNA amplification and real-time data acquisition were performed with an ABI StepOnePlus™ Real-Time PCR System using the following cycle conditions: 1 cycle at 95 °C for 1 min and 95 °C for 15 s, followed by 40 cycles at 60 °C for 1 min. Fluorescence data were analyzed by the ABI StepOnePlus software and expressed as Ct, the number of cycles needed to generate a fluorescent signal above a predefined threshold. The ABI StepOnePlus software set the baseline and threshold values.
RNA Interference Experiment
Cells were transiently transfected with siTauT and siHSF1 using RNAiMAX transfection reagent (Invitrogen Life Tech, Carlsbad, CA, USA) for 48 h. The sequence of siRNA of TauT and HSF1 are as follows: control siRNA (forward) 5′-UUCUCCGAACGUGUCACGUTT-3′, (reverse) 5′-ACGUGACACGUUCGGAGAATT-3′; TauT siRNA (forward) 5′-GAUCUGUCCUUUGUUCUCUUU-3′, (reverse) 5′-AGAGAACAAAGGACAGAUCUU-3′; HSF1 siRNA (forward) 5′-CCUGCAGGUUGUUCAUAAATT-3′, (reverse) 5′-UUUAUGAACAACCUGCAGGTT-3′.
Uptake Study in NSC34 Cell Lines
The [3H] taurine uptake was performed according to the previous report [40]. Cells were washed with extracellular fluid (ECF) buffer consisting of 122 mM NaCl, 25 mM NaHCO3, 3 mM KCl, 1.4 mM CaCl2, 1.2 mM MgSO4, mM K2HPO4, 10 mM D-glucose, and 10 mM HEPES (pH 7.4) at 37 °C. Uptake was initiated by applying ECF buffer containing [3H] taurine (29 nM) in the presence or absence of unlabeled taurine or β-alanine and then incubate at 37 °C for the designated time. Uptake was terminated by the addition of ice-cold ECF buffer. The cells were then solubilized in 1 N NaOH, and radioactivity was measured in a liquid scintillation counter (LS6500; Beckman, Fullerton, CA, USA). Cell to medium ratio (in microliters per milligrams protein) was calculated as follows:
Western Blot Analysis
Cell lysates were prepared from the proposed experimental conditions, subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, and blotted with anti-TauT (E-10: sc-166640, Santa Cruz Biotech, Santa Cruz, CA, USA) and anti-HSF1 (C-5: sc-17756, Santa Cruz Biotech, Santa Cruz, CA, USA) antibody. Protein loading was controlled by probing for α-tubulin (#05–559, Millipore, Billerica, MA, USA) antibody on the same membrane.
Statistical Analysis
The data are presented as the mean±standard error of the mean (SEM). Data analysis was performed by Student’s t test using SigmaPlot 2000 (Systat Software, Chicago, IL, USA). Differences were considered statistically significant when p<0.05.
Supplementary Material
Acknowledgments
This study was supported by the Rare Disease Research Center Grant from Korea Ministry of Health and Welfare (HR), SRC Grant (2010–0029-403) (HR) and WCU Neurocytomics Program Grant (800–20080848) (HR). This study was also supported by the National Research Foundation of Korea (NRF) grant (no. 2011–0030701) (YSK) funded by the Korean government (MEST) and VA Merit Award (JL and NK).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12035-012-8371-9) contains supplementary material, which is available to authorized users.
Contributor Information
Min-Kyung Jung, WCU Neurocytomics Group, Department of Biomedical Sciences, College of Medicine, Seoul National University, Seoul 110-799, South Korea.
Ki Yoon Kim, WCU Neurocytomics Group, Department of Biomedical Sciences, College of Medicine, Seoul National University, Seoul 110-799, South Korea.
Na-Young Lee, Research Center for Cell Fate Control, College of Pharmacy, Sookmyung Women’s University, Seoul 140-742, South Korea.
Young-Sook Kang, Research Center for Cell Fate Control, College of Pharmacy, Sookmyung Women’s University, Seoul 140-742, South Korea.
Yu Jin Hwang, WCU Neurocytomics Group, Department of Biomedical Sciences, College of Medicine, Seoul National University, Seoul 110-799, South Korea.
Yunha Kim, WCU Neurocytomics Group, Department of Biomedical Sciences, College of Medicine, Seoul National University, Seoul 110-799, South Korea.
Jung-Joon Sung, Department of Neurology, Seoul National University Hospital, Seoul 110-799, South Korea.
Ann McKee, Boston VA Healthcare System, Building 1A, Room 105, 150 South Huntington Avenue, Boston, MA 02130, USA; Alzheimer’s Disease Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, 150 South Huntington Avenue, Boston, MA 02118, USA.
Neil Kowall, Boston VA Healthcare System, Building 1A, Room 105, 150 South Huntington Avenue, Boston, MA 02130, USA; Alzheimer’s Disease Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, 150 South Huntington Avenue, Boston, MA 02118, USA.
Junghee Lee, Boston VA Healthcare System, Building 1A, Room 105, 150 South Huntington Avenue, Boston, MA 02130, USA; Alzheimer’s Disease Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, 150 South Huntington Avenue, Boston, MA 02118, USA.
Hoon Ryu, WCU Neurocytomics Group, Department of Biomedical Sciences, College of Medicine, Seoul National University, Seoul 110-799, South Korea; Boston VA Healthcare System, Building 1A, Room 105, 150 South Huntington Avenue, Boston, MA 02130, USA; Alzheimer’s Disease Center, Boston University School of Medicine, Boston, MA 02118, USA; Department of Neurology, Boston University School of Medicine, 150 South Huntington Avenue, Boston, MA 02118, USA.
References
- 1.Rowland LP, Shneider NA (2001) Amyotrophic lateral sclerosis. N Engl J Med 344:1688–1700 [DOI] [PubMed] [Google Scholar]
- 2.Rosen DR (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62 [DOI] [PubMed] [Google Scholar]
- 3.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264:1772–1775 [DOI] [PubMed] [Google Scholar]
- 4.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW (1997) ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18:327–338 [DOI] [PubMed] [Google Scholar]
- 5.Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW (1998) Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science 281:1851–1854 [DOI] [PubMed] [Google Scholar]
- 6.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL (1995) An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14:1105–1116 [DOI] [PubMed] [Google Scholar]
- 7.Festoff BW, Suo Z, Citron BA (2003) Prospects for the pharmacotherapy of amyotrophic lateral sclerosis: old strategies and new paradigms for the third millennium. CNS Drugs 17:699–717 [DOI] [PubMed] [Google Scholar]
- 8.McGeer EG, McGeer PL (2005) Pharmacologic approaches to the treatment of amyotrophic lateral sclerosis. BioDrugs 19:31–37 [DOI] [PubMed] [Google Scholar]
- 9.Rothstein JD (2003) Of mice and men: reconciling preclinical ALS mouse studies and human clinical trials. Ann Neurol 53:423–426 [DOI] [PubMed] [Google Scholar]
- 10.Weiss MD, Weydt P, Carter GT (2004) Current pharmacological management of amyotrophic lateral sclerosis and a role for rational polypharmacy. Expert Opin Pharmacother 5:735–746 [DOI] [PubMed] [Google Scholar]
- 11.Plaitakis A, Constantakakis E, Smith J (1988) The neuroexcito-toxic amino acids glutamate and aspartate are altered in the spinal cord and brain in amyotrophic lateral sclerosis. Ann Neurol 24:446–449 [DOI] [PubMed] [Google Scholar]
- 12.Urushitani M, Kurisu J, Tsukita K, Takahashi R (2002) Proteasomal inhibition by misfolded mutant superoxide dismutase 1 induces selective motor neuron death in familial amyotrophic lateral sclerosis. J Neurochem 83:1030–1042 [DOI] [PubMed] [Google Scholar]
- 13.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW (1995) Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol 38:73–84 [DOI] [PubMed] [Google Scholar]
- 14.Gurney ME, Cutting FB, Zhai P, Andrus PK, Hall ED (1996) Pathogenic mechanisms in familial amyotrophic lateral sclerosis due to mutation of Cu, Zn superoxide dismutase. Pathol Biol (Paris) 44:51–56 [PubMed] [Google Scholar]
- 15.Lee J, Ryu H, Kowall NW (2009) Differential regulation of neuronal and inducible nitric oxide synthase (NOS) in the spinal cord of mutant SOD1 (G93A) ALS mice. Biochem Biophys Res Commun 387:202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Ryu H, Kowall NW (2009) Motor neuronal protection by L-arginine prolongs survival of mutant SOD1 (G93A) ALS mice. Biochem Biophys Res Commun 384:524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshino Y, Koike H, Akai K (1979) Free amino acids in motor cortex of amyotrophic lateral sclerosis. Experientia 35:219–220 [DOI] [PubMed] [Google Scholar]
- 18.Yoshino Y, Iwabuchi S, Akai K (1976) Free amino-acids in the human spinal cord—analysis of anterior, posterior columns and anterior, lateral and posterior funiculi. Rinsho Shinkeigaku 16:74–81 [PubMed] [Google Scholar]
- 19.Iłzecka J, Stelmasiak Z, Solski J, Wawrzycki S, Szpetnar M (2003) Plasma amino acids concentration in amyotrophic lateral sclerosis patients. Amino Acids 25:69–73 [DOI] [PubMed] [Google Scholar]
- 20.Mozaffari MS, Abdelsayed R, Patel C, Schaffer SW (2006) Effects of dietary salt and fat on taurine excretion in healthy and diseased rats. Adv Exp Med Biol 583:173–180 [DOI] [PubMed] [Google Scholar]
- 21.Martin GP, el-Hariri LM, Marriott C (1992) Bile salt- and lysophosphatidylcholine-induced membrane damage in human erythrocytes. J Pharm Pharmacol 44:646–650 [DOI] [PubMed] [Google Scholar]
- 22.Bitoun M, Tappaz M (2000) Gene expression of taurine transporter and taurine biosynthetic enzymes in hyperosmotic states: a comparative study with the expression of the genes involved in the accumulation of other osmolytes. Adv Exp Med Biol 483:239–248 [DOI] [PubMed] [Google Scholar]
- 23.Bitoun M, Tappaz M (2000) Gene expression of taurine transporter and taurine biosynthetic enzymes in brain of rats with acute or chronic hyperosmotic plasma. A comparative study with gene expression of myo-inositol transporter, betaine transporter and sorbitol biosynthetic enzyme. Brain Res Mol Brain Res 77:10–18 [DOI] [PubMed] [Google Scholar]
- 24.Vitarella D, DiRisio DJ, Kimelberg HK, Aschner M (1994) Potassium and taurine release are highly correlated with regulatory volume decrease in neonatal primary rat astrocyte cultures. J Neurochem 63:1143–1149 [DOI] [PubMed] [Google Scholar]
- 25.Benjamin IJ, McMillan DR (1998) Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res 83:117–132 [DOI] [PubMed] [Google Scholar]
- 26.Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, Rao M, Eagle A, Kammesheidt A, Christensen A, Mendell JR, Burghes AH, Kaspar BK (2011) Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol 29(9):824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith KE, Borden LA, Wang CH, Hartig PR, Branchek TA, Weinshank RL (1992) Cloning and expression of a high affinity taurine transporter from rat brain. Mol Pharmacol 42:563–569 [PubMed] [Google Scholar]
- 28.Bitoun M, Tappaz M (2000) Gene expression of the transporters and biosynthetic enzymes of the osmolytes in astrocyte primary cultures exposed to hyperosmotic conditions. Glia 32:165–176 [PubMed] [Google Scholar]
- 29.Lin CT, Song GX, Wu JY (1985) Is taurine a neurotransmitter in rabbit retina? Brain Res 337:293–298 [DOI] [PubMed] [Google Scholar]
- 30.Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163 [DOI] [PubMed] [Google Scholar]
- 31.Wade JV, Olson JP, Samson FE, Nelson SR, Pazdernik TL (1988) A possible role for taurine in osmoregulation within the brain. J Neurochem 51:740–745 [DOI] [PubMed] [Google Scholar]
- 32.Pan C, Giraldo GS, Prentice H, Wu JY (2010) Taurine protection of PC12 cells against endoplasmic reticulum stress induced by oxidative stress. J Biomed Sci 17(Suppl 1):S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP (1992) Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn 194:209–221 [DOI] [PubMed] [Google Scholar]
- 34.Durham HD, Dahrouge S, Cashman NR (1993) Evaluation of the spinal cord neuron X neuroblastoma hybrid cell line NSC-34 as a model for neurotoxicity testing. Neurotoxicology 14:387–395 [PubMed] [Google Scholar]
- 35.Gomes C, Keller S, Altevogt P, Costa J (2007) Evidence for secretion of Cu, Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci Lett 428:43–46 [DOI] [PubMed] [Google Scholar]
- 36.Gomes C, Palma AS, Almeida R, Regalla M, McCluskey LF, Trojanowski JQ, Costa J (2008) Establishment of a cell model of ALS disease: Golgi apparatus disruption occurs independently from apoptosis. Biotechnol Lett 30:603–610 [DOI] [PubMed] [Google Scholar]
- 37.Ryu H, Lee J, Impey S, Ratan RR, Ferrante RJ (2005) Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proc Natl Acad Sci U S A 102:13915–13920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu G, Meininger CJ (2008) Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol 440:177–189 [DOI] [PubMed] [Google Scholar]
- 40.Kang YS, Ohtsuki S, Takanaga H, Tomi M, Hosoya K, Terasaki T (2002) Regulation of taurine transport at the blood–brain barrier by tumor necrosis factor-alpha, taurine and hypertonicity. J Neurochem 83:1188–1195 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


