Abstract
Background:
Low cardiorespiratory fitness (CRF) is usually observed in people living with HIV (PLWH). The effect of a low-volume high intensity interval training (LV-HIIT) on CRF in HIV+ and HIV- Hispanic women was evaluated in this study.
Setting:
A non-randomized clinical trial with pre and post-test using a LV-HIIT intervention was conducted in the AIDS Clinical Trials Unit (ACTU) and the Puerto Rico Clinical and Translational Research Consortium (PRCTRC) at the University of Puerto Rico Medical Sciences Campus.
Methods:
29 HIV+ and 13 HIV- Hispanic women recruited from community-based programs and clinics, and able to engage in daily physical activities, volunteered to participate. Of these, 20 HIV+ (69%) and 11 HIV- (85%) completed the study and were included in the analyses. LV-HIIT consisted of 6-weeks, 3 days/week, 8-10 high and low intensity intervals on a cycle-ergometer at 80-90% of heart rate reserve. Main outcome measures were CRF (defined as VO2peak), peak workload, and time to peak exercise.
Results:
Average peak workload and time to peak exercise increased after training (P<0.05) in both groups. However, average CRF was significantly higher after training only in the HIV- group. Gains in CRF were observed in 100% of HIV- and 50% of HIV+ women. This was not influenced by exercise testing, habitual physical activity, or anthropometric variables.
Conclusion:
Given the lack of change in CRF observed in the HIV+ group post LV-HIIT intervention, it is important to focus on variations that may occur within groups.
Keywords: cardiorespiratory fitness, VO2peak, exercise workload, time to peak exercise, ventilatory threshold
INTRODUCTION
Life expectancy among people living with HIV (PLWH) is approximating that of the general population [1]. However, chronic illness and reduced functionality disproportionately affect PLWH [2, 3]. For example, cardiovascular disease is the principal cause of death regardless of gender, and its prevalence is increasing in PLWH although it is decreasing in the general population [4, 5].
Low cardiorespiratory fitness (CRF) determined by maximal oxygen consumption (VO2max or VO2peak), predicts cardiovascular disease and all-cause mortality [6–8]. Aerobic exercise improves CRF in healthy and chronic disease populations [9], including PLWH [10]. However, PLWH usually present lower CRF than expected [11–14]. Although there is no consensus regarding the optimal exercise modality to improve CRF in PLWH, continuous or interval aerobic exercise with intensities from 45-80% have been emphasized [10, 15]. Aerobic exercise of higher intensities is also effective improving CRF among PLWH, with better responses when intensities are from 75-90% of VO2peak or heart rate (HR) reserve [16–17].
High intensity interval training (HIIT) is a popular exercise modality combining aerobic and anaerobic energy demands with periods of high intensity interspersed with periods of low intensity or rest [18]. Low volume HIIT (LV-HIIT) is a less time commitment variation of HIIT consisting of low frequency and duration (i.e., 4-12 intervals of 1-4 min duration for 2-12 weeks), but constant-high load intervals (i.e., 80-90% of HR reserve) [18–21]. LV-HIIT has been shown to improve CRF, muscle oxidative metabolism, and glucose control in male patients with coronary artery disease and diabetes [19, 22]. To our knowledge, CRF response to LV-HIIT has not been documented in HIV+ women. Therefore, the purpose of this study was to evaluate the effect of LV-HIIT on CRF among HIV+ and HIV- women.
METHODS
Participants
Twenty-nine HIV+ and 13 HIV- women volunteered for the LV-HIIT intervention. The study was approved by the IRB of the University of Puerto Rico Medical Sciences Campus (UPR-MSC), and registered in clinicaltrials.gov (NCT02962622). Recruitment ocurred by personal contact in clinics and community-based programs. Inclusion criteria for HIV+ women included no opportunistic infections of the central nervous system, no active systemic infection, CD4 count at or above 500 cells/mm3, and HIV viral load less than 200 copies/ml. Inclusion criteria for all participants included being 21 years or older, no uncontrolled hypertension, diabetes, or neurodegenerative diseases; also at least 9th grade education level, and engagement in normal activities but no formal exercise training.
Nine HIV+ and two HIV- participants withdrew because of inability to adhere to the frequency of study visits. Their pre-test results were similar to those who completed the study, except for sedentary behavior (6.7 ± 0.6 vs. 8.3 ± 0.2 hr/day, respectively, P=0.02). No complaints about the exercise intervention were expressed. Twenty HIV+ (69%) and 11 HIV- (85%) women completed all study visits.
Study Protocol
Two pre-intervention visits, 18 LV-HIIT sessions, and 2 post-intervention visits were scheduled for each participant. LV-HIIT sessions were scheduled during working hours on weekdays, and supervised by personnel experienced in exercise training. All pre and post-tests were conducted in the same order.
Pre and post exercise tests were conducted on a Monark® 928E (Sweden) cycle-ergometer starting at 25 Watts (W), increasing 25 W every 2-minutes at 50 rpm cadence until volitional fatigue. Oxygen consumption and ventilation were measured with a COSMED® FitMate Med metabolic system (California, USA) with continuous beat by beat HR (Garmin, USA) and 12-lead ECG monitoring (Edan® SE-12 Express, Edan Instruments, China). Pre and post-training measures also included body weight, bioelectrical impedance (BIA) for body composition (OMRON HBF-510, Kyoto, Japan), height (SECA 213, Hanover, USA) with participants’ head aligned according to the Frankfurt horizontal plane and holding a normal inhalation, waist and hip circumferences (Gulick anthropometric tape, Creative Health Products, USA), and lean body mass (LBM) with full-body DEXA (HOLOGIC Discovery, Ontario, Canada). Waist-worn accelerometer-determined moderate to vigorous physical activity (MVPA) and sedentary time (ActiGraph® GT3X+BT) following a standard 7-day wear time protocol were analyzed with the ActiLife v.6 software (ActiGraph Corp LLC, College Station, FL).
LV-HIIT intervention consisted of 6-weeks, 3-days/week (18 sessions total) on a Monark® 928E cycle ergometer. All sessions began with a 5 min warm-up at 25 W and 50 rmp. During sessions 1-6 (week 1-2), participants completed eight-1-min intervals at 80% of HR reserve interspersed with 1-min recovery for a total of 16 min. During sessions 7-18 (week 3-6), participants completed ten-1-min intervals at 90% of HR reserve interspersed with 1-min recovery for a total of 20 min. Workloads were determined using individual HR responses during the VO2peak test, and were periodically adjusted to help participants reach their prescribed exercise intensity. Cadence was kept at 50 rpm. During recovery, workloads were lowered to 25 W at 50 rpm cadence. At the end of each session, participants completed 5-min cool-down stretching exercises.
Shapiro-Wilk tests for normality were conducted for all variables. T-tests and Wilcoxon-Signed-Rank tests were used to identify differences between groups at pre and post-test, and differences within group from pre to post-test, when appropriate. Percent change in VO2peak from pre to post-test was used to identify those who improved (positive change) and those who did not improve (zero or negative change) after the LV-HIIT intervention. Multiple logistic regression was used to identify factors at pre and post-test that might contribute to changes in VO2peak. Statistical analyses were conducted with STATA 15.1 (STATA Corp LLC, College Station, Texas) using an alfa equal or less than 0.05 for statistical significance.
RESULTS
Participant’s General Characteristics
HIV+ women were on combined antiretroviral therapy (cART), had average CD4 of 774.2 ± 92.2 cells/ml, and 85% had non-detectable viral loads (<20 copies/ml). Age, body mass index (BMI), and percent fat were similar at pre and post-test with no difference between groups. LBM was not different between groups, but was higher at post-test only in HIV- women. Waist circumference and waist to hip ratio (WHR) were higher among HIV+ women compared with HIV- women. MVPA was extremely low, and sedentary time was high in both groups at pre and post-test, without significant group differences (Table 1).
Table 1.
Characteristics of study participants at pre and post-test: mean ± standard error of the mean (95% Confidence Interval)
| Characteristic | HIV+ (n=20) | HIV- (n=11) | ||
|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | |
| Age (yrs) | 46.9 ± 2.2 (42.2-51.5) |
41.6 ± 4.5 (31.5-51.8) |
||
| BMI (kg/m2) | 28.7 ± 1.4 (25.7-31.6) |
28.5 ± 1.4 (25.6-32.5) |
26.8 ± 0.9 (24.7-28.9) |
26.9 ± 1.0 (24.8-29.0) |
| BIA-Fat (%) | 42.5 ± 2.0 (38.3-46.7) |
42.9 ± 2.1 (38.5-47.3) |
41.5 ± 1.7 (37.8-45.3) |
41.5 ± 1.7 (37.7-45.3) |
| DEXA-LBM (kg) | 42.5 ± 2.1 (37.7-47.3) |
42.5 ± 2.1 (37.9-47.1) |
37.1 ± 1.5‡ (33.9-40.4) |
37.7 ± 1.4‡ (34.5-40.8) |
| Waist C (cm) | 98.5 ± 3.1* (92.1-104.9) |
98.1 ± 2.9** (92.0-104.1) |
86.4 ± 2.8* (80.2-92.5) |
85.3 ± 2.8** (80.0-91.6) |
| WHR | 0.93 ± 0.01* (0.90-0.95) |
0.92 ± 0.02** (0.89-0.96) |
0.86 ± 0.02* (0.80-0.91) |
0.84 ± 0.02** (0.79-0.90) |
| MVPA (min/week) | 19.9 ± 2.8 (14.0-25.7) |
21.7 ± 3.4 (14.5-28.9) |
17.1 ± 5.9 (3.8-30.3) |
20.0 ± 6.1 (6.1-33.9) |
| Sedentary (hr/day) | 7.9 ± 0.3 (7.3-8.4) |
8.2 ± 0.3 (7.5-8.8) |
8.4 ± 0.4 (7.4-9.3) |
8.4 ± 0.5 (7.7-9.6) |
| VO2peak (ml·kg−1·min−1) |
19.4 ± 0.78 (17.7-21.0) |
19.7 ± 0.6** (18.4-21.0) |
22.1 ± 1.4 (18.9-25.3) |
24.3 ± 1.5** (21.1-27.6) |
| Workload-peak (W) | 96.3 ± 5.5†
(84.7-107.8) |
108.8 ± 4.9† (98.5-119.0) |
102.3 ± 9.2‡ (81.8-122.8) |
115.9 ± 7.7‡ (98.7-133.2) |
| Time to peak (min) | 7.2 ± 0.4† (6.3-8.0) |
7.8 ± 0.4† (6.9-8.6) |
7.5 ± 0.6‡ (6.1-8.9) |
8.5 ± 0.6‡ (7.1-9.9) |
| HR-peak (bpm) | 149.6 ± 4.1* (141.1-158.1) |
151.3 ± 4.7 (141.4-161.2) |
163.4 ± 5.1* (152.0-174.7) |
163.4 ± 5.0 (152.1-174.6) |
| VEpeak (L/min) | 54.2 ± 3.4 (47.0-61.4) |
56.8 ± 3.9 (48.7-64.9) |
56.3 ± 4.4 (46.5-66.2) |
59.5 ± 4.6 (49.3-69.7) |
| VO2 at VT (mlˑkg−1ˑmin−1) |
14.9 ± 0.61* (13.6-16.2) |
15.5 ± 0.6 (14.4-16.7) |
16.6 ± 0.87* (14.6-18.5) |
17.8 ± 1.0 (15.7-19.9) |
| HR at VT (bpm) | 122.6 ± 3.0*† (116.4-128.8) |
130.1 ± 3.5† (125.1-139.7) |
132.8 ± 3.8* (124.1-141.5) |
138.1 ± 4.5 (128.1-148.1) |
BMI= body mass index, BIA = bioelectrical impedance, DEXA = dual energy x ray absorptiometry, LBM = lean body mass, Waist C = waist circumference, WHR = waist to hip ratio, MVPA = moderate to vigorous physical activity. VO2 = oxygen consumption, HR = heart rate, VE = ventilation, VT = ventilatory threshold.
Between group values with same symbol (* or **) are significantly different (P≤0.05).
Within group values with same symbol († or ‡) are significantly different.
Training Load, Cardiorespiratory Fitness and Other Exercise Testing Parameters
Workload eliciting 80% of HR reserve during weeks 1-2 was 77.0 ± 4.4 W for HIV+ women, and 81.8 ± 7.4 W for HIV- women (P=0.55). Training HR was 120 ± 9 and 125 ± 13 bpm in HIV+ and HIV- women, respectively. Workload during weeks 3-6 was increased (P<0.0001) to elicit 90% of HR reserve (89.0 ± 5.0 W and 94.0 ± 8.3 W, respectively [P=0.58]). Training HR for weeks 3-6 were 132 ± 8 bpm for HIV+ and 142 ± 21 bpm for HIV-, representing approximately 10 beats above the ventilatory threshold.
No between group VO2peak difference was observed at pre-test (Table 1). After training, VO2peak was higher only in HIV- women, with a mean change of 2.24 ± 0.4 compared to 0.35 ± 0.8 mlˑkg−1ˑmin−1 in HIV+ women (P=0.002). Individual changes in CRF showed all HIV- women having higher values compared to pre-test, but 50% of HIV+ women did not change or somewhat lowered CRF values at post-test (Figure 1), suggesting possible non-responsiveness to LV-HIIT. Yet, HIV+ and HIV- women had similar or higher peak workloads at post-test. No anthropometric, exercise testing or physical activity variables at pre and post-test explained VO2peak response difference between groups.
Figure 1.
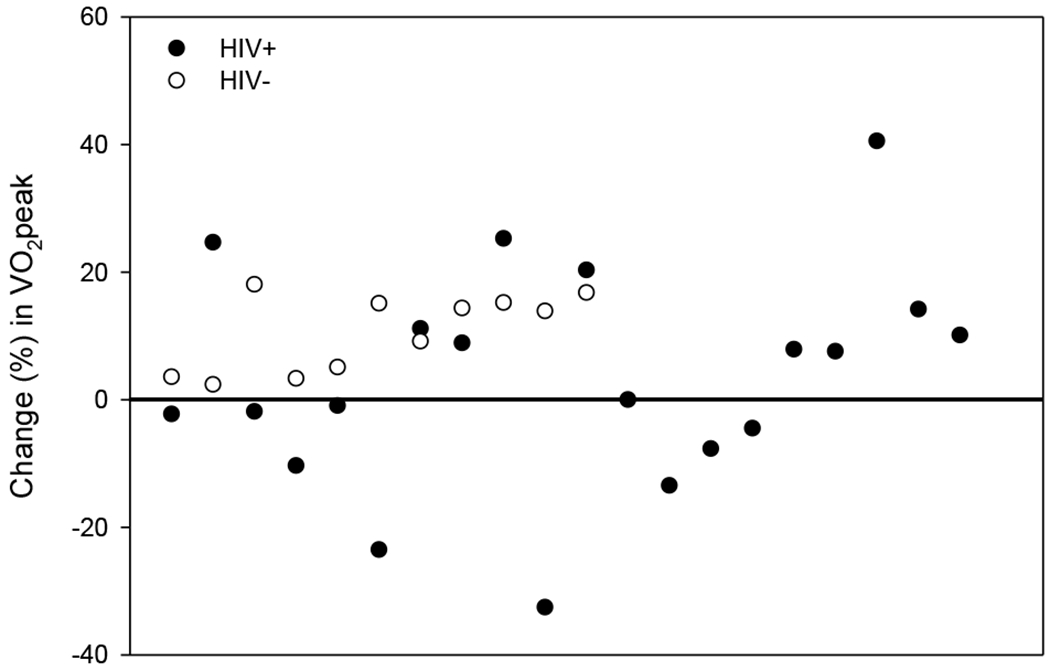
Individual participants’ percent (%) change in VO2peak from pre to post low volume – high intensity interval training (LV-HIIT) intervention. HIV+ participants (closed circles) and HIV- participants (open circles) above the horizontal line had a positive change in CRF after the LV-HIIT intervention.
DISCUSSION
LV-HIIT improved peak exercise time and workload in HIV+ and HIV- women. However, VO2peak was higher at post-test only in HIV- compared to HIV+. All HIV- women but only 50% of HIV+ women showed gains in VO2peak. This is different from studies conducted mostly in HIV+ males, where high intensity exercise significantly improved VO2peak [16, 17].
Studies measuring VO2peak in HIV+ women are scarce. A study using estimates of CRF in PLWH [23] found higher VO2peak for men than women, with values in the lowest range published among PLWH [12, 24]. Those values were lower than our previous [14] and current results among Hispanic PLWH. In the present study, pre and post VO2peak were lower than reference values for age and sex [25], reflecting poor CRF [26]. Potential mitochondrial DNA function and biogenesis impairments due to nucleoside reverse transcriptase inhibitors (NRTI) exposure, and chronic immune activation and inflammation are possible explanations [27–28].
Our findings of individual VO2peak gains in 100% of HIV- but no gains in 50% of HIV+ women, support the idea that some people may not respond to training stimuli. Although parameters for VO2max non-response have been proposed [29–30], there is no consensus regarding the degree of change needed to identify people with limited ability to improve CRF in response to exercise training. Bacon et al. [31] indicated that with high enough training intensity (90% of VO2max) all people can improve CRF with HIIT, even when no individual responses were documented. Interindividual variations in VO2max trainability are expected because the ability for oxygen transport and delivery into active muscles dependends on a series of physiological events and confounders such as sex and age [9]. Moreover, Weatherwax et al. [32] highlighted the importance of individualizing exercise intensity based on ventilatory threshold (VT). They reported that all participants with individualized exercise prescription improved VO2max compared with 60% of those with a non-individualized prescription, suggesting that some individuals may not respond equally to standardized exercise prescriptions.
CRF responders and non-responders in the present study had similar anthropometrics (age, BMI, %fat, waist circumference, WHR), exercise testing outcomes (ventilation, HR, workload, VO2 at VT, HR at VT), habitual physical activity, and sedentary time. In a multiple logistic regression model, none of these variables could explain the differences observed in VO2peak response between HIV+ and HIV- women. We also used coefficient of variation for change in VO2peak in HIV- women (9.2%) to distinguish VO2peak responders from non-responders among HIV+ women. Using this criteria, 70% of HIV+ women were categorized as non-responders even when their HR during training were above the VT.
VO2 at VT is considered a stable submaximal CRF value because, compared with VO2peak, it is more independent from motivation and exercise duration [33]. In HIV+ women, VO2 at VT, similar to VO2peak, was not different from pre to post-test. This was also true in our HIV- group, even when they showed an increase in VO2peak at post-test. This is consistent with Webel et al. [34] who reported no change in VO2 at VT nor in VO2peak after a self-management lifestyle-exercise intervention in HIV+ adults. Also similar to Webel et al. [32], the percent of VO2peak achieved at VT was not different between groups or between tests (Pre and post: HIV+ = 77.3 ± 2.1 and 79.1 ± 2.0%; HIV- = 76.0 ± 2.6 and 74.2 ± 3.2%; P>0.05 in all). Although exercise training is expected to increase the time to VT [35], HIV- women did not change this outcome (5.4 ± 0.5 vs. 6.0 ± 0.5 min [P=0.13]) but HIV+ women improved at post-test (5.1 ± 0.3 vs. 6.0 ± 0.3 min [P=0.01]). This finding suggest that for HIV+ women, time to VT might be a better CRF outcome than VO2peak, VO2 at VT or the percentage of VO2peak at VT.
In conclusion, LV-HIIT was well tolerated and improved exercise capacity in HIV+ and HIV- Hispanic women. However, interindividual variations in CRF must be considered when adopting or recommending LV-HIIT. It is possible that for HIV+ women, LV-HIIT had limited effects on muscle’s capacity for oxygen extraction and/or consumption. Whether this limitation is associated with chronic immune activation, inflammation, and/or long term exposure to NRTI, needs to be clarified. Considering CRF in HIV+ vs. HIV- women at post-test, statistical power appeared adequate (0.85). However, increasing the number of participants is important as more variables, including socioeconomic characteristics, are explored to further explain the differences observed. CRF response to LV-HIIT must be tested in HIV+ men, and testing longer LV-HIIT duration among PLWH is also recommended.
ACKNOWLEDGMENTS
To the staff at UPR-MSC Aids Clinical Trials Unit, Puerto Rico Clinical and Translational Research Consortium, and La Perla de Gran Precio community-based project. The study was supported by NIMHD S21MD001830, R21MH095524, U54MD007587, R25MD007607, UM1AI069415, UPRRP/DEGI, and 5UM1AI0694.
Sources of Support:
NIMHD S21MD001830, R21MH095524, U54MD007587, R25MD007607, UM1AI069415, and UPRRP/DEGI.
Disclosure of Funding:
National Institutes of Health (NIH)
Footnotes
Meeting at which part of the data was presented:
Joint Meeting of the International Society for NeuroVirology (ISNV) and the Society on NeuroImmune Pharmacology (SNIP), Chicago, April 10-14, 2018.
No conflict of interest to disclose
Contributor Information
Farah A. Ramírez-Marrero, University of Puerto Rico, Rio Piedras Campus.
Sigrid Pérez-Frontera, University of Puerto Rico, School of Medicine.
Marcos A. Amalbert-Birriel, University of Puerto Rico, Río Piedras Campus.
Miriam Matos, University of Puerto Rico, School of Medicine.
Jorge Santana-Bagur, University of Puerto Rico, School of Medicine.
Walter R. Frontera, University of Puerto Rico, School of Medicine.
Valerie Wojna, University of Puerto Rico, Medical Sciences Campus.
REFERENCES
- 1.Boender TS, Smit C, Sighem AV, et al. AIDS Therapy Evaluation in the Netherlands (ATHENA) national observational HIV cohort: cohort profile. BMJ Open 2018; 8:e022516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oursler KK, Goulet JL, Crystal S, et al. Association of age and comorbidity with physical function in HIV-infected and uninfected patients: results from the Veterans Aging Cohort Study. AIDS Patient Care STDS 2011; 25(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.High K, Bradley S, Loeb M, et al. A New Paradigm for Clinical Investigation of Infectious Syndromes in Older Adults: Assessing Functional Status as a Risk Factor and Outcome Measure. J Am Geriatr Soc 2005; 53: 528–535. [DOI] [PubMed] [Google Scholar]
- 4.Hanna DB, Ramaswamy C, Kaplan RC, et al. Trends in cardiovascular disease mortality among persons with HIV in New York City, 2001-2012. Clin Infect Dis 2016; 63:1122–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Womack JA, Chang CH, So-Armah KA, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc 2014; 3:e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women A Meta-analysis. JAMA 2009; 301(19):2024–2035. [DOI] [PubMed] [Google Scholar]
- 7.Harber MP, Kaminsky LA, Arena R, et al. Impact of Cardiorespiratory Fitness on All-Cause and Disease-Specific Mortality: Advances Since 2009. Prog Cardiovasc Dis 2017; 60: 11–20. [DOI] [PubMed] [Google Scholar]
- 8.Imboden MT, Harber MP, Whaley MH, et al. The Influence of Change in Cardiorespiratory Fitness With Short-Term Exercise Training on Mortality Risk From The Ball State Adult Fitness Longitudinal Lifestyle Study Mary T. Mayo Clin Proc 2019; 94(8):1406–1414. [DOI] [PubMed] [Google Scholar]
- 9.Joyner MJ, Lundby C. Concepts About V O2max and Trainability Are Context Dependent. Exerc Sport Sci Rev 2018; 46(3):138–43. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien KK, Tynan AM, Nixon SA, et al. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis 2016;16:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes-Neto M, Conceição CS, Ogalha C, et al. Aerobic capacity and health-related quality of life in adult HIV-infected patients with and without lipodystrophy. Braz J Infect Dis 2016; 20(1):76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes-Neto M, Rodríguez I, Lédo AP, et al. Muscle strength and aerobic capacity in HIV-infected patients: A systematic review and meta-analysis. J Aquir Immune Defic Syndr 2018; 79(4): 491–500. [DOI] [PubMed] [Google Scholar]
- 13.Oursler KK, Sorkin JD, Smith BA, et al. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses 2006; 22(11): 1113–1121. [DOI] [PubMed] [Google Scholar]
- 14.Ramírez-Marrero FA, Santana-Bagur JL, Joyner MJ, et al. Metabolic síndrome in relation to cardiorespiratory fitness, active and sedentary behavior in HIV+ Hispanics with and without lipodystrophy. PR Health Sci J 2014; 33(4): 163–169. [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes-Neto M, Conceição CS, Ogalha C, et al. A systematic review of the effects of different types of therapeutic exercise on physiologic and functional measurements in patients with HIV/AIDS. Clinics 2013; 68(8): 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacArthur RD, Levine SD, Birk TJ. Supervised exercise training improves cardiopulmonary fitness in HIV-infected persons. Med Sci Sports and Exerc 1993; 25(6): 684–688. [PubMed] [Google Scholar]
- 17.Oursler KK, Sorkin JD, Ryan AS, et al. A pilot randomized aerobic exercise trial in older HIV-infected men: Insights into strategies for successful aging with HIV. PLoS ONE 2018; 13(6): e0198855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibala MJ, Little JP, MacDonald MJ, et al. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 2012; 590.5: 1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little JP, Gillen JB, Percival ME, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol 2011; 111(6):1554–60. [DOI] [PubMed] [Google Scholar]
- 20.Farias-Junior LF, Macêdo GAD, Browne RAV, et al. Physiological and pshychological responses during low-volume high-intensity interval training sessions with different work-recovery durations. J Sports Sci Med 2019; 18(1): 181–190. [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor JL, Holland DJ, Spathis JG, et al. Guidelines for the delivery and monitoring of high intensity interval training in clinical populations. Prog Cardiovasc Dis 2019; 62: 140–146. [DOI] [PubMed] [Google Scholar]
- 22.Currie KD, Dubberley JB, McKelvie RS, et al. Low-volume, high-intensity interval training in patients with CAD. Med Sci Sports Exerc 2013; 45(8): 1436–1442. [DOI] [PubMed] [Google Scholar]
- 23.Webel AR, Perazzo J, Phillips JC, et al. The relationship between physical activity and cardiorespiratory fitness among people living with Human Immunodeficiency Virus throughout the life span. J Cardiovasc Nurs 2019a; 34(5): 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zech P, Pérez-Chaparro C, Schuch F, et al. Effects of aerobic and resistance exercise on cardiovascular parameters for people living with HIV: a Meta-analysis. J Assoc Nurses AIDS Care 2019, 30(2): 186–205. [DOI] [PubMed] [Google Scholar]
- 25.Loe H, Steinshamn S, Wisløff U (2014). Cardio-Respiratory Reference Data in 4631 Healthy Men and Women 20–90 Years: The HUNT 3 Fitness Study. PLoS ONE 2014, 9(11): e113884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACSM (American College of Sports Medicine). ACSM’s Guidelines for Exercise Testing and Prescription, 10E. Philadelphia: LWW; 2017. [Google Scholar]
- 27.Skinner JS, Jaskólski A, Jaskóska A, et al. Age, sex, race, initial fitness, and response to training: the HERITAGE Family Study. J Appl Physiol 2001, 90(5): 1770–1776. [DOI] [PubMed] [Google Scholar]
- 28.Bouchard C, Sarzynski MA, Rice TK, et al. Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. J Appl Physiol 2011, 110(5): 1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleischman A, Johnsen S, Systrom DM, et al. Effects of a nucleoside reverse teranscriptase inhibitor, staduvine, on glucose disposal and mitochondrial function in muscle of healthy adults. AM J Physiol Endocrionol Metab 2007, 292: E1666–E1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran T, Guardigni V, Pencina KM, et al. Atypical skeletal muscle profiles in Human Immunodeficiency Virus-infected asymptomatic middle-aged adults. Clin Inf Dis 2018, 66(12): 1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bacon AP, Carter RE, Ogle EA, et al. VO2max trainability and high intensity interval training in Humans: A meta-analysis. PLoS ONE 2013; 8(9): e73182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weatherwax RM, Harris NK, Kilding AE, et al. Incidence of VO2max responders to personalized versus standardized exercise prescription. Med Sci Sports Exerc 2019; 51(4): 681–691. [DOI] [PubMed] [Google Scholar]
- 33.Wagner J, Agostini P, Arena R, et al. The role of gas exchange variables in cardiopulmonary exercise testing for risk stratification and management of heart failure with reduced ejection fraction. Am Heart J 2018; 202, 116–126. [DOI] [PubMed] [Google Scholar]
- 34.Webel AR, Jenkins T, Vest M, et al. Cardiorespiratory fitness is associated with inflammation and physical activity in HIV+ adults. AIDS. 2019b; 33: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 35.Pennington C The exercise effect on the anaerobic threshold in response to graded exercise. Int J Health Sci 2015; 3(1): 225–234. [Google Scholar]
- 36.Kohler JJ, Lewis W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ Mol Mutagen 2007; 48(3–4): 166–172. [DOI] [PubMed] [Google Scholar]


