Abstract
Cocaine addiction remains a major public concern throughout the world especially in developed countries. In the last three decades, significant achievements have led to a greater understanding of the signaling pathways involved in the development of cocaine addiction; however, there are no FDA-approved treatments available to reverse or block this brain disease due to either the unsatisfactory therapeutic efficacy or severe side effects. Previous studies have demonstrated that chronic exposure to cocaine elevates levels of cyclic AMP (cAMP) as a neuroadaptative response in reward-related brain regions. Phosphodiesterase 4 (PDE4) inhibitors, which elevate cAMP levels, have been shown to block cocaine-mediated behavioral changes related to psychoactive and reinforcing properties. Unfortunately, previously studied PDE4 inhibitors induce severe side-effects which limit their clinical usage. In this study, we identified a novel PDE4B inhibitor KVA-D-88 with an improved selectivity profile compared to previous compounds (e.g., rolipram). Pharmacokinetic studies have shown this compound is brain penetrant and preferably acts on PDE4B compared to PDE4D in vitro, alluding to less unwanted side effects with KVA-D-88 in vivo. Interestingly, pre-treatment with KVA-D-88 significantly inhibited cocaine-induced hyper-locomotor activity. In cocaine self-administered mice with differential schedules, KVA-D-88 strikingly decreased the number of active nose-pokes, cocaine infusions and reduced the break point. Taken together, our findings demonstrate that this novel PDE4 inhibitor, KVA-D-88, could inhibit cocaine-mediated rewarding effects implying its potential clinical usage for cocaine addiction.
Keywords: PDE4 inhibitors, cocaine, drug addiction, self-administration, cAMP, locomotor activity
Introduction
Drug addiction remains one of the major public health concerns throughout the world especially in developed countries1. Cocaine remains one of the most-abused drugs and has long been known for its addictive properties and its abuse is associated with enormous health and economic burden on both individuals and society2. Although huge achievements have enabled a greater understanding of the neurobiological mechanisms underlying the development of cocaine addiction, there still are no FDA-approved treatments available clinically to prevent or block cocaine addiction3.
Phosphodiesterases (PDEs) are a family of enzymes which regulate the intracellular concentrations of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) which play critical roles in translating extracellular stimuli into various cellular responses4. There are a total of eleven subtypes of PDEs (PDE 1–11) which can be grouped into three categories based on the substrates specificity: PDE4, PDE7, and PDE8 are specific for cAMP; PDE5, PDE6, and PDE9 are specific for cGMP; and the remaining PDEs can hydrolyze both cAMP and cGMP5. Among these PDE members, PDE4 is highly expressed in several reward-related brain regions including the prefrontal cortex (PFc), nucleus accumbens (NAc), ventral tegmental area (VTA) and amygdala6. These brain regions are sensitive to the exposure of drugs of abuse and subsequent drug mediated alterations in the activity of cAMP-mediated pathways in these regions have been identified as drug-associated neuroadaptations underlying drug tolerance and dependence7.
PDE4 is necessary for regulating cAMP levels in the brain and the activity of PDE4 is inherently involved in the rewarding effects induced by multiple drugs of abuse, including cocaine. Previous investigations have demonstrated the therapeutic efficacy of PDE4 inhibitors against multiple drugs of abuse using various rodent models. The PDE4 inhibitor rolipram has been shown to reduce cocaine reward, incentive saliency and seeking behavior in vivo by using multiple behavioral approaches including open field test, conditioned place preference (CPP) and self-administration8. Another inhibitor, ibudilast, and its analog AV1013, reduced methamphetamine (Meth)-induced hyper-locomotor activity and sensitization9. In addition, rolipram and ibudilast were also effective in attenuating Meth-mediated seeking and consumption behaviors10. PDE4 inhibitors were also effective in regulating alcohol intake; the underlying signaling mechanisms involved have been reviewed in detail elsewhere11. Rolipram and Ro 20-1724 reduced alcohol intake without altering total fluid intake, alcohol-induced sedation or alcohol metabolism in mice12. Rolipram also dose-dependently reduced alcohol self-administration and decreased 2-bottle choice alcohol consumption and preference13. Interestingly, rolipram also reduced morphine-induced hyper-locomotor activity and CPP14. However, rolipram did not significantly inhibit heroin self-administration under a fixed ratio 1 (FR1), but decreased the rewarding effects under a progressive ratio (PR) paradigm14.
Although existing PDE4 inhibitors have shown beneficial effects on addiction-related behaviors in rodent models in the context of multiple drugs of abuse, their progression into clinical applications for addicts has been hampered due to severe side effects such as emesis and nausea due to off-target effects15. PDE4 has four subfamilies: PDE4A, PDE4B, PDE4C, PDE4D, and they are differentially expressed in various brain regions16. PDE4A is predominantly expressed in the olfactory system; PDE4B is highly expressed in the mesolimbic dopamine system; PDE4C is largely absent from the rodent brain and only found in limited regions of the human brain; and PDE4D is expressed in many parts of the brain but not in the VTA and NAc16. It has been implied that PDE4B inhibition is responsible for the beneficial effects on drug addiction whereas PDE4D inhibition mediates side-effects in vivo. Thus, the development of a novel, PDE4B preferring inhibitor, devoid of the PDE4D driven side effects, is a promising approach to treat cocaine addiction clinically.
In this study, we aimed to produce a novel isoform-selective inhibitor for PDE4B and investigated its impact on cocaine-mediated behavioral changes. For this purpose, KVA-D-88 was synthesized and subsequent pharmacokinetic studies showed that this drug had higher inhibitory potential for PDE4B than PDE4D and was also brain penetrant. The EC50 of KVA-D-88 to elevate cAMP levels was 0.5 μM, which is 10-fold lower, compared to the EC50 of apremilast in vitro (data collected at BPS Biosciences, San Diego, CA). In behavioral tests, KVA-D-88 was capable of decreasing cocaine-mediated hyper-locomotor activity and hypersensitization in open field tests. In addition, pre-administration of KVA-D-88 strikingly decreased cocaine-mediated seeking and intake behaviors in cocaine self-administered mice. These findings strongly indicate that this novel PDE4B inhibitor could be a potential therapeutic for the treatment of cocaine addiction.
Results
PK studies of KVA-D-88.
In this study, we synthesized a novel PDE4 inhibitor KVA-D-8818, the chemical structure can be seen in Fig. 1A) and characterized its pharmacokinetic parameters in vivo. Mice were administered with KVA-D-88 either through i.v. injection (1 mg/kg) or oral gavage (10 mg/kg) and after varying time periods the blood was collected to determine the plasma concentration of KVA-D-88. Through i.v. injection, the maximum plasma concentration (Cmax) of KVA-D-88 was 898.2 ng/ml. The area under the plasma concentration-time curve (AUC) from time zero to the last measurable concentration (AUClast) was 1382.9 ng/ml and AUC from time zero to infinity (AUC∞) was 1387.9 ng/ml. The half-life for KVA-D-88 (t1/2) was 6.1 hour and the mean residence time (MRT) was 5.7 hour. When KVA-D-88 was administered orally, the time to reach maximum concentration in the blood (Tmax) was 0.25 h. The Cmax in the blood was 2150.5 ng/ml, AUClast = 10818.3 ng/ml and AUC∞ = 10825.9 ng/ml. The half-life for KVA-D-88 (t1/2) was 7.16 hour and the mean residence time (MRT) was 9 h. The summarized PK data are shown in Fig. 1B. We also detected the plasma concentration of KVA-D-88 at various time periods (0 – 72 hour) post-administration and found that KVA-D-88 was still detectable after 48 h administration (Fig. 1C). These data indicated that KVA-D-88 was reasonably stable in vivo through either administration route.
Fig. 1: Pharmacokinetic studies of KVA-D-88 in vivo.
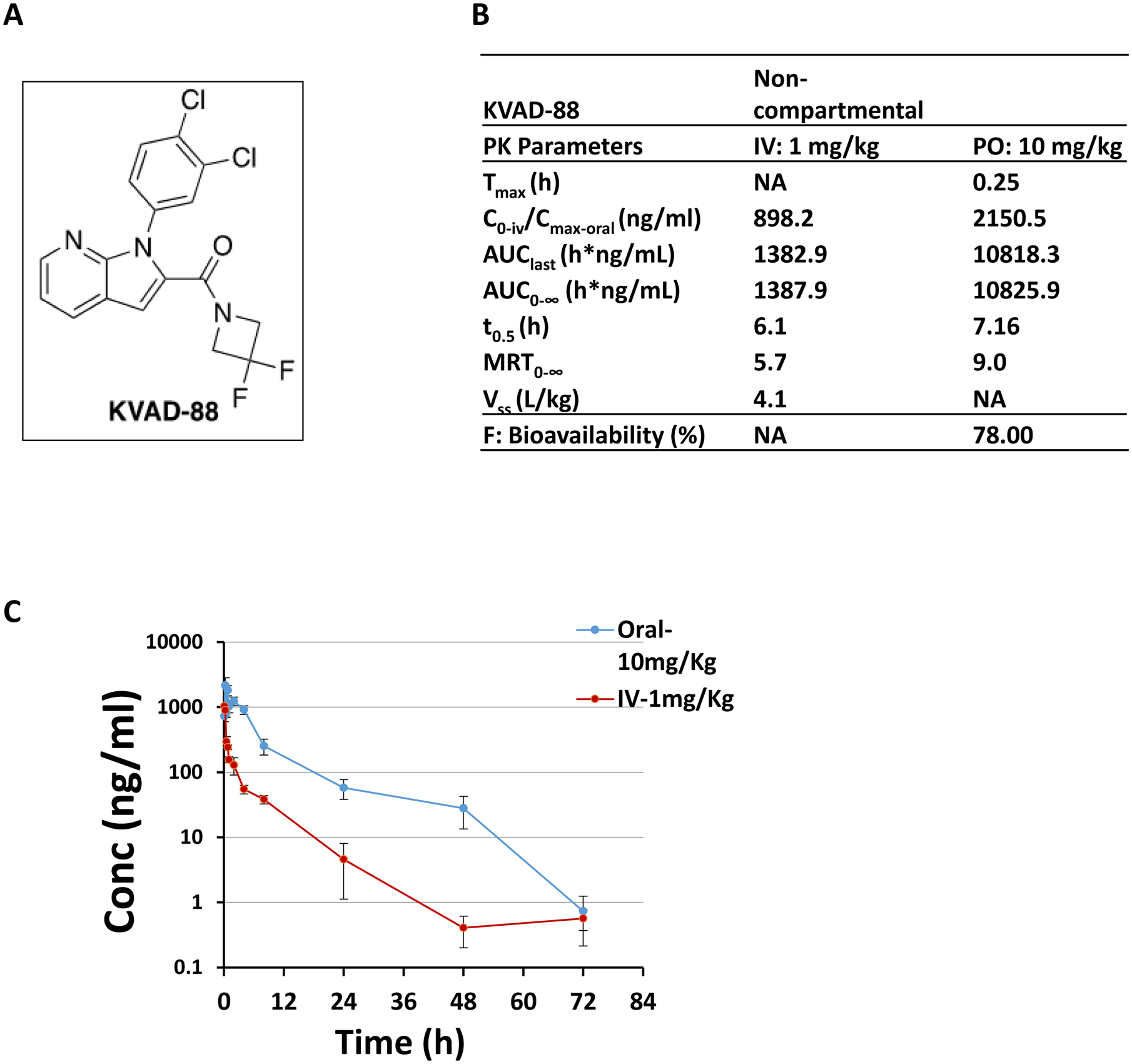
KVA-D-88 was synthesized and administered into mice by either i.v. (1 mg/kg) or oral approach (10 mg/kg). The blood was then collected for determining its pharmacokinetic parameters. (A) Representative molecular structure of KVA-D-88. (B) PK parameters of KVA-D88 in mice with either i.v. injections or oral approach. (C) The concentration-time curve of KVA-D-88 in vivo after either i.v. injections or oral approach.
KVA-D-88 is brain penetrant and shows isoform-preferring inhibition for PDE4.
The next step was to determine whether this new synthesized drug was able to cross the brain blood barrier (BBB) to mediate its biological effects through PDE4 inhibition. Mice were administered with KVA-D-88 (i.p., 10 mg/kg) and 30 min later sacrificed to determine the concentrations of KVA-D-88 in both the blood and the brain. The results showed that the average concentration of KVA-D-88 was 3899.2 ng/ml and 2347.7 ng/g in the blood and the brain, respectively, and the concentration ratio of brain: blood was approximately 0.6 (Kp). (Figs. 2A). These results demonstrated that KVA-D-88 was capable of crossing the BBB. We then determined whether KVA-D-88 had PDE4 isoform-preferring inhibition by comparing its IC50 on PDE4B and PDE4D in vitro. Meanwhile other PDE4 inhibitors rolipram and apremilast were used as controls. Our results showed that the IC50 of KVA-D-88 on PDE4B and PDE4D was 140 and 880 nM, respectively, revealing this new compound has a stronger inhibitory effect on PDE4B than PDE4D. On the contrary, rolipram showed the same IC50 value (110 nM) on PDE4B and PDE4D indicating it had equal inhibition effect on these two isoforms. However, another inhibitor apremilast had preference for PDE4D inhibition (4.5 nM) relative to PDE4B inhibition (12 nM) (Fig. 2B). We also checked the inhibitory effect of KVA-D-88 on other PDE isoforms (supplementary table 1). Taken together, our findings indicated that KVA-D-88 was a preferential PDE4B isoform-preferring inhibitor. We also determined the EC50 of KVA-D-88 on cAMP production induced by forskolin in vitro. As shown in Fig. 2C, the quantity of cAMP was proportionally elevated after increased doses of KVA-D-88 were added into the detection system. A similar trend was also obtained after adding increased doses of apremilast into the detection system (Fig. 2D). Our data revealed that the EC50 of KVA-D-88 and apremilast was 0.5 μM and 5 μM, respectively implying that KVA-D-88 was about 10-fold stronger than apremilast on PDE4 inhibition (Fig. 2E). In summary, our findings showed that KVA-D-88 is brain penetrant, capable of inhibiting PDE4 in vitro and manifests PDE4B isoform-preferring inhibition. All these results promote KVA-D-88 as a potential candidate for the clinical treatment of cocaine addiction.
Fig. 2: Characterization of PDE4 in vivo and in vitro.
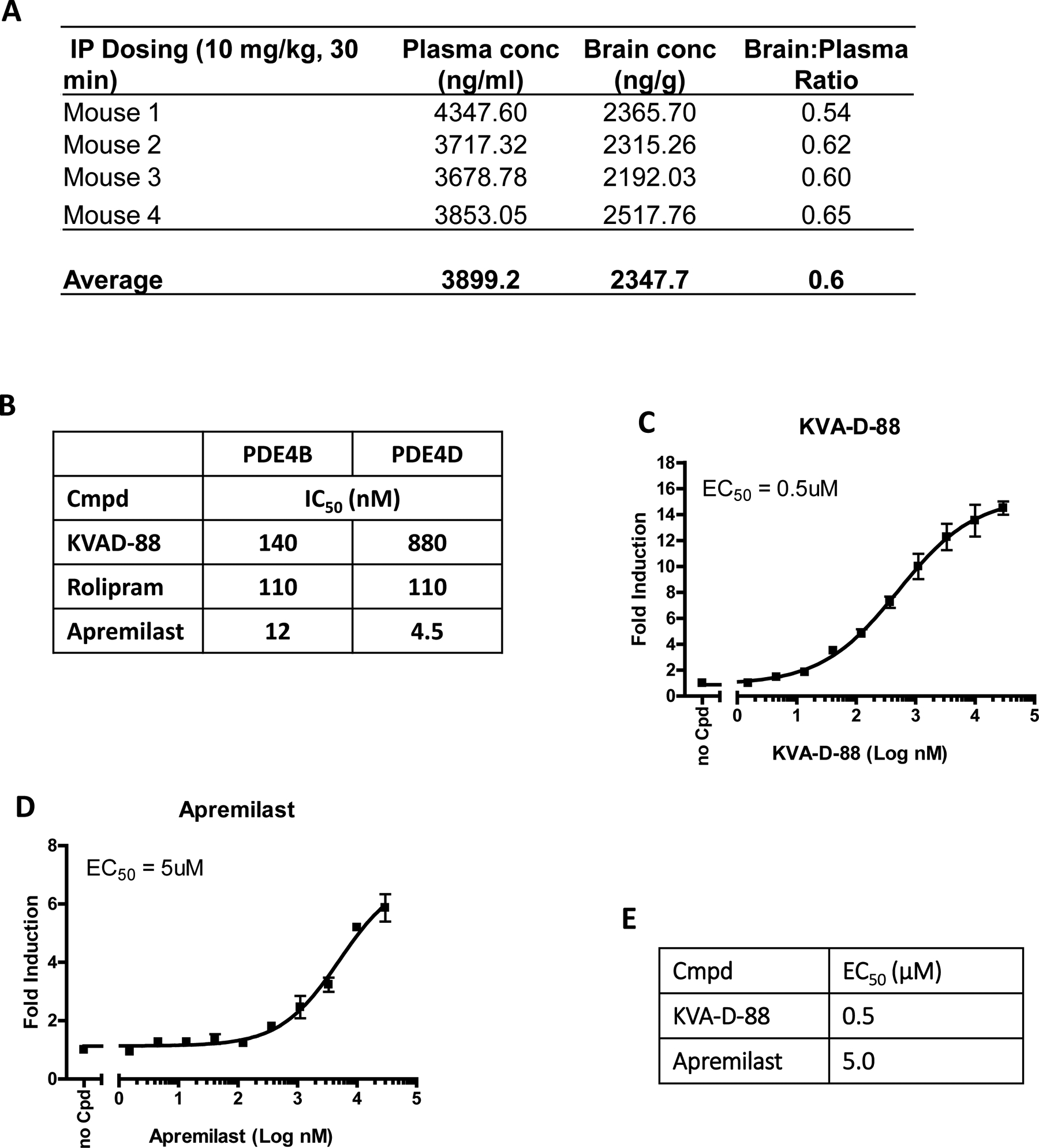
KVA-D-88 was administered into mice (n = 4) by either i.p. injection (10 mg/kg) or p.o. approach (10 mg/kg) and 30 min or 24 hours later, mice were sacrificed for the removal blood and brain. The concentrations of KVA-D-88 were determined in these two tissues to calculate the concentration ratio of brain vs. plasma. (A) Brain and plasma KVA-D-88 concentration 30 min after i.p. injection (n = 4). (B) The IC50 of KVA-D-88 on PDE4B and PDE4D in vitro. HEK293 cells were cultured and transfected with PDE4B1 expression vector, CRE luciferase reporter and a control renilla luciferase vector. The cells were dosed with KVA-D-88 and incubated overnight. Forskolin was added and incubated for 5 – 6 h. A dual luciferase assay was performed for measuring firefly luminescence. The intensity of renilla luminescence was served as internal control. Cell based assays were performed in triplicate at each concentration. The EC50 value was determined by the concentration causing a half-maximal percent activity. (C) The non-linear dose-response curve of KVA-D-88 on PDE4. (D) The non-linear dose-response curve of apremilast on PDE4. (E) The table of EC50 of KVA-D-88 and apremilast on PDE4 in vitro.
The effects of KVA-D-88 on cocaine-mediated hyper-locomotion activity and sensitization.
After characterization of KVA-D-88 both in vitro and in vivo, we sought to explore its effects on reward-related behavioral changes induced by cocaine. Male WT C57/BL6 mice were administered with KVA-D-88 at two doses (5 or 10 mg/kg, i.p.) and 30 min later followed with cocaine injection (20 mg/kg, i.p.). Mice were then immediately put into open field to record their locomotor activity for 45 minutes. As shown in Figs. 3A and 3B, while cocaine significantly increased the locomotor activity, the pre-administration of KVA-D-88 strikingly blocked this upregulation. Interestingly, KVA-D-88 had minimal effects on mouse locomotor activity at basal levels. We next determined whether this inhibitory effect of KVA-D-88 was gender-specific. Similarly, KVA-D-88 at both testing doses also significantly blocked cocaine-mediated hyper-locomotion in female mice (Figs. 3C and 3D, P < 0.05). Cocaine can induce sensitization of locomotor activity with repeated injection. We next explored the effects of KVA-D-88 on cocaine-mediated locomotor sensitization. Mice were injected with KVA-D-88 and 30 min later followed with cocaine injection daily for consecutive 7 days. Every other day, mice were put into the open field apparatus to record their locomotor activities. As seen in Fig. 3E, cocaine significantly increased the locomotor activity with highest upregulation on the 7th day while pre-administration of KVA-D-88 strikingly blocked cocaine-mediated locomotor sensitization. Taken together, our findings demonstrate that KVA-D-88 can significantly inhibit cocaine-mediated psychoactive behavioral changes.
Fig. 3: The effects of KVA-D-88 on cocaine-mediated locomotor activity.
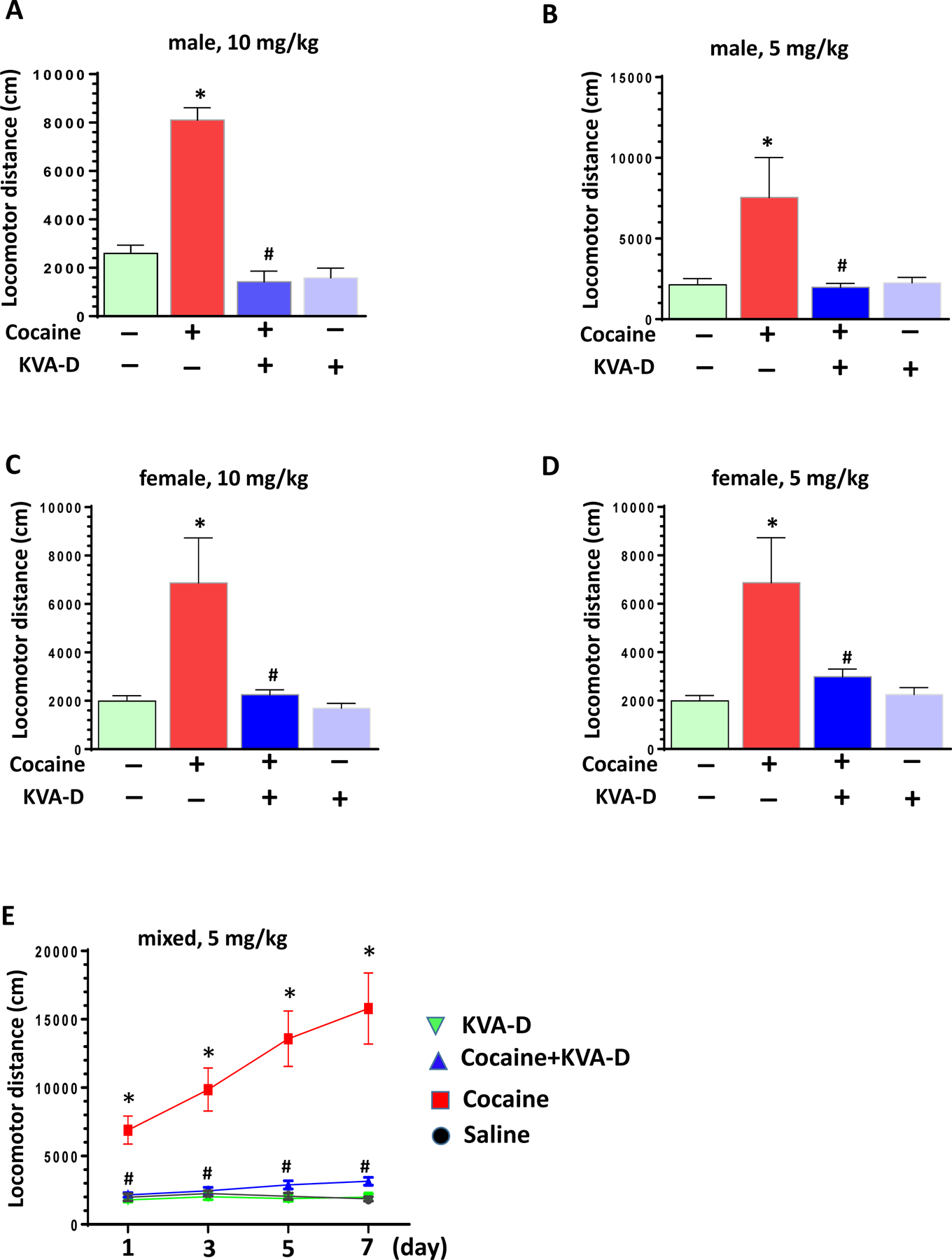
WT mice (both genders, 3 month old) were employed for these experiments. Mice were divided into four groups with various treatments (± KVA-D-88 ± cocaine). Mice were pre-injected with KVA-D-88 (i.p.) 30 min later followed with cocaine injection (20 mg/kg, i.p.). Mice were then immediately put into open field to record their locomotor activity for 45 min. (A) The effects of KVA-D88 (10 mg/kg) on acute locomotion responses in male WT mice (* P < 0.05, cocaine vs. control; # P < 0.05, cocaine + KVA-D-88 vs. cocaine; n = 6 – 8). (B) The effects of KVA-D88 (5 mg/kg) on acute locomotion responses in male WT mice (* P < 0.05, cocaine vs. control; # P < 0.05, cocaine + KVA-D-88 vs. cocaine; n = 6 – 8). (C) The effects of KVA-D88 (10 mg/kg) on acute locomotion responses in female WT mice (* P < 0.05, cocaine vs. control; # P < 0.05, cocaine + KVA-D-88 vs. cocaine; n = 6 – 8). (D) The effects of KVA-D88 (5 mg/kg) on acute locomotion responses in female WT mice (* P < 0.05, cocaine vs. control; # P < 0.05, cocaine + KVA-D-88 vs. cocaine; n = 6 – 8). (E) The effects of KVA-D-88 on cocaine-mediated sensitization on locomotor activity (* P < 0.05; cocaine vs. control, # P < 0.05, cocaine + KVA-D-88 vs. cocaine; n = 6 – 8).
The effects of KVA-D-88 on cocaine-mediated seeking and intake behavior.
Compared to passive cocaine injection into mice, cocaine self-administration is the gold standard model in the field of drug addiction, which mimics the real scenario where humans voluntarily seek and consume cocaine (active route)19. Previous studies have demonstrated that PDE4 inhibitors could inhibit drugs of abuse-mediated seeking and intake behavior8b, 10. To better understand the effects of KVA-D-88 on cocaine-mediated reinforcing effects, we first established a cocaine self-administration mouse model. WT C57/BL6 mice (10 −12 weeks) were subjected to right jugular vein catheterization and after one week of recovery, these mice were put into self-administration cubicles for training. In this 2 h training session, mice received cocaine infusions (1 mg/kg/infusion) by nose-poking the active sensor which is linked to an infusion pump and cue light while no cocaine infusion occurred when nose-poking the inactive sensor. After 7 – 10 days training, mice which stably self-administered cocaine were chosen for subsequent experiments. Our results clearly demonstrated that these mice had a strong preference for the active nose-poke sensor and stably self-administered cocaine implicating the successful establishment of cocaine self-administration (Supplementary Figs. 1A and 1B).
To investigate the effects of KVA-D-88 on cocaine-mediated seeking and intake behavior, cocaine self-administered mice were pre-injected with this inhibitor/vehicle and 30 min later put into the cubicle for 2 hours on FR1 schedule (one cocaine injection per one nose-poke). The numbers of active nose-pokes and cocaine infusions were automatically recorded by the software graphic state 4. Our results clearly demonstrated that KVA-D-88 at both doses of 5 and 2.5 mg/kg significantly decreased the number of cocaine infusions and active nose-pokes (P < 0.05, Figs. 4A – 4D). Also KVA-D-88 strikingly reduced the rate of cocaine self-administration confirming its inhibitory effects on cocaine-mediated intake behavior (P < 0.05, Fig. 4E). We also investigated the dose-response curve of KVA-D-88 on cocaine self-intake. KVA-D-88 was administered into mice with increasing dose: 0.5, 1.0, 2.0, and 4.0 mg/kg for consecutive two sessions and the numbers of active nose poke were calculated as the average for each mouse (n = 4). As shown in supplementary Fig. 2A, KVA-D-88 dose-dependently reduced cocaine self-intake: no inhibitory effect at dose 0.5 and 1.0 mg/kg; at 2 mg/kg, KVA-D-88 showed the inhibitory effects but did not reached the significance. However, significant effects could be observed at dose 4 mg/kg (P < 0.05). Apremilast is a known PDE4 inhibitor decreasing the reward-related behavior induced by alcohol20. We also checked the effects of apremilast cocaine self-intake. At 5 mg/kg, apremilast did not decrease the numbers of cocaine self-intake; while at dose 20 mg/kg, we observed significant its inhibitory effects (supplementary Fig. 2B). Our data demonstrated that KVA-D-88 is almost 10- fold stronger than apremilast on cocaine inhibition which is consistent with our PK studies. The reason for this difference is probably that apremilast is poor on brain penetrance while our KVA-D-88 could easily cross the blood brain barrier. The next step was to investigate the effects of KVA-D-88 on cocaine-mediated reinforcement under different administration schedules. Mice which have stably taken cocaine under FR1 were transited to FR3 schedule (one cocaine injection per every three nose-pokes) for three sessions. As demonstrated in supplementary Fig. 3, the ratio of cocaine self-administration in 2-hour session was significantly reduced in FR3 compared to in FR1 indicating mice need to take more effort to obtain cocaine in FR3 (P < 0.05). Mice were then pre-injected with KVA-D-88 (5 mg/kg) or vehicle and the numbers of active nose-pokes and cocaine infusions were recorded under FR3 schedule (2 h session). As seen in Figs. 5A and 5B, KVA-D-88 significantly decreased the numbers of active nose-poke and cocaine infusions (P < 0.05). Another batch of mice were put under PR schedule with KVA-D-88/vehicle pre-injection for recording their break points (3 h session). Under this schedule, mice had to pay more effort (nose-poking) to get one cocaine injection than last time they obtained. As expected, KVA-D-88 significantly decreased the numbers of break point (Fig. 5C, P < 0.05) indicating this PDE4B inhibitor reduced cocaine-mediated reinforcing effects in vivo.
Fig. 4: The effects of KVA-D-88 on active nose-poking and cocaine infusions in self-administered mice under FR1 schedule.
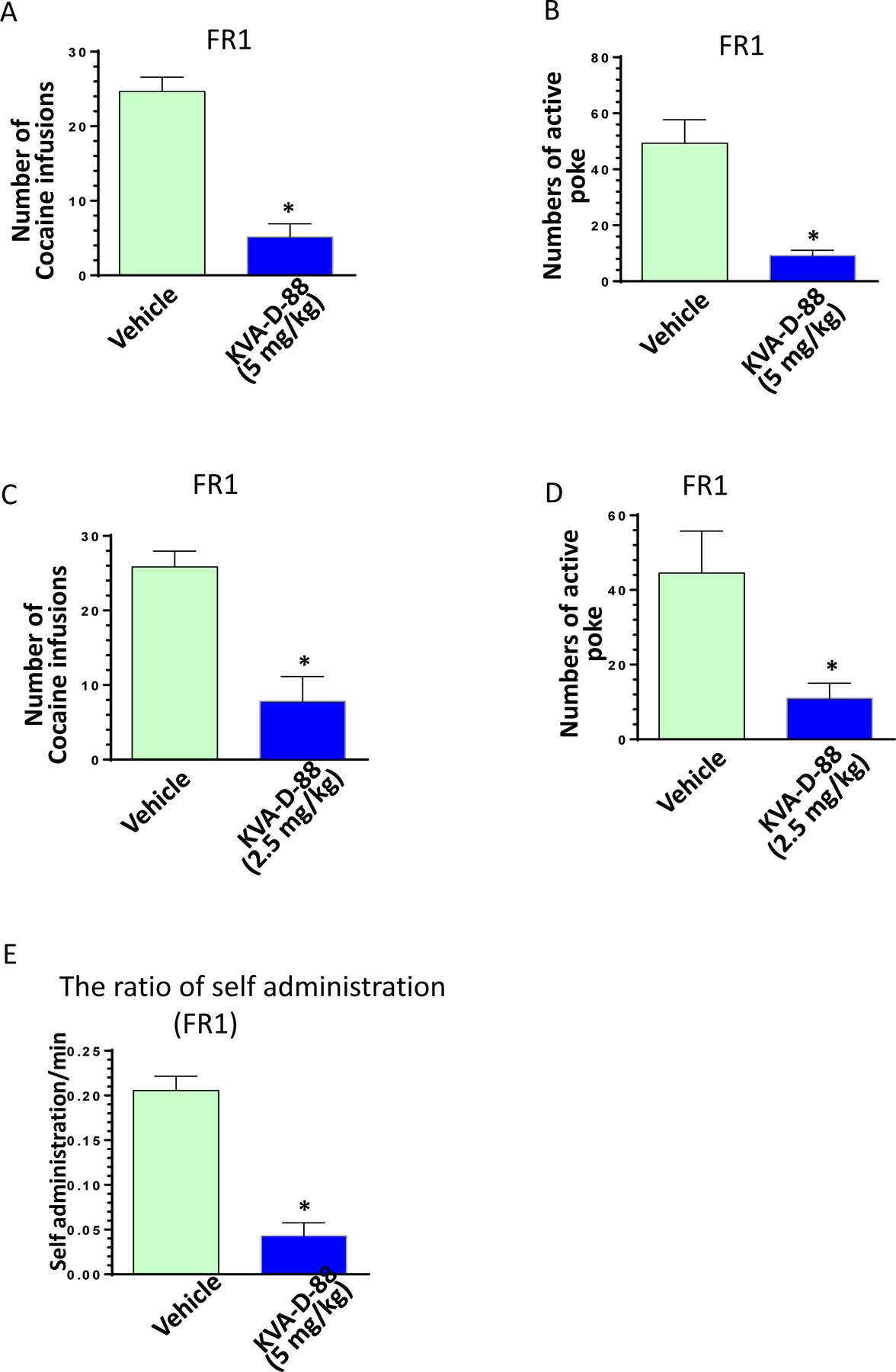
(A) The effect of KVA-D-88 (5 mg/kg) on the numbers of cocaine intake in the self-administered mice under FR1 schedule (P < 0.05, n = 6). (B) The effect of KVA-D-88 (5 mg/kg) on the numbers of active nose-poking in the self-administered mice under FR1 schedule (P < 0.05, n = 6). (C) The effect of KVA-D-88 (2.5 mg/kg) on the numbers of cocaine intake in the self-administered mice under FR1 schedule (P < 0.05, n = 6). (D) The effect of KVA-D-88 (2.5 mg/kg) on the numbers of active nose-poking in the self-administered mice under FR1 schedule (P < 0.05, n = 6). (E) The effect of KVA-D-88 (5.0 mg/kg) on the ratio of cocaine self-administration in the self-administered mice under FR1 schedule (P < 0.05, n = 6).
Fig. 5: The effects of KVA-D-88 on active nose-poking and cocaine infusions in self-administered mice under FR3 or PR schedule.
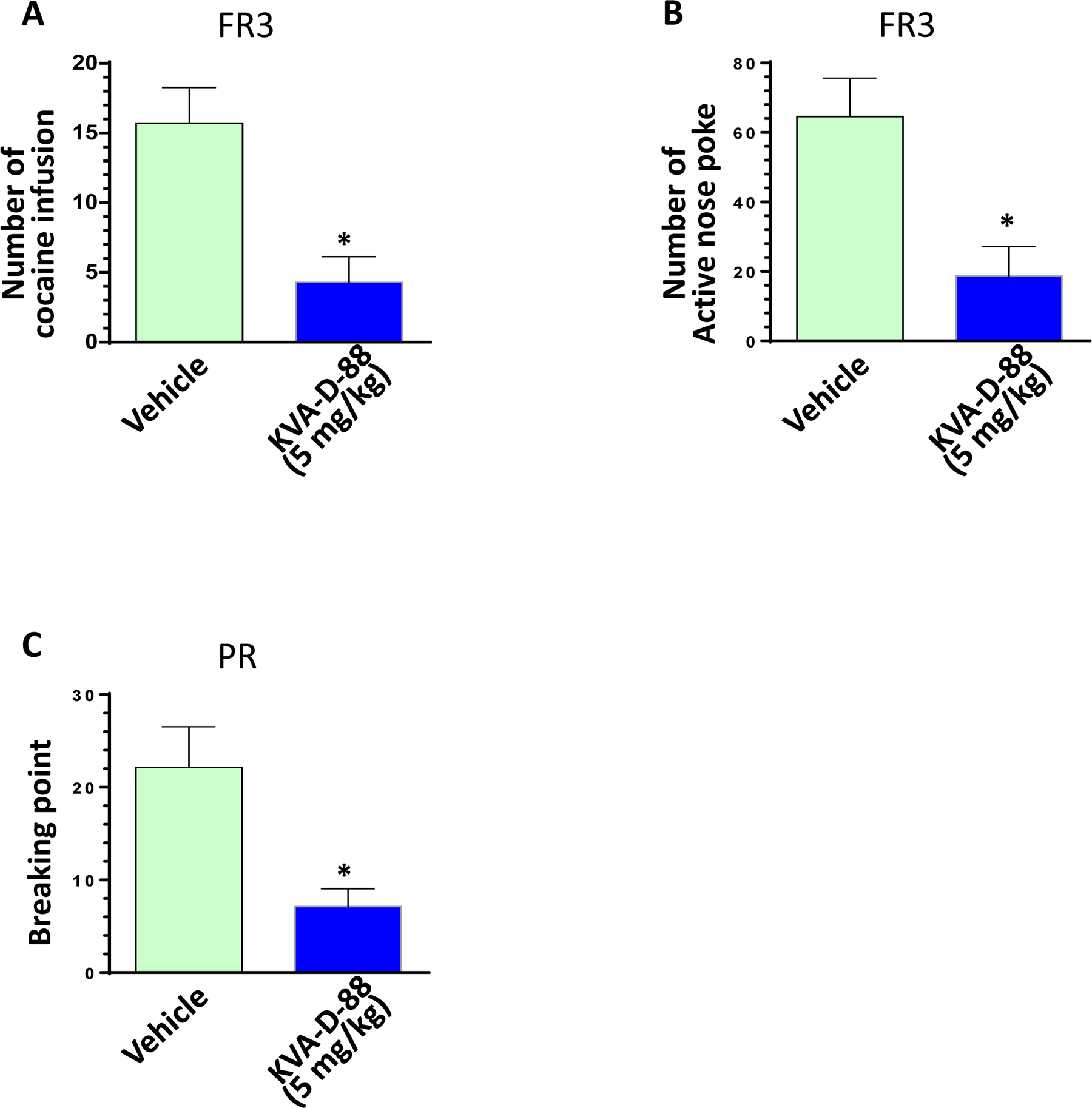
WT mice (3 month old) were trained to develop stable cocaine self-administration on FR1 schedule. Then mice were switched to an FR3 schedule with or without KVA-D-88 pre-injection and the numbers of active nose-poke and cocaine infusions were recorded. Another batch of mice were switched to a PR schedule with or without KVA-D-88 pre-injection and the numbers of active nose-poke and cocaine infusions were recorded. (A) The effect of KVA-D-88 (5 mg/kg) on the numbers of cocaine intake in the self-administered mice under FR3 (P < 0.05, n = 6). (B) The effect of KVA-D-88 (5 mg/kg) on the numbers of active nose-poke in the self-administered mice under FR3 (P < 0.05, n = 6). (C) The effect of KVA-D-88 (5 mg/kg) on breaking point in the self-administered mice under PR (P < 0.05, n = 6).
Discussion
The currently available PDE4 inhibitors are limited in their clinical usage for drug addiction due to their non-selective isoform inhibition which induces severe side-effects in addicts. In this study, we synthesized a novel PDE4 inhibitor KVA-D-88 and PK studies demonstrated that it was table in vivo and effectively increased cAMP production in vitro with preference for PDE4B. Also, KVA-D-88 could significantly block cocaine-mediated hyper-locomotion activity and sensitization by using open-field locomotor tests. Strikingly, this drug was capable of decreasing cocaine-mediated reinforcing effects in self-administered mice. Our findings indicate that KVA-D-88 could be a good candidate utilized in clinic for the treatment of cocaine addiction.
Cocaine induces dramatic alterations in cellular signaling in vivo which underlies the development of cocaine addiction21. The increased activity of cAMP-mediated pathways induced by drugs of abuse including cocaine in brain reward regions was identified about three decades ago22 and is well-accepted as neuroadaptive response involved in drug tolerance and dependence23. Previous studies have shown that the upregulation of cAMP pathway-CREB activation in the NAc either by local infusion of protein kinase A (PKA, downstream effector of cAMP) activators into NAc or by use of viral-mediated gene transfer to overexpress CREB in this region blunted the rewarding effects of opiates and cocaine24. The levels of cAMP are tightly regulated by adenylate cyclase (AC, for synthesis) and PDEs (for degradation) in vivo25. Inhibition on PDEs activity has been developed as a promising therapeutic approach for cocaine addiction in the past decades. Among the PDE family, PDE4 has been shown to be highly expressed in brain reward related regions and specifically degrade cAMP16. Accordingly, several PDE4 inhibitors including rolipram have been tested for the potential therapeutic effects on drug addiction. While these drugs possessed the ability to significantly block drugs of abuse-mediated behavioral changes, they also induced severe side-effects because of off-target effects15. Due to this disadvantage, there is no PDE4 inhibitor available for the treatment of cocaine addiction and there is urgent need for novel PDE4 isoform-selective inhibitors.
In this study, we synthesized a novel PDE4 inhibitor KVA-D-88 and characterized this reagent in vitro and in vivo. The PK results showed that the plasma concentration of KVA-D-88 could reach 2150.5 ng/ml by oral dosing and the half-life of this inhibitor was around 6 – 7 h through either i.v. injection or p.o. approach. These features demonstrated that KVA-D-88 could be easily taken up through both routes and remained highly concentrated for several hours in vivo, indicating that KVA-D-88 was amenable for preclinical studies. In addition, we identified that the concentration ratio (brain vs. plasma) of KVA-D-88 is 0.6 and 1.2 through i.v. and p.o. dosing, respectively, which demonstrated this drug can cross the BBB and enter the brain reaching therapeutically efficacious levels. Entry into the brain is critical for KVA-D-88 to inhibit brain PDE4 activity to mediate its inhibition effects. We realize that the brain KVA-D-88 concentrations were obtained by using the whole brain homogenates. Actually, in various brain regions, the concentrations of KVA-D-88 may be different due to discrete blood vessel distributions or differences in neuronal activity. We will further characterize the brain KVA-D-88 distribution pattern, especially in the reward-related regions such as striatum, mPFc and hippocampus.
PDE4 has four isoforms including PDE4A, 4B, 4C and 4D with differential tissue distribution in vivo16. Generally, PDE4B inhibition is responsible for therapeutic effects on drug addiction and PDE4D inhibition could lead to untoward side-effects17. To decide whether KVA-D-88 possesses isoform-selective inhibition on PDE4, we performed IC50 assays in vitro and found that the IC50 of this drug for PDE4B and PDE4D is 140 and 880 nM, respectively. This result indicated that KVA-D-88 preferably acted on PDE4B at a lower concentration implying it would induce less side effects in vivo. Supporting this assumption, the IC50 of rolipram on PDE4B and PDE4D was the same at 110 nM indicating no preference of rolipram for these two isoforms. We also determined the EC50 of KVA-D-88 on cAMP production in a cell culture system and found that the EC50 concentration of KVA-D-88 was 10-fold lower than that of apremilast indicating that this drug could be used at lower concentrations to achieve its biological effects in vivo. Taken together, our data supported that KVA-D-88 could be an excellent candidate to treat cocaine addiction in the clinic.
Our behavioral studies also supported that KVA-D-88 could be used preclinically for cocaine addiction. In open field tests, KVA-D-88 significantly blocked cocaine-mediated hyper-locomotor after a single cocaine injection as well as the locomotor sensitization of mice with repeated cocaine injections. Strikingly, KVA-D-88 inhibited active nose-pokes and cocaine infusions in cocaine self-administered mice (FR1). Further experiments demonstrated that KVA-D-88 could also inhibit active nose-poking, cocaine infusions and lower break points in cocaine self-administered mice under FR3 and PR schedule, respectively. These results demonstrated that KVA-D-88 was capable of reducing cocaine-mediated reinforcing effects. These findings were consistent with previous investigations that showed that PDE4 inhibitors had therapeutic effects on drug addiction8. Previous studies have shown that rolipram attenuated cocaine-induced locomotor sensitization and conditional place preference in mice26. A possible underlying mechanism for rolipram was that it could prevent cocaine-induced reductions in GABAergic inhibition leading to the restoration of a balance between excitation (E) and inhibition (I) and a normalization of the E/I ratio26. The detailed mechanisms underlying KVA-D-88 inhibition on cocaine-mediated reward properties remain unexplored here but the well-known downstream effectors such as PKA or cAMP response element-binding protein (CREB) will be our focus for further explorations.
Another potential mechanism responsible for KVA-D-88 inhibition could be modulation of neuroimmune signaling. Emerging evidence has indicated that dysregulations in neuroimmune signaling were inherently involved in substance abuse disorders including cocaine addiction27. Cocaine is capable of activating microglia in both in vitro and in vivo models28. Microarray analyses from mouse brains have implicated cocaine-mediated upregulation of the expression of pro-inflammatory mediators such as IL1β, IL6, TNFα & MCP-1 in reward-related regions of the brain including the prefrontal cortex & nucleus accumbens29. Correspondingly, minocycline, an inhibitor of microglial activation, was capable of blocking reward-related behavioral changes induced by abused drugs10, 30. PDE4 inhibitors are well-known as anti-inflammatory reagents and have the ability to inhibit microglial activation and neuroinflammation under various conditions31. For example, ibudilast, a PDE4 inhibitor, decreased drug seeking and intake behaviors in Meth self-administered rats in vivo, where these effects were associated with the decreased neuroinflammation levels10. Also, rolipram blocked LPS-induced Ca2+ increase, ROS production and the upregulation on NLRP3 inflammasome and cleaved caspase-1 in vitro32. So it will be interesting to examine the alterations on neuroimmune signaling in cocaine self-administered mice with/without KVA-D-88 pre-treatment.
In summary, we synthesized a novel PDE4 isoform preferring inhibitor KVA-D-88 and showed its inhibitory effects on cocaine-mediated psychoactive and reinforcing behavioral changes. Our findings show promising preclinical results in attenuating cocaine addiction in rodent models. Further optimization and in vivo studies are currently in progress and the results will be reported in due course.
Method
Animals and reagents:
Wild type C57BL/6J mice were purchased from Jackson Laboratory (Maine, USA). All the animals were housed under conditions of constant temperature and humidity on a 12-h light, 12-h dark cycle, with lights on at 0700 h. Food and water were available ad libitum. All animal procedures were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and the National Institutes of Health. Cocaine hydrochloride (C5776) was purchased from Sigma and freshly dissolved into 0.9% saline prior to use. PDE4 inhibitor KVA-D-88 was synthesized in Dr. Hopkins’s lab.
Pharmacokinetic (PK) study of KVA-D-88:
The small molecule KVA-D-88 was synthesized (Manuscript was accepted, ACS Med. Chem. Lett. 2019, manuscript ID: ml-2019-00369y) and stored (solid format) in a dry brown bottle and freshly dissolved in vehicle composed of DMSO, PEG400, EtOH, cremaphore and PBS (5:30:10:10:5:40%) at various doses for pharmacological and animal experiments. Wild type C57BL/6J mice were administrated with KVA-D-88 either intravenously at 1 mg/kg (i.v.) or orally at 10 mg/kg (p.o.). Blood samples are collected from the facial vein by puncturing the skin using sterile 0.5 mm goldenrod animal lancets. The drops of freely coming blood are collected in EDTA-containing tubes. Bleeding is stopped after collecting ~ 50 ul of blood by pressing the vein with cotton ballBlood was collected at various time points (0 – 72 h) post-administration and the concentrations of KVA-D-88 and various PK parameters were assayed. To investigate the concentration of KVA-D-88 in the brain, the mice were administrated with KVA-D-88 through intraperitoneal injection (i.p.) or oral approach both at a dose of 10 mg/kg. After 30 min (i.p.) mice were sacrificed for the collection of blood and brain tissues to determine the concentration of KVA-D-88.
The EC50 detection of KVA-D-88 in vitro:
HEK293 cells were cultured in growth media (10% fetal bovine serum, 1% Pen-Strep, 1% Non-essential amino acids, 1 mM Na-pyruvate) and seeded at 30,000 cells/well into 96-well microplate. Cells were incubated at 37 °C and 5% CO2 overnight. The following day, the cells were transfected with PDE4B1 expression vector, CRE luciferase reporter and a control Renilla luciferase vector (BPS Bioscience # 79526) using Lipofectamine 2000 and Opti-MEM for 6 h. The media was removed and the cells were dosed with test compounds (KVA-D-88) or controls in 50 μl of fresh growth medium and incubated overnight. The next day, forskolin was added in 5 μl of growth medium to stimulated wells at a final concentration of 10 μM for 5 – 6 h. After treatment, a dual luciferase assay was performed using BPS Bioscience Dual Luciferase assay system (BPS Bioscience # 60683): 55 μl of firefly luciferase reagent per well was added to measure firefly luminescence. Subsequently, another 55 μl /well of Renilla luciferase reagent was added to measure Renilla luminescence. Cell based assays were performed in triplicate at each concentration. To obtain the normalized luciferase activity of CRE reporter, subtract background luminescence then calculate the ratio of firefly luminescence from the CRE reporter to Renilla luminescence from the control Renilla luciferase vector. The normalized luciferase activity data was analyzed using the Graph Prism. The EC50 value was determined by the concentration causing a half-maximal percent activity.
The IC50 determination of KVA-D-88 on PDE4 in vitro:
The IC50 values of KVA-D-88 on PDE4 isoforms were determined by BPS Bioscience, San Diego. In brief, coat proteins (PDE4 isoforms) were added into the plate in a volume of 50 μl (2–5 ng/μl) at 4 °C overnight. The next day, KVA-D-88 was added to the coated plate with varying concentrations followed by addition of the corresponding biotinylated binding partner. The reaction was incubated for 2 h at room temperature. Binding assays were performed in duplicate at each concentration and the plates were read by using Synergy 2 BioTek plate reader. The luminescence data were analyzed using the Graphpad Prism. Percent inhibition was determined by normalizing the data to signal from negative control wells (uncoated wells treated with the biotinylated ligand, set as 100% inhibition) and positive control wells (coated wells treated with the biotinylated ligand in the absence of any inhibitor, set as 0% inhibition). Data for a reference compounds or antibodies are included as a control for inhibition.
Locomotor analysis:
WT mice (both gender, n = 6 – 8) were divided into four groups receiving various treatments: (1) vehicle + saline; (2) vehicle + cocaine; (3) KVA-D-88 + saline; and (4) KVA-D-8 + cocaine. Cocaine was administered at 20 mg/kg (i.p.) and KVA-D-88 was used at two different doses 5 or 10 mg/kg (i.p.). KVA-D-88 was firstly injected into the mice and 30 min later followed with cocaine administration. To investigate the effects of KVA-D-88 on acute locomotor response, immediately following cocaine injection, mice were put into the open field apparatus (Truescan, Coulbourn instrument) to detect locomotor activity for 45 min. The TruScan photobeam activity system consists of a clear arena with infrared sensors located on a ring 3 cm above floor level. There are 16 beams spaced 1 inch apart on the sensor ring that are used to detect movements by mice. These data are automatically relayed to a PC computer and interpreted by software. To investigate the effects of KVA-D-88 on locomotor sensitization, mice were repeatedly injected with KVA-D-88 and cocaine for 7 consecutive days and the locomotor responses were recorded every other day.
Self-administration:
(a) Surgery procedure:
WT mice (10 – 12 weeks, both genders) were implanted with permanently indwelling catheters (Plastics one) into the right jugular vein under a combination of ketamine hydrochloride (100 mg/mg, i. p.) and xylazine (10 mg/mg, i.p.) anesthesia. The catheter is tied to the vein with surgical silk and is passed subcutaneously to the back of the mouse where the catheter is affixed to a small plastic pedestal (26 G, Plastics One Company). After the surgery, the catheters were flushed daily with heparin (30 IU/ml) to avoid clotting. Mice were allowed at least 7 days of recovery in their home before the start of the experiments.
(b) The establishment of cocaine self-administered mice:
Mice were individually put into sound-mitigation cubicles (Coulbourne Instrument) for the training of self-administered cocaine (1.0 mg/kg/infusion). Mice were reinforced for nose-poking the cue-paired (active) sensor by delivery of intravenous cocaine in 5 second with 20 μl solution while nose-poking the inactive sensor resulted in no consequence. A timeout period of 15 seconds followed each drug injection. All responses were recorded automatically using a computer interface and Graphic State Notation 4 software (Coulbourne Instrument). Mice were trained to self-administer cocaine during one daily session under a fixed ratio 1 (FR1) schedule. Under this schedule, mice would get one cocaine infusion by performing one active nose-poking. Each session lasted for a maximum of 2 h or until the mice received 30 cocaine infusions to avoid overdose. The acquisition period was conducted for 7 – 10 days to help mice perform stable cocaine self-intake under the following criteria: (1) the ratio of active poke vs. inactive poke is above 2 : 1; (2) the minimum of cocaine infusions is above 10; and (3) the variation for cocaine intake for consecutive three days is below 20%. After the establishment of cocaine intake, mice were under an FR1 schedule for an additional one week of cocaine self-administration (daily 2 h sessions, 1.0 mg/kg/infusion). Mice were flushed before and after each session and after the last session, mice were administered with ketamine to affirm the patency of catheter setup.
(c) The effects of KVA-D-88 on cocaine-mediated seeking and taking behavior.
KVA-D-88/vehicle was injected into cocaine self-administered mice (i.p.) and 30 min later, mice were put into cubicle for 2 h under FR1 schedule. The numbers of active nose-poke and cocaine infusions were recorded. Mice with vehicle injection served as controls. Another batch of mice were under FR3 schedule. Under this schedule, mice would get one cocaine infusion on every three active nose-poking. Three days later, mice with/without KVA-D-88 injection were put into cubicle to record the number of active nose-poke and cocaine infusions. To investigate the effects of KVA-D-88 on reinforcing effects induced by cocaine, a third batch of established self-administered mice were under PR schedule. The PR schedule followed the sequence: 1, 2, 4, 6, 9, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, etc. The response requirement for its reinforcement was given by R(i) = [5e0.2i − 5] and the session time is 3 hours. Breakpoint is operationally defined as the final ratio in effect (i.e., response requirement) when the last injection was delivered in this session33.
Statistical analysis:
The results were presented as means ± SEM. For comparisons between two groups an unpaired two-tailed student’s T-test was used; for comparing multiple groups, one-way ANOVA analysis followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means was used. All statistical tests were performed with GraphPad Prism (La Jolla, CA, USA). Probability levels of < 0.05 were considered statistically significant. A minimum of four biological replicates were used for all experiments.
Safety.
The authors claimed there was no unexpected, new, and/or significant hazards or risks associated with the reported work.
Supplementary Material
Acknowledgement:
This work was supported by NIH NIDA grants (R01 DA043138, DA047156) and UNMC Nebraska Center for Substance Abuse Research (NCSAR) pilot award. We are very thankful for self-administration technical support from Miss Danielle Arena (Tufts University). Thanks for Shannon E. Callen for critical reading. Receptor binding profiles was generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2018-00023-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA.
Footnotes
Supporting information:
The inhibitory effects of KVA-D-88 on PDE4 isoforms; the establishment of cocaine self-administered mice; the dose-curve effects of PDE4 inhibitors on cocaine self-intake; and the effects of different schedule on the ratio of cocaine self-administration in vivo.
Competing financial interests:
The authors declare no competing financial interests.
Reference
- 1.Peacock A; Leung J; Larney S; Colledge S; Hickman M; Rehm J; Giovino GA; West R; Hall W; Griffiths P; Ali R; Gowing L; Marsden J; Ferrari AJ; Grebely J; Farrell M; Degenhardt L, Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018, 113 (10), 1905–1926. [DOI] [PubMed] [Google Scholar]
- 2.Milano G; Saenz E; Clark N; Busse A; Gale J; Campello G; Mattfeld E; Maalouf W; Heikkila H; Martelli A; Morales B; Gerra G, Report on the International Workshop on Drug Prevention and Treatment in Rural Settings Organized by United Nation Office on Drugs and Crime (UNODC) and World Health Organization (WHO). Subst Use Misuse 2017, 52 (13), 1801–1807. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz J; Saxon AJ, Medications to treat cocaine use disorders: current options. Curr Opin Psychiatry 2019, 32 (4), 275–281. [DOI] [PubMed] [Google Scholar]
- 4.(a) Hansen RT 3rd; Zhang HT, The Past, Present, and Future of Phosphodiesterase-4 Modulation for Age-Induced Memory Loss. Adv Neurobiol 2017, 17, 169–199; [DOI] [PubMed] [Google Scholar]; (b) Bolger GB, The PDE4 cAMP-Specific Phosphodiesterases: Targets for Drugs with Antidepressant and Memory-Enhancing Action. Adv Neurobiol 2017, 17, 63–102. [DOI] [PubMed] [Google Scholar]
- 5.(a) Lugnier C, Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther 2006, 109 (3), 366–98; [DOI] [PubMed] [Google Scholar]; (b) Zhang HT, Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des 2009, 15 (14), 1688–98. [DOI] [PubMed] [Google Scholar]
- 6.Olsen CM; Liu QS, Phosphodiesterase 4 inhibitors and drugs of abuse: current knowledge and therapeutic opportunities. Front Biol (Beijing) 2016, 11 (5), 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen RT; Feng WY; Liang JH; Zhang HT, Role of phosphodiesterase 4-mediated cyclic AMP signaling in pharmacotherapy for substance dependence. Curr Pharm Des 2015, 21 (3), 355–64. [DOI] [PubMed] [Google Scholar]
- 8.(a) Janes AC; Kantak KM; Cherry JA, The involvement of type IV phosphodiesterases in cocaine-induced sensitization and subsequent pERK expression in the mouse nucleus accumbens. Psychopharmacology (Berl) 2009, 206 (2), 177–85; [DOI] [PubMed] [Google Scholar]; (b) Knapp CM; Foye MM; Ciraulo DA; Kornetsky C, The type IV phosphodiesterase inhibitors, Ro 20-1724 and rolipram, block the initiation of cocaine self-administration. Pharmacol Biochem Behav 1999, 62 (1), 151–8; [DOI] [PubMed] [Google Scholar]; (c) Thompson BE; Sachs BD; Kantak KM; Cherry JA, The Type IV phosphodiesterase inhibitor rolipram interferes with drug-induced conditioned place preference but not immediate early gene induction in mice. Eur J Neurosci 2004, 19 (9), 2561–8. [DOI] [PubMed] [Google Scholar]
- 9.Snider SE; Vunck SA; van den Oord EJ; Adkins DE; McClay JL; Beardsley PM, The glial cell modulators, ibudilast and its amino analog, AV1013, attenuate methamphetamine locomotor activity and its sensitization in mice. Eur J Pharmacol 2012, 679 (1–3), 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snider SE; Hendrick ES; Beardsley PM, Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol 2013, 701 (1–3), 124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logrip ML, Phosphodiesterase regulation of alcohol drinking in rodents. Alcohol 2015, 49 (8), 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu W; Lu T; Chen A; Huang Y; Hansen R; Chandler LJ; Zhang HT, Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology (Berl) 2011, 218 (2), 331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen RT; Zhang M; Qin WJ; Liu Q; Wang WP; Lawrence AJ; Zhang HT; Liang JH, The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol seeking and consumption in alcohol-preferring Fawn-Hooded rats. Alcohol Clin Exp Res 2012, 36 (12), 2157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori T; Baba J; Ichimaru Y; Suzuki T, Effects of rolipram, a selective inhibitor of phosphodiesterase 4, on hyperlocomotion induced by several abused drugs in mice. Jpn J Pharmacol 2000, 83 (2), 113–8. [DOI] [PubMed] [Google Scholar]
- 15.Hansen RT 3rd; Zhang HT, Phosphodiesterase-4 modulation as a potential therapeutic for cognitive loss in pathological and non-pathological aging: possibilities and pitfalls. Curr Pharm Des 2015, 21 (3), 291–302. [DOI] [PubMed] [Google Scholar]
- 16.(a) Johansson EM; Reyes-Irisarri E; Mengod G, Comparison of cAMP-specific phosphodiesterase mRNAs distribution in mouse and rat brain. Neurosci Lett 2012, 525 (1), 1–6; [DOI] [PubMed] [Google Scholar]; (b) Lakics V; Karran EH; Boess FG, Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology 2010, 59 (6), 367–74. [DOI] [PubMed] [Google Scholar]
- 17.Mori F; Perez-Torres S; De Caro R; Porzionato A; Macchi V; Beleta J; Gavalda A; Palacios JM; Mengod G, The human area postrema and other nuclei related to the emetic reflex express cAMP phosphodiesterases 4B and 4D. J Chem Neuroanat 2010, 40 (1), 36–42. [DOI] [PubMed] [Google Scholar]
- 18.Vadukoot AK; Sharma S; Aretz CD; Kumar S; Gautam N; Alnouti Y; Aldrich AL; Heim CE; Kielian T; Hopkins CR ACS Med. Chem. Lett 2020, Article ASAP. DOI: 10.1021/acsmedchemlett.9b00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Spanagel R, Animal models of addiction. Dialogues Clin Neurosci 2017, 19 (3), 247–258; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Aarde SM; Taffe MA, Predicting the Abuse Liability of Entactogen-Class, New and Emerging Psychoactive Substances via Preclinical Models of Drug Self-administration. Curr Top Behav Neurosci 2017, 32, 145–164. [DOI] [PubMed] [Google Scholar]
- 20.(a) Blednov YA; Da Costa AJ; Tarbox T; Ponomareva O; Messing RO; Harris RA, Apremilast Alters Behavioral Responses to Ethanol in Mice: I. Reduced Consumption and Preference. Alcohol Clin Exp Res 2018, 42 (5), 926–938; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Blednov YA; Da Costa AJ; Harris RA; Messing RO, Apremilast Alters Behavioral Responses to Ethanol in Mice: II. Increased Sedation, Intoxication, and Reduced Acute Functional Tolerance. Alcohol Clin Exp Res 2018, 42 (5), 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Hamilton PJ; Nestler EJ, Epigenetics and addiction. Curr Opin Neurobiol 2019, 59, 128–136; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nestler EJ; Luscher C, The Molecular Basis of Drug Addiction: Linking Epigenetic to Synaptic and Circuit Mechanisms. Neuron 2019, 102 (1), 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terwilliger RZ; Beitner-Johnson D; Sevarino KA; Crain SM; Nestler EJ, A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res 1991, 548 (1–2), 100–10. [DOI] [PubMed] [Google Scholar]
- 23.(a) Sakai N; Thome J; Newton SS; Chen J; Kelz MB; Steffen C; Nestler EJ; Duman RS, Inducible and brain region-specific CREB transgenic mice. Mol Pharmacol 2002, 61 (6), 1453–64; [DOI] [PubMed] [Google Scholar]; (b) Dong Y; Green T; Saal D; Marie H; Neve R; Nestler EJ; Malenka RC, CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci 2006, 9 (4), 475–7. [DOI] [PubMed] [Google Scholar]
- 24.(a) Miserendino MJ; Nestler EJ, Behavioral sensitization to cocaine: modulation by the cyclic AMP system in the nucleus accumbens. Brain Res 1995, 674 (2), 299–306; [DOI] [PubMed] [Google Scholar]; (b) Carlezon WA Jr.; Thome J; Olson VG; Lane-Ladd SB; Brodkin ES; Hiroi N; Duman RS; Neve RL; Nestler EJ, Regulation of cocaine reward by CREB. Science 1998, 282 (5397), 2272–5; [DOI] [PubMed] [Google Scholar]; (c) Barrot M; Olivier JD; Perrotti LI; DiLeone RJ; Berton O; Eisch AJ; Impey S; Storm DR; Neve RL; Yin JC; Zachariou V; Nestler EJ, CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A 2002, 99 (17), 11435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Fertig BA; Baillie GS, PDE4-Mediated cAMP Signalling. J Cardiovasc Dev Dis 2018, 5 (1), 8–21; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Page CP; Spina D, Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb Exp Pharmacol 2011, (204), 391–414. [DOI] [PubMed] [Google Scholar]
- 26.(a) Liu X; Zhong P; Vickstrom C; Li Y; Liu QS, PDE4 Inhibition Restores the Balance Between Excitation and Inhibition in VTA Dopamine Neurons Disrupted by Repeated In Vivo Cocaine Exposure. Neuropsychopharmacology 2017, 42 (10), 1991–1999; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhong P; Wang W; Yu F; Nazari M; Liu X; Liu QS, Phosphodiesterase 4 inhibition impairs cocaine-induced inhibitory synaptic plasticity and conditioned place preference. Neuropsychopharmacology 2012, 37 (11), 2377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.(a) Clark KH; Wiley CA; Bradberry CW, Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res 2013, 23 (2), 174–88; [DOI] [PubMed] [Google Scholar]; (b) Cui C; Shurtleff D; Harris RA, Neuroimmune mechanisms of alcohol and drug addiction. Int Rev Neurobiol 2014, 118, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(a) Liao K; Guo M; Niu F; Yang L; Callen SE; Buch S, Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation 2016, 13, 33; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guo ML; Liao K; Periyasamy P; Yang L; Cai Y; Callen SE; Buch S, Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 2015, 11 (7), 995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(a) Ahmed SH; Lutjens R; van der Stap LD; Lekic D; Romano-Spica V; Morales M; Koob GF; Repunte-Canonigo V; Sanna PP, Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A 2005, 102 (32), 11533–8; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Piechota M; Korostynski M; Solecki W; Gieryk A; Slezak M; Bilecki W; Ziolkowska B; Kostrzewa E; Cymerman I; Swiech L; Jaworski J; Przewlocki R, The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum. Genome Biol 2010, 11 (5), R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(a) Attarzadeh-Yazdi G; Arezoomandan R; Haghparast A, Minocycline, an antibiotic with inhibitory effect on microglial activation, attenuates the maintenance and reinstatement of methamphetamine-seeking behavior in rat. Prog Neuropsychopharmacol Biol Psychiatry 2014, 53, 142–8; [DOI] [PubMed] [Google Scholar]; (b) Fujita Y; Kunitachi S; Iyo M; Hashimoto K, The antibiotic minocycline prevents methamphetamine-induced rewarding effects in mice. Pharmacol Biochem Behav 2012, 101 (2), 303–6; [DOI] [PubMed] [Google Scholar]; (c) Zhang L; Kitaichi K; Fujimoto Y; Nakayama H; Shimizu E; Iyo M; Hashimoto K, Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry 2006, 30 (8), 1381–93; [DOI] [PubMed] [Google Scholar]; (d) Chen H; Manev H, Effects of minocycline on cocaine sensitization and phosphorylation of GluR1 receptors in 5-lipoxygenase deficient mice. Neuropharmacology 2011, 60 (7–8), 1058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.(a) You T; Cheng Y; Zhong J; Bi B; Zeng B; Zheng W; Wang H; Xu J, Roflupram, a Phosphodiesterase 4 Inhibitior, Suppresses Inflammasome Activation through Autophagy in Microglial Cells. ACS Chem Neurosci 2017, 8 (11), 2381–2392; [DOI] [PubMed] [Google Scholar]; (b) Hedde JR; Hanks AN; Schmidt CJ; Hughes ZA, The isozyme selective phosphodiesterase-4 inhibitor, ABI-4, attenuates the effects of lipopolysaccharide in human cells and rodent models of peripheral and CNS inflammation. Brain Behav Immun 2017, 64, 285–295; [DOI] [PubMed] [Google Scholar]; (c) Pearse DD; Hughes ZA, PDE4B as a microglia target to reduce neuroinflammation. Glia 2016, 64 (10), 1698–709. [DOI] [PubMed] [Google Scholar]
- 32.Lee DU; Shin DM; Hong JH, The Regulatory Role of Rolipram on Inflammatory Mediators and Cholinergic/Adrenergic Stimulation-Induced Signals in Isolated Primary Mouse Submandibular Gland Cells. Mediators Inflamm 2016, 2016, 3745961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.(a) Vassoler FM; Toorie AM; Byrnes EM, Increased cocaine reward in offspring of females exposed to morphine during adolescence. Psychopharmacology (Berl) 2019, 236 (4), 1261–1272; [DOI] [PubMed] [Google Scholar]; (b) Hitchcock LN; Raybuck JD; Wood MA; Lattal KM, Effects of a histone deacetylase 3 inhibitor on extinction and reinstatement of cocaine self-administration in rats. Psychopharmacology (Berl) 2019, 236 (1), 517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


