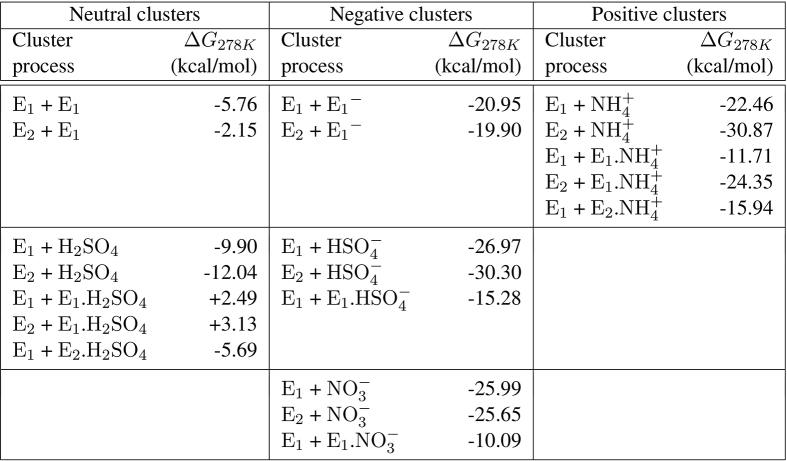
Extended Data Table 1.
Quantum chemical calculations of ELVOC cluster formation energies
Formation Gibbs free energies at 278 K, ΔG278 K, for neutral, negatively charged and positively charged ELVOC clusters. The cluster processes indicate the incident E1/E2 vapour molecule + the target cluster. Quantum chemical calculations made at other temperatures (not shown) indicate that the binding energies strengthen by −1.0 kcal mol−1 per 20 K reduction in temperature. The uncertainty in the calculated energies is less than 2 kcal mol−1. Our calculations indicate the following approximate order for different functional groups to contribute to the cluster binding energies involving 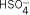 or H2SO4 (starting with the strongest): (i) carboxylic acids, R–C(=O)–OH; (ii) hydroxyls, R–OH; (iii) hydroperoxy acids, R–C(=O)–O–OH; (iv) hydroperoxides, R–O–OH; and (v) carbonyls, R–(R′–)C=O. In the case of
or H2SO4 (starting with the strongest): (i) carboxylic acids, R–C(=O)–OH; (ii) hydroxyls, R–OH; (iii) hydroperoxy acids, R–C(=O)–O–OH; (iv) hydroperoxides, R–O–OH; and (v) carbonyls, R–(R′–)C=O. In the case of 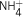 , the main interacting group is carbonyl, independently of which other groups are attached to it; therefore ammonium will form stronger clusters with carboxylic acids, hydroperoxy acids or carbonyls than it will with hydroxyls or hydroperoxides.
, the main interacting group is carbonyl, independently of which other groups are attached to it; therefore ammonium will form stronger clusters with carboxylic acids, hydroperoxy acids or carbonyls than it will with hydroxyls or hydroperoxides.