Abstract
Background:
Since December 2019, coronavirus (COVID-19) spread throughout the world. The high rate of infection and its unknown nature led specialists to report the condition of patients. The aim of this study is to systematically review of symptoms, laboratory and radiologic findings, treatment, and outcomes of patients with COVID-19.
Materials and Methods:
Databases such as PubMed, Embase, Scopus, Web of Science, Google Scholar, and Cochrane were searched. Finally, 46 articles were appropriate for the aim of the study. After quality evaluation, the necessary data were extracted and meta-analysis was performed.
Results:
4858 articles were retrieved until March 30, 2020. After screening, the full-text of 46 articles was assessed. Of the reported cases, 31.7% had no comorbidities, 21.4% had high blood pressure, 70.6% had fever, and lymphopenia was reported in 55.2% of patients. For 16% bilateral patchy shadowing in radiography and for 51% ground-glass opacity was reported. Outcomes were remarkable for recover to death.
Conclusion:
COVID-19 leads to healthcare problems for countries. Nonspecific symptoms have made it difficult for differential diagnoses without computed tomography-scan or corona Test, but they are not available in many countries. Therefore, this systematic review can help health care staff to make decisions based on symptoms, treatments, and outcomes..
Keywords: COVID-19, coronavirus, meta-analysis, SARS-CoV-2
INTRODUCTION
Recently, a family of viruses with developed and special genome called human coronaviruses has been responsible for a large number of respiratory system diseases. Currently, these viruses are known as one of the main causes of severe respiratory diseases such as bronchitis and bronchiolitis and pneumonia in children, young people, and adults.[1] Six types of coronaviruses have already been identified.[2] Over the past two decades, many people have died from these viruses, ranging from 10% severe acute respiratory syndrome-related coronavirus (SARS-CoV) to 37% Middle East respiratory syndrome-related coronavirus (MERS-CoV).[3] Furthermore, these viruses are known as nosocomial infection agents and impose exorbitant costs to health systems.[1]
Despite the world's familiarity with these types of viruses, in December 2019, a series of cases of pneumonia with unknown etiology emerged in Wuhan, China, which the early symptoms were greatly similar to viral pneumonia. However, a closer examination and analysis of lower lung samples revealed that a type of coronavirus (nCOV) is the cause of the symptoms. This virus is named new coronavirus 2019 or COVID-19 by the WHO.[3] This time, the issue was quite different from previous times. The concern was not related to the mortality rate (MR) from the virus, rather it was about the high rate of its transmission. Furthermore, it was not known how to deal with this disease because of a lack of knowledge.
Despite the lower MR of this virus in comparison with its families, it is worrying due to its high prevalence and contagion so that the number of deaths and infections is very remarkable. Among 1,039,158 people have been infected with this virus in the world until April 4, 2020, 55,163 participants of them died.[4]
Coronaviruses exhibit high resistance in the environment. This feature has made it difficult to control and prevent the disease.[1] Furthermore, the rapid spread and transmission of this virus have caused worldwide concern. Until now more than 200 countries reported to have been infected by this virus and many patients have died.[5] On the other hand, according to the Centers for Disease Control and Prevention report, the incubation period of 2019-nCov is 2–14 days and has recently mentioned this period can also be very momentous in the virus transmission.[6]
Due to the mentioned features of this virus, this worldwide outbreak calls for faster and wisely control by countries.[7] For more effective control and prevention, recognizing the sign and symptoms of the patients in different periods of illness and isolating them plays an important role.[8] However, a variety of symptoms have been mentioned so far, some only just have been added such as Anosmia, hyposmia, and ageusia.[9,10] Furthermore, symptoms of fever, dry cough, and fatigue in the early stages without the typical symptoms of acute respiratory disease, and later pneumonia-like symptoms, gastrointestinal symptoms such as diarrhea with virus excretion (up to several weeks) and in some cases, central nervous system involvement such as multiple sclerosis has been reported.[1] Although some of the clinical manifestations of SARS, MERS, and COVID appears to be similar, distinct symptoms have also been reported in some patients, so faster differential diagnosis is required for treatment.[3,11,12,13]
Laboratory findings of patients infected by COVID-19 showed lymphopenia and leukopenia. Furthermore, findings of chest computed tomography (CT) scan showed bilateral abnormalities in lung lobes, which are very similar to symptoms of influenza and other respiratory viruses. This pattern made it more difficult to early differential diagnosis.[3,13] Other laboratory findings showed increased prothrombin time, increased D-dimer, increased liver enzymes, and increased cardiac enzymes in some cases. Pro calcitonin level was reported mostly normal, although these reports were contradictory in different studies.[13,14,15]
Although viral diseases usually have an overestimated MR in the early stages, issues are different this time. MRs are rising rapidly as a result of severe contagion, world outbreak, and lack of differential diagnosis and lack of appropriate and specific treatment.[15]
However, many countries are facing lack of facilities such as laboratory testing and sampling, hence priority is given only to subjects with very severe symptoms while recognizing symptoms and their incidence and frequency can have an effective role in the subsequent control of the disease. Since various symptoms have been mentioned in the studies, a systematic review is required to provide a conclusion for health and policymakers.
Likewise, there is no specific treatment for the disease so far, and various countries are trying different drugs and sometimes combinations therapy.[13,14] The efficacy of these drugs has not been systematically investigated by using patient-centered outcomes such as MR, discharge of hospital, and remission. Therefore, this study was conducted with the aim of recognizing the specific symptoms of COVID-19 as well as its treatments and outcomes as a systematic review with meta-analysis.
METHODS
Data sources and search strategy
Based on the PRISMA guide[16] (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), we used an evidence-based model for framing a PICO question model (PICO: Participants, Intervention, Comparison, and Outcomes). The questions posed was the following: What are the symptoms of patients with patients with COVID-19? What are laboratory and radiologic findings in patients with COVID-19? What are the treatments for COVID-19? What are the outcomes of patients with COVID-19? (P) Participants: Patients with COVID-19.(I) Intervention: Treatments performed in patients with COVID-19.© Comparison: Not applicable. (O) Outcomes: Hospitalization, recovery, death.
Since the primary purpose of this study was to conclude of the articles that listed the symptoms or treatments of COVID-19, all valid databases were searched. A broad search was attempted and the search restrictions were moved to include the maximum number of articles in the study without missing any valuable and related article. Then, duplicate entering studies from various databases were removed. After that, all of the titles were read and unrelated articles were deleted.
We search databases- PubMed, Embase, Scopus, Google Scholar, web of sciences, and Cochrane with keywords included coronavirus, COVID 19, symptoms, signs, treatments, outcomes. No time limits were set for searches. However, since the emergence of the disease was in January 2020; the first articles were found on this time; and no studies had been done before. Therefore, all studies before that time were excluded after evaluating the titles. Diagram 1 shows the flow of assessing studies. Selection criteria and data extraction. Due to the emergence of the disease and to obtain the maximum possible knowledge, there was no restriction on the type of articles in the searching level. However, all the descriptive and analytical studies as well as the observationally reported interventional studies were included in the study. The search keywords and how each site was searched along with the number of articles are listed in Table 1. All studies were included until March 30, 2020.
Diagram 1.
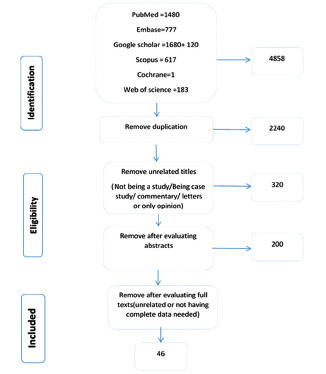
Flow diagram of literature search and study selection (PRISMA flow chart)
Table 1.
Databases and the results of searches
| Databases | Search results |
|---|---|
| PubMed | Search (corona virus [Title]) OR COVID 19[Title]=918 |
| Search (treatment [Title/Abstract]) AND ((corona virus [Title]) OR COVID 19?[Title])=97 | |
| Search (corona virus [Title]) OR COVID 19[Title]) AND symptom [Title/Abstract]=22 | |
| Embase | ‘corona virus’:ab, ti AND (symptoms: ab, ti OR signs: ab, ti)=41 |
| (‘corona virus’:ti OR ‘covid 19’:ti) AND (symptoms: ab, ti OR signs: ab, ti)=67 | |
| Google scholar | allintitle: “corona virus”=113 (limit to after 2019) |
| allintitle: “corona virus”=103 (without citation) (limit to after 2019) | |
| allintitle: symptoms “covid 19”=15 (limit to after 2019) | |
| allintitle: covid-19=1680 (limit to after 2019) | |
| Scopus | (TITLE-ABS-KEY (“covid 19”) OR TITLE-ABS-KEY (“corona virus”))=617 (year: 2020) |
| Cochrane from Ovid | “corona virus”.m_titl=0 |
| COVID 19.m_titl=0 | |
| “corona virus”.mp. [mp=ti, ot, ab, tx, kw, ct]=3 | |
| Cochrane | 1 COVID 19 in Title Abstract Keyword |
| 9 corona viruses in Title Abstract Keyword (not related) | |
| Web of science | TITLE: (“corona virus”) OR TITLE: (covid-19)=183 |
COVID 19=Coronavirus disease 2019
All articles were assessed based on authors, place of study, sample number, study design, patient characteristics, and symptoms. Furthermore, laboratory findings and treatments used for patients were evaluated completely. Studies lacking the essential components required by the current study objective were excluded from the systematic review; such as being a case report; not mention the definitive diagnosis of COVID-19 with either laboratory tests or definitive symptoms. Furthermore, some articles were removed because they have not assessed symptoms entirely or only, they mentioned one or two nonspecific symptoms.
Quality assessment
Finally, all of 46 remaining articles were reviewed by two reviewers (J. N, A, D) independently and screened studies to identify all potentially eligible studies using the JBI Quality Assessment Tool, whose ratings are included in the Supplementary Table 1.
Supplementary Table 1.
JBI quality assessment tool
| ID | Author name | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Weiliang Cao | ✓ | ✓ | ✓ | ✓ | no | ✓ | ✓ | ✓ | ✓ | 8 |
| 2 | Nanshan Chen | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 3 | Wei jie Guan | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 4 | Zhiliang Hu | ✓ | ✓ | ✓ | ✓ | No | No | ✓ | ✓ | ✓ | 7 |
| 5 | Chaolin Huang | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 6 | Ying Huang | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 7 | Lei Liu | ✓ | ✓ | No | UN | ✓ | ✓ | ✓ | ✓ | ✓ | 7 |
| 8 | Zuojiong Gong | ✓ | ✓ | No | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| 9 | Ying Liang | ✓ | ✓ | ✓ | ✓ | UN | ✓ | ✓ | ✓ | ✓ | 8 |
| 10 | Hong Jian Zhang | ✓ | ✓ | ✓ | No | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| 11 | Ling Mao | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 12 | Guo Qing Qian | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 13 | Jun Liu | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 14 | Jianlei Cao | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 15 | Sibylle Bernard Stoecklin | UN | UN | UN | UN | UN | UN | UN | UN | UN | 0 |
| 16 | Simon Petrie | UN | UN | UN | UN | UN | UN | UN | UN | UN | 0 |
| 17 | DaweiWang | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 18 | Hongcui Cao | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 19 | Wenjie Yang | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 20 | Bicheng Zhang | ✓ | ✓ | ✓ | UN | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| 21 | Matthew Arentz | ✓ | ✓ | ✓ | UN | ✓ | UN | ✓ | ✓ | ✓ | 7 |
| 22 | Pingzheng Mo | ✓ | ✓ | ✓ | ✓ | ✓ | UN | ✓ | ✓ | ✓ | 8 |
| 23 | Yihui Huang | ✓ | ✓ | ✓ | UN | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| 24 | Kunhua Li | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 25 | Shuchang Zhou | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 26 | Jin jin Zhang | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 27 | Jiong Wu, | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 28 | ZhongliangWang | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 29 | SijiaTian | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 30 | Suxin Wan | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 31 | Yuan Xue | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 32 | Wen Zhao | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 33 | Tao Yao | ✓ | ✓ | ✓ | No | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| 34 | De JIN | ✓ | ✓ | ✓ | No | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| 35 | Suochen Tian | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 36 | Sakiko Tabata | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 37 | Jie Liu | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 38 | Jiaming Zhang | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 39 | Chin Ion Lei | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 40 | Tao Chen | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 41 | Yuhong Chen | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 42 | peng peng | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 43 | Zhibing Lu | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 44 | Wen Hsin Hsih | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 45 | Shohei Inui | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 46 | Yida Yang | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
UN=Unknown; ✓=Yes; Q1=Was the sample frame appropriate to address the target population?; Q2=Were study participants sampled in an appropriate way?; Q3=Was the sample size adequate?; Q4=Were the study subjects and the setting described in detail?; Q5=Was the data analysis conducted with sufficient coverage of the identified sample?; Q6=Were valid methods used for the identification of the condition?; Q7=Was the condition measured in a standard, reliable way for all participants?; Q8=Was there appropriate statistical analysis?; Q9=Was the response rate adequate, and if not, was the low response rate managed appropriately?; JBI= Joanna Briggs Institute
Statistical analysis
In this stage, data extracted from all articles were entered into STATA Version 16.0 software (StataCorp LLC Production, College Station, Texas, USA) software for meta-analysis and providing general conclusions about the symptoms and treatments. It should be noted that the heterogeneity of the studies was evaluated according to different symptoms, laboratory findings and treatments, and most of the criteria were heterogeneous in the studies. The Q and tests were used to investigate heterogeneity. Since the articles are heterogeneous, the random model has been used by the maximum likelihood estimation method. To describe each of the signs and symptoms, the ratio and confidence interval (CI) of 95% have been reported.
RESULTS
In this systematic review, 46 articles remained after the final evaluation.[3,12,13,14,15,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] The majority of articles were case series (73.3%), 25.7% were descriptive retrospective and <1% were descriptive prospective or epidemiological reports. Although most of the articles were originated in China, other articles–about 5%-were from countries such as Japan, the USA, Australia, France, and Taiwan. Besides, 44.5% of articles were published in March 2020. The characteristics of the studies have presented in Table 2.
Table 2.
Baseline characteristics of all the studies included in the meta-analysis
| Author | Title | Type of study and number of samples | Incubation period (days) | Treatment | Detail | Outcome | Complications | Detection method |
|---|---|---|---|---|---|---|---|---|
| Chen N | Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China | Descriptive study retrospective single center n=99 |
- | Oxygen therapy 75 Mechanical ventilation: Noninvasive (ie, face mask) 13 Invasive 4 CRRT 9 ECMO 3 Antibiotic 70 Antifungal 15 Antiviral 75 Glucocorticoids 19 IVIG 27 |
Cephalosporins, quinolones, carbapenems, tigecycline against methicillin-resistant Staphylococcus aureus, linezolid, and antifungal drugs The duration of antibiotic treatment was 3-17 days (median 5 days [3-7]) Methylprednisolone sodium succinate, methylprednisolone, and dexamethasone for 3-15 days (median 5 [3-7]) |
Hospitalized 57 Discharged 31 Died 11 |
ARDS 25 AKI 3 Septic shock 4 VAP 1 |
RT-PCR |
| Weiliang Cao | Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang, Hubei | Retrospective study n=128 |
- | - | - | - | - | RT-PCR |
| Wei-jie Guan | Clinical characteristics of 2019 novel coronavirus infection in China | Retrospective study n=1099 |
Median (3) Range (0-24) |
Oxygen therapy 418 Mechanical ventilation 67 Invasive 24 Noninvasive 56 IV antibiotics 632 Oseltamivir 393 Antifungal 30 Systemic corticosteroids 204 ECMO 5 CRRT 9 IV IgG 143 |
Maximal daily dose of corticosteroids (mg/kg) 1.5 (0.7-40.0) |
Discharged 55 Death 15 Recovered 9 Hospitalized 1029 |
ICU admitted 55 Septic shock 11 Acute respiratory distress syndrome 37 AKI 6 DIC 1 Rhabdomyolysis 1 Pneumonia 869 |
RT-PCR |
| Zhiliang Hu | Clinical Characteristics of 24 Asymptomatic Infections with COVID-19 Screened among Close Contacts in Nanjing, China | Case series n=24 |
8 (6-9) | Antiviral 21 Antibiotics and Antifungal 1 IVIG 3 Interferon atomization 24 Corticosteroids 0 Mechanical ventilation 0 |
Lopinavir/ritonavir Darunavir/cobicistat |
Hospitalized 15 Discharge 9 No death |
18 cases (75.0%) had the virus cleared admission to ICU 0 | RT-PCR |
| Chaolin Huang | Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China | Prospectively n=41 |
- | O2 therapy: Nasal 27 NIV 10 Invasive mechanical ventilation 2 ECMO 2 Antiviral 38 Antibiotic 41 Glucocorticoid 9 |
- | Hospitalized 7 Discharged 28 Died 6 |
ARDS 12 AKI 3 Shock 3 Acute cardiac injury 5 Secondary infection 4 |
RT-PCR |
| Ying Huang | Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China | Retrospectivesingle- centered study n=36 |
- | O2 therapy 35 Mechanical ventilation Noninvasive 19 Invasive 9 Antibiotic 36 Antiviral 35 Glucocorticoids 25 IVIG 20 α-IFN 6 |
- | Dead 36 | ARDS 36 Electrolyte disturbance 16 Acute renal injury 1 |
RT-PCR |
| Lei Liu | Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing?China | Retrospective, single-center case series n=51 |
14 | Oseltamivir (po) 7 Interferon (po) 51 Kaletra (po) 51 Thymopentin (IM) 48 Traditional Chinese medicine decoction (po) 28 Antibiotic 11 IV IgG 4 High-flow oxygen 8 noninvasive ventilation 6 Invasive ventilation 1 |
- | Discharged 50 Died 1 |
Average hospitalization day was 12 days |
laboratory confirmed |
| Zuojiong Gong1 | Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China | Retrospective review of medical records n=25 |
Mean±SD 10.56±4.42 days |
- | - | - | - | RT-PCR |
| Jie Xu | Prevalence and clinical features of 2019 novel COVID-19 in the Fever Clinic of a teaching hospital in Beijing | A single-center, retrospective study n=21 |
2-10 days | - | - | - | - | RT-PCR |
| Hong-Jian Zhang | Epidemiological and Clinical Characteristics of 124 Elderly Outpatients with COVID-19 in Wuhan, China | Retrospective study n=124 |
7-11 | - | - | - | - | RT-PCR |
| Bo Hu | Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China | A retrospective case series study n=214 |
- | - | - | - | - | RT-PCR |
| Xiao-Min Chen | Epidemiologic and Clinical Characteristics of 91 Hospitalized Patients with COVID-19 in Zhejiang, China | Retrospective case series n=91 |
6 (3-8) days | - | - | Remained in hospital: 60 (65.93) Discharged: 31 (34.07) Died: 0 ICU: 9 (9.89) |
- | RT-PCR |
| Jun Liu | Clinical characteristics and treatment of patients infected with COVID-19 in Shishou, China | Single-center case series n=89 |
- | Noninvasive ventilation: 31 (35%) IMV: 4 (4%) |
IFN: 89 (100%) 85 (96%) were treated with moxifloxacin Other antibiotics: 4 (4%) Immunoglobulins: 35 (39%) Lopinavir/ritonavir 84 (94%) Other antivirals: 5 (6%) Methylprednisolone: 35 (39%) |
At present, of the 89 patients admitted, 16 have been discharged, 1 has died, 2 have deteriorated, and the remaining patients have improved or stabilized | ICU: 35 Non-ICU: 53 |
RT-PCR |
| Jianlei Cao | Clinical features and short-term outcomes of 18 patients with COVID-19 in intensive care unit | Retrospective case series n=102 |
3 (2-6) days | Antiviral: 100 (98.0) Antibiotic: 101 (99.0) Glucocorticoid: 51 (50.0) Immunoglobulin: 11 (10.8) Chinese medicine: 3 (2.9) Oxygen: 76 (74.5) NIV: 5 (4.9) IMV: 14 (13.7) ECMO: 3 (2.9) CRRT: 6 (5.9) |
- | Hospital admission: 6 Discharge: 85 Died: 17 |
MODS: 10 ARDS: 1 Cardiac arrest: 4 Respiratory failure: 2 |
laboratory- confirmed |
| Sibylle Bernard Stoecklin, France | First cases of COVID-19 in France: Surveillance, investigations and control measures, January 2020 | Case series n=3 |
- | - | - | Death: 0 Hospitalized: 3 Discharge: 2 |
- | RT-PCR |
| Simon Petrie, Australia |
2019-nCoV acute respiratory disease, Australia Epidemiology Report | Epidemiology report n=12 |
- | - | - | Death: 0 Hospitalized 12 |
ICU admitted 1 | PCR |
| Zhiyong Peng | Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China | Single-center case series n=138 |
- | Antiviral therapy: 124 (89.9) Glucocorticoid therapy: 62 (44.9) CKRT: 2 (1.45) Oxygen inhalation: 106 (76.81) NIV: 15 (10.9) IMV: 17 (12.32) ECMO: 4 (2.9) |
Oseltamivir: 124 (89.9%) Moxifloxacin: 89 (64.4%) Ceftriaxone: 34 (24.6%) Azithromycin: 25 (18.1%) glucocorticoid therapy: 62 (44.9%) |
34.1% were discharged 6 died (4.3%) 61.6% hospitalized |
ICU: 36 Non-ICU: 102 |
RT-PCR |
| Jian Wu | Clinical Characteristics of Imported Cases of COVID-19 in Jiangsu Province | Multicenter descriptive retrospective n=80 |
- | Oxygen therapy 36 Immunoglobulin therapy 16 Chinese medicine 3 Antibiotic treatment 73 Antiviral treatment 80 Hormone therapy 12 |
All patients were treated empirically with a single antibiotic, mainly moxifloxacin. The duration was 3-12 days. All patients received ribavirin antiviral therapy for 3-12 days | Hospitalized 61 Discharged: 721 Died 0 |
- | RT-PCR |
| Wenjie Yang | Clinical characteristics and imaging manifestations of the 2019 novel COVID-19 | Retrospective multi-center cohort study n=149 |
- | Oxygen therapy: 134 Antibiotic: 34 Antifungal: 0 Antiviral: 140 Interferon: 144 Glucocorticoids: 5 Immunoglobulin: 19 |
- | Hospitalized 76 Discharged: 73 Died 0 |
- | RT-PCR |
| Bicheng Zhang | Clinical characteristics of 82 death cases with COVID-19 | Cohort n=82 |
5-10 days | Antibiotics: 82 Corticosteroids: 29 Anti-virus: 82 Oxygen therapy: 82 Mechanical ventilation: 33 |
- | Death: 82 | ICU: 14 | RT-PCR |
| Matt Arentz, USA | Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State |
Case series n=21 |
- | Oxygen therapy 1 Invasive 15 Non-invasive 4 |
- | Hospitalization 10 Death 11 |
ARDS 20 Septic shock 4 Cardiac injury 7 AKI 4 Hepatic injury 3 |
RT-PCR |
| Pingzheng Mo | Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China | Descriptive Retrospective n=155 |
- | Oxygen therapy 102 Invasive 36 IVIG 9 α-IFN 30 Antiviral treatment 45 Arbidol 31 Lopinavir 27 Thymalfasin 11 |
- | - | - | laboratory confirmation |
| Yihui Huang | Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis | Descriptive Retrospective n=34 |
--- | Oxygen therapy 25 Invasive 3 Non-invasive 2 Antibiotic therapy 31 Antiviral treatment 41 Glucocorticoids 21 Lopinavir/ritonavi 9 |
--- | --- | ICU care 8 No-ICU car 26 |
laboratory confirmation |
| Kunhua Li | The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia | Descriptive retrospective n=83 |
- | - | - | - | - | --- |
| Shuchang Zhou | CT Features of COVID-19 Pneumonia in 62 Patients in Wuhan, China | Descriptive retrospective n=65 |
- | - | - | - | - | Laboratory confirmed and the CT |
| Jin-jin Zhang | Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China | Descriptive retrospective n=140 |
- | In this study, data in regard to the treatment and outcome of these patients were not finalized, since most of these patients are remaining hospitalized | - | - | - | RT-PCR |
| Jiong Wu | Chest CT Findings in Patients with COVID-19 and its Relationship with Clinical Features | Descriptive retrospective n=80 |
- | - | - | - | - | RT-PCR |
| Z Wang | Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China | Case series n=69 |
- | Oxygen therapy 43 Antibiotic therapy 66 Antiviral treatment 36 Arbidol 36 Glucocorticoids 10 |
- | Discharge 18 Hospitalization 44 Death 5 |
- | RT-PCR |
| S Tian | Characteristics of COVID-19 infection in Beijing.” J Infect 80 (4): 401-406 | Descriptive Retrospective n=262 |
- | - | - | Discharge 45 Hospitalization 214 Recovered 3 |
- | RT-PCR |
| Suxin Wan | Clinical Features and Treatment of COVID-19 Patients in Northeast Chongqing | Descriptive retrospective n=135 |
Oxygen therapy 90 IMV 1 NIV 34 Antibiotic therapy 59 Antiviral treatment 135 Glucocorticoids 36 |
- | Discharge 15 Hospitalization 150 Death 1 |
ARDS 21 Septic shock 1 Cardiac 10 |
RT-PCR |
|
| Tianmin Xu | Clinical Features and Dynamics of Viral Load in Imported and Non-imported Patients with COVID-19 | Descriptive retrospective n=51 |
4-14 | - | - | - | - | RT-PCR |
| Aixin Li | Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: A retrospective cohort study | Retrospective cohort study n=77 |
4 (3-7) | - | - | Discharged 64 Hospitalization 8 Death 5 |
Nonsevere 57 Severe 20 Any 28 ARDS 3 Shock 1 Acute heart failure 2 AKI 2 Liver dysfunction 25 MODS 1 Secondary infection 3 |
RT-PCR |
| Tao Yao | Clinical characteristics of 55 cases of deaths with COVID-19 pneumonia in Wuhan, China | Retrospective case series n=55 |
- | Antifungal therapy 2 Antibiotic therapy 53 Glucocorticoid therapy 35 IVIG therapy 39 CRRT 4 Invasive mechanical ventilation 12 ECMO 1 |
55 patients received antiviral therapy for 5-14 days, and all of them received arbidole, 38 received Oseltamivir 10 received ribavirin, 1 received Lopinavir and ritonavir. | Death 55 | - | RT-PCR |
| De JIN | Clinical findings of 100 Mild Cases of COVID-19 in Wuhan: A | Retrospective, single center study n=100 |
- | Oxygen therapy 58 NIV 10 No respiratory support 32 Antibiotic treatment 95 Antiviral treatment 100 Antifungal treatment 1 Glucocorticoids 59 IVIG therapy 43 Chinese herbal medicine 67 |
All patients received Oseltamivir (75 mg/twice daily), Ganciclovir Sodium (5 mg/kg), Ribavirin (500 mg/twice daily), Arbidol hydrochloride (200 mg/twice daily), recombinant human interferon-alpha-2b (300/IU), Lopinavir (200 mg/day), Ganciclovir (600 mg/twice daily) and Traditional Chinese Medicine (200 ml/twice daily). 12 (12%) patients were treated with a single antiviral treatment, and 88 (88%) patients were given combination therapy The antibiotics used were Lacefofax, Moxifloxacin, Piracillin and Tazobactam, Azithromycin, Imipenem and Cilastatin, Voriconazole, Levofloxacin, Cefoperazone and Sulbactam, Meropenem and Minocycline |
Hospitalized 96 Discharged 1 Died 33 |
- | RT-PCR |
| Tiejun Wu | Clinical characteristics and reasons of different duration from onset to release from quarantine for patients with COVID-19 Outside Hubei province, China |
Descriptive retrospective n=37 |
- | Antibiotics 27 Antifungal drugs 1 Antiviral drugs 37 Glucocorticoids 8 Albumin 12 immunoglobulin 7 Thymosin 24 Oxygen therapy 15 Chinese Medicine 37 |
Daily dose of Glucocorticoids 40 mg 6/8 80 mg 1/8 120 mg 1/8 IV antibiotics 17/27 Oral antibiotics 10/27 Two antiviral 25/37 Three antiviral 12/37 |
- | Mild 5 Moderate 30 Severe 1 Critical 1 Complications 1 ARDS 2 |
RT-PCR |
| Kazuo Imai, Japan | Non-severe vs severe symptomatic COVID-19: 104 cases from the outbreak 1 on the cruise ship 2 “Diamond Princess” in Japan | Descriptive Retrospective n=104 |
- | Oxygen therapy 13 Mechanical ventilation 1 |
- | Died 0 Recovered 104 |
- | RT-PCR |
| Fan Yang | Epidemiological, Clinical Characteristics and Outcome of Medical Staff Infected with COVID-19 in Wuhan, China: A Retrospective Case Series Analysis | A Retrospective Case Series Analysis n=64 |
- | Oxygen therapy 34 Electrocardiograph monitoring 9 Antibiotics treatment 55 Antiviral treatment 64 Traditional Chinese medicine 13 Immune globulin 23 Thymosin 33 |
- | Hospital discharge 34 Continued hospitalization 30 Death 0 |
- | RT-PCR |
| Xiaoyan Ming | Association of Cardiovascular Manifestations with In-hospital Outcomes in Patients with COVID-19: A Hospital Staff Data | Retrospective, single-center case series n=41 |
- | Oxygen therapy: 23 ECMO 1 Antibiotics treatment: 39 Antiviral treatment 40 Glucocorticoids 32 Traditional Chinese medicine 8 Immune globulin 33 Thymosin 16 Kaletra 16 |
- | - | ARDS 2 Septic shock 20 Hepatic 8 Infection 20 |
RT-PCR |
| Iek Long Lo Macau |
Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau |
Retrospective study n=10 |
- | Oxygen therapy: 4 Antibiotics treatment: 10 Antiviral treatment 10 Glucocorticoids 3 |
- | Hospitalization 10 Discharge 5 |
Mild 2 Moderate 4 Severe 4 Critical 0 |
RT-PCR |
| Tao Chen | Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study | Retrospective study n=274 |
- | Oxygen therapy: 251 Antibiotics treatment: 249 Antiviral therapy 236 Glucocorticoid therapy 217 Immunoglobulin 54 IFN 89 CRRT 3 ECMO 1 |
- | Death 113 Recovered 161 |
ARDS 196 Sepsis 179 AKI 29 Liver injury 13 DIC 21 Electrolyte 93 Cardiac 132 Shock 46 |
RT-PCR |
| Yuhong Chen | Clinical characteristics and current treatment of critically ill patients with COVID-19 outside Wuhan, China | Multicenter, retrospective, observational n=37 |
- | Oxygen therapy: 24 Antibiotics treatment: 33 Antiviral therapy 37 Glucocorticoid therapy 41 Chinese medicine 35 Immunoglobulin 19 CRRT 2 Thymosin 32 Kaletra 34 |
- | - | Cardiac 6 AKI 4 Hepatic 1 |
laboratory confirmation |
| Peng peng | Treatment Outcomes, Influence Factors of 116 Hospitalized COVID-19 Patients with Longer/Prolonged Treatment Course in Wuhan, China | Single center retrospective observational study n=116 |
- | Oxygen therapy: 83 Antibiotics treatment 109 Antiviral therapy 116 Glucocorticoid therapy 94 Immunoglobulin 58 ECMO 5 |
- | Recovered 72 | None-severe 87 Severe 29 Any 49 Shock 5 ARDS 38 AKI 21 Cardiac 32 VAP 30 |
RT-PCR |
| Tao Guo | Cardiovascular Implications of Fatal Outcomes of Patients with COVID-19 | Retrospective single-center case series n=187 |
- | Antivirus 166 Antibiotic 183 Glucocorticoid 106 Immune globulin 21 Mechanical ventilation 45 |
- | Death 43 | ARDS 46 Coagulopathy 42 Liver injury 19 Kidney injury 18 Cardiac 11 |
Interim guidance of the WHO |
| Vietnam | Featuring COVID-19 cases via screening symptomatic patients with epidemiologic link during flu season in a medical center of central Taiwan |
Retrospective n=2 |
- | Not mentioned | - | Hospitalization 2 | Not mentioned | RT-PCR |
| Japan | Chest CT Findings in Cases from the Cruise Ship “Diamond Princess” with COVID-19 | Retrospective n=112 |
- | Not mentioned | - | Not mentioned | Not mentioned | RT-PCR |
| Yida Yang | Epidemiological, clinical and virologic characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms | Retrospective n=5 |
5 | Antibiotic 277 Antivirus 546 Glucocorticoid 74 |
- | ICU admit 17 | Liver injury 64 ARDS 17 Shock 2 |
RT-PCR |
NIV=Noninvasive ventilation; IMV=Invasive mechanical ventilation; CRRT=Continuous renal replacement therapy; ECMO=Extracorporeal membrane oxygenation; IV=Intravenous; IVIG=IV immunoglobulin; ARDS=Acute respiratory distress syndrome; AKI=Acute kidney injury; VAP=Ventilator-associated pneumonia; RT-PCR=Real time reverse transcription polymerase chain reaction; ICU=Intensive care unit; COVID 19=Coronavirus disease 2019; α-IFN=Alpha interferon; SARS-CoV-2=Severe acute respiratory syndrome-related coronavirus-2; CT=Computed tomography; DIC=Disseminated intravascular coagulation; MODS=Multiple organ dysfunction syndrome
The total number of patients reported in these articles was 5570. Slightly more than half of the patients were male (52.74%); and the mean age of the patients was 53.2 (CI: 50.17–56.33) year. The incubation period was reported between 0 and 24 days. Half of the patients reported contact with Chinese people, 15.56% had contact with patients, and 5.94% were exposed to the Huanan seafood market. Moreover, only 1.79% mentioned that they haven't had any contact.
It should be noted that only some articles have dealt with coexisting disorders or clinical characteristics. For example, 16 articles cited coexisting disorders, in which 38% (n = 2969) subjects were present, of which 31.7% had no coexisting disorders. About 21.4% of patients had hypertension (CI = 0.18–0.27); 10.8% had endocrine disorder especially diabetes mellitus (CI = 0.009–0.13); 8.1% had cardiovascular disease (CI = 0.08–0.13); 3.8% had chronic obstructive pulmonary disease or other respiratory diseases (CI = 0.03–0.05); 4.7% had cerebrovascular disease (CI = 0.04–0.07);12.6% had a history of previous surgery (CI = 0.02–0.10); and 6.3% had digestive system disease.
Clinical characteristics
The highest clinical characteristic (about 96% of the total number of patients surveyed) assessed was coughs. They reported that 60.5% of subjects (CI = 0.53–0.63) had dry coughs. However, expectoration and Sputum production was reported in 23.3% (CI = 0.18–0.31). As well as, about 15.8% reported chest pain (n = 225/1419). In this section, the sensitivity of the symptoms was not considered and only the prevalence of symptoms in patients was examined.
The second clinical characteristic (about 94%, equivalent to 5234 people) studied was the temperature which 70.6% of them fever was reported. However, only 5% of subjects had fever >39°. The forest plot of fever is shown in Figure 1.
Figure 1.
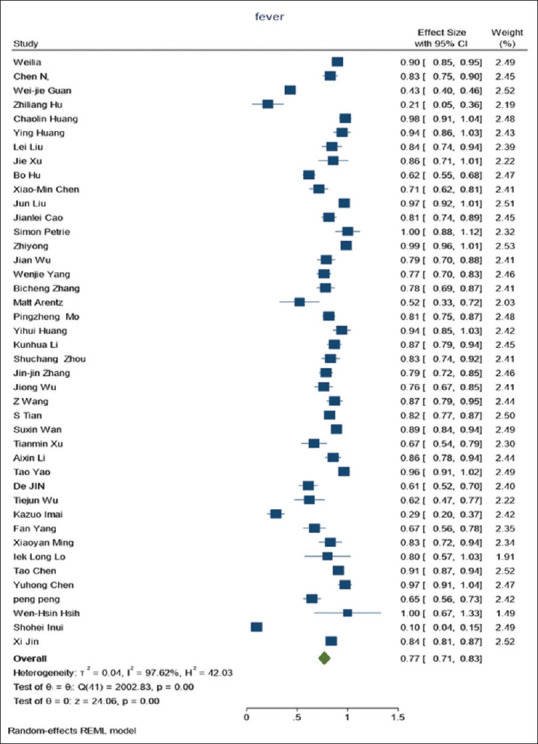
Forest plot of fever
Moreover, 21.1% had a fever <37.5°. Feeling ill, malaise or severe fatigue and weakness had 35.7% prevalence. Shortness of breath/dyspnea existed only in 22.2%. Poor appetite and anorexia were prevalent in about one fourth of patients (23.6%), although, this symptom was examined in only 24% of subjects. Also, 18.5% reported myalgia and 11.1% had headache. Sore throat or pharyngalgia was about 12.2%.
Other signs and symptoms were rigor/chill (12%); diarrhoea/loose bowel movement (9.5%); rhinorrhoea/nasal congestion or sneezing/snotty nose (6.9%); nausea/vomiting (7.5%); dizziness (10.1%). Confusion/loss of consciousness was another important symptom that only assessed 7.7% of patients and 11.65% reported it. Other symptoms reported were tonsils well (2.09%); hmoptysis (1.8%); and hypogeusia/hyposmia (5.1%).
Other signs and symptoms were examined in few patients or they had a lower prevalence including conjunctivitis, abdominal pain, back pain, ataxia, and wheeze. The results of the sensitivity analysis are shown in Supplementary Table 2.
Supplementary Table 2.
Egger test (publication bias)
| Variable | B1 | SE | Z | P |
|---|---|---|---|---|
| High CRP | 1.22 | 2.971 | 0.41 | 0.681 |
| GGO | −2.14 | 3.929 | −0.54 | 0.586 |
| Fever | −2.60 | 1.546 | −1.68 | 0.092 |
SE=Standard error; GGO=Ground glass opacity; CRP=C-reactive protein
Laboratory findings
One of the most important laboratory findings in COVID-19 is SpO2. However, only one study has reported this measure with 82 samples that 32.9% had Spo2 between 85% and 94% and 10.1% had Spo2 <85. No one had normal Spo2.
WBC counts were among the most important laboratory criteria; studies reported 58.7% of samples had normal range (3.5–9.5 count, ×109/L) and 24% <3.5 and 10.2% more than 9.5. For half of the samples were reported lymphocytes counts in the normal range (1.1–3.2 count, ×109/L) and 55.2% below normal and only 13.7% above normal. Half of the patients had normal platelet counts, 21.9% had less than normal; and 5.5% (38/681) had platelet more than >350.
As well, ten articles measured Creatine kinase-about 2000 patients-and reported that 10% have been above creatine kinase normal range (171 U/L). In 15 articles which evaluated 2276 patients reported that D-dimer of 26.5% of patients has been >500 mg/l., but an article reported D-dimer in a normal range when they measured 51 people.
Criteria such as erythrocyte sedimentation rate (ESR), Fibrinogen, creatine kinase MB, PT, PTT, BUN, Na, K, and Chloride were reported in a small number of articles, the abnormal rate of which was not noteworthy. Other laboratory findings are shown in Table 3. The forest plot of C-reactive protein has displayed in Figure 2.
Table 3.
Laboratory findings and abnormalities in patients with coronavirus disease 2019
| Laboratory findings (normal range) | Number of patients examined | Number with the condition | Percent | Proportion | CI | I 2 | P |
|---|---|---|---|---|---|---|---|
| Pro calcitonin (<0.5 ng/mL) | |||||||
| NL | 534 | 233 | 43.6 | 0.39 | 0.11-0.68 | 99.28 | <0.001 |
| ≥0.5 | 2563 | 376 | 14.6 | 0.27 | 0.22-0.32 | 98.01 | <0.001 |
| Total bilirubin (5-21 mmol/L) | |||||||
| NL | 100 | 100 | 100 | ||||
| ≤5 | 149 | 7 | 4.6 | 0.05 | 0.02-0.09 | ||
| >21 | 1553 | 125 | 8.0 | 0.09 | 0.05-0.14 | 88.24 | <0.001 |
| Alanine aminotransferase (9-50 U/L) | |||||||
| NL | 94 | 70 | 74.4 | 0.75 | 0.66-0.84 | ||
| ≤9 | 256 | 9 | 3.3 | 0.03 | 0.00-0.07 | 48.20 | 0.15 |
| >50 | 2439 | 406 | 16.6 | 0.17 | 0.13-0.20 | 81.74 | <0.001 |
| Aspartate aminotransferase (15-40 U/L) | |||||||
| NL | 135 | 82 | 60.7 | 0.61 | 0.53-0.69 | 0 | 0.80 |
| ≤15 | 226 | 114 | 50.4 | 0.39 | 0.35-0.44 | ||
| >40 | 2607 | 499 | 19.1 | 0.22 | 0.17-0.27 | 90.63 | <0.001 |
| Albumin (3.4-5.4 g/dL) | |||||||
| ≤3.4 | 653 | 296 | 45.3 | 0.52 | 0.26-0.77 | 99.07 | <0.001 |
| >5.4 | 249 | 20 | 8.0 | 0.03 | 0.01-0.05 | ||
| Fibrinogen (2-4 g/L) | |||||||
| ≤2 | 91 | 3 | 3.2 | 0.03 | 0.01-0.09 | ||
| >4 | 128 | 26 | 20.3 | 0.18 | 0.12-0.25 | ||
| LDH (125-243 U/L) | |||||||
| NL | 41 | 11 | 26.8 | 0.27 | 0.16-0.42 | ||
| ≤125 | 0 | 0 | 0 | ||||
| >243 | 2361 | 802 | 33.9 | 0.43 | 0.31-0.55 | 97.35 | <0.001 |
| Creatine kinase (<171 U/L) | |||||||
| NL | 92 | 78 | 84.7 | 0.66 | 0.51-0.78 | ||
| <171 | 248 | 42 | 16.9 | 0.16 | 0.11-0.20 | ||
| ≥171 | 1959 | 200 | 10.2 | 0.12 | 0.08-0.16 | 85.40 | <0.001 |
| Haemoglobin (115-150 g/L) | |||||||
| NL | 290 | 193 | 66.5 | 0.55 | 0.23-0.87 | 96.75 | <0.001 |
| ≤115 | 644 | 253 | 39.2 | 0.42 | 0.29-0.55 | 92.55 | <0.001 |
| Creatinine (64-104 µmol/L) | |||||||
| NL | 25 | 7 | 28 | 0.28 | 0.14-0.48 | ||
| ≤64 | 434 | 60 | 13.8 | 0.16 | 0.06-0.25 | 92.69 | <0.001 |
| >104 | 980 | 140 | 14.2 | 0.13 | 0.08-0.19 | 90.51 | <0.001 |
LDH=Lactate dehydrogenase; CI=Confidence interval
Figure 2.
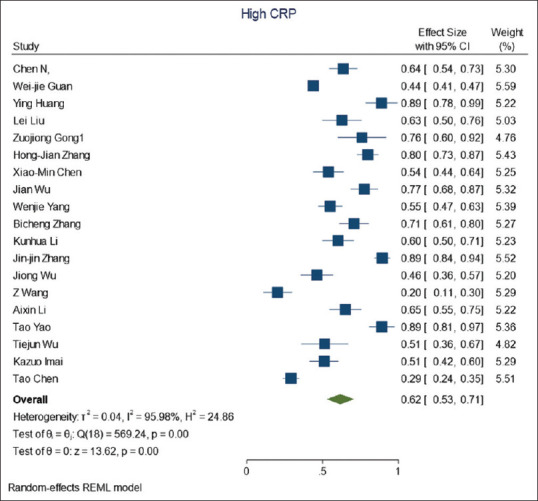
Forest plot of C-reactive protein
Radiologic findings
Because one of the organs involved in the COVID-19 disease is the lungs, chest radiography and CT-Scan can show the involvement of this tissue. Therefore, some studies have addressed this issue in addition to laboratory findings and symptoms. However, various studies showed different features. Three studies reported bilateral patchy shadowing in 16% (193/1202) in radiology and 18 studies showed Ground glass opacity (GGO) on 51.6% (1579/3055) patients in the CT-scan. The forest plot of GGO is presented in Figure 3. Other radiologic features are shown in Table 4.
Figure 3.
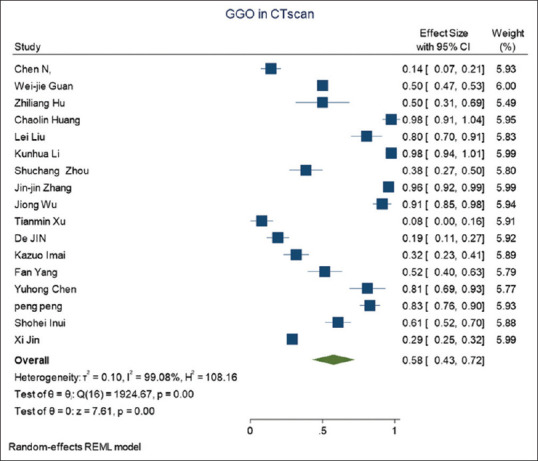
Forest plot of ground-glass opacity in computed tomography scan
Table 4.
Radiologic findings in patients with coronavirus disease 2019
| Radiologic findings | Number of patients examined | Number with the condition | Percent | Proportion | CI | I 2 | P |
|---|---|---|---|---|---|---|---|
| Abnormalities on chest radiograph | |||||||
| Bilateral patchy shadowing | 1202 | 193 | 16.0 | 0.09 | 0.08-0.11 | ||
| Local patchy shadowing | 1313 | 78 | 5.9 | 0.02 | 0.01-0.03 | ||
| Ground-glass opacity | 1120 | 65 | 5.8 | 0.05 | 0.04-0.06 | ||
| Interstitial abnormalities | 1101 | 13 | 1.1 | 0.01 | 0.00-0.02 | ||
| Normal | 674 | 76 | 11.2 | 0.10 | 0.04-0.16 | 35.59 | 0.21 |
| Abnormalities on chest CT | |||||||
| GGO1 | 3055 | 1579 | 51.6 | 0.58 | 0.42-0.73 | 99.26 | <0.001 |
| Bilateral patchy shadowing | 1539 | 904 | 58.7 | 0.75 | 0.47-1.03 | 99.13 | <0.001 |
| Local Patchy shadowing | 1324 | 437 | 33.0 | 0.16 | 0.00-0.31 | 96.52 | <0.001 |
| Bilateral pneumonia | 1730 | 1086 | 62.7 | 0.73 | 0.60-0.87 | 97.83 | <0.001 |
| Unilateral pneumonia | 1258 | 247 | 19.6 | 0.18 | 0.12-0.24 | 87.20 | <0.001 |
| Pulmonary consolidation or exudation | 484 | 190 | 39.2 | 0.38 | 0.23-0.53 | 93.26 | <0.001 |
| Interstitial abnormalities | 1335 | 173 | 12.9 | 0.10 | 0.01-0.19 | 96.63 | <0.001 |
GOO=Ground glass opacity; CI=Confidence interval; CT=Computed tomography
Treatment
COVID-19 is an emergent disease and no specific treatment has been well-known for it, therefore different drugs were used in combination. Oxygen therapy is the first and popular treatment as mentioned Spo2 is decreased in the patients. Antiviral such as oseltamivir, lopinavir/ritonavir (Kaletra), darunavir/cobicistat, and Arbidol were used. Other medications used for COVID-19 are shown in Table 5.
Table 5.
Treatments used for patients with coronavirus disease 2019
| Treatment | Number of patients examined | Number of patients with this condition | Percent | Proportion | CI | I 2 | P |
|---|---|---|---|---|---|---|---|
| Oxygen therapy | 2994 | 1726 | 57.6 | 0.59 | 0.47-0.71 | 98.48 | <0.001 |
| NIV | 2361 | 293 | 12.4 | 0.12 | 0.09-0.15 | 93.22 | <0.001 |
| IMV | 2807 | 262 | 9.3 | 0.14 | 0.09-0.19 | 92.10 | <0.001 |
| Antibiotic | 3411 | 2212 | 64.8 | 0.72 | 0.61-0.83 | 99.19 | <0.001 |
| Moxifloxacin | 37 | 26 | 70.2 | 0.70 | 0.54-0.83 | ||
| Levofloxacin | 37 | 8 | 21.6 | 0.22 | 0.11-0.37 | ||
| Ceftriaxone | 37 | 2 | 5.4 | 0.05 | 0.01-0.18 | ||
| Linezolid | 37 | 2 | 5.4 | 0.05 | 0.01-0.18 | ||
| Carbapenems | 37 | 6 | 16.2 | 0.16 | 0.08-0.31 | ||
| Antiviral | 2581 | 2284 | 88.4 | 0.87 | 0.80-0.94 | 97.03 | <0.001 |
| Oseltamivir | 1228 | 444 | 36.1 | 0.40 | 0.09-0.71 | 98.64 | <0.001 |
| Lopinavir/Rritonavir (Kaletra) | 342 | 158 | 2.83 | 0.52 | 0.17-0.88 | 98.31 | <0.001 |
| Darunavir/Cobicistat | 24 | 21 | 87.5 | 0.88 | 0.69-0.96 | ||
| Arbidol | 261 | 93 | 35.6 | 0.47 | 0.16-0.78 | 96.09 | <0.001 |
| Other treatments | |||||||
| Thymosin/Thymopentin | 264 | 164 | 2.94 | 0.62 | 0.40-0.84 | 95.20 | <0.001 |
| Antifungal | 1459 | 57 | 3.9 | 0.04 | 0.02-0.07 | 75.40 | <0.001 |
| Glucocorticoids | 3629 | 1188 | 32.7 | 0.41 | 0.30-0.52 | 98.63 | <0.001 |
| Chines medicine | 432 | 191 | 44.2 | 0.43 | 0.08-0.79 | 99.20 | <0.001 |
| IVIG | 2589 | 526 | 20.3 | 0.30 | 0.23-0.38 | 96.10 | <0.001 |
| α-IFN | 689 | 344 | 49.9 | 0.41 | 0.05-0.88 | 99.64 | <0.001 |
| CRRT | 1702 | 29 | 1.7 | 0.02 | 0.00-0.03 | 61.88 | 0.02 |
| ECMO | 1863 | 22 | 1.1 | 0.01 | 0.00-0.02 | 41.62 | 0.10 |
NIV=Noninvasive ventilation; IMV=Invasive mechanical ventilation; CRRT=Continuous renal replacement therapy; ECMO=Extracorporeal membrane oxygenation; IVIG=Intravenous immunoglobulin; α-IFN=Alfa interferon; CI=Computed tomography
Complications
Although medical staff have tried to provide the best available treatment for patients with COVID-19 severe complications that happened in some of the patients, and has even led to mortality. Some of the most important complications mentioned in the articles are: 22.9% acute respiratory injury/acute respiratory distress syndrome (448/1995); 11.2% septic shock (93/826); 35.1% electrolyte disorder (109/310); 13.6% Disseminated intravascular coagulation (63/461); 10.3% liver disorder (133/1288), and 14.4% ventilator-associated pneumonia (31/215).
Outcomes
Different outcomes have been recorded after getting the disease in the studies including discharge 23% (549/2381); hospitalization 76.9% (1767/2297); recovery 24.1% (493/2042); death 12.4% (311/2502).
DISCUSSION
This study investigated systematically physical symptoms and signs, as well as laboratory and radiological findings and treatment in published studies on patients with COVID-19. Despite the new emergence and lack of confirmed knowledge of the symptoms of the disease and the ways of treatment, many studies have been done on patients with COVID-19 during their physical examination and treatment. In addition to their therapeutic duties, specialists from different countries have provided detailed reports on the patients' condition and their outcomes, as evidenced by the publication of several thousand articles in this field. Despite a lot of articles in this regard, it was tried to observe all of the principles and procedures of systematic review with high quality; so that the studies were evaluated carefully in terms of quality and relevance with the intention of the studies that remained for the final review be valid and invaluable. Hence, 46 articles remained in the final stage. These articles were all reviewed for quality and they were worthy to study.
Slightly more than half of the patients were male. However, studies were heterogeneous (CI = 0.49–0.54; P < 0.001). Given that men have social attendance and COVID-19 is contagious, this can be a possible sensible explanation. Contact with Chinese people was the most reason of transmission, which was expected due to the initial emergence of the disease in China and the most of published papers were from this country.
In this study, most of the studies confirmed the presence of comorbidities in patients with COVID-19. The comorbidities were confirmed not only in COVID-19 but also in previous influenza viral infections such as MERS and H1N1.[58,59]
The most common symptoms reported were dry cough and fever. These symptoms and the extent of involvement contain important messages. The fact that fever has been reported in two-thirds of patients, and only one-tenth of patients had a fever above 39°C, raises doubts about the use of temperature to separate and quarantine of patients.
Many of the other symptoms are not specific to COVID-19, and they are similar to other virus infections in this family and other viruses that make it difficult to differentiate. However, it is recommended that protective measures be taken even in suspicious cases and the symptoms be considered corona to prevent further spread. Symptoms such as feeling ill, malaise or severe fatigue or weakness, and dyspnea are also seen in other flu and pneumonia.[60,61,62] Sore throat or pharyngalgia and rhinorrhea, Nasal congestion, sneezing and snotty nose, as well as rigor/chill are also nonspecific symptoms and have been reported with other influenza and common cold.[63,64,65]
Myalgia and headache have also been reported in other pneumonia with other origin.[66] Furthermore, symptoms such as dizziness and confusion/loss of consciousness have been reported before the emergence of COVID-19.[67,68,69] Poor appetite, anorexia, nausea/vomiting, and diarrhea/loose bowel movement are also common symptoms of other viral infections and pneumonia.[70,71,72]
Laboratory findings suggest that arterial blood saturation is significantly reduced. This finding has also been observed in other pneumonia due to lung tissue damage.[73] Both lymphopenia and lymphocytosis were found in patients. Meanwhile, half of the patient had lymphopenia, while slightly more than one-tenth had Lymphocytosis. This discrepancy can go back to the time of measurement. As the disease progresses, the risk of lymphopenia increases. Some studies confirmed that lymphopenia is a frequent finding in hospitalized patients with community-acquired pneumonia (CAP), affecting approximately 50% of the patients[74] and it is associated with a deregulated immune response, increased severity, and mortality.[75] Some authors suggested that is lymphocytosis evidence of active inflammation in pneumonia.
Although other studies have shown that monocyte was positively correlated with ESR and negatively with body temperature,[76] in the current studies, this correlation has not been evaluated.
The study revealed the platelet counts of one-fifth of evaluated people were lower than normal; it has been noted in other studies so that a study indicated that an increase in mean platelet measurements during admission can predict the prognoses of patients with pneumonia and related to poor outcomes.[77] However, in the current study, only 5.5% had more platelet.
Creatine kinase was also reported to be high in 10% of those measured. This is consistent with other infections that lead to pneumonia.[78] D-dimer was measured in only one article; however, more than a quarter of patients had a high rate of that. However, a study has shown in CAP patients, a D-dimer >2 μg/mL was risk factor associated with in-hospital mortality.[79]
Radiological findings included chest X-ray (CXR) and CT scan of patients in current studies have shown lung tissue involvement. Chest CT scan can be performed after the detection of abnormalities in CXR. Combination of radiological findings with clinical manifestations can lead to better clinical judgment.[80] In current studies, some have shown bilateral patchy shadowing or GGO in CT scans of the lungs. In a multicentre study, this feature has also been confirmed. It showed a mixed and diverse pattern with both lung parenchyma and the Interstitial involved.[81] Another modality is chest CT-scan. It can be ordered in suspected cases with typical symptoms at the first step, or it can be performed after the detection of any abnormalities in CXR.
Therapies in the studies indicated that specialists have used various combination therapies in addition to oxygen therapy. They were usually a combination of antiviral, antibiotic, and miscellaneous treatments. Nevertheless, more specific treatments have been given for other pneumonia or viral infections because of more comprehensive knowledge and broader research.[82,83]
CONCLUSIONS
Up to the present, the studies of COVID-19 usually have been observational, and experts have reported them along with their medical prescriptions. Nevertheless, research is ongoing and new signs and symptoms of the disease are being identified. However, the results of the current study could be useful because it showed the most popular symptoms and the validity of them for identifying and isolating patients. Because some symptoms, such as fever, occur in only two-thirds of people, they are not a good measure of isolation and more measures should be done. Although CT scan is a valid test for detecting the typical pattern of COVID-19 pneumonia, in the early stages of the disease is not recommended since in this period CT scan of lungs may be completely normal. Furthermore, in other forms of COVID-19 which affect organs other than the respiratory system, CT scan is not a valuable diagnostic test.
Further studies in European countries, the United States, and Asia are needed to identify new dimensions of the disease; therefore, systematic reviews can be done regularly.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Geller C, Varbanov M, Duval RE. Human coronaviruses: Insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4:3044–68. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Q, Song Y, Shi M, Cheng Y, Zhang W, Xia XQ. Inferring the hosts of coronavirus using dual statistical models based on nucleotide composition? Sci Rep. 2015;5:17155. doi: 10.1038/srep17155. doi: 10.1038/srep17155; https://www.nature.com/articles/srep17155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus Disease (COVID-2019) Situation Report –88. [Last accessed on 2020 Apr 17]. Available from: https://www.who.int/emergencies/diseases/novelcoronavirus-2019/situation-reports/
- 5.Countries Where COVID-19 Has Spread 2020. [Last accessed on 2020 Apr 10]. Available from: https://www.worldometers.info/coronavirus/countries-wherecoronavirus-has-spread/
- 6.Kobayashi T, Jung SM, Linton NM, Kinoshita R, Hayashi K, Miyama T, et al. Communicating the risk of death from novel coronavirus disease (COVID-19). In: Multidisciplinary. J Clin Med. 2020;9:580. doi: 10.3390/jcm9020580. doi: 10.3390/jcm9020580; https://www.mdpi.com/2077-0383/9/2/580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters A, Vetter P, Guitart C, Lotfinejad N, Pittet D. Understanding the emerging coronavirus: What it means for health security and infection prevention. J Hosp Infect. 2020;104:440–8. doi: 10.1016/j.jhin.2020.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen K, Yang Y, Wang T, Zhao D, Jiang Y, Jin R, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: Experts' consensus statement. World J Pediatr. 2020;16:223–31. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagheri SHR, Asghari AM, Farhadi M, Shamshiri AR, Kabir A, Kamrava SK, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. [Last accessed on 2020 Apr 12];Med J Islam Repub Iran. 2020 34:62. doi: 10.34171/mjiri.34.62. doi: 10.34171/mjiri.34.62. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7500422/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recalcati S. Cutaneous manifestations in COVID-19: A first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–3. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 11.Al-Tawfiq JA, Zumla A, Memish ZA. Travel implications of emerging coronaviruses: SARS and MERS-CoV. Travel Med Infect Dis. 2014;12:422–8. doi: 10.1016/j.tmaid.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian S, Chang Z, Wang Y, Wu M, Zhang W, Zhou G, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–6. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80:401–6. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutton B, Catalá-Lépez F, Moher D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med Clin (Barc) 2016;147:262–6. doi: 10.1016/j.medcli.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 17.Countries Where COVID-19 has Spread. 2020. Apr 04, [Last accessed on 2020 Apr 09]. Available from: https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/
- 18.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–4. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao J, Hu X, Cheng W, Yu L, Tu WJ, Liu Q. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 2020;46:851–3. doi: 10.1007/s00134-020-05987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao W. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang, Hubei. medRxiv. 2020 [Preprint]. doi: 10.1101/2020.02.23.20026963. [Google Scholar]
- 21.Chany C, Moscovici O, Lebon P, Rousset S. Association of coronavirus infection with neonatal necrotizing enterocolitis. Pediatrics. 1982;69:209–14. [PubMed] [Google Scholar]
- 22.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan Q, Guo G, Ren Y, Shang H, Du J, Li M, et al. Treatment outcomes, influence factors of 116 hospitalized COVID-19 patients with longer/prolonged treatment course in Wuhan, China. Lancet. 2020:116. [Preprint]. doi: 10.2139/ssrn.3550017. [Google Scholar]
- 24.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsih WH, Cheng MY, Ho MW, Chou CH, Lin PC, Chi CY, et al. Featuring COVID-19 cases via screening symptomatic patients with epidemiologic link during flu season in a medical center of central Taiwan. J Microbiol Immunol Infect. 2020;53:459–66. doi: 10.1016/j.jmii.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–11. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Tu M, Wang S, Chen S, Zhou W, Chen D, et al. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Travel Med Infect Dis. 2020;4:101606. doi: 10.1016/j.tmaid.2020.101606. [Epub 1477-8939]. doi: 10.1016/j.tmaid.2020.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Zhou H, Yang R, Xu Y, Feng X, Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv. 2020 [Preprint]. doi: 10.1101/2020.02.27.20029009. [Google Scholar]
- 29.Inui S, Fujikawa A, Jitsu M, Kunishima N, Watanabe S, Suzuki Y, et al. Chest CT findings in cases from the cruise ship "Diamond Princess" with coronavirus disease 2019 (COVID-19) [Last accessed on 2020 Apr 10];Radiology. 2020 22:e204002. doi: 10.1148/ryct.2020204002. doi: 10.1148/ryct.2020200110. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7233452/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jian-Ya G. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv. 2020 [Preprint]. doi: 10.1101/2020.02.20.20025536. [Google Scholar]
- 31.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–9. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55:327–31. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Wang L, Yan S, Yang F, Xiang L, Zhu J, et al. Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128–32. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang Y, Liang J, Zhou Q, Li X, Lin F, Deng Z, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–9. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y, Ji C, Weng W, Xu P, Hu Y, Liang W, et al. Epidemiological and Clinical Characteristics of 124 Elderly Outpatients with COVID-19 in Wuhan, China. medRxiv. 2020 [Preprint]. doi: 10.1101/2020.03.09.20033118. [Google Scholar]
- 36.Liu J, Ouyang L, Guo P, sheng Wu H, Fu P, liang Chen Y, et al. Epidemiological, clinical characteristics and outcome of medical staff infected with COVID-19 in Wuhan, China: A retrospective case series analysis. medRxiv. 2020 [Preprint]. doi: 10.1101/2020.03.09.20033118. [Google Scholar]
- 37.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo IL, Lio CF, Cheong HH, Lei CI, Cheong TH, Zhong X, et al. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16:1698–707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–90. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa270. doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian GQ, Yang NB, Ding F, Ma AH, Wang ZY, Shen YF, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM. 2020;113:474–81. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin X, Qiu S, Yuan Y, Zong Y, Tuo Z, Li J, et al. Clinical Characteristics and Treatment of Patients Infected with COVID-19 in Shishou, China. China. 2020 [Preprint]. doi: 10.2139/ssrn.3541147. [Google Scholar]
- 43.Bernard Stoecklin S, Rolland P, Silue Y, Mailles A, Campese C, Simondon A, et al. First cases of coronavirus disease 2019 (COVID-19) in France: Surveillance, investigations and control measures, January 2020? Euro Surveill. 2020;25:2000094. doi: 10.2807/1560-7917.ES.2020.25.6.2000094. doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabata S, Imai K, Kawano S, Ikeda M, Kodama T, Miyoshi K, et al. Non-severe vs severe symptomatic COVID-19: 104 cases from the outbreak on the cruise Ship'Diamond Princess' in Japan. medRxiv. 2020 [Preprint]. doi: 10.1101/2020.03.18.20038125. [Google Scholar]
- 45.2019-nCoV National Incident Room Surveillance Team. 2019-nCoV acute respiratory disease, Australia: Epidemiology Report 1 (Reporting week 26 January-1 February 2020). Commun Dis Intell (2018) [Last accessed on 2020 Apr 10];2020 6:44. doi: 10.33321/cdi.2020.44.13. doi: 10.33321/cdi.2020.44.13. Retrieved from: https://pubmed.ncbi.nlm.nih.gov/32027812/; https://www1.health.gov.au/internet/main/publishing.nsf/ Content/1D03BCB527F40C8BCA258503000302EB/$File/2019_ncov_acute_respiratory_disease_epidemiology_ report_1_reporting_week_26_january_2020.pdf. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–77. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei L, Jin D, Zhang J, Wang B, Sun M, Li X, et al. Clinical findings of 100 mild cases of COVID-19 in Wuhan: A descriptive study. SSRN. 2020. [Last accessed on 2020 Apr 17]. p. 3551332. [Preprint]. Available from: https://pesquisa.bvsalud.org/global-literature-on-novel coronavirus-2019-ncov/resource/en/ppcovidwho-631 .
- 48.Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: A multicenter descriptive study. Clin Infect Dis. 2020;71:706–12. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L, et al. Chest CT findings in patients with corona virus disease 2019 and its relationship with clinical features. Invest Radiol. 2020;10:257–61. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu T, Chen C, Zhu Z, Cui M, Chen C, Dai H, et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis. 2020;94:68–71. doi: 10.1016/j.ijid.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–93. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao T, Gao Y, Cui Q, Shen J, Peng B, Chen Y, et al. Clinical characteristics of 55 cases of deaths with COVID-19 pneumonia in Wuhan, China: Retrospective Case Series. Lancet. 2020 doi: 10.1186/s12879-020-05423-7. [Preprint ]. doi: 10.2139/ssrn.3550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang B, Zhou X, Qiu Y, Feng F, Feng J, Jia Y, et al. Clinical characteristics of 82 death cases with COVID-19? PLoS ONE. 2020;15:e0235458. doi: 10.1371/journal.pone.0235458. doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–41. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 55.Zhao W, Yu S, Zha X, Wang N, Pang Q, Li T, et al. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: A retrospective cohort study. medRxiv. 2020 [Preprint]. doi: 10.1101/2020.03.13.20035436. [Google Scholar]
- 56.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214:1–8. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 58.Brender N. Palgrave Macmillan, London: Springer; 2014. Cases comparison: Outlook on H1N1 influenza pandemic and conclusions. In: Global Risk Governance in Health; pp. 166–95. [Google Scholar]
- 59.Javed A. IntechOpen. Chroneos, London: 2017. [Last accessed on 2020 Apr 17]. Pneumonia of viral etiologies. In: Contemporary Topics of Pneumonia. Available from: https://www.intechopen.com/books/contemporary-topicsof-pneumonia/pneumonia-of-viral-etiologies . [Google Scholar]
- 60.Garibaldi BT, Danoff SK. Symptom-based management of the idiopathic interstitial pneumonia. Respirology. 2016;21:1357–65. doi: 10.1111/resp.12649. [DOI] [PubMed] [Google Scholar]
- 61.Seki M, Fuke R, Oikawa N, Hariu M, Watanabe Y. Association of influenza with severe pneumonia/empyema in the community, hospital, and healthcare-associated setting. Respir Med Case Rep. 2016;19:1–4. doi: 10.1016/j.rmcr.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nichols L. Pneumonia as a trigger for atrial fibrillation. J Rural Med. 2017;12:146–8. doi: 10.2185/jrm.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang XM, Hu S, Hu CH, Hu XY, Yu YX, Wang YF, et al. Chest imaging of H7N9 subtype of human avian influenza. Radiol Infect Dis. 2015;1:51–6. doi: 10.1016/j.jrid.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Werkhoven CH, Huijts SM, Postma DF, Oosterheert JJ, Bonten MJ. Predictors of bacteraemia in patients with suspected community-acquired pneumonia. PLoS One. 2015;10:1–12. doi: 10.1371/journal.pone.0143817. doi: 10.1371/journal.pone.0143817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanamsagar MH, Sherwani AM. Upper respiratory tract infection and evidence based medicine – A Review. Int J Physiol Nutrit Phys Educ. 2019;4:1506–8. [Google Scholar]
- 66.Sharma L, Losier A, Tolbert T, Dela Cruz CS, Marion CR. Atypical pneumonia: Updates on legionella, chlamydophila, and mycoplasma pneumonia. Clin Chest Med. 2017;38:45–58. doi: 10.1016/j.ccm.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martínez-González J, Robles-Arias C, Rodríguez-Cintrén W. Rapidly progressive and almost lethal pneumonia. P R Health Sci J. 2017;36:41–3. [PubMed] [Google Scholar]
- 68.Chaudhary M, Ayub SG, Mir MA, Protocol G. Comparative efficacy and safety analysis of CSE-1034: An open labeled phase III study in community acquired pneumonia. J Infect Public Health. 2018;11:691–7. doi: 10.1016/j.jiph.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Ferrer M, Travierso C, Cilloniz C, Gabarrus A, Ranzani OT, Polverino E, et al. Severe community-acquired pneumonia: Characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS One. 2018;13:1–14. doi: 10.1371/journal.pone.0191721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos C, Oliveira RC, Serra P, Baptista JP, Sousa E, Casanova P, et al. Pathophysiology of acute fibrinous and organizing pneumonia – Clinical and morphological spectra. Pathophysiology. 2019;26:213–7. doi: 10.1016/j.pathophys.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cilléniz C, Rodríguez-Hurtado D, Torres A. Characteristics and management of community-acquired pneumonia in the era of global aging. Med Sci (Basel) 2018;6:35. doi: 10.3390/medsci6020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiang R, Tang Q, Chen XQ, Li MY, Yang MX, Yun X, et al. Effects of zinc combined with probiotics on antibiotic-associated diarrhea secondary to childhood pneumonia. J Trop Pediatr. 2019;65:421–6. doi: 10.1093/tropej/fmy069. [DOI] [PubMed] [Google Scholar]
- 73.Tse CF, Chan YYF, Poon KM, Lui CT. Clinical prediction rule to predict pneumonia in adult presented with acute febrile respiratory illness. Am J Emerg Med. 2019;37:1433–8. doi: 10.1016/j.ajem.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 74.Bermejo-Martin JF, Cilloniz C, Mendez R, Almansa R, Gabarrus A, Ceccato A, et al. Lymphopenic Community Acquired Pneumonia (L-CAP), an immunological phenotype associated with higher risk of mortality. EBioMedicine. 2017;24:231–6. doi: 10.1016/j.ebiom.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Méndez R, Menéndez R, Amara-Elori I, Feced L, Piré A, Ramírez P, et al. Lymphopenic community-acquired pneumonia is associated with a dysregulated immune response and increased severity and mortality. J Infect. 2019;78:423–31. doi: 10.1016/j.jinf.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Huang Y, Liu A, Liang L, Jiang J, Luo H, Deng W, et al. Diagnostic value of blood parameters for community-acquired pneumonia. Int Immunopharmacol. 2018;64:10–5. doi: 10.1016/j.intimp.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 77.Lee JH, Park M, Han S, Hwang JJ, Park SH, Park SY. An increase in mean platelet volume during admission can predict the prognoses of patients with pneumonia in the intensive care unit: A retrospective study? PLoS One. 2018;13:e0208715. doi: 10.1371/journal.pone.0208715. doi: 10.1371/journal.pone.0208715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tao RJ, Luo XL, Xu W, Mao B, Dai RX, Li CW, et al. Viral infection in community acquired pneumonia patients with fever: A prospective observational study. J Thorac Dis. 2018;10:4387–95. doi: 10.21037/jtd.2018.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dai RX, Kong QH, Mao B, Xu W, Tao RJ, Wang XR, et al. The mortality risk factor of community acquired pneumonia patients with chronic obstructive pulmonary disease: A retrospective cohort study. BMC Pulm Med. 2018;18:12. doi: 10.1186/s12890-018-0587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shirani K, Sheikhbahaei E, Torkpour Z, Ghadiri Nejad M, Kamyab Moghadas B, Ghasemi M, et al. A narrative review of COVID-19: The new pandemic disease. Iran J Med Sci. 2020;45:233–49. doi: 10.30476/ijms.2020.85869.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis. 2020;71:706–12. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maqbool K, Sadiq S, Mir AW, Bashir N, Bashir N. Influenza A, H1N1 and H3N2 experience at a tertiary care ICU. Int J. 2018;1:56. [Google Scholar]
- 83.Maruyama T, Fujisawa T, Suga S, Nakamura H, Nagao M, Taniguchi K, et al. Outcomes and prognostic features of patients with influenza requiring hospitalization and receiving early antiviral therapy: A prospective multicenter cohort study. Chest. 2016;149:526–34. doi: 10.1378/chest.14-2768. [DOI] [PubMed] [Google Scholar]


