Abstract
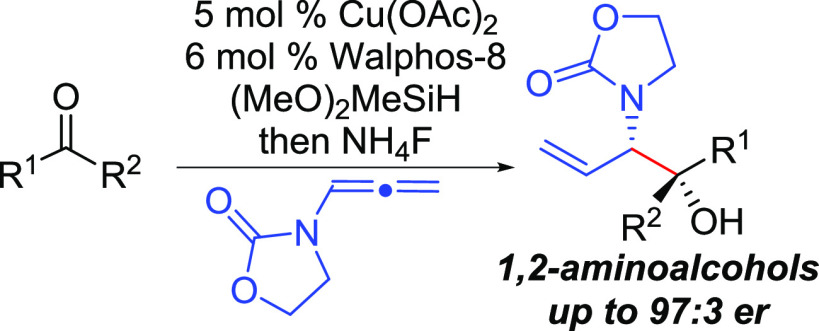
Herein, we report the development of a catalytic enantioselective addition of N-substituted allyl equivalents to ketone electrophiles through use of Cu-catalyzed reductive coupling to access important chiral 1,2-aminoalcohol synthons in high levels of regio-, diastereo-, and enantioselectivity. Factors affecting enantioinduction are discussed including the identification of a reversible ketone allylation step that has not been previously reported in Cu-catalyzed reductive coupling.
Chiral vicinal aminoalcohols (4, Figure 1) are ubiquitous in nature and represent an important class of biologically active compounds for applications in medicine and the pharmaceutical industry.1 A recent survey claims >300,000 compounds, > 2,000 natural products, and >80 Food and Drug Administration approved drugs contain the 1,2-aminoalcohol fragment.2 Therefore, asymmetric synthetic methods for the efficient preparation of the 1,2-aminoalcohol motif are needed to provide access to these valuable compounds.1b−1d,3 However, asymmetric preparation of 4 through the retrosynthetic disconnection highlighted in Figure 1 is challenging because the electron-withdrawing nature of the amino- and hydroxyl-substituents create a polarity profile where the reacting carbon atoms are both positively charged requiring the need for a formal cross-electrophile coupling.4 As a result, common polar processes prevalent in synthetic chemistry defined by the reaction between an electrophilic and nucleophilic species are not immediately amenable to the preparation of 4 since neither reacting carbon atom is nucleophilic.4 To circumvent this issue, strategies for reversing the polarity of common functional groups to enable these types of bond disconnection strategies are important methods for synthetic organic chemistry and have been defined as umpolung.4 For example, the Henry5 reaction between nitroalkanes and carbonyl electrophiles allows for the preparation of 1,2-aminoalcohols through a polar two-electron umpolung process. More recently, nonpolar radical based methods have emerged;6 however, enantioselective variants are few.7
Figure 1.
Aminoallylation strategies to 1,2-aminoalcohols.
In the context of an umpolung route toward aminoalcohols 4, aminoallylation of a carbonyl electrophile 2 by a nucleophilic amino-substituted allymetal reagent 1 represents a powerful technique for the generation of 1,2-aminoalcohol 3 containing alkene functionality that may be utilized in further synthetic manipulations. Indeed, carbonyl allylation chemistries have been extensively developed for the preparation of chiral homoallylic alcohols;8 however, the application of amino-substituted organometallic reagent 1 in analogous chemistry has been underdeveloped.2,9−11 While Barrett9 originally reported an asymmetric process using the stoichiometric preparation of chiral boron reagents of 1 (M = B), only recently have catalytic asymmetric variants emerged to promote the catalytic generation of 1.2,10,11 For example, Krische2a (Figure 1B) recently disclosed an enantioselective Ir-catalyzed aminoallylation of aldehydes through hydrogen transfer from alcohol 5 to allenamide 6 generating the necessary α,γ-aminoanion nucleophile (1, M = Ir) and the aldehyde electrophile. Additionally, our group10a recently reported an orthogonal method for the aminoallylation of ketone electrophiles enabled by Cu-catalyzed reductive coupling12,13 (Figure 1C). Here, the readily available Evans-auxiliary derived chiral allenamide 9 was employed for stereochemical control affording high diastereoselectivies.10a,11 While this method is practical due to the low cost of the Evans auxiliary, we appreciated the fact that absolute stereochemical control by a chiral Cu-catalyst with an achiral allenamide would increase atom efficiency (Figure 1D). Significantly, enantioselective metal catalyzed aminoallylation of ketone electrophiles is unknown and can be more challenging than aldehydes due to the decreased reactivity and steric differentiation of ketones versus aldehydes. Herein we report the development of the first Cu-catalyzed enantioselective aminoallylation of ketone electrophiles.
Initial studies focused on identifying an appropriate chiral ligand scaffold to afford high diastereo- and enantioselectivity in the reductive coupling of ketones and achiral allenamide 11 (Table 1, entries 1–7).14 Importantly, in all cases, variable amounts of carbonate migration product 13a were formed from internal trapping of the Cu-alkoxide11,14 intermediate with only single diastereomers of 12a and 13a observed. Of the ligands studied, W8 was identified as the best candidate providing the desired branched reaction product 12a in good enantioselectivity as a single diastereomer (entry 5). Interestingly, (S,S)-Ph-BPE (entry 1) and J11 (entry 7) were inferior to W8 despite their widespread use in Cu-catalyzed reductive coupling reactions.12,13
Table 1. Chiral Ligand Surveya.
| entry | ligand | % 12ab | 12a:13ab | er 12ac |
|---|---|---|---|---|
| 1 | (S,S)-Ph-BPE | 64 | 89:11 | 20:80 |
| 2 | (R)-BINAP | 82 | 83:17 | 18:82 |
| 3 | (R)-Segphos | 51 | 86:14 | 30:70 |
| 4 | W3 | 64 | 90:10 | 57:43 |
| 5 | W8 | 77 | 81:19 | 93:7d |
| 6 | J6 | 58 | 91:9 | 15:85 |
| 7 | J11 | 60 | 78:22 | 28:72 |
| 8e | W8 | 71 | 87:13 | 87:13 |
| 9f | W8 | 58 | >99:1 | 88:12 |
| 10f,g | W8 | 61 | 90:10 | 91:9 |
| 11h | W8 | 45i | 52:48j | 85:15i,k |
| 12f,h | W8 | 50i | >99:1j | 97:3 |
| 13f,g,h | W8 | 77i | >99:1j | 96:4 |
1a (0.25 mmol), 11 (0.375 mmol), and 0.50 mmol Me(MeO)2SiH in 0.5 mL of toluene. In all cases, a single diastereomer of 12a and 13a was obtained (1H NMR spectroscopic analysis). See the Supporting Information for additional details.
Determined by 1H NMR spectroscopy on the unpurified reaction mixture using dimethylfumarate as standard.
Value determined by chiral HPLC analysis.
Er of 13a was 50:50.
Using 10 equiv of silane.
2 equiv of t-BuOH added.
PhCF3 used as solvent.
Propiophenone (8b) used in place of 8a.
Value for 12b.
Ratio of 12b:13b.
Compounds 12 and 13 were produced in differing enantiopurities (Table 1, entries 5 and 11); one explanation consistent with this outcome is reversibility in the allylcupration.14 Reversible allylation15 in metal catalyzed reductive coupling reactions has not been identified prior to our work,11,16 and this issue would have significant ramifications on catalyst stereocontrol. For instance, the enantiopurity of product 12 would be dependent on the subsequent rate of silylation vs carbonate migration of the intermediate Cu-alkoxide formed after allylcupration leading to catalyst turnover if the allylcupration step was reversible.14 Along these lines, and in an effort to improve enantioselection, we reasoned that enantioselectivities may be improved if the rate of trapping of the Cu-alkoxide intermediate formed after allylcupration could be increased relative to carbonate migration. This may be achieved either through an increased silylation rate or by use of protic additives capable of quenching the Cu-alkoxide by protonolysis17 (e.g., t-BuOH). In this regard, examination of alternate silane reducing agents or reaction solvents led to no improvements.14 Use of excess silane (Table 1, entry 8) reduced the amount of 13a formed but also reduced the enantiopurity of 12a. Addition of t-BuOH as a proton donor generally mitigated the formation of 13 but afforded reduced yields presumably due to competitive protonation of the N-allyl(Cu) nucleophile (entries 5 vs 9/10 and 11 vs 12/13). This effect was more pronounced when a more sterically demanding ketone was used (propiophenone (8b), entries 11–13), and use of PhCF3 as solvent led to improved yields (entry 12 vs 13). The large amounts of 13 formed in the absence of t-BuOH with 8b are consistent with an increased rate of carbonate migration due to an enhanced Thorpe–Ingold effect.
With optimized conditions in hand (Table 1, entries 5 and 13), the ketone scope was examined (Scheme 1). Notably, the Me-group of 8 could be replaced with increased substitution providing products in high enantioselectivities (12a–12c), which can often be challenging due to the decreased steric bias of the two ketone substituents. Para-substitution of the ketone Ph-group generally led to a decrease in enantioselectivities (12d–12j); however, enantiopurity could be improved through the use of t-BuOH as an additive (Method B). Here, electron-poor ketones (8d–e) afforded good yields whereas electron-rich ketones (8f–i) provided poor yields with Method B due to incomplete conversion from competitive protonation of the allenamide, likely due to the reduced rate of addition to these less electrophilic ketones. Interestingly, ketones with meta-substitution (8k–o) returned to typical enantioselection levels as was obtained with 8a; however, addition of t-BuOH led to reduced er with the exception of the bromo derivative 12m. For ketones containing both meta- and para-substitution, the para-trend was stronger affording products with moderate enantioselectivities (12i,j). Smaller ketones bearing 5-membered heterocycles (12p–r) generally afforded good yields and enantioselectivities without the formation of 13 presumably due to increased silylation rates with these less hindered ketones. A cyclic ketone (8s) afforded a moderate yield due to large amounts of the linear product being formed. A ketone lacking prochirality (8t) afforded reduced enantioselection while 8u afforded only linear product l-12u. Finally, ortho-substitution was not tolerated providing large amounts of 13 in poor enantioselectivity along with the linear isomer when 2′-methoxyacetophenone was used.
Scheme 1. Enantioselective Cu-Catalyzed Reductive Coupling.
Reaction utilizes 0.25 mmol of ketone; see the Supporting Information.
Yield and er of 13 after converting the mixture of 12/13 to 13 with NaH.
58% yield of liner isomer also isolated.
A working model to rationalize the observed absolute and relative stereochemistry obtained in these reactions is highlighted in Scheme 2. The quadrant diagram given for the (W8)Cu-catalyst (14) is proposed based off of a (W1)PdCl2 crystal structure.18 Analysis of the X-ray structure suggests that the northern hemisphere of the complex is sterically more encumbered over the southern hemisphere due to the axial-like orientation of the two Ph-groups. When this information is applied to 14, a tetrahedral geometry is expected.11−13 Furthermore, replacement of the PPh2-group of W1 with the PCy2 moiety would result in the eastern hemisphere of 14 having increased steric hindrance relative to the western hemisphere containing the P(Ar)2 group. These effects create the quadrant diagram shown for 14 suggesting the southwest quadrant as the most “open.” Mechanistically,10,11 hydrocupration of allenamide 11 is expected to provide linear oxazolidinone-substituted allyl ligands that may afford an equilibrating mixture of E and Z isomers due to inhibition of oxazolidinone complexation to Cu by the chelating nature of W8.19 However, the Z-isomer, as in 15, may lead to an energetically lower pathway since the oxazolidinone group resides in the most open quadrant. Additionally, hydrocupration of 11 from (W8)Cu–H complex 14 would be expected to have the smaller hydride ligand in the northern hemisphere of the catalyst (La = H) with the allenamide approaching from the south (i.e., Lb) to minimize steric strain. From 15, subsequent complexation of the ketone electrophile and nucleophilic addition to the re-face occurs preferentially since the large substituent (RL) resides in the less sterically hindered northwestern quadrant over the P(Ar)2 group and the small substituent (Rs) in the more sterically hindered northeast quadrant over the P-Cy-substituent (TS-1 quadrant-view). A side-view of TS-1 shows this pathway to be chairlike10,11,13 having the RL-group of the ketone pseudoequatorial. This analysis correctly predicts the observed stereochemical outcome. These arguments assume that stereoinduction is controlled by ketone allylcupration. However, the overall enantioselectivity obtained will be a function of the relative rates of stereoisomers in the allylcupration event and the subsequent silylation or carbonate migration steps of 16 for catalyst turnover due to the reversibility of the allylcupration step. This phenomenon accounts for the variability in enantioselectivity obtained when utilizing diverse ketone electrophiles (Scheme 1). Furthermore, when t-BuOH is added, an additional catalyst turnover event through protonolysis of 16(17,20) is possible that can affect the stereoconvergence of 16 resulting in modulation of the enantioinduction in these processes.
Scheme 2. Working Mechanistic Stereochemical Model.

To gain experimental evidence for the reversibility of ketone allylcupration, we attempted to regenerate intermediate 16 from 12a by treatment with an LCuOtBu catalyst, but only rearrangement product 13a was observed.14 Therefore, b-17 was prepared using the described methodology and an analogous experiment was performed utilizing (W8)CuOtBu prepared in situ from CuI, KOtBu, and W8 (Scheme 3a). Gratifyingly, ketone 8a was observed in addition to the linear allylation product (l-17) and protonation products 18 and 19. Additionally, recovered b-17 had reduced enantiopurity. Together, these results offer strong evidence in support of a reversible ketone allylcupration in Cu-catalyzed reductive coupling reactions of allenamides.10,11,14
Scheme 3. Mechanistic Experiments and Applications.
In regards to the synthetic utility of the present methodology, the process was scaled to 1.0 mmol scale providing 12b in good yield with high enantiopurity as a single diastereomer, and W8 could be recovered and recycled with identical results (Scheme 3b). Reaction products 12 were generally highly crystalline and could be recrystallized to single enantiomers.14 Unmasking of the amino group of 12a was achieved by carbonate migration to 13a followed by a three-step telescoped sequence performed without purification of intermediates through tosylation, elimination with DBU/NaI,21 and hydrolysis of the resultant enamide to afford 20 (Scheme 3c). Aminoalcohol 21 is then obtained from hydrolysis of 20.10a
In conclusion, we have disclosed an enantio- and diastereoselective aminoallylation of ketones by Cu-catalyzed reductive coupling to access useful chiral protected 1,2-aminoalcohols. Identification of an unprecedented reversible ketone addition step that appears to be unique to N-carbamoyl substituted aminoallylation intermediates derived from the hydrometalation of allenamides having important implications on asymmetric induction was reported. Future investigation into the unique reactivity of these systems for asymmetric synthesis is ongoing.
Acknowledgments
Startup funding from the Virginia Commonwealth University and the Bill and Melinda Gates Foundation (The Medicines for All Institute, Grant Number OPP#1176590) is gratefully acknowledged. M.D.E. acknowledges the NSF for a summer REU fellowship (CHE-1851916). We thank Dr. Joseph Turner (VCU) for assistance in collecting HRMS data.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c02258.
Detailed experimental procedures, compound characterization data, chiral HPLC traces, and copies of NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Davison E. K.; Sperry J. Natural Products with Heteroatom-Rich Ring Systems. J. Nat. Prod. 2017, 80, 3060–3079. 10.1021/acs.jnatprod.7b00575. [DOI] [PubMed] [Google Scholar]; b Gupta P.; Mahajan N. Biocatalytic Approaches Towards the Stereoselective Synthesis of Vicinal Amino Alcohols. New J. Chem. 2018, 42, 12296–12327. 10.1039/C8NJ00485D. [DOI] [Google Scholar]; c Wetzel S.; Schuffenhauer A.; Roggo S.; Ertl P.; Waldmann H. Chimia 2007, 61, 355–360. 10.2533/chimia.2007.355. [DOI] [Google Scholar]; d Sehl T.; Maugeri Z.; Rother D. Multi-Step Synthesis Strategies Towards 1,2-Amino Alcohols with Special Emphasis on Phenylpropanolamines. J. Mol. Catal. B: Enzym. 2015, 114, 65–71. 10.1016/j.molcatb.2014.12.005. [DOI] [Google Scholar]; e Mushtaq S.; Abbasi B. H.; Uzair B.; Abbasi R. Natural Products as Reservoirs of Novel Therapertic Agents. EXCLI Journal 2018, 17, 420–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Spielmann K.; Xiang M.; Schwartz L. A.; Krische M. J. Direct Conversion of Primary Alcohols to 1,2-Amino Alcohols: Enantioselective Iridium-Catalyzed Carbonyl Reductive Coupling of Phthalimido-Allene via Hydrogen Auto-Transfer. J. Am. Chem. Soc. 2019, 141, 14136–14141. 10.1021/jacs.9b08715. [DOI] [PMC free article] [PubMed] [Google Scholar]; For nonenantioselective variants, see:; b Skucas E.; Zbieg J. R.; Krische M. J. anti-Aminoallylation of Aldehydes via Ruthenium-Catalyzed Transfer Hydrogenative Coupling of Sulfonamido Allenes: 1,2-Aminoalcohols. J. Am. Chem. Soc. 2009, 131, 5054–5055. 10.1021/ja900827p. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zbieg J. R.; Mcinturff E. L.; Krische M. J. Allenamide Hydro-Hydroxyalkylation: 1,2-Amino Alcohols via Ruthenium-Catalyzed Carbonyl anti-Aminoallylation. Org. Lett. 2010, 12, 2514–2516. 10.1021/ol1007235. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhang W.; Chen W.; Xiao H.; Krische M. J. Carbonyl anti-(α-Amino)allylation via Ruthenium Catalyzed Hydrogen Autotransfer: Use of an Acetylenic Pyrrole as an Allylmetal Pronucleophile. Org. Lett. 2017, 19, 4876–4879. 10.1021/acs.orglett.7b02336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected reviews, see:; a Bergmeier S. C. The Synthesis of Vicinal Amino Alcohols. Tetrahedron 2000, 56, 2561–2576. 10.1016/S0040-4020(00)00149-6. [DOI] [Google Scholar]; b Burchak O. N.; Py S. Reductive Cross-Coupling Reactions (RCCR) Between C=N and C=O for β-Amino Alcohol Synthesis. Tetrahedron 2009, 65, 7333–7356. 10.1016/j.tet.2009.06.003. [DOI] [Google Scholar]; c Karjalainen O. K.; Koskinen A. M. P. Diastereoselective Synthesis of Vicinal Amino Alcohols. Org. Biomol. Chem. 2012, 10, 4311–4326. 10.1039/c2ob25357g. [DOI] [PubMed] [Google Scholar]
- a Seebach D. Methods of Reactivity Umpolung. Angew. Chem., Int. Ed. Engl. 1979, 18, 239–336. 10.1002/anie.197902393. [DOI] [Google Scholar]; b Evans D. A.; Andrews G. C. Allylic Sulfoxides: Useful Intermediates in Organic Synthesis. Acc. Chem. Res. 1974, 7, 147–155. 10.1021/ar50077a004. [DOI] [Google Scholar]
- For reviews, see:; a Palomo C.; Oiarbide M.; Laso A. Recent Advances in the Catalytic Asymmetric Nitroaldol (Henry) Reaction. Eur. J. Org. Chem. 2007, 2007, 2561–2574. 10.1002/ejoc.200700021. [DOI] [Google Scholar]; b Blay G.; Hernandez-Olmos V.; Pedro J. R. Development of New N,N-Ligands for the Enantioselective Copper(II)-Catalyzed Henry Reaction. Synlett 2011, 2011, 1195–1211. 10.1055/s-0030-1260558. [DOI] [Google Scholar]; c Matsunaga S.; Shibasaki M. Recent Advances in Cooperative Bimetallic Asymmetric Catalysis: Dinuclear Schiff Base Complexes. Chem. Commun. 2014, 50, 1044–1057. 10.1039/C3CC47587E. [DOI] [PubMed] [Google Scholar]; d Zhang S.; Li Y.; Xu Y.; Wang Z. Recent Progress in Copper Catalyzed Asymmetric Henry Reaction. Chin. Chem. Lett. 2018, 29, 873–883. 10.1016/j.cclet.2017.10.001. [DOI] [Google Scholar]
- a Schwarz J. L.; Kleinmans R.; Paulisch T. O.; Glorius F. 1,2-Amino Alcohols via Cr/Photoredox Dual-Catalyzed Addition of α-Amino Carbanion Equivalents to Carbonyls. J. Am. Chem. Soc. 2020, 142 (5), 2168–2174. 10.1021/jacs.9b12053. [DOI] [PubMed] [Google Scholar]; b Wang R.; Ma M.; Gong X.; Fan X.; Walsh P. J. Reductive Cross-Coupling of Aldehydes and Imines Mediated by Visible Light Photoredox Catalysis. Org. Lett. 2019, 21, 27–31. 10.1021/acs.orglett.8b03394. [DOI] [PubMed] [Google Scholar]
- Kim Y. J.; Lee Y. S.; Choi Y.; Ryu D. H. Enantioselective 1,2-Addition of α-Aminoalkyl Radical to Aldehydes via Visible-Light Photoredox Initiated Chiral Oxazaborolidinium Ion Catalysis. ACS Catal. 2020, 10, 10585–10591. 10.1021/acscatal.0c02443. [DOI] [Google Scholar]
- Reviews:; a Denmark S. E.; Fu J. Catalytic Enantioselective Addition of Allylic Organometallic Reagents to Aldehydes and Ketones. Chem. Rev. 2003, 103, 2763–2793. 10.1021/cr020050h. [DOI] [PubMed] [Google Scholar]; b Yus M.; González-Gómez J. C.; Foubelo F. Catalytic Enantioselective Allylation of Carbonyl Compounds and Imines. Chem. Rev. 2011, 111, 7774–7854. 10.1021/cr1004474. [DOI] [PubMed] [Google Scholar]; c Yus M.; González-Gómez J. C.; Foubelo F. Diastereoselective Allylation of Carbonyl Compounds and Imines: Application to the Synthesis of Natural Products. Chem. Rev. 2013, 113, 5595–5698. 10.1021/cr400008h. [DOI] [PubMed] [Google Scholar]; d Holmes M.; Schwartz L. A.; Krische M. J. Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev. 2018, 118, 6026–6052. 10.1021/acs.chemrev.8b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Barrett A. G. M.; Seefeld M. A. B-[(E)-3-(Diphenylamino)allyl]diisopinocampheylborane: An Excellent Reagent for the Stereoselective Synthesis fo anti-β-Diphenylamino Alcohols. J. Chem. Soc., Chem. Commun. 1993, 339–341. 10.1039/C39930000339. [DOI] [Google Scholar]; b Barrett A. G. M.; Seefeld M. A. The Use of B-[(E)-3-(Diphenylamino)allyl]diisopinocompheylborane as a Reagent for the Stereoselective Synthesis of anti-β-Diphenylamino Alcohols and trans-1-Diphenylamino-2-(1-hydroxylalkyl)-cyclopropanes. Tetrahedron 1993, 49, 7857–7870. 10.1016/S0040-4020(01)88011-X. [DOI] [Google Scholar]
- a Gargaro S. L.; Klake R. K.; Burns K. L.; Elele S. O.; Gentry S. L.; Sieber J. D. Access to a Catalytically Generated Umpolung Reagent through the Use of Cu-Catalyzed Reductive Coupling of Ketones and Allenes for the Synthesis of Chiral Vicinal Aminoalcohol Synthons. Org. Lett. 2019, 21, 9753–9758. 10.1021/acs.orglett.9b03937. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Klake R. K.; Gargaro S. L.; Gentry S. L.; Elele S. O.; Sieber J. D. Org. Lett. 2019, 21, 7992–7998. 10.1021/acs.orglett.9b02973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For an analogous method employing imines, see:Agrawal T.; Martin R. T.; Collins S.; Wilhelm Z.; Edwards M. D.; Gutierrez O.; Sieber J. D. Access to Chiral Diamine Derivatives through Stereoselective Cu-Catalyzed Reductive Coupling of Imines and Allenamides. J. Org. Chem. 2021, 86, 5026–5046. 10.1021/acs.joc.0c02971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For other recent Cu-catalyzed reductive coupling methods not proceeding by aminoallylation, see:; a Li K.; Shao X.; Tseng L.; Malcolmson S. J. 2-Azadienes as Reagents for Preparing Chiral Amines: Synthesis of 1,2-Amino Tertiary Alcohols by Cu-Catalyzed Enantioselective Reductive Couplings with Ketones. J. Am. Chem. Soc. 2018, 140, 598–601. 10.1021/jacs.7b12213. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shao X.; Li K.; Malcolmson S. J. Enantioselective Synthesis of anti-1,2-Diamines by Cu-Catalyzed Reductive Couplings of Azadienes with Aldimines and Ketimnes. J. Am. Chem. Soc. 2018, 140, 7083–7087. 10.1021/jacs.8b04750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent examples of Cu-catalyzed reductive allylation, see:; a Tsai E. Y.; Liu R. Y.; Yang Y.; Buchwald S. L. A Regio- and Enantioselective CuH-Catalyzed Ketone Allylation with Terminal Allenes. J. Am. Chem. Soc. 2018, 140, 2007–2011. 10.1021/jacs.7b12271. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Liu R. Y.; Zhou Y.; Yang Y.; Buchwald S. L. Enantioselective Allylation Using Allene, a Petroleum Cracking Byproduct. J. Am. Chem. Soc. 2019, 141, 2251–2256. 10.1021/jacs.8b13907. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Liu T. Y.; Yang Y.; Buchwald S. L. Regiodivergent and Diastereoselective CuH-Catalyzed Allylation of Imines with Terminal Allenes. Angew. Chem., Int. Ed. 2016, 55, 14077–14080. 10.1002/anie.201608446. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Li C.; Liu R. Y.; Jesikiewicz L. T.; Yang Y.; Liu P.; Buchwald S. L. CuH-Catalyzed Enantioselective Ketone Allylation with 1,3-Dienes: Scope, Mechanism, and Applications. J. Am. Chem. Soc. 2019, 141, 5062–5070. 10.1021/jacs.9b01784. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Li C.; Shin K.; Liu R. Y.; Buchwald S. L. Engaging Aldehydes in CuH-Catalyzed Reductive Coupling Reactions: Stereoselective Allylation with Unactivated 1,3-Diene Pronucleophiles. Angew. Chem., Int. Ed. 2019, 58, 17074–17080. 10.1002/anie.201911008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See the Supporting Information for additional details/data.
- Recent review:Nogi K.; Yorimitsu H. Carbon-Carbon Bond Cleavage at Allylic Postions: Retro-allylation and Deallylation. Chem. Rev. 2021, 121, 345–364. 10.1021/acs.chemrev.0c00157. [DOI] [PubMed] [Google Scholar]
- Only a single non-enantioselective example of a Cu-catalyzed retro-allylation process is known requiring 80–110 °C; see:Sai M.; Yorimitsu H.; Oshima K. Allyl-, Allenyl-, and Propargyl-Transfer Reactions through Cleavage of C-C Bonds Catalyzed by an N-Heterocyclic Carbene/Copper Complex: Synthesis of Multisubstituted Pyrroles. Angew. Chem., Int. Ed. 2011, 50, 3294–3298. 10.1002/anie.201100631. [DOI] [PubMed] [Google Scholar]
- For examples of Cu-catalysis turnover by protonolysis with alcohol additives, see:; a Ascic E.; Buchwald S. L. Highly Diastereo- and Enantioselective CuH-Catalyzed Synthesis of 2,3-Disubstituted Indolines. J. Am. Chem. Soc. 2015, 137, 4666–4669. 10.1021/jacs.5b02316. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang S.; del Pozo J.; Romiti F.; Mu Y.; Torker S.; Hoveyda A. H. Delayed Catalyst Function Enables Direct Enantioselective Conversion of Nitriles to NH2-amines. Science 2019, 364, 45–51. 10.1126/science.aaw4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox A. F.; Rheingold A. L.; Golen J. A.; Kassel W. S.; Nataro C. Taniaphos and Walphos Ligands: Oxidative Electrochemistry and Complexation. Synthesis, Characterization, Oxidative Electrochemistry, and X-ray Structures of [(Taniaphos/Walphos)MCl2] (M = Pd or Pt). Inorg. Chim. Acta 2008, 361, 3283–3293. 10.1016/j.ica.2007.09.031. [DOI] [Google Scholar]
- The allylcopper complexes are represented as η1 rather than η3 (i.e., π-allyl) based on a number of computational studies that predict the η1 form to be more stable. See, refs (11 and 13c−13e).
- Protonolysis of LCu(allyl) species by chairlike transition states is reported; see:; a Jang H.; Jung B.; Hoveyda A. H. Catalytic Enantioselective Protoboration of Disubstituted Allenes. Access to Alkenylboron Compounds in High Enantiomeric Purity. Org. Lett. 2014, 16, 4658–4661. 10.1021/ol5022417. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Meng F.; Jung B.; Haeffner F.; Hoveyda A. H. NHC-Cu-Catalyzed Protoboration of Monosubstituted Allenes. Ligand-Controlled Site Selectivity, Application to Synthesis and Mechanism. Org. Lett. 2013, 15, 1414–1417. 10.1021/ol4004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phukan P.; Bauer M.; Maier M. E. Facile Generation of Alkenes and Dienes from Tosylates. Synthesis 2003, 2003, 1324–1328. 10.1055/s-2003-40210. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.