Abstract
Background and aims
To systematically address all the relevant evidence of the association between high-density lipoprotein cholesterol (HDL-C) and COVID-19 infection.
Methods
We searched PubMed, PubMed Central and medRxiv databases (up to May 2021) for studies related to HDL-C and COVID-19 infection. A qualitative synthesis of published prospective and retrospective studies for the role of low HDL-C levels on COVID-19 infection severity was performed.
Results
Thirty-three studies (6 prospective, 27 retrospective) including 11,918 COVID-19 patients were eligible for the systematic review. Twelve studies compared HDL-C levels on admission in COVID-19 patients with healthy controls. In these 12 studies, COVID-19 patients had significantly lower HDL-C levels on admission compared with that of healthy controls. Twenty-eight studies observed the HDL-C levels among COVID-19 diagnosed patients, to establish the role of low HDL-C values in the prognosis of the infection. Twenty-four studies showed a correlation between low HDL-C levels with disease severity, while only 4 studies showed no association.
Conclusions
Low HDL-C levels should be added in the list of the others well-known risk factors for COVID-19 severity.
Keywords: COVID-19, SARS-COV-2, High-density lipoprotein cholesterol, HDL-C, Severity
Highlights
-
•
Patients with COVID-19 have lower HDL-C levels compared with healthy controls.
-
•
Low HDL-C levels consist a poor prognostic factor for COVID-19 severity.
-
•
HDL-C levels represent a cheap, well standardized, and easy to perform, biomarker.
-
•
Improving HDL-C functionality may ameliorate COVID-19 related complications.
-
•
Current evidence is limited by low quality studies.
1. Introduction
The coronavirus disease 2019 (COVID-19) is caused by a novel strain of coronavirus, named as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1]. The first case of a patient infected by the SARS-CoV-2, causing viral pneumonia, was presented in December 2019 in the city of Wuhan in China [2]. The World Health Organization (WHO) declared coronavirus disease a global pandemic in March 2020 and an international public health emergency issue. As of May 18th, 2021, there have been 163,312,429 confirmed cases of COVID-19, including 3,386,825 deaths worldwide [World Health Organization, Coronavirus disease (COVID-19) outbreak (https://covid19.who.int/accessed 18 May 2021)] [3].
The estimated mortality rate for COVID-19 is about 2.3% [4] among the confirmed cases. Until recently, studies have demonstrated that disease's worse prognosis is associated with patients' older age and comorbidity status [5]. However, studies have brought to the surface that other factors, including patients' laboratory data, can play an additional predictive role in COVID-19 prognosis [[5], [6], [7]]. In particular, low absolute count of lymphocytes, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), D-dimer levels, lactate dehydrogenase (LDH), troponin, ferritin and creatinine phosphokinase (CK) at onset are associated with disease progression and poor outcomes [5,6].
The majority of patients with mild to moderate disease have a better prognosis, than those who are classified as severe to critical cases, concerning mortality rates [8]. Since the COVID-19 has nowadays become a global threat to public health, an urge has been generated to interpret early patients' laboratory values and to identify those with severe disease and a poorer prognosis, in order to facilitate the appropriate supportive care and improve the recovery rate. As such, cheap and easy to perform biomarkers like lipid levels could be useful in clinical practice, especially in those settings where more sophisticated or expensive biomarkers cannot be performed, like in underdeveloped countries with poor resources. So far, little is known about the association between disease severity and lipid levels. Critical illness is well known to be accompanied by low levels of total cholesterol (TCHOL) and apolipoprotein B [9]. These alterations are attributed to increased production of certain cytokines and may suggest worse prognosis and increased mortality. Low high-density lipoprotein cholesterol (HDL-C) levels may be seen in several infectious diseases [9]. Of note, HDL-C levels can get rapidly decreased in septic conditions [10,11].
Our aim is to assess the role of HDL-C levels on COVID-19 infection in a systematic way.
2. Materials and methods
2.1. Literature search
We searched PubMed, PubMed Central and medRxiv databases up to 18th of May 2021 using the keywords (HDL-Cholesterol OR high-density lipoprotein cholesterol OR HDL) AND (COVID-19 OR SARS-COV-2 OR Coronavirus).
2.2. Study design
We performed a qualitative synthesis of published prospective or retrospective studies to evaluate whether low HDL-C levels are associated with COVID-19 infection severity.
2.3. Data extraction
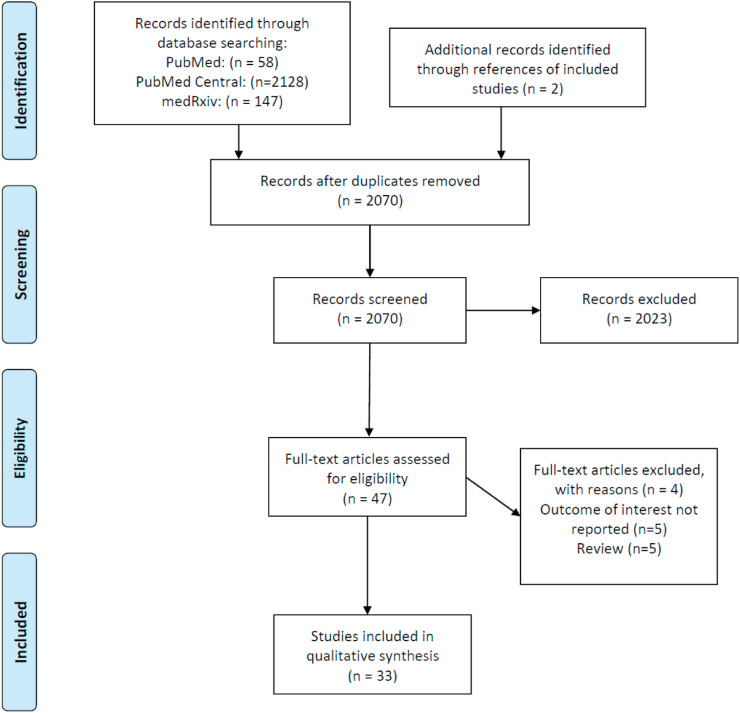
Studies were independently and thoroughly examined by two investigators (APA, AP) and studies' characteristics were extracted. Any discrepancy between the reviewers was resolved by consensus. For the review of our analysis, data extraction was performed with adherence to Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA model, Fig. 1) [12]. Original articles are included in the present systematic review. We additionally assessed the references of these articles, as well as reviews on the topic to identify for any missed relevant studies.
Fig. 1.
PRISMA flow diagram of articles related to High-Density Lipoprotein Cholesterol and COVID-19 infection.
3. Results
Thirty-three studies, with a total of 11,918 COVID-19 patients, were included in our systematic review (Table 1). Twenty-three of them were retrieved from PubMed database, 8 from medRxiv database, 1 from PubMed Central and 1 from Social Science Research Network (SSRN). The studies retrieved from medRxiv and SSRN are preprints and have not been peer-reviewed. Among all 33 studies, 27 were retrospective, while only 6 were conducted prospectively. Twelve studies compared the HDL-C levels on admission in COVID-19 patients with controls (Table 2). In all these 12 studies, COVID-19 patients had significantly lower HDL-C levels on admission compared with that of healthy controls. Twenty-eight studies observed the HDL-C levels among COVID-19 diagnosed patients, to establish any correlation between HDL-C values and prognosis of the disease (Table 3). Among them, 23 studies showed a negative correlation between HDL-C levels and disease severity, while only 1 study showed a positive correlation, and 4 studies showed no association.
Table 1.
Characteristics of all included studies.
| First author, Year, (PMID) | Country | Study Design | COVID-19 Patients (n) | Controls (n) | HDL-C levels (mg/dL) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Controls | COVID-19 | Mild | Severe | Critical | |||||
| Zhoo et al., 2021 (33833388) |
China | Retrospective | 268 | 42.5 (33.5–50.2) | 42.5 (34.7–50.2) | 34.7 (27.0–50.2) | |||
| Masana et al., 2021 (33785815) | Spain | Retrospective | 297 | 30.9 (24.7–40.1) | 33.9 (27.8–41.7) | 28.2 (22.8–37.8) | |||
| Hilser et al., 2021 (33667465) | USA | Retrospective | 968 | 3087 | 53 ± 19 | 50 ± 18 | |||
| Li et al., 2021 (33639119) |
China | Retrospective | 424 | 34.8 (30.9–42.5) | 34.8 (30.9–42.5) Survivors | 30.9 (23.2–34.8) Deceased |
|||
| Huang et al., 2021 (33571404) |
China | Retrospective | 218 | 76 | 58.8 ± 21.3 | 39.4 ± 10.8 | 44.5 ± 10.4 | 32.1 ± 64.6 | |
| Turgay Yildirim et al., 2021 (33524862) | Turkey | Retrospective | 139 | 44.0 (32.5–77.0) Survivors |
28.5 (21.5–32.0) Deceased |
||||
| Begue et al., 2021 (33504824) |
France | Prospective | 8 | 16 | 56.5 (51.8–65.4) | 29.8 (19–32.1) | |||
| Salari et al., 2021 (33449333) |
Iran | Retrospective | 250 | 35.00 ± 13.00 Survivors |
35.00 ± 19.00 Deceased |
||||
| Sun et al., 2021 (33344516) |
China | Prospective | 99 | 45.6 (38.7–54.9) | 36.4 (28.6–43.3) | ||||
| Ouyang et al., 2020 (33308159) |
China | Retrospective | 107 | 41.4 Survivors |
27.1 Deceased |
||||
| Qin et al., 2020 (33282265) |
China | Retrospective | 248 | 34.4 ± 14.3 | 33.3 ± 12 | 39.8 ± 19 | |||
| Zhang et al., 2020 (33122929) |
China | Retrospective | 98 | 38.7 (30.9–42.5) Non -ICU |
30.9 (19.3–38.7) ICU |
||||
| 34.8 (30.9–42.5) Survivors |
27 (19.3–38.7) Deceased |
||||||||
| Fan et al., 2020 (32320740) |
China | Retrospective | 21 | 31 | 51.4 (38.7–65.7) | 42.5 (30.9–54.1) | 42.5 (30.9–54.1) | 34.8 (30.9–34.8) | |
| Wei et al., 2020 (32430154) |
China | Retrospective | 597 | 50 | 52 (40–65) | 49 (41–58) | 50 (42–59) | 50 (41–59) | 36 (29–43) |
| Huang et al., 2020 (32567003) |
China | Retrospective | 2623 | – | 36.7 (30.9–44.9) | 33.3 (28.2–40.6) | 29.8 (21.7–35.6) Deceased |
||
| Hu et al., 2020 (32653486) |
China | Retrospective | 114 | 80 | 49.1 (46.8–54.5) | 41.8 (36–41.8) | 46.8 (39.4–57.2) | 39 (34–46.4) | |
| Wang et al., 2020 (32677918) |
China | Retrospective | 143 | – | 34.8 (30.9–46.4) | 42.5 (34.8–50.2) | 34.8 (27–38.7) | ||
| Ressaire et al., 2020 (32866665) |
France | Retrospective | 31 | – | 27.8 (23.6–36) | 32.9 (18.2–50.3) | 25.9 (24–42.5) | ||
| Wang et al., 2020 (32892746) |
China | Retrospective | 228 | 1140 | 53 (47.2–58.4) | 30.2 (25.5–37.5) | 30.6 (26.7–37.5) | 26.7 (22.8–36.7) | |
| Tanaka et al., 2020 (32970772) |
France | Prospective | 48 | – | 27 (19.3–34.8) | ||||
| Zhang et al., 2020 (32636061) | China | Retrospective | 74 | – | 39.8 (33.3–48.3) | 41.8 (37.1–49.5) | 35.6 (28.6–46.4) | ||
| Xue et al., 2020 (33045571) |
China | Retrospective | 114 | 37.9 (30.2–46) | 40.2 (30.9–48) | 34 (28.6–44) | |||
| Han et al., 2020 (33028689) |
China | Retrospective | 52 | 48 (36.7–56) | 32.9 (29.4–45.6) | ||||
| Malik et al., 2021 |
Pakistan | Prospective | 1067 | 688 | 47 (36–52) | 45 (37–50) | 46 (41–50) | 40 (37–46) | |
| Peng et. Al, 2020 |
China | Retrospective | 861 | 1108 | 58.5 ± 12.4 | 46.3 ± 12.5 | 50.7 ± 11.7 | 43.1 ± 11.6 | 34.3 ± 9.3 |
| Nie et al., 2020 |
China | Retrospective | 97 | – | 40.6 (36–58) | 34 (31.3–42.5) | |||
| Aparisi et al., 2020 |
Spain | Retrospective | 654 | – | Before admission 51.6 (41.7–62.1) |
Survivors 52.6 (43–64) |
Non-survivors 47.2 (37.5–60) |
||
| Admission 34 (28–43) |
34 (28.3–43) | 33.5 (27–44) | |||||||
| 7th day 33 (26.5–41.6) |
34 (28–41.8) | 27 (19.1–37) | |||||||
| Osuna-Ramos et al., 2020 |
Mexico | Prospective | 102 | 30 | 41.43 ± 15.01 | 29.9 ± 12.19 | 30 (22–36.5) | 26 (16–43) | |
| Rigatti et al., 2020 |
United States | Retrospective | 1520 | 48505 | 56.1 (46.2–67.9) | 55 (46.2–65.7) | |||
| Zhang et al., 2020 |
China | Retrospective | 43 | – | 41.8 ± 9.3 | 42.5 ± 7.7 | |||
| Chen et al., 2020 |
China | Retrospective | 291 | – | 30.2 (25.5–37.1) | 30.6 (26.7–37.5) | 25.9 (22.4–36.4) | ||
| Hu et al., 2020 |
China | Prospective | 71 | 80 | 56.8 ± 1.2 | 45.6 ± 1.2 | |||
| Alcántara-Alonso et al., 2021 |
Mexico | Retrospective | 43 | 29.5 (10.4–76.5) | 39.9 (20.1–51.6) | 27.6 (19.3–63.5) | 22.15(10.4–56.5) | ||
COVID-19: coronavirus disease 2019; HDL-C: High-Density Lipoprotein Cholesterol; ICU: Intensive Care Unit.
Table 2.
Characteristics of the included studies using control group.
| First author, Year |
Country | Study Design | COVID-19 Patients (n) | Controls (n) | HDL-C levels (mg/dL) On admission |
Statistical Significance between groups | |
|---|---|---|---|---|---|---|---|
| Controls | COVID-19 | ||||||
| Hilser et al., 2021 | USA | Retrospective | 968 | 3087 | 53 ± 19 | 50 ± 18 | p < 0.001 |
| Huang et al., 2021 |
China | Retrospective | 218 | 76 | 58.8 ± 21.3 | 39.4 ± 10.8 | p < 0.05 |
| Begue et al., 2021 |
France | Prospective | 8 | 16 | 56.5 (51.8–65.4) | 29.8 (19–32.1) | p < 0.0001 |
| Fan et al., 2020 | China | Retrospective | 21 | 31 | 51.4 (38.7–65.7) | 42.5 (30.9–54.1) | p < 0.05 |
| Wei et al., 2020 | China | Retrospective | 597 | 50 | 52 (40–65) | 49 (41–58) | p < 0.05 |
| Hu et al., 2020 |
China | Retrospective | 114 | 80 | 49.1 (46.8–54.5) | 41.8 (36–41.8) | p < 0.001 |
| Malik et al., 2021 |
Pakistan | Prospective | 1067 | 688 | 47 (36–52) | NA | p = 0.047 |
| Wang et al., 2020 |
China | Retrospective | 228 | 1140 | 53 (47.2–58.4) | 30.2 (25.5–37.5) | p < 0.001 |
| Peng et. Al, 2020 |
China | Retrospective | 861 | 1108 | 58.5 ± 12.4 | 46.3 ± 12.5 | p < 0.05 |
| Osuna-Ramos et al., 2020 | Mexico | Prospective | 102 | 30 | 41.43 ± 15.01 | 29.9 ± 12.19 | p = 0.0002 |
| Rigatti et al., 2020 |
United States | Retrospective | 1520 | 48505 | 56.1 (46.2–67.9) | 55 (46.2–65.7) | p = 0.014 |
| Hu et al., 2020 |
China | Prospective | 71 | 80 | 56.8 ± 1.2 | 45.6 ± 1.2 | p < 0.001 |
COVID-19: coronavirus disease 2019; HDL-C: High-Density Lipoprotein Cholesterol; NA: Not Available.
Table 3.
Characteristics of the included studies evaluating COVID-19 severity.
| First author, Year | Country | Study Design | COVID-19 Patients (n) | HDL-C levels (mg/dL) |
COVID-19 Severity Correlation |
||
|---|---|---|---|---|---|---|---|
| Mild | Severe | Critical | |||||
| Zhoo et al., 2021 | China | Retrospective | 268 | 42.5 (34.7–50.2) | 34.7 (27.0–50.2) | p = 0.03 | |
| Masana et al., 2021 | Spain | Retrospective | 297 | 33.9 (27.8–41.7) | 28.2 (22.8–37.8) | p < 0.001 | |
| Li et al., 2021 | China | Retrospective | 424 | 34.8 (30.9–42.5) Survivors | 30.9 (23.2–34.8) Deceased | p = 0.001 | |
| Huang et al., 2021 | China | Retrospective | 218 | 44.5 ± 10.4 | 32.1 ± 64.6 | p < 0.05 | |
| Turgay Yildirim et al., 2021 | Turkey | Retrospective | 139 | 44.0 (32.5–77.0) Survivors |
28.5 (21.5–32.0) Deceased |
p < 0.001 | |
| Salari et al., 2021 | Iran | Retrospective | 250 | 35.00 ± 13.00 Survivors |
35.00 ± 19.00 Deceased |
p = NS | |
| Sun et al., 2021 | China | Prospective | 99 | 45.6 (38.7–54.9) | 36.4 (28.6–43.3) | p < 0.001 | |
| Ouyang et al., 2020 | China | Retrospective | 107 | 41.4 Survivors |
27.1 Deceased |
P = 0.006 | |
| Qin et al., 2020 | China | Retrospective | 248 | 33.3 ± 12 | 39.8 ± 19 | p < 0.001 | |
| Zhang et al., 2020 | China | Retrospective | 98 | 38.7 (30.9–42.5) Non -ICU |
30.9 (19.3–38.7) ICU |
p = 0.007 | |
| 34.8 (30.9–42.5) Survivors |
27 (19.3–38.7) Deceased |
p = 0.001 | |||||
| Fan et al., 2020 | China | Retrospective | 21 | 42.5 (30.9–54.1) | 34.8 (30.9–34.8) | p = 0.03 | |
| Wei et al., 2020 | China | Retrospective | 597 | 50 (42–59) | 50 (41–59) | 36 (29–43) | p < 0.05 |
| Huang et al., 2020 | China | Retrospective | 2623 | 36.7 (30.9–44.9) | 33.3 (28.2–40.6) | 29.8 (21.7–35.6) Deceased |
p < 0.001 |
| Zhang et al., 2020 | China | Retrospective | 74 | 41.8 (37.1–49.5) | 35.6 (28.6–46.4) | p < 0.05 | |
| Xue et al., 2020 | China | Retrospective | 114 | 40.2 (30.9–48) | 34 (28.6–44) | p = 0.017 | |
| Han et al., 2020 | China | Retrospective | 52 | 48 (36.7–56) | 32.9 (29.4–45.6) | p = 0.002 | |
| Hu et al., 2020 | China | Retrospective | 114 | 46.8 (39.4–57.2) | 39 (34–46.4) | p < 0.001 | |
| Wang et al., 2020 | China | Retrospective | 143 | 42.5 (34.8–50.2) | 34.8 (27–38.7) | p < 0.05 | |
| Ressaire et al., 2020 | France | Retrospective | 31 | 32.9 (18.2–50.3) | 25.9 (24–42.5) | p = NS | |
| Wang et al., 2020 | China | Retrospective | 228 | 30.6 (26.7–37.5) | 26.7 (22.8–36.7) | p < 0.001 | |
| Malik et al., 2021 | Pakistan | Prospective | 1067 | 45 (37–50) | 46 (41–50) | 40 (37–46) | p < 0.01 |
| Peng et. Al, 2020 | China | Retrospective | 861 | 46.3 ± 12.5 | 50.7 ± 11.7 | 43.1 ± 11.6 | p < 0.05 |
| Nie et al., 2020 | China | Retrospective | 97 | 40.6 (36–58) | 34 (31.3–42.5) | p < 0.05 | |
| Aparisi et al., 2020 | Spain | Retrospective | 654 | 34 (28–41.8) | 27 (19.1–37) | p = 0.011 | |
| Zhang et al., 2020 | China | Retrospective | 43 | 41.8 ± 9.3 | 42.5 ± 7.7 | p = NS | |
| Chen et al., 2020 | China | Retrospective | 291 | 30.6 (26.7–37.5) | 25.9 (22.4–36.4) | p = 0.009 | |
| Osuna-Ramos et al., 2020 | Mexico | Prospective | 102 | 30 (22–36.5) | 26 (16–43) | p = NS | |
| Alcántara-Alonso et al., 2021 | Mexico | Retrospective | 43 | 39.9 (20.1–51.6) | 27.6 (19.3–63.5) | 22.15 (10.4–56.5) | p = 0.018 |
COVID-19: coronavirus disease 2019; HDL-C: High-Density Lipoprotein Cholesterol; ICU: Intensive Care Unit; NS: non-significant.
3.1. PubMed studies
3.1.1. Prospective
In an observational cohort study, the association of lipid profile with disease severity and mortality in 99 COVID-19 patients was assessed [13]. The authors found that HDL-C and apoA1 levels were significantly lower in the severe disease group, with mortality cases showing the lowest levels (p < 0.0001). Furthermore, HDL-C (OR: 0.643, 95% CI: 0.456–0.906, p = 0.012) and apoA1 levels (OR: 0.651, 95% CI: 0.456–0.929, p = 0.018) were independently associated with disease severity. Notably, apoA1 and HDL-C levels were negatively correlated with both admission levels and highest concentrations of CRP and interleukin-6, indicating a strong association with the inflammatory status [13].
Tanaka et al. study was conducted among 48 ICU COVID-19 positive patients [14]. Upon admission, HDL-C levels were low [27.1 mg/dL (19.3–34.8)], but throughout disease improvement, HDL-C levels during the 28-study period increased compared with admission (p = 0.024). The HDL levels also returned within their normal reference levels in survivors' group. Importantly however, there was no relationship between HDL-C and LDL-C values with mortality (log-rank p = 0.554 and p = 0.083, respectively). In addition, no differences in HDL-C and LDL-C levels between survivors and non-survivors were observed over time [14].
In a monocentric study conducted in a surgical intensive care unit (ICU) in France, 8 patients admitted for sepsis due to COVID-19 were enrolled [15]. HDL-C levels of these patients were significantly lower on admission [29.8 mg/dL (18.9–32.1)] compared with that of 16 control healthy subjects [56.5 mg/dL (51.8–65.4)]. Regarding the rest lipid profile, TCHOL, LDL-C and apoA1 levels were lower (p = 0.0016, p = 0.0081 and p < 0.0001, respectively) and triglyceride levels were higher (p = 0.0152) in severe COVID-19 ICU patients when compared with controls [15].
3.1.2. Retrospective
In a population-based study from Hong Kong, among 268 COVID-19 patients, 43 met the primary outcome (composite intensive care unit admission, need for intubation or death) [16]. Those patients had significantly lower HDL-C levels [34.7 mg/dL (27.0–50.2)] compared with that of 225 non-severe cases [42.5 mg/dL (34.7–50.2)], (p = 0.03). Similar results regarding LDL-C were observed (p < 0.0001) [16].
In a cross-sectional retrospective study, the lipid profile during hospitalization was observed [17]. Among 297 COVID-19 patients, HDL-C levels were significantly higher in 149 patients with mild disease [33.9 mg/dL (27.8–41.7)] compared with that of 148 patients with severe disease [28.2 mg/dL (22.8–37.8)], (p < 0.001). On the contrary, triglyceride levels were significantly higher in patients with severe disease [171.5 mg/dL (122.9–254.6)] compared with patients with mild disease [142.3 mg/dL (99.9–192.7)], (p < 0.001). The authors concluded that severe outcome was associated with lower HDL-C and higher triglyceride levels [17].
A recent study, using data from the UK Biobank, investigated the relationship between severity of COVID-19 and HDL-C levels [18]. Among included cases, 968 COVID-19 patients had significantly lower HDL-C levels (50 ± 18 mg/dL) compared with that of 3087 unmatched hospital-based controls C (53 ± 19 mg/dL), (p < 0.001). Of importance, a 10 mg/dl increase in serum HDL-C levels was associated with significantly (p = 0.0004) reduced risk of SARS-CoV-2 infection [odds ratio (OR), 0.90; 95% CI), 0.85–0.95], identifying HDL-C levels as a clinical risk factor for SARS-CoV-2 infection [18].
A retrospective, single-center, observational study included 424 patients with severe COVID-19 infection (390 survivors, 34 non-survivors) [19]. HDL-C levels were significantly lower on admission in non-survivors [30.9 mg/dL (23.2–34.8)] compared with that of survivors group [34.8 mg/dL (30.9–42.5)], (p = 0.001). Similar results were observed regarding apoA1 levels. The authors concluded that low concentrations of HDL-C [AUC: 0.33 (95% CI:0.23–0.43, p = 0.001) and apoA1 levels [AUC: 0.28 (95% CI: 0.20–0.36, p < 0.001)] at admission were significantly associated with increased disease severity [19].
In a cross-sectional retrospective study, Huang et al. recruited 86 severe COVID-19 patients, 132 non-severe COVID-19 patients and 76 healthy individuals as control group [20]. Serum HDL-C levels in COVID-19 group (39.5 ± 10.8 mg/dL) were significantly lower compared with the control group (58.8 ± 21.3 mg/dL), (p < 0.05). Notably, HDL-C levels in patients with severe COVID-19 (32.1 ± 64.6 mg/dL) were significantly lower than that in non-severe COVID-19 patients (44.5 ± 10.5 mg/dL), (p < 0.05). The authors concluded that HDL-C represents a poor prognostic factor in COVID-19 disease severity [20].
In another retrospective study conducted in Turkey, the atherogenic index of plasma (AIP), calculated as the base 10 logarithm of the triglyceride to HDL-C ratio, was found to consist an early biomarker to predict pneumonia, intubation and intensive care need in 139 COVID-19 patients [21]. Among them, 26 patients died. The AIP in the deceased group was significantly higher compared with that in the survivor group (p < 0.001). Of note, the deceased group had more patients with hypertension (p = 0.006), diabetes mellitus (p = 0.002), cardiovascular diseases (p = 0.001) and chronic renal failure (p = 0.003) compared with the survivor group [21]. Regarding lipid profile, TCHOL, HDL-C and LDL-C levels were lower (p = 0.004, p < 0.001, p < 0.001, respectively) and triglyceride levels were higher (p < 0.001) in deceased patients when compared with survivors. More specific, HDL-C levels in deceased patients were 28.5 (21.5–32.0) mg/dL while in survivors were significantly higher 44.0 (32.5–77.0) mg/dL, (p < 0.001) [21].
Among 107 COVID-19 inpatients that were included in a retrospective analysis, 82 survived while 25 died [22]. Although no difference was observed in HDL-C levels on admission between survivors and non-survivors (36.7 vs 33.3 mg/dL, p = 0.323), on their last tests this difference became profound (41.4 vs 30.6 mg/dL, p = 0.006) [22].
A retrospective case-control study conducted in Iran included 250 COVID-19 patients [23]. No significant difference on HDL-C levels was observed between 103 deceased and 147 survived patients. Of note, higher levels of TCHOL [Odds ratio (OR) = 2.55; 95% CI = 1.19–5.45] and LDL (OR = 2.27; 95% CI = 1.07–4.79) as well as elevated triglycerides (OR = 5.14; 95% CI = 2.28–11.56), were identified as independent risk factors of COVID-19 mortality, while no significant association was detected regarding HDL-C [23].
Qin et al., observed the lipid profiles and their alterations in hospitalized patients with COVID-19 pneumonia in 248 patients [24]. In this retrospective study, the authors reported that HDL-C levels were significantly higher in the 74 severe cases (39.8 ± 18.9 mg/dL) compared with 174 common cases on admission (33.3 ± 12.0 mg/dL) (p < 0.001). Notably, among 68 severe cases, HDL-C levels were lower in 33 patients who needed hospitalization for more than 29 days compared with the 35 severe cases that needed hospitalization for less than 29 days (p < 0.05). Regarding LDL-C, common cases exhibited lower levels (85.1 ± 35.2 mg/dL) compared with that of severe cases (77.0 ± 29.0 mg/dL), (p = 0.024). The authors concluded that an increase in LDL-C and HDL-C during hospitalization of severe COVID-19 cases seems to predict disease recovery [24].
Fan et al. study included 21 COVID-19 infected patients [25]. Comparison was made between them and a control group, which included 31 healthy non-COVID-19 individuals. While HDL-C in healthy subjects ranged among normal levels [54.1 mg/dL (38.7–65.8)], COVID-19 subjects' HDL-C levels followed a significant decrease [pre-infection: 54.1 mg/dL (38.7–69.6); on admission: 42.5 mg/dL (30.9–54.1), p = 0.03], and remained low during disease's progression [46.4 mg/dL (38.7–54.1) and on discharge [38.7 mg/dL (38.7–54.1)]. Likewise, HDL-C levels among patients who passed away (n = 4) decreased steadily until death [on admission: 34.8 mg/dL (30.9–34.8), disease progression: 46.4 mg/dL (34.8–38.7), death: 23.2 mg/dL (19.3–27.1)] [25].
In another study, a total of 98 COVID-19 positive patients were enrolled and divided to ICU/non-ICU and survivors/non-survivors groups [26]. Disease severity was associated with decreased HDL-C levels, since the 46 patients in the non-ICU group had significantly lower HDL-C levels [38.7 (30.9–42.5) mg/dL] compared with the 52 patients in the ICU group [HDL-C = 30.9 (19.3–38.7) mg/dL, p < 0.05]. Similarly, disease mortality was associated with decreased HDL-C levels, since the 62 survivors had significantly higher HDL-C levels [34.8 (30.9–42.5) mg/dL] compared with the 36 non-survivors [HDL-C = 27.1 (19.3–38.7) mg/dL, p < 0.05]. All in all, the authors concluded that the TG/HDL-C ratio, was independently correlated with myocardial injury, heart failure, disease severity (needing ICU treatment), and mortality in COVID-19 patients [26].
Another study compared the laboratory findings of 50 healthy subjects with those of 597 COVID-19 positive patients, among which, 171 were severe and 32 referred as critical cases on admission [27]. COVID-19 cases presented with significantly decreased HDL values [49 mg/dL (41–58)] on admission compared with the control group [52 mg/dL (40–65), p < 0.05]. Notably, HDL-C levels were significantly decreased among critical cases of the COVID-19 cohort on admission [36 mg/dL (29, 43)] compared with the HDL-C levels of the healthy subjects [52 mg/dL (40, 65)], of the mild cases [50 mg/dL (42, 59)] and of the severe ones [HDL-C = 50 mg/dL (41, 59). Regarding LDL-C and TCHOL levels, COVID-19 patients had significantly lower levels on admission when compared with normal subjects (p < 0.001). In addition, there were significant and gradual decreases in LDL-C (p < 0.02) and TCHOL (p < 0.05) levels [mild: 91 mg/dL (76, 104), 173 mg/dL (148, 203), severe: 86 mg/dL (69, 102), 167 mg/dL (138, 197); critical: 69 mg/dL (48, 81), 125 mg/dL (95, 162), respectively, across the three groups. Fatality rate was noted in 2.2% (n = 13) (21). The authors concluded that as lipid profile progressively becomes worse, so does the disease severity [27].
Hu et al. collected laboratory values from 114 positive COVID-19 patients on admission and compared them with 80 age-matched healthy controls [28]. Compared with the control group [49.1 mg/dL (46.8–54.5)], infected patients presented with significantly lower HDL-C levels [41.8 mg/dL (36.0–41.8), p < 0.001]. Notably, HDL-C levels among severe COVID-19 cases [39.1 mg/dL (34.0–46.4)] were also lower when compared with the mild group [46.8 mg/dL (39.4–57.2), p < 0.001]. The authors concluded that decreased serum HDL-C is associated with COVID-19 infection severity [28].
In Huang et al. study, data from 2623 clinically confirmed adult COVID-19 patients was extracted, among which, 2008 (76.6%) were diagnosed as non-critically and 615 (23.4%) as critically ill patients [29]. Median HDL-C levels were remarkably lower among the critically ill group [33.3 mg/dL (0.73–1.05)] compared with the non-critical one [36.7 mg/dL (30.9–44.9), p < 0.001], while the death group presented with the lowest HDL values [29.8 mg/dL (21.7–35.6)] [29].
In Wang et al. study, a total of 143 COVID-19 positive patients were enrolled and divided to mild/moderate and severe/critical groups [30]. Disease severity was associated with decreased HDL-C levels (r = −0.362, p = 0.000), since the 71 patients in the severe/critical group had significantly lower HDL-C levels [34.8 mg/dL (27.1–38.7)] compared with the 72 patients in the mild/moderate group [42.5 mg/dL (34.8–50.3), p = 0.000] [30].
Ressaire et al. examined the lipid profile of 31 out of 54 COVID-19 patients admitted to the ICU [31]. On admission, total patients' HDL-C levels were lower than the normal reference [27.8 mg/dL (23.6–40.0)]. Notably, among those, non-survivors (n = 7) had lower values of HDL levels [25.9 mg/dL (24.0–42.5)] compared with the 24 patients in the survivor group [32.9 mg/dL (18.2–50.3), p < 0.28]. Although no significant difference on HDL-C levels was observed, LDL-C and TCHOL levels were significantly lower in non-survivors compared with survivors (p = 0.004 and p = 0.007, respectively) [31].
Wang et al. compared HDL-C levels of 1140 healthy individuals with 228 confirmed COVID-19 cases, as well as levels between severe and non-severe COVID-19 patients [32]. Baseline median HDL-C levels upon admission of all COVID-19 patients was significantly lower [30.2 mg/dL (25.5–37.5), p < 0.001] in comparison with the control group [53.0 mg/dL (47.2–58.4)]. Of note, severe COVID-19 cases also presented with significantly lower median levels of HDL [26.7 mg/dL (22.8–36.7)], than the non-severe cases [30.6 (26.7–37.5), p = 0.032]. Thus, the authors concluded that decreased serum HDL-C were associated with COVID-19 disease severity [32].
Zhang et al. included 74 diabetic patients who were diagnosed with COVID-19 infection. Among those, 47 (63.5%) were characterized as non-severe and 27 (36.5%) as severe cases [33]. While the HDL-C levels of all COVID-19 patients was not among normal values [39.8 mg/dL (32.3–48.3)], mean HDL-C levels of severe cases were statistically lower [HDL-C = 35.6 mg/dL (28.6–46.4)] than that of the non-severe ones [41.8 mg/dL (37.1–49.5), p < 0.021] [33].
Xue et al. included 114 patients with positive serology COVID-19 test [34]. Out of these, 58 patients were severe cases (including 5 cases of critically ill patients) and 56 mild to moderate cases. Mean HDL-C levels of COVID-19 patients was low [37.9 mg/dL (30.2–46.0)]. Severe cases and critically ill patients had significantly lower HDL-C levels [34.0 mg/dL (29.0–44.1] compared with the mild to moderate cases [40.2 mg/dL (30.9–48.0), p = 0.017] (29). This study additionally suggested that beyond the lipid profile, novel biomarkers such as high sensitivity C-reactive protein-prealbumin ratio (HsCPAR), high sensitivity C-reactive protein-albumin ratio (HsCAR) and prognostic nutritional index (PNI) may serve as accurate predictors of disease severity [34].
Han et al. collected samples from 52 COVID-19 positive individuals, divided them into symptomatic and asymptomatic groups [35]. Concerning HDL-C levels, while asymptomatic patients had their values among a normal range [48.0 mg/dL (36.7–56.1)], a great decrease was observed among symptomatic ones [32.9 mg/dL (29.4–45.6)], p = 0.002]. These results, in combination with other biomarkers and panels, suggested that asymptomatic COVID-19 individuals presented with normal clinical indicators, whereas the symptomatic ones were not [35].
3.2. Other databases
Similar results were shown in the 8 studies that were retrieved from medRxiv database [[36], [37], [38], [39], [40], [41], [42], [43]], the 1 from PubMed Central [44] and the 1 from Social Science Research Network (SSRN) [45]. The studies retrieved from medRxiv and SSRN are preprints and have not been peer-reviewed. The results of the above studies are summarized in Table 1, Table 2, Table 3.
In brief, in a recent prospective study that included 1067 COVID-19 patients (mild: 319, moderate: 391, critical: 357) and 688 healthy adults as controls, the authors suggested that HDL-C consist a predictor of COVID-19 progression severity [AUC: 0.448 (95% CI: 0.301–0.353, p < 0.01)], as well as an independent risk factor for COVID-19 [B: 0.143 ± 0.029, p < 0.01) [36].
In another study that included 97 COVID-19 hospitalized patients, Nie et al. found that apart from HDL-C (r = −0.332, p = 0.024), the severity of COVID-19 was also negatively correlated with serum total protein (r = −0.422, p = 0.004), serum albumin (r = −0.351, p = 0.017) and ApoA1 (r = −0.325, p = 0.028) [39].
4. Discussion
This systematic review focuses on the association of HDL-C with COVID-19 infection. Our results suggest that low HDL-C levels consist a poor prognostic factor for COVID-19 severity.
Several well-known poor prognostic factors for COVID-19 infection, such as low absolute lymphocyte count and high levels of C-reactive protein (CRP), D-dimers, troponin, lactate dehydrogenase (LDH), creatine kinase (CK), and ferritin have been previously reported. Additionally, more sophisticated biomarkers such as several interleukin levels are of prognostic value [46]. We now present the evidence regarding HDL-C levels in a systematic way, since it represents a biomarker easy to perform across the universe, is well standardized and is cheap compared to many of the previously reported biomarkers, which are not easy to perform especially in poor resource settings.
A recent analysis that used data of 317,306 (171,466 women) participants from the UK Biobank showed that high HDL-C levels were associated with a lower risk of hospitalization due to COVID-19 infection [47]. Similarly, data from 9005 UK Biobank participants (1508 positive for COVID-19 infection) showed that elevated plasma HDL-C, as well as elevated ApoA1 levels, were associated with a reduced odds of testing positive for SARS-CoV-2 (OR = 0.845, 95% CI = 0.788–0.907) [48]. Moreover, in another genetic and exposure analysis that included 7362 subjects from the UK Biobank (1485 positive for COVID-19 infection) COVID-19 participants had lower HDL-C levels compared with non-COVID-19 participants [49]. In addition, a significant correlation between HDL and genetic factors (single-nucleotide polymorphisms) was observed [49].
Low HDL-C levels may be attributed to inherited disorders or secondary causes [9]. Inherited forms include familial hypoalphalipoproteinemia, Tangier disease or Lecithine:cholesterol acylotransferase (LCAT) deficiency, while secondary causes of low HDL-C levels include cigarette smoking, obesity, low-fat diet, hyperthyroidism, diabetes, chronic kidney disease, malignancies, paraproteinemias, several drugs and infections [9,50].
Regarding infections, low HDL-C levels may be attributed to several infectious diseases, such as visceral leishmaniasis [51], severe leptospirosis [52], acute brucellosis [53], pulmonary tuberculosis [54], tick-borne encephalitis [55], as wells as chronic hepatitis B and C [56,57]. Furthermore, HDL-C levels can get rapidly decreased in septic conditions [10,11].
The mechanisms by which COVID-19 infection reduces HDL-C levels, as well as HDL functionality are not clearly defined. It seems that the “cytokine storm” underlying COVID-19 infection produces immune-mediated inflammatory dyslipoproteinemia, leading to low HDL-C levels [58]. In particular, elevated levels of interleukin-6 and other cytokines in COVID-19 infection may inhibit the apolipoprotein AI synthesis, which results to decreased HDL-C levels. In addition, the “cytokine storm” increases the activity of secretory phospholipase A2 (sPLA2) and endothelial cell lipase, enzymes that metabolize key HDL constituents and eventually leading to decreased HDL-C levels [59]. Furthermore, low HDL-C levels can be partially explained by the increased hepatic production of serum amyloid A (SAA) levels during inflammation [58]. SAA can displace apolipoprotein AI and AII from the HDL surface to produce an SAA-enriched HDL, which is catabolized at a faster rate compared to the normal HDL particle. Moreover, various cytokines during inflammation, decrease the activity of lipoprotein lipase (LPL), thus reducing peripheral clearance of very low density lipoproteins (VLDLs) [59]. This results in elevation of triglycerides and triglyceride-rich lipoproteins (such as VLDLs) leading to an increase of triglyceride content within HDL particles. The triglyceride-rich HDLs are then hydrolysed by the hepatic lipase and the LPL producing small HDLs. Small HDLs lead to increased breakdown of HDL, resulting in decreased HDL-C levels [59]. Of importance, initial COVID-19 data have even indicated that healthy infected individuals display abnormal liver function tests, suggesting a possible direct implication of SARS-CoV-2 in liver damage [30]. As a result, liver dysfunction itself is associated with low to very low HDL-C levels, which, according to recent data, is strongly associated with mortality [60]. The above findings suggest that HDL protein composition and function are altered in COVID-19 patients, implying that not only quantity but also quality of HDL matters [61,62]. Notably, shotgun proteomics was performed on HDL fractions and their endothelial protective effect was tested on human umbilical vein endothelial cells [15]. Study results showed that HDL particles from ICU severe COVID-19 patients were characterized by quantitative and qualitative abnormalities compared to those isolated from healthy subjects [15]. Those dysfunctional HDL particles were characterized by a loss of protective effect towards endothelial cells in inflammatory conditions [63]. These findings could play a role also in the treatment of the acute phase of COVID-19 infection [64]. Proposed interventions to either improve HDL functionality, such as increasing LCAT activity mentioned above, or replenishment with functional HDL or relevant apolipoproteins, such as ApoA-I mimetic peptides could be beneficial for the management of SARSCoV-2 related complications [58]. Interestingly, a recent study in 115 COVID-19 patients with SARS-COV-2 nucleic acid positive time exceeding 14 days showed that HDL-C reduction was an independent risk factor of the prolonged nucleic acid turning negative time, suggesting that HDL-C plays a role in virus clearance from the human body [65].
Importantly, HDLs have been shown to be a part of the innate immune system [59]. HDL can buffer toxic materials derived from infection and inflammation by moderating the inflammatory burst. We suggest that this protective mechanism is minimized with the low HDL-C concentrations we find in COVID-19. Hence, low HDL levels are not only a side effect of inflammation and infection, but also contribute to poor disease progression.
Several limitations are present in this systematic review. Most of the studies are of low methodological quality, with the majority being retrospective and a significant percentage of them been reported without peer review process. Small sample sizes, no clear assessing for confounders and heterogeneous reporting further limit the quality of the evidence base. Finally, we were not able to perform a meta-analysis as metrics such as OR were not reported in the vast majority of the studies.
5. Conclusion
After taking the above into consideration, it is clear that HDL-C changes are not only a marker of nutritional status or hypercatabolism but represent a strong and reliable inflammation biomarker and may play a role in the management of COVID-19 infected patients. Large-scale clinical studies are needed to reach more robust conclusions regarding the clinical significance of low HDL-C levels as a predictor of COVID-19 severity. Future studies should consider including lipid profile in COVID-19 infected patients. In addition, these studies should also address if there are common features involved in the pathogenesis of low HDL-C in COVID-19 infection, as little is known in this field. Although promising, there is uncertainty if low HDL-C levels represent a marker of COVID-19 severity based on the existing evidence. However, we strongly suggest that COVID-19 patients may benefit from monitoring more closely their lipid profile.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
Agouridis AP conceptualized and designed the study, participated in data acquisition, extraction and interpretation, prepared tables, wrote and drafted the initial manuscript and approved the final manuscript as submitted; Pagkali A and Rizos EC participated in data analysis and interpretation, reviewed and revised the manuscript and approved the final manuscript as submitted; Zintzaras E and Ntzani EE verified the analytical methods, performed all the relevant calculations and technical details of the systematic review, reviewed and revised the initial manuscript and approved the final manuscript as submitted;
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
Dr. Aris P. Agouridis would like to acknowledge the training he received in the Postgraduate Programme (MSc) “Research Methodology in Biomedicine, Biostatistics and Clinical Bioinformatics at University of Thessaly”.
References
- 1.Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L., et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J. 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Coronavirus disease (COVID-19) outbreak. https://covid19.who.int/ Available online. (18 May)
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Li S., Liu J., Liang B., Wang X., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh K., Mittal S., Gollapudi S., Butzmann A., Kumar J., et al. A meta-analysis of SARS-CoV-2 patients identifies the combinatorial significance of D-dimer, C-reactive protein, lymphocyte, and neutrophil values as a predictor of disease severity. Int J Lab Hematol. 2021;43:324–328. doi: 10.1111/ijlh.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. 2020. 41. 145,151. [DOI]
- 9.Moutzouri E., Elisaf M., Liberopoulos E.N. Hypocholesterolemia. Curr Vasc Pharmacol. 2011;9:200–212. doi: 10.2174/157016111794519354. [DOI] [PubMed] [Google Scholar]
- 10.van Leeuwen H.J., Heezius E.C., Dallinga G.M., van Strijp J.A., Verhoef J., et al. Lipoprotein metabolism in patients with severe sepsis. Crit Care Med. 2003;31:1359–1366. doi: 10.1097/01.CCM.0000059724.08290.51. [DOI] [PubMed] [Google Scholar]
- 11.Meilhac O., Tanaka S., Couret D. High-density lipoproteins are bug scavengers. Biomolecules. 2020;10 doi: 10.3390/biom10040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 13.Sun J.T., Chen Z., Nie P., Ge H., Shen L., et al. Lipid profile features and their associations with disease severity and mortality in patients with COVID-19. Front Cardiovasc Med. 2020;7:584987. doi: 10.3389/fcvm.2020.584987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka S., De Tymowski C., Assadi M., Zappella N., Jean-Baptiste S., et al. Lipoprotein concentrations over time in the intensive care unit COVID-19 patients: results from the ApoCOVID study. PloS One. 2020;15 doi: 10.1371/journal.pone.0239573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begue F., Tanaka S., Mouktadi Z., Rondeau P., Veeren B., et al. Altered high-density lipoprotein composition and functions during severe COVID-19. Sci Rep. 2021;11:2291. doi: 10.1038/s41598-021-81638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J., Lee S., Wang X., Li Y., Wu W.K.K., et al. Development of a multivariable prediction model for severe COVID-19 disease: a population-based study from Hong Kong. NPJ Digit Med. 2021;4:66. doi: 10.1038/s41746-021-00433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masana L., Correig E., Ibarretxe D., Anoro E., Arroyo J.A., et al. Low HDL and high triglycerides predict COVID-19 severity. Sci Rep. 2021;11:7217. doi: 10.1038/s41598-021-86747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilser J.R., Han Y., Biswas S., Gukasyan J., Cai Z., et al. Association of serum HDL-cholesterol and apolipoprotein A1 levels with risk of severe SARS-CoV-2 infection. J Lipid Res. 2021;62:100061. doi: 10.1016/j.jlr.2021.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Zhang Y., Lu R., Dai M., Shen M., et al. Lipid metabolism changes in patients with severe COVID-19. Clin Chim Acta. 2021;517:66–73. doi: 10.1016/j.cca.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S., Zhou C., Yuan Z., Xiao H., Wu X. The clinical value of high-density lipoprotein in the evaluation of new coronavirus pneumonia. Adv Clin Exp Med. 2021;30:153–156. doi: 10.17219/acem/130606. [DOI] [PubMed] [Google Scholar]
- 21.Turgay Yildirim O., Kaya S. The atherogenic index of plasma as a predictor of mortality in patients with COVID-19. Heart Lung. 2021;50:329–333. doi: 10.1016/j.hrtlng.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouyang S.M., Zhu H.Q., Xie Y.N., Zou Z.S., Zuo H.M., et al. Temporal changes in laboratory markers of survivors and non-survivors of adult inpatients with COVID-19. BMC Infect Dis. 2020;20:952. doi: 10.1186/s12879-020-05678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salari A., Mahdavi-Roshan M., Ghorbani Z., Mortazavi S.S., Naghshbandi M., et al. An investigation of risk factors of in-hospital death due to COVID-19: a case-control study in Rasht, Iran. Ir J Med Sci. 2021 doi: 10.1007/s11845-020-02455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin C., Minghan H., Ziwen Z., Yukun L. Alteration of lipid profile and value of lipids in the prediction of the length of hospital stay in COVID-19 pneumonia patients. Food Sci Nutr. 2020;8:6144–6152. doi: 10.1002/fsn3.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan J., Wang H., Ye G., Cao X., Xu X., et al. Letter to the Editor: low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism. 2020;107:154243. doi: 10.1016/j.metabol.2020.154243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B., Dong C., Li S., Song X., Wei W., et al. Triglyceride to high-density lipoprotein cholesterol ratio is an important determinant of cardiovascular risk and poor prognosis in coronavirus disease-19: a retrospective case series study. Diabetes Metab Syndr Obes. 2020;13:3925–3936. doi: 10.2147/DMSO.S268992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei X., Zeng W., Su J., Wan H., Yu X., et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. 2020;14:297–304. doi: 10.1016/j.jacl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X., Chen D., Wu L., He G., Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin Chim Acta. 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W., Li C., Wang Z., Wang H., Zhou N., et al. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci China Life Sci. 2020;63:1678–1687. doi: 10.1007/s11427-020-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D., Li R., Wang J., Jiang Q., Gao C., et al. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. BMC Infect Dis. 2020;20:519. doi: 10.1186/s12879-020-05242-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ressaire Q., Dudoignon E., Moreno N., Coutrot M., Depret F. Low total cholesterol blood level is correlated with pulmonary severity in COVID-19 critical ill patients. Anaesth Crit Care Pain Med. 2020;39:733–735. doi: 10.1016/j.accpm.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G., Zhang Q., Zhao X., Dong H., Wu C., et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis. 2020;19:204. doi: 10.1186/s12944-020-01382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q., Wei Y., Chen M., Wan Q., Chen X. Clinical analysis of risk factors for severe COVID-19 patients with type 2 diabetes. J Diabet Complicat. 2020;34:107666. doi: 10.1016/j.jdiacomp.2020.107666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue G., Gan X., Wu Z., Xie D., Xiong Y., et al. Novel serological biomarkers for inflammation in predicting disease severity in patients with COVID-19. Int Immunopharm. 2020;89:107065. doi: 10.1016/j.intimp.2020.107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han H., Xu Z., Cheng X., Zhong Y., Yuan L., et al. Descriptive, retrospective study of the clinical characteristics of asymptomatic COVID-19 patients. mSphere. 2020;5 doi: 10.1128/mSphere.00922-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik J., Laique T., Ishaq U., Ashraf A., Malik A., et al. Effect of COVID-19 on lipid profile and its correlation with acute phase reactants. medRxiv. 2021:2021. doi: 10.1101/2021.04.13.21255142. 04.13.21255142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osuna-Ramos J.F., Rendón-Aguilar H., Jesús-González L.A.D., Reyes-Ruiz J.M., Espinoza-Ortega A.M., et al. Serum lipid profile changes and their clinical diagnostic significance in COVID-19 Mexican Patients. medRxiv. 2020 doi: 10.1101/2020.08.24.20169789. 2020.08.24.20169789. [DOI] [Google Scholar]
- 38.Peng Y., Wan L., Fan C., Zhang P., Wang X., et al. medRxiv; 2020. Cholesterol metabolism—impacts on SARS-CoV-2 infection prognosis. 2020.04.16.20068528. [DOI] [Google Scholar]
- 39.Nie S., Zhao X., Zhao K., Zhang Z., Zhang Z., et al. medRxiv; 2020. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study. 2020.03.24.20042283. [DOI] [Google Scholar]
- 40.Aparisi Á., Iglesias-Echeverría C., Ybarra-Falcón C., Cusácovich I., Uribarri A., et al. medRxiv; 2020. Low-density lipoprotein cholesterol levels are associated with poor clinical outcomes in COVID-19. 2020.10.06.20207092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rigatti S.J., Stout R. medRxiv; 2020. SARS-CoV-2 antibody prevalence and association with routine laboratory values in a life insurance applicant population. 2020.09.09.20191296. [DOI] [Google Scholar]
- 42.Zhang H., Wang X., Fu Z., Luo M., Zhang Z., et al. Potential factors for prediction of disease severity of COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.03.20.20039818. 2020.03.20.20039818. [DOI] [Google Scholar]
- 43.Chen X., Zheng F., Qing Y., Ding S., Yang D., et al. medRxiv; 2020. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. 2020.03.03.20030353. [DOI] [Google Scholar]
- 44.Alcántara-Alonso E., Molinar-Ramos F., González-López J.A., Alcántara-Alonso V., Muñoz-Pérez M.A., et al. High triglyceride to HDL-cholesterol ratio as a biochemical marker of severe outcomes in COVID-19 patients. Clin Nutr ESPEN. 2021 doi: 10.1016/j.clnesp.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu X., Chen D., Wu L., He G., Ye W. Low serum cholesterol level among patients with COVID-19 infection in Wenzhou, China. SSRN. 2020. https://ssrncom/abstract=3544826 or http://dxdoiorg/102139/ssrn3544826
- 46.Izcovich A., Ragusa M.A., Tortosa F., Lavena Marzio M.A., Agnoletti C., et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PloS One. 2020;15 doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lassale C., Hamer M., Hernaez A., Gale C.R., Batty G.D. medRxiv; 2021. High density lipoprotein cholesterol and risk of subsequent COVID-19 hospitalisation: the UK Biobank study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scalsky R.J., Desai K., Chen Y.J., O'Connell J.R., Perry J.A., et al. medRxiv; 2020. Baseline cardiometabolic profiles and SARS-CoV-2 risk in the UK Biobank. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Yang H., Li S., Li W.D., Wang J., et al. Association analysis framework of genetic and exposure risks for COVID-19 in middle-aged and elderly adults. Mech Ageing Dev. 2021;194:111433. doi: 10.1016/j.mad.2021.111433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolovou G.D., Mikhailidis D.P., Anagnostopoulou K.K., Daskalopoulou S.S., Cokkinos D.V. Tangier disease four decades of research: a reflection of the importance of HDL. Curr Med Chem. 2006;13:771–782. doi: 10.2174/092986706776055580. [DOI] [PubMed] [Google Scholar]
- 51.Agouridis A.P., Liberopoulos E.N., Kostapanos M.S., Elisaf M.S. New-onset extremely low levels of high-density lipoprotein cholesterol. J Clin Lipidol. 2012;6:593–595. doi: 10.1016/j.jacl.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Liberopoulos E., Apostolou F., Elisaf M. Serum lipid profile in patients with severe leptospirosis. Nephrol Dial Transplant. 2004;19:1328–1329. doi: 10.1093/ndt/gfh054. author reply 9-30. [DOI] [PubMed] [Google Scholar]
- 53.Apostolou F., Gazi I.F., Kostoula A., Tellis C.C., Tselepis A.D., et al. Persistence of an atherogenic lipid profile after treatment of acute infection with Brucella. J Lipid Res. 2009;50:2532–2539. doi: 10.1194/jlr.P900063-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deniz O., Gumus S., Yaman H., Ciftci F., Ors F., et al. Serum total cholesterol, HDL-C and LDL-C concentrations significantly correlate with the radiological extent of disease and the degree of smear positivity in patients with pulmonary tuberculosis. Clin Biochem. 2007;40:162–166. doi: 10.1016/j.clinbiochem.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 55.Garmashova N.V., Tuzikov F.V., Tuzikova N.A., Tyshkevich O.B., Doronin B.M., et al. Characteristics of lipids imbalance in patients with tick-borne encephalitis. Nucleos Nucleot Nucleic Acids. 2004;23:1003–1007. doi: 10.1081/NCN-200026055. [DOI] [PubMed] [Google Scholar]
- 56.Su T.C., Lee Y.T., Cheng T.J., Chien H.P., Wang J.D. Chronic hepatitis B virus infection and dyslipidemia. J Formos Med Assoc. 2004;103:286–291. [PubMed] [Google Scholar]
- 57.Corey K.E., Kane E., Munroe C., Barlow L.L., Zheng H., et al. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology. 2009;50:1030–1037. doi: 10.1002/hep.23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorokin A.V., Karathanasis S.K., Yang Z.H., Freeman L., Kotani K., et al. COVID-19-Associated dyslipidemia: implications for mechanism of impaired resolution and novel therapeutic approaches. Faseb J. 2020;34:9843–9853. doi: 10.1096/fj.202001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filippas-Ntekouan S., Liberopoulos E., Elisaf M. Lipid testing in infectious diseases: possible role in diagnosis and prognosis. Infection. 2017;45:575–588. doi: 10.1007/s15010-017-1022-3. [DOI] [PubMed] [Google Scholar]
- 60.Trieb M., Rainer F., Stadlbauer V., Douschan P., Horvath A., et al. HDL-related biomarkers are robust predictors of survival in patients with chronic liver failure. J Hepatol. 2020;73:113–120. doi: 10.1016/j.jhep.2020.01.026. [DOI] [PubMed] [Google Scholar]
- 61.Agouridis A.P., Banach M., Mikhailidis D.P. Dysfunctional high-density lipoprotein: not only quantity but first of all quality? Arch Med Sci. 2015;11:230–231. doi: 10.5114/aoms.2015.49816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katsiki N., Athyros V.G., Karagiannis A., Mikhailidis D.P. High-density lipoprotein, vascular risk, cancer and infection: a case of quantity and quality? Curr Med Chem. 2014;21:2917–2926. doi: 10.2174/0929867321666140303152132. [DOI] [PubMed] [Google Scholar]
- 63.Otocka-Kmiecik A., Mikhailidis D.P., Nicholls S.J., Davidson M., Rysz J., et al. Dysfunctional H.D.L. A novel important diagnostic and therapeutic target in cardiovascular disease? Prog Lipid Res. 2012;51:314–324. doi: 10.1016/j.plipres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Athyros V.G., Katsiki N., Karagiannis A., Mikhailidis D.P. Should raising high-density lipoprotein cholesterol be a matter of debate? J Cardiovasc Med. 2012;13:254–259. doi: 10.2459/JCM.0b013e3283522422. [DOI] [PubMed] [Google Scholar]
- 65.Ding X., Zhang J., Liu L., Yuan X., Zang X., et al. High-density lipoprotein cholesterol as a factor affecting virus clearance in covid-19 patients. Respir Med. 2020;175:106218. doi: 10.1016/j.rmed.2020.106218. [DOI] [PMC free article] [PubMed] [Google Scholar]