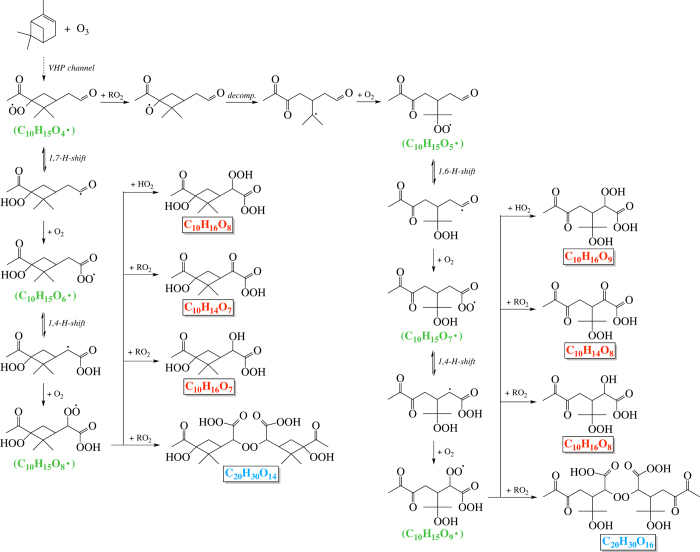
Extended Data Figure 3. Proposed mechanism for the formation of the E1 and E2 surrogates via peroxy radical formation.
The proposed scheme for the formation of the ELVOC monomer (C10H14O7) and dimer (C20H30O14) surrogates selected for quantum chemical calculations (Extended Data Fig. 7) is based on recently established autoxidation mechanisms for a series of cycloalkane + O3 systems16,18,19,20,73,74. Peroxy radicals in the figure are indicated by a green label, E1 by a red label and E2 by a blue label. Addition of ozone to the double bond of α-pinene produces two carbonyl-substituted Criegee biradicals. The energy-rich Criegee biradical is either collisionally stabilized, or isomerizes via 1,4-H-shift to a vinylhydroperoxide (VHP), which then decomposes to yield an OH· and an alkenoxy radical. The alkenoxy radical reacts with O2, leading to a peroxy radical, which is the potential precursor to a sequence of autoxidation reactions leading to the formation of HOMs16. Here the peroxy radical C10H15O4· is chosen as the starting point for HOM formation. The first intramolecular hydrogen abstraction is likely to take place at the aldehydic carbon from the opposite side of the peroxy group, although the rigid four-carbon-atom ring could hinder bending of the structure. For the cis configuration where the peroxy group and the aldehydic hydrogen are on the same side of the cyclobutyl ring, the 1,7-H shift rate is calculated20 to be 0.14 s−1, which initiates the autoxidation chemistry on a fast timescale compared to the HOM lifetime resulting from loss to the CLOUD chamber walls (about 900 s). The resultant acylic radical undergoes rapid O2 addition, leading to an -OOH functionalized peroxyacyl radical (C10H15O6·). The second intramolecular hydrogen abstraction is expected to proceed at the carbon atom in the α position of the peroxyacyl group via 1,4-H isomerization. The resultant C10H15O8· terminates by known reactions of peroxy radicals (HO2· or RO2· under the present experimental conditions), producing a spectrum of HOM monomers that includes the E1 surrogate, C10H14O7. The homogeneous recombination of two peroxy radicals via elimination of O2 produces the covalently bound dimer C20H30O14 chosen as the E2 surrogate. Alternatively, C10H15O8· can undergo further autoxidation, if sufficiently labile hydrogen atoms are available, leading to the observed closed-shell monomers with ≥9 O (Fig. 1). The self/cross-reaction of the C10H15O4· peroxy radical produces an alkoxy radical, which decomposes rapidly, leading to a carbonyl-functionalized peroxy radical (C10H15O5·). This peroxy radical is another potential starting structure for HOM formation. The carbon-ring-opening reaction pathway, while increasing the steric availability of the H atom, might be a slow step. The effective formation rate of the C=O-functionalized peroxy radical is calculated to be less than about 10−3 s−1, which is comparable to its wall deposition rate. The timescale with respect to the subsequent autoxidation reaction, on the other hand, is expected to be of the order of seconds, by analogy with that for branched-chain peroxy radicals73. The unbalanced sources and sinks potentially account for the low signals of peroxy radicals with odd oxygen numbers (for example, C10H15O5, C10H15O7 and C10H15O9). The autoxidation process of C10H15O5· is presumed to proceed by an autoxidative reaction pathway similar to that for C10H15O4·, eventually leading to the spectrum of HOM monomers and dimers observed in the CLOUD chamber. Except for the autoxidation channel, all the peroxy radicals are still subject to well-established reactions such as R(′)O2 + RO2/HO2, which are potentially important if the reaction rate is comparable to that for the autoxidation channel.