Abstract
In patients with autoimmune diabetes no significant differences were observed in glucose control, expressed as time in range evaluated by continuous glucose monitoring comparing the 3 days after Sars-Cov2 vaccine with the 14 days preceding the vaccine.
Keywords: Sars-Cov-2, Vaccination, Glucose control, Diabetes, CGM
1. Introduction
The outbreak of coronavirus disease 2019 (COVID-19) at the beginning of 2020 has resulted in a huge impact around the world. Direct effects of COVID-19 were seen in terms of worse outcomes especially in vulnerable people as patients with diabetes mellitus (DM) [1], [2]. Further, the indirect effects caused by lockdown measures adopted to contrast the pandemic have also impacted on diabetes management [3], [4].
The development of effective vaccines against the Sars-Cov2 has been greeted with enthusiasm as a possible ultimate solution to overcome the pandemic [5]. Vaccination in Italy began at the end of 2020, first with health care providers, followed by the elderly and then by extremely vulnerable people. Amongst vulnerable subjects, patients with autoimmune diabetes (AD) were also accounted for. The most common side effects of vaccines are pain at the injection site, fever, headache and fatigue in the 24–48 h following vaccination [5]. For this reason, we hypothesized that the stress caused by the side effects of vaccination, mainly due to systemic reaction as fever and fatigue, together with the inflammatory response occurring after vaccination due to the immune system activation, could impact glucose control in patients with AD. Therefore, the aim of this report was to assess the short-term effects of Sars-Cov2 vaccination on glucose control in patients with AD.
2. Material and methods
To evaluate the effects of vaccination on glucose control, defined according to the international consensus on time in range (TIR) [6], we performed an analysis of continuous glucose monitoring (CGM) data comparing the 3 days after the date of vaccine with the 14 days preceding the vaccine, for both the first and second dose of mRNA vaccine BNT162b2 (Comirnaty). As primary aim we evaluated differences in TIR after the second dose of vaccine compared to TIR before the second dose of vaccine. As secondary aims, we explored change in TIR after the first dose of vaccine compared to TIR before the first dose of vaccine. We further evaluated change in time above range (TAR), time below range (TBR), glycaemic variability expressed by coefficient variation (CV) and standard deviation (SD) after the first and after the second dose of vaccination. A period of 3 days-time after vaccination was chosen because the major side effects due to vaccine injections were reported in the 24–48 h after the injection [5].
We enrolled patients with autoimmune diabetes that agreed to undergo to Sars-Cov2 vaccination, we excluded patients that were unable to upload data on online platforms or that were not using CGM during the vaccination.
The study was 90% powered to detect a significant change in TIR with Wilcoxon signed-rank test (alpha level: 0.05) after compared to before the second dose of vaccine. Distributions of variables were tested for normality with the Shapiro-Wilk test. Differences between pre and post-vaccination variables were tested using the Wilcoxon signed-rank test. IBM SPSS Statistics v.21 software was used for data analysis and Prism 9.0 software was used for graphical representations. The study was performed in accordance with the Declaration of Helsinki, and the study procedures were approved by the institutions’ ethics committees (ref 6382/2021).
3. Results
We retrospectively enrolled 35 subjects with AD (60% male, median [25th-75th percentile] age 36 [27–51] years, disease duration 18 [11–29] years, BMI 24.2 [23.0–25.8] kg/m2, HbA1c 7.6 [6.9–8.0]%), treated with closed-loop (31%) and open-loop CSII or multiple daily injections (MDI) of insulin (69%).
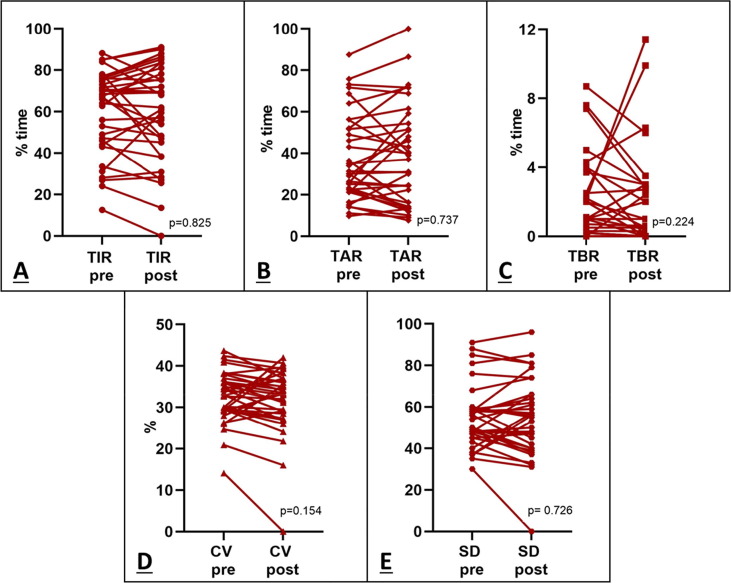
No significant differences in TIR between after and before the second vaccination dose were found (67.5[46.0–76.0]% Vs 62.0[45.2–78.2]%, p = 0.825). Further, no significant differences were observed for the other variables: TAR (30.0[22.0–52.0]% Vs 37.0[16.0–54.4]%, p = 0.737), TBR (1.0[0.2–2.5]% Vs 0.5[0.0–3.0]%, p = 0.224), CV (33.0[29.4–37.0]% Vs 33.5[28.5–37.0]%, p = 0.154) and SD (54.0[45.0–59.0]% Vs 40.0[56.0–66.0]%, p = 0.726), as shown in Fig. 1 .
Fig. 1.
Effect of second dose of Sars-Cov2 vaccine on glycaemic control. No significant changes in TIR (A), TAR(B), TBR (C), CV (D) and SD (E) were observed before and after the second dose of vaccine in patients with AD.
Similar results were obtained analyzing data before and after the first dose of the vaccine. To understand if the closed-loop system could have influenced the results by automatically correcting possible hyperglycaemia or suspending insulin infusion to avoid possible hypoglycaemic events, we divided patients that used closed-loop system compared to patients that used open-loop system or MDI. Again, we did not observe any significant difference in TIR, TAR, TBR, CV and SD between pre and post CGM data collected during the two doses of vaccine in both groups (data available upon request).
Focusing on data gathered, both patients that showed the most relevant increase of TBR after the second dose, as seen in Fig. 1-C, were not on a closed-loop system and for this reason we can speculate that they struggle most to overcome hypoglycaemic events, without the predictive discontinuation of insulin. No other specific differences were found between these patients and the other subjects enrolled.
4. Discussion
This is the first report that showed an overall safety profile of Sars-Cov2 vaccination on glycaemic control in patients with AD. Several studies analysed the impact of Covid-19 [1], [7], [8] and of lock-down measures to contrast the pandemic on patients with DM [3], [4], but, to our knowledge, no studies have been carried out to explore the effect of Sars-Cov2 vaccination on blood glucose concentration in patients with DM. It could be hypothesized that vaccination, throughout immune system activation and side effects, could impact glycaemic control of people with AD. However, the results of this report showed an overall safety profile of Sars-Cov2 vaccination on patients with AD, adding useful information to health care providers to manage people with diabetes that have to receive the vaccination in the future. Further, this is also the first time that direct and acute effects of vaccination on glucose control were investigated in people with AD in the following days after vaccination, paving the path to new studies investigating the impact of vaccination on glucose control in these patients taking advantage of CGM systems. The generalizability of our findings is limited by the low sample size and by the single-centre designs. Patients enrolled were affected by autoimmune diabetes and showed an overall young age and a normal BMI, for this reason the results may not be generalizable in an elderly and obese population. Further, studies with a longer follow-up should investigate the late effects of vaccination on blood glucose levels in the weeks following the injection for side-effects that may be developed in longer time. In conclusion, data on CGM in patients with AD suggests that Sars-Cov2 vaccination is safe and that patients could detect a possible increase in glycaemic values but it is not clinically significant.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Maddaloni E, D’Onofrio L, Alessandri F, Mignogna C, Leto G, Pascarella G, et al. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: A multicentre retrospective study (CoViDiab II). Cardiovasc Diabetol 2020;19. https://doi.org/10.1186/s12933-020-01140-2. [DOI] [PMC free article] [PubMed]
- 2.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol 2020. https://doi.org/10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed]
- 3.D’Onofrio L., Pieralice S., Maddaloni E., Mignogna C., Sterpetti S., Coraggio L., et al. Effects of the COVID-19 lockdown on glycaemic control in subjects with type 2 diabetes: the glycalock study. Diabetes Obes Metab. 2021 doi: 10.1111/dom.14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maddaloni E, Coraggio L, Pieralice S, Carlone A, Pozzilli P, Buzzetti R. Effects of COVID-19 Lockdown on Glucose Control: Continuous Glucose Monitoring Data From People With Diabetes on Intensive Insulin Therapy. Diabetes Care 2020;43:e86–7. https://doi.org/10.2337/dc20-0954. [DOI] [PubMed]
- 5.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603–15. https://doi.org/10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed]
- 6.Danne T, Garg S, Peters AL, Buse JB, Mathieu C, Pettus JH, et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care 2019. https://doi.org/10.2337/dc18-2316. [DOI] [PMC free article] [PubMed]
- 7.Maddaloni E, D’Onofrio L, Alessandri F, Mignogna C, Leto G, Coraggio L, et al. Clinical features of patients with type 2 diabetes with and without Covid-19: A case control study (CoViDiab I). Diabetes Res Clin Pract 2020;169. https://doi.org/10.1016/j.diabres.2020.108454. [DOI] [PMC free article] [PubMed]
- 8.Israelsen S.B., Pottegård A., Sandholdt H., Madsbad S., Thomsen R.W., Benfield T. Comparable COVID-19 outcomes with current use of GLP-1 receptor agonists, DPP-4 inhibitors or SGLT-2 inhibitors among patients with diabetes who tested positive for SARS-CoV-2. Diabetes, Obes Metab. 2021;23:1397–1401. doi: 10.1111/dom.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]