Abstract
A small colony of zebrafish (Danio rerio) experienced 30% acute mortality within a few days after receipt from a commercial source. A few fish presented with small areas of raised scales or tissue necrosis, primarily near the caudal peduncle. Edwardsiella ictaluri (E. ictaluri) was identified by real-time PCR of pooled zebrafish and swabs of the pre-filter and fine filter pads, with subsequent sequence analysis. E. ictaluri is most commonly associated with an enteric septicemia in catfish species and can have significant economic impact on commercial catfish fisheries. However, several references report naturally occurring E. ictaluri infection of nonictalurid fishes, including zebrafish. Ours is the first report demonstrating the use of environmental sampling to identify E. ictaluri in a zebrafish colony by real-time PCR. Moreover, our report indicates that E. ictaluri is a relevant disease for institutions using zebrafish as research species and emphasizes the importance of carefully considering importation and quarantine practices.
Abbreviations: ESC, enteric septicemia of catfish
Edwardsiella ictaluri (E. ictaluri) is a gram-negative facultative intracellular bacterium, known primarily for its economic impact in catfish (Ictalurus spp.) aquaculture in the United States. E. ictaluri is the causative agent for Enteric Septicemia of Catfish (ESC), or Hole-in-the-Head disease of catfish, and is one of the most commonly reported diseases by US catfish producers.6,17,22,25 The significant economic impact of ESC has driven ongoing research and development of various vaccines administered through immersion and feeding.17,22,39 Disease transmission among fish occurs by direct contact through the fecal-oral route, nasal passages, and gills.6,12,17 In catfish, E. ictaluri infection can present as areas of hemorrhage around the base of fins, skin ulceration in various locations, bulging eyes, and a distended abdomen, with mortality of 10 to 50% in populations of pond-raised channel catfish (Ictalurus punctatus).6,12
Nonictalurid fish that are susceptible to spontaneous infection are phylogenetically diverse. These species of fish include: Ayu (Plecoglossus altevelis),34 Bengal danios (Devario devario),38 green knifefish (Eigemannia virescens),16 a red-bellied piranha (Pygocentris nattereri),19 Nile tilapia (Oreochromis niloticus),37 and hybrid red tilapia (Oreochromis sp.).7 Naturally occurring epizootics have been reported in 3 laboratory zebrafish colonies,12 and since 2013 IDEXX BioAnalytics has identified E. ictaluri as the cause of morbidity and mortality in zebrafish colonies from 6 institutions. Clinical presentation of edwardsiellosis caused by E. ictaluri in zebrafish can include tissue necrosis, abdominal distention, general lethargy, raised scales, and skin hemorrhage, although acute mortality without clinical signs is also common.12,26 The disease is generally systemic. A number of organs can be affected including the kidney, spleen, and brain with large quantities of bacteria present, often located within macrophages. 12
Experimental E. ictaluri infections have also been described in many nonictalurid hosts such as rainbow trout (Oncorhynchus mykiss), Chinook salmon (Oncorhynchus tshawytscha),3 and blue tilapia (Oreochromis aureus).28 Zebrafish have been used as an experimental model for ESC.14,26,33,36
This article describes an outbreak of Edwardsiella ictaluri in zebrafish purchased for use in undergraduate studies. The diagnosis was based on clinical signs, identification of E. ictaluri by real-time PCR in both clinically diseased fish and environmental samples from the tank filter, and sequence analysis. To our knowledge, this is the first report demonstrating the use of environmental sampling to identify Edwardsiella ictaluri in a colony of zebrafish.
Case Report
An undergraduate laboratory received 24 zebrafish from a commercial vendor in February 2020 and introduced them as a single group into a single unpopulated static 75.7 L glass aquarium. One fish was found dead 2 d after arrival; the death was attributed to shipping and transport stress. However, by 6 d after arrival, 5 fish were observed to have raised scales and small areas of tissue necrosis, mainly near the caudal peduncle. These fish were subsequently isolated into a separate quarantine tank. The fish in the quarantine tank (Batch A) were found dead the next morning and were stored in a −20°C freezer, along with 2 additional fish that were found dead in the primary tank. Batch A were submitted to IDEXX BioAnalytics (Columbia, MO) as a pooled sample for real-time PCR testing for a panel of infectious agents. At that time, the remaining fish did not show any apparent abnormal behaviors or exhibit any clinical signs. However, 4 more zebrafish were found dead over the following week. On 19 d after arrival, the remaining 12 fish were euthanized; none of them showed abnormal behaviors or physical clinical signs at euthanasia. DLAR obtained 4 of these zebrafish; 2 of them (Batch B) were submitted to IDEXX BioAnalytics (Columbia, MO) for microbiologic culture and real-time PCR analysis, along with swabs of the feed and the pre-filter and fine filter pads. The other 2 zebrafish (Batch C) were submitted to the DLAR in house diagnostic laboratory for necropsy and histologic evaluation.
After euthanasia of this group of fish, the tank and related equipment were sanitized, and a new group of zebrafish was obtained from another investigator at our institution as replacements. These fish showed no signs of disease throughout the study and were euthanized after laboratory use at the end of the semester as originally planned.
Materials and Methods
Animals.
Upon receipt, male zebrafish (n = 24) were placed into a single 75.7 L (20-gallon) static glass aquarium with a gravel floor substrate. The water source was municipal city water treated by reverse osmosis and deionization and then adjusted to appropriate salinity and conductivity with 60 mg/L of Instant Ocean (Blacksburg, VA). Fish were maintained on a 14:10 light:dark cycle with 10% to 15% water exchanged weekly. Water temperature and pH were monitored daily, with ammonia, nitrate, and nitrite levels monitored on a weekly basis. Accepted ranges for those parameters were 26 to 30 °C, 7.2 to 7.4, less than 0.02 mg/L, less than 35 mg/L, and less than 0.2 mg/L respectively. The aquarium was equipped with air stone aeration and a canister filter (EHIEM Aquatics Group, Germany) with media to provide adequate surface area for nitrifying bacteria performing biologic filtration. Fish were fed dry fish flakes (Tetra, Blacksburg VA) once per day.
The affected zebrafish were purchased from a commercial vendor that provides biological supplies to high school and undergraduate classes and sells zebrafish to research laboratories. Zebrafish were housed in a classroom setting, as they were approved for use in teaching and research laboratories in an undergraduate biology class. A total of 4 tanks were used in the classroom, and tank populations were separated by source and day of arrival. The colonies were intended for short-term use, as the studies on this protocol were initiated and completed within a single semester by the biology students. All procedures were performed in accordance with the guidelines set forth by the Guide for the Care, and Use of Laboratory Animals,15 All animal use at our facility is covered by protocols approved by the Duke University Medical Center (DUMC) Institutional Animal Care and Use Committee (IACUC). DUMC is fully AAALAC-accredited and maintains a Public Health Service Animal Welfare Assurance.
Necropsy and Histopathology.
Live zebrafish were euthanized just prior to necropsy by immersion in MS-222 (Tricaine methanosulfonate, 500 mg/kg in the water, Syndel, Fernandale, WA) buffered to a pH of 7.0-7.5 in accordance with the American Veterinary Medical Association 2020 euthanasia guidelines18 and Duke IACUC euthanasia policies. Two fish (Batch C) were submitted to the Duke Division of Laboratory Animal Resources (DLAR) for assessment. A gross necropsy was performed on 2 of the zebrafish; this included internal and external examination of fish and collection of gill and fin clippings for wet mount preparations. Gill arches and lamellae were snipped on one side of the fish using iris tenotomy scissors. Samples were placed on a clean histology slide and covered with a coverslip. Slides were examined under light microscopy for the presence of bacteria, fungi, protozoa and other parasites.
For the wet mount, a drop of fish tank water was placed on a clean histology slide. The skin behind the fins, on the caudal peduncle and under the mandible were gently scraped with a scalpel blade and sample placed on the slide. A coverslip was added, and the slide was examined under light microscopy for the presence of bacteria, fungi, protozoa and other parasites. A similar technique was used for wet mount preparation of the fins.
For fixed tissue, a lengthwise incision was made through the body wall from the anal fin along the belly to the gill chamber, exposing the coelomic cavity and viscera to fixation. Zebrafish were fixed in 10% buffered formalin (VWR International, PA) for 3 d, then cut in half longitudinally and embedded in paraffin as 2 whole body longitudinal sections. Serial, 5 µm thick whole body longitudinal sections were performed 150 µm apart and stained with hematoxylin and eosin for microscopic evaluation by a veterinary pathologist.
Microbiologic analysis.
Skin and trunk kidney were cultured from euthanized zebrafish (Batch B) at IDEXX BioAnalytics and streaked for culture and isolation onto BBL Trypticase Soy Agar with 5% sheep blood (TSA II; Becton Dickinson), BBL Chocolate II Agar (Becton Dickinson), BBL Hektoen Enteric Agar (Becton Dickinson), and Tryptone Yeast Extract Salts (TYES) Agar, which was prepared inhouse according to a published formulation.13 Cultures were incubated aerobically at 22 °C for 5 to 7 d. Representative bacterial colonies were harvested for proteomic analysis using a previously described direct transfer method.27 Bacteria were overlaid with 1 μL of a saturated solution of α-cyano-4-hydroxycinnamic acid in 50% acetonitrile, and 2.5% trifluoroacetic acid (Matrix HCCA, Bruker Daltronics, Billerica, MA). The matrix was subsequently allowed to dry, and bacteria were analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using a mass spectrometer (Microflex, Bruker Daltronics) and flexControl software (Bruker Daltronics). Bacterial identification was based on automated analysis by MALDI BioTyper software (Bruker Daltronics), which compared the spectra for each isolate with an integrated reference spectral database.
Molecular analysis.
Total nucleic acids were extracted from 2 pools of whole zebrafish (Batch A and Batch B) and separately for filter swabs and feed samples for real-time PCR analysis to detect a wide array of infectious agents including Edwardsiella ictaluri, Flavobacterium columnare, Ichthyophthirius multifiliis, Infectious spleen and kidney necrosis virus (ISKNV), Mycobacterium abscessus, M. chelonae, M. fortuitum, M. gordonae, M. haemophilum, M. marinum, M. peregrinum, M. saopaulense, Myxidium streisingeri, Piscinoodinium pillulare, Pleistophora hyphessobryconis, Pseudocapillaria tomentosa, Pseudoloma neurophilia, and a recently described virus, Zebrafish picornavirus.1
Batch A comprised 5 frozen fish from the acute, early mortalities, and Batch B included 2 fish from the final colony cull. The canister filter of the original tank was opened to allow access to both the prefilter and fine filter pads, which were swabbed and and analyzed separately. The interior of the feed bottle was also swabbed. Nucleic acid extractions were performed using a commercially available platform according to the manufacturer’s protocol (NucleoMag VET Kit; Macherey-Nagel GmbH and KG, Düren, Germany). The Tetro cDNA Synthesis Kit (Bioline, London, United Kingdom) was used to synthesize cDNA. Real-time PCR assay hydrolysis probe and primers were designed using PrimerExpress version 3.0 software (Applied BioAnalytics; Waltham, MA) based on the IDEXX Laboratories proprietary service platform using the genome sequences available in GenBank. Analysis was performed at IDEXX BioAnalytics (Columbia, MO) using standard primer and probe concentrations and the master mix LightCycler 480 Probes Master (Roche Applied Science, Indianapolis, IN) in a commercially available instrument (LightCycler 480; Roche Applied Science). Real-time PCR assay-specific positive and negative controls were included in all runs of the assays. Hydrolysis-probe–based real-time PCR assays targeting a eukaryotic gene (18S rRNA) or bacterial gene (16S rRNA) were used to ensure nucleic acid recovery and the absence of PCR inhibition. Positive real-time PCR results were confirmed by sequence analysis of the amplicon. Sequence analysis was also performed using previously published Edwardsiella sequencing primers and cycling conditions.9 Primers targeting DNA gyrase subunit A (gyrA) were GyrAF (5′-AGCGCCTTGTACTCATCCAG-3′) and GyrAR (5′-TGGTGCATGAGATCCCCTAT-3′), and primers targeting DNA gyrase subunit B (gyrB) were GyrBF (5′-CCCTGTCTGAAAAGCTGGAG-3′) and GyrBR (5′-CTCGTTCATCAGCGACTCAA-3′).9 The resulting amplicons were sequenced in both directions using Sanger methodology (GENEWIZ, South Plainfield, NJ), assembled into contigs using Sequencher software (Gene Codes Corporation), and compared with GenBank sequences using BLAST software (National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/).
Results
Necropsy findings.
Gill, skin, and fin clippings from the 2 zebrafish submitted to DLAR from the final cull (Batch C) were examined by wet mount and were negative for bacteria, fungi, protozoa, and other external parasites. External examination of Batch B and Batch C fish revealed a small, irregular, 1 to 2 mm diameter, slightly gelatinous and hemorrhagic area at the base of the tail and rare small hemorrhages caudal to the operculum (Figure 1). The remainder of the gross necropsy observations were unremarkable.
Figure 1.

Gross image of a zebrafish displaying a small, irregular, 1-2 mm diameter, slightly gelatinous and hemorrhagic area at the base of the tail (black arrow) and rare small hemorrhages caudal to the operculum (white arrow).
Histopathology.
Two zebrafish from Batch C were examined histologically. No evidence of edwardsiellosis or other significant lesions was observed, except that several digenetic trematode metacercaria were encysted in the muscles and other tissues, including the esophagus, the base of the heart, and tail, with various levels of inflammatory infiltrate composed of macrophages and lymphocytes (Figure 2).
Figure 2.
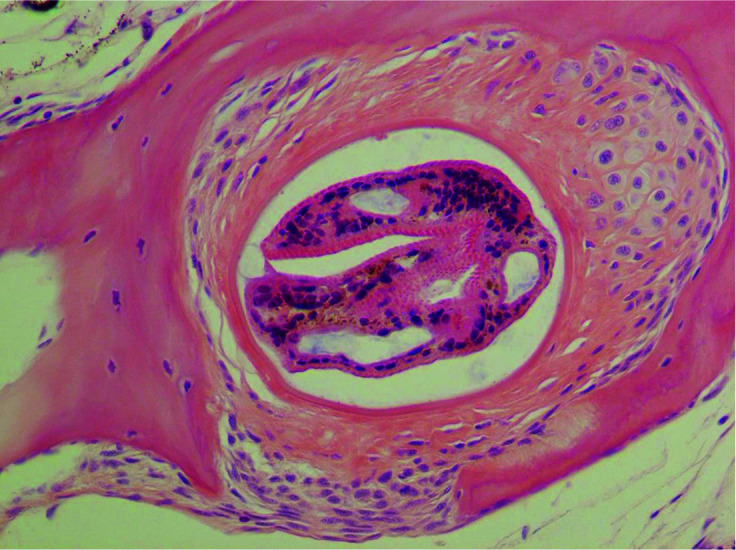
Photomicrograph of encysted metacercaria in zebrafish tissue.
Molecular analysis and microbiology (Table 1).
Table 1.
Molecular analysis
| Sample | Date | E ictaluri PCR result |
|---|---|---|
| Batch A (5 fish) | February 25 | + |
| Pre-Filter pad | March 23 | + |
| Fine Filter pad | March 23 | + |
| Feed | March 23 | — |
| Batch B (2 fish) | March 23 | — |
The pooled Batch A zebrafish sample tested strongly positive for E. ictaluri by real-time PCR, and the positive result was confirmed by sequence analysis of the amplicon. Other than the positive E. ictaluri result, the Batch A samples were negative for all the other infectious agents included in the test panel. The second set of zebrafish (Batch B) was negative for E. ictaluri by microbial culture. Both the Batch B zebrafish and feed samples were also negative for E. ictaluri by real-time PCR; however, the filter swabs tested positive for E. ictaluri by real-time PCR (assays performed by IDEXX Laboratories are validated to detect 10 or fewer template copies per reaction). Additional confirmation was obtained by Edwardsiella-specific conventional PCR and sequence analysis of 2 genes: gyrA and gyrB. Sequence analysis of gyrA from the pooled Batch A zebrafish sample displayed 100% identity to E. ictaluri over 593 bases. Similarly, sequence analysis of gyrB from the pooled Batch A zebrafish sample displayed 100% identity to E. ictaluri over 615 bases. Edwardsiella-specific sequence analysis of gyrB from the filter swabs displayed 100% identity to E. ictaluri over 623 bases. Sequence analysis of gyrA from the filter swabs was unsuccessful.
Discussion
Zebrafish are the most common finfish used in the research setting and are susceptible to infection with E. ictaluri. Here, we report an outbreak of E. ictaluri in an aquarium housing a shipment of 24 zebrafish purchased from a company that supplies biologic materials and organisms primarily to high school and undergraduate classrooms for teaching purposes, as well as to research institutions. Biologic supply companies have been described as high-risk vendors for laboratory zebrafish along with wholesalers, pet stores, and multispecies aquaculture facilities that do not practice colony health monitoring and lack adequate biosecurity practices.4 A surprising number of investigators continue to use zebrafish from high-risk vendors for biomedical research, even though several reports link high-risk vendors to introduction of zebrafish pathogens.2,12,29,35
The educational use and management of the zebrafish described in this report is unique for our institution. The fish were purchased for short-term use by undergraduate biology students during a one-semester class. The fish were not being bred, and the shipments of fish were managed with an “all-in all-out” approach. The allocated space for aquaria was limited to four 75.7 L tanks located on a classroom table, and the total zebrafish population was generally less than 100 fish. Although new batches of zebrafish did undergo basic quarantine and acclimation after receipt, they did not undergo the standard, more rigorous quarantine practices used for our large, long-term, zebrafish colonies. Fortunately, because the tanks were populated in an “all-in all-out” system with no transfers among tanks, none of the other tanks in the room experienced any morbidity or mortality.
The standard quarantine procedures for the long-term colonies at our institution include the following steps: 1) all incoming embryos or adult fish immediately enter a quarantine room; 2) only progeny from embryos produced in quarantine by the imported adult fish and surface disinfected using both sodium hypochlorite and iodine can enter the main zebrafish colonies; 3) once subsequent generations or required data from the imported fish have been obtained, the original imported fish are euthanized according to approved protocol methods; and 4) the quarantine room has a dedicated supply of tanks, nets, food, filters, etc.; these items are not removed from the room for any reason. The fourth requirement is enforced because equipment that has been in contact with quarantined fish or systems can act as a fomite and thereby introduce pathogens into the main holding areas. The Zebrafish International Resource Center (ZIRC) uses similar quarantine practices, in addition to receiving or cryopreserving sperm and performing in vitro fertilization with colony eggs.21 With regard to the latter practices, a recent study found that E. ictaluri and other zebrafish pathogens survived various cryopreservation and freezing protocols, although the study did not evaluate the infectivity and transmission of the surviving organisms.24
The acute morbidity and mortality described in this case study were consistent with the previously described epizootic course of edwardsiellosis in zebrafish.12,26 Strongly positive real-time PCR-positive results from the homogenized pool of Batch A fish identified Edwardsiella ictaluri, which was confirmed by sequence analysis of the amplicon and by sequence analysis of 2 additional bacterial genes, gyrA and gyrB. We could not perform histopathology on the fish from Batch A because they died acutely and were frozen by the lab. By week 4, only 12 fish remained in the 75.7 L tank. These fish were clinically normal and continued to be free of any physical or behavioral abnormalities. The 2 fish that we examined histologically were obtained from these late surviving, clinically normal fish; these 2 fish had no histologic evidence of E. ictaluri infection. The surviving fish in this case report may not have been exposed to a minimal infectious dose, as fewer than one zebrafish per liter remained in the tank after the initial acute episode of mortality; these fish were quickly removed to avoid cannibalism. In contrast, laboratory zebrafish are commonly housed at densities ranging from 5 to 10 fish/L. A previous report found that lower density of catfish after harvest reduced the odds of having an ESC event.6 Furthermore, one or more of the surviving fish may have arrived as asymptomatic carriers from the population of origin. Molecular analysis of various types of environmental samples has been used to detect E. ictaluri in commercial aquaculture8 and to detect other pathogens in laboratory zebrafish.5,20,23 However, ours is the first report demonstrating the use of environmental sampling to detect E. ictaluri in zebrafish. In our case study, the environmental sample provided corroboration of the diagnosis. In other situations, environmental testing may provide evidence that counters an absence of histopathology findings or low numbers of affected animals in a population.
We recognize that some residual E. ictaluri material may have been present in the filters from previous tank populations. However, the combination of clinical disease, acute deaths, the timing of the findings, and positive PCR from fish samples leads us to conclude that all of our findings arose from a single related event. When questioned, the biological supply company stated that they had not experienced any unexpected mortality.
E. ictaluri was probably present in a few of the initial 24 fish received and, after shipping stress, resulted in an outbreak and acute mortality. As in mammals, acute or chronic husbandry-related stress in zebrafish gives rise to elevated cortisol levels, resulting in a stress leukogram and increased susceptibility to pathogens.11,30–32 The presence of encysted metacercaria in some of the same zebrafish that were affected by E. ictaluri suggests that they were reared in outdoor ponds with exposure to snails, birds, and/or other hosts that would allow continuation of the trematode life cycle. The Edwardsiella outbreak further indicates that this commercial source of zebrafish lacks adequate biosecurity practices for laboratory zebrafish. As a result of this experience, the teaching lab now obtains fish from biosecure research colonies in our own institution. These inhouse facilities employ stringent quarantine and bioexclusion measures, and no other outbreaks have occurred since this case. The implementation of standard practices in rodent research, including approved vendor lists, diligent adherence to proven quarantine practices, routine health surveillance, and work and materials flow that protect the colony health status are all essential to the biosecurity of research zebrafish colonies, allowing protection of zebrafish health, human health, and scientific validity and reproducibility.
Acknowledgments
We thank Shannon Primm for technical assistance with sequence analysis. We thank Emily Ozdowski for sample collection assistance. Marcus J Crim is an employee of IDEXX BioAnalytics, a division of IDEXX Laboratories, a company that provides veterinary diagnostics.
References
- 1.Altan E, Kubiski SV, Boros A, Reuter G, Sadeghi M, Deng X, Creighton EK, Crim MJ, Delwart E.2019. A highly divergent picornavirus infecting the gut epithelia of zebrafish (Danio rerio) in research institutions worldwide. Zebrafish 16: 291– 299. 10.1089/zeb.2018.1710. [DOI] [PubMed] [Google Scholar]
- 2.Binesh CP.2013. Mortality due to viral nervous necrosis in zebrafish Danio rerio and goldfish Carassius auratus . Dis Aquat Organ 104: 257– 260. 10.3354/dao02605. [DOI] [PubMed] [Google Scholar]
- 3.Chatla K, Gaunt P, Petrie-Hanson L, Hohn C, Ford L, Hanson L.2014. Zebrafish (Danio rerio) bioassay for visceral toxicosis of catfish and botulinum neurotoxin serotype E. J Vet Diagn Invest 26: 240– 245. 10.1177/1040638713519642. [DOI] [PubMed] [Google Scholar]
- 4.Crim MJ.2020. Viral diseases, p 509– 526. Chapter 42. In: Cartner S, Eisen JS, Farmer S, Guillemin K, Kent ML, Sanders GE, editors. The zebrafish in biomedical research: biology, husbandry, diseases, and research applications. San Diego (CA: ): Academic Press. 10.1016/B978-0-12-812431-4.00042-7 [DOI] [Google Scholar]
- 5.Crim MJ, Lawrence C, Livingston RS, Rakitin A, Hurley SJ, Riley LK.2017. Comparison of antemortem and environmental samples for zebrafish health monitoring and quarantine. J Am Assoc Lab Anim Sci 56: 412– 424. [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham FL, Jack SW, Hardin D, Wills RW.2014. Risk factors associated with enteric septicemia of catfish on Mississippi commercial catfish farms. J Aquat Anim Health 26: 84– 90. 10.1080/08997659.2014.886635. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Senapin S, Jeamkunakorn C, Nguyen V, Nguyen N, Rodkhum C, Khunrae P, Rattanarojpong T.2019. Natural occurrence of edwardsiellosis caused by Edwardsiella ictaluri in farmed hybrid red tilapia (Oreochromis sp.) in Southeast Asia. Aquaculture 499: 17– 23. 10.1016/j.aquaculture.2018.09.007. [DOI] [Google Scholar]
- 8.Griffin MJ, Mauel MJ, Greenway TE, Khoo LH, Wise DJ.2011. A real-time polymerase chain reaction assay for quantification of Edwardsiella ictaluri in catfish pond water and genetic homogeneity of diagnostic case isolates from Mississippi. J Aquat Anim Health 23: 178– 188. 10.1080/08997659.2011.637006. [DOI] [PubMed] [Google Scholar]
- 9.Griffin MJ, Quiniou SM, Cody T, Tabuchi M, Ware C, Cipriano RC, Mauel MJ, Soto E.2013. Comparative analysis of Edwardsiella isolates from fish in the eastern United States identifies two distinct genetic taxa amongst organisms phenotypically classified as E. tarda . Vet Microbiol 165: 358– 372. 10.1016/j.vetmic.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Griffin MJ, Reichley SR, Greenway TE, Quiniou SM, Ware C, Gao DX, Gaunt PS, Yanong RP, Pouder DB, Hawke JP, Soto E.2016. Comparison of Edwardsiella ictaluri isolates from different hosts and geographic origins. J Fish Dis 39: 947– 969. 10.1111/jfd.12431. [DOI] [PubMed] [Google Scholar]
- 11.Grzelak AK, Davis DJ, Caraker SM, Crim MJ, Spitsbergen JM, Wiedmeyer CE.2017. Stress leukogram induced by acute and chronic stress in zebrafish (Danio rerio). Comp Med 67: 263– 269. [PMC free article] [PubMed] [Google Scholar]
- 12.Hawke JP, Kent M, Rogge M, Baumgartner W, Wiles J, Shelley J, Savolainen LC, Wagner R, Murray K, Peterson TS.2013. Edwardsiellosis caused by Edwardsiella ictaluri in laboratory populations of zebrafish Danio rerio . J Aquat Anim Health 25: 171– 183. 10.1080/08997659.2013.782226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heil N, editor. 2009. National wild fish health survey—laboratory procedures manual. Warm Springs (GA): US Fish and Wildlife Service. [Google Scholar]
- 14.Hohn C, Petrie-Hanson L.2012. Rag1–/– mutant zebrafish demonstrate specific protection following bacterial re-exposure. PLoS One 7: 1– 10. 10.1371/journal.pone.0044451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute for Laboratory Animal Research. 2010. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 16.Kent ML, Lyons JM. [Internet]. 1982. Edwardsiella ictaluri in the Green knifefish, Eigemannia virescens . Fish health news: A service to the field of fish health research, vol 2. [Cited 15 June 2021]. Available at: https://books.google.com/books?id=ZxZc6hBjZYcC. [Google Scholar]
- 17.Kumar G, Byars TS, Greenway TE, Aarattuthodiyil S, Khoo LH, Griffin MJ, Wise DJ.2019. Economic assessment of commercial-scale Edwardsiella ictaluri vaccine trials in US catfish industry. Aquac Econ Manag 23: 254– 275. 10.1080/13657305.2019.1632392. [DOI] [Google Scholar]
- 18.Leary S, Underwood W, Anthony R, Cartner S, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin M, Meyer R, Miller D, Shearer J, Turner T, Yanong R.2020. AVMA guidelines for the euthanasia of animals: 2020 Edition. Schaumburg (IL): AVMA American Veterinary Medical Association. [Google Scholar]
- 19.Matteucci G, Di Giuseppe M, Faraci L, Luparello M, Binanti D.2018. Bacterial ulcerative dermatitis in a piranha (Pygocentrus nattereri) fed with fresh fish diet. J Exot Pet Med 27: 23– 26. 10.1053/j.jepm.2017.11.005. [DOI] [Google Scholar]
- 20.Miller M, Sabrautzki S, Beyerlein A, Brielmeier M.2019. Combining fish and environmental PCR for diagnostics of diseased laboratory zebrafish in recirculating systems. PLoS One 14: 1–9. 10.1371/journal.pone.0222360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray KN, Varga ZM, Kent ML.2016. Biosecurity and health monitoring at the zebrafish international resource center. Zebrafish 13 Suppl 1: S30– S38. 10.1089/zeb.2015.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nho SW, Abdelhamed H, Karsi A, Lawrence ML.2017. Improving safety of a live attenuated Edwardsiella ictaluri vaccine against enteric septicemia of catfish and evaluation of efficacy. Vet Microbiol 210: 83– 90. 10.1016/j.vetmic.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Norris L, Lawler N, Hunkapiller A, Mulrooney DM, Kent ML, Sanders JL.2020. Detection of the parasitic nematode, Pseudocapillaria tomentosa, in zebrafish tissues and environmental DNA in research aquaria. J Fish Dis 43: 1087– 1095. 10.1111/jfd.13220. [DOI] [PubMed] [Google Scholar]
- 24.Norris LJ, Watral V, Kent ML.2018. Survival of bacterial and parasitic pathogens from zebrafish (Danio rerio) after cryopreservation and thawing. Zebrafish 15: 188– 201. 10.1089/zeb.2017.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterman MA, Posadas BC.2019. Direct economic impact of fish diseases on the East Mississippi catfish industry. N Am J Aquac 81: 222– 229. 10.1002/naaq.10090. [DOI] [Google Scholar]
- 26.Petrie-Hanson L, Romano CL, Mackey RB, Khosravi P, Hohn CM, Boyle CR.2007. Evaluation of zebrafish Danio rerio as a model for enteric septicemia of catfish (ESC). J Aquat Anim Health 19: 151– 158. 10.1577/H06-026.1. [DOI] [PubMed] [Google Scholar]
- 27.Philips BH, Crim MJ, Hankenson FC, Steffen EK, Klein PS, Brice AK, Carty AJ.2015. Evaluation of Presurgical skin preparation agents in African clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci 54: 788– 798. [PMC free article] [PubMed] [Google Scholar]
- 28.Plumb J, Sanchez D.1983. Susceptibility of five species of fish to Edwardsiella ictaluri . J Fish Dis 6: 261– 266. 10.1111/j.1365-2761.1983.tb00075.x. [DOI] [Google Scholar]
- 29.Pullium JK, Dillehay DL, Webb S.1999. High mortality in zebrafish (Danio rerio). Contemp Top Lab Anim Sci 38: 80– 83. [PubMed] [Google Scholar]
- 30.Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB.2009. Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture 297: 157– 162. 10.1016/j.aquaculture.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsay JM, Watral V, Schreck CB, Kent ML.2009. Husbandry stress exacerbates mycobacterial infections in adult zebrafish, Danio rerio (Hamilton). J Fish Dis 32: 931– 941. 10.1111/j.1365-2761.2009.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsay JM, Watral V, Schreck CB, Kent ML.2009. Pseudoloma neurophilia (Microsporidia) infections in zebrafish Danio rerio: effects of stress on survival, growth, and reproduction. Dis Aquat Organ 88: 69– 84. 10.3354/dao02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rendueles O, Ferrières L, Frétaud M, Bégaud E, Herbomel P, Levraud JP, Ghigo JM.2012. A new zebrafish model of oro-intestinal pathogen colonization reveals a key role for adhesion in protection by probiotic bacteria. PLoS Pathog 8: 1– 17. 10.1371/journal.ppat.1002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakai T, Kamaishi T, Sano M, Tensha K, Arima T, Iida Y, Nagai T, Nakai T, Iida T.2008. Outbreaks of Edwardsiella ictaluri infection in ayu Plecoglossus altivelis in Japanese rivers. Fish Pathol 43: 152– 157. 10.3147/jsfp.43.152. [DOI] [Google Scholar]
- 35.Sanders JL, Lawrence C, Nichols DK, Brubaker JF, Peterson TS, Murray KN, Kent ML.2010. Pleistophora hyphessobryconis (Microsporidia) infecting zebrafish Danio rerio in research facilities. Dis Aquat Organ 91: 47– 56. 10.3354/dao02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santander J, Martin T, Loh A, Pohlenz C, Gatlin DM, Curtiss R.2013. Mechanisms of intrinsic resistance to antimicrobial peptides of Edwardsiella ictaluri and its influence on fish gut inflammation and virulence. Microbiology (Reading) 159: 1471– 1486. 10.1099/mic.0.066639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soto E, Griffin M, Arauz M, Riofrio A, Martinez A, Cabrejos ME.2012. Edwardsiella ictaluri as the causative agent of mortality in cultured Nile tilapia. J Aquat Anim Health 24: 81– 90. 10.1080/08997659.2012.675931. [DOI] [PubMed] [Google Scholar]
- 38.Waltman W, Shotts E, Blazer V.1985. Recovery of Edwardsiella ictaluri from danio (Danio devario). Aquaculture 46: 63– 66. 10.1016/0044-8486(85)90176-0. [DOI] [Google Scholar]
- 39.Wise DJ, Greenway TE, Byars TS, Griffin MJ, Khoo LH.2015. Oral vaccination of channel catfish against enteric septicemia of catfish using a live attenuated Edwardsiella ictaluri isolate. J Aquat Anim Health 27: 135– 143. 10.1080/08997659.2015.1032440. [DOI] [PubMed] [Google Scholar]


