Abstract
Background: As immune checkpoint inhibitors (ICIs) transition to the forefront of cancer treatment, a better understanding of immune related adverse events (IRAEs) is essential to promote safe clinical practice. Dermatologic adverse events are the most common IRAEs and can lead to drug withdrawal and decreased quality of life. This meta-analysis aimed to investigate the risk of the most prevalent dermatologic adverse events (pruritus and rash) among various ICI treatment regimens.
Methods: A systematic search of electronic databases was performed to identify qualified randomized controlled trials (RCTs). Data for any grade and high grade pruritus and rash were extracted for meta-analysis. Two reviewers independently assessed methodological quality. The relative risk summary and 95% confidence interval were calculated.
Results: 50 RCTs involving 29941 patients were analyzed. The risk of pruritus (2.15 and 4.21 relative risk respectively) and rash (1.61 and 3.89 relative risk respectively) developing from CTLA-4 or PD-1/-L1 inhibitor were increased compared to placebo, but this effect was not dose-dependent. PD-1/-L1 plus CTLA-4 inhibitor was associated with increased risk of pruritus (1.76 and 0.98 relative risk respectively) and rash (1.72 and 1.37 relative risk respectively) compared to either monotherapy. Compared with CTLA-4 inhibitor, PD-1/-L1 inhibitor had a significantly decreased risk of pruritus and rash in both monotherapy and combination therapy (0.65 and 0.29 relative risk respectively). No significant difference was found between PD-1/-L1 inhibitor combined with chemotherapy and PD-1/-L1 monotherapy in any grade and high grade rash (0.84 and 1.43 relative risk respectively). In subgroup analyses, PD-1 inhibitor was associated with reduced risk of pruritus and rash compared to PD-L1 inhibitor.
Conclusion: Our meta-analysis demonstrates a better safety profile for PD-1/-L1 inhibitor compared to CTLA-4 inhibitor in terms of pruritus and rash among both monotherapy and multiple combination therapies. PD-L1 inhibitor may contribute to an increased risk of pruritus and rash compared to PD-1 inhibitor.
Keywords: meta-analysis, checkpoint inhibitors, combination immunotherapy, immune-related adverse events, dermatologic adverse events
Introduction
The application of immune checkpoint inhibitors (ICIs) is a significant milestone for clinical strategies in cancer. Due to increased activation of the immune system, ICIs can cause a spectrum of IRAEs that affect multiple organ systems and can even lead to death (Fausto et al., 2020). Dermatologic toxicities appear to be the most prevalent IRAEs, both with Programmed cell death protein 1/Programmed cell death-ligand 1 (PD-1/PD-L1) inhibitor and Cytotoxic T lymphocyte associate protein 4 (CTLA-4) inhibitor, and occur in more than a third of patients treated with ICI monotherapy (Sibaud et al., 2016). Consequently, decreased quality of life due to dermatologic adverse events may contribute to unnecessary drug withdrawal by patients. Additionally, management of serious dermatologic adverse events, including oral and topical steroids, may result in reduced drug efficacy (Geisler et al., 2020). Among dermatologic IRAEs manifestations, pruritus and rash are the most common (Boutros et al., 2016; Ellis et al., 2020; Geisler et al., 2020). Indeed, clinical studies demonstrate that pruritus may occur in 11–21% of patients treated with anti-PD-1/-L1 inhibitor, 24.4–35.4% of patients treated with CTLA-4 inhibitor, and 33.2–47% of patients in dual CTLA-4/PD-1 blockade (Geisler et al., 2020; Nishijima et al., 2017; Sibaud et al., 2016). For rash, incidence ranges as high as 20% for patients receiving PD-1 inhibitor, 14–26% for patients receiving CTLA-4 inhibitor, and 28.4–55% for patients receiving dual anti-CTLA-4/PD-1 blockade therapy (Geisler et al., 2020; Sibaud et al., 2016). Therefore, to balance the benefits and risks among multiple ICI treatment patterns in clinical strategy, an improved understanding of dermatologic IRAEs is essential (Collins et al., 2017; Ellis et al., 2020).
Combination immunotherapy has become a popular treatment option due to its superior clinical efficacy. However, ICI combination therapy is associated with toxic effects resulting from unbalanced activation of the immune system (Da et al., 2020). As mentioned above, combination of anti-CTLA-4 and anti-PD-1 therapy is associated with more frequent, more severe, and earlier dermatologic IRAEs compared to monotherapy (Almutairi et al., 2020; Sibaud et al., 2016). However, few studies have assessed dermatologic adverse events resulting from various ICI treatment regimens. Although previous meta-analysis (Nishijima et al., 2017; Yang et al., 2019) evaluated the incidence of selected dermatologic and mucosal adverse effects associated with PD-1/-L1 inhibitors, the authors included chemotherapy and ipilimumab as the only control arms. Other studies investigated the incidence and risk of IRAEs (including dermatologic adverse events) due to ICI monotherapy and combination therapy (Almutairi et al., 2020; Velasco et al., 2017; Wang et al., 2021), yet the patients included in their analysis were limited to a single tumor such as melanoma or lung cancer. Moreover, direct comparisons of the risk of dermatologic IRAEs between combination therapy and ICI monotherapy are lacking due to a dearth of head-to-head clinical trials. Therefore, a better understanding of dermatologic adverse events in this context is still needed. In the current study, we focused on the two most common dermatologic adverse events, pruritus and rash (Braun et al., 2020; Golian et al., 2016), in patients receiving ICI monotherapies and combination therapies including chemotherapy, targeted therapy, and other ICI treatment regimens. All the data used in this meta-analysis are derived from published literature and clinical trials.
Materials and Methods
Search Strategy and Eligibility Criteria
Two investigators (Yang Ge and Hui-Yun Zhang) independently searched PubMed, Embase, Web of Science, and the Cochrane Library. The last search was performed on January 20, 2020. The following terms were used: (Nivolumab or Opdivo or ONO-4538 or ONO 4538 MDX-1106 or BMS-936558 or pembrolizumab or lambrolizumab or Keytruda or cemiplimab or Pidilizumab or camrelizumab or SHR-1210 or JS001 or sintilimab or Durvalumab or MEDI4736 or atezolizumab or avelumab or Bavencio or tremelimumab or ticilimumab or Ipilimumab) and (Carcinoma or Neoplasia or Tumor or Cancer or Malignancy) and randomized controlled trials.
The following inclusion criteria were used: 1) studies included either ICI monotherapy or ICI combination therapy with chemotherapy/targeted therapy/ICIs in patients diagnosed with solid tumor; 2) studies investigated the following dermatologic adverse events: pruritus and rash; 3) randomized controlled clinical trials published in English. The following exclusion criteria were used: 1) phase I clinical trials; 2) studies without related data; 3) studies reporting dermatologic adverse events which are not related to ICIs; 3) editorials, letters, case reports, expert opinions, or reviews; and 4) duplicate publications.
Data Extraction and Quality Assessment
The following information was extracted from each eligible study: first author, publication year, number of patients, cancer type, National Clinical Trial (NCT) number, randomization, trial phase, line of therapy, treatment, events of pruritus and rash in intervention and control arms (any grade and high grade). Our identification of any grade and high grade IRAEs was based on the Common Terminology Criteria for Adverse Events (CTCAE): “any grade” referred to CTCAE grades 1–5; “low grade” referred to CTCAE grades 1–2; “high grade” referred to CTCAE grades 3–5. The dosage of ICIs was also extracted to investigate if high dose ICIs are associated with increased IRAEs. Less than or equal to 3 mg/kg of PD-1/CTLA-4 was identified as “low dose”, while greater than or equal to 10 mg/kg was identified as “high dose”. The extraction was performed by two investigators (Yang Ge and Huiyun Zhang) independently and any controversies were resolved by discussion.
Quality assessment was performed using Review Manager 5.3. Risk of bias for the eligible study was evaluated according to following items recommended by the Cochrane Collaboration: randomization, allocation concealment blinding of participant, blinding of outcome assessors, incomplete outcome data, selective reporting, and other bias.
Statistical Analysis
We conducted the meta-analysis using Review Manager 5.3. Risk ratio (RR) and 95% confidence interval (95% CI) were applied to evaluate the risk of pruritus and rash for both experimental and control arms. Relative risk ratio (RRR) with 95% CIs between different treatment regimens were calculated using RRs and 95% CIs. Heterogeneity was tested by the I2 and Q test. When p > 0.1 and I2 ≤ 50%, it was considered to indicate no significant heterogeneity and the fixed-effect model was applied. Otherwise, the random-effects model was applied. Begg’s and Egger’s tests were performed using Stata 16.0 to estimate publication bias. Subgroup analyses were performed to explore the sources of heterogeneity according to the different ICI class and tumor types.
Results
Search Results and Study Characteristics
14,819 publications were initially identified from the database and plus 11 from other sources. After excluding duplicates, 13,777 publications were assessed for review of title and abstract. 336 articles were further assessed for full-text review. Finally, 50 RCTs (n = 29,941 patients) were included in this meta-analysis ( Figure 1 ). Most of the included studies involved patients with melanoma (N = 15) and none small cell lung carcinoma (NSCLC) (N = 12). The others were focused on renal cell carcinoma (RCC) (N = 5), head and neck squamous cell carcinoma (HNSCC) (N = 4), small cell lung cancer (SCLC) (N = 3), gastric cancer or gastro-oesophageal junction cancer (GC/GOJC) (N = 3), prostate cancer (N = 2), urothelial cancer (UC) (N = 2), malignant mesothelioma (N = 1), triple-negative breast cancer (TNBC) (N = 1), hepatocellular carcinoma (HCC) (N = 1), and pancreatic cancer (N = 1). Details of characteristics of the included studies are shown in Table 1.
FIGURE 1.
PRISMA flow chart of study selection.
TABLE 1.
Characteristics of the included studies.
| NCT | Author | Year | Cancer type | Phase | Line | Blinding | Treatment regimen | No. of patients | No. of pruritus events | No. of rash events | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade | High grade | Any grade | High grade | |||||||||
| 00289640 | Wolchok et al. (2010) | 2010 | Melanoma | 2 | >1 | Double-blind | Ipilimumab 10 mg/kg q3w | 71 | 23 | 2 | 16 | 0 |
| Ipilimumab 3 mg/kg Q3w | 71 | 15 | 1 | 17 | 1 | |||||||
| Ipilimumab 0.3 mg/kg Q3w | 72 | 2 | 0 | 3 | 0 | |||||||
| 00324155 | C. Robert et al. (2011) | 2011 | Melanoma | 3 | 1 | Double-blind | Ipilimumab (10 mg/kg) + dacarbazine (850 mg/m2 of body-surface area)given at weeks 1, 4, 7, and 10 | 247 | 66 | 5 | 55 | 3 |
| Placebo (10 mg/kg) + dacarbazine (850 mg/m2of body-surface area) given at weeks 1, 4, 7, and 10 | 251 | 15 | 0 | 12 | 0 | |||||||
| 00527735 | Reck et al. (2013) | 2013 | SCLC | 2 | 1 | Double-blind | Ipilimumab plus chemotherapy | 84 | 55 | 5 | 43 | 2 |
| Placebo plus chemotherapy | 44 | 2 | 0 | 5 | 0 | |||||||
| 00257205 | Ribas et al. (2013) | 2013 | Melanoma | 3 | 1 | None | Tremelimumab (15 mg/kg once every 90 days) | 325 | 100 | 3 | 106 | 7 |
| Chemotherapy | 319 | 16 | 0 | 17 | 1 | |||||||
| 00861614 | Kwon et al. (2014) | 2014 | Prostate cancer | 3 | >1 | Double-blind | Ipilimumab 10 mg/kg Q3W | 393 | 80 | 1 | 68 | 2 |
| Placebo | 396 | 15 | 0 | 16 | 0 | |||||||
| 01354431 | Motzer et al. (2015b) | 2015 | Clear-cell renal cell carcinoma | 2 | >1 | Double-blind | Nivolumab 0.3 mg/kg q3w | 59 | 6 | 0 | 5 | 0 |
| Nivolumab 2 mg/kg q3w | 54 | 5 | 1 | 4 | 0 | |||||||
| Nivolumab 10 mg/kg q3w | 54 | 6 | 0 | 7 | 0 | |||||||
| 00636168 | Eggermont et al. (2015) | 2015 | Melanoma | 3 | Adjuvant | Double-blind | Ipilimumab 10 mg/kg q3w | 471 | 187 | 11 | 162 | 52 |
| Placebo | 474 | 51 | 0 | 6 | 0 | |||||||
| 01642004 | Brahmer et al. (2015) | 2015 | NSCLC | 3 | >1 | None | Nivolumab 3 mg/kg Q2W | 131 | 3 | 0 | 5 | 0 |
| Docetaxel 75 mg/m^2 Q3W | 129 | 0 | 0 | 8 | 2 | |||||||
| 01668784 | Motzer et al. (2015a) | 2015 | RCC | 3 | >1 | None | Nivolumab 3 mg/kg Q2W | 406 | 57 | 39 | 41 | 2 |
| Everolimus 10 mg QD | 397 | 0 | 0 | 79 | 3 | |||||||
| 01673867 | Borghaei et al. (2015) | 2015 | NSCLC | 3 | >1 | None | Nivolumab 3 mg/kg Q2W | 287 | 24 | 0 | 27 | 1 |
| Docetaxel 75 mg/m^2 Q3W | 268 | 4 | 0 | 8 | 0 | |||||||
| 01704287 | Ribas et al. (2015) | 2015 | Melanoma | 2 | >1 | Double-blind | Pembrolizumab 10 mg/kg Q3w | 179 | 42 | 0 | 18 | 0 |
| Pembrolizumab 2 mg/kg Q3w | 178 | 37 | 0 | 21 | 0 | |||||||
| Chemotherapy | 171 | 6 | 0 | 8 | 0 | |||||||
| 01721746 | Weber et al. (2015) | 2015 | Melanoma | 3 | >1 | None | Nivolumab | 268 | 43 | 0 | 25 | 1 |
| Chemotherapy | 102 | 2 | 0 | 5 | 0 | |||||||
| 01721772 | Robert et al. (2015a) | 2015 | Melanoma | 3 | 1 | Double-blind | Nivolumab 3 mg/kg Q2W | 206 | 35 | 1 | 31 | 1 |
| Dacarbazine 1,000 mg/m^2 Q3W | 205 | 11 | 0 | 6 | 0 | |||||||
| 01844505 | Larkin et al. (2015) | 2015 | Melanoma | 3 | 1 | Double-blind | Ipilimumab 3 mg/kg Q3W for four cycles | 311 | 110 | 1 | 65 | 5 |
| Nivolumab 1 mg/kg + ipilimumab 3 mg/kg Q3W | 313 | 104 | 6 | 89 | 9 | |||||||
| Nivolumab 3 mg/kg Q2W | 313 | 59 | 0 | 68 | 1 | |||||||
| 01866319 | Robert et al. (2015b) | 2015 | Melanoma | 3 | ≥1 | None | Ipilimumab 3 mg/kg Q3w | 256 | 65 | 1 | 37 | 2 |
| Pembrolizumab 10 mg/kg Q2w | 278 | 40 | 0 | 41 | 0 | |||||||
| Pembrolizumab 10 mg/kg Q3w | 277 | 39 | 0 | 37 | 0 | |||||||
| 01927419 | Postow et al. (2015b) | 2015 | Melanoma | 2 | 1 | Double-blind | Nivolumab 1 mg/kg + ipilimumab 3 mg/kg Q3W for four cycles | 94 | 33 | 1 | 39 | 5 |
| Placebo 1 mg/kg + ipilimumab 3 mg/kg Q3W | 46 | 13 | 0 | 12 | 0 | |||||||
| 01057810 | Beer et al. (2016) | 2016 | Prostate cancer | 3 | 1 | Double-blind | Ipilimumab 10 mg/kg q3w | 399 | 109 | 1 | 132 | 10 |
| Placebo | 199 | 14 | 1 | 15 | 0 | |||||||
| 01450761 | Reck et al. (2016) | 2016 | SCLC | 3 | 1 | None | Etoposide andplatinum (cisplatin or carboplatin) plus ipilimumab 10 mg/kg q3w | 154 | 55 | 3 | 90 | 8 |
| Etoposide andplatinum (cisplatin or carboplatin) plus placebo 10 mg/kg q3w | 150 | 8 | 0 | 12 | 0 | |||||||
| 01905657 | Herbst et al. (2016) | 2016 | NSCLC | 2/3 | >1 | None | Pembrolizumab 10 mg/kg, Q3w | 343 | 32 | 0 | 44 | 1 |
| Pembrolizumab 2 mg/kg, Q3w | 339 | 25 | 0 | 29 | 1 | |||||||
| Docetaxel 75 mg/m2 every 3 weeks | 309 | 5 | 1 | 14 | 0 | |||||||
| 02039674 | Langer et al. (2016) | 2016 | NSCLC | 2 | 1 | None | Pembrolizumab 200 mg + pemetrexed 500 mg/m2 + carboplatin area under curve 5 mg/ml q3w | 59 | 7 | 0 | 16 | 1 |
| Pemetrexed 500 mg/m2 + carboplatin AUC 5 mg/ml per min | 62 | 2 | 0 | 9 | 0 | |||||||
| 02105636 | Ferris et al. (2016) | 2016 | HNC | 3 | >1 | None | Nivolumab 3 mg/kg Q2W | 236 | 17 | 0 | 18 | 0 |
| Standard therapy | 111 | 0 | 0 | 5 | 1 | |||||||
| 01285609 | Govindan et al. (2017) | 2017 | NSCLC | 3 | >1 | Double-blind | Paclitaxel and carboplatin plus blinded ipilimumab 10 mg/kg q3w | 388 | 56 | 4 | 67 | 8 |
| Placebo plus chemotherapy | 361 | 8 | 0 | 14 | 0 | |||||||
| 01515189 | Ascierto et al. (2017) | 2017 | Melanoma | 3 | ≥1 | Double-blind | Ipilimumab 3 mg/kg Q3w | 362 | 82 | 2 | 95 | 5 |
| Ipilimumab 10 mg/kg Q3w | 364 | 81 | 2 | 5 | 2 | |||||||
| 01843374 | Maio et al. (2017) | 2017 | Malignant mesothelioma | 2 | >1 | Double-blind | Tremelimumab 10 mg/kg Q4w | 380 | 103 | 3 | 79 | 2 |
| Placebo | 189 | 15 | 0 | 13 | 0 | |||||||
| 02041533 | Carbone et al. (2017) | 2017 | NSCLC | 3 | 1 | None | Nivolumab 3 mg/kg Q2W | 267 | 22 | 0 | 26 | 2 |
| Investigator’s choice chemotherapy Q3W | 263 | 7 | 1 | 15 | 1 | |||||||
| 02125461 | Antonia et al. (2017) | 2017 | NSCLC | 3 | >1 | Double-blind | Durvalumab (10 mg per kilogram of body weight) q2w | 475 | 33 | 0 | 37 | 1 |
| Placebo q2w | 234 | 5 | 0 | 13 | 0 | |||||||
| 02256436 | Bellmunt et al. (2017) | 2017 | UC | 3 | >1 | None | Pembrolizumab 200 mg q3w | 266 | 52 | 0 | NA | NA |
| Chemotherapy | 255 | 7 | 1 | NA | NA | |||||||
| 02267343 | Kang et al. (2017) | 2017 | GC/GOJC | 3 | >1 | Double-blind | 3 mg/kg nivolumab Q2W | 330 | 30 | 0 | 19 | 5 |
| Placebo | 161 | 9 | 0 | 0 | 0 | |||||||
| 02388906 | Weber et al. (2017) | 2017 | Melanoma | 3 | 1 | Double-blind | Ipilimumab 10 mg/kg Q3W | 453 | 152 | 5 | 133 | 14 |
| Nivolumab 3 mg/kg Q2W | 452 | 105 | 0 | 90 | 5 | |||||||
| 01928394 | Janjigian et al. (2018) | 2018 | Esophagogastric cancer | 2 | >1 | None | Nivolumab 3 mg/kg + ipilimumab 1 mg/kg Q3W | 52 | 12 | 0 | 8 | 0 |
| Nivolumab 1 mg/kg + ipilimumab 3 mg/kg Q3W | 49 | 9 | 1 | 10 | 0 | |||||||
| Nivolumab 3 mg/kg Q2W | 59 | 10 | 0 | 5 | 0 | |||||||
| 02302807 | Powles et al. (2018) | 2018 | Urothelial bladder cancer | 3 | >1 | None | Atezolizumab 1,200 mg Q3W | 459 | 59 | NA | 40 | NA |
| Chemotherapy | 443 | 14 | NA | 21 | NA | |||||||
| 02362594 | Eggermont et al. (2018) | 2018 | Melanoma | 3 | Adjuvant | Double-blind | Pembrolizumab 200 mg q3w | 509 | 90 | 0 | 82 | 1 |
| Placebo | 502 | 51 | 0 | 52 | 0 | |||||||
| 02366143 | Socinski et al. (2018) | 2018 | NSCLC | 3 | 1 | None | Atezolizumab 1,200 mg plus bevacizumab plus carboplatin plus paclitaxel | 393 | NA | NA | 52 | 5 |
| Bevacizumab plus carboplatin plus paclitaxel | 394 | NA | NA | 20 | 0 | |||||||
| 02374242 | Long et al. (2018) | 2018 | Melanoma | 2 | ≥1 | None | Nivolumab 1 mg/kg + ipilimumab 3 mg/kg q3w | 35 | 13 | 0 | 22 | 4 |
| Nivolumab 3 mg/kg q2w | 25 | 2 | 0 | 5 | 0 | |||||||
| 02425891 | Schmid et al. (2018) | 2018 | TNBC | 3 | 1 | Double-blind | Atezolizumab plus nab-paclitaxel | 452 | 46 | 0 | 59 | 2 |
| Placebo plus nab-paclitaxel | 438 | 36 | 0 | 54 | 2 | |||||||
| 02477826 | Hellmann et al. (2018) | 2018 | Lung cancer | 3 | 1 | None | Nivolumab 3 mg/kg Q2w + ipilimumab 1 mg/kg Q6W | 576 | 81 | 3 | 96 | 9 |
| Nivolumab 240 mg Q2W | 391 | 30 | 0 | 43 | 3 | |||||||
| Chemotherapy | 570 | 5 | 0 | 29 | 0 | |||||||
| 02578680 | Gandhi et al. (2018) | 2018 | NSCLC | 3 | 1 | Double-blind | Pembrolizumab 200 mg q3w + carboplatin/cisplatin 75 mg/kg/m2 q3w + pemetrexed 5 mg/kg/m2 q3w | 405 | 55 | NA | 109 | NA |
| placebo200 mg q3w + carboplatin/cisplatin 75 mg/kg/m2 q3w + pemetrexed 5 mg/kg/m2 q3w | 202 | 22 | NA | 28 | NA | |||||||
| 02763579 | Horn et al. (2018) | 2018 | SCLC | 3 | 1 | Double-blind | Atezolizumab plus chemotherapy | 198 | NA | NA | 37 | 4 |
| Placebo plus chemotherapy | 196 | NA | NA | 20 | 0 | |||||||
| 02775435 | Paz-Ares et al. (2018) | 2018 | NSCLC | 3 | 1 | Double-blind | Pembrolizumab plus chemotherapy | 278 | 40 | NA | 47 | NA |
| Placebo plus chemotherapy | 280 | 25 | NA | 32 | NA | |||||||
| 02220894 | Mok et al. (2019) | 2019 | NSCLC | 3 | 1 | None | Pembrolizumab 200 mg q3w | 636 | 46 | 2 | 46 | 3 |
| Chemotherapy | 615 | 15 | 0 | 27 | 0 | |||||||
| 02252042 | Cohen et al. (2019) | 2019 | HNC | 3 | >1 | None | Pembrolizumab 200 mg q3w | 246 | 12 | 0 | 19 | 1 |
| Chemotherapy | 234 | 16 | 2 | 34 | 1 | |||||||
| 02358031 | Burtness et al. (2019) | 2019 | HNSCC | 3 | 1 | None | Pembrolizumab 200 mg every 3 weeks | 330 | NA | NA | 25 | 2 |
| Pembrolizumab 200 mg every 3 weeks + carboplatin (5 mg/m2)/cisplatin (100 mg/m2) + 5-fluorouracil (1,000 mg/m2 per day for 4 consecutive days) q3w | 276 | NA | NA | 23 | 1 | |||||||
| .Cetuximab (400 mg/m2 loading dose, then 250 mg/m2 qw)+carboplatin (5 mg/m2)/cisplatin (100 mg/m2) + 5-fluorouracil (1,000 mg/m2 per day for 4 consecutive days) q3w | 287 | NA | NA | 101 | 17 | |||||||
| 02319044 | Siu et al. (2019) | 2019 | HNSCC | 2 | >1 | None | Durvalumab 20 mg/kg Q4w plus tremelimumab 1 mg/kg Q4w for 4 cycles, durvalumab 10 mg/kg Q2W | 133 | 5 | NA | 9 | NA |
| Durvalumab 10 mg/kg Q2w for 4 cycles, durvalumab 10 mg/kg Q2W | 65 | 5 | NA | 1 | NA | |||||||
| Tremelimumab 10 mg/kg Q4w for 7 cycles, tremelimumab 10 mg/kg Q12w for 2 cycles | 65 | 3 | NA | 5 | NA | |||||||
| 02420821 | Rini et al. (2019b) | 2019 | RCC | 3 | 1 | None | Atezolizumab 1200 mg plus bevacizumab 15 mg/kg Q3W | 451 | 85 | 0 | 70 | 3 |
| Sunitinib 50 mg QD | 446 | 22 | 0 | 53 | 2 | |||||||
| 02558894 | O'Reilly et al. (2019) | 2019 | Pancreatic ductal carcinoma | 2 | >1 | None | Durvalumab (1,500 mg every 4 weeks) | 33 | 2 | 0 | NA | NA |
| Durvalumab (1,500 mg every 4 weeks) plus tremelimumab (75 mg every 4 weeks) | 32 | 1 | 0 | NA | NA | |||||||
| 02569242 | Kato et al. (2019) | 2019 | Oesophageal squamous cell carcinoma | 3 | >1 | None | Nivolumab 240 mg Q2W | 209 | NA | NA | 23 | 1 |
| Chemotherapy | 208 | NA | NA | 31 | 2 | |||||||
| 02684006 | Motzer et al. (2019) | 2019 | RCC | 3 | 1 | None | Avelumab (10 mg per kilogram of body weight) q2w + axitinib (5 mg) orally twice daily | 434 | 53 | 0 | 54 | 2 |
| Sunitinib (50 mg) orally once daily | 439 | 19 | 0 | 42 | 2 | |||||||
| 02702401 | Finn. et al. (2019) | 2019 | HCC | 3 | >1 | Double-blind | Pembrolizumab 200 mg q3w | 279 | 37 | 1 | 23 | 1 |
| Placebo | 134 | 6 | 0 | 3 | 0 | |||||||
| 02714218 | Celeste et al. (2019) | 2019 | Melanoma | 3 | 1 | Double-blind | Nivolumab 1 mg/kg + ipilimumab 3 mg/kg Q3W | 178 | 47 | 0 | 47 | 0 |
| Nivolumab 3 mg/kg + ipilimumab 1 mg/kg Q3W | 180 | 43 | 1 | 31 | 0 | |||||||
| 02853331 | Rini et al. (2019a) | 2019 | RCC | 3 | 1 | None | Pembrolizumab plus axitinib | 429 | 53 | 1 | 46 | 1 |
| Sunitinib | 425 | 18 | 0 | 38 | 1 | |||||||
Incidence of Pruritus/Rash Associated With Immune Checkpoint Inhibitor Monotherapy or Combination Therapy
Immune Checkpoint Inhibitors Monotherapy Vs Placebo
A total of four studies including 2,624 patients were assessed in this analysis. When comparing PD-1/-L1 inhibitor with placebo, the RR was 2.15 (95% CI 1.60-2.89, p < 0.00001) (Supplementary Figure 1A) for any grade pruritus. For high grade pruritus, RR could not be assessed because less than 3 RCTs were available. For rash, the RRs were 1.61 (95% CI 1.24-2.11, p = 0.0004) (Supplementary Figure 1B) and 1.87 (95% CI 0.30-11.56, p = 0.50), for any grade and high grade respectively (Supplementary Figure 1C). A similar result was found when comparing CTLA-4 inhibitor with placebo. The RRs were 4.21 (95% CI 3.48-5.10, p < 0.00001) (Supplementary Figure 2A) and 5.57 (95% CI 1.77-17.48, p = 0.003) (Supplementary Figure 2B) for any grade and high grade pruritus respectively. For rash, the RRs were 3.89 (95% CI 3.21-4.72, p < 0.00001) (Supplementary Figure 2C) and 7.37 (95% CI 2.24, 24.25, p = 0.001) for any grade and high grade respectively (Supplementary Figure 2D).
Programmed Cell Death Protein 1/Programmed Cell Death-Ligand 1 Inhibitor Vs Chemotherapy
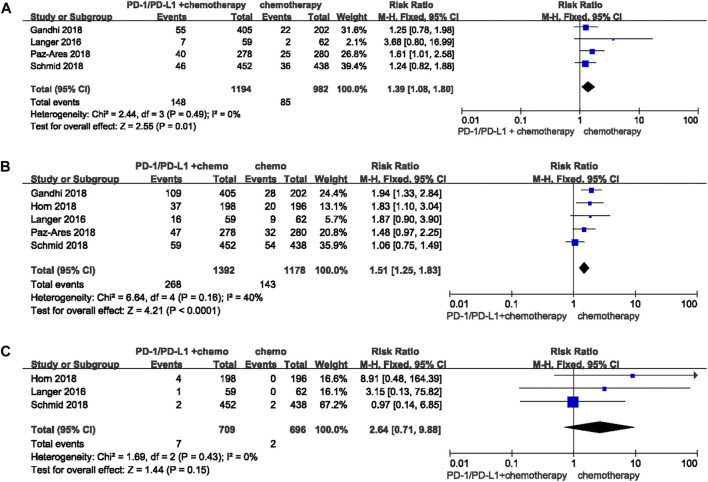
To make a comparison between PD-1/-L1 inhibitor and chemotherapy, 8,107 patients from 13 studies were included. The RRs for any grade and high grade pruritus were 4.67 (95% CI 3.66–5.95, p < 0.00001) (Figure 2A) and 0.66 (95% CI 0.24-1.85 p = 0.43), respectively (Figure 2B). For rash, the RRs were 1.61 (95% CI 1.12-2.30, p = 0.009) (Figure 2C) and 1.48 (95% CI 0.72-3.05, p = 0.28) (Figure 2D) for any grade and high grade, respectively.
FIGURE 2.
Forest plots of the relative risks and 95% CIs for pruritus and rash after PD-1/-L1 inhibitor compared to chemotherapy. (A) any grade pruritus; (B) high grade pruritus; (C) any grade rash; (D) high grade rash.
Programmed Cell Death Protein 1/Programmed cell Death-Ligand 1 Vs CTLA-4 Inhibitor
To investigate the difference in pruritus and rash between PD-1/-L1 inhibitor and CTLA-4 inhibitor, four studies with 2,370 patients were included. RRs for any grade and high grade pruritus developed after PD-1/-L1 inhibitor treatment were 0.65 (95%CI 0.56-0.75, p < 0.00001) (Supplementary Figure 3A) and 0.15 (95%CI 0.03-0.89, p = 0.04) (Supplementary Figure 3B) respectively compared to CTLA-4 inhibitor treatment. For rash the RRs were 1.06 (95%CI 0.85-1.34, p = 0.60) (Supplementary Figure 3C) and 0.29 (95%CI 0.12-0.68, p = 0.005) for any grade and high grade respectively (Supplementary Figure 3D).
High Dose Vs Low Dose Programmed Cell Death Protein 1/Programmed Cell Death-Ligand 1 Inhibitor
In this section, five qualifying studies with 2,015 patients total were analyzed. Compared to low dose groups, RRs for any grade pruritus and any grade rash developed after high dose PD-1/PD-L1 inhibitor therapy were 0.84 (95%CI 0.63-1.14, p = 0.26) (Supplementary Figure 4A) and 0.79 (95%CI 0.56-1.11, p = 0.17) respectively (Supplementary Figure 4B).
Immune Checkpoint Inhibitors Combination Chemotherapy Vs Chemotherapy Alone
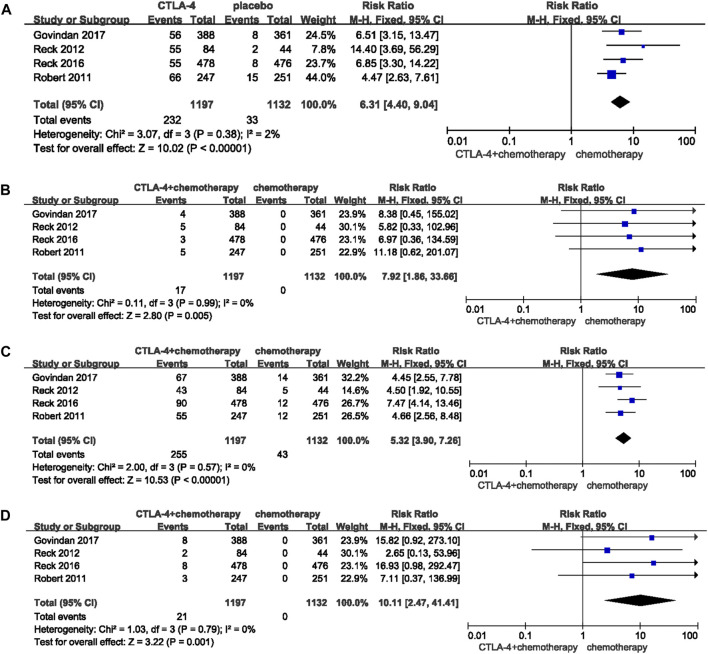
Nine studies with 4,899 patients were suitable for this analysis. When compared with chemotherapy alone, RRs were 1.39 (95%CI 1.08-1.80, p = 0.01) (Figure 3A) and 1.51 (95%CI 1.25-1.83, p < 0.0001) (Figure 3B) for any grade pruritus and any grade rash developed after PD-1/-L1 inhibitor combined with chemotherapy. RR for high grade rash was 2.64 (95%CI 0.71-9.88, p = 0.15) (Figure 3C). Data was not sufficient for comparison of high grade pruritus between PD-1/-L1 plus chemotherapy and chemotherapy. Studies available included four RCTs reporting an any grade pruritus group, two of which did not report data for high grade pruritus. No patients in the remaining two studies were reported to have experienced high grade pruritus. Similarly, the combination of CTLA-4 inhibitor and chemotherapy increased the risk of pruritus and rash compared with chemotherapy [any grade pruritus RR:6.31 (95%CI 4.40-9.04, p < 0.00001) (Figure 4A); high grade pruritus RR:7.92 (95%CI 1.86-33.66, p = 0.005) (Figure 4B); any grade rash RR:5.32 (95%CI 3.90-7.26, p < 0.00001) (Figure 4C); and high grade rash RR:10.11 (95%CI 2.47–41.41, p = 0.001) (Figure 4D)].
FIGURE 3.
Forest plots of the relative risks and 95% CIs for pruritus and rash in comparison of PD-1/-L1 plus chenotherapy and chemotherapy. (A) any grade pruritus; (B) any grade rash; (C) high grade rash.
FIGURE 4.
Forest plots of the relative risks and 95% CIs for pruritus and rash in comparison of CTLA-4 plus chenotherapy and chemotherapy. (A) any grade pruritus; (B) high grade pruritus; (C) any grade rash; (D) high grade rash.
Programmed Cell Death Protein 1/Programmed Cell Death-Ligand 1 Inhibitor Combined With Targeted Therapy Vs Targeted Therapy Alone
Three studies with 2,624 patients were included in this section. Compared to targeted therapy, RR for any grade pruritus associated with PD-1/-L1 inhibitor combined with targeted therapy was 3.22 (95% CI 2.43-4.27, p < 0.00001) (Figure 5A). RRs for any grade and high grade rash were 1.24 (95% CI 1.00-1.55, p = 0.05) (Figure 5B) and 1.20 (95% CI 0.37-3.91, p = 0.77) respectively (Figure 5C).
FIGURE 5.
Forest plots of the relative risks and 95% CIs for pruritus and rash in comparison of PD-1/-L1 inhibitor plus targeted therapy and targeted therapy alone. (A) any grade pruritus; (B) any grade rash; (C) high grade rash.
Programmed Cell Death Protein 1/Programmed Cell Death-Ligand 1 and Cytotoxic T Lymphocyte Associate Protein 4 Inhibitor Combination Therapy Vs Monotherapy
1,878 patients in five studies were included in the comparison between PD-1/PD-L1 plus CTLA-4 inhibitor and PD-/PD-L1 inhibitor alone. Compared to PD-1/-L1 inhibitor monotherapy, PD-1/-L1 inhibitor plus CTLA-4 inhibitor was associated with increased risk of pruritus and rash [any grade pruritus RR:1.76 (95% CI 1.42-2.18, p < 0.00001) (Figure 6A), high grade pruritus RR: 6.05 (95% CI 1.17-31.33, p = 0.03) (Figure 6B), any grade rash RR:1.72 (95% CI 1.29-2.31, p = 0.0003) (Figure 6C), high grade rash RR:3.89 (95% CI 1.45-10.42, p = 0.007) (Figure 6D)].
FIGURE 6.
Forest plots of the relative risks and 95% CIs for pruritus and rash in comparison of combined immunotherapy and either monotherapy: (A) any grade pruritus for PD-1/-L1 plus CTLA-4 inhibitor compared to PD-1/-L1 inhibitor; (B) high grade pruritus for PD-1/-L1 plus CTLA-4 inhibitor compared to PD-1/-L1 inhibitor; (C) any grade rash for PD-1/-L1 plus CTLA-4 inhibitor compared to PD-1/-L1 inhibitor; (D) high grade rash for PD-1/-L1 plus CTLA-4 inhibitor compared to PD-1/-L1 inhibitor; (E) any grade pruritus for PD-1/-L1 plus CTLA-4 inhibitor compared to CTLA-4 inhibitor; (F) any grade rash for PD-1/-L1 plus CTLA-4 inhibitor compared to CTLA-4 inhibitor.
For comparison of PD-1/-L1 plus CTLA-4 inhibitor to CTLA-4 inhibitor monotherapy, we included four studies with 1,813 patients total. Only any grade rash was more frequent in patients administered CTLA-4 inhibitor combined with PD-1/-L1 inhibitor, in comparison to CTLA-4 inhibitor monotherapy [any grade pruritus RR:0.98 (95% CI 0.80-1.19, p = 0.81) (Figure 6E), any grade rash RR:1.37 (95% CI 1.07-1.74, p = 0.01) (Figure 6F)]. Data for high grade pruritus and high grade rash are not reported because only two studies identified included these categories, which was not sufficient for a qualified meta-analysis.
Programmed Cell Death Protein 1/Programmed Cell Death-Ligand 1 Inhibitor Combination Chemotherapy Vs Programmed Cell Death Protein 1/Programmed Cell Death-Ligand 1 Monotherapy or Cytotoxic T Lymphocyte Associate Protein 4 Inhibitor Combination Chemotherapy
16,039 patients from 25 studies were included in this analysis. Compared to PD-1/-L1 inhibitor monotherapy, relative risk ratios (RRRs) for any grade and high grade rash developed during PD-1/-L1 inhibitor treatment combined with chemotherapy were not significantly increased (RRR for any grade pruritus was 0.30 (95% CI 0.21-0.42, p < 0.00001), RRR for any grade rash was 0.84 (95% CI 0.61-1.15, p = 0.28), RRR for high grade rash was 1.43 (95% CI 0.46-4.40, p = 0.54). A comparison between PD-1/-L1 combination chemotherapy and CTLA-4 combination chemotherapy was also conducted. PD-1/-L1 plus chemotherapy was associated with decreased risk of any grade pruritus and any grade rash, compared to CTLA-4 plus chemotherapy. RRR for any grade pruritus was 0.22 (95% CI 0.14-0.49, p < 0.00001), RRR for any grade rash was 0.29 (95% CI 0.19–0.43, p < 0.00001), and RRR for high grade rash was 0.25 (95% CI 0.04-1.73, p = 0.08) (Table 2).
TABLE 2.
Relative risk ratios of treatment regimen differences for the risk of pruritus and rash.
| Treatment scheme | No. of trials | Any-grade pruritus | No. of trials | Any-grade rash | No. of trials | 3–5 grade pruritus | No. of trials | 3–5 grade rash | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR (95%CI) | p | RR (95%CI) | p | RR (95%CI) | RR (95%CI) | RR (95%CI) | p | |||||
| A:PD-1/L1+chemotherapyVS chemotherapy | 4 | 1.39 (1.08, 1.80) | 0.01 | 5 | 1.53 (1.19, 1.98) | 0.001 | 4 | NA | NA | 5 | 2.64 (0.82, 4.16) | 0.15 |
| B: PD-1/L1 VS chemotherapy | 13 | 4.67 (3.66, 5.95) | <0.00001 | 12 | 1.82 (1.52, 2.19) | <0.00001 | 13 | 0.86 (0.28, 2.66) | 0.43 | 12 | 1.85 (0.54, 2.57) | 0.69 |
| RRR (A VS B) | — | 0.30 (0.21, 0.42) | <0.00001 | RRR (A VS B) | 0.84 (0.61, 1.15) | 0.28 | — | NA | NA | RRR (A VS B) | 1.43 (0.46, 4.40) | 0.54 |
| Treatment scheme | No. of trials | Any-grade pruritus | No. of trials | Any-grade rash | No. of trials | 3–5 grade pruritus | No. of trials | 3–5 grade rash | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR (95%CI) | p | RR (95%CI) | p | RR (95%CI) | RR (95%CI) | RR (95%CI) | p | |||||
| C: PD-1/L1+chemotherapy VS chemotherapy | 3 | 1.39 (1.08, 1.80) | 0.01 | 3 | 1.53 (1.19, 1.98) | 0.001 | 3 | NA | NA | 3 | 2.64 (0.71, 9.88) | 0.15 |
| D: CTLA-4+chemotherapy VS chemotherapy | 14 | 6.31 (4.40, 9.04) | <0.00001 | 14 | 5.32 (3.90, 7.28) | <0.00001 | 14 | 7.92 (1.86, 33.65) | 0.005 | 14 | 10.11 (2.47, 41.41) | 0.001 |
| RRR (C VS D) | — | 0.22 (0.14, 0.49) | <0.00001 | RRR (C VS D) | 0.29 (0.19, 0.43) | <0.00001 | — | NA | NA | RRR (C VS D) | 0.25 (0.04, 1.73) | 0.08 |
Subgroup Analyses
Programmed Cell Death Protein 1 Vs Programmed Cell Death-Ligand 1 Inhibitor
Subgroup analysis was performed to identify the relative impact of PD-1 and PD-L1 inhibitor on pruritus and rash. 20,769 patients from 42 studies were included in this analysis. Risks of any grade pruritus (RR: 1.93 (95% CI 1.40-2.67) p < 0.00001 Supplementary Figure 5A) and any grade rash [RR: 1.28 (95% CI 1.03-1.58) p < 0.00001 Supplementary Figure 5B] developed during PD-1 inhibitor therapy were decreased compared to PD-L1 inhibitor. When assessing high grade rash between PD-1 inhibitor and PD-L1 inhibitor therapies, no statistically significant difference was found [RR: 0.67 (95% CI 0.39-1.17) p = 0.46 Supplementary Figure 5C].
Tumor Type Subgroup Analysis
43 studies with 24,871 patients were included in this subgroup analysis. Cancer type stratification demonstrated HNSCC has a lower risk for any grade pruritus and rash, compared to all cancer types. RRs for any grade pruritus: 1.08 (95% CI 0.26-4.38, p = 0.94), high grade pruritus: 0.19 (95% CI 0.01-3.94), any grade rash: 0.49 (95% CI 0.20-1.15, p = 0.001), high grade rash: 0.18 (95% CI 0.05-0.58, p = 0.004). The RRs for any grade pruritus did not reach the statistical cutoff for significance (Supplementary Figures 6A–D).
Sensitivity Analysis and Publication Bias
Risk of bias graph and risk of bias summary are shown in Supplementary Figure 7 and Supplementary Figure 8. Sensitivity analysis showed that no single study could significantly affect the aggregated estimates (Supplementary Figure 9). However, there was mild asymmetry for RRs of pruritus and rash (Supplementary Figure 10). The Egger’s test (Supplementary Figure 11) shown some evidence of publication bias for pruritus (p = 0.005/p = 0.006) and high grade rash (p = 0.001), while the Begg’s test revealed no evidence of publication bias (Supplementary Figure 12).
Discussion
With the growing number of patients receiving ICIs, there is significant need to understand associated adverse events in order to improve therapy management. In clinical practice ICIs have shown significant efficiency in multiple tumors, both as mono-and combination therapies. The unique ICI mechanism of action (Sibaud et al., 2016) is also accompanied with a series of IRAEs, which are distinguishable from traditional adverse effects of cancer treatment. Dermatological reactions, especially pruritus and rash, are some of the most common IRAEs, and can severely affect the quality of life and psychological well-being of patients (Sibaud et al., 2016). High grade rash can impact ICI treatment efficacy through dose-limiting effects or even result in treatment discontinuation (Geisler et al., 2020). To achieve better clinical efficacy, ICI combination therapy has become more commonly used. However, few studies have been conducted to assess the risk of dermatological-specific IRAEs among multiple treatment patterns. To our knowledge, the current study is the first comprehensive assessment of the relative risk of pruritus and rash among various ICI treatment regimens.
We first compared ICI monotherapy to placebo, and both PD-1/-L1 and CTLA-4 inhibitor were associated with increased risk of any grade pruritus and rash. Notably, CTLA-4 inhibitor was associated with higher risk of high grade pruritus and rash. A comparison between PD-1/-L1 inhibitor and CTLA-4 inhibitor monotherapy was also conducted. RRs for pruritus and rash developed after PD-1/-L1 inhibitor were decreased compared to CTLA-4 inhibitor, which is in line with the current mainstream consensus that CTLA-4 inhibitor is more likely to lead to pruritus and rash (Almutairi et al., 2020; Geisler et al., 2020; Hansen et al., 2017; Sibaud et al., 2016).
Whether the risk of developing pruritus and rash correlated with different dose regimens of immune checkpoint inhibitor is an important area of focus given issues regarding patient quality of life and treatment discontinuation. Previous studies have shown no significant correlation between PD-1/-L1 inhibitor dosage and incidence of pruritus and rash (Hansen et al., 2017; Robert et al., 2014). On the contrary, a retrospective study suggested that the frequency of IRAEs (pruritus and rash included) developed after Ipilimumab increased with dose. Another study reached a similar conclusion (Golian et al., 2016) that cutaneous IRAEs related to ipilimumab are dose-related. In the current study, compared with the low dose group, RRs for any grade pruritus and rash developed after PD-1/-L1 inhibitor in the high dose group were not significantly increased. The corresponding comparison between CTLA-4 inhibitor high dose and low dose group could not be carried out because of insufficient data. Overall, given the discrepancies among findings in studies assessing dose-dependencyof rash and pruritus, further efforts should be made to investigate the problem and instruct clinical application, both in terms of mechanism and clinical research.
In order to increase the percentage of patients benefiting from ICI treatment and reduce the occurrence of IRAEs, efforts are currently being made to combine current ICIs with new checkpoint inhibitors or other treatment methods to achieve synergistic effects (Kon and Benhar, 2019). In clinical practice, PD-1/-L1 and CTLA-4 inhibitor are being combined with other anti-cancer drugs including chemotherapy, targeted therapy, radiotherapy and other immunotherapies. Although traditionally regarded as immunosuppressive agents, some preclinical studies have shown that chemotherapy may have immune-stimulatory properties (Postow et al., 2015a). Some studies indicate combination chemotherapy leads to more general adverse events (Lynch et al., 2012; Wang et al., 2021), while other studies report severe side effects (Chamoto et al., 2020) . We used the relative risk ratio (RRR) to indirectly compare the risk of pruritus and rash. RRR was used to compare PD-1/PD-L1 inhibitor monotherapy with combined chemotherapy based on PD-1/PD-L1 inhibitor, and showed that the risk of pruritus, but not rash, was increased (Table 2). These results suggest that PD-1/-L1 inhibitor combined with chemotherapy may have a tolerable dermatologic adverse profile in terms of pruritus and rash, indicating that increased efficacy through combining ICIs with chemotherapy may be feasible. Targeted therapies for oncogenic signaling pathways are also attractive partners in combination with immune checkpoint blockade (Postow et al., 2015a). Unfortunately, only 2 RCTs comparing PD-1/-L1 inhibitor and targeted therapy resulted from our database search, and RRR for PD-1/PD-L1 inhibitor plus targeted therapy compared to PD-1/-L1 monotherapy could not be calculated. When more data becomes available, further analysis of this aspect may provide useful information.
Since CTLA-4 inhibitor monotherapy showed increased risk of pruritus and rash relative to PD-1/-L1 inhibitor according to our data, RRR was calculated to investigate the difference between PD-1/-L1 plus chemotherapy and CTLA-4 plus chemotherapy. When contrasted with PD-1/-L1 inhibitor combination chemotherapy, CTLA-4 inhibitor combination chemotherapy was associated with a much higher risk of pruritus and rash (Table 2). The mechanism leading to this is not yet fully understood. The major physiological role of CTLA-4 seems to be through distinct effects on the two main subsets of cluster of differentiation four positive (CD4+) T cells: down modulation of helper T cell activity and enhancement of regulatory T (Treg) cell immunosuppressive activity (Bylicki et al., 2020; Cancela et al., 2020; Peggs et al., 2009). Blockade of the PD-1 pathway may enhance antitumor immune responses by diminishing the number and/or suppressive activity of intratumoral Treg cells (Arigami et al., 2020). It is thought that PD-1 predominantly regulates effector T cell activity within tissue and tumors, whereas CTLA-4 predominantly regulates T cell activation (Arigami et al., 2020). Although dermatologic adverse events observed with ICIs used in combination are more frequent, more severe, and longer lasting (Sibaud et al., 2016), combination immunotherapy has more extensive clinical applications due to improved efficacy. Therefore, our data suggest that PD-1/-L1 inhibitor may be preferable in patients who have suffered from previous dematologic problems. Moreover, in the case of severe dermatologic IRAEs with CTLA-4 therapy, re-challenge with an agent of a different class may be a good treatment strategy.
Subgroup analysis was performed to investigate if there was any difference in the incidence of pruritus and rash between PD-1 and PD-L1 inhibitor. Based on the known interactions of PD-1 ligands, PD-1 antibodies may have different biological activities than PD-L1 antibodies. PD-1 antibodies prevent PD-1 from interacting with PD-L1 and Programmed cell death-ligand 2(PD-L2), but do not prevent the interaction between PD-L1 and Cluster of differentiation 80(CD80). In contrast, most PD-L1 antibodies prevent the interaction between PD-L1 and CD80 and between PD-L1 and PD-1, but not the interaction between PD-1 and PD-L2. Therefore, it is possible that depending on which interaction predominates in a particular cancer, PD-1 and PD-L1 antibodies may not have redundant activity (Arigami et al., 2020). Results from subgroup analysis showed that any grade pruritus and rash developed from PD-1 inhibitor were decreased compared to PD-L1 inhibitor, while the comparison in high grade (3–5) rash did not reach a statistically significant level. Therefore, PD-1 inhibitor may be recommended in terms of decreased dermatologic adverse events (pruritus and rash) for clinical applications. In cancer type subgroup analysis, we found that patients with HNSCC may have better tolerability overall as evidenced by a lower risk for any grade pruritus. Since only 1 RCT of HNSCC was included in high grade subgroup, more efforts are needed to validate this observation.
Our study has some notable strengths. To the best of our knowledge, this is the first and most comprehensive analysis that investigated the risk of pruritus and rash among different ICI treatment regiments in multiple solid tumors. In addition, the 50 clinical trials included in our meta-analysis were all highly qualified randomized control trails, which supports the credibility of our study. Morever, we investigated the risk of not only all grade but also high grade pruritus and rash, for the management of these two side effects of differing severity. Finally, since head-to-head comparison of PD-1/PD-L1 inhibitor combination therapies and PD-1/PD-L1 inhibitor alone were not available, we used the relative risk ratio (RRR) to indirectly compare the risk of pruritus and rash. The results of our RRR analysis indicate that the added skin toxicity of chemotherapy is manageable in combination immunotherapy, which may have clinical implications.
This meta-analysis also has some limitations. Mild heterogeneity was found among the included studies. The heterogeneity may result from differences in cancer type, line of therapy, follow-up time, or other unspecified factors. Study design, blinding, dosage and frequency of drug administration in both intervention and control arm could also have resulted in heterogeneity. Thus, we utilized the random-effect model and subgroup analyses for high heterogeneity to explore possible variation in the outcomes of the included studies. What`s more, since patients included in our meta-analysis were from RCTs with strict inclusion criteria, risk of pruritus and rash could be underestimated because of their better health condition, compared with patients in real world application.
Conclusion
In summary, we identified that PD-1/-L1 inhibitor is associated with decreased risk of pruritus and rash in comparison to CTLA-4 inhibitor in both monotherapy and combined immunotherapy regimens. Additionally, pruritus and rash developed from PD-1/-L1 inhibitor are not dose-dependent. Moreover, compared to PD-1/-L1 inhibitor alone, the combination of chemotherapy with PD-1/-L1 inhibitor may not significantly increase the risk of pruritus and rash. As the most prevalent and obvious IRAEs, dermatologic adverse events such as rash and pruritus should be further studied to help manage such events and enhance patient benefits from ICI therapy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
HZ and YG collected and analyzed the data and wrote the article. HZ prepared the figures and tables. JY and NW modified the article. YG and JY provided the idea. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.640099/full#supplementary-material
References
- Almutairi A. R., McBride A., Slack M., Erstad B. L., Abraham I. (2020). Potential Immune-Related Adverse Events Associated with Monotherapy and Combination Therapy of Ipilimumab, Nivolumab, and Pembrolizumab for Advanced Melanoma: A Systematic Review and Meta-Analysis. Front. Oncol. 10, 91. 10.3389/fonc.2020.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia S. J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., et al. (2017). Durvalumab after Chemoradiotherapy in Stage III Non-small-cell Lung Cancer. N. Engl. J. Med. 377 (20), 1919–1929. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- Arigami T., Matsushita D., Okubo K., Yanagita S., Ehi K., Sasaki K., et al. (2020). Response Rate and Prognostic Impact of Salvage Chemotherapy after Nivolumab in Patients with Advanced Gastric Cancer. Oncology 98 (9), 630–636. 10.1159/000507219 [DOI] [PubMed] [Google Scholar]
- Ascierto P. A., Del Vecchio M., Robert C., Mackiewicz A., Chiarion-Sileni V., Arance A., et al. (2017). Ipilimumab 10 Mg/kg versus ipilimumab 3 Mg/kg in patients with unresectable or Metastatic Melanoma: a randomised, double-blind, Multicentre, phase 3 trial. Lancet Oncol. 18 (5), 611–622. 10.1016/s1470-2045(17)30231-0 [DOI] [PubMed] [Google Scholar]
- Beer M., T., Kwon D. E., Drake G. C., Fizazi K., Logothetis C., Gravis G., et al. (2016). Randomized, Double-Blind, Phase III Trial of Ipilimumab versus Placebo in Asymptomatic or Minimally Symptomatic Patients with Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 35 (1), 40–47. 10.1200/JCO.2016.69.1584 [DOI] [PubMed] [Google Scholar]
- Bellmunt J., de Wit R., Vaughn D. J., Fradet Y., Lee J. L., Fong L., et al. (2017). Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 376 (11), 1015–1026. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghaei H., Paz-Ares L., Horn L., Spigel D. R., Steins M., Ready N. E., et al. (2015). Nivolumab versus Docetaxel in Advanced Nonsquamous Non-small-cell Lung Cancer. N. Engl. J. Med. 373 (17), 1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros C. T., Routier A., Lambotte E., Ladurie O., Carbonnel F. L., Izzeddine F., et al. (2016). Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13 (8), 473–486. 10.1038/nrclinonc.2016.58 [DOI] [PubMed] [Google Scholar]
- Brahmer J., Reckamp K. L., Baas P., Crino L., Eberhardt W. E., Poddubskaya E., et al. (2015). Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-small-cell Lung Cancer. N. Engl. J. Med. 373 (2), 123–135. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D. A., Hou Y., Bakouny Z., Ficial M., Sant' Angelo M., Forman J., et al. (2020). Interplay of somatic alterations and immune infiltration Modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat. Med. 26 (6), 909–918. 10.1038/s41591-020-0839-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtness Barbara., Kevin J Harrington R. G., Soulières Denis., Tahara Makoto., de Castro Gilberto., Jr, Psyrri Amanda., et al. (2019). Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or Metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394 (10212), 1915–1928. 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- Bylicki O., Guisier F., Monnet I., Doubre H., Gervais R., Janicot H., et al. (2020). Efficacy and safety of programmed cell-death-protein-1 and its ligand inhibitors in pretreated patients with epidermal growth-factor receptor-Mutated or anaplastic lymphoma kinase-translocated lung adenocarcinoma. Medicine (Baltimore) 99 (3), e18726. 10.1097/md.0000000000018726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela D. B., Gómez-De Rueda F., Antolinos Pérez M. J., Jiménez-Morales A., López-Hidalgo J. L. (2020). Acute coronary syndrome and recurrent colitis as immune-related adverse events in a lung cancer patient. J. Oncol. Pharm. Pract. 26 (1), 252–255. 10.1177/1078155219865596 [DOI] [PubMed] [Google Scholar]
- Carbone D. P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., et al. (2017). First-Line Nivolumab in Stage IV or Recurrent Non-small-cell Lung Cancer. N. Engl. J. Med. 376 (25), 2415–2426. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste L. e., Meyer Nicolas., Mortier Laurent., Marquez-Rodas Ivan., Robert Caroline., Rutkowski Piotr., et al. (2019). Evaluation of Two Dosing Regimens for Nivolumab in Combination with Ipilimumab in Patients with Advanced Melanoma: Results from the Phase IIIb/IV CheckMate 511 Trial. J. Clin. Oncol. 37 (11), 867–875. 10.1200/JCO.18.01998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoto K., Hatae R., Honjo T. (2020). Current issues and perspectives in PD-1 blockade cancer immunotherapy. Int. J. Clin. Oncol. 25 (5), 790–800. 10.1007/s10147-019-01588-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. E. W., Soulières D., Le Tourneau C., Dinis J., Licitra L., Ahn M.-J., et al. (2019). Pembrolizumab versus Methotrexate, docetaxel, or cetuximab for recurrent or Metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. The Lancet 393 (10167), 156–167. 10.1016/s0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- Collins L. K., Chapman M. S., Carter J. B., Samie F. H. (2017). Cutaneous adverse effects of the immune checkpoint inhibitors. Curr. Probl. Cancer 41 (2), 125–128. 10.1016/j.currproblcancer.2016.12.001 [DOI] [PubMed] [Google Scholar]
- Da L., Teng Y., Wang N., Zaguirre K., Liu Y., Yali Q., et al. (2020). Organ-Specific Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitor Monotherapy versus Combination Therapy in Cancer: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 10, 1671. 10.3389/fphar.2019.01671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont A. M. M., Blank Christian. U., Mandala Mario., Henrik Schmidt. (2018). Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 378 (19), 1789–1801. 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- Eggermont A. M. M., Chiarion-Sileni V., Grob J.-J., Dummer R., Wolchok J. D., Schmidt M. D., et al. (2015). Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III Melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 16 (5), 522–530. 10.1016/S1470-2045(15)70122-1 [DOI] [PubMed] [Google Scholar]
- Ellis S. R., Vierra A. T., Millsop J. W., Lacouture M. E., Kiuru M. (2020). Dermatologic toxicities to immune checkpoint inhibitor therapy: A review of histopathologic features. J. Am. Acad. Dermatol. 83 (4), 1130–1143. 10.1016/j.jaad.2020.04.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto P., Giulia Grizzi M. G., Ghidini Antonio., Ratti §Margherita., Panni Stefano., Cabiddu Mary., et al. (2020). Immune-related Adverse Events and Survival in Solid Tumors Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Immunother. 43 (1), 1–7. 10.1097/CJI.0000000000000300 [DOI] [PubMed] [Google Scholar]
- Ferris R. L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A. D., Licitra L., et al. (2016). Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 375 (19), 1856–1867. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R. S., Ryoo Baek-Yeol., Philippe Merle., Kudo Masatoshi., Mohamed Bouattour., Lim Ho. Yeong., et al. (2019). Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized,Double-Blind, Phase III Trial. J. Clin. Oncol. 38 (3), 193–202. 10.1200/JCO.19.01307 [DOI] [PubMed] [Google Scholar]
- Gandhi L., Rodriguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., et al. (2018). Pembrolizumab plus Chemotherapy in Metastatic Non-small-cell Lung Cancer. N. Engl. J. Med. 378 (22), 2078–2092. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- Geisler A. N., Phillips G. S., Barrios D. M., Wu J., Leung D. Y. M., Moy A. P., et al. (2020). CME Part II: Immune checkpoint inhibitor-related dermatologic adverse events. J. Am. Acad. Dermatol. 83 (5), 1255–1268. 10.1016/j.jaad.2020.03.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golian d., E., Kwong B. Y., Swetter S. M., Pugliese S. B. (2016). Cutaneous Complications of Targeted Melanoma Therapy. Curr. Treat. Options. Oncol. 17 (11), 57. 10.1007/s11864-016-0434-0 [DOI] [PubMed] [Google Scholar]
- Govindan R., Szczesna Aleksandra., Ahn Myung-Ju., Schneider Claus-Peter., Mella Pablo. Fernando. Gonzalez., Barlesi Fabrice., et al. (2017). Phase III Trial of Ipilimumab Combined with Paclitaxel and Carboplatin in Advanced Squamous Non–small-cell Lung Cancer. JOURNAL CLINICAL ONCOLOGY 35 (30), 3449–3457. 10.1200/JCO.2016.71.7629 [DOI] [PubMed] [Google Scholar]
- Hansen A. R., Khoja L., Day D., Chen T. W. W., Siu L. L. (2017). Tumor- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann. Oncol. 28 (10), 2377–2385. 10.1093/annonc/mdx286 [DOI] [PubMed] [Google Scholar]
- Hellmann M. D., Ciuleanu T. E., Pluzanski A., Lee J. S., Otterson G. A., Audigier-Valette C., et al. (2018). Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 378 (22), 2093–2104. 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R. S., Baas P., Kim D.-W., Felip E., Pérez-Gracia J. L., Han J.-Y., et al. (2016). Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet 387 (10027), 1540–1550. 10.1016/s0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- Horn L., Mansfield A. S., Szczesna A., Havel L., Krzakowski M., Hochmair M. J., et al. (2018). First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 379 (23), 2220–2229. 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- Janjigian Y. Y., Bendell Johanna., Calvo Emiliano., Kim Joseph. W., Ascierto Paolo. A., Sharma Padmanee., et al. (2018). CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients with Metastatic Esophagogastric Cancer. JOURNAL CLINICAL ONCOLOGY 36 (28), 2836–2844. 10.1200/JCO.2017.76.6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.-K., Boku N., Satoh T., Ryu M.-H., Chao Y., Kato K., et al. (2017). Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet 390 (10111), 2461–2471. 10.1016/s0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- Kato K., Cho B. C., Takahashi M., Okada M., Lin C.-Y., Chin K., et al. (2019). Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a Multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20 (11), 1506–1517. 10.1016/s1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- Kon E., Benhar I. (2019). Immune checkpoint inhibitor combinations: Current efforts and important aspects for success. Drug Resist. Updat 45, 13–29. 10.1016/j.drup.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Kwon E. D., Drake C. G., Scher H. I., Fizazi K., Bossi A., van den Eertwegh A. J. M., et al. (2014). Ipilimumab versus placebo after radiotherapy in patients with Metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a Multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15 (7), 700–712. 10.1016/s1470-2045(14)70189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer C. J., Gadgeel S. M., Borghaei H., Papadimitrakopoulou V. A., Patnaik A., Powell S. F., et al. (2016). Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 17 (11), 1497–1508. 10.1016/s1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J. J., Cowey C. L., Lao C. D., et al. (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 373 (1), 23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long G. V., Atkinson V., Lo S., Sandhu S., Guminski A. D., Brown M. P., et al. (2018). Combination nivolumab and ipilimumab or nivolumab alone in Melanoma brain Metastases: a Multicentre randomised phase 2 study. Lancet Oncol. 19 (5), 672–681. 10.1016/s1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- Lynch T. J., Bondarenko I., Luft A., Serwatowski P., Barlesi F., Chacko R., et al. (2012). Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, Multicenter phase II study. J. Clin. Oncol. 30 (17), 2046–2054. 10.1200/JCO.2011.38.4032 [DOI] [PubMed] [Google Scholar]
- Maio M., Scherpereel A., Calabrò L., Aerts J., Perez S. C., Bearz A., et al. (2017). Tremelimumab as second-line or third-line treatment in relapsed Malignant Mesothelioma (DETERMINE): a Multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 18 (9), 1261–1273. 10.1016/s1470-2045(17)30446-1 [DOI] [PubMed] [Google Scholar]
- Mok T. S. K., Wu Y.-L., Kudaba I., Kowalski D. M., Cho B. C., Turna H. Z., et al. (2019). Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or Metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. The Lancet 393 (10183), 1819–1830. 10.1016/s0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- Motzer R. J., Rini B. I., McDermott D. F., George S., Hammers H. J., Srinivas S., et al. (2015b). Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 373 (19), 1803–1813. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R. J., Rini B. I., McDermott D. F., Redman B. G., Kuzel T. M., Harrison M. R., et al. (2015a). Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J. Clin. Oncol. 33 (13), 1430–1437. 10.1200/jco.2014.59.0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer Robert. J., Konstantin Penkov J. H., Rini Brian., Albiges Laurence., Campbell Matthew. T., Venugopal Balaji., et al. (2019). Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 380 (12), 1103–1115. 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima T. F., Shachar S. S., Nyrop K. A., Muss H. B. (2017). Safety and Tolerability of PD-1/pd-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist 22 (4), 470–479. 10.1634/theoncologist.2016-0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly E. M., Oh D. Y., Dhani N., Renouf D. J., Lee M. A., Sun W., et al. (2019). Durvalumab with or without Tremelimumab for Patients with Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 5 (10), 1431–1438. 10.1001/jamaoncol.2019.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares L., Luft A., Vicente D., Tafreshi A., Gumus M., Mazieres J., et al. (2018). Pembrolizumab plus Chemotherapy for Squamous Non-small-cell Lung Cancer. N. Engl. J. Med. 379 (21), 2040–2051. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- Peggs K. S., Quezada S. A., Chambers C. A., Korman A. J., Allison J. P. (2009). Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206 (8), 1717–1725. 10.1084/jem.20082492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow A., M., Callahan M. K., Wolchok J. D. (2015a). Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 33 (17), 1974–1982. 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow A., M., Chesney Jason., Anna C., Pavlick D. O., Robert C. (2015b). Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 372, 2006–2017. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T., Durán I., van der Heijden M. S., Loriot Y., Vogelzang N. J., De Giorgi U., et al. (2018). Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or Metastatic urothelial carcinoma (IMvigor211): a Multicentre, open-label, phase 3 randomised controlled trial. The Lancet 391 (10122), 748–757. 10.1016/s0140-6736(17)33297-x [DOI] [PubMed] [Google Scholar]
- Reck M., Bondarenko I., Luft A., Serwatowski P., Barlesi F., Chacko R., et al. (2013). Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, Multicenter phase 2 trial. Ann. Oncol. 24 (1), 75–83. 10.1093/annonc/mds213 [DOI] [PubMed] [Google Scholar]
- Reck M., Luft A., Szczesna A., Havel L., Kim S. W., Akerley W., et al. (2016). Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 34 (31), 3740–3748. 10.1200/jco.2016.67.6601 [DOI] [PubMed] [Google Scholar]
- Ribas A., Kefford R., Marshall M. A., Punt C. J., Haanen J. B., Marmol M., et al. (2013). Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced Melanoma. J. Clin. Oncol. 31 (5), 616–622. 10.1200/jco.2012.44.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Puzanov Igor., Dummer Reinhard., Schadendorf Dirk., Hamid Omid., Robert Caroline., et al. (2015). Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory Melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 16 (8), 908–918. 10.1016/s1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini B. I., Plimack E. R., Stus V., Gafanov R., Hawkins R., Nosov D., et al. (2019a). Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 380 (12), 1116–1127. 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- Rini Brian. I., Thomas Powles M. B. A., Escudier Bernard., McDermott David. F., Suarez Cristina., Bracarda Sergio., et al. (2019b). Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated Metastatic renal cell carcinoma (IMmotion151): a Multicentre, open-label, phase 3, randomised controlled trial. Lancet 393 (10189), 2404–2415. 10.1016/S0140-6736(19)30723-8 [DOI] [PubMed] [Google Scholar]
- Robert Caroline., Schachter Jacob., Long Georgina. V., Arance Ana., Grob Jean. Jacques. (2015b). Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 372 (26), 2521–2532. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- Robert C., Ribas A., Wolchok J. D., Hodi F. S., Hamid O., Kefford R., et al. (2014). Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced Melanoma: a randomised dose-comparison cohort of a phase 1 trial. The Lancet 384 (9948), 1109–1117. 10.1016/s0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- Robert C., Thomas Luc., Bondarenko Igor., O’Day Steven., Weber Jeffrey., Garbe Claus., et al. (2011). Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 364 (26), 2517–2526. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- Robert Caroline., Long Georgina. V., Brady Benjamin. (2015a). Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 372 (4), 320–330. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- Schmid P., Adams S., Rugo H. S., Schneeweiss A., Barrios C. H., Iwata H., et al. (2018). Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 379 (22), 2108–2121. 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- Sibaud V., Meyer N., Lamant L., Vigarios E., Mazieres J., Delord J. P. (2016). Dermatologic complications of anti-PD-1/pd-L1 immune checkpoint antibodies. Curr. Opin. Oncol. 28 (4), 254–263. 10.1097/CCO.0000000000000290 [DOI] [PubMed] [Google Scholar]
- Siu L. L., Even C., Mesia R., Remenar E., Daste A., Delord J. P., et al. (2019). Safety and Efficacy of Durvalumab with or without Tremelimumab in Patients with PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol. 5 (2), 195–203. 10.1001/jamaoncol.2018.4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski M. A., Jotte R. M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N., et al. (2018). Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 378 (24), 2288–2301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- Velasco G. D., Je Y., Bosse D., Awad M. M., Ott P. A., Moreira R. B., et al. (2017). Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/pd-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 5 (4), 312–318. 10.1158/2326-6066.CIR-16-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Liang H., Wang W., Zhao S., Cai X., Zhao Y., et al. (2021). Immune-related adverse events of a PD-L1 inhibitor plus chemotherapy versus a PD-L1 inhibitor alone in first-line treatment for advanced non-small cell lung cancer: A Meta-analysis of randomized control trials. Cancer 127 (5), 777–786. 10.1002/cncr.33270 [DOI] [PubMed] [Google Scholar]
- Weber J., Mandala M., Del Vecchio M., Gogas H. J., Arance A. M., Cowey C. L., et al. (2017). Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 377 (19), 1824–1835. 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- Weber J. S., D'Angelo S. P., Minor D., Hodi F. S., Gutzmer R., Neyns B., et al. (2015). Nivolumab versus chemotherapy in patients with advanced Melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16 (4), 375–384. 10.1016/s1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- Wolchok J. D., Neyns B., Linette G., Negrier S., Lutzky J., Thomas L., et al. (2010). Ipilimumab Monotherapy in patients with pretreated advanced Melanoma: a randomised, double-blind, Multicentre, phase 2, dose-ranging study. Lancet Oncol. 11 (2), 155–164. 10.1016/s1470-2045(09)70334-1 [DOI] [PubMed] [Google Scholar]
- Yang W. L., Li S., Yang Q. (2019). Risk of dermatologic and Mucosal adverse events associated with PD-1/pd-L1 inhibitors in cancer patients: A Meta-analysis of randomized controlled trials. Medicine (Baltimore) 98 (20), e15731. 10.1097/MD.0000000000015731 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.