Abstract
Synchrony can be defined as the precise coordination between independent individuals, and this behaviour is more enigmatic when it is imperfect. The traditional theoretical explanation for imperfect synchronous courtship is that it arises as a by-product of the competition between males to broadcast leading signals to attract female attention. This competition is considered an evolutionary stable strategy maintained through sexual selection. However, previous studies have revealed that leading signals are not honest indicators of male quality. We studied imperfect courtship synchrony in fiddler crabs to mainly test whether (i) signal leadership and rate are defined by male quality and (ii) signal leadership generates synchrony. Fiddler crab males wave their enlarged claws during courtship, and females prefer leading males—displaying ahead of their neighbour(s). We filmed groups of waving males in the field to detect how often individuals were leaders and if they engaged in synchrony. Overall, we found that courtship effort is not directly related to male size, a general proxy for quality. Contrary to the long-standing assumption, we also revealed that leadership is not directly related to group synchrony, but faster wave rate correlates with both leadership and synchrony.
This article is part of the theme issue ‘Synchrony and rhythm interaction: from the brain to behavioural ecology’.
Keywords: fiddler crab, sexual selection, courtship, wave display, evolutionary stable strategy
1. Introduction
Animal synchrony is a ubiquitous phenomenon in nature. It occurs when independent individuals self-organize and coordinate [1]. Synchrony in courtship signals, in particular, is a special type of animal synchrony in which concerted signals arise from two non-mutually exclusive explanations: either adaptively or incidentally [2,3]. In adaptive synchrony, all individuals participating in the synchrony benefit. Synchrony may help deter natural enemies, such as acoustically orienting predators that eavesdrop on prey courtship signals [4]. Synchrony may also serve to broadcast species identity to conspecific females that recognize the species' rhythm [5], and enhance group attractiveness as it increases signal intensity [3,6]. Conversely, incidental synchrony is fundamentally a by-product of sexual selection. Females are biased to perceive and prefer males that give signals that stand out, and synchrony results from the competition between males to broadcast leading signals [7]. To achieve leadership, a male first inhibits its signal upon perceiving a rival's leading signal and then resets his own [7,8]. Under this mechanism, competition for leadership between closely matched males generates almost simultaneous or completely alternated signals between individuals [6,8]. Given the competition scenario, this type of synchrony is inherently incidental and imperfect.
Based on the evidence of the inhibitory-resetting mechanism and female preference, studies have extensively claimed that incidental synchrony is an emergent consequence of the competition for leadership [9–12]. The imperfect synchrony that arises from male–male competition is considered an evolutionary stable strategy maintained by female preference [7]. Leading signals produced by males should, therefore, be honest indicators of male quality, where vigorous males would achieve leadership more often. This explanation only works if the signal is condition-dependent; males in good condition can honestly signal their quality to females by increasing signal intensity. However, empirical studies repeatedly fail to find clear evidence for this explanation [13–16], and whether signal leadership relates to male quality remains to be determined in many taxa.
Fiddler crabs are an exceptional case of courtship synchrony because it is the only known group to present visual and motion-based synchrony [17]. Males possess an enlarged claw that has a dual function: as a weapon and signal in territory disputes with other males, and as a courtship signal for females [18] (electronic supplementary material, video S1). Male wave displays are stereotyped and species-specific, indicating strong sexual selection in the group [19]. Synchronous waving is widely considered incidental with females of many species preferring males that produce leading waves [17]. The cooperative explanation for synchrony has been refuted a few times in this group because imperfect synchrony is a poor strategy to avoid predators [17,20]. In addition, in some species, females do not preferentially approach males in aggregations [21] and males probably do not synchronize to enhance conspicuousness to distant females because synchrony only occurs when a female is close [9]. However, there are a few exceptions. A species from Panama, Leptuca saltitanta, displays in synchrony, but females do not prefer leading waves. Males may synchronize either in the presence or absence of females, which is also true for the Australian species, Austruca perplexa [22]. Additionally, females generally prefer males that wave at a faster rate compared to his neighbour(s). This preference for faster waves can also lead to the emergence of synchrony but is often overlooked [17,23]. Although female preference for leading signals is considered the main explanation for the emergence of synchrony in fiddler crabs, to our knowledge no study to date has looked for a relationship between male quality and the leadership role and if leadership is a precursor for synchrony. Moreover, while the explanation that competition for leadership can generate synchrony is largely accepted in all synchronous taxa, empirical research is still needed to address other courtship and social elements to fully understand synchrony emergence.
In this study, we make systematic field observations to investigate how a sequence of aspects from the biology of a fiddler crab species directly and indirectly correlates with male leadership and synchrony. More specifically, we aim to reveal whether the level of competition (number of courting males), male quality (size), risk-taking (walked distance between the burrow and the female) and courtship effort (wave rate) are related to the likelihood of achieving leadership and synchronizing with rivals. Although the study explores many possible relationships between these aspects, we mainly hypothesized that: (i) males that are larger and face low levels of competition take higher risks during courtship, which would lead to waving at faster rates and achieve leadership; (ii) faster wave rate and leadership are direct indicators of male quality (size); and (iii) following the traditional theory, leadership and wave rate are directly correlated with synchrony.
2. Methods
(a) . Study species
We observed Austruca mjoebergi fiddler crabs in the wild at East Point Reserve, Darwin, Australia (12°24′18″ S;130°49′45″ E). This species occurs in dense, mixed-sex populations on the mudflats. Most individuals own a burrow and defend the territory around it. Once a female is ready to mate, she leaves her burrow in search of a male [18]. As she wanders in the mudflat assessing male courtship displays, she walks through males' territories and they join in and drop out of a group that forms around her (electronic supplementary material, video S1). When she stops to assess waving males, a clear group with particular individuals surrounds her. Natural mate choice occurs quickly (average 18 s; electronic supplementary material, table S1), as wandering females risk dehydration and predation [18,20]. Once a female finds an attractive male, she will then assess his burrow (by tapping her legs at the burrow's entrance) before entering the burrow and mating with him [18].
Male A. mjoebergi crabs synchronize when waving to a nearby female. Females prefer males that are closer to her, but further away from other males. They also prefer leading and higher amplitude waves, displayed at fast rates [9,21,24]. The level of competition that males face is linked to group size, where the male's relative size would represent his advantage from his neighbours. Although size may not be a proxy for male quality in other animal taxa, it relates to mating success in fiddler crabs and, in part, quality [25,26]. Courtship is energetically costly and larger males tend to court more and are preferred by females [25,26]. Larger males also win more fights over territory [27], and because burrow quality (temperature) is central for egg incubation, it is important to own a burrow in a prime location [28].
(b) . Data collection
We filmed 60 independent groups of waving males under natural conditions in the field during the mating seasons of 2015 and 2019. Prior to filming, we first found groups of active males by observing waving, fighting and feeding behaviours. We then captured a mate-searching female that was at least 4 m away from the observed male group and tethered her to a nail by a 2 cm long cotton thread glued to the carapace. To stimulate courtship, we placed the female near the active males by inserting the nail into the mud at least 10 cm from one of the males' burrows. Following this, males would form a group, defined by individuals that could see the female (maximum distance for visual recognition is 32 cm [29]) and interacted with each other while competing for the female's attention through courtship. Group size naturally ranged from 2 to 7 individuals in this species, which has been observed in other synchronously waving fiddler crab species [17].
We recorded each group directly from above with a video camera (JVC GZ-EX355BAA) supported on a tripod. We placed a small ruler on the ground to measure distances and male sizes in the videos. From the recorded videos, we selected a period of 20 s (natural female choice duration; electronic supplementary material, table S1) with the highest activity to use for further analysis. We discarded a recording if any of the males did not engage in courtship behaviour, a more distant neighbour (over 32 cm) joined in later or a wandering crab approached the group. We tested 56 distinct females in total. Between trials females were kept in individual plastic cups filled with approximately 5 ml seawater and kept in the shade to reduce stress. Females were released back onto the mudflats once they had participated in up to three recording attempts (on the same day as capture).
(c) . Data extraction
To investigate how aspects of the biology of A. mjoebergi directly and indirectly correlate with male leadership and synchrony, we observed the videos frame-by-frame at a rate of 30 frames s−1 using the software digilite (J. Hemmi and R. Parker—The MathWorks, Inc., Natick, MA, USA). We observed each male in the group separately to avoid potential bias from observing the neighbouring males’ activity. To quantify male activity, we captured the precise timing of the two stages of the displays (wave start and apex) and obtained: (i) wave rate (courtship effort), (ii) leadership, and (iii) synchrony. We also counted the number of males in the group (level of competition), and by using the ruler in the videos, we converted pixels into centimetres and obtained: (i) the average distances between the female, the male and his burrow (risk-taking) and (ii) males' sizes (quality) by measuring carapace width.
We considered two important spatial measures in our analyses. First, the distance that the male travels from his burrow (hereafter referred to as male-to-burrow) was used as a measure of risk-taking (by leaving the burrow unattended to approach and court the female). Second, the distance between a male's burrow and the female (hereafter referred to as female-to-burrow) was an imposed distance created by the researcher when placing the female on the ground (figure 1a). These spatial measures cannot be replaced by the distance between the male and the female, because it disregards the imposed female-to-burrow distance.
Figure 1.

Graphical representations of the study's methods. (a) A male fiddler crab waving to a female: red arrow indicates the distance between the male's burrow and the female (female-to-burrow); blue arrow indicates the distance between the male and his burrow (male-to-burrow). (b) (i) A group of five males, represented by the colours 1-blue, 2-red, 3-yellow, 4-green, 5-purple (y-axis), waving for 20 s (x-axis). The horizontal black bars indicate the duration of each wave from start to apex. The vertical bars intersecting the horizontal black bars show the time intervals when at least two individuals overlapped their waves, and the colour indicates the identity of the leader. When a dot is next to a male's number on the y-axis, it indicates that other males entered in synchrony with that male. The black arrows indicate a few common scenarios: (I) non-overlapping waving of male 1, (II) simultaneous waving of males 2 and 5, (III) male 1 as a leader, (IV) simultaneous waving start of males 3 and 4, but male 3 reaches apex first. (ii) A circular graph representing the phase angles of each male in relation to all other males in the group. Values range from 180° (alternating waves) to 0° = 360° (simultaneous waves).
Given that males are under competition imposed by their own group, we normalized the distances (female-to-burrow and male-to-burrow), male size and wave rate in relation to the values of the other individuals in the group. We calculated how much each measurement deviated from the group average, that is, mi–<mg>, where mi is the measure of an individual and <mg> is the average among all individuals within the same group. This allowed us to compare individuals from distinct groups, where positive or negative values indicate that the measurement is above or below the average, respectively.
We determined leadership when at least two individuals overlapped their waves (from start to apex—the most conspicuous and distinguishable moments of the display [9,30,31]) and when there was a unique wave per individual. Thus, if a male waves after the wave apex of a previous male and reaches its own wave apex before the wave start of a following male, it is a lone waver. However, when males overlap their waves, the leader is defined when a male starts waving prior to at least another male, and not during the wave of any other male. A male gives a following wave when it starts within the wave start and apex of any male (leader or another follower). If males start at the same time, the first male to reach apex is the leader. If males start and reach wave apex at the same time, both are leaders (figure 1b). Next, we counted the number of times that each male was a leader over the total number of overlapping wave events. However, the number of leadership events of a given male will depend on group size. For example, the proportion of leadership of each male by chance would be 50% in a group of two and 33% in a group of three males. Thus, we accounted for the effect of group size by first evaluating how much the individual participation deviated from random participation, di = pi − 1/ng, where pi is the percentage of leadership events performed by a male i whose group is composed by ng males. However, this deviation could still be affected by group size. If a male leads all the time, its deviation would be di = 0.5 in a group of two males and di = 0.67 in a group of three males. Thus, we normalized di by the maximum possible deviation (1 − 1/ng), ending with: d′i = di/(1 − 1/ng), and the leadership normalization ranging from 1 to −1. Negative values indicate that a male leads less than expected by chance given its group size, positive values indicates that a male leads more than expected by chance and zero exactly as expected by chance.
Synchrony was defined by signalling adjustments between the members of a group [1,9], where individuals do not need to display at the same time to be synchronized, but they need to keep an adjusted rhythm (i.e. the time delay). This concept is employed to measure synchrony in fiddler crabs [9,23,32] and we used it in the present study. We measured the wave delay as the set of phase angles that would result from the wave delay of male A in relation to male B and vice-versa. We then confirmed that A is synchronized with B if the wave delay was constant (phase angles not uniformly distributed) by applying Rayleigh's test [32]. We highlight that the phase angles of A in relation to B can differ from B in relation to A. Thus, each male has a set of independent phase angles where it is possible that male A is in synchrony with B, and B is not in synchrony with A. For our analysis, we were interested in evaluating if all other males in the group synchronize with a given individual. To do this, we combined all phase angles obtained from each other male in relation to that individual and tested for uniformity. The output of the analysis is binary (yes or no, with p < 0.05) where each male gets a value, meaning that other males are synchronized or not with the given individual. Although males from larger groups have a larger list of phase angles, the number of phase angles does not alter the chances of presenting a uniform (asynchrony) or non-uniform (synchrony) distribution (electronic supplementary material, figure S1).
We defined synchrony as the non-uniformity of phase angles, because it is a more accurate approach as opposed to counting simultaneous signals. In addition, determining a threshold that defines signals in synchrony (i.e. phase angle of 30°, 40° or 50°) is arbitrary and imprecise. Nevertheless, synchrony (non-uniform phase angles) in fiddler crabs is also characterized by simultaneous signals (around 0° phase angles, see Results) [9,30] and, therefore, follows the traditional definition.
(d) . Statistical analyses
To understand how the level of competition, male quality, risk-taking and courtship effort affect leadership and generate synchrony, we ran structural equation models (SEMs) using the R-package piecewiseSEM [33]. SEMs are a regression-based approach where variables can function as both predictor and response within a single multivariate model. This approach is useful to identify indirect effects through a series of sequential and interconnected models proposed at once. Given that some of our response variables were not normally distributed, we fit SEMs through the d-separation approach. This approach translates a directed acyclic graph (i.e. box-and-arrow diagram indicating directed linkages) into a set of linear equations that are evaluated independently, allowing for the inclusion of non-normally distributed response variables and random effects to account for non-independence in the data. The way that variables are related results in a set of direct and indirect links whose effects will be estimated, except for relationships between variables that are not specified. The non-specified relationships among variables are called independence claims and represent hypotheses of independence [34]. By testing the validity of the set of independence claims, we test for the fit of the entire model. In the d-separation approach, model fit is tested through Fisher's C-test, where the null hypothesis is that the set of independence claims is true. In this way, failing to reject the null hypothesis implies that the set of independence claims fitted through the SEM is not statistically different from the true set, and the proposed SEM represents a reliable causal structure to explain the relationships among the variables [34]. The hypothesized model structure was supported by Fisher's C-test (C6df = 7.194; p = 0.303) and implemented with the following equations for each response variable:
-
1.
distance male-to-burrow = distance female-to-burrow + male size + group size;
-
2.
wave rate = distance male-to-burrow + distance female-to-burrow + male size + group size;
-
3.
leadership = distance male-to-burrow + male size + wave rate; and
-
4.
synchrony = distance male-to-burrow + male size + group size + wave rate + leadership.
The Fisher's C-test indicated that female-to-burrow is not independent from synchrony. However, the addition of this direct relationship did not improve considerably the power of the individual model (electronic supplementary material, file S1) and we opted for the simpler model structure above. The response variables contain the values of each male separately. Given that the behaviour of each male is linked to the behaviour of the other individuals in the group, we considered video identity as a random effect for all the independent equations. The last equation has a binomial error distribution and logit link function. It was fitted as a binomial generalized linear mixed model (GLMM), where synchrony is coded as 1-synchrony and 0-asynchrony. All other equations have an approximate Gaussian distribution and were fitted as linear mixed models (LMM). All models were fitted through the package lme4 [35]. The assumptions of each independent mixed equation were accessed through residual plots and tested for normality, variance and presence of outliers using the DHARMa package [36].
3. Results
The results from our structural model are illustrated in figure 2 with estimates of the significant relationships (all relationships depicted in the electronic supplementary material, figure S2) and our main hypotheses indicated in Roman numerals. Detailed results of each equation are listed below.
Figure 2.

Structural equation model (SEM) results. Arrows depict the hypothesized causal relationships of the SEM, and boxes represent predictor and response variables, in which boxes receiving arrow heads are response variables and boxes sending arrows are predictor variables. Solid arrows depict significant effects and dashed arrows represent non-significant effects. Black and grey lines represent positive effects, green and light green lines represent negative effects. Numbers next to solid arrows are non-standardized effects. Numbers inside boxes are conditional R2 values of each independent equation. The hypotheses listed in the introduction are indicated in blue Roman numerals near arrowheads.
(a) . Male-to-burrow distance
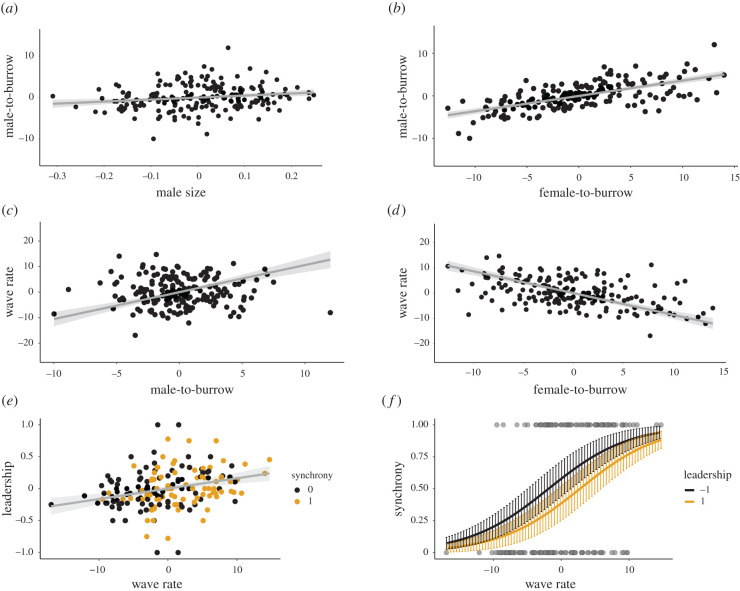
Larger males take higher risks by travelling further from their burrows (male-to-burrow) (estimate: 4.888 ± 1.517; p = 0.002; table 1; figure 3a). Males travel further away from their burrows when they are also further away from the female (female-to-burrow) (0.359 ± 0.030; p < 0.001; table 1; figure 3b). However, the effect of male quality (size) on male-to-burrow distance is weaker than that of female-to-burrow distance (standard estimate: 0.643 versus 0.175; table 1; figure 3a,b). Lastly, males take the same relative risks regardless of the competition imposed by their group size (0 ± 0.126; p = 1; table 1).
Table 1.
Direct effects of the causal relationships in the global model. (Columns represent response and predictor variables for each independent equation, LMM and GLMM estimated effects ± standard error, standard estimates and p-values.)
| response | predictor | estimate | std. estimate | p-values |
|---|---|---|---|---|
| male-to-burrow | male size | 4.888 ± 1.517 | 0.175 | 0.002 |
| male-to-burrow | group size | 0 ± 0.126 | 0 | 1.000 |
| male-to-burrow | female-to-burrow | 0.359 ± 0.0304 | 0.643 | 0 |
| wave rate | female-to-burrow | −0.86 ± 0.076 | −0.846 | 0 |
| wave rate | male-to-burrow | 1.056 ± 0.138 | 0.581 | 0 |
| wave rate | group size | 0 ± 0.238 | 0 | 1.000 |
| wave rate | male size | −4.309 ± 2.939 | −0.085 | 0.145 |
| leadership | male-to-burrow | −0.009 ± 0.007 | −0.085 | 0.230 |
| leadership | male size | 0.356 ± 0.196 | 0.128 | 0.072 |
| leadership | wave rate | 0.016 ± 0.004 | 0.296 | 0 |
| synchrony | group size | 0.369 ± 0.321 | — | 0.249 |
| synchrony | leadership | −0.744 ± 0.785 | — | 0.344 |
| synchrony | wave rate | 0.287 ± 0.06 | — | 0 |
| synchrony | male-to-burrow | −0.124 ± 0.076 | — | 0.103 |
| synchrony | male size | −1.012 ± 2.104 | — | 0.631 |
Figure 3.
Main predicted effects for each independent fitted equation. Lines represent the predicted effect and 95% confidence interval of (a) male size on the male's distance from its burrows; (b) female distance on the male's distance from its burrows; (c) male distance from burrow on wave rate; (d) female distance from the male's burrow on male's wave rate; (e) male wave rate on the occurrence of leadership where colours depict points with (1) or without (0) synchrony; and (f) predicted effect and standard error of male's wave rate on the probability of synchrony events, each line represents the extreme levels of leadership—(1) leads more and (−1) less than expected by chance. All variables are relative measurements and do not have units.
(b) . Wave rate
Males that take greater risks by travelling further away from their burrow to approach the female (male-to-burrow), invest more into courtship effort (wave rate) (1.056 ± 0.138; p < 0.001; table 1; figure 3c). However, as the initial distance from the female (female-to-burrow) is large, males wave at lower rates (−0.86 ± 0.076; p < 0.001; table 1; figure 3d). As with male-to-burrow distance, when female-to-burrow distance is large, males also tend to travel further away from their burrows. Thus, the total effect of the female-to-burrow distance on wave rate is the sum of its direct and indirect effects through the male-to-burrow distance (total effect: −0.481; electronic supplementary material, table S2). Moreover, male size does not affect courtship effort (−4.309 ± 2.939; p = 0.145) and males also wave at the same relative rate regardless of their group size (0 ± 0.238; p = 1; table 1).
(c) . Leadership
Males waving at faster rates tended to be leaders (0.016 ± 0.004; p < 0.001; table 1; figure 3e). However, neither the distance between a male and his burrow (male-to-burrow) (−0.009 ± 0.007; p = 0.23; table 1), nor his size affect leadership (0.356 ± 0.196; p = 0.072; table 1).
(d) . Synchrony
Synchrony was characterized not only by non-uniform phase angles distributions, but also by small values of phase angles, meaning that synchronous males waved almost simultaneously (electronic supplementary material figure S3). Males with faster wave rates are more likely to be in synchrony with their neighbours (0.287 ± 0.06; p < 0.001; table 1; figure 3f). However, the proportion of times a male leads does not influence his probability of synchronizing (−0.744 ± 0.785; p = 0.344; table 1; figure 3f). In addition, neither group size (0.369 ± 0.321; p = 0.249), male-to-burrow distance (−0.124 ± 0.076; p = 0.103), nor male size (−1.012 ± 2.104; p = 0.631; table 1) directly affect the probability of a male synchronizing with his neighbours.
To further validate our results, we re-ran the analyses with 55 groups randomly selected 10 times. None of the previous significant results changed or were influenced by outliers (group 27). However, male size had a significant positive effect and male-to-burrow had a marginally significant negative effect on leadership in two of the subsets (electronic supplementary material, file S2). Thus, these relationships must be interpreted with caution.
4. Discussion
In the present paper, we provide a comprehensive assessment of a series of interconnected effects that may produce imperfect courtship synchrony in a synchronously signalling fiddler crab species. Our results confirm previous findings and reveal unprecedented information about the core mechanisms underlying this epiphenomenon. Surprisingly, we found that the level of competition mediated by group size does not affect courtship. Nevertheless, larger males did take greater risks by travelling further from their burrows to approach a female, and in turn, had higher courtship effort (wave rate) and leadership. However, male quality (size) did not directly correlate with courtship effort, leadership or even synchrony. Finally, the likelihood of leadership and group synchrony were predicted by wave rate, but leadership had a lesser role in synchronous courtship than expected.
(a) . No link between group size and courtship
The number of neighbours did not affect synchronous courtship in A. mjoebergi fiddler crabs. This supports previous work on a South American species, Leptuca leptodactyla, although synchrony was somewhat favoured in lower densities [30]. Our findings also revealed that the number of males in a group did not affect either relative courtship effort or risk-taking. A male's success may partly rely on the competitive ability of neighbours and fiddler crabs actively select their neighbours through territorial combats [37]. Thus, selecting the number of neighbours may have a negligible effect on courtship and, in part, mating success. However, we should take caution with this interpretation as wave rate and male-to-burrow distance are relative measures.
(b) . Indirect effects of male quality
Studies of synchronous, acoustic-signalling taxa have examined proximate relationships between male quality and signal leadership by testing several indicators of male quality [13,14,16]. In Neoconocephalus katydids, leadership is highly repeatable, but is not heritable, and is unrelated to male morphology, fecundity or condition [13,15]. Similarly, in our study we found no direct link between male size with courtship effort and leadership (figure 2). It is unclear why females prefer fast wave rate and leading waves if these qualities do not directly relate to male size, which is condition-dependent and partially linked to fitness benefits. A possible explanation is that female preference for leading waves is driven by a pre-existing sensory bias [38]. However, we found that larger males were more likely to approach the female (figure 3a) and, in turn, invest more into courtship (figure 3c). Longer distances between the male's burrow and a female motivates him to approach the female (figure 3b), and consequently travel away from his burrow, and this trend is more pronounced in larger males. Thus, we show that male size indirectly links to courtship. However, future studies are needed to investigate more precise indicators of male condition and reproductive success and if males can be habitual leaders.
According to the handicap hypothesis, high-quality males produce costly signals evidencing a relationship between male signal and quality [39]. Indeed, an indirect link between male quality and leadership has been previously revealed in other taxa, as leadership can be related to other signal characteristics that demand energetic investment [7,16]. Our results show that leadership is linked to fast signalling rates (figure 3e). These are two courtship elements that are also correlated in synchronously signalling insects and anurans [14,15,16,40]. Leadership could, therefore, be costly for males in terms of higher energetic demands, meaning only males in good condition can produce leading waves. Both wave rate and leadership are preferred traits for female A. mjoebergi [41], and other fiddler crab species (Austruca occidentalis, [42]; A. perplexa, [22]). However, female L. saltitanta fiddler crabs prefer only fast wave rates and not males that produce leading waves [22]. Thus, we suggest that the preference for leadership has evolved after the preference for fast wave rate.
(c) . Rethinking imperfect synchrony emergence
Imperfect synchrony was first confirmed when researchers discovered that male katydids employ an inhibitory-resetting strategy to give leading chirps [7]. The resulting competition observed both in playback experiments and computer simulations generated almost simultaneous signalling between individuals [7]. By combining evidence of female preference for leading signals, the traditional theoretical explanation for imperfect synchrony is that it arises incidentally from the competition for leadership [7,10]. Moreover, by employing the inhibitory-resetting mechanism, males also tend to give fast rate signals, because they are less often inhibited [14,23]. In this study, we complement this information by demonstrating that synchrony is not a direct outcome of the leadership role as previously thought (figure 2). Instead, synchrony is correlated with fast wave rates, where males participate more often in simultaneous waving events and produce extra waves in-between [22] (figure 3f; electronic supplementary material, figure S3). However, when trying to achieve leadership by increasing wave rate, males will strongly synchronize with rivals (figure 2). Thus, the theory that leadership generates synchrony is not entirety inaccurate.
Given that the main mechanism for synchrony emergence is wave rate, a group of competitive males would engage in a faster rate and, as a consequence, more likely synchronize. Thus, if females prefer fast wave rates and leadership, they should be more attracted to such competitive groups in synchrony. This is true in the bushcricket, Mecopoda elongata, where females are attracted to synchronous groups of males because together they amplify their signal. However, females also prefer leading chirps and fast chirp rate, evidencing the competitive element of that interaction [3]. Evidence that synchrony in fiddler crabs is adaptive is in fact observed in L. saltitanta and A. perplexa that present synchrony in the absence of females [22]. Indeed, synchrony may have a partially adaptive role in A. mjoebergi, because females are attracted to distant males if there is one leading male followed by neighbours in synchrony (L. Harrison and P. Backwell 2021, personal communication).
5. Conclusion
Extensive research has repeatedly investigated courtship synchrony, but many aspects of this phenomenon remain to be understood. By exploring a series of interconnected effects in synchronous courtship in fiddler crabs, we unveil important aspects about imperfect synchrony that were once previously overlooked. This study emphasizes the importance of basic research through systematic field observations and detailed behavioural data collection.
Acknowledgements
We thank the North Australian Research Unit for facilities; Patricia R.Y. Backwell for fieldwork access and equipment use; Lauren Harrison, Michael Greenfield and Marcio R. Pie for comments; Lauren Harrison and Gabriela Melo for providing supplementary data and assistance with fieldwork. ASBL thanks her children's grandmother for helping her with the children during the COVID-19 pandemic and enabling her to carry out this work.
Ethics
This project was approved by the Australian National University Animal Ethics Committee (A2018/44) and the research was conducted under the permit DCC2322876—Darwin City Council. Females were kept in individual cups under the shade when not being used. After the experiment females were released in a separate area of the mudflat to avoid recapture. Handling was limited and no crabs were injured during the study.
Data accessibility
The computing codes and datasets supporting this article have been uploaded as part of the electronic supplementary material [43].
Authors' contributions
D.M.P. designed the study, collected and analysed the data, and prepared the manuscript. S.B.L.A. and C.L.K. designed the study, analysed the data and prepared the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES)—Finance code 001 to D.M.P. and C.L.K.
References
- 1.Pikovsky A, Rosenblum M, Kurths J. 2001. Synchronization: a universal concept in nonlinear sciences, vol. 12. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Greenfield MD. 1994. Cooperation and conflict in the evolution of signal interactions. Annu. Rev. Ecol. Syst. 25, 97-126. ( 10.1146/annurev.es.25.110194.000525) [DOI] [Google Scholar]
- 3.Hartbauer M, Haitzinger L, Kainz M, Römer H. 2014. Competition and cooperation in a synchronous bushcricket chorus. R. Soc. Open Sci. 1, 140167. ( 10.1098/rsos.140167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuttle MD, Ryan MJ. 1982. The role of synchronized calling, ambient light, and ambient noise, in anti-bat-predator behavior of a treefrog. Behav. Ecol. Sociobiol. 11, 125-131. ( 10.1007/BF00300101) [DOI] [Google Scholar]
- 5.Moiseff A, Copeland J. 2010. Firefly synchrony: a behavioral strategy to minimize visual clutter. Science 329, 181-181. ( 10.1126/science.1190421) [DOI] [PubMed] [Google Scholar]
- 6.Greenfield MD. 1994. Synchronous and alternation choruses in insects and anurans: common mechanisms and diverse functions. Am. Zool. 34, 605-615. ( 10.1093/icb/34.6.605) [DOI] [Google Scholar]
- 7.Greenfield MD, Roizen I. 1993. Katydid synchronous chorusing is an evolutionarily stable outcome of female choice. Nature 363, 618-620. ( 10.1038/364618a0) [DOI] [Google Scholar]
- 8.Greenfield MD, Marin-Cudraz T, Party V. 2017. Evolution of synchronies in insect choruses. Biol. J. Linnean Soc. 122, 487-504. ( 10.1093/biolinnean/blx096) [DOI] [Google Scholar]
- 9.Reaney LT, Sims RA, Sims SWM, Jennions MD, Backwell PRY. 2008. Experiments with robots explain synchronized courtship in fiddler crabs. Curr. Biol. 18, 62-63. ( 10.1016/j.cub.2007.11.047) [DOI] [PubMed] [Google Scholar]
- 10.Greenfield MD. 1997. Acoustic communication in Orthoptera. In The bionomics of grasshoppers, katydids and their kin (eds Gangwere SK, Muralirangan MC, Muralirangan M), pp. 197-230. Wallingford, UK: CAB International. [Google Scholar]
- 11.Greenfield MD, Esquer-Garrigos Y, Streiff R, Party V. 2016. Animal choruses emerge from receiver psychology. Sci. Rep. 6, 34369. ( 10.1038/srep34369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Party V, Streiff R, Marin-Cudraz T, Greenfield MD. 2015. Group synchrony and alternation as an emergent property: elaborate chorus structure in a bushcricket is an incidental by-product of female preference for leading calls. Behav. Ecol. Sociobiol. 69, 1957-1973. ( 10.1007/s00265-015-2008-8) [DOI] [Google Scholar]
- 13.Murphy MA, Schul J. 2017. Does leadership indicate male quality in Neoconocephalus katydids? Behav. Ecol. Sociobiol. 71, 22. ( 10.1007/s00265-016-2253-5) [DOI] [Google Scholar]
- 14.Hartbauer M, Kratzer S, Römer H. 2006. Chirp rate is independent of male condition in a synchronising bushcricket. J. Insect. Physiol. 52, 221-230. ( 10.1016/j.jinsphys.2005.10.006) [DOI] [PubMed] [Google Scholar]
- 15.Murphy MA, Gerhardt HC, Schul J. 2017. Leader preference in Neoconocephalus ensiger katydids: a female preference for a nonheritable male trait. J. Evol. Biol. 30, 2222-2229. ( 10.1111/jeb.13188) [DOI] [PubMed] [Google Scholar]
- 16.Richardson C, Lena JP, Joly P, Lengagne T. 2008. Are leaders good mates? A study of call timing and male quality in a chorus situation. Anim. Behav. 76, 1487-1495. ( 10.1016/j.anbehav.2008.06.019) [DOI] [Google Scholar]
- 17.Backwell PRY. 2018. Synchronous waving in fiddler crabs: a review. Cur. Zool. 65, 83-88. ( 10.1093/cz/zoy053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane J. 1975. Fiddler crabs of the world. Ocypodidae: genus Uca. Princeton, NJ: Princeton University Press. [Google Scholar]
- 19.Perez DM, Rosenberg MS, Pie MR. 2012. The evolution of waving displays in fiddler crabs (Uca spp., Crustacea: Ocypodidae). Biol. J. Linnean Soc. 106, 307-315. ( 10.1111/j.1095-8312.2012.01860.x) [DOI] [Google Scholar]
- 20.Perez DM, Christy JH, Backwell PRY. 2016. Choosing a mate in a high predation environment: female preference in the fiddler crab Uca terpsichores. Ecol. Evol. 6, 7443-7450. ( 10.1002/ece3.2510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez DM, Backwell PRY. 2019. Male spacing and female choice in a fiddler crab. Behav. Ecol. 30, 1769-1774. [Google Scholar]
- 22.Backwell PRY, Jennions MD, Wada K, Murai M, Christy JH. 2006. Synchronous waving in two species of fiddler crabs. Acta Ethol. 9, 22-25. ( 10.1007/s10211-005-0009-8) [DOI] [Google Scholar]
- 23.Perez DM, Crisigiovanni EL, Pie MR, Rorato AC, Lopes SR, Araujo SBL. 2020. Ecology and signal structure drive the evolution of synchronous displays. Evolution 74, 434-446. ( 10.1111/evo.13841) [DOI] [PubMed] [Google Scholar]
- 24.Booksmythe I, Detto T, Backwell PRY. 2008. Female fiddler crabs settle for less: the travel costs of mate choice. Anim. Behav. 76, 1775-1781. ( 10.1016/j.anbehav.2008.07.022) [DOI] [Google Scholar]
- 25.Callander S, Jennions MD, Backwell PRY. 2012. The effect of claw size and wave rate on female choice in a fiddler crab. J. Ethol. 30, 151-155. ( 10.1007/s10164-011-0309-6) [DOI] [Google Scholar]
- 26.Mowles SL, Jennions MD, Backwell PRY. 2017. Multimodal communication in courting fiddler crabs reveals male performance capacities. R. Soc. Open Sci. 4, 161093. ( 10.1098/rsos.161093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez DM, Heatwole SJ, Morrell LJ, Backwell PRY. 2015. Handedness in fiddler crab fights. Anim. Behav. 110, 99-104. ( 10.1016/j.anbehav.2015.09.012) [DOI] [Google Scholar]
- 28.Chou C-C, Perez DM, Johns S, Gardner R, Kerr KA, Head ML, McCullough EL, Backwell PRY. 2019. Staying cool: the importance of shade availability for tropical ectotherms. Behav. Ecol. Sociobiol. 73, 106. ( 10.1007/s00265-019-2721-9) [DOI] [Google Scholar]
- 29.How MJ, Zeil J, Hemmi JM. 2007. Differences in context and function of two distinct waving displays in the fiddler crab, Uca perplexa (Decapoda: Ocypodidae). Behav. Ecol. Sociobiol. 62, 137-148. ( 10.1007/s00265-007-0448-5) [DOI] [Google Scholar]
- 30.Perez DM, Backwell PRY. 2019. Selection for conspicuous visual signals in a fiddler crab. Behav. Ecol. Sociobiol. 73, 61. ( 10.1007/s00265-019-2670-3) [DOI] [Google Scholar]
- 31.Perez DM, Backwell PRY. 2020. The functions of multiple visual signals in a fiddler crab. Ethology 126, 455-462. ( 10.1111/eth.12993) [DOI] [Google Scholar]
- 32.Rorato AC, Araujo SBL, Perez DM, Pie MR. 2017. Social cues affect synchronization of male waving displays in a fiddler crab (Crustacea: Ocypodidae). Anim. Behav. 126, 293-300. ( 10.1016/j.anbehav.2017.02.014) [DOI] [Google Scholar]
- 33.Lefcheck JS. 2016. piecewiseSEM: piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573-579. ( 10.1111/2041-210X.12512) [DOI] [Google Scholar]
- 34.Shipley B. 2000. A new inferential test for path models based on directed acyclic graphs. Struct. Equ. Model. 7, 206-218. ( 10.1207/S15328007SEM0702_4) [DOI] [Google Scholar]
- 35.Bates D, Maechler M, Bolker B, Walker S.. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 36.Hartig F. 2020. DHARMa: residual diagnostics for hierarchical (multi-level / mixed) regression models. R package version 0.3.2.0. See https://CRAN.R-project.org/package=DHARMa.
- 37.Booksmythe I, Jennions MD, Backwell PRY. 2010. Investigating the ‘dear enemy’ phenomenon in the territory defence of the fiddler crab, Uca mjoebergi. Anim. Behav. 79, 419-423. ( 10.1016/j.anbehav.2009.11.020) [DOI] [Google Scholar]
- 38.Land M, Layne J. 1995. The visual control of behaviour in fiddler crabs. I. Resolution, thresholds and the role of the horizon. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 177, 81-90. ( 10.1007/BF00243400) [DOI] [Google Scholar]
- 39.Zahavi A. 1975. Mate selection: a selection for a handicap. J. Theor. Biol. 53, 205-214. ( 10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 40.Hartbauer M, Kratzer S, Steiner K, Römer H. 2005. Mechanisms for synchrony and alternation in song interactions of the bushcricket Mecopoda elongata (Tettigoniidae: Orthoptera). J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 191, 75-188 ( 10.1007/s00359-004-0586-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mowles SL, Jennions MD, Backwell PRY. 2018. Robotic crabs reveal that female fiddler crabs are sensitive to changes in male display rate. Biol. Lett. 14, 20170695. ( 10.1098/rsbl.2017.0695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Backwell PRY, Jennions MD, Christy JH, Passmore JH. 1999. Female choice in the synchronously waving fiddler crab Uca annulipes. Ethology 105, 415-421. ( 10.1046/j.1439-0310.1999.00387.x) [DOI] [Google Scholar]
- 43.Perez DM, Klunk CL, Araujo SBL. 2021. Imperfect synchrony in animal displays: why does it occur and what is the true role of leadership? Figshare. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The computing codes and datasets supporting this article have been uploaded as part of the electronic supplementary material [43].