Abstract
Purpose
To determine the effect of metformin on early Nd:YAG laser treatment for posterior capsule opacification (PCO) and to explore a molecular mechanism to explain a possible protective effect of metformin against PCO.
Methods
We conducted: 1) a retrospective cohort study of patient eyes undergoing phacoemulsification at our institution; and 2) laboratory investigation of the effect of metformin on the behavior of lens epithelial cells in the context of an animal model for PCO. Population-averaged Cox proportional hazards modeling was used to estimate risk for time to Nd:YAG. For laboratory studies, expression of markers for epithelial-to-mesenchymal transition (EMT) implicated in PCO pathogenesis was measured in tissue culture and following extracapsular lens extraction in a mouse model.
Results
The rate of Nd:YAG laser capsulotomy was 13.1% among the 9798 eyes. Both metformin use and diabetes were protective factors for Nd:YAG laser capsulotomy in univariate analysis. However, in multivariable analysis with nondiabetics as the reference group, only metformin use among diabetics was significantly protective of Nd:YAG (hazard ratio: 0.68, 95% CI: 0.54–0.85, P = 0.0008), while eyes of patients with diabetes without metformin use did not significantly differ (P = 0.5026). Treatment of lens epithelial cells with metformin reduced the level of the EMT markers ⍺-SMA and pERK induced by TGF-β2. Similarly, metformin treatment reduced ⍺-SMA expression in lens epithelial cells following extracapsular lens extraction in a mouse model.
Conclusions
The protective effect of metformin against early Nd:YAG may relate to its ability to downregulate EMT in residual lens epithelial cells that otherwise trend toward myofibroblast development and PCO.
Keywords: YAG, posterior capsule opacification, cataract surgery, metformin
Cataract surgery is the most common surgical procedure in the United States with approximately three million cases annually. While this surgery is typically safe with very low rates of intraoperative complications, undesirable postoperative events can occur, including the onset and progression of visually disabling posterior capsular opacification (PCO). PCO develops, in part, from proliferation and migration of lens epithelial cells left from the lens extraction process.1 Following surgery, epithelial cells undergo a process of epithelial-to-mesenchymal transition (EMT), resulting in cell migration and deposition of extracellular matrix components which induce contraction and wrinkling of the lens capsule. PCO can reduce visual acuity when the visual axis is involved.2 The incidence of PCO for uncomplicated senile cataract surgeries increases over time and is reportedly as high as 50% to as low as <5%.3 Restoration of a clear visual axis, most often accomplished by use of a Nd:YAG laser to create a capsulotomy, is typically successful and uneventful. However, complications of YAG capsulotomy can lead to retinal detachment, damage to the intraocular lens implant, cystoid macular edema, and increased intraocular pressure.4 Furthermore, it is typically more difficult to perform future IOL exchange on eyes treated with YAG capsulotomy to correct for dissatisfaction with vision or IOL adjustment. In addition to these medical issues, Nd:YAG laser is a financial burden for both patients and insurers, and access to the procedure can be an obstacle in developing countries.5 Given the relatively high incidence of PCO and the potential undesirable outcomes from YAG capsulotomy, there is considerable interest in discovery of therapeutic agents and IOL design modifications to reduce the need for YAG therapy.
Although many studies have examined intraocular lens (IOL) material and design as risk factors for PCO,6–13 only a few have assessed demographics and clinical characteristics as risk factors for PCO and the need for Nd:YAG capsulotomy.14–19 Younger age and female gender are risk factors for Nd:YAG,14,17 and lower IOL power has also been found to increase the risk.17,18 Some studies have demonstrated lower incidence of PCO in diabetics when compared to nondiabetics,20,21 yet other studies have shown a higher incidence of PCO in patients with diabetes.16,19,22,23
Studies have demonstrated TGF-β plays an important role in molecular signaling leading to EMT.24 Numerous factors have been identified to suppress TGF-β-dependent molecular signaling in EMT, including inhibitors of aldose reductase, as well as additional factors that regulate signaling downstream from the TGF-β receptor.25 However, no study has yet examined related medications, such as metformin, and its impact on Nd:YAG laser capsulotomy.
A recently published case-control study demonstrated a dose-response relationship of metformin as a protective factor for the development of age-related macular degeneration (AMD).26 Metformin is one of the most widely prescribed medications in patients with type 2 diabetes, where it improves insulin sensitivity and reduces hepatic gluconeogenesis to affect an overall improvement in metabolic control. The primary purpose of the present translational study was firstly, to explore the effect of metformin use on the occurrence of early Nd: YAG laser capsulotomy using a prospective cohort of cataract surgeries from an academic institution. Secondly, we tested the hypothesis that metformin reduces epithelial-to-mesenchymal transition of lens epithelial cells in vitro as well as in an in vivo animal model of lens extraction.
Materials and Methods
Cohort Study
Part one of this research is a retrospective cohort study of phacoemulsification cataract surgeries performed at the University of Colorado Denver Sue Anschutz-Rodgers Eye Center. All cataract surgeries from January 1, 2014, through December 31, 2018, included in the institution's Cataract Outcomes Registry are included in the present study. Details of this registry are further described in Miller et al.27 In brief, data from the medical chart regarding demographic characteristics, medical history, ocular comorbidities, preoperative ocular measures, surgical events, and postoperative outcomes are entered into a secure web-based RedCAP system by trained research assistants. For the present study, eyes among patients < 55 years old, eyes that had traumatic cataracts, and eyes with an anterior chamber intraocular lens or no intraocular lens inserted were excluded. The study was adherent to the guidelines of the Declaration of Helsinki and approved by the Colorado Multiple Institutional Review Board (COMIRB).
Surgeries were performed using the standard phacoemulsification technique using clear corneal incisions. Femtosecond laser and intraoperative aberrometry were utilized based on surgeon and patient preference. Surgery was performed under topical anesthesia unless otherwise dictated by patient comorbidities. At the conclusion of the surgery, moxifloxacin was injected into the anterior chamber for infection prophylaxis. Postoperatively, patients were typically prescribed either difluprednate 0.05% twice daily for two weeks and nepafenac 0.1% once daily for two weeks, or prednisolone acetate 1% and ketorolac 0.5% four times daily with a taper of one drop per week for four weeks.
Statistical Analysis for Cohort Study
The main outcome of the cohort study was development of PCO to the extent that an Nd:YAG laser capsulotomy was performed at one of our clinic locations within six years of cataract surgery. Medical records for all patients included in the cohort were reviewed for Nd:YAG procedure code (CPT 66821) through April 1, 2020. Follow-up time for each patient eye was calculated as the difference in days between date of cataract surgery and date of Nd:YAG for those patients who underwent this procedure, and date of surgery and the last day of medical record review (April 1, 2020) for patients who did not undergo Nd:YAG laser capsulotomy.
Associations between early Nd:YAG capsulotomy and potential risk factors included demographic and clinical characteristics. Demographic factors examined included gender, race/ethnicity, and age. Clinical variables included use of metformin, patient history of diabetes, and history of autoimmune disease. Ocular comorbidities included history of uveitis, glaucoma, combined surgeries with vitrectomy at the time of phacoemulsification, complex surgeries, and complicated surgeries. Complicated surgeries included one or more of the following: posterior capsule rupture, vitreous loss, retained lens, choroidal hemorrhage, iris trauma, and zonular dialysis. Complex surgeries were defined as cases requiring one or more of the following: Malyugin ring expansion device, iris hooks, stretch pupilloplasty, anterior capsule staining with trypan blue or capsular support devices such as capsular tension ring or capsular hooks. Surgeon level was categorized as attending or resident. Lens types were grouped into monofocal, monofocal toric, or multifocal. The vast majority of monofocal lenses implanted at our institution include: ZCB00 (Johnson & Johnson, Santa Ana, CA, USA), SN60WF/SA60WF (Alcon, Fort Worth, TX, USA), or MX60 (Bausch and Lomb, Bridgewater, NJ, USA). Most monofocal torics implanted are SA6ATx (Alcon) or ZCTxxx (Johnson & Johnson). The majority of lenses categorized for our analysis as multifocal included ZXR00, ZXTxx, ZKB00, or ZMB00 (Johnson & Johnson).
Basic frequencies were used to describe categorical variables and the incidence rate of the main outcome Nd:YAG laser capsulotomy. Kaplan-Meier estimates were used to assess incidence rates of Nd:YAG capsulotomy for each of the first six years of follow-up. Kaplan-Meier curves are also presented to illustrate Nd:YAG rates by metformin use and diabetic status: diabetic patients on metformin, diabetic patients not on metformin, and patients not having either. Curves are presented for these three groups stratified by age group, an important factor associated with Nd:YAG. Univariate and multivariable Cox proportional hazards models were used to examine associations of potential predictors of the outcome with sandwich estimators to account for the intrapatient correlation of some patients having two eyes included in the analysis. Hazards ratios (HRs) are used as relative measures of associations between patient groups since patients have variable follow-up time. Patients on metformin who did not have diabetes were excluded from multivariable and stratified analyses. Variables with P values < 0.05 were included in the multivariable analysis. SAS version 9.4 was used for the retrospective cohort study analysis (SAS Institute, Cary, NC, USA).
Tissue Culture Studies
Lens epithelial cells in tissue culture were used to examine the effect of metformin (N,N-dimethylbiguanide; Sigma-Aldrich, St. Louis, MO, USA) on the EMT response to exposure to TGF-β2. Primary mouse lens epithelial cells (LEC) were purified from an aldose reductase (AKR1B1) transgenic mouse model previously characterized for elevated risk of diabetic cataract formation.25,28 Lenses were dissected from four- to six-week-old mouse eyes and the capsule-epithelium removed by careful dissection from the lens mass. LECs were detached by incubating capsular bags in PBS containing 0.05% Trypsin-EDTA (ATCC, Manassas, VA, USA) at 37°C for eight minutes. LECs were recovered by centrifugation and seeding on a 60 mm plate. Cultures were expanded by incubation at 37°C in the presence of 5% CO2 in DMEM (Corning Cellgro, Manassas, VA, USA), 10% Fetal Bovine Serum (Gemini Bio products, West Sacramento, CA, USA), 100U penicillin, 100 µg streptomycin (Corning) and 0.25 µg amphotericinB (Lonza Biosciences, Basel, Switzerland) per ml for three to five weeks. Cells were replated and cultured for 24 hours before treatment with either 3 mM metformin (Sigma-Aldrich) alone or with TGF-β2 at 10 ng/ml (Novus Biologicals, Centennial, CO, USA) in serum-free medium for 48 hours. Cells were then harvested with RIPA lysis buffer containing proteinase inhibitors (Pierce Biotechnology, Rockford, IL, USA). Protein content in cell lysates was determined using the BCA Protein Assay Reagent (Thermo Scientific, Waltham, MA, USA). For Western blot analysis, equal amounts of proteins were separated by SDS-PAGE precast gels (Bio-Rad, Hercules, MA, USA) and transferred to PVDF membrane (GE, Piscataway, NJ, USA). Membranes were blocked with 5% nonfat dry milk in TBST buffer and probed with antibodies to the following markers: Gapdh (1:1000 dilution, Santa Cruz, CA, USA), α-SMA (1:1000 dilution, Abcam, Cambridge, MA, USA), extracellular signal-regulated kinase 1 and 2 (ERK 1/2) and phospho ERK/2 (1:1000 dilution, Cell Signaling Technology, Danvers, MA, USA). Blots were incubated with primary antibody overnight at 4°C, then probed with secondary antibodies conjugated with horseradish peroxidase at room temperature for one hour. Immune complexes were visualized by enhanced chemiluminescence and imaging with Bio-Rad ChemiDoc XRS+ imaging system (Bio-Rad), with signal intensity quantified using ImageLab Software Version 3.0. Statistical analysis was performed using GraphPad Prism version 5.03 (GraphPad Software, La Jolla, CA, USA). The relative expression of α-SMA was normalized to GAPDH and pERK expression was normalized to total ERK. Relative fold change of α-SMA and pERK were analyzed by 1-way ANOVA with Tukey's multiple comparison test. Data are mean ± standard error of the mean (SEM) of three experiments.
Extracapsular Lens Extraction in a Mouse Model
We used Mus musculus strain C57BL/6 to measure the impact of metformin treatment on the wound response to lens extraction. Transgenic mice on C57BL/6 background, designed for overexpression of human aldose reductase (strain PAR40), have been described previously.28 Metformin was given ad lib in drinking water at 0.2 mg/mL to give a dose range of approximately 26 to 70 mg/kg/day beginning 48 hours prior to surgery and continuing during five postsurgical days until tissue collection for analysis. We followed a previously described method for lens extraction,29 with slight modifications, as follows. Animals were anesthetised using 90 mg/kg ketamine (VetOne, Cambridge, ON, Canada), 10 mg/kg xylazine (VetOne) and given 1 mg/kg buprenorphine SR (ZooPharm, Laramie, WY, USA). The pupils were then dilated by topical application of one drop of 0.2% tropicamide (Akron, Lake Forest, IL, USA), 0.5% phenylephrine (Akron) and the cornea anesthetised with one drop of ophthalmic proparacaine (Alcon, Fort Worth, TX, USA) and 5% ophthalmic betadine (Alcon). Extracapsular lens extraction (ECLE) was performed by making an incision through the cornea and then the lens capsule using a 1 mm slit scalpel (Alcon) followed by hydrodissection of the lens fiber mass using a bent cannula (Alcon). The anterior chamber was reinflated using viscoat (Alcon) and the cornea opening was sealed using Resure (Ocular Therapeutix, Bedford, MA, USA). The animals were allowed to recover for five days and then the lens capsule was recovered for RNA analysis or the whole globe was fixed for immunohistochemistry studies.
Quantitative RT-PCR
Lens capsules were kept in a solution of Qiazol (Qiagen, Austin, TX, USA): chloroform (Sigma) and stored at −80°C prior to RNA extraction using an RNeasy micro kit (Qiagen) according to the manufacturer's instructions. Complementary DNA was created using iScript reverse transcription supermix for RT-qPCR (Bio-Rad) and the qPCR was performed with iTaq Universal SYBR green supermix (Bio-Rad). Primers for αSMA were forward 5′-CTGTTATAGGTGGTTTCGTGGA-3′, reverse 5′-GAGCTACGAACTGCCTGAC-3′ (Integrated DNA Technologies, Coralville, IA, USA) and GAPDH were 5′-AATGGTGAAGGTCGGTGTG-3′, reverse 5′-GTGGAGTCATACTGGAACATGTAG-3′ (Integrated DNA Technologies) using the CFX (Bio-Rad). Each sample was performed in triplicate.
Immunohistochemistry
Whole globes were fixed in 4% paraformaldehyde for 10 minutes at 4°C followed by four hours per each sucrose (Fisher, Waltham, MA, USA) gradient 10%, 20%, 30% and then frozen into optimal cutting temperature (OCT, Tissue Tek, Torrance, CA, USA). The sections were stained with 1:500 αSMA A488 (Abcam, catalog# Abcam ab202295) diluted in 1% BSA (Sigma) and 0.1% tween 20 (Fisher) in saline for one hr at room temperature. The slides were then washed and counterstained with fluoromount (Electron Microscope Systems, Hatfield, PA, USA) containing DAPI. Slides were imaged on a confocal microscope Nikon Eclipse Ti (Nikon, Tokyo, Japan) using NIS Elements software version 5.20.02 (Nikon).
Statistical Analysis of Laboratory Studies
Statistical analyses were performed using GraphPad Prism software version 5.03 (GraphPad Software) using unpaired two-tailed Student's t-test.
Results
Cohort Study
After exclusions of patients under age 55 years (n = 904), traumatic cataracts (n = 25), ACIOLs (n = 24), and surgeries with no lens inserted (n = 11), a total of 9798 eyes that underwent phacoemulsification at our institution were included in the analytic dataset. Demographic variables are shown in Table 1. Patient eyes were 58.9% female, 74.0% White, and the average age was 71.1 years (SD: 7.9). The overall rate of Nd:YAG laser capsulotomy was 13.1% (n = 1283 eyes). The mean time to Nd:YAG was 630 (SD 469) days after cataract surgery. Based on Kaplan-Meier estimates the incidence rate of Nd:YAG laser capsulotomy increased each follow-up year from 5.0% at one year, to 12.0% at three years, and 17.1% at five years. Incidence of having an Nd:YAG during the follow-up time were higher among females (14.4% vs. 11.2% for males, P < 0.0001) and Whites (14.4%) versus other race/ethnic groups. Younger age was a strong predictor of having had Nd:YAG laser capsulotomy both as a continuous and categorical variable.
Table 1.
Demographic Predictors of Time to YAG Following Cataract Surgery, N = 9798
| Time to YAG | ||||
|---|---|---|---|---|
| Characteristic | Total (Column %) | YAG # (Row %) | HR (95% CI) | P Value |
| Total | 9798 | 1283 (13.1%) | — | — |
| Gender | ||||
| Male | 4028 (41.1%) | 451 (11.2%) | Reference | — |
| Female | 5770 (58.9%) | 832 (14.4%) | 1.33 (1.16–1.52) | <0.0001 |
| Race/ethnicity | ||||
| White | 7255 (74.0%) | 1045 (14.4%) | Reference | — |
| Hispanic | 795 (8.1%) | 78 (9.8%) | 0.66 (0.51–0.86) | 0.0022 |
| African American | 831 (8.5%) | 68 (8.2%) | 0.53 (0.40–0.71) | <0.0001 |
| Asian | 422 (4.3%) | 42 (10.0%) | 0.67 (0.47–0.96) | 0.0305 |
| Other | 271 (2.8%) | 19 (7.0%) | 0.48 (0.28–0.83) | 0.0080 |
| Unknown | 224 (2.3%) | 31 (13.8%) | 1.05 (0.67–1.63) | 0.8366 |
| Age, years | ||||
| Mean (SD) | 71.1 (7.9) | 69.3 (7.6) | 0.97 (0.96–0.98) | <0.0001 |
| Age group, years | ||||
| 55–64 | 2304 (23.5%) | 381 (16.5%) | Reference | — |
| 65–75 | 4834 (49.3%) | 663 (13.7%) | 0.84 (0.72–0.97) | 0.0198 |
| 76+ | 2660 (27.2%) | 239 (9.0%) | 0.52 (0.42–0.62) | <0.0001 |
Potential clinical characteristics assessed for their associations with having a Nd:YAG are shown in Table 2. Metformin use, diabetes, and complex surgery were all protective of later requiring a Nd:YAG. Almost all of patients taking metformin had diabetes (with the exception of eight patients), and a little over half, 54.8%, of the 2347 patient eyes with diabetes were on metformin. In univariate analysis, eyes of patients on metformin had a lower hazard of Nd:YAG compared to patients not on metformin (HR: 0.63, 95% CI: 0.50–0.79, P < 0.0001), and patients with diabetes also had a lower hazard (HR: 0.68, 95% CI: 0.57–0.80, P < 0.0001). Multifocal lenses (P < 0.0001) and surgeries combined with vitrectomy (P = 0.0304) had higher hazards of Nd:YAG. Whereas, patient eyes with complex surgeries (P < 0.0001), increasing IOL power (P < 0.0001), and worse preoperative best-corrected visual acuity (BCVA) (P = 0.0010) were inversely associated with the hazard for Nd:YAG laser capsulotomy during the study period.
Table 2.
Clinical Characteristics of Time to YAG Following Cataract Surgery
| Time to YAG | ||||
|---|---|---|---|---|
| Characteristic | Total (Column %) | YAG # (Row %) | HR (95% CI) | P Value |
| Metformin | ||||
| Yes | 1296 (13.2%) | 114 (8.9%) | 0.63 (0.50–0.79) | <0.0001 |
| No | 8502 (86.8%) | 1169 (13.8%) | Reference | — |
| Diabetes | ||||
| Yes | 2347 (24.0%) | 231 (9.8%) | 0.68 (0.57–0.80) | <0.0001 |
| No | 7451 (76.0%) | 1052 (14.1%) | Reference | — |
| Autoimmune disease | ||||
| Yes | 861 (8.8%) | 130 (15.0%) | 1.20 (0.97–1.49) | 0.0937 |
| No | 8937 (91.2%) | 1153 (12.9%) | Reference | — |
| History of uveitis | ||||
| Yes | 132 (1.4%) | 22 (16.7%) | 1.51 (0.90–2.55) | 0.1213 |
| No | 9666 (98.6%) | 1261 (13.0%) | Reference | — |
| Glaucoma | ||||
| Yes | 1423 (14.5%) | 196 (13.8%) | 1.00 (0.85–1.20) | 0.9425 |
| No | 8370 (85.5%) | 1087 (13.0%) | Reference | — |
| Combined surgery with vitrectomy | ||||
| Yes | 131 (1.3%) | 24 (18.3%) | 1.59 (1.05–2.42) | 0.0304 |
| No | 9667 (98.3%) | 1259 (13.0%) | Reference | — |
| Complex surgery | ||||
| Yes | 1421 (14.5%) | 129 (9.1%) | 0.65 (0.53–0.79) | <0.0001 |
| No | 8377 (85.5%) | 1154 (13.8%) | Reference | — |
| Type of lens | ||||
| Monofocal | 8730 (89.1%) | 1069 (12.2%) | Reference | — |
| Multifocal | 440 (4.5%) | 130 (29.6%) | 3.22 (2.55–4.06) | <0.0001 |
| Toric | 628 (6.4%) | 84 (13.4%) | 1.12 (0.87–1.43) | 0.3920 |
| Surgeon | ||||
| Attending | 9192 (94.1%) | 1210 (13.2%) | Reference | — |
| Resident | 577 (5.9%) | 72 (12.5%) | 0.82 (0.64–1.06) | 0.1223 |
| Missing | 29 | |||
| Complicated surgery* | ||||
| Yes | 107 (1.1%) | 17 (15.9%) | 1.22 (0.75–1.99) | 0.4252 |
| No | 9691 (98.9%) | 1266 (13.1%) | Reference | — |
| Prolonged steroids | ||||
| Yes | 885 (9.3%) | 125 (14.1%) | 0.92 (0.76–1.12) | 0.4095 |
| No | 8613 (90.7%) | 1141 (13.2%) | Reference | — |
| Missing | 300 | |||
| No YAG | YAG | |||
| Mean (SD) | Mean (SD) | HR (95% CI) | P Value | |
| IOL Power, diopters | 19.7 (4.0) | 18.7 (4.4) | 0.95 (0.94–0.97) | <0.0001 |
| Preoperative BCVA, logMAR | 0.36 (0.47) | 0.31 (0.39) | 0.77 (0.65–0.90) | 0.0010 |
Complicated surgery includes choroidal hemorrhage, iris trauma, posterior capsule rupture, vitreous loss, retained lens, and zonular dialysis.
As expected, our three patient groups of diabetic patients on metformin, diabetic patients not on metformin, and not having either differed in terms of several variables. For example, nondiabetic patients were more likely to be female (60.3%) than diabetic patients on and not on metformin (57.7% and 50.1%, respectively) and nondiabetic patients were less likely to have complex surgery (12.9%) than both diabetic groups (17.8% and 21.8%, respectively). Multivariable analysis to account for these confounding variables is shown in Table 3 and demonstrated that diabetics not on metformin did not have a significantly different hazard from nondiabetics, whereas diabetics on metformin were significantly protective compared to nondiabetics (HR: 0.68, 95% CI: 0.54–0.85, P = 0.0008). Other risk factors that were significant in the multivariable model were female gender, decreasing age, and multifocal versus monofocal lens. Kaplan-Meier estimates of Nd:YAG for three patient groups of metformin and diabetes, diabetes and no metformin, and neither diabetes nor metformin are shown in Figure 1 by age group. These figures show consistently lower rates of Nd:YAG laser capsulotomy for patients with metformin use (blue curves).
Table 3.
Multivariable Predictors of Time to YAG Following Cataract Surgery
| Characteristic | Hazard Ratio | 95% Confidence Interval | P Value* |
|---|---|---|---|
| Metformin and diabetes | 0.68 | 0.54–0.85 | 0.0008 |
| Diabetes, no metformin | 0.93 | 0.74–1.16 | 0.5026 |
| Neither diabetes nor metformin | Reference | — | — |
| Female | 1.32 | 1.15–1.51 | <0.0001 |
| Race/ethnicity | |||
| White | Reference | — | — |
| Hispanic | 0.76 | 0.58–1.00 | 0.0477 |
| African American | 0.61 | 0.45–0.82 | 0.0012 |
| Asian | 0.75 | 0.53–1.07 | 0.1158 |
| Other | 0.51 | 0.29–0.88 | 0.0154 |
| Unknown | 1.03 | 0.66–1.61 | 0.8832 |
| Combined w/vitrectomy | 1.86 | 1.21–2.86 | 0.0045 |
| Complex surgery | 0.81 | 0.66–0.99 | 0.0440 |
| Type of lens | |||
| Monofocal | Reference | — | — |
| Multifocal | 2.63 | 2.08–3.33 | <0.0001 |
| Toric | 0.94 | 0.73–1.21 | 0.6149 |
| Age, years | 0.97 | 0.96–0.98 | <0.0001 |
| IOL power, diopters | 0.96 | 0.95–0.98 | <0.0001 |
| Preoperative Logmar, logMAR | 0.85 | 0.73–0.99 | 0.0343 |
Figure 1.
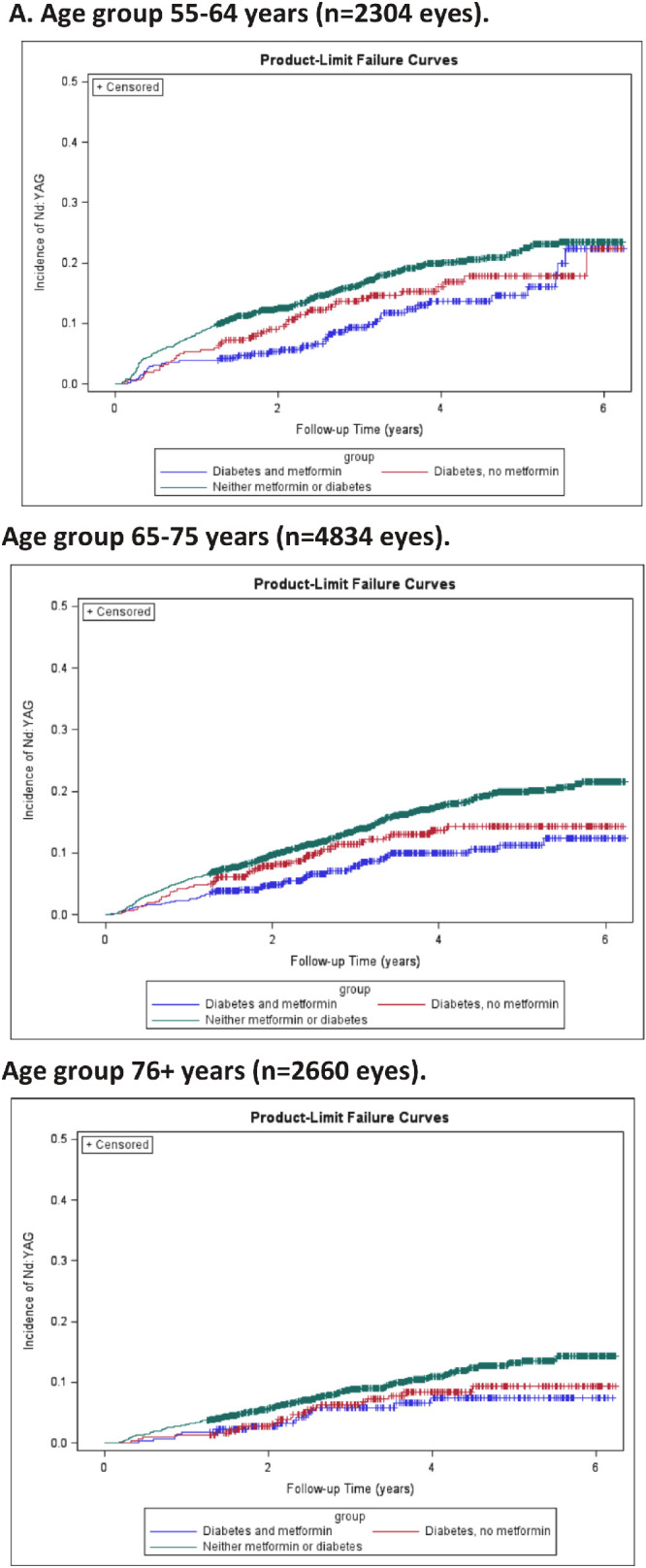
Unadjusted incidence rate of Nd:YAG laser following cataract surgery by (1A) monofocal and multifocal lens types and (1B) age category 55–64, 65–75, and 76+ years for three study groups of patients eyes: 1) Metformin and diabetes (blue); 2) Diabetes, no metformin (red); and 3) Neither metformin or diabetes (green).
Effect of Metformin on EMT Markers in Tissue Culture Cells
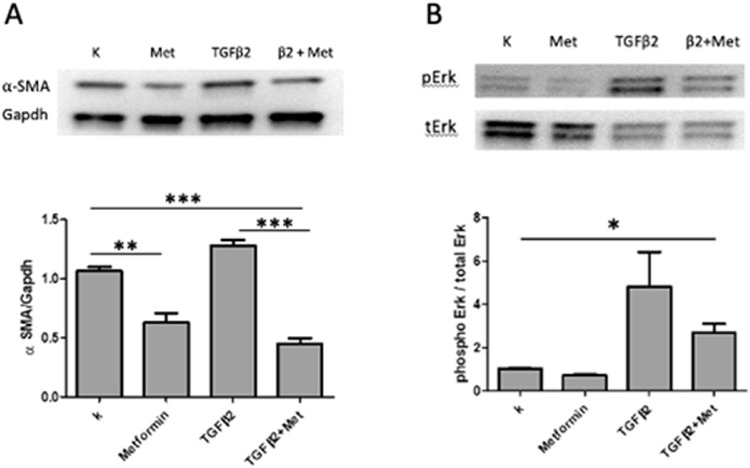
EMT is considered a hallmark feature marking the early stages of lens changes leading to PCO. We conducted a lens tissue culture study as an initial test of whether metformin exposure had an effect on EMT in lens cells. The levels of ⍺-smooth muscle actin (⍺-SMA) and phospho-ERK (extracellular signal-regulated kinase), well-recognized markers of cells undergoing epithelial-to-mesenchymal transition, and thus indicators of early steps in the pathogenesis of posterior capsule opacification, were measured in lens epithelial cells following exposure to TGF-β2 either in the presence or absence of metformin. As shown in Figure 2, addition of metformin to culture media reduced the level of ⍺-SMA expression in LEC in the absence (P < 0.01) or presence (P < 0.001) of TGF-β2. Similarly, metformin blunted phospho-ERK levels in LEC incubated with TGF-β2 (P < 0.01).
Figure 2.
Metformin suppresses TGF-β2-induced expression of ⍺-SMA and ERK activation. Mouse primary lens epithelial cells were treated with metformin (3 mM) with or without TGF-β2 for 48 hours. Western blot analysis shown significant inhibitory effect of metformin on α-SMA expression (A) and ERK activation (B) in the presence of TGF-β2. The relative expression of α-SMA was normalized to Gapdh and pERK expression was normalized to total ERK as well. Relative fold-change of α-SMA and pERK were analyzed by 1-way ANOVA with Tukey's multiple comparison test. Data are mean ± SEM of three experiments (n = 3), *P < 0.05, **P < 0.01, and ***P < 0.001.
Effect of Metformin on αSMA Expression in a Mouse Model of Extracapsular Lens Extraction
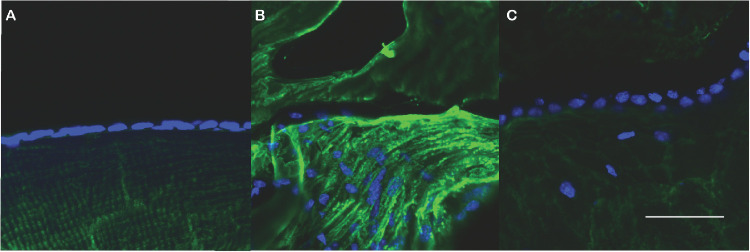
To assess the impact of metformin on EMT in lens in vivo, we used qRT-PCR to measure the abundance of αSMA gene transcripts in lens capsules recovered from animals five days following extracapsular lens extraction. As shown in Figure 3, αSMA transcript levels were elevated approximately threefold following extracapsular lens extraction (P < 0.001) in comparison with capsules from unoperated control mice. However, treatment of mice with metformin in drinking water for 48 hours prior to surgery essentially normalized the abundance of αSMA gene transcripts following lens extraction. Immunostaining for αSMA confirmed these patterns. Strong staining for αSMA, observed in capsules five days following lens extraction, was essentially at background levels in capsules of metformin-treated mice five days following lens extraction and in unoperated control mice (Fig. 4).
Figure 3.
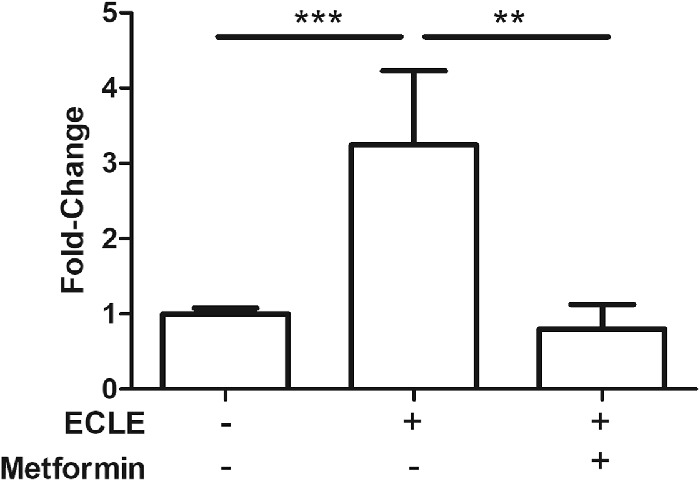
Metformin suppresses TGF-β2-induced activation of ⍺-SMA gene expression. Fold-change of αSMA gene transcript levels measured by qRT-PCR. Metformin was given ad lib in the drinking water for 48 hrs prior to extracapsular lens extraction (ECLE) surgery and continued for five days after surgery. Data are mean ± SEM of three experiments (n = 3), **P < 0.01, and ***P < 0.001.
Figure 4.
Metformin suppresses αSMA expression in lens following lens extraction. Lens cryosections were immunostained for αSMA (green) and nuclei (blue). In comparison to lens capsule of unoperated eye (panel A), ECLE surgery induces robust staining for αSMA (panel B) unless mice are treated with metformin (panel C).
Discussion
This translational study identified metformin as a protective factor for early Nd:YAG laser capsulotomy in our analysis of a large retrospective cohort of patients undergoing cataract surgery. Furthermore, we demonstrated that metformin can decrease the EMT in vitro and in vivo in the mouse lens capsule, which is an early cellular transformational process involved in the development of PCO. Metformin may inhibit TGF-β-induced EMT in exposed lens epithelial cells, thereby decreasing PCO formation. Accordingly, this may explain why diabetic patients taking metformin in our study were found to be at significantly lower risk for development of PCO and subsequent YAG capsulotomy as compared to cataract patients not on metformin or nondiabetic patients.
Many mechanisms of action have been identified to explain the therapeutic effects of metformin in the context of type 2 diabetes.30 Chief among possible mechanisms of action is the ability of metformin to stimulate AMP-activated protein kinase (AMPK), a key energy sensing enzyme that is activated by elevations in AMP/ATP ratio. While AMPK activity is reduced in diabetes mellitus, its activation in response to therapeutic agents such as metformin helps to improve metabolic control. Activation of AMPK is also reported to inhibit TGF-β-induced EMT and myofibroblast activation in kidney and lung fibrosis,31,32 presumably by interfering with the ability of activated and nuclear-translocated Smad3 to effect transcriptional activation of target genes such as the EMT marker αSMA. Work from our lab and others has demonstrated that exposure of LEC to TGF-β results in activation of Smad3 and upregulation of αSMA expression.25 Therefore, we consider it likely that reduced levels of TGF-β-induced αSMA expression in LEC cultures exposed to metformin may well follow a similar mechanism as seen in kidney proximal tubule epithelial cells and mesangial cells. Given that the basal level of αSMA expression is relatively high in LECs derived from our mouse model, we recognize that the metformin-mediated reduction in αSMA from a level modestly elevated by TGF-β must be interpreted with caution33. It is possible that metformin acts through redundant mechanisms to suppress EMT. For example, Lasiste and coworkers recently reported on the ability of metformin to suppress the induction of EMT markers in a virally transformed human lens-derived cell line after exposure to both TGF-β and an unspecified isoform of fibroblast growth factor.34 Given that other signaling pathways have been shown to affect growth and proliferation of lens epithelial cells, including those influenced by extracellular matrix components including fibronectin,35,36 it is possible that the inhibiting effect of metformin against lens changes leading to the need for YAG capsulotomy acts at one or more steps in these regulatory pathways. Indeed, contraction of the extracellular matrix, which may not be functionally linked to signaling downstream from the TGF-β receptor, may be an important readout on the therapeutic effect of metformin. Metformin may also exert therapeutic effects through AMPK-independent mechanisms involving inhibition of mitochondrial complex I activity.37
The duration of metformin exposure necessary to achieve protection against the need for Nd:YAG capsulotomy is not yet known. In our studies, the protective effects of metformin were observed in animals receiving the drug for two days preceding lens extraction and continuously thereafter for five days before tissue analysis. Future studies will test whether initiation of metformin therapy after the onset of PCO symptoms will halt or even reverse fibrotic changes to the lens capsule.
Several other populations in our study were also associated with decreased Nd:YAG capsulotomy rate in the multivariable analysis. Our study supports previously published findings that female gender, younger age, lower IOL power, and better preoperative visual acuity are associated with increased Nd:YAG capsulotomy rate. Gender differences in YAG rate may be related to women more frequently seeking medical advice and higher levels of symptom reporting.14 Better preoperative visual acuity has been proposed as a risk factor for YAG as it seems very plausible that those who are interested in cataract surgery sooner due to visual symptoms would also be patients who note decline in vision due to PCO sooner as well.14,17 Lower IOL powers in axial myopes may be more prone to PCO formation due to mechanical properties of larger capsular bags forming less PCO-limiting capsular adhesion.17,18 Greater proliferative ability of LECs in younger patients may be responsible for increased PCO in the younger population, as well as a possibility of more demanding visual needs.17 Multifocal lens implantation has also previously been associated with increased Nd:YAG capsulotomy rate as compared to monofocal lenses, likely due to a combination of visual needs of the patient and interaction between the multifocal optics and visual obscuration generated by the PCO.38,39
The racial differences in YAG capsulotomy rate amongst Hispanics and African Americans, as well as decreased YAG rate in complex surgeries and increased YAG rate in combined cataract surgery with vitrectomy have not previously been explored to our knowledge and warrant further investigation. It is unclear whether racial differences represent differences in access to care, or cultural variations in visual expectations following cataract surgery. We initially hypothesized that eyes that underwent complex surgery were more likely to undergo a prolonged course of steroids following surgery, which may impact PCO formation, however, the hazard ratio for this group was below one, indicating steroid use a less likely contributor. Combined phacoemulsification and vitrectomy surgeries were more likely to undergo YAG capsulotomy. It is unclear whether this is secondary to changes in the molecular environment of the vitreous cavity in these eyes that could affect the rate of PCO formation, or greater involvement of ophthalmologic care for eyes with coexisting retinal pathology with attempts to maximize vision.
The retrospective nature of this study has inherent limitations of potential confounders that are not accounted for in the analysis or data capture. In addition, patients who are more likely to return to clinic because of other visual issues are more likely to have an Nd:YAG laser capsulotomy procedure. These procedures can also be performed outside our institution's clinics, which would not be captured in our dataset. However, findings from an unpublished study at our institution found that very few patients (n = 14) included in our study received Nd:YAG capsulotomies at outside Colorado clinics, suggesting that most patients return to our clinics for cataract-related care.
Further studies of larger cohorts are warranted to validate these findings from our single academic institution and to examine the association of metformin as a protective factor for Nd:YAG in various subpopulations. Future studies are also required to more fully understand how metformin may interfere with onset and development of PCO. The data in this study generated in vivo and in vitro using mouse lens capsule suggest that the effect of metformin on PCO development is not dependent on the presence of diabetes. However, virtually all patients in our clinical study used metformin in the context of treatment for diabetes. Although diabetes was eliminated as a covariate in PCO opacification, we cannot predict whether metformin would also have a protective effect if used in nondiabetic patients, nor can we comment on the length of metformin therapy that might be useful after cataract surgery in nondiabetics. Further research on this protective effect of metformin would be important to determine if its use may be optimized as a therapeutic for PCO prevention in a broader population.
Acknowledgments
Supported in part by NIH grant EY028147, an Unrestricted Research grant to the Department of Ophthalmology from Research to Prevent Blindness, Inc., New York, NY, and the Frederic C. Hamilton Macular Degeneration Center and the Colorado Clinical & Translational Sciences Institute (CCSTI) with the Development and Informatics Service Center (DISC) from NIH/NCRR.
Diclosure: J.L. Patnaik, None; K.L. Christopher, None; M.G. Pedler, None; B. Shieh, None; C.C. Petrash, None; B.D. Wagner, None; N. Mandava, None; A.M. Lynch, None; A.G. Palestine, None; J.M. Petrash, None
References
- 1.Wormstone IM.The human capsular bag model of posterior capsule opacification. Eye (Lond). 2020; 34: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buehl W, Sacu S, Findl O.. Association between intensity of posterior capsule opacification and visual acuity. J Cataract Refract Surg. 2005; 31: 543–547. [DOI] [PubMed] [Google Scholar]
- 3.Raj SM, Vasavada AR, Johar SR, Vasavada VA, Vasavada VA.. Post-operative capsular opacification: a review. Int J Biomed Sci. 2007; 3: 237–250. [PMC free article] [PubMed] [Google Scholar]
- 4.Karahan E, Er D, Kaynak S.. An overview of Nd:YAG laser capsulotomy. Med Hypothesis Discov Innov Ophthalmol. 2014; 3: 45–50. [PMC free article] [PubMed] [Google Scholar]
- 5.Awasthi N, Guo S, Wagner BJ.. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009; 127: 555–562. [DOI] [PubMed] [Google Scholar]
- 6.Stordahl PB, Drolsum L.. A comparison of Nd:YAG capsulotomy rate in two different intraocular lenses: AcrySof and Stabibag. Acta Ophthalmol Scand. 2003; 81: 326–330. [DOI] [PubMed] [Google Scholar]
- 7.Schriefl SM, Menapace R, Stifter E, Zaruba D, Leydolt C.. Posterior capsule opacification and neodymium:YAG laser capsulotomy rates with 2 microincision intraocular lenses: four-year results. J Cataract Refract Surg. 2015; 41: 956–963. [DOI] [PubMed] [Google Scholar]
- 8.Georgopoulos M, Findl O, Menapace R, Buehl W, Wirtitsch M, Rainer G.. Influence of intraocular lens material on regeneratory posterior capsule opacification after neodymium:YAG laser capsulotomy. J Cataract Refract Surg. 2003; 29: 1560–1565. [DOI] [PubMed] [Google Scholar]
- 9.Ursell PG, Dhariwal M, Majirska K, et al.. Three-year incidence of Nd:YAG capsulotomy and posterior capsule opacification and its relationship to monofocal acrylic IOL biomaterial: a UK real world evidence study. Eye (Lond). 2018; 32: 1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biber JM, Sandoval HP, Trivedi RH, de Castro LEF, French JW, Solomon KD.. Comparison of the incidence and visual significance of posterior capsule opacification between multifocal spherical, monofocal spherical, and monofocal aspheric intraocular lenses. J Cataract Refract Surg. 2009; 35: 1234–1238. [DOI] [PubMed] [Google Scholar]
- 11.Buehl W, Findl O.. Effect of intraocular lens design on posterior capsule opacification. J Cataract Refract Surg. 2008; 34: 1976–1985. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier L, Lafuma A, Laurendeau C, Berdeaux G.. Neodymium:YAG laser rates after bilateral implantation of hydrophobic or hydrophilic multifocal intraocular lenses: twenty-four month retrospective comparative study. J Cataract Refract Surg. 2010; 36: 1195–1200. [DOI] [PubMed] [Google Scholar]
- 13.Leydolt C, Schartmuller D, Schwarzenbacher L, Schranz M, Schriefl S, Menapace R.. Comparison of posterior capsule opacification development with 2 single-piece intraocular lens types. J Cataract Refract Surg. 2017; 43: 774–780. [DOI] [PubMed] [Google Scholar]
- 14.Ando H, Ando N, Oshika T.. Cumulative probability of neodymium: YAG laser posterior capsulotomy after phacoemulsification. J Cataract Refract Surg. 2003; 29: 2148–2154. [DOI] [PubMed] [Google Scholar]
- 15.Baratz KH, Cook BE, Hodge DO.. Probability of Nd:YAG laser capsulotomy after cataract surgery in Olmsted County, Minnesota. Am J Ophthalmol. 2001; 131: 161–166. [DOI] [PubMed] [Google Scholar]
- 16.Elgohary MA, Dowler JG.. Incidence and risk factors of Nd:YAG capsulotomy after phacoemulsification in non-diabetic and diabetic patients. Clin Exp Ophthalmol. 2006; 34: 526–534. [DOI] [PubMed] [Google Scholar]
- 17.Lindholm JM, Laine I, Tuuminen R.. Five-year cumulative incidence and risk factors of Nd:YAG capsulotomy in 10 044 hydrophobic acrylic 1-piece and 3-piece intraocular lenses. Am J Ophthalmol. 2019; 200: 218–223. [DOI] [PubMed] [Google Scholar]
- 18.Lindholm JM, Laine I, Tuuminen R.. Intraocular lens power, myopia, and the risk of Nd:YAG capsulotomy after 15,375 cataract surgeries. J Clin Med. 2020; 9(10): 3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Tong N, Pan L, et al.. Retrospective analyses of potential risk factors for posterior capsule opacification after cataract surgery. J Ophthalmol. 2018; 2018: 9089285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaczek A, Zetterstrom C.. Posterior capsule opacification after phacoemulsification in patients with diabetes mellitus. J Cataract Refract Surg. 1999; 25: 233–237. [DOI] [PubMed] [Google Scholar]
- 21.Knorz MC, Soltau JB, Seiberth V, Lorger C.. Incidence of posterior capsule opacification after extracapsular cataract extraction in diabetic patients. Metab Pediatr Syst Ophthalmol (1985). 1991; 14: 57–58. [PubMed] [Google Scholar]
- 22.Ionides A, Dowler JG, Hykin PG, Rosen PH, Hamilton AM.. Posterior capsule opacification following diabetic extracapsular cataract extraction. Eye (Lond). 1994; 8(Pt 5): 535–537. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi K, Hayashi H, Nakao F, Hayashi F.. Posterior capsule opacification after cataract surgery in patients with diabetes mellitus. Am J Ophthalmol. 2002; 134: 10–16. [DOI] [PubMed] [Google Scholar]
- 24.Wormstone IM, Tamiya S, Anderson I, Duncan G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci. 2002; 43: 2301–2308. [PubMed] [Google Scholar]
- 25.Chang KC, Petrash JM.. Aldose reductase mediates transforming growth factor beta2 (TGF-beta2)-induced migration and epithelial-to-mesenchymal transition of lens-derived epithelial cells. Invest Ophthalmol Vis Sci. 2015; 56: 4198–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blitzer AL, Ham SA, Colby KA, Skondra D.. Association of metformin use with age-related macular degeneration: a case-control study. JAMA Ophthalmol. 2021; 139(3): 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller DC, Christopher KL, Patnaik JL, et al.. Posterior capsule rupture during cataract surgery in eyes receiving intravitreal anti-VEGF injections. Curr Eye Res. 2021; 46(2): 179–184. [DOI] [PubMed] [Google Scholar]
- 28.Snow A, Shieh B, Chang KC, et al.. Aldose reductase expression as a risk factor for cataract. Chem Biol Interact. 2015; 234: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zukin LM, Pedler MG, Groman-Lupa S, Pantcheva M, Ammar DA, Petrash JM.. Aldose reductase inhibition prevents development of posterior capsular opacification in an in vivo model of cataract surgery. Invest Ophthalmol Vis Sci. 2018; 59: 3591–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B.. Metformin: from mechanisms of action to therapies. Cell Metab. 2014; 20: 953–966. [DOI] [PubMed] [Google Scholar]
- 31.Thakur S, Viswanadhapalli S, Kopp JB, et al.. Activation of AMP-activated protein kinase prevents TGF-beta1-induced epithelial-mesenchymal transition and myofibroblast activation. Am J Pathol. 2015; 185: 2168–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra R, Cool BL, Laderoute KR, Foretz M, Viollet B, Simonson MS.. AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J Biol Chem. 2008; 283: 10461–10469. [DOI] [PubMed] [Google Scholar]
- 33.Chang KC, Shieh B, Petrash JM.. Influence of aldose reductase on epithelial-to-mesenchymal transition signaling in lens epithelial cells. Chem Biol Interact. 2017; 276: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasiste JM, Zoroquiain P, Miyamoto D, Burnier MN.. Metformin activity in an in vitro model of posterior capsule opacification. Vision Pan-America. 2018; 17: 105–112. [Google Scholar]
- 35.VanSlyke JK, Boswell BA, Musil LS.. Fibronectin regulates growth factor signaling and cell differentiation in primary lens cells. J Cell Sci. 2018; 131(22): jcs217240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shihan MH, Kanwar M, Wang Y, Jackson EE, Faranda AP, Duncan MK.. Fibronectin has multifunctional roles in posterior capsular opacification (PCO). Matrix Biol. 2020; 90: 79–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheaton WW, Weinberg SE, Hamanaka RB, et al.. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014; 3: e02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran DB, Vargas V, Potvin R.. Neodymium:YAG capsulotomy rates associated with femtosecond laser-assisted versus manual cataract surgery. J Cataract Refract Surg. 2016; 42: 1470–1476. [DOI] [PubMed] [Google Scholar]
- 39.Shah VC, Russo C, Cannon R, Davidson R, Taravella MJ.. Incidence of Nd:YAG capsulotomy after implantation of AcrySof multifocal and monofocal intraocular lenses: a case controlled study. J Refract Surg. 2010; 26: 565–568. [DOI] [PubMed] [Google Scholar]