Abstract
Often referred to as the silent killer, ovarian cancer is the most lethal gynecologic malignancy. This disease rarely shows any physical symptoms until late stages and no known biomarkers are available for early detection. Because ovarian cancer is rarely detected early, the physiology behind the initiation, progression, treatment, and prevention of this disease remains largely unclear. Over the past 2 decades, the laying hen has emerged as a model that naturally develops epithelial ovarian cancer that is both pathologically and histologically similar to that of the human form of the disease. Different molecular signatures found in human ovarian cancer have also been identified in chicken ovarian cancer including increased CA125 and elevated E-cadherin expression, among others. Chemoprevention studies conducted in this model have shown that decreased ovulation and inflammation are associated with decreased incidence of ovarian cancer development. The purpose of this article is to review the major studies performed in laying hen model of ovarian cancer and discuss how these studies shape our current understanding of the pathophysiology, prevention and treatment of epithelial ovarian cancer.
Abbreviations: AA, arachidonic acid; CTNNB1, beta-catenin; CA125, cancer antigen-125; COX, cyclooxygenase enzymes; DR6, death receptor 6; E-cad, E-cadherin; EC, endometroid; EOC, epithelial ovarian cancer; HGSOC, high-grade serous; MPA, medroxyprogesterone acetate; OM3FA, omega 3 fatty acids; OSE, ovarian surface epithelia; PCOS, poly-cystic ovarian syndrome; PR, progesterone receptor; PCNA, proliferating cell nuclear antigen; PGE2, prostaglandin E2; RO, Restricted Ovulators; SELENBP1, selenium-binding protein; VEGF, vascular endothelial growth factor
Ovarian cancer is the leading cause of death among female gynecologic malignancies, with a 47% 5 y relative survival rate.154 Early detection of the disease is necessary for decreasing the high mortality rate. However, early detection is difficult due to the lack of known specific biomarkers and clinically detectable symptoms until the tumor reaches at an advanced stage. The disease has multiple subtypes. Epithelial ovarian cancer (EOC) is the most common type of ovarian cancer, accounting for about 90% of all reported cases.127,164 EOC is commonly subdivided into 5 histotypes: high-grade serous (HGSOC), low-grade serous, mucinous, endometroid (EC), and clear cell. The histotypes differ in terms of tumor cell morphology, severity, systemic effect, and response to treatment. Among the different subtypes, HGSOC accounts for about 70% of cases of EOC observed in women. HGSOC has a higher mitotic index and is a more aggressive form of cancer with a worse prognosis. HGSOC and low-grade serous histotypes exhibit distinctly different presentations of the disease82,166 and demand different treatment modalities. EC (10% to 20%), mucinous (5% to 20%), and clear cell (3% to 10%) histotypes are less common forms of the disease. The subtypes of EOC also differ in terms of 5 y survival rates of patients; that is, HGSOC (20% to 35%), EC (40% to 63%), mucinous (40% to 69%), and clear cell (35% to 50%).20,76,148
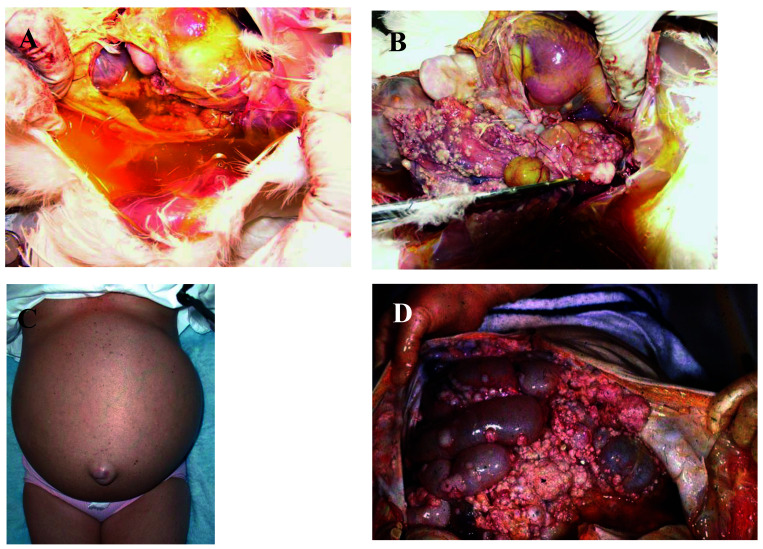
Developing a representative animal model for EOC has been challenging due to the histologic and pathologic differences among different subtypes of EOC. While developing a reliable animal model is challenging due to the vast complexity and limited understanding of the origin of the disease, laying hens naturally develop EOC that is histopathologically very similar to the human form of the disease (Figure 1).15 All the different human ovarian cancer histotypes have been observed in laying hen ovarian cancer (Figure 2). In addition, the presentation of the disease in chickens is remarkably similar to the human form of the disease, with early-stage ovarian cancer in laying hens having similar precursor lesions as occur in women.15 The laying hen develops ovarian cancer spontaneously, allowing analysis of early events and investigation into the natural course of the disease, as tumors can be examined as they progress from normal to late-stage ovarian carcinoma. The gross appearance of these stages is shown in Figure 3.
Figure 1.
Gross pathologic presentation of chicken compared with human ovarian cancer. The remarkably similar presentation in hens (A,B) and women (C,D) at the gross anatomic level with profuse abdominal ascites and peritoneal dissemination of metastasis. A) Ascites in abdominal cavity chicken with advanced ovarian cancer (photo credit: DB Hales); (B) Chicken ovarian cancer with extensive peritoneal dissemination of metastasis (photo credit: DB Hales); (C) Distended abdomen from ascites fluid accumulation in woman with ovarian cancer (http://www.pathguy.com/bryanlee/ovca.html) (D) Human ovarian cancer with extensive peritoneal dissemination of metastasis (http://www.pathguy.com/bryanlee/ovca.html).
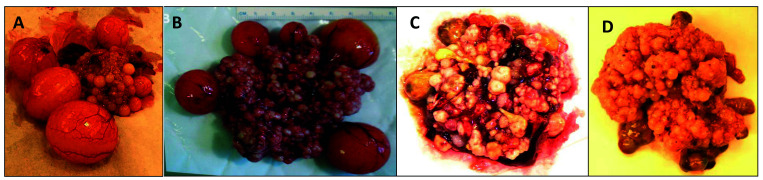
Figure 2.
Gross anatomic appearance of different stages of ovarian cancer in the chicken The progression from the normal hen ovary to late-stage metastatic ovarian cancer. (A) Normal chicken ovary showing hierarchal clutch of developing follicles and postovulatory follicle; (B) Stage 1 ovarian cancer, confined to ovary with vascularized follicles; (C) Stage 2/3 ovarian cancer, metastasis locally to peritoneal cavity with ascites; (D) Stage 4 ovarian cancer, late stage with metastasis to lung and liver with extensive ascites (photo credits: DB Hales).
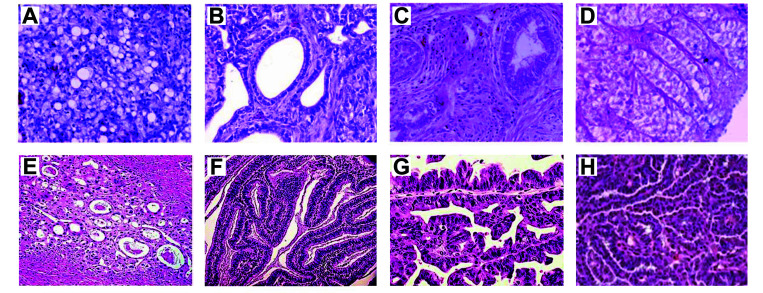
Figure 3.
Histologic subtypes in chicken compared with human ovarian cancers. H and E staining of formalin fixed paraffin embedded tissues from hens with ovarian cancer (A through D) and women (E through G). (A) Chicken clear cell carcinoma; (B) Chicken endometrioid carcinoma; (C) Chicken mucinous adenocarcinoma; (D) Chicken serous papillary adenocarcinoma (photo credits: DB Hales). (E) Human clear cell carcinoma; (F) Human endometrioid carcinoma; (G) Human mucinous cystadenocarcinoma; (H) Human serous adenocarcinoma (https://www.womenshealthsection.com).
Over the past 2 decades, the laying hen has emerged as a valuable experimental model for EOC, in addition to other in vivo models such as Patient-Derived Xenografts (PDX) and Genetically Engineered Mouse Models (GEMMs). Comparison of the hen model with other animal models has been reviewed elsewhere.72 Modern-day laying hens, such as the white leghorn, have been selected from their ancestor red jungle fowl57 for decreased broodiness and persistent ovulation, resulting in approximately one egg per day, if proper nutrition and light-dark cycles are maintained. Daily rupture and consequent repair of the ovarian surface epithelia (OSE) due to the persistent ovulation promotes potential error during rapid DNA replication. This increases the probability of oncogenic mutations, ultimately leading to neoplasia.137 Inflammation resulting from continuous ovulation also promotes the natural development of EOC.81 By the age of 2.5 to 3 y, laying hens have undergone a similar number of ovulations as a perimenopausal woman. The risk of ovarian cancer in white leghorn hens in this time (4%) is similar to the lifetime risk of ovarian cancer in women (0.35% to 8.8%).125 By the age of 4 to 6 y, the risk of ovarian cancer in hens rises to 30% to 60%.54 The incidence of ovarian carcinoma in the hens, however, depends on the age, genetic strain,80 and the egg-laying frequency of the specific breed.54 The common white leghorn hen has routinely been employed in chicken ovarian cancer studies. On average, hens are exposed to 17 h of light per day, with lights turned on at 0500 h and turned off at 2200 h. The laying hen model of EOC does present some considerable challenges. Despite its great utility for research, the model is still used mainly by agricultural poultry scientists and a small number of ovarian cancer researchers.
Comprehensive and proper vivarium support is required to conduct large-scale cancer prevention studies. Only a few facilities are available for biomedical chicken research, including University of Illinois Urbana-Champaign, Cornell University, Penn State University, NC State, Auburn University, and MS State University. Another difficulty is a lack of available antibodies specific for chicken antigens. Because of the structural dissimilarities between most human proteins and murine antigens to their chicken counterparts, cross-reactivity of available antibodies is also limited. The entire chicken genome was sequenced in 2004;78 however, the chromosomal locus of many key genes, such as p53, are still unknown. Overall, humans and chickens share about 60% of genetic commonality, whereas humans and rats share about 88% of their genes. Specific pathway-mutated strains of chickens are not yet available, limiting the ability to study key pathways in carcinogenesis and prevention of cancer using this model. Although all 5 different subtypes of ovarian cancer are present in hens, their most predominant subtype is different from women. Close to 70% of women diagnosed with ovarian cancer have serous EOC, while the predominant subtype reported in hens is endometrioid.15 However, these comparisons are complicated because observations of cancer in hens consist of both early and late stages of the disease, wherein women, most of the data is from late stage and aggressive ovarian carcinoma.
The spontaneous onset of ovarian cancer and the histologic and pathologic similarities to the human form of the disease make laying hens an excellent model for continued research on EOC. To date, a large number of studies have been performed on laying hens. Here we have divided the current studies into 2 groups— (A) studies that have described the molecular presentation of EOC to be similar to that in women; (Table 1) and (B) chemoprevention studies performed on large cohorts of laying hens (Table 2). The purpose of this review is to outline the major studies conducted using the laying hen model for EOC, and to describe how the research has advanced the field of ovarian cancer.
Table 1.
Studies investigating key molecular signatures in laying hen ovarian cancer
| Author | Year | Significance | Key molecular targets | Citation |
| Haritani and colleagues. | 1984 | Investigating ovarian tumors for key gene signatures | Ovalbumin | 71 |
| Rodriguez-Burford and colleagues. | 2001 | Investigating expressions of clinically important prognostic markers in cancerous hens | CA125, cytokeratin AE1/AE3, pan cytokeratin, Lewis Y, CEA, Tag 72, PCNA, EGFR, erbB-2, p27, TGF{α}, Ki-67, MUC1, and MUC2 | 135 |
| Giles and colleagues. | 2004, 2006 | Investigating ovarian tumors for key gene signatures | Ovalbumin, PR, PCNA, Vimentin | 62, 63 |
| Jackson and colleagues. | 2007 | CA125 expression in hen ovarian tumors | CA125 | 79 |
| Stammer and colleagues. | 2008 | SELENBP1 downregulation in hen ovarian tumors | SELENBP1 | 149 |
| Hales and colleagues. | 2008 | Cyclooxygenase expressions in hen ovarian tumors | COX1, COX2, PGE2 | 67 |
| Urick and colleagues. | 2008-2009 | VEGF expression in cultured ascites cells from hen ovarian tumors | VEGF | 160, 161 |
| Ansenberger and colleagues. | 2009 | Elevation of E-cadherin in hen ovarian tumors | E-cad | 6 |
| Hakim and colleagues. | 2009 | Investigating oncogenic mutations in hen ovarian tumors | p53, K-ras, H-ras | 66 |
| Zhuge and colleagues. | 2009 | CYP1B1 levels in chicken ovarian tumors | CYP1B1 | 175 |
| Seo and colleagues. | 2010 | Upregulation of Claudin-10 in hen ovarian tumors | Claudin-10 | 145 |
| Trevino and colleagues. | 2010 | Investigating ovarian tumors for key gene signatures | Ovalbumin, Pax2, SerpinB3, OVM, LTF, RD | 157 |
| Choi and colleagues. | 2011 | Upregulation of MMP-3 in hen ovarian tumor stroma | MMP-3 | 28 |
| Barua and colleagues. | 2012 | Upregulation of DR6 in hen ovarian tumors | DR6 | 16 |
| Lee and colleagues. | 2012-2014 | Upregulation of DNA methylation in hen ovarian tumors | DNMT1, DNMT3A, DNMT3B, SPP1, SERPINB11, SERPINB13 |
94, 101, 103, 104 |
| Lim and colleagues. | 2013-2014 | Key genes upregulated in endometrioid hen tumors | AvBD-11, CTNNB1, Wnt4 | 102, 11, 100 |
| Bradaric and colleagues. | 2013 | Investigating immune cells in hen ovarian tumors | 23 | |
| Ma and colleagues. | 2014 | Identifying unique proteins from proteomic profiling | F2 thrombin, ITIH2 | 106 |
| Hales and colleagues. | 2014 | Key genes upregulated in hen ovarian tumors | PAX2, MSX2, FOXA2, EN1 | 68 |
| Parada and colleagues, | 2017 | Unique ganglioside expressed in hen ovarian tumors | NeuGcGM3 | 124 |
Table 2.
Ovarian cancer prevention studies using laying hen model
| Author | Year | Significance | Citation |
| Barnes and colleagues. | 2002 | Medroxyprogesterone study | 14 |
| Johnson and colleagues. | 2006 | Different genetic strain of laying hens (C strain and K strain) | 80 |
| Urick and colleagues. | 2009 | Dietary aspirin in laying hens | 161 |
| Giles and colleagues. | 2010 | Restricted Ovulator strain | 61 |
| Carver and colleagues. | 2011 | Calorie-restricted hens | 25 |
| Eilati and colleagues. | 2012-2013 | Dietary flaxseed in laying hens | 43, 44, 45 |
| Trevino and colleagues. | 2012 | Oral contraceptives in laying hens | 156 |
| Rodriguez and colleagues. | 2013 | Calorie-restricted hens with or without Vitamin D and progestin | 136 |
| Mocka and colleagues. | 2017 | p53 stabilizer CP-31398 in laying hens | 112 |
Etiology of the disease: current hypotheses
To understand, diagnose, treat, and prevent any malignancy requires the determination of the origin of the tumor. Unlike many other epithelial carcinomas such as colon and cervical cancer, which have well-defined precursor lesions, the cell of origin for EOC is poorly understood. An emerging body of evidence suggests an important role for the fallopian tube in initiating serous ovarian cancer.35,86 The proposed tubal hypothesis states that some ovarian cancers may arise from the fimbriae or the distal fallopian tube in women. Embryologically, OSE originates from the coelomic epithelium, whereas the fallopian tube, uterus and cervix originate from the paramesonephric (Müllerian) ducts. Neoplastic OSE is present in Müllerian-like tissues in which the epithelium is not of Müllerian origin, supporting this hypothesis. The tubal hypothesis classifies ovarian carcinoma in 2 groups, ‘Type I’ are low-grade cancers often lacking p53 mutations and originate from the ovaries; ‘Type II’ are aggressive high-grade carcinomas that arise from the fallopian tube with mutated p53 in over 90% of all cases reported.27,88,142
Among multiple contributing factors for carcinogenesis, chronic inflammation seems to have a significant role, giving rise to the inflammation hypothesis.116,122 A prolonged and sustained inflammatory response is well established as a potent activator of tumor growth and invasion.4,12,13 The ovulatory process involves the rupture of the OSE, which triggers a strong inflammatory reaction due to the wound healing response; cyclic wounding and healing exacerbate the inflammation, with infiltrating leukocytes, production of inflammatory cytokines, and a marked upregulation in major inflammatory signaling pathways. The group of activated inflammatory molecules during ovulation include IL8, CCL2 and CCL5/RANTES and is similar to those activated during EOC.55 OSE is a continuum of the peritoneal lining, unlike most other organs in the peritoneal cavity.7 Therefore, the OSE is also exposed to any environmental or xenobiotic stress present in the peritoneum, which are likely to be extremely inflammatory in nature.
The gonadotropin hypothesis33 of ovarian cancer arose from early observations of an increased incidence of ovarian cancer in rodents after transplanting their ovaries to their spleen. This tumorigenesis was attributed to elevated pituitary gonadotropin levels caused by disruption of negative feedback of estrogen to the pituitary gland.167 Ovarian tumors did not form if one ovary was left intact while the other was transplanted to the spleen.19 Exposure to carcinogens or X-irradiation that causes loss of functional oocytes and ovarian failure also induced ovarian cancer in animals.107,114 The mouse strain ((C57BL/6J x C3H/HeJ)F1 WxWv), which is congenitally deficient in or has few functional oocytes, also rapidly develops ovarian cancer.114,152 These models all have increased gonadotropin release due to disruption of the hypothalamus-pituitary-ovarian negative feedback loop. Tumor development could be reduced or prevented by suppressing gonadotropin in these animals.21,105 Ectopic expression of hormone receptors (that is, receptors for GnRH-I/II,29,48 activin,110,150 inhibin,56 estrogen,74,92,128 progesterone3,92,95 and androgen,26,69,92) has also been reported in EOC. The gonadotropin hypothesis proposes that the surge of gonadotropins due to lack of gonadal negative feedback in menopause and/or premature ovarian failure contributes to the development and progression of EOC.
The incessant ovulation hypothesis,51 which arose in 1971, is based on an analysis of epidemiologic data from patients and animals and postulates that continuous ovulation causes continuous damage to the OSE.51 (Table 3) This damage triggers rapid wound healing and the generation of an immense inflammatory burden on the ovaries, increasing the likelihood of oncogenic mutations and carcinogenesis. While repairing the ovulation wound, the OSE often forms an indentation from retraction of corpus albicans or disintegration of a cystic follicle.129 Such indentations lead to the deposition of the surface epithelium into the cavity of a corpus luteum, resulting in the formation of inclusion cysts that remain in the ovarian stroma. Presence of inclusion cysts in the contralateral ovary in women with ovarian cancer has provided strong evidence that cysts could play a major role in the development of ovarian cancer.111 Ovulation frequency is reportedly higher in the right ovary than in the left ovary126 and studies have also reported a higher propensity for the right ovary to develop ovarian cancer.34 Ovulation-inducing agents (clomiphene and gonadotropins) are suggested risk factors for developing ovarian cancer.5,91,132,138,163 In contrast, reducing the total number of ovulations has reduced the risk of getting ovarian cancer. Oral contraceptive users have about a 30% lower risk of getting EOC than do nonusers due to decreased ovulatory events.22,113,158 Also, each additional pregnancy after the first reduces the risk of getting EOC by 10% to 16%.70,134 Thus, the incessant ovulation hypothesis encompasses other pre/coexisting hypothesis for onset of ovarian cancer, such as the gonadotropin hypothesis32,133 and the inflammation hypothesis.4,12,13,49 However, some evidence contradicts the hypothesis that gradually accumulating postovulatory, benign inclusion cysts leads to the onset of a malignant carcinoma.119,131,139 Although patients suffering from Poly-Cystic Ovarian Syndrome (PCOS) have a high number of inclusion cysts in their ovaries,131 the high number of cysts is not positively correlated with increased ovulation; in fact, PCOS patients are predominately hypo-ovulating and are subfertile.
Table 3.
Key hypotheses describing the origin of ovarian cancer
| Hypotheses | Main features | Key citations |
| Tubal hypothesis | OSE is not embryologically derived from Mullerian ducts, however ovarian neoplasms present Mullerian features. This suggests that OC originates from the epithelium of the fallopian tube where p53 signatures have been observed and the disease later migrates onto the ovarian surface. | 35, 27, 86, 89, 142 |
| Inflammation hypothesis | Prolonged and sustained inflammation in the ovary gives rise to neoplastic changes. Ovulation is an inflammatory process. The OSE is also exposed to the peritoneal cavity and therefore is exposed to environmental and xenobiotic stress. | 4, 13, 55 |
| Gonadotropin hypothesis | Factors that induce gonadotropin release from the pituitary (such as loss of negative feedback by estrogens to the pituitary) induce ovarian carcinogenesis. | 19, 21, 105, 107, 118, 167 |
| Incessant Ovulation hypothesis | Continuous ovulation and consequential rapid wound healing of the OSE results in an immense inflammatory burden on the ovaries and can trigger oncogenic changes. Ovarian cancer incidence can also be positively correlated with the number of ovulations. This hypothesis combines elements of both the inflammation and gonadotropin hypotheses. | 34, 51, 52, 111, 126 |
The specific etiology of ovarian cancer is still not resolved, perhaps predominantly because ovarian cancer is rarely detected in early stages, and therefore, the early molecular events that promoting the neoplastic changes are unknown.
Investigating key molecular signatures in laying hen ovarian cancer: similarities between the chicken model and human EOC
Detection of ovarian cancer is a challenge, primarily because of the lack of sensitive predictive biomarkers. Cancer antigen-125 (CA125)/mucin-16 (MUC-16) has been one of the earliest developed biomarkers for EOC.17 While detection of the gradually elevating serum level of CA125 glycoprotein has long been used as a diagnostic marker for ovarian cancer,83 CA125 levels cannot be used to distinguish ovarian cancer from other cancers. A previous study18 reported that approximately 29% of patients with nongynecological cancer presented with elevated serum CA125. In laying hens, 2 studies have investigated CA125 expression; one study found that about 90% of ovarian tumors in laying hens express CA125,79 whereas the other did not detect CA125 in chicken ovarian tumors, using the same CA125 antibody.135 The first study79 used a high-temperature antigen retrieval method, whereas the second,135 which failed to detect CA125, used a low-temperature antigen retrieval method for CA125 immunostaining. This difference might be the key reason for these contradicting results. The second study also investigated expression of several other prognostic markers that were routinely used for clinical evaluation of ovarian cancer in women; of these, they found expression of cytokeratin AE1/AE3, pan cytokeratin, Lewis Y, CEA, Tag 72, PCNA, EGFR, erbB-2, p27 and TGF α to be positive in chicken ovarian tumors, while Ki-67, Muc1 and Muc2 were not detected in the hens.135 More recently, one study159 reported have shown Ki-67 positive staining and have expanded the number of known angiogenic and proliferation markers with chicken-cross reactive antibodies.159 A separate report106 conducted a shotgun proteomic analysis using combinational peptide ligand libraries (CPLL)-LC-MSMS workflow of chicken blood proteins and identified 264 unique proteins. From the unique proteins identified, 10 potential biomarkers were selected through semiquantitative spectral counting analysis; of these, interalpha inhibitor heavy chain (ITIH2) and F2 thrombin were found to be elevated in hens with cancer, as compared with normal hens. The human homolog of F2 thrombin, prothrombin fragment F258 and another human heavy chain of ITIH4174 are both reported to be elevated in women with ovarian cancer. These studies allowed the identification of similar key biomarkers of EOC in laying hens and women.
One of the unique features in ovarian cancer is an upregulation of a transmembrane cell-cell adhesion glycoprotein, E-cadherin (E-cad), which is expressed by the normal oviductal and endometrial surface epithelia but is not found in the ovarian stroma.7,60 During its early neoplastic changes, the OSE undergoes a conformational change from cuboidal to columnar epithelial cells, which also occurs in prostate cancer. During these neoplastic changes, E-cad is reported to be gradually elevated in women.8,144 Another report found that E-cad is significantly upregulated in ovarian cancer in laying hens.6 In addition, E-cad is expressed in the granulosa cell layer around the follicles in normal ovary, around the inclusion cysts in the early neoplastic ovaries, and throughout the cancerous epithelium in late-stage ovarian tumors.6 They found that the metastasized secondary tumors in the peritoneal cavity of hens form similar glandular structures with high E-cad expression.6 Recently, an ectopic miR-200 expression in OSE cells in 3D culture was reported to stabilize the formation of inclusion cysts with a subsequent increase in E-cad expression.30 Several gene expression studies have also reported upregulation of E-cad in chicken ovarian tumors.64,153,157 One report assessed gene expression and performed a bioinformatic analysis of chicken ovarian tumors and reported that key genes upregulated in chicken ovarian cancer included PAX2, MSX2, FOXA2 and EN1.68 All of these upregulated genes are involved in controlling branching morphogenesis during gland development, and parallel upregulation of E-cad and miR-200 family members.68 At about the same time, another publication reported that the junction adherence molecule Claudin-10 is elevated in chicken ovarian cancer as also occurs in women.145
One of the most intensively studied tumor suppressor genes is TP53. This gene codes for the p53 protein and is often referred to as the ‘guardian of the genome’. Over 50% of all human cancers have a mutant TP53 gene.121 In ovarian cancer, TP53 is mutated in about 95% of HGSOC.24 This exclusive feature of HGSOC is widely accepted for prognostic distinction of this subtype.9,141 One report66 analyzed the TP53 mutation in chickens in 2 flocks from a previously-reported calorie-restricted chicken study.25 This TP53 study66 performed gene expression analysis from 172 4 y old white leghorn hens, grouped as calorie-restricted (n = 102) (flock A) and normal diet (n = 70) (flock B). Flock A birds had a significantly lower number of ovulations and a lower incidence of ovarian cancer. Gene expression analysis revealed that 48% of the chicken ovarian tumors had a mutated p53 gene, 14% in flock A and 96% in flock B. This was a striking resemblance to the human form of ovarian cancer in which p53 mutations correlate with the number of lifetime ovulations in women.140,168 This study66 also reported that most of the p53 mutations were found in the proline-rich and DNA-binding domains (82 of 90 mutations), similar to previous reports in women.66 The type of mutation also differed between the 2 flocks of hens. All flock A mutations (14) were found within the DNA-binding domain and only 0.7% (1 out of 14) were a missense mutation, whereas 93% of flock B mutations (71 out of 76) were missense mutations, and all mutations were found within the proline-rich domain. Most of the flock B mutations (76%) involved a change in an aliphatic amino acid at position 62 (Ala) or 72 (Thr) into a proline. However, the effect of these mutations on p53 function is not known. This study66 also found very few K-ras mutations (1.2%) and no H-ras mutations in these birds. Ras mutations in women are also extremely rare in aggressive ovarian carcinomas.36,108,118,171 Positive Her-2/neu staining was reported in 53% of hen ovarian adenocarcinomas, which was similar to that reported in women.117 Together, these findings reveal a similar oncogenic mutational landscape between the laying hens and human disease.
Selenium-binding protein (SELENBP1) downregulation has been observed in women with ovarian cancer.77 Because selenium is an essential micronutrient involved in reducing cell proliferation and promoting apoptosis in tumor cells, a marked decrease in SELENBP1 has been positively correlated with cancer progression in women.96 A previous study found that SELENBP1 is downregulated in all histotypes of ovarian cancer in laying hens.149 In normal, noncancerous chicken ovaries, SELENBP1 is strongly expressed at or near the surface epithelium. This expression was significantly lower in tumors and early neoplastic lesions, indicating another significant similarity between the laying hen and human ovarian cancer.96
Several other studies have supported the use of the chicken model to study ovarian cancer. One article29 reported an upregulation of matrix metalloproteinase 3 in the chicken ovarian tumor stroma, as had earlier been reported in human ovarian cancer.115 Another group16 reported elevated expression of Death receptor 6 (DR6), a receptor that mediates suppression of antitumor activities, in ovarian tumors in hens. DR6 expression found in serum and microvessels was low in normal hens, and gradually increased with ovarian cancer progression into the late stages of the disease. DR6 expression was also reported to be elevated in women with ovarian cancer.143,146 Other articles identified that an avian homolog of the β-defensin (AvBD-11),102 β-catenin (CTNNB1)11 and Wnt4100 were expressed abundantly in the glandular epithelium of the endometrioid type of ovarian cancer in hens. In women with ovarian cancer, human β-defensin (hBD) has been reported to influence vasculogenesis under the influence of VEGF-A.31 Human EOC has been reported to express mutated CTNNB189,109 and Wnt4 has also been positively associated with a higher risk of ovarian cancer in women173 A separate report found an increase in de novo DNA methylation in chicken ovarian tumors and an upregulation of DNMT1, DNMT3A and DNMT3B.94 Other studies reported that a secreted phosphoprotein (SPP1)101 and 2 serine proteinase inhibitors, SERPINB11104 and SERPINB3103 are significantly upregulated in ovarian cancer. All of these factors (that is, DNMT1, DNMT3A, DNMT3B,1,2 SPP1,172 SERPINB11 and SERPINB13)10 were also found to be elevated in women with ovarian cancer. Another article64 investigated differential gene expression patterns in localized and metastasized hen ovarian tumors and normal ovarian epithelium samples. A class comparison analysis with the human ovarian cancer microarray GEO database (GSE6008) revealed that the altered gene expression pattern in laying hen EOC is very similar (approximately 78%) to that in women.64
Studies involving immune system activation to target and kill cancer cells have been revolutionized since the discovery of immune checkpoint inhibitors. T-cells express immune checkpoint receptors such as CTLA-4 and PD-1on their surface; these receptors send an “off’ signal upon binding with their ligands, rendering the T-cell inactive. CTLA-4 binds with the B7 ligand on antigen-presenting cells with a higher affinity than CD28, impeding the co-stimulating signal for T-cell activation. PD-1 binds with PD-L1 ligand, which is expressed by many tumor cells and M2-like macrophages. Inhibiting the checkpoint inhibitors prevents the inactivation of T-cells, allowing them to attack the tumor cells more effectively. However, despite initial success in melanoma and lung cancer, many solid tumors have shown formidable resistance against the immune checkpoint inhibition therapy. The tumor microenvironment substantially aids this resistance, leading to an increasing interest in investigating how the tumor and its microenvironment harness host anti-tumor immunity. The immune system in chickens and humans are reported to be very similar,39,50 although chickens lack IgE and IgD immunoglobins,130 and the MHC regions of chickens are simpler and more compact than those of humans.84,85 A report conducted to investigate the association of immune cells with ovarian cancer showed that the immune cell content and locations in early to late ovarian cancer is similar in laying hens and women.23 This work also provided evidence that CD4+ helper T-cells were less prevalent than CD8+ cytotoxic T-lymphocytes and B cells in both normal and cancerous ovaries. B cells were found in the stroma and were not associated with the follicles. These findings were similar to the immune landscape seen in women with ovarian tumors.155 Another study reported that chicken ovarian tumors uniquely express a NeuGcGM3 ganglioside that is not expressed in normal ovarian tissue.124 NeuGcGM3 ganglioside has also been reported to be highly expressed in women with breast and ovarian cancer.90,120
Other studies in laying hens have further supported the oviductal origin theory of ovarian cancer. One of the earlier studies that suggested an oviductal origin of ovarian cancer used tumor tissues that were collected from 12 hens with ovarian adenocarcinoma, immunostained with ovalbumin, and all found to have positive staining,71 Another group analyzed 16 hen ovarian adenocarcinomas with or without oviductal involvement and 9 normal ovarian tissues for expression of ovalbumin, proliferating cell nuclear antigen (PCNA) and progesterone receptor (PR).63 All ovarian cancer samples were positive for ovalbumin and PCNA, yet ovalbumin was absent in normal OSE. Progesterone receptor was present in 9 of 14 ovarian tumors. A follow up study reported that ovarian tumors strongly express cytokeratin and PCNA and weakly express vimentin in the gland-like regions of the tumors; normal OSE expressed cytokeratin, PCNA and PR.62 Another group performed a microarray-based gene expression study in laying hens and found by functional annotation analysis that the top 25 genes altered in the ovarian tumors are related to the oviduct.157 Of them, OVAL (Ovalbumin/SerpinB14), Paired-box 2 (Pax2), SerpinB3 (important in promoting EMT), OVM (Ovomucoid/SPIK7), LTF (lactotransferrin), and RD (riboflavin binding protein) were expressed in the hen in both early and late-stage ovarian cancer, but not in normal OSE. Another important observation was that all these altered genes, like several other oviduct-related genes, are driven by estradiol.157 The expression of these oviductal genes is also involved in human ovarian carcinoma. One of these genes, Pax8, is a well-established marker for ovarian tumors in women and promotes tumor cell growth and differentiation (chickens do not have a Pax8 gene).97,99 The laying hen studies have not helped to resolve the controversy surrounding the ovarian cancer cell of origin; however, the presence of the same controversy in the laying hens further endorses the model as a reliable equivalent to the human form of ovarian cancer.
Cancer prevention studies using the laying hen
The first large cohort study that connected the number of ovulations with the onset of ovarian cancer in laying hens monitored egg production in 3 different flocks of 466 white-leghorn hens.54 The flocks were grouped in 2 y, 3 y and 4 y or older birds, and the incidence of EOC in those birds were 9%, 19% and 39%, respectively. The study also monitored incidences of oviductal adenocarcinoma, granulosa cell tumors, Sertoli cell tumors and other tumor types but only EOC was positively correlated with age across the flocks. The observations of the study and the incessant ovulation hypothesis proposed by others, led to a focus on the impact of ovulation and inflammation in ovarian cancer incidence.52
Prostaglandins are bioactive lipids that are produced from arachidonic acid (AA) through the action of the cyclooxygenase (COX) enzymes. Humans have 2 isoforms of COX: COX1, which is constitutively expressed in most cells and tissues, and COX2, which is inducible by various inflammatory stimuli. Upregulation of COX2 has been reported in many cancers.40,147 COX1 is expressed in the laying hen by the OSE, granulosa cell layer, cortical interstitium, and postovulatory follicles in normal ovaries, while spreading largely to the tumor stroma with ovarian cancer.67 However, expression of COX2 in the hen ovary increases with age yet is not affected by the onset of cancer.67 To investigate the effect of inhibition of the COX1 and COX2 enzymes on ovarian cancer cells, cultured ascitic cells were collected from cancerous chickens and treated with aspirin, sc-560 (a specific COX1 inhibitor) or ns-398 (a specific COX2 inhibitor).160 The data showed an increase in vascular endothelial growth factor (VEGF) expression in the ascitic cells, theca cells in normal ovaries, and the glandular areas of the tumor. VEGF levels in the peritoneal ascites was higher than in tumors. Aspirin and sc-560 significantly decreased the cell growth and VEGF production but ns-398 did not in the OVCAR-3 ovarian cancer cell line. These findings supported the previous observations that, unlike most carcinomas, COX1 is upregulated37,38,65,87,93,98,162 and COX2 is downregulated or remains unaltered in ovarian cancer.169 The proliferation of the ascites cells and subsequent VEGF production are dependent on COX1 but not on COX2. In a study of the effects of dietary aspirin in laying hens,161 hens from 3 different age groups were fed either a control diet or a diet supplemented with 0.1% aspirin for one year. Dietary aspirin did not decrease the incidence but decreased the stage or severity of ovarian cancer. That study further found that dietary aspirin significantly decreased the liver prostaglandin E2 (PGE2) levels in the birds, which indicated an inhibition of the systemic activity of the COX enzymes. An increase in PGE2, and other prostanoids (TxB2) has also been reported in women with ovarian cancer.73,170
Eicosanoids such as PGE2, produced from AA (omega-6 fatty acids) are proinflammatory. In contrast, eicosanoids derived from omega 3 fatty acids (OM3FA) have antiinflammatory properties. Dietary ingestion of OM3FA is well known to increase their incorporation to cell membranes and therefore affect the inflammatory response. A cancer-preventative study in laying hens evaluated dietary supplementation of flaxseed, the richest plant source of OM3FA (mostly α-linolenic acid).59 Feeding a 10% flaxseed supplemented diet to 2.5 y old hens for one year was associated with a decrease in severity of ovarian cancer.44 The concentration of PGE2 and COX2 expressions in the ovaries were both significantly lower with flaxseed diet, whereas the concentration of OM3FA in yolks more than doubled. This study44 also reported that long-term consumption (4 y) of 10% flaxseed decreases both the severity and incidence of ovarian cancer. Long-term flaxseed consumption significantly diminishes PGE2 and COX2 levels in chicken ovaries, protecting them from ovarian cancer.43,45 Another study showed that a flaxseed supplemented diet decreased PGE2 concentration and COX2 expression in the ovaries in a dose-dependent manner, accompanied by dose-dependent increases in the OM3FA/OM6FA ratio.41 Dietary supplementation with fish oil (rich in OM3FA, mostly eicosapentanoic acid) has been shown to downregulate expression of COX1, COX2, and PGE2 levels in chicken ovaries,46 suggesting that antiinflammatory actions of the omega-3 fatty acids are pivotal in targeting the prostaglandin biosynthesis pathway and thereby hindering the onset of ovarian cancer. Dietary flaxseed has also been reported to alter the estrogen metabolism pathways, inducing the CYP1A1 pathway and enhancing systemic production of the 2-methoxyestradiol metabolite while suppressing the CYP1B1 and CYP3A4 pathways.41,42 In addition, CYP1B1 levels in chicken ovarian tumors are significantly higher than in the age-matched normal chicken ovaries,175 which also supports an alteration in the estrogen metabolism pathway in chicken ovarian cancer. The flaxseed diet also activated the MAPK pathways (p38 MAPK and Erk-1/2) in the ovaries, which may be protective against ovarian carcinogenesis.42,123
Ovarian cancer was analyzed59 in a unique, previously developed hyperlipidemic strain of white leghorns referred to as “Restricted Ovulators” (RO).75 The RO strain has a mutated VLDL receptor; VLDL protein is necessary for oocytic uptake of major yolk precursor macromolecules such as VLDL and vitellogenin-2. Because of this mutation, oocytes fail to mature and lack a typical follicular hierarchy.47 This failure results in massive lipid accumulation in blood plasma, making the hens hyperlipidemic, hypoprogesteronemic and hyperestrogenemic. The study monitored egg-laying and ovarian cancer incidence in 31 RO and 33 WT hens (around 4 y of age at euthanasia) over a period of 972 d and found that only 3% of the RO hens developed ovarian cancer as compared with about 27% of the WT hens.61–63 The RO birds laid significantly fewer eggs than did wildtype birds. Another report made similar observations.54 Another observation from this study54 was that the RO birds had a significantly higher plasma estradiol concentration.
A study of 800 white leghorn hens used caloric restriction to suppress ovulation.25 In this 2 y study, a normal caloric diet formulated for daily egg-production was compared with a diet designed to cause a 55% decrease in dietary energy consumption. Calorie-restricted birds had significantly lower body weight, produced about 64% fewer eggs and developed 23% less cancer of the reproductive tract. In chickens on the normal diet, 26% had ovarian cancer, as compared with only 6% of the calorie-restricted birds. These data support the hypothesis that reduction in ovulation reduces the incidence of carcinogenesis.
A subsequent study divided around 2,400 birds into 6 groups: regular diet, calorie-restricted diet, calorie-restricted supplemented with vitamin D3, the progestin levonorgestrel, progestin provera, or a combination of levonorgestrel and vitamin D3.136 Ovulation rate did not change in the calorie-restricted groups, except that the levonorgestrel treated birds had fewer ovulatory events than did the other birds. Overall, progestin-treated birds had fewer reproductive tract cancers, including ovarian cancers, than did the other groups, whereas vitamin D3 did not affect cancer incidence. However, the study concluded that suppressing ovulation (with progestins) reduces the incidence of ovarian cancer.136 These findings were consistent with previous data from a study that administered medroxyprogesterone acetate (MPA; 100 mg dose), a progestin-only contraceptive, to 3 y old hens in 3 intervals over 16 mo.14 The data showed a 15% reduction in all reproductive tract adenocarcinoma in the treated group as compared with the control. Egg-laying frequency was also reduced after MPA administration.
Suppressing ovulation by use of contraceptives and subsequent prevention of ovarian carcinogenesis was studied using oral contraceptives in laying hens.156 In this study, 231 one-y-old white leghorns were divided into control and 3 treatment groups; progestin (MPA) alone, estradiol (compudose implant) alone, and a combination of both progestin and estradiol. Hens were euthanized after 16 mo and the recorded incidence of ovarian cancer in the 4 groups were as follows: control (19%), estradiol only (19%), progestin only (4%) and combination of progestin and estradiol (2%). Thus, in combination with estradiol, the efficacy of progestins improved, resulting in a further decrease the incidence of ovarian cancer and egg-laying frequency. Estradiol alone did not alter either egg-laying frequency or ovarian cancer onset. However, estradiol alone also did not increase ovarian cancer incidence. Another study that monitored ovarian cancer onset in 2 strains of domestic hens derived from a similar genetic background found that one strain (C strain) had a higher incidence of ovarian neoplasm than the other (K strain).80 C strain birds had a higher plasma estradiol levels and a lower α-inhibin level (both in plasma and the granulosa cell layer of the ovaries) than did the K strain. RO birds also had higher plasma estradiol levels and a lower rate of ovarian cancer onset. These combined observations suggest that, unlike breast cancer, increased exposure to estradiol probably does not have a role in inducing ovarian cancer.
Another dietary chemoprevention study in the laying hens was performed112 with a p53 stabilizing compound CP-31398.53,165 CP-31398 is a styryl quinazoline compound151 that is suggested to act in a chaperone-like manner, binding to newly synthesized p53 protein and maintaining its proper folding and wild-type conformation.165 In this study, CP-31398 was fed to approximately 1.5 y-old hens for 94 wk in low (100 ppm), moderate (200 ppm) and high (300 ppm) doses.112 Dietary CP-31398 in moderate and high doses significantly lowered onset of ovarian cancer (approximately 77% lower) as compared with the low-dose and control group. The moderate and high dose birds also had significantly lower feed consumption, body weight and number of eggs laid than did the low dose-fed and control group hens.
The ability to perform such chemopreventive studies on a model that is spontaneous and histopathologically similar to women is probably the greatest virtue of the laying hen model in ovarian cancer research. In addition, the possibility of performing long-term longitudinal analysis for potential biomarkers and studying key oncogenic molecular changes during early stages of carcinogenesis also makes it an extremely advantageous research model.
Perspectives
The advantages of the spontaneous development of EOC in hens likely transcend the challenges of using the laying hen model. The model is ideal for studying the progression of the disease. Similar to women, EOC in laying hens is an age-related disease, which contributes to the advantages of this model. Most of the large cohort studies in hens were able to describe early tumors that could only be identified histologically because the ovary was functional and appeared to be normal on gross inspection. These early neoplasms provide critical information that is likely relevant to elucidating the onset and early development of ovarian tumors in women. Studying the early events in carcinogenesis is an excellent way to identify biomarkers for early detection of the disease. Other indisputable benefits of using the laying hen as a model for EOC is the short generation time and the ability to perform large scale screening for chemoprevention trials. The laying hen provides the ovarian cancer research community with the critical resource of a natural experimental model, and with the current integrated multi-omics technologies, the laying hen remains as the most promising model system to harness key epidemiologic and molecular signatures to fight EOC.
References
- 1.Ahluwalia A, Hurteau JA, Bigsby RM, Nephew KP.2001.DNA methylation in ovarian cancer. II. Expression of DNA methyltransferases in ovarian cancer cell lines and normal ovarian epithelial cells.Gynecol Oncol 82:299–304. 10.1006/gyno.2001.6284. [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia A, Yan P, Hurteau JA, Bigsby RM, Jung SH, Huang TH, Nephew KP.2001.DNA methylation and ovarian cancer. I. Analysis of CpG island hypermethylation in human ovarian cancer using differential methylation hybridization.Gynecol Oncol 82:261–268. 10.1006/gyno.2001.6291. [DOI] [PubMed] [Google Scholar]
- 3.Akahira J, Suzuki T, Ito K, Kaneko C, Darnel AD, Moriya T, Okamura K, Yaegashi N, Sasano H.2002.Differential expression of progesterone receptor isoforms A and B in the normal ovary, and in benign, borderline, and malignant ovarian tumors.Jpn J Cancer Res 93:807–815. 10.1111/j.1349-7006.2002.tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A.2008.Pathways connecting inflammation and cancer.Curr Opin Genet Dev 18:3–10. 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Anderson SM, Dimitrievich E.1996.Ovulation induction for infertility is it safe or not? S D J Med 49:419–421. [PubMed] [Google Scholar]
- 6.Ansenberger K, Zhuge Y, Lagman JA, Richards C, Barua A, Bahr JM, Hales DB.2009.E-cadherin expression in ovarian cancer in the laying hen, Gallus domesticus, compared to human ovarian cancer.Gynecol Oncol 113:362–369. 10.1016/j.ygyno.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC.2001.Ovarian surface epithelium: biology, endocrinology, and pathology.Endocr Rev 22:255–288. 10.1210/er.22.2.255. [DOI] [PubMed] [Google Scholar]
- 8.Auersperg N, Woo MM, Gilks CB.2008.The origin of ovarian carcinomas: a developmental view.Gynecol Oncol 110:452–454. 10.1016/j.ygyno.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Ayadi L, Chaabouni S, Khabir A, Amouri H, Makni S, Guermazi M, Frikha M, Boudawara TS.2010.Correlation between immunohistochemical biomarkers expression and prognosis of ovarian carcinomas in tunisian patients.World J Oncol 1:118–128. 10.4021/wjon2010.06.213w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badola S, Spurling H, Robison K, Fedyk ER, Silverman GA, Strayle J, Kapeller R, Tsu CA.2006.Correlation of serpin-protease expression by comparative analysis of real-time PCR profiling data.Genomics 88:173–184. 10.1016/j.ygeno.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Bae SM, Lim W, Jeong W, Lee JY, Kim J, Han JY, Bazer FW, Song G.2014.Hormonal regulation of beta-catenin during development of the avian oviduct and its expression in epithelial cell-derived ovarian carcinogenesis.Mol Cell Endocrinol 382:46–54. 10.1016/j.mce.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Balkwill F, Coussens LM.2004.Cancer: an inflammatory link.Nature 431:405–406. 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 13.Balkwill F, Mantovani A.2001.Inflammation and cancer: back to Virchow? Lancet 357:539–545. 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 14.Barnes MN, Berry WD, Straughn JM, Kirby TO, Leath CA, Huh WK, Grizzle WE, Partridge EE.2002.A pilot study of ovarian cancer chemoprevention using medroxyprogesterone acetate in an avian model of spontaneous ovarian carcinogenesis.Gynecol Oncol 87:57–63. 10.1006/gyno.2002.6806. [DOI] [PubMed] [Google Scholar]
- 15.Barua A, Bitterman P, Abramowicz JS, Dirks AL, Bahr JM, Hales DB, Bradaric MJ, Edassery SL, Rotmensch J, Luborsky JL.2009.Histopathology of ovarian tumors in laying hens: a preclinical model of human ovarian cancer.Int J Gynecol Cancer 19:531–539. 10.1111/IGC.0b013e3181a41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barua A, Yellapa A, Bahr JM, Abramowicz JS, Edassery SL, Basu S, Rotmensch J, Bitterman P.2012.Expression of death receptor 6 by ovarian tumors in laying hens, a preclinical model of spontaneous ovarian cancer.Transl Oncol 5:260–268. 10.1593/tlo.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC.1981.Reactivity of a monoclonal antibody with human ovarian carcinoma.J Clin Invest 68:1331–1337. 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bast RC, Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker L, Zurawski VR, Jr, Knapp RC.1983.A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer.N Engl J Med 309:883–887. 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 19.Biskind GR, Biskind MS.1948.Atrophy of ovaries transplanted to the spleen in unilaterally castrated rats; proliferative changes following subsequent removal of intact ovary.Science 108:137–138. 10.1126/science.108.2797.137. [DOI] [PubMed] [Google Scholar]
- 20.Björkholm E, Pettersson F, Einhorn N, Krebs I, Nilsson B, Tjernberg B.1982.Long-term follow-up and prognostic factors in ovarian carcinoma. The radiumhemmet series 1958 to 1973.Acta Radiol Oncol 21:413–419. 10.3109/02841868209134321. [DOI] [PubMed] [Google Scholar]
- 21.Blaakaer J, Baeksted M, Micic S, Albrectsen P, Rygaard J, Bock J.1995.Gonadotropin-releasing hormone agonist suppression of ovarian tumorigenesis in mice of the Wx/Wv genotype.Biol Reprod 53:775–779. 10.1095/biolreprod53.4.775. [DOI] [PubMed] [Google Scholar]
- 22.Bosetti C, Negri E, Trichopoulos D, Franceschi S, Beral V, Tzonou A, Parazzini F, Greggi S, La Vecchia C.2002.Long-term effects of oral contraceptives on ovarian cancer risk.Int J Cancer 102:262–265. 10.1002/ijc.10696. [DOI] [PubMed] [Google Scholar]
- 23.Bradaric MJ, Penumatsa K, Barua A, Edassery SL, Yu Y, Abramowicz JS, Bahr JM, Luborsky JL.2013.Immune cells in the normal ovary and spontaneous ovarian tumors in the laying hen (Gallus domesticus) model of human ovarian cancer.PLoS One 8:1–10. 10.1371/journal.pone.0074147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research Network. 2011. Integrated genomic analyses of ovarian carcinoma.Nature 474:609–615. 10.1038/nature10166. Erratum in: Nature 2012.490:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carver DK, Barnes HJ, Anderson KE, Petitte JN, Whitaker R, Berchuck A, Rodriguez GC.2011.Reduction of ovarian and oviductal cancers in calorie-restricted laying chickens.Cancer Prev Res (Phila) 4:562–567. 10.1158/1940-6207.CAPR-10-0294. [DOI] [PubMed] [Google Scholar]
- 26.Chadha S, Rao BR, Slotman BJ, van Vroonhoven CC, van der Kwast TH.1993.An immunohistochemical evaluation of androgen and progesterone receptors in ovarian tumors.Hum Pathol 24:90–95. 10.1016/0046-8177(93)90067-Q. [DOI] [PubMed] [Google Scholar]
- 27.Cho KR, Shih Ie M .2009. Ovarian cancer.Annu Rev Pathol 4:287–313. 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JW, Ahn SE, Rengaraj D, Seo HW, Lim W, Song G, Han JY.2011.Matrix metalloproteinase 3 is a stromal marker for chicken ovarian cancer.Oncol Lett 2:1047–1051. 10.3892/ol.2011.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi KC, Auersperg N, Leung PC.2001.Expression and antiproliferative effect of a second form of gonadotropin-releasing hormone in normal and neoplastic ovarian surface epithelial cells.J Clin Endocrinol Metab 86:5075–5078. 10.1210/jcem.86.10.8100. [DOI] [PubMed] [Google Scholar]
- 30.Choi PW, So WW, Yang J, Liu S, Tong KK, Kwan KM, Kwok JS, Tsui SKW, Ng SK, Hales KH, Hales DB, Welch WR, Crum CP, Fong WP, Berkowitz RS, Ng SW.2020.MicroRNA-200 family governs ovarian inclusion cyst formation and mode of ovarian cancer spread.Oncogene 39:4045–4060. 10.1038/s41388-020-1264-x. [DOI] [PubMed] [Google Scholar]
- 31.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G.2004.Tumor-infiltrating dendritic cell precursors recruited by a β-defensin contribute to vasculogenesis under the influence of Vegf-A.Nat Med 10:950–958. 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 32.Cramer DW, Hutchison GB, Welch WR, Scully RE, Ryan KJ.1983.Determinants of ovarian cancer risk. I. Reproductive experiences and family history.J Natl Cancer Inst 71:711–716. [PubMed] [Google Scholar]
- 33.Cramer DW, Welch WR.1983.Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis.J Natl Cancer Inst 71:717–721.PubMed [PubMed] [Google Scholar]
- 34.Cruickshank DJ.1990.Aetiological importance of ovulation in epithelial ovarian cancer: a population based study.BMJ 301:524–525. 10.1136/bmj.301.6751.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y.2007.The distal fallopian tube: a new model for pelvic serous carcinogenesis.Curr Opin Obstet Gynecol 19:3–9. 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 36.Cuatrecasas M, Erill N, Musulen E, Costa I, Matias-Guiu X, Prat J.1998.K-ras mutations in nonmucinous ovarian epithelial tumors: a molecular analysis and clinicopathologic study of 144 patients.Cancer 82:1088–1095.. [DOI] [PubMed] [Google Scholar]
- 37.Daikoku T, Tranguch S, Trofimova IN, Dinulescu DM, Jacks T, Nikitin AY, Connolly DC, Dey SK.2006.Cyclooxygenase-1 is overexpressed in multiple genetically engineered mouse models of epithelial ovarian cancer.Cancer Res 66:2527–2531. 10.1158/0008-5472.CAN-05-4063. [DOI] [PubMed] [Google Scholar]
- 38.Daikoku T, Wang D, Tranguch S, Morrow JD, Orsulic S, DuBois RN, Dey SK.2005.Cyclooxygenase-1 is a potential target for prevention and treatment of ovarian epithelial cancer.Cancer Res 65:3735–3744. 10.1158/0008-5472.CAN-04-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davison TF.2003.The immunologists’ debt to the chicken.Br Poult Sci 44:6–21. 10.1080/0007166031000085364. [DOI] [PubMed] [Google Scholar]
- 40.Denkert C, Köbel M, Pest S, Koch I, Berger S, Schwabe M, Siegert A, Reles A, Klosterhalfen B, Hauptmann S.2002.Expression of cyclooxygenase 2 is an independent prognostic factor in human ovarian carcinoma.Am J Pathol 160:893–903. 10.1016/S0002-9440(10)64912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dikshit A, Gomes Filho MA, Eilati E, McGee S, Small C, Gao C, Klug T, Hales DB.2015.Flaxseed reduces the pro-carcinogenic micro-environment in the ovaries of normal hens by altering the PG and oestrogen pathways in a dose-dependent manner.Br J Nutr 113:1384–1395. 10.1017/S000711451500029X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dikshit A, Hales K, Hales DB.2017.Whole flaxseed diet alters estrogen metabolism to promote 2-methoxtestradiol-induced apoptosis in hen ovarian cancer.J Nutr Biochem 42:117–125. 10.1016/j.jnutbio.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eilati E, Bahr JM, Hales DB.2013.Long term consumption of flaxseed enriched diet decreased ovarian cancer incidence and prostaglandin E(2)in hens.Gynecol Oncol 130:620–628. 10.1016/j.ygyno.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eilati E, Hales K, Zhuge Y, Ansenberger Fricano K, Yu R, van Breemen RB, Hales DB.2013.Flaxseed enriched diet-mediated reduction in ovarian cancer severity is correlated to the reduction of prostaglandin E(2) in laying hen ovaries.Prostaglandins Leukot Essent Fatty Acids 89:179–187. 10.1016/j.plefa.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eilati E, Pan L, Bahr JM, Hales DB.2012.Age dependent increase in prostaglandin pathway coincides with onset of ovarian cancer in laying hens.Prostaglandins Leukot Essent Fatty Acids 87:177–184. 10.1016/j.plefa.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eilati E, Small CC, McGee SR, Kurrey NK, Hales DB.2013.Anti-inflammatory effects of fish oil in ovaries of laying hens target prostaglandin pathways.Lipids Health Dis 12: 1–12. 10.1186/1476-511X-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elkin RG, Bauer R, Schneider WJ.2012.The restricted ovulator chicken strain: an oviparous vertebrate model of reproductive dysfunction caused by a gene defect affecting an oocyte-specific receptor.Anim Reprod Sci 136:1–13. 10.1016/j.anireprosci.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emons G, Ortmann O, Becker M, Irmer G, Springer B, Laun R, Holzel F, Schulz KD, Schally AV.1993.High affinity binding and direct antiproliferative effects of LHRH analogues in human ovarian cancer cell lines.Cancer Res 53:5439–5446. [PubMed] [Google Scholar]
- 49.Erdman SE, Poutahidis T.2010.Cancer inflammation and regulatory T cells.Int J Cancer 127:768–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erf GF.2004.Cell-mediated immunity in poultry.Poult Sci 83:580–590. 10.1093/ps/83.4.580. [DOI] [PubMed] [Google Scholar]
- 51.Fathalla MF.1971.Incessant ovulation—a factor in ovarian neoplasia? Lancet 298:163. 10.1016/S0140-6736(71)92335-X. [DOI] [PubMed] [Google Scholar]
- 52.Fathalla MF. 2013.Incessant ovulation and ovarian cancer—a hypothesis re-visited.Facts Views Vis Obgyn 5:292–297. PubMed [PMC free article] [PubMed] [Google Scholar]
- 53.Foster BA, Coffey HA, Morin MJ, Rastinejad F.1999.Pharmacological rescue of mutant p53 conformation and function.Science 286:2507–2510. 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- 54.Fredrickson TN.1987.Ovarian tumors of the hen.Environ Health Perspect 73:35–51. 10.1289/ehp.877335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freedman RS, Deavers M, Liu J, Wang E.2004. Peritoneal inflammation—a microenvironment for Epithelial Ovarian Cancer (EOC). J Transl Med 2: 1–10. 10.1186/1479-5876-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuller PJ, Zumpe ET, Chu S, Mamers P, Burger HG.2002.Inhibin-activin receptor subunit gene expression in ovarian tumors.J Clin Endocrinol Metab 87:1395–1401. 10.1210/jcem.87.3.8340. [DOI] [PubMed] [Google Scholar]
- 57.Fumihito A, Miyake T, Sumi S, Takada M, Ohno S, Kondo N.1994.One subspecies of the red junglefowl (Gallus gallus gallus) suffices as the matriarchic ancestor of all domestic breeds.Proc Natl Acad Sci USA 91:12505–12509. 10.1073/pnas.91.26.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gadducci A, Marrai R, Baicchi U, Gagetti O, Facchini V, Genazzani AR.1996.Prothrombin fragment F1 + 2 and thrombin-antithrombin III complex (TAT) plasma levels in patients with gynecological cancer.Gynecol Oncol 61:215–217. 10.1006/gyno.1996.0127. [DOI] [PubMed] [Google Scholar]
- 59.Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM.2006.n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits.Am J Clin Nutr 836 Suppl:1526S–1535S. 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- 60.Geiger B, Ayalon O.1992.Cadherins.Annu Rev Cell Biol 8:307–332. 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- 61.Giles JR, Elkin RG, Trevino LS, Urick ME, Ramachandran R, Johnson PA.2010.The restricted ovulator chicken: a unique animal model for investigating the etiology of ovarian cancer.Int J Gynecol Cancer 20:738–744. 10.1111/IGC.0b013e3181da2c49. [DOI] [PubMed] [Google Scholar]
- 62.Giles JR, Olson LM, Johnson PA.2006.Characterization of ovarian surface epithelial cells from the hen: a unique model for ovarian cancer.Exp Biol Med (Maywood) 231:1718–1725. 10.1177/153537020623101108. [DOI] [PubMed] [Google Scholar]
- 63.Giles JR, Shivaprasad HL, Johnson PA.2004.Ovarian tumor expression of an oviductal protein in the hen: a model for human serous ovarian adenocarcinoma.Gynecol Oncol 95:530–533. 10.1016/j.ygyno.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez Bosquet J, Peedicayil A, Maguire J, Chien J, Rodriguez GC, Whitaker R, Petitte JN, Anderson KE, Barnes HJ, Shridhar V, Cliby WA.2011.Comparison of gene expression patterns between avian and human ovarian cancers.Gynecol Oncol 120:256–264. 10.1016/j.ygyno.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 65.Gupta RA, Tejada LV, Tong BJ, Das SK, Morrow JD, Dey SK, DuBois RN.2003.Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer.Cancer Res 63:906–911. [PubMed] [Google Scholar]
- 66.Hakim AA, Barry CP, Barnes HJ, Anderson KE, Petitte J, Whitaker R, Lancaster JM, Wenham RM, Carver DK, Turbov J, Berchuck A, Kopelovich L, Rodriguez GC.2009.Ovarian adenocarcinomas in the laying hen and women share similar alterations in p53, ras, and HER-2/neu.Cancer Prev Res (Phila) 2:114–121. 10.1158/1940-6207.CAPR-08-0065. [DOI] [PubMed] [Google Scholar]
- 67.Hales DB, Zhuge Y, Lagman JA, Ansenberger K, Mahon C, Barua A, Luborsky JL, Bahr JM.2008.Cyclooxygenases expression and distribution in the normal ovary and their role in ovarian cancer in the domestic hen (Gallus domesticus).Endocrine 33:235–244. 10.1007/s12020-008-9080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hales KH, Speckman SC, Kurrey NK, Hales DB.2014.Uncovering molecular events associated with the chemosuppressive effects of flaxseed: a microarray analysis of the laying hen model of ovarian cancer.BMC Genomics 15: 1–14. 10.1186/1471-2164-15-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamilton TC, Davies P, Griffiths K.1981.Androgen and oestrogen binding in cytosols of human ovarian tumours.J Endocrinol 90:421–431. 10.1677/joe.0.0900421. [DOI] [PubMed] [Google Scholar]
- 70.Hankinson SE, Colditz GA, Hunter DJ, Willett WC, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE.1995.A prospective study of reproductive factors and risk of epithelial ovarian cancer.Cancer 76:284–290.. [DOI] [PubMed] [Google Scholar]
- 71.Haritani M, Kajigaya H, Akashi T, Kamemura M, Tanahara N, Umeda M, Sugiyama M, Isoda M, Kato C.1984.A study on the origin of adenocarcinoma in fowls using immunohistological technique.Avian Dis 28:1130–1134. 10.2307/1590291. [DOI] [PubMed] [Google Scholar]
- 72.Hasan N, Ohman AW, Dinulescu DM.2015.The promise and challenge of ovarian cancer models.Transl Cancer Res 4:14–28. 10.3978/j.issn.2218-676X.2015.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heinonen PK, Metsä-Ketelä T.1984.Prostaglandin and thromboxane production in ovarian cancer tissue.Gynecol Obstet Invest 18:225–229. 10.1159/000299085. [DOI] [PubMed] [Google Scholar]
- 74.Hillier SG, Anderson RA, Williams AR, Tetsuka M.1998.Expression of oestrogen receptor alpha and beta in cultured human ovarian surface epithelial cells.Mol Hum Reprod 4:811–815. 10.1093/molehr/4.8.811. [DOI] [PubMed] [Google Scholar]
- 75.Ho KJ, Lawrence WD, Lewis LA, Liu LB, Taylor CB.1974.Hereditary hyperlipidemia in nonlaying chickens.Arch Pathol 98:161–172. [PubMed] [Google Scholar]
- 76.Högberg T, Kågedal B.1993.Monitoring CA 125 serum levels during early chemotherapy is an excellent prognostic method in advanced ovarian cancer.Gynecol Oncol 48:413–414. [PubMed] [Google Scholar]
- 77.Huang KC, Park DC, Ng SK, Lee JY, Ni X, Ng WC, Bandera CA, Welch WR, Berkowitz RS, Mok SC, Ng SW.2006.Selenium binding protein 1 in ovarian cancer.Int J Cancer 118:2433–2440. 10.1002/ijc.21671. [DOI] [PubMed] [Google Scholar]
- 78.International Chicken Genome Sequencing Constortium. 2004.Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution.Nature 432:695–716. 10.1038/nature03154. Erratum in Nature 2005 433:777. [DOI] [PubMed] [Google Scholar]
- 79.Jackson E, Anderson K, Ashwell C, Petitte J, Mozdziak PE.2007.CA125 expression in spontaneous ovarian adenocarcinomas from laying hens.Gynecol Oncol 104:192–198. 10.1016/j.ygyno.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 80.Johnson PA, Giles JR.2006.Use of genetic strains of chickens in studies of ovarian cancer.Poult Sci 85:246–250. 10.1093/ps/85.2.246. [DOI] [PubMed] [Google Scholar]
- 81.Johnson PA, Giles JR.2013.The hen as a model of ovarian cancer.Nat Rev Cancer 13:432–436. 10.1038/nrc3535. [DOI] [PubMed] [Google Scholar]
- 82.Kaldawy A, Segev Y, Lavie O, Auslender R, Sopik V, Narod SA.2016.Low-grade serous ovarian cancer: A review.Gynecol Oncol 143:433–438. 10.1016/j.ygyno.2016.08.320. [DOI] [PubMed] [Google Scholar]
- 83.Kaneko SJ, Gerasimova T, Smith ST, Lloyd KO, Suzumori K, Young SR.2003.CA125 and UQCRFS1 fish studies of ovarian carcinoma.Gynecol Oncol 90:29–36. 10.1016/S0090-8258(03)00144-6. [DOI] [PubMed] [Google Scholar]
- 84.Kaufman J, Jacob J, Shaw I, Walker B, Milne S, Beck S, Salomonsen J.1999.Gene organisation determines evolution of function in the chicken MHC.Immunol Rev 167:101–117. 10.1111/j.1600-065X.1999.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 85.Kaufman J, Milne S, Gobel TW, Walker BA, Jacob JP, Auffray C, Zoorob R, Beck S.1999.The chicken B locus is a minimal essential major histocompatibility complex.Nature 401:923–925. 10.1038/44856. [DOI] [PubMed] [Google Scholar]
- 86.Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM.2012.High-grade serous ovarian cancer arises from fallopian tube in a mouse model.Proc Natl Acad Sci USA 109:3921–3926. 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kino Y, Kojima F, Kiguchi K, Igarashi R, Ishizuka B, Kawai S.2005.Prostaglandin E2 production in ovarian cancer cell lines is regulated by cyclooxygenase-1, not cyclooxygenase-2.Prostaglandins Leukot Essent Fatty Acids 73:103–111. 10.1016/j.plefa.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 88.Kurman RJ, Shih Ie M.2010.The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory.Am J Surg Pathol 34:433–443. 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurman RJ, Shih Ie M.2011.Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm.Hum Pathol 42:918–931. 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Labrada M, Clavell M, Bebelagua Y, Leon J, Alonso DF, Gabri MR, Veloso RC, Verez V, Fernandez LE.2010.Direct validation of NGcGM3 ganglioside as a new target for cancer immunotherapy.Expert Opin Biol Ther 10:153–162. 10.1517/14712590903443084. [DOI] [PubMed] [Google Scholar]
- 91.Lacey JV, Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C.2002.Menopausal hormone replacement therapy and risk of ovarian cancer.JAMA 288:334–341. 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 92.Lau KM, Mok SC, Ho SM.1999.Expression of human estrogen receptor-α and -β, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells.Proc Natl Acad Sci USA 96:5722–5727. 10.1073/pnas.96.10.5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee G, Ng HT.1995.Clinical evaluations of a new ovarian cancer marker, COX-1.Int J Gynaecol Obstet 49Suppl:S27–S32. 10.1016/0020-7292(95)02406-3. [DOI] [PubMed] [Google Scholar]
- 94.Lee JY, Jeong W, Lim W, Lim CH, Bae SM, Kim J, Bazer FW, Song G.2013.Hypermethylation and post-transcriptional regulation of DNA methyltransferases in the ovarian carcinomas of the laying hen.PLoS One 8:1–7. 10.1371/journal.pone.0061658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee P, Rosen DG, Zhu C, Silva EG, Liu J.2005.Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer.Gynecol Oncol 96:671–677. 10.1016/j.ygyno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 96.Letavayová L, Vlcková V, Brozmanová J.2006.Selenium: from cancer prevention to DNA damage.Toxicology 227:1–14. 10.1016/j.tox.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 97.Li CG, Nyman JE, Braithwaite AW, Eccles MR.2011.PAX8 promotes tumor cell growth by transcriptionally regulating E2F1 and stabilizing RB protein.Oncogene 30:4824–4834. 10.1038/onc.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li S, Miner K, Fannin R, Carl Barrett J, Davis BJ.2004.Cyclooxygenase-1 and 2 in normal and malignant human ovarian epithelium.Gynecol Oncol 92:622–627. 10.1016/j.ygyno.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 99.Liliac L, Carcangiu ML, Canevari S, Caruntu ID, Ciobanu Apostol DG, Danciu M, Onofriescu M, Amalinei C.2013.The value of PAX8 and WT1 molecules in ovarian cancer diagnosis.Rom J Morphol Embryol 54:17–27. [PubMed] [Google Scholar]
- 100.Lim CH, Lim W, Jeong W, Lee JY, Bae SM, Kim J, Han JY, Bazer FW, Song G.2013.Avian WNT4 in the female reproductive tracts: potential role of oviduct development and ovarian carcinogenesis.PLoS One 8:1–9. 10.1371/journal.pone.0065935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lim W, Jeong W, Kim J, Ka H, Bazer FW, Han JY, Song G.2012.Differential expression of secreted phosphoprotein 1 in response to estradiol-17β and in ovarian tumors in chickens.Biochem Biophys Res Commun 422:494–500. 10.1016/j.bbrc.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 102.Lim W, Jeong W, Kim J, Yoshimura Y, Bazer FW, Han JY, Song G.2013.Expression and regulation of beta-defensin 11 in the oviduct in response to estrogen and in ovarian tumors of chickens.Mol Cell Endocrinol 366:1–8. 10.1016/j.mce.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 103.Lim W, Kim HS, Jeong W, Ahn SE, Kim J, Kim YB, Kim MA, Kim MK, Chung HH, Song YS, Bazer FW, Han JY, Song G. 2012SERPINB3 in the chicken model of ovarian cancer: a prognostic factor for platinum resistance and survival in patients with epithelial ovarian cancer.PLoS One 7:1–10. 10.1371/journal.pone.0049869. Erratum 2013. PLoS One [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lim W, Kim JH, Ahn SE, Jeong W, Kim J, Bazer FW, Han JY, Song G.2012.Avian SERPINB11 gene: a marker for ovarian endometrioid cancer in chickens.Exp Biol Med (Maywood) 237:150–159. 10.1258/ebm.2011.011250. [DOI] [PubMed] [Google Scholar]
- 105.Lux-Lantos VA, Thyssen SM, Chamson A, Libertun C.1995.Effect of a gonadotropin releasing hormone analog on an experimental ovarian tumor: direct and indirect actions.Life Sci 57:291–300. 10.1016/0024-3205(95)00272-8. [DOI] [PubMed] [Google Scholar]
- 106.Ma Y, Sun Z, de Matos R, Zhang J, Odunsi K, Lin B.2014.Towards an animal model of ovarian cancer: cataloging chicken blood proteins using combinatorial peptide ligand libraries coupled with shotgun proteomic analysis for translational research.OMICS 18:280–297. 10.1089/omi.2013.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marchant J.1961.The effect of hypophysectomy on the development of ovarian tumours in mice treated with dimethylbenzanthracene.Br J Cancer 15:821–827. 10.1038/bjc.1961.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matias-Guiu X, Prat J.1998.Molecular pathology of ovarian carcinomas.Virchows Arch 433:103–111. 10.1007/s004280050224. [DOI] [PubMed] [Google Scholar]
- 109.McConechy MK, Anglesio MS, Kalloger SE, Yang W, Senz J, Chow C, Heravi-Moussavi A, Morin GB, Mes-Masson AM, Australian Ovarian Cancer Study Group, Carey MS, McAlpine JN, Kwon JS, Prentice LM, Boyd N, Shah SP, Gilks CB, Huntsman DG.2011.Subtype-specific mutation of PPP2R1A in endometrial and ovarian carcinomas.J Pathol 223:567–573. 10.1002/path.2848. [DOI] [PubMed] [Google Scholar]
- 110.Minegishi T, Kameda T, Hirakawa T, Abe K, Tano M, Ibuki Y.2000.Expression of gonadotropin and activin receptor messenger ribonucleic acid in human ovarian epithelial neoplasms.Clin Cancer Res 6:2764–2770. [PubMed] [Google Scholar]
- 111.Mittal KR, Zeleniuch-Jacquotte A, Cooper JL, Demopoulos RI.1993.Contralateral ovary in unilateral ovarian carcinoma: a search for preneoplastic lesions.Int J Gynecol Pathol 12:59–63. 10.1097/00004347-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 112.Mocka EH, Stern RA, Fletcher OJ, Anderson KE, Petitte JN, Mozdziak PE.2017.Chemoprevention of spontaneous ovarian cancer in the domestic hen.Poult Sci 96:1901–1909. 10.3382/ps/pew422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Modugno F, Ness RB, Allen GO, Schildkraut JM, Davis FG, Goodman MT.2004.Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis.Am J Obstet Gynecol 191:733–740. 10.1016/j.ajog.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 114.Murphy ED. 1972.Hyperplastic and early neoplastic changes in the ovaries of mice after genic deletion of germ cells.J Natl Cancer Inst 48:1283–1295. [PubMed] [Google Scholar]
- 115.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM.2000.Matrix metalloproteinases: biologic activity and clinical implications.J Clin Oncol 18:1135–1149. 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 116.Ness RB, Cottreau C.1999.Possible role of ovarian epithelial inflammation in ovarian cancer.J Natl Cancer Inst 91:1459–1467. 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 117.Nielsen JS, Jakobsen E, Holund B, Bertelsen K, Jakobsen A.2004.Prognostic significance of p53, Her-2, and EGFR overexpression in borderline and epithelial ovarian cancer.Int J Gynecol Cancer 14:1086–1096. 10.1111/j.1048-891X.2004.14606.x. [DOI] [PubMed] [Google Scholar]
- 118.O’Neill CJ, Deavers MT, Malpica A, Foster H, McCluggage WG.2005.An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms.Am J Surg Pathol 29:1034–1041. [PubMed] [Google Scholar]
- 119.Okamura H, Katabuchi H.2001.Detailed morphology of human ovarian surface epithelium focusing on its metaplastic and neoplastic capability.Ital J Anat Embryol 1062 Suppl 2:263–276. [PubMed] [Google Scholar]
- 120.Oliva JP, Valdés Z, Casacó A, Pimentel G, Gonzalez J, Alvarez I, Osorio M, Velazco M, Figueroa M, Ortiz R, Escobar X, Orozco M, Cruz J, Franco S, Diaz M, Roque L, Carr A, Vazquez AM, Mateos C, Rubio MC, Perez R, Fernández LE.2006.Clinical evidences of GM3 (NeuGc) ganglioside expression in human breast cancer using the 14F7 monoclonal antibody labelled with (99m)Tc.Breast Cancer Res Treat 96:115–121. 10.1007/s10549-005-9064-0. [DOI] [PubMed] [Google Scholar]
- 121.Olivier M, Hollstein M, Hainaut P.2010.TP53 mutations in human cancers: origins, consequences, and clinical use.Cold Spring Harb Perspect Biol 2:1–17. 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ose J, Schock H, Tjønneland A, Hansen L, Overvad K, Dossus L, Clavel-Chapelon F, Baglietto L, Boeing H, Trichopolou A, Benetou V, Lagiou P, Masala G, Tagliabue G, Tumino R, Sacerdote C, Mattiello A, As Bueno-de-Mesquita H B, Peeters P H M, Onland-Moret NC, Weiderpass E, Gram IT, Sánchez S, Obon-Santacana M, Sànchez-Pérez M-J, Larrañaga N, Huerta Castaño JM, Ardanaz E, Brändstedt J, Lundin E, Idahl A, Travis RC, Khaw K-T, Rinaldi S, Romieu I, Merritt MA, Gunter MJ, Riboli E, Kaaks R, Fortner RT.2015.Inflammatory markers and risk of epithelial ovarian cancer by tumor subtypes: The epic cohort.Cancer Epidemiol Biomarkers Prev 24:951–961. 10.1158/1055-9965.EPI-14-1279-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pal P, Hales K, Petrik J, Hales DB.2019.Pro-apoptotic and anti-angiogenic actions of 2-methoxyestradiol and docosahexaenoic acid, the biologically derived active compounds from flaxseed diet, in preventing ovarian cancer.J Ovarian Res 12: 1–16. 10.1186/s13048-019-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parada AC, Palomo AG, Hales DB, Hales K, Fernandez LE.2017.Detection and Characterization by immunohistochemistry of the NGcGM3 ganglioside in the chicken animal model of ovarian cancer.Journal of Oncology Translational Research 3:1–3. 10.4172/2476-2261.1000123 [DOI] [Google Scholar]
- 125.Pearce CL, Stram DO, Ness RB, Stram DA, Roman LD, Templeman C, Lee AW, Menon U, Fasching PA, McAlpine JN, Doherty JA, Modugno F, Schildkraut JM, Rossing MA, Huntsman DG, Wu AH, Berchuck A, Pike MC, Pharoah PDP.2015.Population distribution of lifetime risk of ovarian cancer in the United States.Cancer Epidemiol Biomarkers Prev 24:671–676. 10.1158/1055-9965.EPI-14-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Potashnik G, Insler V, Meizner I, Sternberg M.1987.Frequency, sequence, and side of ovulation in women menstruating normally.Br Med J (Clin Res Ed) 294:219–219. 10.1136/bmj.294.6566.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prat J.2012.New insights into ovarian cancer pathology.Ann Oncol 23Suppl 10:x111–x117. 10.1093/annonc/mds300. [DOI] [PubMed] [Google Scholar]
- 128.Pujol P, Rey JM, Nirde P, Roger P, Gastaldi M, Laffargue F, Rochefort H, Maudelonde T.1998.Differential expression of estrogen receptor-α and -β messenger RNAs as a potential marker of ovarian carcinogenesis.Cancer Res 58:5367–5373. [PubMed] [Google Scholar]
- 129.Radisavljevic SV.1977.The pathogenesis of ovarian inclusion cysts and cystomas.Obstet Gynecol 49:424–429. [PubMed] [Google Scholar]
- 130.Ratcliffe MJH.2006.Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development.Dev Comp Immunol 30:101–118. 10.1016/j.dci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 131.Resta L, Russo S, Colucci GA, Prat J.1993.Morphologic precursors of ovarian epithelial tumors.Obstet Gynecol 82:181–186. [PubMed] [Google Scholar]
- 132.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, Weiderpass E, Persson IR.2002.Hormone replacement therapy and the risk of invasive epithelial ovarian cancer in Swedish women.J Natl Cancer Inst 94:497–504. 10.1093/jnci/94.7.497. [DOI] [PubMed] [Google Scholar]
- 133.Risch HA.1998.Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone.J Natl Cancer Inst 90:1774–1786. 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 134.Risch HA, Marrett LD, Jain M, Howe GR.1996.Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study.Am J Epidemiol 144:363–372. 10.1093/oxfordjournals.aje.a008937. [DOI] [PubMed] [Google Scholar]
- 135.Rodriguez-Burford C, Barnes MN, Berry W, Partridge EE, Grizzle WE.2001.Immunohistochemical expression of molecular markers in an avian model: a potential model for preclinical evaluation of agents for ovarian cancer chemoprevention.Gynecol Oncol 81:373–379. 10.1006/gyno.2001.6191. [DOI] [PubMed] [Google Scholar]
- 136.Rodriguez GC, Barnes HJ, Anderson KE, Whitaker RS, Berchuck A, Petitte JN, Lancaster JM, Wenham RM, Turbov JM, Day R, Maxwell GL, Carver DK.2013.Evidence of a chemopreventive effect of progestin unrelated to ovulation on reproductive tract cancers in the egg-laying hen.Cancer Prev Res (Phila) 6:1283–1292. 10.1158/1940-6207.CAPR-12-0426. [DOI] [PubMed] [Google Scholar]
- 137.Rosales-Nieves AE, González-Reyes A.2014.Genetics and mechanisms of ovarian cancer: parallels between Drosophila and humans.Semin Cell Dev Biol 28:104–109. 10.1016/j.semcdb.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 138.Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG.1994.Ovarian tumors in a cohort of infertile women.N Engl J Med 331:771–776. 10.1056/NEJM199409223311204. [DOI] [PubMed] [Google Scholar]
- 139.Salazar H, Godwin AK, Daly MB, Laub PB, Hogan WM, Rosenblum N, Boente MP, Lynch HT, Hamilton TC.1996.Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies.J Natl Cancer Inst 88:1810–1820. 10.1093/jnci/88.24.1810. [DOI] [PubMed] [Google Scholar]
- 140.Saleemuddin A, Folkins AK, Garrett L, Garber J, Muto MG, Crum CP, Tworoger S.2008.Risk factors for a serous cancer precursor (“p53 signature”) in women with inherited BRCA mutations.Gynecol Oncol 111:226–232. 10.1016/j.ygyno.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sallum LF, Andrade L, Ramalho S, Ferracini AC, de Andrade Natal R, Brito ABC, Sarian LO, Derchain S.2018.WT1, p53 and p16 expression in the diagnosis of low- and high-grade serous ovarian carcinomas and their relation to prognosis.Oncotarget 9:15818–15827. 10.18632/oncotarget.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salvador S, Gilks B, Köbel M, Huntsman D, Rosen B, Miller D.2009.The fallopian tube: primary site of most pelvic high-grade serous carcinomas.Int J Gynecol Cancer 19:58–64. 10.1111/IGC.0b013e318199009c. [DOI] [PubMed] [Google Scholar]
- 143.Sasaroli D, Gimotty PA, Pathak HB, Hammond R, Kougioumtzidou E, Katsaros D, Buckanovich R, Devarajan K, Sandaltzopoulos R, Godwin AK, Scholler N, Coukos G.2011.Novel surface targets and serum biomarkers from the ovarian cancer vasculature.Cancer Biol Ther 12:169–180. 10.4161/cbt.12.3.16260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A, Kenny HA, Peter ME, Ramakrishnan V, Yamada SD, Lengyel E.2008.Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target.Cancer Res 68:2329–2339. 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Seo HW, Rengaraj D, Choi JW, Ahn SE, Song YS, Song G, Han JY.2010.Claudin 10 is a glandular epithelial marker in the chicken model as human epithelial ovarian cancer.Int J Gynecol Cancer 20:1465–1473. [PubMed] [Google Scholar]
- 146.Shi B, Bao J, Liu Y, Shi J.2018.Death receptor 6 promotes ovarian cancer cell migration through KIF11.FEBS Open Bio 8:1497–1507. 10.1002/2211-5463.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Smith WL.2005.Cyclooxygenases, peroxide tone and the allure of fish oil.Curr Opin Cell Biol 17:174–182. 10.1016/j.ceb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 148.Sorbe B, Frankendal B, Veress B.1982.Importance of histologic grading in the prognosis of epithelial ovarian carcinoma.Obstet Gynecol 59:576–582. [PubMed] [Google Scholar]
- 149.Stammer K, Edassery SL, Barua A, Bitterman P, Bahr JM, Hales DB, Luborsky JL.2008.Selenium-Binding Protein 1 expression in ovaries and ovarian tumors in the laying hen, a spontaneous model of human ovarian cancer.Gynecol Oncol 109:115–121. 10.1016/j.ygyno.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Steller MD, Shaw TJ, Vanderhyden BC, Ethier JF.2005.Inhibin resistance is associated with aggressive tumorigenicity of ovarian cancer cells.Mol Cancer Res 3:50–61. [PubMed] [Google Scholar]
- 151.Tang X, Zhu Y, Han L, Kim AL, Kopelovich L, Bickers DR, Athar M.2007.CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice.J Clin Invest 117:3753–3764. 10.1172/JCI32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Terada N, Kitamura Y, Namiki M, Kuroda H, Ito M, Takatsuka D, Matsumoto K.1984.Production of androgens and estrogens by tubular adenomas which developed in ovaries of mutant mice of Sl/Slt genotype.Cancer Res 44:1827–1830. [PubMed] [Google Scholar]
- 153.Tiwari A, Hadley JA, Hendricks GL, 3rd, Elkin RG, Cooper T, Ramachandran R.2013.Characterization of ascites-derived ovarian tumor cells from spontaneously occurring ovarian tumors of the chicken: evidence for E-cadherin upregulation.PLoS One 8:1–18. 10.1371/journal.pone.0057582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL.2018.Ovarian cancer statistics, 2018.CA Cancer J Clin 68:284–296. 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Torres MP, Ponnusamy MP, Lakshmanan I, Batra SK.2009.Immunopathogenesis of ovarian cancer.Minerva Med 100:385–400. [PubMed] [Google Scholar]
- 156.Treviño LS, Buckles EL, Johnson PA.2011.Oral contraceptives decrease the prevalence of ovarian cancer in the hen.Cancer Prev Res (Phila) 5:343–349. 10.1158/1940-6207.CAPR-11-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Treviño LS, Giles JR, Wang W, Urick ME, Johnson PA.2010.Gene expression profiling reveals differentially expressed genes in ovarian cancer of the hen: support for oviductal origin? Horm Cancer 1:177–186. 10.1007/s12672-010-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tung KH, Wilkens LR, Wu AH, McDuffie K, Nomura AM, Kolonel LN, Terada KY, Goodman MT.2005.Effect of anovulation factors on pre- and postmenopausal ovarian cancer risk: revisiting the incessant ovulation hypothesis.Am J Epidemiol 161:321–329. 10.1093/aje/kwi046. [DOI] [PubMed] [Google Scholar]
- 159.Uloza V, Kuzminienė A, Palubinskienė J, Balnytė I, Ulozienė I, Valančiūtė A.2017.Model of human recurrent respiratory papilloma on chicken embryo chorioallantoic membrane for tumor angiogenesis research.Histol Histopathol 32:699–710. [DOI] [PubMed] [Google Scholar]
- 160.Urick ME, Giles JR, Johnson PA.2008.VEGF expression and the effect of NSAIDs on ascites cell proliferation in the hen model of ovarian cancer.Gynecol Oncol 110:418–424. 10.1016/j.ygyno.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 161.Urick ME, Giles JR, Johnson PA.2009.Dietary aspirin decreases the stage of ovarian cancer in the hen.Gynecol Oncol 112:166–170. 10.1016/j.ygyno.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 162.Urick ME, Johnson PA.2006.Cyclooxygenase 1 and 2 mRNA and protein expression in the Gallus domesticus model of ovarian cancer.Gynecol Oncol 103:673–678. 10.1016/j.ygyno.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 163.Whittmore AS, Harris R, Itnyre J.1992.Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group.Am J Epidemiol 136:1184–1203. 10.1093/oxfordjournals.aje.a116427. [DOI] [PubMed] [Google Scholar]
- 164.Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A,Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group. 2007. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol Oncol 106:69–74. 10.1016/j.ygyno.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 165.Wischhusen J, Naumann U, Ohgaki H, Rastinejad F, Weller M.2003.CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death.Oncogene 22:8233–8245. 10.1038/sj.onc.1207198. [DOI] [PubMed] [Google Scholar]
- 166.Wong KK, Lu KH, Malpica A, Bodurka DC, Shvartsman HS, Schmandt RE, Thornton AD, Deavers MT, Silva EG, Gershenson DM.2007.Significantly greater expression of ER, PR, and ECAD in advanced-stage low-grade ovarian serous carcinoma as revealed by immunohistochemical analysis.Int J Gynecol Pathol 26:404–409. 10.1097/pgp.0b013e31803025cd. [DOI] [PubMed] [Google Scholar]
- 167.Yakushiji M, Imamura M, Masumoto R, Kato T.1981.Development of experimental tumors in the ovary of a rat after transplantation into spleen.Kurume Med J 28:45–52. 10.2739/kurumemedj.28.45. [DOI] [PubMed] [Google Scholar]
- 168.Yang HP, Murphy KR, Pfeiffer RM, George N, Garcia-Closas M, Lissowska J, Brinton LA, Wentzensen N.2016.Lifetime number of ovulatory cycles and risks of ovarian and endometrial cancer among postmenopausal women.Am J Epidemiol 183:800–814. 10.1093/aje/kwv308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang WL, Roland IH, Godwin AK, Xu XX.2005.Loss of TNF-α-regulated COX-2 expression in ovarian cancer cells.Oncogene 24:7991–8002. 10.1038/sj.onc.1208943. [DOI] [PubMed] [Google Scholar]
- 170.Ylikorkala O, Kauppila A, Viinikka L.1983.Prostacyclin and thromboxane in ovarian cancer: effect of cytostatics and prostaglandin synthesis inhibitors.Gynecol Oncol 16:340–345. 10.1016/0090-8258(83)90160-9. [DOI] [PubMed] [Google Scholar]
- 171.Young T, Mei F, Liu J, Bast RC, Jr, Kurosky A, Cheng X.2005.Proteomics analysis of H-RAS-mediated oncogenic transformation in a genetically defined human ovarian cancer model.Oncogene 24:6174–6184. 10.1038/sj.onc.1208753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zeng B, Zhou M, Wu H, Xiong Z.2018.SPP1 promotes ovarian cancer progression via Integrin beta1/FAK/AKT signaling pathway.Onco Targets Ther 11:1333–1343. 10.2147/OTT.S154215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang J, Zhang P, Shen Y, Yang M, Zou H, Liu H.2018.Relationship of WNT4 gene with the risk of epithelial ovarian cancer: A Han Chinese population-based association study.Genet Test Mol Biomarkers 22:686–692. 10.1089/gtmb.2018.0157. [DOI] [PubMed] [Google Scholar]
- 174.Zhang Z, Bast RC, Jr, Yu Y, Li J, Sokoll LJ, Rai AJ, Rosenzweig JM, Cameron B, Wang YY, Meng XY, Berchuck A, Van Haaften-Day C, Hacker NF, de Bruijn HW, van der Zee AG, Jacobs IJ, Fung ET, Chan DW.2004.Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer.Cancer Res 64:5882–5890. 10.1158/0008-5472.CAN-04-0746. [DOI] [PubMed] [Google Scholar]
- 175.Zhuge Y, Lagman JA, Ansenberger K, Mahon CJ, Daikoku T, Dey SK, Bahr JM, Hales DB.2009.CYP1B1 expression in ovarian cancer in the laying hen Gallus domesticus.Gynecol Oncol 112:171–178. 10.1016/j.ygyno.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]