Abstract
Background:
Smoking cessation has been reported to benefit patients even after a diagnosis of lung cancer. We studied the smoking behavior of patients who participated in a phase 3 trial of adjuvant therapy following resection of stages IB-IIIA non-small cell lung cancer (NSCLC).
Methods:
ECOG-ACRIN 1505 was conducted to determine whether the addition of bevacizumab to adjuvant chemotherapy would improve overall survival (OS) for patients with early stage NSCLC. Studying the association between smoking status and OS was a secondary endpoint. Patients completed a questionnaire about their smoking habits at baseline, 3, 6, 9 and 12 months.
Results:
1501 patients were enrolled and 99.8%, 95%, 94%, 93%, and 93% responded to the questionnaire at baseline, 3, 6, 9 and 12 months, respectively. 90% reported a current or previous history of cigarette smoking. 60% of non-smokers at enrollment reported smoking after diagnosis (before randomization); however, 1% of them reported smoking at 12 months. 94% of respondents smoked none/fewer cigarettes daily at 12 months. The incidence of grades 3-5 toxicity on treatment was 68%, 76%, and 72% in never, former, and current smokers, respectively (p=0.05). The disease-free survival (DFS) for never-smokers relative to current and former smokers was (HR 0.93, p=0.64, HR 1.05, p=0.72), and OS was (adjusted HR for death 0.54, p=0.005, adjusted HR for death 0.68, p=0.03), respectively.
Conclusion:
This is the first comprehensive, prospective report of smoking habits in NSCLC patients from a phase III early stage trial. There was a high rate of smoking reduction and cessation following study entry. DFS did not differ significantly between smokers and never smokers, though there were less grade 3-5 toxicities and more favorable OS in never-smokers.
Introduction
The link between tobacco use and cancer development has been well established, and continues to be responsible for approximately 30% of all cancer-related deaths.1 Lung cancer is among the most common tobacco-driven tumors, as 85% of all patients diagnosed with lung cancer have a smoking history.2 Fortunately, decades of public health initiatives in the US have led to decreased tobacco usage. However, the adverse effect of tobacco smoking is not limited just to the risk of lung cancer development. In cancer patients, the 2014 Report of the Surgeon General found an approximate 50% median increase in mortality for patients reporting current smoking with a lesser effect noted in patients reporting former smoking.3 Retrospective studies have reported that patients who continue smoking following the diagnosis of lung cancer have worse outcomes. Patients who continue smoking at the time of diagnosis have a significantly decreased performance status at both 6 and 12 months, regardless of other factors such as stage or comorbidities.4 They also experience increased pain levels over former or never smokers.5 Additionally, patients with NSCLC who continue to use tobacco after diagnosis have a decreased overall survival (OS) compared to non-smoking NSCLC patients.6, 7 However, lung cancer clinical trials have not adequately performed prospective assessments evaluating the patterns of tobacco use and cessation and the effects on outcomes.8, 9
ECOG-ACRIN 1505 was a randomized, phase 3 trial of platinum doublet chemotherapy with or without bevacizumab for resected stages IB, II and IIIA NSCLC. The study noted no difference in OS (HR 0.99, 95% CI 0.82-1.19) or DFS (HR 0.99, 95% CI: 0.86–1.15) between the two treatment arms.10 We utilized prospectively collected smoking assessments to evaluate the following endpoints: smoking pattern (smoking habit throughout 1 year of follow-up), patient characteristics (demographics and treatment compliance) and outcomes (disease-free survival (DFS), OS, and toxicity) in this patient cohort according to baseline smoking status.
Methods
ECOG-ACRIN 1505 enrolled patients with stages IB (<= 4cm), II and IIIA NSCLC following surgical resection. The trial methods have been published previously.10 The first and last patient were enrolled on July 19 2007 and October 17 2013. The cutoff date of the data presented here is October 20th, 2015 and the median follow-up (95% CI) is 50.4 (47.8, 52.9) months. Studying the patterns of tobacco use and association between smoking status and outcome was a secondary endpoint in the study. Patients completed a questionnaire about their smoking habits at baseline, 3, 6, 9 and 12 months after study entry. Per protocol, the association of baseline smoking status with overall survival was to be evaluated. The baseline questionnaire asked about patients’ smoking history including age when patient started smoking consistently, current smoking status, and average number of cigarettes smoked per day since first exposure. The follow-up surveys asked about current smoking status, habit after lung cancer diagnosis and number of cigarettes smoked. Both baseline and follow-up smoking surveys contained a section for NicAlert test result for patients who reported that they were not a current cigarette smoker. Per protocol, the questionnaires were mandatory for all patients; the NicAlert test was optional, however these tests were not completed due to lack of funding.
Smoking status was derived from the baseline smoking survey and patients were grouped into 3 categories: (1) Never Smoker (patients who responded ‘No’ to “Has the patient ever smoked during his/her entire life?”) (2) Former Smoker (patients that reported ‘Yes’ to “Has the patient ever smoked during his/her entire life?” and that further reported ‘No’ to “Does the patient currently Smoke?”) and (3) Current Smoker (patients that responded ‘Yes’ to “Does the patient currently Smoke?”). The terms current/former/never smoker relate to smoking status at the time of trial enrollment unless otherwise defined. Differences in smoking pattern, toxicity, and patient characteristics by smoking status were evaluated using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Log-rank tests were used to assess differences in time-to-event outcomes by smoking status, and Cox modeling was used to estimate hazard ratios. OS was defined as the time from randomization to death from any cause, with censoring defined as the last date of follow-up. DFS was defined as time of randomization to disease recurrence, new lung cancer primary, second lung cancer primary, or death, whichever occurred first, with censoring defined at the date of the last disease assessment. Randomization was stratified by (1) type of chemotherapy, (2) 6th edition AJCC stage, (3) histology, and (4) gender.6 No adjustments have been made for multiple comparisons and all p-values are two-sided with significance defined at the 0.05 level.
Results
Table 1 presents the patients baseline smoking information. Questionnaire compliance among patients with defined smoking status was 99.8% (1479/1482) at baseline. Supplemental Figure 1 shows questionnaire completion adjusted by survival status at 3, 6, 9, and 12 months. The survey completion rate was 93% at 12 months. In total, 90% of respondents reported having a smoking history, with a median starting age of 17 years and a median age of stopping of 55 years. At the time of study enrollment, 160 (11%) reported current smoking, 1167 (79%) reported former smoking, and 155 (10%) reported never smoking. Of patients reporting former smoking at trial enrollment, 689 (60%) reported smoking between diagnosis and trial enrollment (Table 1).
Table 1.
Baseline Smoking Survey Results
| Variable | N |
|---|---|
| Total number of patients enrolled to E1505 | 1501 |
| Number of patients with baseline smoking status | 1482 (99%) |
| Ever smoked? | |
| Yes | 1344 (90%) |
| No | 154 (10%) |
| Missing/Unknown/Refused | 3 |
| Median age started smoking [years], (Q1, Q3) | 17 (15,19) |
| Median age stopped smoking [years], (Q1, Q3) | 55 (46-62) |
| Number of cigarettes/day, (Q1, Q3) | 20 (20,30) |
| Current smoking at enrollment? | |
| Yes | 160 (12%) |
| No | 1176 (88%) |
| Missing/Unknown/Refused | 165 |
| If former smoker at enrollment, reported smoking after diagnosis, but before trial enrollment? | |
| Yes | 689 (60%) |
| No | 467 (40%) |
| Missing/Unknown/Refused | 20 |
| Smoke cigars? | |
| Yes [median # cigars/day] | 48 (4%) [2] |
| No | 1275 (96%) |
| Missing/Unknown/Refused | 178 |
Women constituted a greater percentage (74%) of never smokers than either current (58%) or former (46%) smokers (p < 0.001). Current smokers had a significantly lower body mass index (BMI) than either former or never smokers (23.9 vs. 26.9 vs. 27.1 (p < 0.001), respectively) and trended towards greater body weight loss (weight loss > 5%, 28% vs. 21% and 15%, p=0.06, respectively). There were no differences in dose reductions for chemotherapy (p=0.75) or bevacizumab (p=0.95) between smokers and never smokers. The median number of chemotherapy cycles was similar (4 cycles) for current, former, and never smokers (Table 2).
Table 2.
Demographic and Treatment Characteristics by Baseline Smoking Status at Trial Enrollment
| Variable | Never –smoker N=155 |
Former- smoker*,x N=1167 |
Current- smokert N=160 |
P-value | |
|---|---|---|---|---|---|
| Median Age (Yrs) | 59 | 62 | 56 | p < 0.001 | |
| Female | 114 (74%) | 539 (46%) | 92 (58%) | p < 0.001 | |
| Race | p < 0.001 | ||||
| White | 114 (76%) | 1043 (90%) | 131 (83%) | ||
| Black | 20 (13%) | 84 (7%) | 25 (16%) | ||
| Asian | 17 (11%) | 18 (2%) | 1 (<1%) | ||
| Hawaiian | - | 5 (<1%) | - | ||
| Native American | - | 5 (<1%) | 1 (<1%) | ||
| Missing/Unknown/Not reported | 4 | 12 | 2 | ||
| Ethnicity | p = 0.004 | ||||
| Hispanic | 12 (8%) | 33 (3%) | 3 (2%) | ||
| Non-Hispanic | 138 (92%) | 1064 (97%) | 150 (98%) | ||
| Missing/Unknown/Not reported | 5 | 70 | 7 | ||
| >5% Weight loss in Previous 6 Months | 23 (15%) | 239 (21%) | 44 (28%) | p = 0.02 | |
| Median BMI | 27.1 | 26.9 | 23.9 | p < 0.001 | |
| Months from Surgery to Randomization (median, IQR) | 1.63 [1.45, 1.94] | 1.68 [1.45, 2.04] | 1.77 [1.51, 2.14] | p = 0.054 | |
| Study Assigned Treatment Arm | p = 0.76 | ||||
| Arm A (Chemo.) | 81 (52%) | 581 (50%) | 77 (48%) | ||
| Arm B (Chemo. + Bev.) | 74 (48%) | 586 (50%) | 83 (52%) | ||
| Bevacizumab Dose Modifications | No | 24 | 194 | 29 | p = 0.95 |
| Yes | 49 | 381 | 50 | ||
| Total | 73 | 575 | 79 | ||
| Chemotherapy Dose Modifications | No | 65 | 521 | 70 | p = 0.75 |
| Yes | 89 | 624 | 86 | ||
| Total | 154 | 1145 | 156 | ||
| Median number of Bevacizumab cycles | 8 | 9 | 12 | p = 0.36 | |
| Median number of Chemotherapy cycle | 4 | 4 | 4 | p = 0.29 |
Quit smoking prior to study enrollment
9 patients among 1176 patients who reported not smoking at baseline were never smokers who were not expected to complete the details about baseline smoking status.
17 patients among 1344 patients who reported ever smoking in their lifetime did not complete the question about smoking at baseline and could therefore not be classified as current or former smokers.
We next sought to define the smoking patterns of patients and how they changed over the course of treatment. For the 12 months that patients were followed and completed questionnaires, the overall number of patients who smoked did not significantly change (Supplemental Figure 2). Of the 60% of patients who reported continued smoking after their lung cancer diagnosis but reported not smoking at the time of enrollment, 1% reported smoking at 12 months. At the 12-month mark, 94% of patients reported that they were smoking none/fewer cigarettes than at baseline, while only 4% report smoking more (Table 3). 87% of patients reported smoking none/less than 10 cigarettes per day at 12 months.
Table 3.
Smoking Habits at Follow-Up Timepoints by Baseline Smoking Status at Trial Enrollment
| Variable | Category | Baseline Smoking Status, n (%) | Total | ||
|---|---|---|---|---|---|
| Never (n=155) |
Former (n=1167) |
Current (n=160) |
(n=1482) | ||
| Smoking Habit, 3 mos. | Stopped smoking | 1 (2.3) | 217 (40.3) | 15 (11.1) | 233 (32.5) |
| No change, still does not smoke | 42 (97.7) | 280 (52.0) | 1 (0.7) | 323 (45.1) | |
| No change, smoke the same amount | - | 4 (0.7) | 20 (14.8) | 24 (3.4) | |
| Smokes more cigarettes now (per day) | - | 10 (1.9) | 3 (2.2) | 13 (1.8) | |
| Smokes fewer cigarettes now (per day) | 27 (5.0) | 96 (71.1) | 123 (17.2) | ||
| Unknown/missing | 112 | 629 | 25 | 766 | |
| Smoking Habit, 6 mos. | Stopped smoking | 2 (5.9) | 174 (36.7) | 13 (10.6) | 189 (30.0) |
| No change, still does not smoke | 32 (94.1) | 238 (50.2) | 3 (2.4) | 273 (43.3) | |
| No change, smoke the same amount | - | 8 (1.7) | 15 (12.2) | 23 (3.7) | |
| Smokes more cigarettes now (per day) | - | 10 (2.1) | 4 (3.3) | 14 (2.2) | |
| Smokes fewer cigarettes now (per day) | 44 (9.3) | 88 (71.5) | 132 (20.9) | ||
| Unknown/missing | 121 | 693 | 37 | 851 | |
| Smoking Habit, 9 mos. | Stopped smoking | 2 (5.0) | 147 (34.3) | 11 (9.3) | 160 (27.3) |
| No change, still does not smoke | 38 (95.0) | 210 (49.1) | 3 (2.5) | 251 (42.8) | |
| No change, smoke the same amount | - | 4 (0.9) | 17 (14.4) | 21 (3.6) | |
| Smokes more cigarettes now (per day) | - | 16 (3.7) | 4 (3.4) | 20 (3.4) | |
| Smokes fewer cigarettes now (per day) | 51 (11.9) | 83 (70.3) | 134 (22.9) | ||
| Unknown/missing | 115 | 739 | 42 | 896 | |
| Smoking Habit,12 mos. | Stopped smoking | 1 (2.8) | 134 (31.8) | 13 (11.2) | 148 (25.8) |
| No change, still does not smoke | 35 (97.2) | 212 (50.2) | 3 (2.6) | 250 (43.6) | |
| No change, smoke the same amount | - | 6 (1.4) | 11 (9.5) | 17 (3.0) | |
| Smokes more cigarettes now (per day) | - | 15 (3.6) | 5 (4.3) | 20 (3.5) | |
| Smokes fewer cigarettes now (per day) | 55 (13.0) | 84 (72.4) | 139 (24.2) | ||
| Unknown/missing | 119 | 745 | 44 | 908 | |
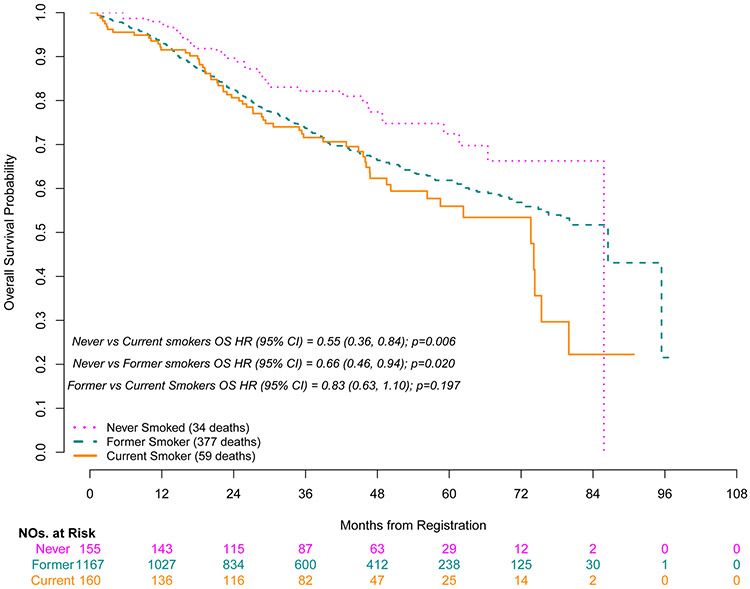
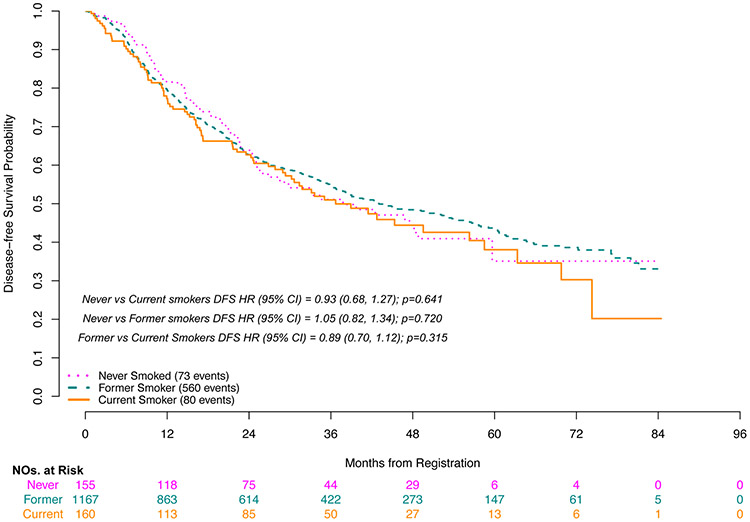
Grade 3-5 toxicities during adjuvant therapy occurred more frequently in the current/former group than in never smokers (72%/77% vs. 68%, p=0.05, respectively). Grade 3-5 lung infections were documented in 4.4% of current smokers and were not reported in never smokers (p=0.02). Grade 3-5 anemia was also lowest in never smokers vs current/former smokers (3% vs. 5%/8%, p=0.02, respectively) (Table 4). Never smokers had improved OS compared to former smokers (HR for death 0.66, p=0.02) and current smokers (HR for death 0.55, p=0.006) (Figure 1). Smoking remained an independent predictor of OS in a multivariable Cox model after adjusting for other known prognostic factors; never smokers had improved OS compared to former smokers (adjusted HR for death 0.68, p=0.03) and current smokers (adjusted HR for death 0.54, p=0.005) (Table 5). However, there were no significant differences seen in DFS for never smokers when compared to either current or former smokers (HR 0.93, p=0.64, HR 1.05, p=0.72, respectively) (Figure 2). Sensitivity analyses of these time to event analyses modeling smoking status as a time-varying covariate yielded nearly identical results (Supplemental Table S1).
Table 4.
Salient Treatment-related Toxicity by Smoking Status at Trial Enrollment
| Toxicity | Never | Former | Current | P-value | |||
|---|---|---|---|---|---|---|---|
| G 3/4 | G5 | G 3/4 | G5 | G3/4 | G5 | ||
| Anemia | 2.5% | - | 8.1% | - | 5.1% | - | 0.02 |
| Neutropenia | 29.9% | - | 37.4% | - | 23.7% | - | 0.001 |
| Dyspnea | - | - | 5.8% | - | 5.8% | 0.001 | |
| Lung infection | - | - | 2.9% | 0.1% | 3.8% | 0.6% | 0.02 |
| Mucositis | 1.3% | - | 0.8% | - | 3.2% | - | 0.03 |
| Worst Degree | 68.2% | - | 74.2% | 2.3% | 67.3% | 4.5% | 0.05 |
Figure 1.
Overall Survival by Baseline Smoking Status
Table 5.
Multivariable Cox model of OS. Variables considered for inclusion in the full model of hazard ratios for death include those shown in the final model below, as well as BMI, performance status and histology; the latter 3 variables were excluded from the final model as they were no longer statistically significant at the 0.05 level.
| Variable | HR | p-value | 95% CI |
|---|---|---|---|
| Never vs. Current Smokers | 0.54 | 0.005 | 0.35-0.83 |
| Never vs. Former Smokers | 0.68 | 0.03 | 0.47-0.97 |
| Age | 1.02 | 0.007 | 1.00-1.03 |
| Male vs. Female | 1.2 | 0.05 | 1.00-1.45 |
| Stage IIIA disease vs Stage I/II | 1.75 | <0.001 | 1.45-2.11 |
| Weight loss in prior 6 mos > 10% vs. not | 1.4 | 0.04 | 1.01-1.94 |
Figure 2.
Disease-Free Survival by Baseline Smoking Status
Discussion
Smoking patterns following the diagnosis and treatment of early stage NSCLC have not been well-defined, relying mostly on small observational or retrospective data. One systematic review found that the percentage of patients who continued to smoke after a lung cancer diagnosis ranged from 6% to 83%.6 More detailed patient tobacco usage data are needed in order to better educate patients and clinicians, as well as to design smoking cessation programs. These data are particularly important for patients with early stage disease where 5-year survival varies between 25%-90% after surgery, varying by stage.11
The negative effects of tobacco use on cancer patients quality of life, response to treatment, and survival has now been well described as summarized in the 2014 Report of the Surgeon General.3 However, to our knowledge, our study is the first report on the smoking habits of patients with early stage NSCLC in a large, randomized clinical trial. Consistent with the known epidemiology of NSCLC, the vast majority of patients enrolled were either current or former tobacco users. Encouragingly, there was a high rate of smoking cessation after the diagnosis of lung cancer and those patients who did smoke consumed fewer cigarettes. Though abstinence from tobacco use is associated with the greatest improvement in quality of life and OS in the general population, reduction of tobacco use has also been shown to improve these outcomes.12, 13
Current/former smokers in our study had greater weight loss and lower BMIs than non-smokers, which likely contributed to their worse survival and toxicity outcomes. Lung cancer patients of all stages who presented with weight loss have been reported to have decreased OS compared to patients who did not present with weight loss (6.4 vs. 9.2 months, p<0.001, respectively).14 The patients on ECOG-ACRIN 1505 all underwent surgery for their early stage lung cancer, and the association between poor nutrition and worse outcomes is well defined in this population.15, 16 The correlation between low BMI and weight loss, tobacco smoking, and poorer outcomes highlight the need to address these issues at diagnosis in order to maximize outcomes for lung cancer patients.
The severe consequences from lung infections associated with cigarette smoking are likely due to a variety of inflammatory processes.17, 18 While on study, none of the patients in the never smoker group developed grade 3 or higher lung infections, vs. 3% and 4.5% of former smokers and current smokers, respectively. This included several grade 5 events. Overall, there were no grade 5 treatment related events overall for never smokers. This finding highlights real consequences of continuing to smoke after a lung cancer diagnosis and should be discussed by the patient and clinician when starting treatment. While it was relatively rare, some patients relapsed and restarted smoking after starting treatment, highlighting the need to continue to support patients in their cessation efforts.
Additionally, there was significant overall survival benefit for never smokers, which was even more pronounced when compared to patients who continued to smoke on treatment. While we demonstrated these survival benefits in a prospective clinical trial, other retrospective studies have shown that a greater pack-year smoking history and continued tobacco use worsens prognosis for patients treated with curative intent.6, 19, 20 The survival benefit of smoking cessation for advanced stage patients is understudied and warrants further investigation. We found worse OS for smokers and an absence of a difference in disease-free survival, which was likely related to the clinical co-morbidities caused by smoking and the difficulties they cause during cancer treatment and recovery.21 Also, NSCLC in never smokers may have a different molecular biology than the NSCLC of smokers, such as harboring an EGFR or ALK mutation, contributing to a difference in outcomes, but mutation status was not available for this study. Finally, when the study started in 2005, the presently available standard questionnaires to obtain smoking data were not available. Therefore, the ECOG thoracic committee members developed the questionnaire that was used for the 1505 study.
Despite the evaluation of smoking habits and outcomes in an early stage lung cancer population, there are some limitations to our study. The most accurate way to assessment of cigarette smoking status is with biochemical confirmation. Though ECOG-ACRIN 1505 protocol had included an optional biochemical verification step, it was not performed since the study did not provide reimbursement for the test. Therefore, our findings are subject to the risk of reporting bias associated with surveys, especially on sensitive topics such as smoking. However, we demonstrated an excellent compliance with the in-depth smoking survey. We believe this allows us to make accurate conclusions about the data. In addition, smoking data collection was limited to the 12 months following trial enrollment. Finally, data were not collected regarding tobacco cessation efforts made by the patient and health care providers.
Our study showed that smoking after diagnosis and at the time of systemic therapy (trial enrollment) significantly affected overall survival/mortality and highlights that smoking cessation should be a focus for patients diagnosed with lung cancer. Reassuringly, our study demonstrated that a large number of patients stopped smoking after diagnosis, and those who continued smoked fewer cigarettes. The benefits of smoking cessation after diagnosis are not just limited to lower toxicity and improved survival, but also have been shown to be linked to improved quality of life. 7, 22, 23 Future lung cancer clinical trials should collect detailed, longitudinal information about tobacco utilization, especially in other treatment settings such as definitive chemoradiation and palliative systemic therapy. We believe tobacco cessation interventions should be incorporated into clinical trial design. Focusing on tobacco cessation in clinical trials may translate to real world practice and has the potential to make a significant difference for patients diagnosed with lung cancer.
Supplementary Material
Supplemental Figure 1. Proportion of Questionnaire Respondents at Follow–up Timepoints. Questionnaire completion adjusted by survival status at 3, 6, 9, and 12 months, plus a 7-day window. The purpose of adjusted follow-up was to ensure that the denominator accounts for patients who were alive and still being followed in the study at those time points while the 7-day window was applied to control for possible delay in survey reporting.
Supplemental Figure 2. Smoking Behavior Post-Registration Among True Survey Respondents (excluding missing/unknowns and including patients that responded ‘yes’ or ‘no’ to the smoking status question).
Acknowledgments
This study was coordinated by ECOG-ACRIN (Peter O’Dwyer, M.D., Chair) and supported in part by Public Health Service Grants CA180820, CA180888, CA180821, & CA180863.
Footnotes
Conflict of Interest Statement:
Dr Gandara reports Consultant and honorarium from Roche-Genentech.
Dr Steuer reports advisory board honorarium from Abbvie, Lilly, Bergen Bio, Armo
Dr Wakelee reports grants form Gilead, Personal Fees from AztraZeneca, Xcovery, Janssen, Daiichi Sankyo, Helsinn, Mirat
Dr. Ramalingam reports grants and other from Amgen, other from Abbvie, grants and other from Astra Zeneca, grants and other from BMS, other from Genentech, other from Roche, grants and other from Merck, grants and other from Takeda, grants from Tesaro, grants from Advaxis
The other Authors report no relevant COI
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3: 733–744. [DOI] [PubMed] [Google Scholar]
- 2.Samet JM, Avila-Tang E, Boffetta P, et al. LUNG CANCER IN NEVER SMOKERS: CLINICAL EPIDEMIOLOGY AND ENVIRONMENTAL RISK FACTORS. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15: 5626–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Chronic Disease P, Health Promotion Office on S, Health. Reports of the Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US), 2014. [Google Scholar]
- 4.Baser S, Shannon VR, Eapen GA, et al. Smoking cessation after diagnosis of lung cancer is associated with a beneficial effect on performance status. Chest. 2006;130: 1784–1790. [DOI] [PubMed] [Google Scholar]
- 5.Daniel M, Keefe FJ, Lyna P, et al. Persistent Smoking after a Diagnosis of Lung Cancer is Associated with Higher Reported Pain Levels. The journal of pain : official journal of the American Pain Society. 2009;10: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. Bmj. 2010;340: b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cataldo JK, Dubey S, Prochaska JJ. Smoking cessation: an integral part of lung cancer treatment. Oncology. 2010;78: 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters EN, Torres E, Toll BA, et al. Tobacco assessment in actively accruing National Cancer Institute Cooperative Group Program Clinical Trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30: 2869–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters EN, Warren GW, Sloan JA, Marshall JR. Tobacco assessment in completed lung cancer treatment trials. Cancer. 2016;122: 3260–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakelee HA, Dahlberg SE, Keller SM, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bugge AS, Lund MB, Valberg M, Brustugun OT, Solberg S, Kongerud J. Cause-specific death after surgical resection for early-stage non-small-cell lung cancer. Eur J Cardiothorac Surg. 2017. [DOI] [PubMed] [Google Scholar]
- 12.Gerber Y, Myers V, Goldbourt U. Smoking reduction at midlife and lifetime mortality risk in men: a prospective cohort study. Am J Epidemiol. 2012;175: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 13.Bolliger CT, Zellweger J-P, Danielsson T, et al. Influence of long-term smoking reduction on health risk markers and quality of life. Nicotine & Tobacco Research. 2002;4: 433–439. [DOI] [PubMed] [Google Scholar]
- 14.Yang R, Cheung MC, Pedroso FE, Byrne MM, Koniaris LG, Zimmers TA. Obesity and Weight Loss at Presentation of Lung Cancer are Associated with Opposite Effects on Survival. The Journal of surgical research. 2011;170: e75–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busch E, Verazin G, Antkowiak JG, Driscoll D, Takita H. Pulmonary complications in patients undergoing thoracotomy for lung carcinoma. Chest. 1994;105: 760–766. [DOI] [PubMed] [Google Scholar]
- 16.Fiorelli A, Vicidomini G, Mazzella A, et al. The influence of body mass index and weight loss on outcome of elderly patients undergoing lung cancer resection. Thorac Cardiovasc Surg. 2014;62: 578–587. [DOI] [PubMed] [Google Scholar]
- 17.Kang M-J, Lee CG, Lee J-Y, et al. Cigarette smoke selectively enhances viral PAMP– and virus-induced pulmonary innate immune and remodeling responses in mice. The Journal of Clinical Investigation. 2008;118: 2771–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maestrelli P, Saetta M, Mapp CE, Fabbri LM. Remodeling in response to infection and injury. Airway inflammation and hypersecretion of mucus in smoking subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164: S76–80. [DOI] [PubMed] [Google Scholar]
- 19.Fujisawa T, Iizasa T, Saitoh Y, et al. Smoking Before Surgery Predicts Poor Long-Term Survival in Patients With Stage I Non–Small-Cell Lung Carcinomas. Journal of Clinical Oncology. 1999;17: 2086–2086. [DOI] [PubMed] [Google Scholar]
- 20.Fox JL, Rosenzweig KE, Ostroff JS. The effect of smoking status on survival following radiation therapy for non-small cell lung cancer. Lung Cancer. 2004;44: 287–293. [DOI] [PubMed] [Google Scholar]
- 21.Morgan G, Schnoll RA, Alfano CM, et al. National cancer institute conference on treating tobacco dependence at cancer centers. J Oncol Pract. 2011;7: 178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126: 1733–1741. [DOI] [PubMed] [Google Scholar]
- 23.Myrdal G, Valtysdottir S, Lambe M, Stahle E. Quality of life following lung cancer surgery. Thorax. 2003;58: 194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Proportion of Questionnaire Respondents at Follow–up Timepoints. Questionnaire completion adjusted by survival status at 3, 6, 9, and 12 months, plus a 7-day window. The purpose of adjusted follow-up was to ensure that the denominator accounts for patients who were alive and still being followed in the study at those time points while the 7-day window was applied to control for possible delay in survey reporting.
Supplemental Figure 2. Smoking Behavior Post-Registration Among True Survey Respondents (excluding missing/unknowns and including patients that responded ‘yes’ or ‘no’ to the smoking status question).