Abstract
This is a case report of a uterine cancer with the International Federation of Gynecology and Obstetrics staging 3c2 with the initial clinical presentation of postmenopausal vaginal bleeding in August 2015. Endometrium biopsy showed invasive nests of poorly differentiated grade 3 endometrioid adenocarcinoma. The patient received robotic surgery including total hysterectomy, bilateral salpingo-oophorectomy, bilateral pelvic lymph node dissection, para-aortic lymph node dissection, and washing cytology. The final pathology showed an endometrioid carcinoma with myometrium invasion up to 85% and para-aortic and pelvic lymph nodes invasion. The patient received six courses of adjuvant chemotherapy (paclitaxel and carboplatin) with concurrent chemoradiotherapy after the surgery. Later, immunotherapy with Picibanil (OK-432) and interleukin-2 (IL-2) was given, and cancer did not recur for 34 months until tumor recurrence at the liver dome and bilateral lung was noted by positron-emission tomography scan in July 2018. The patient received laparoscopic surgery for intra-abdominal tumor excision in December 2018, and the tumor found extended to the right diaphragm, liver surface, omentum, bilateral flank to pelvic peritoneum, Douglas pouch, and upper rectum. We continued the immunotherapy with OK-432, IL-2, Aldara cream (imiquimod), and later on, virotherapy (human papillomavirus vaccine). The immune risk profiles showed T–cells' proliferation and alteration of the Th1/Th2 activation after immunotherapy and virotherapy. Proctectomy with colon-anal anastomosis and cytoreduction surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) (doxorubicin and paclitaxel) was performed in January 2019. After the surgery, the patient received chemotherapy (topotecan, paclitaxel, lipodox, and carboplatin) and continued the immunotherapy. The immune risk profiles showed CD4, CD4/CD8 increase after HIPEC and immunotherapy. The patient continued the therapy until May 2020.
Keywords: Advanced uterine cancer, immunotherapy, virotherapy
INTRODUCTION
Endometrial cancer (EC) is one of the most common gynecologic cancers in developed countries. The incidence is 14.7 per 100,000 and mortality rate is 2.3 per 100,000 people.[1] The most common clinical feature related to this disease is the abnormal vaginal bleeding. More than 66.9% of patients with EC are diagnosed at a local stage, which leads to an overall favorable prognosis with 80%–85% in a 5-year overall survival rate.[2] However, when it comes to a more advanced stage, the prognosis is much unfavorable regardless of treatments.
Therapeutic strategies are decided according to the risk of disease recurrence. To patients with high-risk EC, adjuvant chemotherapy and radiation therapy is thought to be beneficial for prevention of distant metastasis. There are current and emerging treatment options including immunomodulating agents and/or immune checkpoint inhibitors to add on. However, of which evidences supported to treat advanced uterine cancer are quite limited, although immunotherapy may be potentially beneficial to patients' outcomes.
CASE REPORT
A 53-year-old woman, gravida 5, para 2, and menopause for 2 years, presented with postmenopausal vaginal bleeding for 1 month in September 2015. We performed hysteroscopy and found endometrial neoplasm with neovascularization in the uterine cavity. Transcervical resectoscope was performed, and pathology showed invasive nests of poorly differentiated grade 3 endometrioid adenocarcinoma. Serological examination showed carcinoembryonic antigen of 5.72 ng/mL and cancer antigen 125 was 42.9 U/ml. The patient received robotic surgery including total hysterectomy, bilateral salpingo-oophorectomy, bilateral pelvic lymph node dissection, para-aortic lymph node dissection, and washing cytology in October 2015. Final pathology showed endometrioid carcinoma with myometrium invasion up to 85% and para-aortic and pelvic lymph node invasion, staged as International Federation of Gynecology and Obstetrics Staging 3c2. An immunohistochemical study showed both positive estrogen receptor (6F11) and progesterone receptor (1A6).
After the operation, six courses of adjuvant chemotherapy with paclitaxel and carboplatin every 3 weeks were given, and the patient also received concurrent chemoradiotherapy. Later, immunotherapy with Picibanil (OK-432) and interleukin-2 (IL-2) was given, and immune risk profiles were checked before and after immunotherapy [Figure 1]. The immune risk profiles showed a significant increase of CD25 (2.9 fold) and CD4+CD25 (2.6 folds). Human leukocyte antigen (HLA)-DR+, CD28, and CD4/CD8 showed a slight increase. CD11b, NK cell, CD8+CD28−, and natural killer T cell (NKT) showed a decrease.
Figure 1.
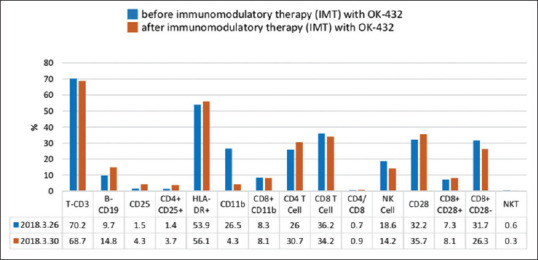
Immune risk profiles comparison before and after immunomodulatory therapy with OK-432
No tumor recurrence for 34 months was noted until tumor recurrence at the liver dome and lung metastasis was noted by positron-emission tomography scan in July 2018. We performed laparoscopic surgery in December 2018 for tumor excision, and it showed that the tumor had extended to the right diaphragm, liver surface, omentum, bilateral flank to pelvic peritoneum, Douglas pouch, and upper rectum. We continued the immunotherapy with OK-432, IL-2, Aldara cream (imiquimod), and virotherapy (human papillomavirus vaccine). We compared the immune risk profiles before and after immunotherapy and virotherapy [Figure 2]. The immune risk profiles showed HLA-DR+ and CD4/CD8 increase after OK-432, whereas HLA-DR+ and CD11b showed further increase after virotherapy.
Figure 2.

Immune risk profiles comparison before and after immunomodulatory therapy with OK-432 and virotherapy
Proctectomy with colon-anal anastomosis and cytoreduction surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) (doxorubicin and paclitaxel) was performed on January 2019. After the surgery, the patient received chemotherapy (topotecan, paclitaxel, lipodox, and carboplatin) and continued the immunotherapy. The immune risk profiles showed CD4 and CD4/CD8 increase after HIPEC and immunotherapy [Table 1]. The patient continued the therapy until May 2020.
Table 1.
Immune risk profiles comparison before and after hyperthermic intraperitoneal chemotherapy and immunotherapy
| Before (HIPEC) and immunotherapy | 1 week after (HIPEC) and immunotherapy | 3 months after (HIPEC) and immunotherapy | |
|---|---|---|---|
| T-CD3 | 67.4 | 76.7 | 79.2 |
| B-CD19 | 16.9 | 6.5 | 7.0 |
| CD25 | 7.6 | 3.6 | 2.7 |
| CD4+CD25+ | 5.8 | 3.1 | 2.6 |
| HLA-DR+ | 62.2 | 54.5 | 42.2 |
| CD3+DR+ | 33.8 | 41 | 32.2 |
| CD11b+ | 22.3 | 19.3 | 24.8 |
| CD8+CD11b+ | 5.1 | 5.4 | 7.3 |
| CD4 T cell | 28.9 | 33.3 | 40 |
| CD8 T cell | 31.6 | 40.3 | 37.7 |
| CD4/CD8 | 0.9 | 0.8 | 1.1 |
| NK-cell | 12.8 | 18.2 | 15.7 |
| CD28 | 34.9 | 32.2 | 37.9 |
| NKT | 3.4 | 6.0 | 7.2 |
| CD3+CD279+ | 6.3 | 0.5 | 0.4 |
| CD154+ | 5.0 | 0.5 | 0.6 |
| CD152 | - | - | <0.1 |
HIPEC: Hyperthermic intraperitoneal chemotherapy, NKT: Natural killer T cell
DISCUSSION
Immunotherapeutic strategies seek to bolster tumor-directed immune responses through tumor antigen selection in vaccine-based approaches, reinvigorate antitumor responses using immune checkpoint inhibitors, and expand T-cell populations using adoptive cellular therapy.[3]
OK-432 was used subcutaneously as an injected bacterium-extracted materials to trigger the skin Langerhans cells to recruit T-cells to secrete signal 3, such as tumor necrosis factor-alpha (TNF-α). In our case, TNF-α significantly increased from 6.6 pg/ml to 179 pg/ml after OK-432. It was reported to induce many cytokines including IL-1, IL-2, interferon-A, TNF-, IL-6, IL-8, granulocyte-colony stimulating factor, granulocyte-macrophage colony-stimulating factor, IL-12, and IL-18.[4,5] The mechanism includes directly inducing tumor lysis, altering cell cycle, recruiting cytotoxic cell, stimulating immune cell proliferation, or helping recognize the tumor antigen. Macrophages and dendritic cells exposure with OK-432 produce IL-12 and IL-18, which shifted the T- and B-cells' balance to Th-1 cell dominant. Theoretically, it is more likely to kill tumor cells for Th-1 activation.
Aldara cream (imiquimod) can activate toll-like receptor 7 which has been shown to induce many kinds of cytokines that shift immune response to the Th1 pathway.[6] Both OK-432 and Aldara cream showed potency to activate our immune system but different in some ways. Evidently, CD4 cells increased after OK-432 treatment, whereas CD8 cells increased after Aldara cream. In our case, OK-432 is unable to upregulate CD11b+ presentation, whereas Aldara cream is able to upregulate CD11b+ presentation. CD25 increased by 2.9 folds after OK-432 treatment but decreased after Aldara cream. HLA-DR was upregulated after both OK-432 and Aldara cream treatment. The changes after Aldara cream treatment are comparable with another previous study.[6]
The activation of naive precursor Th (pTh) cells requires two signals.[7,8] The generation of signal 1 alone leads to the inactivation of the pTh cell. By this reason, expressing the cytotoxic T-lymphocyte-associated protein 4 and downregulating CD28 allow the tumor cell to escape immune surveillance. Anti-IL-2-resistant tumor cell can also downregulate CD28 signal to survive.[9] In our case, CD28 was upregulated with OK-432 treatment but not in Aldara cream. The result was different from another case report in which CD28 also increased after Aldara cream.[6] The possible reason is that the patient received a long-term OK-432 treatment before Aldara cream.
NKT can enhance immune surveillance.[9] NKT is reported to be helpful in killing tumor cells as macrophage and NK cell. NKT recognizes antigens presented by CD1d; rather, major histocompatibility complex was recognized by other T-cell. It was crucial in killing anti-PD-1-resistant tumors when CD8 antigen-specific effect is exhausted.[10] In our case, NKT increased after OK-432 treatment in December 2018 but not in March 2018. The effect of Aldara cream on NKT was not obvious in our case; however, increased NKT has been reported after Aldara cream treatment in the previous study.[6] The possible reason is due to the memory effect after OK-432.
The limitation of this study includes the fact that the immune risk profiles can be affected by different physical conditions of the patient, status of the disease, or different treatment methods (surgery, chemotherapy, etc.). Although some different data of the immune risk profiles were noted compared to the previous study, the benefit of immunotherapy was still observed.
Cancer immunotherapy is evolving quickly in recent years, investigation using a combination of conventional and immune-based treatment modalities is ongoing. Immunotherapy does not only activate our innate immune system to kill tumor cells but also considered whether cancer cells escape from our immune surveillance and conventional treatment.[11] Further development of effective biomarker strategies to identify patients most likely to benefit from the use of immunotherapy is necessary. We do not only aim to prolong a patient's life span but also strike a balance between life quality, complications, and cost to improve care in advanced uterine cancer patients.
Ethical approval
This study was approved by the institutional review board of the Chang Gung Memorial Hospital, Taoyuan, Taiwan (approval number: 201508973B0D001).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initial will not be published and due efforts will be made to conceal the identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The study was supported by grants CMRPG3H1771 (Cheng-Tao Lin), which were provided by Chang Gung Memorial Hospital (Taoyuan, Taiwan).
Conflicts of interest
Prof. Chyi-Long Lee, an editorial board member at Gynecology and Minimally Invasive Therapy, had no role in the peer review process of or decision to publish this article. The other authors declared no conflicts of interest in writing this paper.
REFERENCES
- 1.Bestvina CM, Fleming GF. Chemotherapy for endometrial cancer in adjuvant and advanced disease settings. Oncologist. 2016;21:1250–9. doi: 10.1634/theoncologist.2016-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Endometrial Cancer. [Last accessed on 2018 Jul 11]. Available from: https://seer.cancer.gov/statfacts/html/corp.html .
- 3.Lynam S, Lugade AA, Odunsi K. Immunotherapy for gynecologic cancer: Current applications and future directions. Clin Obstet Gynecol. 2020;63:48–63. doi: 10.1097/GRF.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsnes C, Stavang H, Brokstad K, Olofsson J, Aarstad HJ. Chemokines are secreted by monocytes following OK-432 (lyophilized Streptococcus pyogenes) stimulation. BMC Immunol. 2009;10:6. doi: 10.1186/1471-2172-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alas QM, Lin CT. Immunotherapy with subcutaneous injection of immunomodulatory agent (OK-432) elicits durable response in locally advanced or relapsed cervical cancer. Gynecol Minim Invasive Ther. 2019;8:80–2. doi: 10.4103/GMIT.GMIT_1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin YH, Chu FC, Peng HH, Weng H, Lin CT. Efficacy of imiquimod cream administered intraperitoneally for ovarian metastases in colorectal cancer. Gynecol Obstet (Sunnyvale) 2016;6:12. [Google Scholar]
- 7.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A. 1999;96:185–90. doi: 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Nieuwenhuijze A, Liston A. The molecular control of regulatory T cell induction. Prog Md Biol Transt Sci. 2015;136:69–7. doi: 10.1016/bs.pmbts.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Nouroz F, Bibi F, Noreen S, Masood N. Natural killer cells enhance the immune surveillance of cancer. Egypt J Med Hum. 2016;17:149–54. [Google Scholar]
- 10.Bae EA, Seo H, Kim BS, Choi J, Jeon I, Shin KS, et al. Activation of NKT cells in an anti-PD-1 – Resistant tumor model enhances antitumor immunity by reinvigorating exhausted CD8 T cells. Cancer Res. 2018;78:5315–26. doi: 10.1158/0008-5472.CAN-18-0734. [DOI] [PubMed] [Google Scholar]
- 11.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185–98. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]


