ABSTRACT
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the COVID-19 pandemic, responsible for millions of deaths globally. Even with effective vaccines, SARS-CoV-2 will likely maintain a hold in the human population through gaps in efficacy, percent vaccinated, and arising new strains. Therefore, understanding how SARS-CoV-2 causes widespread tissue damage and the development of targeted pharmacological treatments will be critical in fighting this virus and preparing for future outbreaks. Herein, we summarize the progress made thus far by using in vitro or in vivo models to investigate individual SARS-CoV-2 proteins and their pathogenic mechanisms. We have grouped the SARS-CoV-2 proteins into three categories: host entry, self-acting, and host interacting. This review focuses on the self-acting and host-interacting SARS-CoV-2 proteins and summarizes current knowledge on how these proteins promote virus replication and disrupt host systems, as well as drugs that target the virus and virus interacting host proteins. Encouragingly, many of these drugs are currently in clinical trials for the treatment of COVID-19. Future coronavirus outbreaks will most likely be caused by new virus strains that evade vaccine protection through mutations in entry proteins. Therefore, study of individual self-acting and host-interacting SARS-CoV-2 proteins for targeted therapeutic interventions is not only essential for fighting COVID-19 but also valuable against future coronavirus outbreaks.
KEYWORDS: SARS-CoV-2, COVID-19, drug development, individual virus proteins, virulence factors, virus-host interactions, Nsp1, Nsp6, Orf3a, Orf6, Orf7a
INTRODUCTION
Coronaviruses (CoVs) have been circulating among the human population for centuries and cause a portion of the common colds (human CoV HKU1 [hCoV-HKU1], hCoV-OC43, hCoV-229E, and hCoV-NL63). However, more recently, three CoVs have caused serious threats to global human health: severe acute respiratory syndrome coronavirus (SARS-CoV; 2002 to 2003) and Middle East respiratory syndrome coronavirus (MERS-CoV; 2012 to present) outbreaks and the ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; 2019 to present) pandemic. These coronaviruses share many features; however, marked differences in sequence, structure, and function have been identified. In order to understand how these viruses cause disease and to identify the most effective targets for therapeutic intervention, we need to identify the sites of virus-host interaction and their impact on host systems. Studies of individual virus proteins will be crucial to accomplish this.
Virus-encoded proteins, similar to those from higher-order organisms, carry out specialized functions, with shared and unique features found across virus families. Because viruses are dependent on host mechanisms for replication, these specializations are visible in virus-host interactions as well, including various tactics to manipulate host systems for immune evasion. For human immunodeficiency virus (HIV) (Vpr, Tat, and Nef proteins), influenza virus (M2 and NS1 proteins), and Zika virus (ZIKV) (NS4A protein), to name just a few human viruses, studies of individual virus proteins have revealed a tremendous amount about specific virus-host interactions, effects on host pathways, and resulting detrimental functional consequences in tissue-specific pathology (1–7). These studies have revealed the importance of individual virus proteins to pathogenicity by implicating host pathways enlisted to facilitate the virus replication cycle and to promote virus gene expression, hijacking of the host proteasome function, evasion of the host immune response (NF-κB; JAK/signal transducer and activator of transcription [STAT] signaling), and the role of host endocytic and secretory trafficking pathways, as well as the contribution of host-modifying factors. More importantly, these findings have provided a foundation for the development of new therapeutics driven by pharmacological inhibitors of specific virus-host interactions. The first of these compounds are entering clinical trials (for example, GS-6207, which targets the HIV-1 capsid protein).
COVID-19, the disease caused by SARS-CoV-2 infection, is notable for its detrimental effect on multiple organs and tissues. In-depth studies of how individual virus proteins disrupt host pathways will be crucial to identify the targets most effective for countering tissue damage and to develop inhibitors of these specific virus-host interactions for therapeutic intervention. In addition, these studies would provide insight into the differences between viral strains, e.g., why SARS-CoV-2 is so virulent, and aid in preparing for a future outbreak. This review covers studies available through PubMed prior to mid-February 2021.
SARS-CoV-2 PROTEOME
Genomic organization.
The SARS-CoV-2 genome encodes 28 confirmed proteins (Fig. 1). Open reading frame 1ab (Orf1ab), by far the largest gene, is located at the 5′ end and encodes polyproteins PP1ab and PP1a. These polyproteins are cleaved into 16 nonstructural proteins (Nsp1 to Nsp16). The remaining 3′ sequence contains genes encoding the four structural proteins (spike [S], envelope [E], membrane [M], and nucleocapsid [N]) and eight accessory proteins (Orf3a, Orf3b, Orf6, Orf7a, Orf7b, Orf8, Orf9b, [Orf9c lies outside verified SARS-CoV-2 open reading frame] and Orf10). Notably, sequence conservation among the three most pathogenic human CoVs is not uniform across the genome. Overall conservation is highest for the polyprotein Nsp proteins, while the genome sequence encoding the accessory factors (Orfs) diverges decidedly (8). MERS-CoV (C lineage beta-CoV) shows most differences and even has fewer Orfs than SARS-CoV and SARS-CoV-2 (B lineage beta-CoV). These accessory factors typically lack well-defined domain structures, which leaves their functions largely unresolved and suggests that they might hold the key to the properties unique to each virus. Based on the literature on SARS-CoV-2 protein functions and interaction with host cells, we grouped the virus proteins into three categories: host entry, self-acting, and host interacting. We review each group and focus on individual SARS-CoV-2 proteins that can be targeted either directly by drugs or through inhibiting their host interactions.
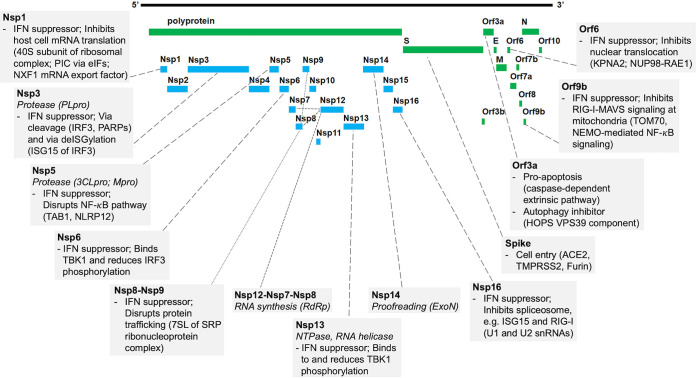
FIG 1.
SARS-CoV-2 protein functions and host interactions. Shown is a graphic representation of the SARS-CoV-2 proteome. Boxes connected to proteins indicate the virus transcription and translation functions (italics) (summary of Table 1) and known human protein interaction with their effect on human pathways (summary of Table 2). Abbreviations: 3CLpro, 3-chymotrypsin-like protease; 7SL, RNA component of SRP ribonucleoprotein complex; ACE2, angiotensin I-converting enzyme 2; eIFs, eukaryotic translation initiation factors; ExoN, exoribonuclease; HOPS, homotypic fusion and protein sorting complex; IFN, interferon; IRF3, interferon regulatory factor 3; ISG, interferon-stimulated gene; KPNA2, karyopherin alpha 2; MAVS, mitochondrial antiviral-signaling protein; Mpro, main protease; NEMO, NF-κB essential modulator; NLRP12, NLR family pyrin domain containing 12; NUP98, nuclear pore complex protein 98; NXF1, nuclear RNA export factor 1; PARPs, poly(ADP-ribosyl) polymerases; PIC, ribosomal preinitiation complex; PLpro, papain-like protease; RAE1, RNA export 1; RdRp, RNA-dependent RNA polymerase; RIG-I, retinoic acid-inducible gene I; SRP, signal recognition particle; TAB1, TGF-β-activated kinase 1 (MAP3K7) binding protein 1; TBK1, TANK binding kinase 1; TMPRSS2, transmembrane serine protease 2; TOM70, translocase of outer membrane 70 (subunit of mitochondrial import receptor); VPS39, vacuolar protein sorting 39 (subunit of HOPS complex).
Host entry: virus spike protein and host factors to gain cell entry.
The interaction of the SARS-CoV-2 spike with the host cell receptor at the host membrane has been a topic of great interest, especially in the context of transmission and infectivity. Due to its wide coverage in the literature, we keep it brief here and instead focus in more detail on the intracellular virus-host interactions and their pathogenic effects on host systems. The virus employs interaction of its spike protein with host plasma membrane receptors and proteases to facilitate membrane fusion and gain host cell entry (Fig. 1). The spike proteins of both SARS-CoV and SARS-CoV-2 use the host angiotensin I-converting enzyme 2 (ACE2) receptor, while the MERS-CoV spike interacts with the host dipeptidyl peptidase (DPP4; also known as CD26) receptor to do this (9). The spike protein is generally considered well preserved; however, SARS-CoV-2 spike protein binding affinity for the ACE2 receptor is significantly stronger than that of its SARS-CoV counterpart, which has been attributed to a structural rearrangement (10). Moreover, numerous mutations have been identified in the SARS-CoV-2 spike protein since the start of the pandemic, just over a year ago (11, 12). Both SARS-CoV-2 and SARS-CoV make use of the host transmembrane protease serine 2 (TMPRSS2) for cleavage of the spike protein trimer at the S1/S2 site upon virus-host cell contact to engage the necessary conformational change prior to cell entry (13). In addition, SARS-CoV-2 contains a furin cleavage site. This site is new to the B-lineage hCoVs, which include SARS-CoV, but has been known from other highly virulent human viruses (such as influenza, measles, mumps, and Ebola viruses and HIV-1) (14, 15). In fact, it has been shown that loss of this furin cleavage site reduces SARS-CoV-2 pathogenesis in vitro (16). Furthermore, it was shown this furin-cleaved site provides a substrate for neuropilin-1 (NRP1), which acts as a cofactor for ACE2-mediated fusion (17). NRP1 is highly expressed in pulmonary and olfactory tissues, and infected olfactory epithelial cells in COVID-19 patients were shown to be NRP1 positive (17). These data suggest that NRP1 could act as a crucial cofactor for virus entry in cells with low ACE2 expression, like olfactory epithelium, thereby enhancing SARS-CoV-2 tropism (i.e., ability to infect different cell types and tissues).
Self-acting: SARS-CoV-2 proteins dedicated to virus translation and replication.
Viruses greatly depend on host systems for translation and replication; therefore, many of their proteins have evolved to interact with host proteins. These interactions target specific host proteins in order to hijack host pathways and create a favorable cellular environment. However, the virus genome also encodes several proteins specialized in virus replication. These carry out the virus-focused tasks of mRNA transcription, translation, and encapsulation (Table 1; Fig. 1). Among these replication-focused proteins are papain-like cysteine protease (PLpro; encoded within the Nsp3 gene) and 3-chymotrypsin-like protease (3CLpro; also known as main protease [Mpro]; encoded by the Nsp5 gene). PLpro cleaves Nsp1, Nsp2, and Nsp3 on Orf1a, while 3CLpro hydrolyzes the remaining Nsp4 to -16 units on the 1a and 1ab SARS-CoV-2 polyproteins. This step is essential for maturation of the nonstructural proteins into functional proteins for virus replication and transcription, as well as to suppress the host immune system. Furthermore, this process enables the virus to carry a larger genome (18–21). Nsp13 possesses NTPase and RNA helicase activities; consequently, it facilitates many steps of viral RNA processing, including replication, transcription, translation, and encapsidation (22). Another key virus-encoded factor for replication is the RNA-dependent RNA polymerase (RdRp) complex, which is responsible for the synthesis of viral RNA. The SARS-CoV-2 complex consists of Nsp12, which forms the catalytic power core (although it is highly inefficient when by itself), and the Nsp7 and Nsp8 cofactors, which greatly boost its polymerase activity (23). Nsp14 provides proofreading services during RNA replication. It detects and then removes any mismatched nucleotides using its exoribonuclease (ExoN) function, which possibly also contributes to virus RNA recombination (24). The latter is a common strategy among viruses to generate new strains that are better at evading the host immune response, less susceptible to vaccines, or might cross over to new host species.
TABLE 1.
SARS-CoV-2 proteins for virus transcription and translationa
| SARS-CoV-2 protein(s) | Protein/complex role | Function | Compared to SARS-CoV | Model system(s)b | Reference(s) |
|---|---|---|---|---|---|
| Nsp3 (PLpro) | Papain-like cysteine protease (PLpro) | Proteolytic cleavage of Nsp1 to -3 of Orf1a for functional maturation | Inhibitors specific for SARS-CoV PLpro activity, similarly, showed efficacy against SARS-CoV-2 PLpro | In vitro; cell lines, HEK293T and Vero CCL-81; cell-free system, Escherichia coli | 20 |
| Nsp5 (3CLpro) | 3-Chymotrypsin-like protease (3CLpro), also known as main protease (Mpro) | Proteolytic cleavage of Orf1a and Orf1ab polyproteins for functional maturation of virus Nsp proteins (Nsp4 to -16), including itself | Compound (GRL-0496) effectively inhibits both SARS-CoV and SARS-CoV-2 3CLpro activity; anti-SARS-CoV 3CLpro antiserum cross-reactive with SARS-CoV-2 3CLpro | In vitro; cell lines, HEK293T(/17), VeroE6, and Caco-2 (ACE2/TMPRSS2) | 19, 21 |
| Nsp12-Nsp7-Nsp8 (polymerase complex) | RdRp complex | Nsp12 is the catalytic subunit with RdRp (RNA synthesis) activity. Nsp7 and Nsp8 cofactors are required to boost efficiency. | Reduced RNA synthesis activity and lower thermostability than with the SARS-CoV core polymerase complex | Cryo-EM | 23, 54, 55 |
| Nsp13 | NTPase and RNA helicase activities | Viral RNA processing at many steps (replication, transcription, translation, and encapsidation) | Highly similar sequences; both have NTPase and RNA helicase activities; activity of both can be inhibited by bismuth salts in a dose-dependent manner | Cell-free system, Escherichia coli | 22 |
| Nsp14 | Proofreading exoribonuclease (ExoN) + RNA recombination | RNA proofreading during virus replication (resistance to nucleoside analogs) and RNA recombination, which drives immune—and vaccine—evasion (generation of subgenomic mRNAs, DVG, and novel strain emergence) | Similar recombination networks (pattern of recombination junctions across the genomes and flanking nucleotide sequences, including DVG junctions, as well as types of recombined RNAs generated) | In vitro; cell lines, DBT-9, Vero CCL-81, 7and VeroE6 | 24 |
Abbreviations: RdRp, RNA-dependent RNA polymerase; cryo-EM, cryo-electron microscopy; DVG, defective viral genome.
DBT-9, delayed brain tumor, murine astrocytoma clone 9 cell line; HEK293T, human embryonic kidney cell line; VeroE6/Vero CCL-81, African green monkey (Cercopithecus aethiops) kidney cell lines.
Host interacting: SARS-CoV-2 proteins targeting host proteins and the effects on host systems.
Viruses carry relatively small genomes and largely depend on host cell machinery for their survival. Therefore, the vast majority of virus proteins, including some of the ones dedicated to virus transcription and translation described above, have evolved to interact with specific host proteins (Table 2; Fig. 1). Unlike the spike-ACE2/TMPRSS2/furin interaction to gain host cell entry, the majority of virus-host interactions take place intracellularly and aim to gain control over host cell systems. Host processes are employed for virus replication (transcription/translation and related protein trafficking), to create a virus-favorable environment (shutting down host systems to divert cellular resources to virus replication) and evasion of the host immune response.
TABLE 2.
SARS-CoV-2 proteins interacting with intracellular host pathwaysa
| SARS-CoV-2 protein | Virus-host interaction | Effects on hostb | Model system(s) | Reference(s) |
|---|---|---|---|---|
| Nsp1 | 40S subunit of ribosomal complex; ribosomal PIC via eIFs; blocking mRNA entry channel | mRNA translation machinery; inhibition of host cell mRNA translation; disruption of retinoic acid-inducible gene I-dependent innate immune responses (reduced IFN-β and IL-8 gene expression) | In vitro; cell lines, HEK293T, HeLa, HEK293-EBNA, VeroE6, HEK-Blue-ISG, A549, Calu-3, HAP1, and H1299; cell-free system, Escherichia coli | 25 – 29 |
| mRNA export factor NXF1; disrupts NXF1 interaction with mRNA export adaptors and nuclear pore complex | mRNA translation machinery; inhibition of host cell mRNA translation | In vitro; cell lines, HEK293T and VeroE6 | 30 | |
| Nsp3 (PLpro) | IRF3l; reduced deISGylation (ISG15) of IRF3; also, cleavage of IRF3 | Innate immune response (IFN); indirect (deISGylation) and direct (cleavage) effects on the IRF3 pathways; strongly inhibits IRF3 activity and results in complete suppression of the type I IFN response | In vitro, cell lines, HeLa, A549, Caco-2, and 293T-ACE2; cell-free system, Leishmania tarentolae | 34, 35 |
| PARPs; macrodomain part of Nsp3 is an (ADP-ribosyl) hydrolase | Innate immune response (IFN); cleaves protein-ADPr bond to counteract host antiviral PARPs, which are induced during the IFN immune response | Cell-free system, Escherichia coli | 36, 37 | |
| Nsp5 (3CLpro) | TAB1; cleavage of TAB1 results in loss of C terminus | Innate immune response (proinflammatory cytokines); cleaved TAB1 leads to reduced TAK1 activation, decreased NF-κB signaling, and decreased cytokine production | In vitro; cell line, 293T-ACE2; cell-free system, Leishmania tarentolae | 34 |
| NLRP12; cleavage of NLRP12 (two cleavage sites) | Innate immune response (proinflammatory cytokines); NLRP12 inhibits NF-κB; cleaved NLRP12 leads to increased NF-κB signaling and thus increased cytokine production and disrupts assembly of the NLRP12/NLRP3 inflammasome, increasing pro-caspase-1 cleavage and thus IL-1β release; together, they might underly hypercytokinesis | In vitro; cell line, 293T-ACE2; cell-free system, Leishmania tarentolae | 34 | |
| Nsp6 | TBK1 | Innate immune response (IFN); TBK1 binding suppresses IRF3 phosphorylation, which leads to reduced IFN production | In vitro; cell lines, HEK293T, Vero CCL-81, BHK-21, Huh-7, and A549 | 38 |
| Related host interactor undetermined | Related host pathway undetermined. Nsp6 induced cytotoxicity in vitro, and in flies induced early lethality, reduced trachea (lung equivalent) branching, muscle defects and reduced muscular mitochondria | In vivo (Drosophila); in vitro; cell line, HEK293T | 42, 46 | |
| Nsp8-Nsp9 | 7SL RNA component of the signal recognition particle (SRP); directly binds 7SL RNA of the SRP | Innate immune response (IFN); disrupts global membrane protein trafficking and protein integration into the cell membrane; SRP-dependent transport is key to the IFN immune response (reduced SRP function resulted in a reduced IFN response) | In vitro; cell lines, HEK293T, Calu-3 (SARS-CoV-2), and VeroE6 (SARS-CoV-2) | 25 |
| Nsp13 | TBK1 | Innate immune response (IFN); TBK1 binding and suppression of TBK1 phosphorylation, which leads to reduced IFN production | In vitro; cell lines, HEK293T, Vero CCL-81, BHK-21, Huh-7, and A549 | 38 |
| Nsp16 | U1 and U2 snRNA components of the spliceosome; directly binds U1 and U2 snRNAs | Innate immune response (IFN); inhibits global mRNA splicing; reduces host innate immune response via increased intron retention in IFN-responsive genes (e.g., ISG15 and RIG-I genes) | In vitro; cell lines, HEK293T and Calu-3 (SARS-CoV-2) | 25 |
| Orf3a | Extrinsic apoptotic pathway | Apoptosis (immune response?); induces cleavage/activation of caspase-8 (Bcl-2 of intrinsic pathway not affected); activated caspase-8 truncates Bid to tBid (increased), which induces release cytochrome c; this leads to apoptosome formation and caspase-9 cleavage/activation and ultimately apoptosis | In vitro; cell lines, HEK293T, VeroE6, and HepG2 | 39 |
| Components VPS39 of HOPS complex; directly binds HOPS VPS39 component | Immune response (autophagy); binding HOPS VPS39 prevents formation of the autophagosomal STX17-SNAP29-VAMP8 SNARE complex, thus blocking autophagy and enabling virus to evade lysosomal degradation | In vitro; cell lines, HeLa with human ACE2 (SARS-CoV-2) and HEK293T | 40 | |
| Orf6 | KPNA2 | Innate immune response (IFN); binds KPNA2 and binds C-terminal domain of NUP98-RAE1 nuclear pore complex, which blocks docking of karyopherin/importin cargo-receptor complex and prevents STAT1 and STAT2 nuclear translocation, thereby inhibiting the IFN immune response; in flies, Orf6 induced early lethality, reduced trachea (lung equivalent) branching, muscle defects, and reduced muscular mitochondria | In vivo (Drosophila) | 38, 41 |
| NUP98-RAE1 nuclear pore complex; directly interacts with NUP98-RAE1 nuclear pore complexc | In vitro; primary cells, HBEC; cell lines, VeroE6, Calu-3, HEK293T, liver-derived (Huh7 and Hep3B), lung-derived (NCI-H2110 A549, NCI-H1975), HRT-18G, and BHK-21J | 42, 46 | ||
| Orf7a | Related host interactor undetermined | Related host pathway undetermined. Nsp6 induced cytotoxicity in vitro, and in flies induced early lethality, reduced trachea (lung equivalent) branching, muscle defects, and reduced muscular mitochondria |
In vivo (Drosophila) In vitro; cell line, HEK293T |
42, 46 |
| Orf9b | TOM70; interacts with TOM70 | Innate immune response (IFN); mitochondrial, TOM70, and IFN immune response | In vitro; cell line, HEK293T | 43, 47 |
| NEMO; targets NEMO, an essential modulator of NF-κB, and inhibits its activation by K63-linked polyubiquitination in response to viral infection | Innate immune response (IFN); disrupts NEMO-mediated NF-κB signaling-induced IFN immune response | In vitro; primary cells, HPAEpiC; cell lines, VeroE60, HEK293T, Caco-2, BEAS-2B, Calu-3, and NCI-H1299 | 48 |
Results in bold are results of in vivo study. 3CLpro, 3-chymotrypsin-like protease; IFN, interferon; PLpro, papain-like cysteine protease; PIC, preinitiation complex; eIFs, eukaryotic translation initiation factors; IRF3, interferon regulatory factor 3; PARPs, poly(ADP-ribosyl) polymerases; snRNA, small nuclear RNA; HOPS, homotypic fusion and protein sorting. Cell lines: A549, human lung epithelial cell line; BEAS-2B, human nontumorigenic lung epithelial cell line; BHK-21J, baby hamster kidney cell line; Caco-2, human colorectal adenocarcinoma cell line; Calu-3, human lung epithelial cell line; H1299, immortalized human lung cancer cell line; HAP1, human leukemia-derived near-haploid adherent fibroblast-like cell line; HBEC, primary human bronchial epithelial cells; HEK293T, human embryonic kidney cell line; HeLa (Henrietta Lacks), immortalized human cervical cancer cell line; Hep3B/HepG2, human hepatocellular (liver) carcinoma cell line; HPAEpiC, primary human pulmonary alveolar epithelial cells; HRT-18G, human colorectal adenocarcinoma cell line; Huh7, human hepatoblastoma cell line; NCI-H1299, human lung adenocarcinoma cell line; NCI-H1975, human lung-derived cell line; NCI-H2110, human lung-derived cell line; VeroE6/Vero CCL-81, African green monkey kidney cell line.
Boldfacing refers to the Drosophila in vivo studies described in reference 46.
The information for KPNA2 in "Effects on host," "Model system(s), and "Reference(s)" also applies to NUP98-RAE1.
(i) Nsp1.
One of the first virus genes translated is the Nsp1 gene. SARS-CoV Nsp1 is known to be a potent inhibitor of the interferon (IFN)-mediated innate immune response, to disrupt the cell cycle and to suppress host gene expression. Therefore, it has been a protein of great interest for SARS-CoV-2 studies. The Nsp1 protein also inhibits host gene expression; it does this by binding the host 40S subunit in ribosomal complexes, thereby blocking the mRNA entry channel, which inhibits the host mRNA translation machinery, which consequently disrupts the retinoic acid-inducible gene I-dependent innate immune responses (reduced IFN-β and interleukin 8 [IL-8] gene expression) (25–27). In addition, several eukaryotic translation initiation factors (eIFs) have been shown to allosterically modulate SARS-CoV-2 Nsp1 interaction with ribosomal preinitiation complexes (PICs) in the absence of mRNA, which provides another means to arrest host protein synthesis (28). And it has been shown that Nsp1 prevents physiological conformation of the 48S PIC by restricting rotation of the ribosome head domain (29). Further, it has been recently reported that SARS-CoV-2 Nsp1 directly binds the mRNA export factor NXF1 (30). This interferes with NXF1 interactions with mRNA export adaptors and the nuclear pore complex, effectively blocking host mRNA export. Thus, it seems that SARS-CoV-2 Nsp1 exerts multiple complementary strategies to take control of host cell systems.
(ii) Nsp3.
Like its MERS-CoV and SARS-CoV counterparts, SARS-CoV-2 Nsp3 possesses deubiquitinating/deISGylating protease activity in addition to its PLpro (31–33). These provide another virus tactic to repress the host innate immune response, marked by reduced cytokine and chemokine production. SARS-CoV-2 Nsp3 has been shown to cleave ISG15 from interferon-regulatory factor 3 (IRF3), thereby inhibiting the host type I IFN (IFN-I) immune response (34, 35). Another strategy by which Nsp3 suppresses the host immune response is through countering poly(ADP-ribosyl) polymerases (PARPs). PARPs are among the first actors of the immune pathway, and several human PARPs are known to induce the IFN innate immune response. They carry out ADP-ribosylation of proteins and nucleic acids in order to establish an antiviral environment. In turn, viruses gain (ADP-ribosyl) hydrolase activity, which cleaves the host protein ADPr bond, to oppose this host protective mechanism. The macrodomain encoded by the SARS-CoV and SARS-CoV-2 Nsp3 gene is responsible for this antagonistic cleavage against PARPs (36, 37).
(iii) Nsp5.
The SARS-CoV-2 Nsp5 gene encodes a 3C-like protease (3CLpro) which is crucial for virus replication by cleaving the Orf1a/ab polyprotein into functional Nsp units. And, similar to Nsp3 (PLpro), it has been shown to also cleave highly select host proteins: transforming growth factor β (TGF-β)-activated kinase 1 (MAP3K7) binding protein 1 (TAB1) and NLR family pyrin domain containing 12 (NLRP12) (34). TGF-β, IL-1, and tumor necrosis factor alpha (TNF-α) respond to viral infection and activate the TAB1/2/3/TAK1 complex. 3CLpro cleavage of TAB1 results in loss of its C terminus and disrupts complex formation. This results in reduced TGF-β-activated kinase 1 (TAK1) and subsequent diminished NF-κB and MAP kinase signaling, ultimately reducing cytokine release (34). NLRP12 is an inhibitor of NF-κB; cleavage by SARS-CoV-2 3CLpro releases this brake, leading to increased NF-κB signaling and increased production of proinflammatory cytokines (34). In addition, NLRP12 cleavage disrupts inflammasome NLRP3/12 formation and might induce cleavage of pro-caspase-1, thereby enhancing release of IL-1β (34).
(iv) Nsp6 and Nsp13.
Evading the IFN-mediated early immune signaling pathway is a common strategy among viruses. Multiple studies reported screens to identify the individual SARS-CoV-2 proteins that antagonize IFN pathway signaling. These have implicated multiple virus proteins but typically have not investigated the virus-host interactions or pathways through which this antagonistic effect is conveyed. That being said, one study recognized two distinct mechanisms aimed at evading the IFN immune response: antagonism of IFN-I production and suppression of IFN-I signaling (38). The study identified several SARS-CoV-2 proteins that have IFN antagonistic capability, including Nsp1, Nsp6, Nsp12, Orf3a, Orf6, Orf7a, Orf7b, and M. Further, it was demonstrated that Nsp6 binds TANK binding kinase 1 (TBK1) and inhibits IRF3 phosphorylation (no effect on TBK1 phosphorylation), and Nsp13 was shown to bind TBK1 and inhibit TBK1 phosphorylation; both actions result in diminished IFN-I production. In addition, Nsp6 and Nsp13 were shown to inhibit IFN signaling by suppressing signal transducer and activator of transcription 1 and 2 (STAT1 and STAT2) phosphorylation, although the mechanism through which this occurs is not yet known (38).
(v) Nsp8 and Nsp9.
Nsp8 and Nsp9 each bind specific regions of the 7SL component of the signal recognition particle (SRP) complex. SRP binds the 80S ribosome and is responsible for signal peptide recognition, SRP-receptor binding, and ribosome translocation. Nsp8 interacts with the binding site for SRP54 and with 28S rRNA, a component of the 60S subunit of the ribosome (25). Nsp9 binds to the SRP19 binding site, which is responsible for proper folding and assembly of the SRP; this includes adequate loading of SRP54 (25). Together, SARS-CoV-2 Nsp8 and Nsp9 suppress the IFN immune response to viral infection through disrupting SRP-mediated trafficking, upon which it is dependent (25).
(vi) Nsp16.
SARS-CoV-2 Nsp16 binds host U1 and U2 small nuclear RNA (snRNA) components of the spliceosome (25). This was shown to disrupt spliceosome functioning and to result in global reduction of mRNA splicing (25). Several viruses are known to target the spliceosome as a tactic to evade the host immune response. Indeed, Nsp16-mediated intron retention in several IFN-responsive genes, including the ISG15 and RIG-I genes, was found to reduce IFN signaling in response to SARS-CoV-2 infection (25), yet another means by which SARS-CoV-2 hampers the host early innate immune response.
(vii) Orf3a.
SARS-CoV Orf3a has been known to induce apoptosis in cells, and it was shown that SARS-CoV-2 Orf3a could similarly induce caspase-dependent apoptosis (39). SARS-CoV-2 Orf3a does this through the extrinsic apoptotic pathway, and its apoptotic activity could be significantly suppressed by caspase inhibitors. Interestingly, Orf3a plasma membrane localization was required for its apoptotic activity for SARS-CoV-2- but not SARS-CoV-encoded protein, which indicates that the viruses use different mechanisms (39). The authors suggest that the reduced Orf3a apoptotic activity in SARS-CoV-2, compared to that in SARS-CoV, and therewith the likely reduced apoptosis-mediated immune response following infection, might contribute to the mild symptoms early on and asymptomatic transmissions observed in SARS-CoV-2.
SNARE complexes are essential to intracellular vesicular transport by mediating vesicle fusion to organelle or plasma membranes. Various viruses are known to disrupt SNARE complex formation, leading to accumulated autophagosomes/amphisomes and possibly facilitation of lysosome-mediated extracellular release of virus particles following replication. Interestingly, the SNARE proteins targeted to gain control of this process vary by virus. SARS-CoV-2 Orf3a disrupts homotypic fusion and protein sorting (HOPS)-mediated formation of the host STX17-SNAP29-VAMP8 SNARE complex (40). Orf3a directly interacts with the VPS39 component of HOPS; this sends it to the late endosomes, where VPS39 accumulates. And indirectly, Orf3a dysregulates the interactions of VPS39 with VPS11 and VPS18 (increased) and with VPS16-VPS33a (decreased) (40). Together, these Orf3a interferences result in diminished host autophagy. Similarly, SARS-CoV-2 M, Orf7a, and Nsp6 proteins reduced autophagy, the last doing so by limiting autophagosome size (40). Interestingly, in the same study, SARS-CoV Orf3a was not able to bind HOPS or block autophagy activity, suggesting that this feature is distinct to the SARS-CoV-2 Orf3a protein (40).
(viii) Orf6.
SARS-CoV-2 Orf6 is a potent antagonist of the IFN immune response. It antagonizes IFN production through selective binding to karyopherin alpha 2 (KPNA2) to block IRF3 nuclear transport and by suppressing IFN signaling through inhibition of STAT1 nuclear translocation (38). Another report showed that Orf6 binding to the nuclear pore complex protein 98 (NUP98)-RAE1 complex prevents STAT nuclear import. This interaction further disrupts docking of the cargo KPNA1-KPNB1 complexes at the nuclear pore (41). Together, these actions inhibit the IFN-mediated innate immune response. We have shown that SARS-CoV-2 Orf6 expression is cytotoxic in an in vitro model (42). Further, using affinity purification and mass spectrometry, we identified a network of host proteins interacting with Orf6. Gene Ontology (GO) analysis revealed that these proteins were involved in a variety of processes, including translation initiation, viral gene expression, antigen processing and presentation, and IL-1-mediated signaling, and in the spliceosome. Further, they were enriched for nuclear transport-related proteins (nucleoporins, importins, and exportins), including RAE1, several NUPs, and XPO1, thus strengthening previous findings of interaction of SARS-CoV-2 Orf6 with the host nuclear pore complex (41–44). Moreover, we found that the SARS-CoV-2 virus-human host protein interaction network is highly conserved all the way from flies (45, 46). This enabled us to study SARS-CoV-2 Orf6 in vivo, using a Drosophila model, which showed that Orf6 causes early lethality, similar to the in vitro findings. Examination of tissue-specific pathogenic effects caused by Orf6 revealed reduced tracheal (fly equivalent of lung) branching, as well as muscle weakness with decreased presence of mitochondria (46).
(ix) Orf9b.
RIG-I like receptors regulate a signaling complex on the mitochondrial outer membrane (including the adaptor proteins MAVS, TRAF3, TRAF6, and TOM70) responsible for activating the antiviral innate immune response and starting IFN production. SARS-CoV-2 Orf9b is another virus-encoded IFN antagonist. It antagonizes IFN in two ways, via TOM70 at the mitochondria (47) and via NEMO (48). Regarding the first, Orf9b localizes at the mitochondrial membrane, where it interacts with TOM70, which significantly reduces IFN activation (47). SARS-CoV and SARS-CoV-2 Orf9b proteins have similar binding affinities for TOM70, suggesting that this might be a shared mechanism among coronaviruses. Regarding the second (NEMO), immediately following virus infection, Orf9b rapidly accumulates and inhibits RIG-I-MAVS signaling-mediated IFN production. It does so by promoting MAVS degradation and subverting its downstream TBK1-IRF3 and IKK-α/β/γ–NF-κB signaling pathways (48). Note that SARS-CoV-2 Orf9b targets the IKK regulator subunit NEMO, which is essential for NF-κB but not IRF3 activation. As described above, SARS-CoV-2 Nsp3 protein disrupts the IRF3-regulated immune response (34, 35), and both Nsp6 and Nsp13 act upon TBK1-IRF3 as well (38). In fact, SARS-CoV-2 employs multiple strategies simultaneously (Nsp3, Nsp6 plus Nsp13, Nsp8-Nsp9, Nsp16, Orf6, and Orf9b) to ensure a nearly complete ablation of IFN signaling. This has been observed even during the later-stage cytokine storm.
TARGETED DRUG DEVELOPMENT FOR SARS-CoV-2 PROTEINS OR THEIR HOST INTERACTIONS
Drug host entry: vaccines and inhibitors targeting virus spike or the interaction with host factors to prevent cell entry.
Not surprisingly, a huge amount of effort has been put into developing an effective vaccine in order to stem the SARS-CoV-2 pandemic. An alternative strategy to prevent infection is to block interaction between the virus spike protein with the host receptor. Drugs targeting the SARS-CoV-2 spike interaction with the host ACE2 entry receptor or the host factors, like TMPRSS2 and furin that facilitate the entry process, have been of great interest. Therefore, camostat (mesylate) and nafamostat (mesylate), both established TMPRSS2 inhibitors, were immediately identified as promising candidates (43). Indeed, early on it was shown that camostat mesylate attenuated SARS-CoV-2 cell entry in vitro (49). Another study proposed captopril, an ACE inhibitor, based on the spike-host protein interaction network (43). All three compounds are currently in clinical trials for the treatment of COVID-19. A different study showed that ipomoeassin F, a small molecule, inhibits Sec61-mediated endoplasmic reticulum membrane translocation, thereby prohibiting interaction between the virus spike and Orf8 proteins with the host ACE2 receptor (50). It is undeniable that vaccines and other means of prevention will be invaluable in the arms race with human viruses. Unfortunately, vaccines cannot provide complete immunity and viruses, especially their spike proteins, continue to mutate and develop new strains better equipped at evading host protective immune systems. Many new SARS-CoV-2 variants have been detected globally throughout this pandemic, including variants of concern (VOCs). Notably, several spike mutations have derived multiple times independently (12). Multiple reports in high-profile journals have been published recently on how effective current vaccines are at providing protection against these new variants. One study looked virus resistance to neutralization by the Pfizer-BioNTech (BNT162b2) or Moderna (mRNA-1273) vaccine following one or two doses (11). Encouragingly, the study found that neutralization was largely preserved against the newly emerging variant. However, the B.1.351 (South African origin) and B.1.1.28 (also known as 501Y.V2) lineage-derived variant P.1 (Brazilian origin) showed significantly reduced neutralization, even in the fully vaccinated group. Both manufacturers are currently developing boosters to expand protection against these new variants. In addition, to prepare for breaches of vaccine protection and new outbreaks in the human population, we need drugs that can minimize virus-caused damage in the host. SARS-CoV-2 is notorious for affecting so many different host tissues, which can culminate in system-wide shutdown as the disease (COVID-19) progresses. To be most effective, these drugs would be designed to target specific virus proteins, those self-acting and essential for virus replication processes and host-interacting ones that are most pathogenic through disrupting host pathways. Based on the studies of individual virus proteins described above, as well as virus proteome-wide host interaction networks, many such therapeutics have been proposed, multiple have been tested in the same in vitro systems used to identify them, and several are currently in clinical trials for the treatment of COVID-19 (Tables 3 and 4).
TABLE 3.
Inhibitors of SARS-CoV-2 transcription and translation
| Drug group | Examplesa | Virus protein | Host protein | Pathway | Reference(s) |
|---|---|---|---|---|---|
| PLpro inhibitors | rac5c (less effective, rac3j and rac3k), GRL-0617, novel inhibitors VIR250 and VIR251 | PLpro (Nsp3) | NAb | Protease; virus mRNA maturation | 20, 33, 35, 51 |
| 3CLpro inhibitors | GRL-0496 (inhibitor SARS-CoV 3CLpro), GC376 (inhibitor SARS-CoV 3CLpro), lopinavir/ritonavir, ASC09F, GC813, and SK80 | 3CLpro (Nsp5) | NA | Protease; virus mRNA maturation | 19, 21, 52 |
| Nucleotide/nucleoside analog inhibitors | Remdesivir, faripiravir, galidesivir, and penciclovir | Polymerase complex (Nsp12-Nsp7-Nsp8) | NA | Polymerase; virus RNA synthesis | 23, 54 |
| Remdesivir, ribavirin, 5-fluorouracil, and β-d-N4-hydroxycytidine (NHC, EIDD 1931/2801) | Exoribonuclease (Nsp14) | Exoribonuclease; virus RNA synthesis (proofreading and recombination) | 24 | ||
| Bismuth salts | Bismuth-peptic complex and ranitidine bismuth citrate | NTPase and RNA helicase (Nsp13) | NA | RNA processing; virus replication, transcription, translation, and encapsidation | 22 |
Bold indicates that the compound is in a clinical trial for treatment of COVID-19.
NA, not applicable.
TABLE 4.
Inhibitors of SARS-CoV-2 host protein-protein interactions
| Druga | Drug class | Virus protein | Host protein | Pathway | Reference(s) |
|---|---|---|---|---|---|
| Amiodarone | Nsp6 | SIGMAR1 | 43 | ||
| Amodiaquine | Antimalarial | Nsp6 | SIGMAR1 | 43 | |
| (Hydroxy)chloroquine | Antimalarial | Nsp6 | SIGMAR1 | 8, 43 | |
| Chlorpromazine | Typical antipsychotic | Nsp6 | SIGMAR1 | 43 | |
| Propranolol | Nsp6 | SIGMAR1 | 43 | ||
| Tamoxifen | Nsp6 | SIGMAR1 | 43 | ||
| GRL-0617 | Nsp3 (PLpro) | Immune response (IFN; NF-κB) | 35 | ||
| Indomethacin | Anti-inflammatory (NSAID) | Nsp7 | PTGES-2 | 8, 43 | |
| Metformin | Nsp7/Orf9c | NDUFs | 8 | ||
| Fostamatinib | Tyrosine kinase inhibitor | Nsp13 | MARK2 | 58 | |
| Ribavirin | Nsp14 | IMPDH2 | 8 | ||
| Ruxolitinib | Orf9b | MARK2/3 | |||
| Selinexor | Orf6 (Nsp4–Nsp9) | XPO1; NUP98-RAE1 | Immune response (IFN); nuclear export inhibitor | 8, 42, 46 | |
| Tocilizumab | Nsp10 | NF-κB-repressing factor | Immune response; IL-6 inhibitor | 44 |
All the compounds listed are in clinical trials for treatment of COVID-19.
Drug self-acting: inhibitors targeting the viral proteins dedicated to virus replication processes.
Due to their essential nature, the virus proteins specialized in virus replication (mRNA transcription, translation, and encapsulation) tend to be highly conserved, whereas, for example, proteins tasked with immune evasion have to constantly adapt to counteract new host tactics. In addition, inhibiting virus-specific proteins is less likely to lead to inadvertent side effects since the host systems would not carry homologous proteins. Together, these characteristics have made these virus replication-dedicated proteins of great interest as potential therapeutic targets (Table 3).
Many PLpro (Nsp3) inhibitors have been developed over the years; however, one study found that these were, in general, unsuccessful at inhibiting SARS-CoV-2 PLpro activity (20), except for the compounds known to be effective inhibitors of SARS-CoV PLpro, which showed similar efficacy against SARS-CoV-2 PLpro (20), as did noncovalent SARS-CoV PLpro-specific inhibitor GRL-0617 (35, 51). In addition to these existing inhibitors, two novel compounds (VIR250 and VIR251) have been shown to specifically inhibit protease activity of SARS-CoV and SARS-CoV-2 but not MERS-CoV (33). The Nsp5 gene encodes the main protease (Mpro; 3CLpro); due to its importance in viral protein maturation for SARS-CoV-2 and many other viruses, it has been a therapeutic target of great interest for a long time. These have included a wide variety of potential SARS-CoV and MERS-CoV 3CLpro inhibitors (52). Urged on by the pandemic, efforts to develop inhibitors for SARS-CoV-2 3CLpro have included drug repurposing. GRL-0496 and GC376 are known inhibitors of SARS-CoV 3CLpro and were shown to be effective in vitro (dose dependent) against 3CLpro activity following SARS-CoV-2 infection (19, 21). In addition, several existing 3CLpro inhibitors are currently in (pre)clinical trials for the treatment of COVID-19, including pyrithiobac derivatives, unsymmetrical aromatic disulfides and SK80 (SARS), GC813 (MERS), and GC376, a peptidomimetic inhibitor and a neuraminidase inhibitor analog (SARS and MERS), as well as anti-HIV protease inhibitors (lopinavir/ritonavir and ASC09F, a fixed-dose combination of ASC09 and ritonavir, combined with oseltamivir) (52). Structural studies, molecular docking, virtual screens, and artificial intelligence are employed to predict binding affinity and potential for inhibitory effect in order to guide selection of candidates and design of new compounds (53).
It has been shown in a cell-free system that bismuth salts could inhibit both the NTPase and RNA helicase activities of SARS-CoV-2 Nsp13 in a dose-dependent manner (22). These compounds are in clinical use for the treatment of diarrhea (bacterial infection) and related stomach ulcers; clinical trials to test their efficacy in treating COVID-19 are currently not listed (https://clinicaltrials.gov). Given its essential function to virus survival, disrupting the virus-encoded RdRp has been a strategy for drug design against many viruses. Indeed, many nucleotide/nucleoside analog inhibitors have been designed for this purpose: their incorporation into viral RNA stops further synthesis. Several of these compounds are currently in clinical trials for COVID-19 (23, 54), including remdesivir (55), the only approved therapeutic for the treatment of SARS-CoV-2 infection. In addition, these drugs prohibit SARS-CoV-2 Nsp14 from carrying out its RNA proofreading duties (24). Remdesivir is a broad-spectrum antiviral, and while initial results looked promising for improving outcomes for COVID-19 patients (56), more recent data from a larger study showed little or no effect on disease progression and survival (57). A better molecular understanding of SARS-CoV-2 polymerase (Nsp12-Nsp7-Nsp8) and proofreading exoribonuclease (Nsp14) activities, through studying the individual virus proteins, could inform virus-specific design and thereby the efficacy of nucleotide/nucleoside analog inhibitors for the treatment of COVID-19.
Drug host interacting: inhibitors targeting the virus proteins, or their host factors, in specific virus-host interactions.
Comprehensive proteomics studies that generated extensive virus-host protein-protein interaction networks have contributed greatly to identification of targets of interest and in screening of existing drug libraries for candidate inhibitors (43, 44). One such study found that around 40% of SARS-CoV-2-interacting proteins were associated with host endomembrane compartments or vesicle trafficking pathways (43). The protein networks further revealed SARS-CoV-2 interaction with host proteins representing innate immune pathways, host translation machinery, ubiquitination complex (commonly targeted by viruses for replication and pathogenesis) through interaction with a cullin ubiquitin ligase, and bromodomain proteins (this could interfere with transcriptional process in support of an antiviral response) (43). By overlaying the interaction networks with pharmacological profiles for a library of pharmacological agents, the authors identified 69 compounds targeting 62 of the identified virus-host protein interactions. In vitro testing of these host-directed compounds for antiviral activity showed compounds that inhibit virus-targeted interaction with translation pathways and sigma-1 and sigma-2 receptors to be most promising (43). At this time, several of the candidate compounds are in clinical trials for COVID-19 (https://clinicaltrials.gov): chloroquine targets Nsp6-SIGMAR1, indomethacin targets Nsp7-PTGES2, metformin targets Nsp7/Orf9c-NDUFs, ribavirin targets Nsp14-IMPDH2, ruxolitinib targets Orf9b-MARK2/3, and selinexor targets Nsp4/9/Orf6-NUPs RAE1) (Table 4). In a follow-up study, the same group used a similar approach but overlaid the virus-host protein interaction networks of MERS-CoV, SARS-CoV, and SARS-CoV-2 (8). Of the promising (FDA-approved) candidates following in vitro validation, several are currently in clinical trials for COVID-19 (https://clinicaltrials.gov): indomethacin, an anti-inflammatory (nonsteroidal anti-inflammatory drug [NSAID]) (targets Nsp7–PGES-2), and chlorpromazine (typical antipsychotic), antimalarials (amodiaquine, hydroxychloroquine, and chloroquine), amiodarone, tamoxifen, and propranolol (all target Nsp6-SIGMAR1) (Table 4).
A different group used a similar approach to generate a SARS-CoV-2 human protein-protein network; they further determined global proteome profiles obtained from peripheral blood mononuclear cells (PBMCs) of patients with mild and severe COVID-19 (44). Analysis of the latter revealed that over 350 host proteins had been dysregulated. Proteins involved in neutrophil activation and blood coagulation were upregulated, while downregulated proteins were enriched for mediators of T cell receptor signaling. The authors confirmed the SARS-CoV-2 Nsp10 interaction with NF-κB-repressing factor (NKRF) in HEK293T cells, which was not present in the previously published network (43). This interaction facilitates an IL-8-mediated response and might contribute to the chemotaxis of neutrophils and cytokine storm (hypercytokinemia) observed in COVID-19 patients. Based on these findings, they proposed several drugs—targeting IL-8, IL-6, or the downstream JAK kinases—for pharmacological intervention in COVID-19 (44). Of these, tocilizumab, an IL-6 inhibitor, is currently in a clinical trial for COVID-19 (https://clinicaltrials.gov) (Table 4).
Another group sought to identify the sections of the virus-host protein network most affected by virus interaction, which they termed master regulators (58). Their analysis was based on interactions between SARS-CoV-2 and human host proteins predicted by structural genomics (59). Among the host systems most affected were apoptotic and mitochondrial pathways and downregulation of the ACE2 entry receptor. Based on these findings, the proposed targets of pharmacological intervention included, more broadly, antivirals and anti-inflammatories, recombinant ACE2, TMPRSS2 inhibitors (e.g., camostat mesylate), reviving innate immunity by targeting monocytes/macrophages, and boosting the downregulated apoptotic mechanisms, as well as, more specifically, interrupting the SARS-CoV-2 Nsp13-host MARK2 interaction by using fostamatinib (used to treat rheumatoid arthritis and immune thrombocytopenic purpura) (58). This tyrosine kinase inhibitor is currently in clinical trials for the treatment of COVID-19 (https://clinicaltrials.gov) (Table 4).
Several of the studies that investigated the pathological consequences for host cell pathways caused by individual SARS-CoV-2 proteins (see “Host interacting: SARS-CoV-2 proteins targeting host proteins and the effects on host systems” above) have also proposed and sometimes tested therapeutic inhibitors for the virus-host interactions. Selected ones are currently in clinical trial for the treatment of COVID-19 (Table 4). For the SARS-CoV-2 Nsp3 gene, which encodes PLpro and also possess deubiquitination/deISGylation activity, treatment with GRL-0617 (Table 4), a known SARS-CoV PLpro inhibitor, attenuated both its protease and nonhydrolysis functions, thus reducing Nsp3-mediated cytopathogenic suppression of the host IFN immune response via the IRF3 pathways and by countering PARPs. Furthermore, treatment with GRL-0617 reduced virus replication in infected cells (35). These findings demonstrate the potentially broad range of PLpro inhibitors in targeting Nsp3 functions. GRL-0617 is in COVID-19 clinical trials. The Nsp5 gene encodes the main protease (Mpro; 3CLpro), which is a target of major interest for pharmacological intervention (see “Drug self-acting: inhibitors targeting the viral proteins dedicated to virus replication processes” above and Table 3). Since its direct effect on host TAB1 and NLRP12 proteins is through protease-mediated cleavage, potent SARS-CoV-2 3CLpro inhibitors might similarly block cleavage of host proteins. It appears that no inhibitors have yet been designed or tested to do so; however, given that this Nsp5-NLRP12 interaction might contribute to the devastating cytokine storm (hypercytokinemia) observed in severe COVID-19 patients, this potential intervention warrants further investigation. SARS-CoV-2 Orf6 targets the host nuclear pore complex. Selinexor is an FDA-approved drug that targets XPO1 and inhibits nuclear export at the NUP98-RAE1 complex (43) (Table 4). We have previously shown that treatment with selinexor attenuates Orf6-mediated pathogenic effects (dose dependent) in both in vitro and in vivo systems (42, 46). Interestingly, SARS-CoV-2 Nsp6 and Orf7a induced phenotypes similar to Orf6 in fly; however, these did not improve following treatment with selinexor. These findings suggest that the underlying pathogenic pathways are a unique feature of each individual virus protein. This notion is supported by the virus protein-specific host interaction networks (43, 44).
SARS-CoV-2 is exceptional in its ability to suppress the IFN-mediated innate immune response. As we have seen above (see “Host interacting: SARS-CoV-2 proteins targeting host proteins and the effects on host systems”), it applies multiple strategies simultaneously to nearly abolish the response even at late stages of disease (COVID-19). The extent of this virus effort is also evident from the two SARS-CoV-2 proteomics studies (43, 44). Even though there is limited (∼16%) overlap between the virus-host protein interaction networks reported by each study, the host proteins involved in the innate immune response to viral infections were preserved (44). Several clinical trials on treatment with IFN in COVID-19 patients are under way, and some encouraging preliminary findings have been reported (60). However, it might be more effective to directly target the virus-host interactions that drive this immune suppression, for example, blocking the Nsp8-Nsp9 interaction with the SRP complex or inhibiting both Orf9b (NEMO-NF-κB pathway) and Nsp3 (IRF3 pathway) simultaneously since they act on different branches of a shared host pathway regulating the IFN response. By studying the individual virus proteins and the host pathways favored to carry out their mission, we could identify more efficient drugs to boost the patient immune system.
CONCLUSION
It has been just over a year since SARS-CoV-2 entered the world stage; in that time, the research community has developed multiple vaccines that are currently being rapidly administered to stem this pandemic. This has been accomplished through Herculean effort and unprecedented global data sharing, yet a year is a long time to wait during a raging pandemic with life effectively on hold. Future outbreaks are a matter of when rather than if. Already, mutations in the SARS-CoV-2 spike protein have been linked to increased resistance to some of the vaccines. We need to prepare: on the one hand by developing pan-coronavirus vaccines and on the other by providing clinical staff with the knowledge and medications needed to treat patients effectively as a first response to novel outbreaks. Therefore, in addition to prevention (vaccines), which focuses on virus-host entry proteins, we need to study the role of virus self-acting (dedicated to virus replication processes) and host-interacting proteins. Research into the role of individual SARS-CoV-2 proteins with their host interactors and the pathways driving the multitude of pathogenic effects has already provided a tremendous amount of information. These form the foundation for drug target identification and design. In fact, several lead compounds have already made it into clinical trials for the treatment of COVID-19. This is only the beginning, as these studies will be elemental to giving patients a fighting chance during the next outbreak.
ACKNOWLEDGMENTS
J.V.D.L. and Z.H. drafted and revised this review. Both authors have read and approved the final manuscript and consent to its publication.
This work was supported, in part, by the University of Maryland Baltimore Institute for Clinical and Translational Research (UMB ICTR) COVID-19 Accelerated Translational Incubator Pilot (ATIP) grant to Z.H.
We declare no competing interests.
REFERENCES
- 1.Buffalo CZ, Iwamoto Y, Hurley JH, Ren X. 2019. How HIV Nef proteins hijack membrane traffic to promote infection. J Virol 93:e01322-19. 10.1128/JVI.01322-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua S, Tan HQ, Engelberg D, Lim LHK. 2019. Alternative experimental models for studying influenza proteins, host-virus interactions and anti-influenza drugs. Pharmaceuticals (Basel) 12:147. 10.3390/ph12040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González ME. 2017. The HIV-1 Vpr protein: a multifaceted target for therapeutic intervention. Int J Mol Sci 18:126. 10.3390/ijms18010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harsh S, Fu Y, Kenney E, Han Z, Eleftherianos I. 2020. Zika virus non-structural protein NS4A restricts eye growth in Drosophila through regulation of JAK/STAT signaling. Dis Model Mech 13:dmm040816. 10.1242/dmm.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Link N, Chung H, Jolly A, Withers M, Tepe B, Arenkiel BR, Shah PS, Krogan NJ, Aydin H, Geckinli BB, Tos T, Isikay S, Tuysuz B, Mochida GH, Thomas AX, Clark RD, Mirzaa GM, Lupski JR, Bellen HJ. 2019. Mutations in ANKLE2, a ZIKA virus target, disrupt an asymmetric cell division pathway in Drosophila neuroblasts to cause microcephaly. Dev Cell 51:713–729.e6. 10.1016/j.devcel.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Gordesky-Gold B, Leney-Greene M, Weinbren NL, Tudor M, Cherry S. 2018. Inflammation-induced, STING-dependent autophagy restricts Zika virus infection in the Drosophila brain. Cell Host Microbe 24:57–68.e3. 10.1016/j.chom.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice AP. 2017. The HIV-1 Tat protein: mechanism of action and target for HIV-1 cure strategies. Curr Pharm Des 23:4098–4102. 10.2174/1381612823666170704130635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon DE, Hiatt J, Bouhaddou M, Rezelj VV, Ulferts S, Braberg H, Jureka AS, Obernier K, Guo JZ, Batra J, Kaake RM, Weckstein AR, Owens TW, Gupta M, Pourmal S, Titus EW, Cakir M, Soucheray M, McGregor M, Cakir Z, Jang G, O’Meara MJ, Tummino TA, Zhang Z, Foussard H, Rojc A, Zhou Y, Kuchenov D, Hüttenhain R, Xu J, Eckhardt M, Swaney DL, Fabius JM, Ummadi M, Tutuncuoglu B, Rathore U, Modak M, Haas P, Haas KM, Naing ZZC, Pulido EH, Shi Y, Barrio-Hernandez I, Memon D, Petsalaki E, Dunham A, Marrero MC, Burke D, Koh C, Vallet T, QCRG Structural Biology Consortium, et al. 2020. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science 370:eabe9403. 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z, Lian X, Su X, Wu W, Marraro GA, Zeng Y. 2020. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res 21:224. 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS. 2020. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367:1260–1263. 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, Feldman J, Pavlovic MN, Gregory DJ, Poznansky MC, Sigal A, Schmidt AG, Iafrate AJ, Naranbhai V, Balazs AB. 2021. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 184:2372–2383.e9. 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy KR, Rennick LJ, Nambulli S, Robinson-McCarthy LR, Bain WG, Haidar G, Duprex WP. 2021. Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science 371:1139–1142. 10.1126/science.abf6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann M, Kleine-Weber H, Pohlmann S. 2020. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell 78:779–784.e5. 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. 2020. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res 176:104742. 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stieneke-Gröber A, Vey M, Angliker H, Shaw E, Thomas G, Roberts C, Klenk HD, Garten W. 1992. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J 11:2407–2414. 10.1002/j.1460-2075.1992.tb05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson BA, Xie X, Bailey AL, Kalveram B, Lokugamage KG, Muruato A, Zou J, Zhang X, Juelich T, Smith JK, Zhang L, Bopp N, Schindewolf C, Vu M, Vanderheiden A, Winkler ES, Swetnam D, Plante JA, Aguilar P, Plante KS, Popov V, Lee B, Weaver SC, Suthar MS, Routh AL, Ren P, Ku Z, An Z, Debbink K, Diamond MS, Shi PY, Freiberg AN, Menachery VD. 2021. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature 591:293–299. 10.1038/s41586-021-03237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M, Smura T, Levanov L, Szirovicza L, Tobi A, Kallio-Kokko H, Österlund P, Joensuu M, Meunier FA, Butcher SJ, Winkler MS, Mollenhauer B, Helenius A, Gokce O, Teesalu T, Hepojoki J, Vapalahti O, Stadelmann C, Balistreri G, Simons M. 2020. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370:856–860. 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira JC, Rabeh WM. 2020. Biochemical and biophysical characterization of the main protease, 3-chymotrypsin-like protease (3CLpro) from the novel coronavirus SARS-CoV 2. Sci Rep 10:22200. 10.1038/s41598-020-79357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froggatt HM, Heaton BE, Heaton NS. 2020. Development of a fluorescence-based, high-throughput SARS-CoV-2 3CLpro reporter assay. J Virol 94:e01265-20. 10.1128/JVI.01265-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klemm T, Ebert G, Calleja DJ, Allison CC, Richardson LW, Bernardini JP, Lu BG, Kuchel NW, Grohmann C, Shibata Y, Gan ZY, Cooney JP, Doerflinger M, Au AE, Blackmore TR, van der Heden van Noort GJ, Geurink PP, Ovaa H, Newman J, Riboldi-Tunnicliffe A, Czabotar PE, Mitchell JP, Feltham R, Lechtenberg BC, Lowes KN, Dewson G, Pellegrini M, Lessene G, Komander D. 2020. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J 39:e106275. 10.15252/embj.2020106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien A, Chen DY, Hackbart M, Close BJ, O’Brien TE, Saeed M, Baker SC. 2021. Detecting SARS-CoV-2 3CLpro expression and activity using a polyclonal antiserum and a luciferase-based biosensor. Virology 556:73–78. 10.1016/j.virol.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu T, Huang M, Wu D, Ren Y, Zhang X, Han Y, Mu J, Wang R, Qiu Y, Zhang DY, Zhou X. 2020. SARS-coronavirus-2 Nsp13 possesses NTPase and RNA helicase activities that can be inhibited by bismuth salts. Virol Sin 35:321–329. 10.1007/s12250-020-00242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Q, Peng R, Yuan B, Zhao J, Wang M, Wang X, Wang Q, Sun Y, Fan Z, Qi J, Gao GF, Shi Y. 2020. Structural and biochemical characterization of the nsp12-nsp7-nsp8 core polymerase complex from SARS-CoV-2. Cell Rep 31:107774. 10.1016/j.celrep.2020.107774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gribble J, Stevens LJ, Agostini ML, Anderson-Daniels J, Chappell JD, Lu X, Pruijssers AJ, Routh AL, Denison MR. 2021. The coronavirus proofreading exoribonuclease mediates extensive viral recombination. PLoS Pathog 17:e1009226. 10.1371/journal.ppat.1009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee AK, Blanco MR, Bruce EA, Honson DD, Chen LM, Chow A, Bhat P, Ollikainen N, Quinodoz SA, Loney C, Thai J, Miller ZD, Lin AE, Schmidt MM, Stewart DG, Goldfarb D, De Lorenzo G, Rihn SJ, Voorhees RM, Botten JW, Majumdar D, Guttman M. 2020. SARS-CoV-2 disrupts splicing, translation, and protein trafficking to suppress host defenses. Cell 183:1325–1339.e21. 10.1016/j.cell.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert K, Karousis ED, Jomaa A, Scaiola A, Echeverria B, Gurzeler LA, Leibundgut M, Thiel V, Muhlemann O, Ban N. 2020. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nat Struct Mol Biol 27:959–966. 10.1038/s41594-020-0511-8. [DOI] [PubMed] [Google Scholar]
- 27.Thoms M, Buschauer R, Ameismeier M, Koepke L, Denk T, Hirschenberger M, Kratzat H, Hayn M, Mackens-Kiani T, Cheng J, Straub JH, Stürzel CM, Fröhlich T, Berninghausen O, Becker T, Kirchhoff F, Sparrer KMJ, Beckmann R. 2020. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science 369:1249–1255. 10.1126/science.abc8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapointe CP, Grosely R, Johnson AG, Wang J, Fernandez IS, Puglisi JD. 2021. Dynamic competition between SARS-CoV-2 NSP1 and mRNA on the human ribosome inhibits translation initiation. Proc Natl Acad Sci U S A 118:e2017715118. 10.1073/pnas.2017715118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan S, Peng L, Park JJ, Hu Y, Devarkar SC, Dong MB, Shen Q, Wu S, Chen S, Lomakin IB, Xiong Y. 2020. Nonstructural protein 1 of SARS-CoV-2 is a potent pathogenicity factor redirecting host protein synthesis machinery toward viral RNA. Mol Cell 80:1055–1066.e6. 10.1016/j.molcel.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang K, Miorin L, Makio T, Dehghan I, Gao S, Xie Y, Zhong H, Esparza M, Kehrer T, Kumar A, Hobman TC, Ptak C, Gao B, Minna JD, Chen Z, García-Sastre A, Ren Y, Wozniak RW, Fontoura BMA. 2021. Nsp1 protein of SARS-CoV-2 disrupts the mRNA export machinery to inhibit host gene expression. Sci Adv 7:eabe7386. 10.1126/sciadv.abe7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barretto N, Jukneliene D, Ratia K, Chen Z, Mesecar AD, Baker SC. 2005. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J Virol 79:15189–15198. 10.1128/JVI.79.24.15189-15198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mielech AM, Kilianski A, Baez-Santos YM, Mesecar AD, Baker SC. 2014. MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology 450–451:64–70. 10.1016/j.virol.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rut W, Lv Z, Zmudzinski M, Patchett S, Nayak D, Snipas SJ, El Oualid F, Huang TT, Bekes M, Drag M, Olsen SK. 2020. Activity profiling and structures of inhibitor-bound SARS-CoV-2-PLpro protease provides a framework for anti-COVID-19 drug design. bioRxiv 2020.04.29.068890. [DOI] [PMC free article] [PubMed]
- 34.Moustaqil M, Ollivier E, Chiu HP, Van Tol S, Rudolffi-Soto P, Stevens C, Bhumkar A, Hunter DJB, Freiberg AN, Jacques D, Lee B, Sierecki E, Gambin Y. 2021. SARS-CoV-2 proteases PLpro and 3CLpro cleave IRF3 and critical modulators of inflammatory pathways (NLRP12 and TAB1): implications for disease presentation across species. Emerg Microbes Infect 10:178–195. 10.1080/22221751.2020.1870414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin D, Mukherjee R, Grewe D, Bojkova D, Baek K, Bhattacharya A, Schulz L, Widera M, Mehdipour AR, Tascher G, Geurink PP, Wilhelm A, van der Heden van Noort GJ, Ovaa H, Muller S, Knobeloch KP, Rajalingam K, Schulman BA, Cinatl J, Hummer G, Ciesek S, Dikic I. 2020. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 587:657–662. 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alhammad YMO, Kashipathy MM, Roy A, Gagne JP, McDonald P, Gao P, Nonfoux L, Battaile KP, Johnson DK, Holmstrom ED, Poirier GG, Lovell S, Fehr AR. 2021. The SARS-CoV-2 conserved macrodomain is a mono-ADP-ribosylhydrolase. J Virol 95:e01969-20. 10.1128/JVI.01969-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rack JGM, Zorzini V, Zhu Z, Schuller M, Ahel D, Ahel I. 2020. Viral macrodomains: a structural and evolutionary assessment of the pharmacological potential. Open Biol 10:200237. 10.1098/rsob.200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia H, Cao Z, Xie X, Zhang X, Chen JY, Wang H, Menachery VD, Rajsbaum R, Shi PY. 2020. Evasion of type I interferon by SARS-CoV-2. Cell Rep 33:108234. 10.1016/j.celrep.2020.108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren Y, Shu T, Wu D, Mu J, Wang C, Huang M, Han Y, Zhang XY, Zhou W, Qiu Y, Zhou X. 2020. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell Mol Immunol 17:881–883. 10.1038/s41423-020-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miao G, Zhao H, Li Y, Ji M, Chen Y, Shi Y, Bi Y, Wang P, Zhang H. 2021. ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Dev Cell 56:427–442.e5. 10.1016/j.devcel.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miorin L, Kehrer T, Sanchez-Aparicio MT, Zhang K, Cohen P, Patel RS, Cupic A, Makio T, Mei M, Moreno E, Danziger O, White KM, Rathnasinghe R, Uccellini M, Gao S, Aydillo T, Mena I, Yin X, Martin-Sancho L, Krogan NJ, Chanda SK, Schotsaert M, Wozniak RW, Ren Y, Rosenberg BR, Fontoura BMA, Garcia-Sastre A. 2020. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc Natl Acad Sci U S A 117:28344–28354. 10.1073/pnas.2016650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J-G, Huang W, Lee H, van de Leemput J, Kane MA, Han Z. 2021. Characterization of SARS-CoV-2 proteins reveals Orf6 pathogenicity, subcellular localization, host interactions and attenuation by Selinexor. Cell Biosci 11:58. 10.1186/s13578-021-00568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O’Meara MJ, Rezelj VV, Guo JZ, Swaney DL, Tummino TA, Hüttenhain R, Kaake RM, Richards AL, Tutuncuoglu B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, Polacco BJ, Braberg H, Fabius JM, Eckhardt M, Soucheray M, Bennett MJ, Cakir M, McGregor MJ, Li Q, Meyer B, Roesch F, Vallet T, Mac Kain A, Miorin L, Moreno E, Naing ZZC, Zhou Y, Peng S, Shi Y, Zhang Z, Shen W, Kirby IT, Melnyk JE, Chorba JS, Lou K, Dai SA, Barrio-Hernandez I, Memon D, Hernandez-Armenta C, et al. 2020. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583:459–468. 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Guo M, Tian X, Wang X, Yang X, Wu P, Liu C, Xiao Z, Qu Y, Yin Y, Wang C, Zhang Y, Zhu Z, Liu Z, Peng C, Zhu T, Liang Q. 2020. Virus-host interactome and proteomic survey reveal potential virulence factors influencing SARS-CoV-2 pathogenesis. Med (N Y) 2:99–112.e7. 10.1016/j.medj.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussain M, Jabeen N, Shabbir S, Udin N, Aziz B, Amanullah A, Raza F, Baig AA. 2020. Dataset for homologous proteins in Drosophila melanogaster for SARS-CoV-2/human interactome. Data Brief 32:106082. 10.1016/j.dib.2020.106082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J-Y, Lee J-G, van de Leemput J, Lee H, Han Z. 2021. Functional analysis of SARS-CoV-2 proteins in Drosophila identifies Orf6-induced pathogenic effects with Selinexor as an effective treatment. Cell Biosci 11:59. 10.1186/s13578-021-00567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang HW, Zhang HN, Meng QF, Xie J, Li Y, Chen H, Zheng YX, Wang XN, Qi H, Zhang J, Wang PH, Han ZG, Tao SC. 2020. SARS-CoV-2 Orf9b suppresses type I interferon responses by targeting TOM70. Cell Mol Immunol 17:998–1000. 10.1038/s41423-020-0514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu J, Shi Y, Pan X, Wu S, Hou R, Zhang Y, Zhong T, Tang H, Du W, Wang L, Wo J, Mu J, Qiu Y, Yang K, Zhang L-K, Ye B-C, Qi N. 2021. SARS-CoV-2 ORF9b inhibits RIG-I-MAVS antiviral signaling by interrupting K63-linked ubiquitination of NEMO. Cell Rep 34:108761. 10.1016/j.celrep.2021.108761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271–280.e8. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Keefe S, Roboti P, Duah KB, Zong G, Schneider H, Shi WQ, High S. 2021. Ipomoeassin-F inhibits the in vitro biogenesis of the SARS-CoV-2 spike protein and its host cell membrane receptor. J Cell Sci 134:jcs257758. 10.1242/jcs.257758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu Z, Huang B, Tang J, Liu S, Liu M, Ye Y, Liu Z, Xiong Y, Zhu W, Cao D, Li J, Niu X, Zhou H, Zhao YJ, Zhang G, Huang H. 2021. The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery. Nat Commun 12:488. 10.1038/s41467-020-20718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He J, Hu L, Huang X, Wang C, Zhang Z, Wang Y, Zhang D, Ye W. 2020. Potential of coronavirus 3C-like protease inhibitors for the development of new anti-SARS-CoV-2 drugs: insights from structures of protease and inhibitors. Int J Antimicrob Agents 56:106055. 10.1016/j.ijantimicag.2020.106055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q, Kang C. 2020. Progress in developing inhibitors of SARS-CoV-2 3C-like protease. Microorganisms 8:1250. 10.3390/microorganisms8081250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang WF, Stephen P, Theriault JF, Wang R, Lin SX. 2020. Novel coronavirus polymerase and nucleotidyl-transferase structures: potential to target new outbreaks. J Phys Chem Lett 11:4430–4435. 10.1021/acs.jpclett.0c00571. [DOI] [PubMed] [Google Scholar]
- 55.Wang Q, Wu J, Wang H, Gao Y, Liu Q, Mu A, Ji W, Yan L, Zhu Y, Zhu C, Fang X, Yang X, Huang Y, Gao H, Liu F, Ge J, Sun Q, Yang X, Xu W, Liu Z, Yang H, Lou Z, Jiang B, Guddat LW, Gong P, Rao Z. 2020. Structural basis for RNA replication by the SARS-CoV-2 polymerase. Cell 182:417–428.e13. 10.1016/j.cell.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez DE Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fatkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members. 2020. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med 383:1813–1826. 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.WHO Solidarity Trial Consortium. 2021. Repurposed antiviral drugs for Covid-19—interim WHO Solidarity Trial results. N Engl J Med 384:497–511. 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guzzi PH, Mercatelli D, Ceraolo C, Giorgi FM. 2020. Master regulator analysis of the SARS-CoV-2/human interactome. J Clin Med 9:982. 10.3390/jcm9040982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srinivasan S, Cui H, Gao Z, Liu M, Lu S, Mkandawire W, Narykov O, Sun M, Korkin D. 2020. Structural genomics of SARS-CoV-2 indicates evolutionary conserved functional regions of viral proteins. Viruses 12:360. 10.3390/v12040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Q, Chen V, Shannon CP, Wei XS, Xiang X, Wang X, Wang ZH, Tebbutt SJ, Kollmann TR, Fish EN. 2020. Interferon-alpha2b treatment for COVID-19. Front Immunol 11:1061. 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]