ABSTRACT
Cardiac fibrosis is a hallmark of various heart diseases and ultimately leads to heart failure. Although long noncoding RNA (lncRNA) SNHG20 has been reported to play important roles in various cancers, its function in cardiac fibrosis remains unclear. The expression of SNHG20 and microRNA 335 (miR-335) in heart tissues of angiotensin II-induced mice and angiotensin II-stimulated mouse cardiomyocyte cell line HL-1 were detected by quantitative real-time PCR (qRT-PCR). Cell viability was evaluated by cell counting kit-8 assay. The expression of galectin-3, fibrosis-related proteins (fibronectin, collagen IaI, and α-SMA), and apoptosis-related proteins [cleaved caspase-3 and cleaved poly(ADP-ribose) polymerase (PARP)] was detected by Western blotting. Bioinformatics prediction, luciferase reporter assay, and RNA pulldown assay were performed to determine the relationship between SNHG20 and miR-335 as well as miR-335 and Galectin-3. Gain- and loss-function assays were performed to determine the role of SNHG20/miR-335/Galectin-3 in cardiac fibrosis. SNHG20 was significantly upregulated and miR-335 was downregulated in heart tissues of angiotensin II-treated mice and angiotensin II-stimulated HL-1 cells. Downregulation of SNHG20 effectively enhanced cell viability and decreased cell size of HL-1 cells and the expression levels of fibrosis-related proteins (fibronectin, collagen IaI, and α-SMA) and apoptosis-related proteins (cleaved caspase-3 and cleaved PARP), which were induced by angiotensin II treatment. Furthermore, SNHG20 elevated the expression levels of Galectin-3 by directly regulating miR-335. Our study revealed that downregulation of SNHG20 improved angiotensin II-induced cardiac fibrosis by targeting the miR-335/Galectin-3 axis, suggesting that SNHG20 is a therapeutic target for cardiac fibrosis and hypertrophy.
KEYWORDS: cardiac fibrosis, SNHG20, miR-335, Galectin-3
INTRODUCTION
Cardiac fibrosis is characterized by net accumulation of extracellular matrix (ECM) proteins in the cardiac interstitium, which contributes to the systolic and diastolic dysfunction in a large number of cardiac pathophysiologic conditions (1). As a biomarker of different types of heart disease, cardiac fibrosis can result in heart failure, the major leading cause of morbidity and mortality worldwide (2). Recently, antifibrosis has become a potential and novel therapeutic strategy for cardiovascular disease caused by cardiac fibrosis (3). Therefore, better understanding of the underlying molecular mechanisms of cardiac fibrosis is necessary, which would contribute to the identification of novel therapeutic targets for cardiac fibrosis-caused diseases.
Long noncoding RNAs (lncRNAs) are a class of regulatory RNAs with more than 200 nucleotides in length and lack a protein-coding property (4). Extensive studies have demonstrated that abnormal expression of lncRNAs is often observed in the diseased heart, providing a profound biological function (5, 6). For example, GASL1 is downregulated in chronic heart failure, and overexpression of GASL1 leads to a decreased apoptotic rate of cardiomyocytes under H2O2 treatment (7). The lncRNA AK137033, also named Safe, is elevated in both myocardial infarction and transforming growth factor beta (TGF-β)-induced cardiac fibrosis, and knockdown of Safe prevents TGF-β-induced fibroblast-myofibroblast transition and improves the impaired cardiac function in mice (8). The lncRNA Crnde is negatively correlated with cardiac fibrosis marker genes, and overexpression of Crnde effectively attenuates cardiac fibrosis and improves cardiac function in mice with diabetic cardiomyopathy (9). The lncRNA SOX2OT is highly expressed in mice with heart failure, and knockdown of SOX2OT effectively reduces myocardial injury and collagen deposition in heart failure mice (10). SNHG20, a newly identified lncRNA, has been found to play important roles in various human cancers (11–13). In addition, one previous study found that SNHG20 is closely associated with the process of vasculogenic mimicry (14), suggesting that it also participates in the progression of cardiovascular disease. A recent study found that SNHG20 was associated with pulmonary fibrosis, and SNHG20 promotes silica-induced pulmonary fibrosis through the microRNA 490-3p (miR-490-3p)/TGFBR1 axis (15). Therefore, this study aimed to explore the function of SNHG20 and the underlying mechanisms in cardiac fibrosis.
MicroRNAs (miRNAs) are a class of single-stranded noncoding RNA molecules, approximately 20 to 26 nucleotides in length, and have been demonstrated to inhibit gene expression by directly targeting the 3′ untranslated regions (3′-UTR) of their targeted mRNAs (16). A growing number of studies have shown that miRNAs are involved in diverse key biological processes, including cancer, inflammatory responses, and cellular function (17). Recently, miR-335 has been reported to be associated with the pathophysiological process of myocardial ischemia, and upregulation of miR-335 ameliorates myocardial ischemia reperfusion injury (18). One study revealed that plasma and myocardial tenascin C levels were increased 7 days after myocardial hypertrophy, and both hypoxic and hypertrophic stimuli result in the upregulation of tenascin C; moreover, the increment in tenascin C was accompanied by upregulation of miR-335 (19). These studies suggest that miR-335 participates in the progression of cardiac fibrosis and hypertrophy, and this study aimed to investigate the potential regulatory role of SNHG20 and miR-335 in cardiac fibrosis and hypertrophy.
Galectin-3 (Gal-3), belonging to a specialized family of mammalian sugar-binding proteins (galectins), harbors a highly conserved sequence (20). It has been reported that the expression levels of Gal-3 were significantly increased in heart failure and related to myocardial fibrosis (21). Although collagen is the most abundant ECM protein in the heart, cellular fibronectin is likely to play a pivotal role in cardiac fibrosis (22). In addition, Gal-3 has been found to regulate the expression of fibrosis-related proteins such as fibronectin, collagen IaI, and α-SMA during disease-induced cardiac fibrosis (23–25). However, the regulatory network of Gal-3 in cardiac fibrosis is unclear.
In the present study, we found that SNHG20 was significantly upregulated in heart tissues of angiotensin II (Ang II)-treated mice and angiotensin II-stimulated cardiomyocyte cell line HL-1. Furthermore, inhibition of SNHG20 improved angiotensin II induced cardiac fibrosis in vitro by targeting the miR-335/Galectin-3 axis, suggesting that SNHG20 serves as a therapeutic target for disease-induced cardiac fibrosis and hypertrophy.
RESULTS
SNHG20 was upregulated and miR-335 was downregulated in mice with Ang II-induced cardiac fibrosis and hypertrophy.
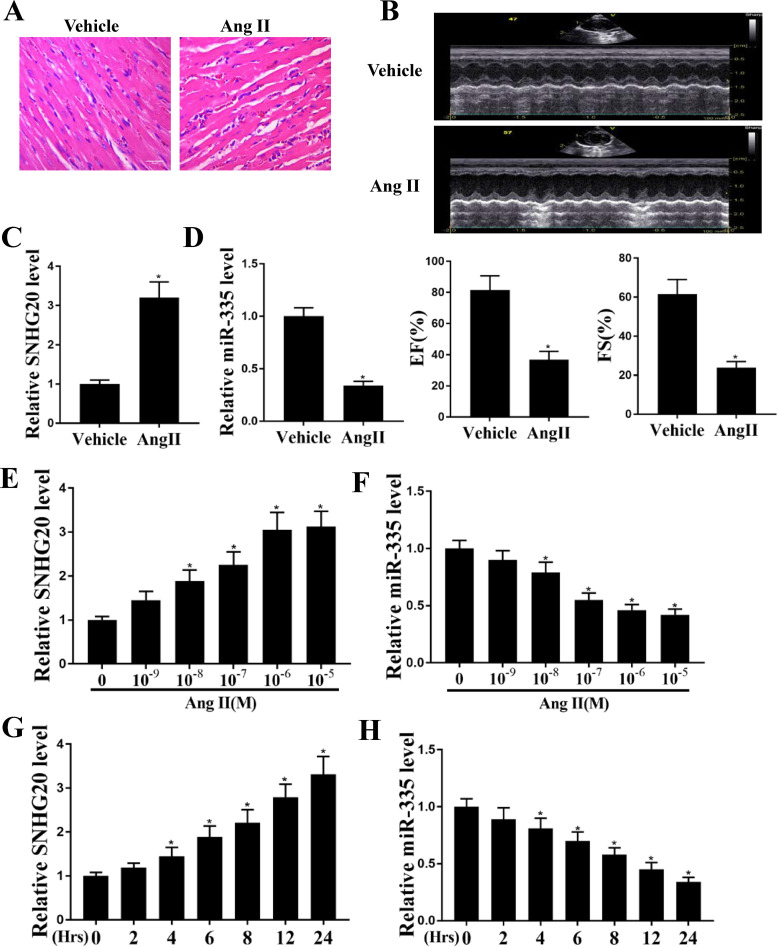
To study the role of SNHG20 in heart failure, a mouse model was used by intraperitoneal injection of Ang II. HE staining showed that cardiomyocyte (CM) cross-sectional area of heart tissues in the Ang II group was larger than that in normal mice (Fig. 1A). Echocardiography examination showed that EF and FS were both significantly decreased in Ang II-induced mice compared with that in the normal group (P < 0.05) (Fig. 1B). These results suggested that the mouse model with Ang II-induced cardiac fibrosis was successfully established. The quantitative real-time PCR (qRT-PCR) assay results showed that the expression levels of SNHG20 were significantly increased and the expression levels of miR-335 were significantly decreased in the heart tissues of Ang II-induced mice compared with that of normal mice (P < 0.05 [Fig. 1C] and P < 0.05 [Fig. 1D]). To further verify this, HL-1 cells were treated with different concentrations of Ang II for 24 h in vitro, and qRT-PCR results showed that SNHG20 was also significantly upregulated and miR-335 was downregulated in HL-1 cells treated with different concentrations of Ang II, except for 10−9 M (P < 0.05 [Fig. 1E] and P < 0.05 [Fig. 1F]). In addition, HL-1 cells were then treated with 10−6 M Ang II for different time periods, and qRT-PCR assay results showed that SNHG20 was still significantly upregulated and miR-335 was downregulated, except for the time point at 2 h (P < 0.05 [Fig. 1G] and P < 0.05 [Fig. 1H]). These results suggested that SNHG20 and miR-335 are associated with cardiac fibrosis and hypertrophy.
FIG 1.
SNHG20 was upregulated and miR-335 was downregulated in mice with Ang II-induced cardiac hypertrophy. (A) HE staining of heart tissues. (B) Echocardiographic assessment. (C and D) The expression of SNHG20 (C) and miR-335 (D) in heart tissues was detected by qRT-PCR. n = 10 mice per group. (E and F) HL-1 cells were treated with different concentrations of Ang II (10−9, 10−8, 10−7, 10−6, and 10−5 M) for 24 h, and the expression of SNHG20 (E) and miR-335 (F) was detected by qRT-PCR. (G and H) HL-1 cells were treated with 10−6 M Ang II for different times (2, 4, 6, 8, 12, and 24 h), and the expression of SNHG20 (G) and miR-335 (H) was detected by qRT-PCR. Each experiment was repeated three times. *, P < 0.05.
The decline of miR-335 by SNHG20.
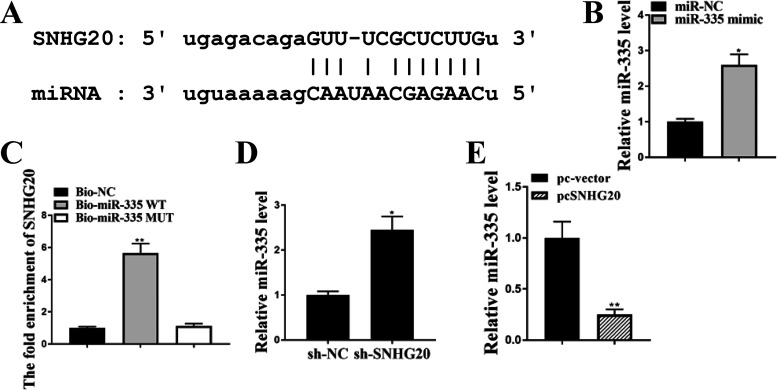
Starbase predicted that there was a putative binding site between SNHG20 and miR-335 (Fig. 2A). To explore their relationship, miR-335 mimics or miR-NC was transfected into HL-1 cells, and qRT-PCR revealed that the expression levels of miR-335 were significantly increased in miR-335 mimics transfected HL-1 cells compared with miR-NC (P < 0.05) (Fig. 2B). RNA pulldown assay demonstrated that SNHG20 was markedly enriched in the Bio-miR-335 WT group compared with Bio-NC (P < 0.01), and there was no significant difference between Bio-miR-335 MUT and Bio-NC (Fig. 2C). Moreover, the expression levels of miR-335 were significantly increased in sh-SNHG20-transfected HL-1 cells compared with sh-NC (P < 0.05) (Fig. 2D). In addition, overexpression of SNHG20 significantly reduced the expression levels of miR335 compared with that in the negative control (pc-vector) (P < 0.01) (Fig. 2E). In addition, we analyzed the copy number of miR-335 per cell in HL-1 cells under SNHG20 knockdown or overexpression, and the results showed that the level of SNHG20 in HL-1 cells was negatively correlated with the level of miR-335 (see Table S1 in the supplemental material). These results indicated that the decline of miR-335 by SNHG20 could be due to target-directed miRNA degradation (TDMD).
FIG 2.
Decline of miR-335 by SNHG20. (A) The putative binding site between miR-335 on the 3′-UTR of SNHG20 was predicted by Starbase. (B and C) HL-1 cells were transfected with miR-335 mimics and miR-NC. (B) The expression of miR-335 in HL-1 cells was detected by qRT-PCR. (C) The enrichment of SNHG20 was detected by RNA pulldown assay. (D) HL-1 cells were transfected with sh-SNHG20 and sh-NC. The expression of miR-335 in HL-1 cells was detected by qRT-PCR. (E) HL-1 cells were transfected with pc-SNHG20 and pc-vector. The expression of miR-335 was evaluated by qRT-PCR. Each experiment was repeated three times. *, P < 0.05; **, P < 0.01.
Downregulation of SNHG20 improved cardiac fibrosis and hypertrophy in Ang II-treated cardiomyocytes in vitro.
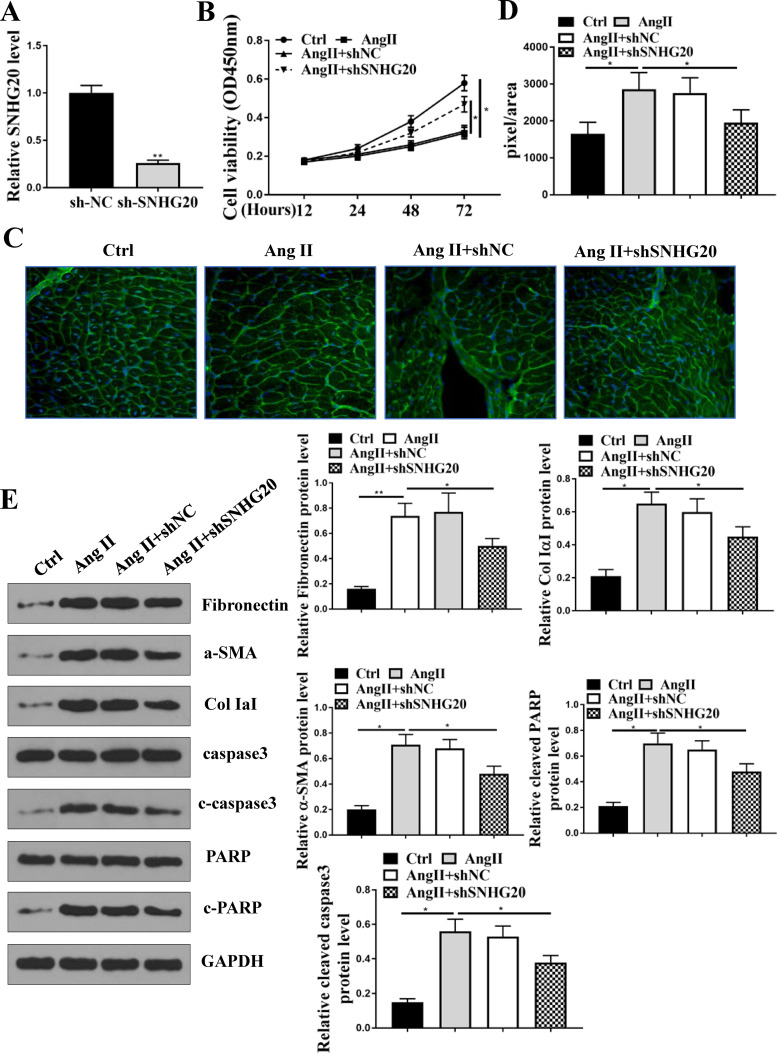
SNHG20 was knocked down in HL-1 cells, and qRT-PCR results showed that the expression of SNHG20 was significantly downregulated in sh-SNHG20-transfected HL-1 cells compared with sh-NC (P < 0.01) (Fig. 3A). Cell counting kit-8 (CCK-8) assay showed that Ang II treatment significantly decreased cell viability of HL-1 cells compared with that in the control group (P < 0.05), and downregulation of SNHG20 obviously attenuated Ang II-induced inhibitory effects on cell viability compared with sh-NC (P < 0.05) (Fig. 3B). WGA staining in HL-1 cells showed that Ang II treatment significantly increased cell size compared with that in the control group (P < 0.05), and downregulation of SNHG20 effectively decreased cell size in Ang II-treated HL-1 cells compared with sh-NC (P < 0.05) (Fig. 3C and D). In addition, Western blot analysis showed that Ang II treatment significantly increased the protein expression levels of fibronectin, collagen IaI, α-SMA, cleaved poly(ADP-ribose) polymerase (PARP), and cleaved aspase-3 in HL-1 cells compared with that in the control group, and this effect was reversed by the transfection of sh-SNHG20 rather than sh-NC (P < 0.05) (Fig. 3E). These results suggested that downregulation of SNHG20 improved cardiac fibrosis and hypertrophy in Ang II-treated cardiomyocyte in vitro.
FIG 3.
Cardioprotective role of downregulation of SNHG20 in Ang II-induced fibrosis in vitro. (A) HL-1 cells were transfected with sh-SNHG20 and sh-NC. The expression of SNHG20 in HL-1 cells. (B to E) HL-1 cells were transfected with sh-SNHG20 and sh-NC and then treated with 10−6 M Ang II for 24 h. (B) Cell viability was evaluated by CCK-8 assay. (C) WGA staining in HL-1 cells. (D) Cell size was determined by WGA staining in HL-1 cells. (E) The protein expression of fibronectin, collagen IaI, α-SMA, cleaved PARP, and cleaved aspase-3 in HL-1 cells was detected by Western blotting. Each experiment was repeated three times. *, P < 0.05.
The effects of SNHG20 in Ang II-induced cardiac fibrosis and hypertrophy in HL-1 cells were mediated by miR-335 in vitro.
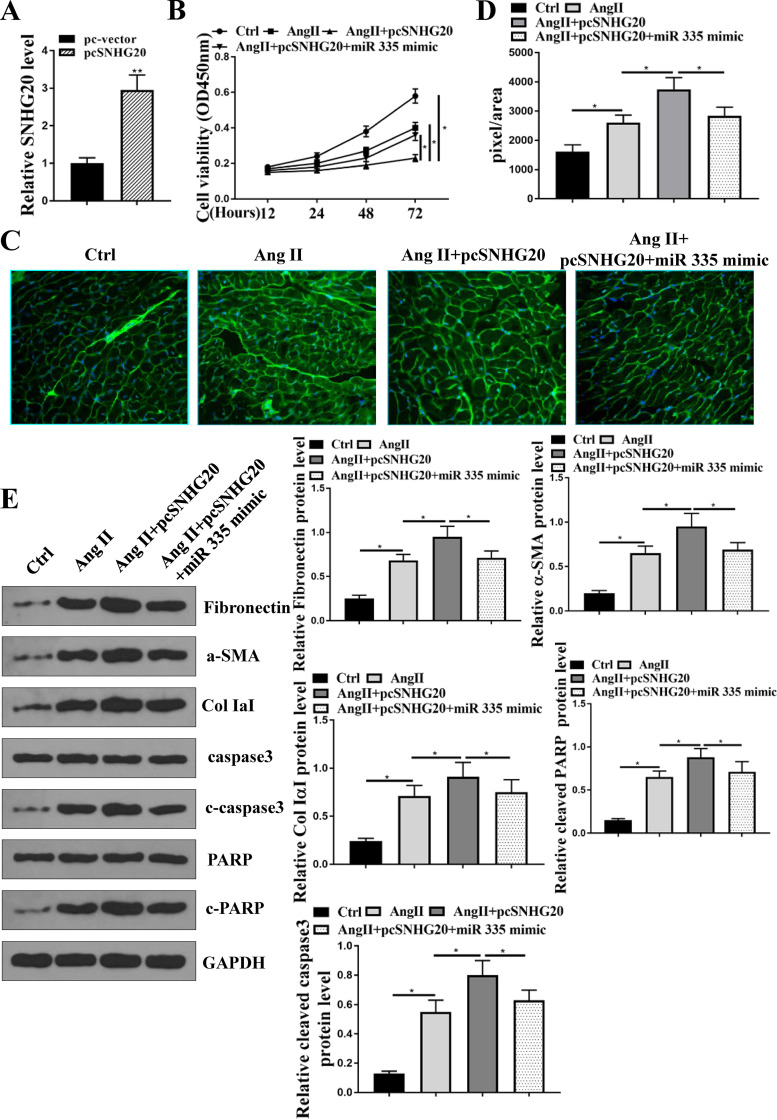
To explore whether the role of SNHG20 was mediated by miR-335, HL-1 cells were transfected with pc-SNHG20 or cotransfected with pc-SNHG20 and miR-335 mimics. The transfection efficiency of pc-SNHG20 was confirmed by qRT-PCR (Fig. 4A), and the results showed that overexpression of SNHG20 significantly increased the expression levels of SNHG20 (P < 0.01). CCK-8 assay showed that compared with the control group, Ang II treatment significantly decreased cell viability (P < 0.05), overexpression of SNHG20 enhanced the inhibitory effect of Ang II on cell viability (P < 0.05), and cotransfection of pc-SNHG20 and miR-335 significantly reversed the effect of pc-SNHG20 in Ang II treatment of HL-1 cells (P < 0.05) (Fig. 4B). WGA staining showed that compared with the control group, Ang II treatment significantly increased cell size (P < 0.05), overexpression of SNHG20 further increased cell size compared with that in the Ang II group (P < 0.05), and the effect of pc-SNHG20 on Ang II-induced cell size was significantly reversed by miR-335 (P < 0.05) (Fig. 4C and D). Western blotting showed that overexpression of SNHG20 further increased the expression levels of fibronectin, collagen IaI, α-SMA, cleaved PARP, and cleaved aspase-3 in Ang II-treated HL-1 cells (P < 0.05), and the effect of pc-SNHG20 in Ang II-treated HL-1 cells was significantly reversed by miR-335 (P < 0.05) (Fig. 4E). These results demonstrated that the effects of SNHG20 in Ang II-induced injury in HL-1 cells were mediated by miR-335.
FIG 4.
Effects of SNHG20 in Ang II-induced cardiac fibrosis on HL-1 cells were mediated by miR-335. (A) HL cells were transfected with pc-SNHG20 and sh-NC, and the expression of SNHG20 was evaluated by qRT-PCR. (B to E) HL-1 cells were transfected with sh-SNHG20, or cotransfected with sh-SNHG20 and miR-335 mimics, and then treated with 10−6 M Ang II for 24 h. (B) Cell viability was assessed by CCK-8 assay. (C) WGA staining in HL-1 cells. (D) Cell size was determined by WGA staining in HL-1 cells. (E) The protein expression of fibronectin, collagen IaI, α-SMA, cleaved PARP, and cleaved aspase-3 in HL-1 cells was detected by Western blotting. Each experiment was repeated three times. *, P < 0.05.
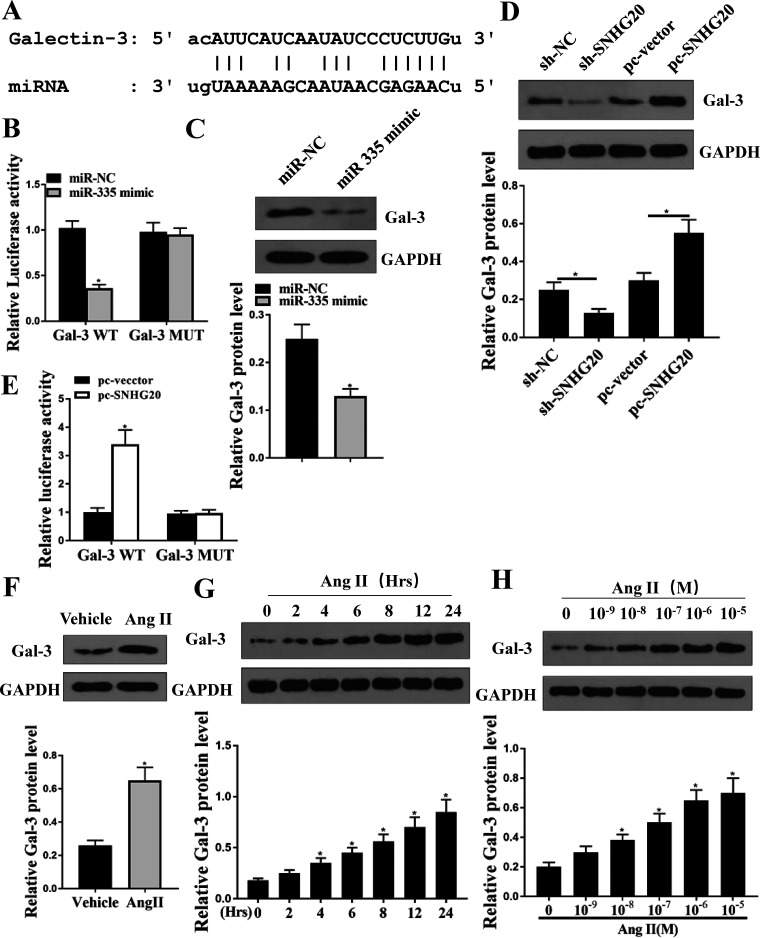
Galectin-3 was the target of miR-335.
To further explore the regulatory mechanism of miR-335, Targetscan was used to predict the potential targets of miR-335, and it showed that there was a putative binding site between miR-335 and the 3′-UTR of Galectin-3 (Fig. 5A). Luciferase reporter assay was performed, and the results showed that miR-335 mimics significantly decreased the relative luciferase activity of Gal-3 WT compared with miR-NC in HL-1 cells (P < 0.05) and exhibited no obvious change on Gal-3 MUT (Fig. 5B). Meanwhile, Western blotting showed that overexpression of miR-335 (miR-335 mimics) in HL-1 cells significantly decreased the protein expression levels of Gal-3 compared with miR-NC (P < 0.05) (Fig. 5C). In addition, downregulation of SNHG20 significantly decreased the protein expression levels of Gal-3 compared with sh-NC in HL-1 cells (P < 0.05), and overexpression of SNHG20 markedly increased the protein expression levels of Gal-3 compared with the negative control (pc-vector) (P < 0.05) (Fig. 5D). In addition, we found that overexpression of SNHG20 significantly increased the relative luciferase activity of Gal-3 WT compared with the negative control (pc-vector) (P < 0.05) but had no obvious effect on Gal-3 MUT (Fig. 5E). Moreover, we found that the expression levels of Gal-3 were significantly increased in heart tissues of Ang II-treated mice compared with that in normal mice (P < 0.05) (Fig. 5F). Furthermore, HL-1 cells were treated with 10−6 M Ang II for different time periods or treated with different concentrations of Ang II for 24 h, and qRT-PCR results showed that the expression levels of Gal-3 were all significantly increased compared with that in the control group, except for 10−6 M Ang II for 2 h and 10−9 M Ang II for 24 h (P < 0.05) (Fig. 5G and H). These results suggested that the effect of SNHG20/miR-335 on Ang II-induced cardiac fibrosis is mediated by galectin-3.
FIG 5.
Galectin-3 was a target of miR-335. (A) The putative binding site between miR-335 and Galectin-3 was predicted by Targetscan. (B) HL-1 cells were cotransfected with miR-335 mimics or miR-NC, and the relative luciferase reporter activity of Gal-3 WT and Gal-3 MUT was measured by the dual-luciferase reporter system. (C) HL-1 cells were transfected with miR-335 mimics or miR-NC, and the protein expression of galectin-3 was detected by Western blotting. (D) HL-1 cells were transfected with sh-SNHG20, pc-SNHG20, and corresponding negative controls, and the protein expression of galectin-3 was detected by Western blotting. (E) HL-1 cells were cotransfected with pc-SNHG20 or pc-vector, and the relative luciferase reporter activity of Gal-3 WT and Gal-3 MUT was measured by the dual-luciferase reporter system. (F) The protein expression of galectin-3 in heart tissues of Ang II-treated mice or normal mice was detected by Western blotting. (G) HL-1 cells were treated with 10−6 M Ang II for different times (2, 4, 6, 8, 12, and 24 h), and the expression of galectin-3 was detected by Western blotting. (H) HL-1 cells were treated with different concentrations of Ang II (10−9, 10−8, 10−7, 10−6, and 10−5 M) for 24 h, and the expression of galectin-3 was detected by Western blotting. Each experiment was repeated three times. *, P < 0.05.
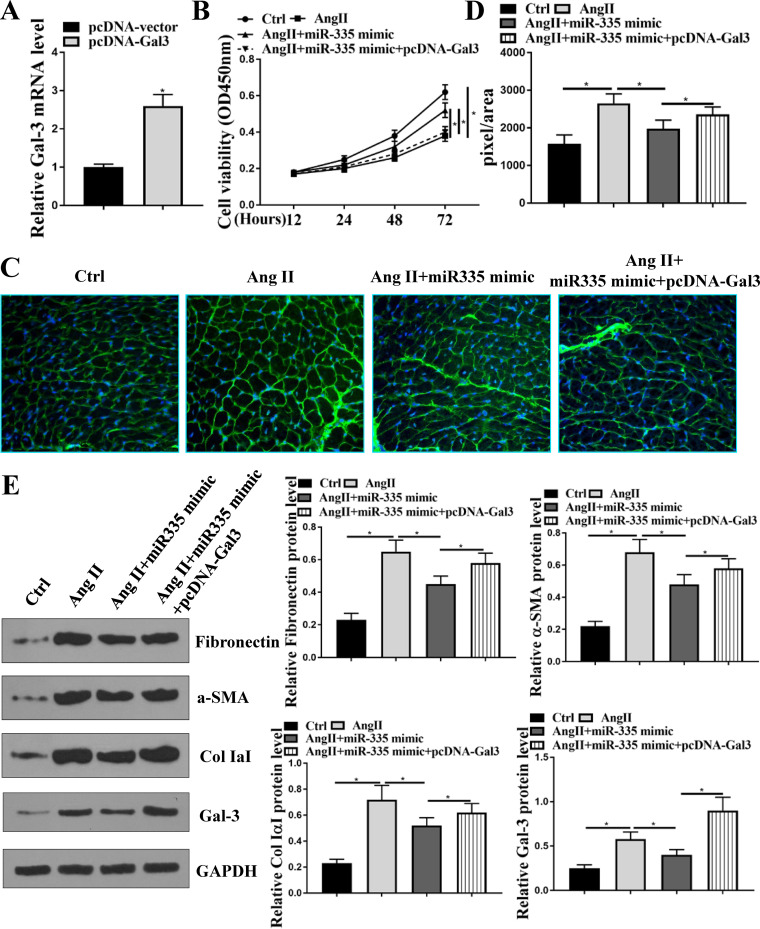
The protective effect of miR-335 in Ang II-induced cardiac fibrosis and hypertrophy was mediated by Galectin-3 in vitro.
To determine whether the effect of miR-335 on Ang II-induced cardiac fibrosis was mediated by galectin-3, HL-1 cells were transfected with pcDNA-Gal-3 or cotransfected with pcDNA-Gal-3 and miR-335 mimics. The expression levels of Gal-3 were significantly increased in HL-1 cells transfected with pcDNA-Gal-3 (P < 0.05) (Fig. 6A). CCK-8 assay revealed that compared with the control group, Ang II treatment significantly decreased cell viability of HL-1 cells (P < 0.05), miR-335 mimics attenuated the inhibitory effect of Ang II on cell viability (P < 0.05), and overexpression of Gal-3 eliminated the protective role of miR-335 mimics on viability of HL-1 cells (P < 0.05) (Fig. 6B). WGA staining showed that miR-335 mimics significantly decreased the size of Ang II-treated HL-1 cells (P < 0.05), and the effect was reversed by the cotransfection of miR-335 and pcDNA-Gal-3 (P < 0.05) (Fig. 6C and D). In addition, overexpression of miR-335 significantly attenuated Ang II-induced elevation of Gal-3 as well as myocytes fibrosis-related proteins, including fibronectin, collagen IaI, and α-SMA in HL-1 cells (P < 0.05), while these inhibitory effects were reversed by cotransfection of miR-335 and pcDNA-Gal-3 (P < 0.05) (Fig. 6E). These results demonstrated that the protective effect of miR-335 on Ang II-induced cardiac fibrosis and hypertrophy was mediated by Gal-3 in vitro.
FIG 6.
Protective effect of miR-335 in Ang II-induced cardiac fibrosis was mediated by Galectin-3 in vitro. (A) HL-1 cells were transfected with pcDNA-Gal-3 and pcDNA-vector, and the expression of Galectin-3 in HL-1 cells was detected by qRT-PCR. (B to E) HL-1 cells were transfected with miR-335 mimics or cotransfected with pcDNA-Gal-3 and miR-335 mimics and then treated with 10−6 M Ang II for 24 h. (B) Cell viability was evaluated by CCK-8 assay. (C) WGA staining in HL-1 cells. (D) Cell size was determined by WGA staining in HL-1 cells. (E) The protein expression of fibronectin, collagen IaI, α-SMA, and Gal-3 in HL-1 cells was detected by Western blotting. Each experiment was repeated three times. *, P < 0.05.
DISCUSSION
Heart failure is a major public health challenge, and its prevalence is increasing due to the aging of the world population (26). Heart failure is typically involved in cardiac remodeling that requires the occurrence of cardiac fibrosis (27). Hence, targeting cardiac fibrosis has emerged as a promising treatment strategy for heart failure, and studies on the molecular mechanisms of fibrosis need to be explored in detail. In this study, we found that SNHG20 was significantly upregulated in heart tissues of angiotensin II-treated mice and angiotensin II-stimulated HL-1 cells. Inhibition of SNHG20 attenuated angiotensin II-induced cardiac fibrosis and hypertrophy. Moreover, our results revealed that the cardiac protective role of knockdown of SNHG20 was mediated by the miR-335/Gal-3 axis, extending the pathogenesis of cardiac fibrosis.
Recently, a large number of lncRNAs have been identified to participate in the process of pathological fibrosis by exerting stimulative or suppressive roles. Wisper, Meg3, and MIAT are three well-known cardiac fibrosis-related lncRNAs exhibiting direct contribution to cardiac fibrosis (28–30). In addition, several lncRNAs, such as FAF, Crnde, and Kcnq1ot1, can attenuate cardiac fibrosis under different pathological fibrosis conditions (9, 31, 32). SNHG20 is identified to act as a tumorigenic lncRNA and has been demonstrated to contribute to the progression of various human cancers, including osteosarcoma (33, 34), prostate cancer (35), glioblastoma (36), laryngeal squamous cell carcinoma (37), and epithelial ovarian cancer (38). However, the role of SNHG20 in cardiac fibrosis remains unclear. Here, we found that SNHG20 was upregulated during angiotensin II-induced fibrosis both in vitro and in vivo. More importantly, angiotensin II treatment significantly decreased HL-1 cell viability and increased cell size, and it enhanced the expression levels of fibrosis-related proteins (fibronectin, collagen IaI, and α-SMA) and apoptosis-related proteins (cleaved caspase-3 and cleaved PARP). This effect was obviously attenuated by knockdown of SNHG20. One previous study found that SNHG20 potentially participated in the process of vasculogenic mimicry in neoplasm (14). Our results further demonstrated that SNHG20 played essential roles not only in cancers but also in cardiac fibrosis. Meanwhile, our study revealed a new function of SNHG20 and facilitated a better understanding of the diverse functions of lncRNAs.
Increasing evidence has demonstrated that miRNAs play crucial roles in the occurrence and development of cardiac fibrosis by directly targeting mRNAs. For example, miR-135a inhibits cardiac fibrosis by inhibiting the TRPM7-collagen production pathway (39). miR-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress (40). miR-21 has been demonstrated to promote cardiac fibrosis after myocardial infarction by targeting Smad7 (41). miR-223 has been identified to enhance cell proliferation, migration, and differentiation in cultured cardiac fibroblasts and then mediate cardiac fibrosis after myocardial infarction, partially through the regulation of RASA1 (42). Although previous studies have revealed that miR-335 was associated with the myocardial ischemia, hypertrophy, and metabolism of the heart (19), its actual function in cardiac fibrosis has not been well studied. Extensive studies have focused on noncoding RNAs, including lncRNAs and miRNAs in cardiovascular diseases, because lncRNAs can exert their important functions by directly sponging miRNAs to inhibit transcription, and lncRNAs and miRNAs may be considered potential biomarkers for the diagnosis and treatment of cardiovascular diseases (43, 44). Here, we found that there was a putative binding site between SNHG20 and miR-335, and the expression of miR-335 was significantly downregulated during angiotensin II-induced fibrosis both in vitro and in vivo. Meanwhile, inhibition of miR-335 obviously reversed the protective effect of sh-SNHG20 in cardiac fibrosis and hypertrophy. We confirmed the potential role of miR-335 in cardiac fibrosis and hypertrophy and further revealed a novel upstream regulatory lncRNA, SNHG20. Previous studies reported that lncRNAs could act as sponges of miRNAs when the lncRNAs were abundant, and sponges did not change the abundance of a microRNA (45). In this study, we found that SNHG20 negatively regulated the expression level of miR-335, suggesting that the decline of miR-335 by SNHG20 is due to target-directed miRNA degradation (TDMD). Interestingly, SNHG20 has been identified to have several target mRNAs, including miR-6516-5p (35), miR-140 (37), and miR-4486 (46). In this study, we also predicted all the potential miRNA targets of SNHG20 and identified 89 miRNAs, including miR-335 (see Table S2 in the supplemental material). Based on this information, whether the identified targets mediated the role of SNHG20 in cardiac fibrosis and hypertrophy should be further determined in subsequent experiments.
It has been reported that galectin-1 directly induces pathological remodeling of the heart and therefore is considered a culprit protein in the occurrence and development of cardiac fibrosis in heart failure (47). In addition, galectin-3 is also a well-known suppressor of apoptosis (48). Gal-3 is identified as a direct target of miRNAs to participate in the progression of human diseases (49, 50). Here, our study revealed that Gal-3 was a target of miR-335, and overexpression of Gal-3 significantly attenuated the protective effect of miR-335 mimics in cardiac fibrosis and hypertrophy and elevated the expression levels of fibrosis-related proteins (fibronectin, collagen IaI, and α-SMA) and apoptosis-related proteins (cleaved caspase-3 and cleaved PARP) inhibited by miR-335 mimics. Taken together, the role of SNHG20/miR-335 in cardiac fibrosis and hypertrophy was partially mediated by Galectin-3.
In addition, to further verify the regulatory axis of SNHG20/miR-335/Gal-3, it is necessary to investigate whether overexpression and silencing of SNHG20 changes the activity of the luciferase reporter linked to the 3′-UTR of galectin-3 with a mutant miR-335 site.
MATERIALS AND METHODS
Cell culture and treatment.
Mouse cardiomyocyte cell line HL-1 (Novobio Inc., Shanghai, China) was used, and cells were cultured in Claycomb medium (Sigma-Aldrich, Merck KGaA) containing 10% fetal bovine serum (FBS; Sigma-Aldrich), 0.1 mM norepinephrine, and 1% penicillin-streptomycin (Nacalai Tesque Inc.) at 37°C with additional 5% CO2. When needed, HL-1 cells were treated with different concentrations of Ang II (Sigma-Aldrich) (10−9, 10−8, 10−7, 10−6, and 10−5 M) for 24 h or treated with 10−6 M Ang II for different time periods (2, 4, 6, 8, 12, and 24 h). To explore the role of SNHG20, HL-1 cells were treated with 10−6 M Ang II for 24 h.
Cell transfection.
Oligonucleotides targeting SNHG20 (sh-SNHG20) and the negative control (sh-NC) were synthesized by RiboBio Inc. (Guangzhou, China) (sh-SNHG20, 5′-GCCACUCACAAGAGUGUAUTT-3′; sh-NC, 5′-GGATACGGAGTACTATAGC-3′). miR-335 mimics and miR-NC (negative control) were also purchased from RiboBio Inc. (Guangzhou, China). The sequences of miR-335 and miR-NC were the following: miR-335, 5′-UUUUUCAUUAUUGCUCCUGACC-3′; miR-NC, 5′-AGGAUGUAUUACCAGUGAUCGG-3′. To overexpress SNHG20 and Galectin-3, the entire full lengths of SNHG20 (2,183 bp) and Galectin-3 were amplified and cloned into pcDNA3.1 expression vector to generate pc-SNHG20 and pcDNA-Gal-3, and the empty vector pcDNA3.1 was used as a negative control (pcDNA-vector). About 50 nM shRNA/miRNA and overexpression vectors (the total amount was 2 μg) were transfected into HL-1 cells using Lipofectamine 3000 (Invitrogen) by following the manufacturer's instructions. Cells were collected for the subsequent experiments at 48 h after transfection.
Animal model.
All animal studies were approved by the Animal Ethics Committee and Laboratory Animal Research Committee of the Fourth Affiliated Hospital of Harbin Medical University. C57BL/6J mice (6 to 7 weeks old, weighing 20 ± 1 g) were purchased from the Shanghai Laboratory Animal Center at the Chinese Academy of Sciences (Shanghai, China). Mice with cardiac fibrosis and hypertrophy were induced through the intraperitoneal injection of Ang II (400 ng/kg of body weight/day; Sigma-Aldrich) to generate the Ang II group, and mice in the control group (Vehicle group) received equal amounts of phosphate-buffered saline (PBS) (n = 10 in each group). After injection for 12 days, mice were anesthetized by intraperitoneal injection of chloral hydrate (350 mg/kg), and cardiac function was assessed. Afterwards, mice were sacrificed using cervical dislocation, and then mouse hearts were collected for the subsequent experiments.
Echocardiographic measurement.
After deep anesthesia, cardiac function of mice was assessed by transthoracic echocardiography with an ultrasound system (Panoview β1500; Cold Spring Biotech, Taiwan, China) equipped with a 30-MHz phased-array transducer. Left ventricular ejection fraction (EF%) and left ventricular fractional shortening (FS%) were calculated in at least 3 consecutive cardiac cycles using Vevo 2100 high-resolution imaging system (Visual Sonics).
Histological analysis.
Mouse hearts of different groups were fixed in 4% paraformaldehyde for 24 h, embedded in paraffin, and cut into 5-μm-thick sections. Heart tissue sections were stained with wheat germ agglutinin (WGA) staining and hematoxylin and eosin (HE) staining to examine histopathology as previously described (51). Cell size was determined by ImageJ software.
Luciferase reporter assay.
Starbase was used to predict the target miRNAs of SNHG20, and Targetscan was applied to predict the target mRNAs of miR-335. The 3′ untranslated region (3′-UTR) of Galectin-3 containing the wild-type (WT) binding sites with miR-335 was amplified from mouse genomic DNA by PCR and cloned into the downstream of the stop codon of the luciferase gene of the pGL3 reporter vector (Promega) to generate the Gal-3 WT. To generate mutated 3′-UTR of Gal-3, the mutation was introduced by a QuikChange II XL site-directed mutagenesis kit (Stratagene) and cloned into pGL3 reporter vector to obtain Gal-3 MUT. The reporter vectors (200 ng/well) then were cotransfected with miR-335 mimics or pc-SNHG20 into HL-1 cells using Lipofectamine 3000 (Invitrogen). After transfection for 48 h, cells were lysed and the relative luciferase activity was detected by a dual-luciferase reporter assay kit (Promega).
RNA pulldown assay.
RNA pulldown assay was performed as previously described (52). In brief, HL-1 cells were transfected with biotinylated WT or MUT of miR-335 against the binding site between SNHG20 and negative control (Bio-NC) (Guangzhou RiboBio Co., Ltd.). Transfected HL-1 cells were lysed by radioimmunoprecipitation assay (RIPA) lysis buffer and incubated with Dynabeads M-280 streptavidin (Invitrogen, CA, USA) at 4°C for 2 h. The bound RNAs then were purified by TRIzol reagent for the detection of enrichment of SNHG20 by qRT-PCR.
CCK-8 assay.
Cell proliferation was measured using cell counting kit-8 (CCK-8; Beyotime, Shanghai, China) by following the manufacturer’s instructions. Briefly, 1 × 105 cells per well were seeded into 96-well plates after transfection and treated with 10−6 M Ang II, and then 10 μl of CCK-8 reagent was added to each well at 12, 24, 48, and 72 h and incubated for 4 h. The absorbance at 450 nm was measured with a microplate reader.
qRT-PCR.
Total RNAs of mice heart tissues or cultured HL-1 cells were extracted using TRIzol reagent (Invitrogen); 2 μg of total RNAs then was reverse transcribed into cDNA using the reverse transcription system kit (TaKaRa). qRT-PCR was performed with a 7500 real-time PCR system (Life Technologies, USA) using SYBR green PCR mix (TaKaRa). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal reference for SNHG20 and U6 for miR-335. Relative expression levels were calculated by the 2-ΔΔCT method. The primers used for PCR in this study were the following: SNHG20 forward, 5′-AGCAACCACTATTTTCTTCC-3′, and reverse, 5′-CCTTGGCGTGTATCTATTTAT-3′; miR-335 forward, 5′-TCAAGAGCAATAACGAAAAATGT-3′, and reverse, 5′-GCGAGCACAGAATTAATACGAC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′; reverse, 5′-AACGCTTCACGAATTTGCGT-3′; Galectin-3 forward, 5′-GCCAACGAGCGGAAAATGG-3′, and reverse, 5′-TCCTTGAGGGTTTGGGTTTCC-3′; and GAPDH forward, 5′-TCROCKCAROCKCTTCCAGG-3′, and reverse, 5′-GATGACCCTTTTGGCTCCC-3′.
Western blotting.
Total proteins of mice heart tissues or HL-1 cells were extracted using RIPA lysis buffer (Beyotime Institute of Biotechnology, China). Approximately equal amounts of protein samples were separated by 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. After blocking in 1% bovine serum albumin (BSA), the membranes were incubated with primary antibodies including anti-galectin-3, antifibronectin, anti-collagen IaI, anti-α-SMA, anti-PARP, anti-caspase-3, and anti-GAPDH at 4°C overnight. All antibodies (Abcam, Cambridge, MA, USA) were diluted at 1:1,000, except for anti-GAPDH at 1:10,000. The next day, the membranes were then incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG at room temperature for 1 h. The protein bands were visualized using the enhanced chemiluminescent (ECL) kit and quantified by Image J software with GAPDH as the internal reference.
Statistical analysis.
All data were analyzed with GraphPad Prism 7 software and presented as the means ± standard deviations (SD). The Student's t test was used for the comparisons between two groups. One-way analysis of variance followed by Tukey's post hoc test was used for multiple comparisons. A P value of <0.05 was considered a statistically significant difference.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Natural Science Foundation of China (81570358), Health and Family Planning Commission of Shanghai Pudong New District, China (PWZxq2017-01 and PWYgy2018-03), and Health and Family Planning Commission of Shanghai, China (ZK2019B25).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Sohns C, Marrouche NF. 2020. Atrial fibrillation and cardiac fibrosis. Eur Heart J 41:1123–1131. 10.1093/eurheartj/ehz786. [DOI] [PubMed] [Google Scholar]
- 2.González A, Schelbert EB, Díez J, Butler J. 2018. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol 71:1696–1706. 10.1016/j.jacc.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Nguyen NB, Pezhouman A, Ardehali R. 2019. Cardiac fibrosis: potential therapeutic targets. Transl Res 209:121–137. 10.1016/j.trsl.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jathar S, Kumar V, Srivastava J, Tripathi V. 2017. Technological developments in lncRNA biology. Adv Exp Med Biol 1008:283–323. 10.1007/978-981-10-5203-3_10. [DOI] [PubMed] [Google Scholar]
- 5.Bär C, Chatterjee S, Thum T. 2016. Long noncoding RNAs in cardiovascular pathology, diagnosis, and therapy. Circulation 134:1484–1499. 10.1161/CIRCULATIONAHA.116.023686. [DOI] [PubMed] [Google Scholar]
- 6.He C, Hu H, Wilson KD, Wu H, Feng J, Xia S, Churko J, Qu K, Chang HY, Wu JC. 2016. Systematic characterization of long noncoding RNAs reveals the contrasting coordination of cis- and trans-molecular regulation in human fetal and adult hearts. Circ Cardiovasc Genet 9:110–118. 10.1161/CIRCGENETICS.115.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng H, Ouyang W, Zhang L, Xiao X, Huang Z, Zhu W. 2019. LncRNA GASL1 is downregulated in chronic heart failure and regulates cardiomyocyte apoptosis. Cell Mol Biol Lett 24:41. 10.1186/s11658-019-0165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao K, Lei W, Wu H, Wu J, Yang Z, Yan S, Lu XA, Li J, Xia X, Han X, Deng W, Zhong G, Zhao ZA, Hu S. 2019. LncRNA-Safe contributes to cardiac fibrosis through Safe-Sfrp2-HuR complex in mouse myocardial infarction. Theranostics 9:7282–7297. 10.7150/thno.33920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng D, Zhang Y, Hu Y, Guan J, Xu L, Xiao W, Zhong Q, Ren C, Lu J, Liang J, Hou J. 2019. Long noncoding RNA Crnde attenuates cardiac fibrosis via Smad3-Crnde negative feedback in diabetic cardiomyopathy. FEBS J 286:1645–1655. 10.1111/febs.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou Y, Liao C, Li H, Yu G. 2020. LncRNA SOX2OT/Smad3 feedback loop promotes myocardial fibrosis in heart failure. IUBMB Life 72:2469–2480. 10.1002/iub.2375. [DOI] [PubMed] [Google Scholar]
- 11.Cui N, Liu J, Xia H, Xu D. 2019. LncRNA SNHG20 contributes to cell proliferation and invasion by upregulating ZFX expression sponging miR-495-3p in gastric cancer. J Cell Biochem 120:3114–3123. 10.1002/jcb.27539. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZX, Zhao Y, Yu Y, Liu N, Zou QX, Liang FH, Cheng KP, Lin FW. 2020. Effects of lncRNA SNHG20 on proliferation and apoptosis of non-small cell lung cancer cells through Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci 24:230–237. 10.26355/eurrev_202001_19915. [DOI] [PubMed] [Google Scholar]
- 13.Guan C, Zhao Y, Wang W, Hu Z, Liu L, Li W, Jiang X. 2020. Knockdown of lncRNA SNHG20 Suppressed the Proliferation of Cholangiocarcinoma by Sponging miR-520f-3p. Cancer Biother Radiopharm 10.1089/cbr.2020.4042. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Xue Y, Liu X, Zheng J, Shen S, Yang C, Chen J, Li Z, Liu L, Ma J, Ma T, Liu Y. 2019. ZRANB2/SNHG20/FOXK1 axis regulates vasculogenic mimicry formation in glioma. J Exp Clin Cancer Res 38:68. 10.1186/s13046-019-1073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng D, Xu Q, Liu Y, Li G, Sun W, Ma D, Ni C. 2021. Long noncoding RNA-SNHG20 promotes silica-induced pulmonary fibrosis by miR-490-3p/TGFBR1 axis. Toxicology 451:152683. 10.1016/j.tox.2021.152683. [DOI] [PubMed] [Google Scholar]
- 16.Michlewski G, Cáceres JF. 2019. Post-transcriptional control of miRNA biogenesis. RNA 25:1–16. 10.1261/rna.068692.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. 2019. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol 234:5451–5465. 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 18.Wu N, Zhang X, Du S, Chen D, Che R. 2018. Upregulation of miR-335 ameliorates myocardial ischemia reperfusion injury via targeting hypoxia inducible factor 1-alpha subunit inhibitor. Am J Transl Res 10:4082–4094. [PMC free article] [PubMed] [Google Scholar]
- 19.Gonçalves IF, Acar E, Costantino S, Szabo PL, Hamza O, Tretter EV, Klein KU, Trojanek S, Abraham D, Paneni F, Hallström S, Kiss A, Podesser BK. 2019. Epigenetic modulation of tenascin C in the heart: implications on myocardial ischemia, hypertrophy and metabolism. J Hypertens 37:1861–1870. 10.1097/HJH.0000000000002097. [DOI] [PubMed] [Google Scholar]
- 20.Nangia-Makker P, Hogan V, Raz A. 2018. Galectin-3 and cancer stemness. Glycobiology 28:172–181. 10.1093/glycob/cwy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-Martínez E, Brugnolaro C, Ibarrola J, Ravassa S, Buonafine M, López B, Fernández-Celis A, Querejeta R, Santamaria E, Fernández-Irigoyen J, Rábago G, Moreno MU, Jaisser F, Díez J, González A, López-Andrés N. 2019. CT-1 (cardiotrophin-1)-Gal-3 (galectin-3) axis in cardiac fibrosis and inflammation. Hypertension 73:602–611. 10.1161/HYPERTENSIONAHA.118.11874. [DOI] [PubMed] [Google Scholar]
- 22.Valiente-Alandi I, Potter SJ, Salvador AM, Schafer AE, Schips T, Carrillo-Salinas F, Gibson AM, Nieman ML, Perkins C, Sargent MA, Huo J, Lorenz JN, DeFalco T, Molkentin JD, Alcaide P, Blaxall BC. 2018. Inhibiting fibronectin attenuates fibrosis and improves cardiac function in a model of heart failure. Circulation 138:1236–1252. 10.1161/CIRCULATIONAHA.118.034609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun M, Jin L, Bai Y, Wang L, Zhao S, Ma C, Ma D. 2019. Fibroblast growth factor 21 protects against pathological cardiac remodeling by modulating galectin-3 expression. J Cell Biochem 120:19529–19540. 10.1002/jcb.29260. [DOI] [PubMed] [Google Scholar]
- 24.Shen H, Wang J, Min J, Xi W, Gao Y, Yin L, Yu Y, Liu K, Xiao J, Zhang YF, Wang ZN. 2018. Activation of TGF-β1/α-SMA/Col I profibrotic pathway in fibroblasts by galectin-3 contributes to atrial fibrosis in experimental models and patients. Cell Physiol Biochem 47:851–863. 10.1159/000490077. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Li S, Hao X, Zhang Y, Deng W. 2019. Perindopril and a galectin-3 inhibitor improve ischemic heart failure in rabbits by reducing Gal-3 expression and myocardial fibrosis. Front Physiol 10:267. 10.3389/fphys.2019.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talman V, Ruskoaho H. 2016. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res 365:563–581. 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacmeister L, Schwarzl M, Warnke S, Stoffers B, Blankenberg S, Westermann D, Lindner D. 2019. Inflammation and fibrosis in murine models of heart failure. Basic Res Cardiol 114:19. 10.1007/s00395-019-0722-5. [DOI] [PubMed] [Google Scholar]
- 28.Piccoli MT, Gupta SK, Viereck J, Foinquinos A, Samolovac S, Kramer FL, Garg A, Remke J, Zimmer K, Batkai S, Thum T. 2017. Inhibition of the cardiac fibroblast-enriched lncRNA Meg3 prevents cardiac fibrosis and diastolic dysfunction. Circ Res 121:575–583. 10.1161/CIRCRESAHA.117.310624. [DOI] [PubMed] [Google Scholar]
- 29.Micheletti R, Plaisance I, Abraham BJ. 2017. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci Transl Med 9:eaai9118. 10.1126/scitranslmed.aai9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu X, Du Y, Shu Y, Gao M, Sun F, Luo S, Yang T, Zhan L, Yuan Y, Chu W, Pan Z, Wang Z, Yang B, Lu Y. 2017. MIAT is a pro-fibrotic long non-coding RNA governing cardiac fibrosis in post-infarct myocardium. Sci Rep 7:42657. 10.1038/srep42657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J, Wang Z, Shi H, Gu L, Wang S, Wang H, Li Y, Wei T, Wang Q, Wang L. 2020. LncRNA FAF inhibits fibrosis induced by angiotensinogen II via the TGFβ1-P-Smad2/3 signalling by targeting FGF9 in cardiac fibroblasts. Biochem Biophys Res Commun 521:814–820. 10.1016/j.bbrc.2019.10.175. [DOI] [PubMed] [Google Scholar]
- 32.Yang F, Qin Y, Lv J, Wang Y, Che H, Chen X, Jiang Y, Li A, Sun X, Yue E, Ren L, Li Y, Bai Y, Wang L. 2018. Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis 9:1000. 10.1038/s41419-018-1029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Luo P, Guo W, Shi Y, Xu D, Zheng H, Jia L. 2018. LncRNA SNHG20 knockdown suppresses the osteosarcoma tumorigenesis through the mitochondrial apoptosis pathway by miR-139/RUNX2 axis. Biochem Biophys Res Commun 503:1927–1933. 10.1016/j.bbrc.2018.07.137. [DOI] [PubMed] [Google Scholar]
- 34.Guo H, Yang S, Li S, Yan M, Li L, Zhang H. 2018. LncRNA SNHG20 promotes cell proliferation and invasion via miR-140-5p-ADAM10 axis in cervical cancer. Biomed Pharmacother 102:749–757. 10.1016/j.biopha.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, Xiao Y, Zhou Y, Zhou Z, Yan W. 2019. lncRNA SNHG20 promotes prostate cancer migration and invasion via targeting the miR-6516-5p/SCGB2A1 axis. Am J Transl Res 11:5162–5169. [PMC free article] [PubMed] [Google Scholar]
- 36.Gao XF, He HQ, Zhu XB, Xie SL, Cao Y. 2019. LncRNA SNHG20 promotes tumorigenesis and cancer stemness in glioblastoma via activating PI3K/Akt/mTOR signaling pathway. Neoplasma 66:532–542. 10.4149/neo_2018_180829N656. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Xu J, Guo YN, Yang BB. 2019. LncRNA SNHG20 promotes the development of laryngeal squamous cell carcinoma by regulating miR-140. Eur Rev Med Pharmacol Sci 23:3401–3409. 10.26355/eurrev_201904_17704. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Dai J, Hou S, Qian Y. 2019. LncRNA SNHG20 predicts a poor prognosis and promotes cell progression in epithelial ovarian cancer. Biosci Rep 39:BSR20182186. 10.1042/BSR20182186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Liu Y, Pan Y, Lu C, Xu H, Wang X, Liu T, Feng K, Tang Y. 2018. MicroRNA-135a inhibits cardiac fibrosis induced by isoproterenol via TRPM7 channel. Biomed Pharmacother 104:252–260. 10.1016/j.biopha.2018.04.157. [DOI] [PubMed] [Google Scholar]
- 40.Yuan J, Liu H, Gao W, Zhang L, Ye Y, Yuan L, Ding Z, Wu J, Kang L, Zhang X, Wang X, Zhang G, Gong H, Sun A, Yang X, Chen R, Cui Z, Ge J, Zou Y. 2018. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics 8:2565–2582. 10.7150/thno.22878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan J, Chen H, Ge D, Xu Y, Xu H, Yang Y, Gu M, Zhou Y, Zhu J, Ge T, Chen Q, Gao Y, Wang Y, Li X, Zhao Y. 2017. Mir-21 promotes cardiac fibrosis after myocardial infarction via targeting Smad7. Cell Physiol Biochem 42:2207–2219. 10.1159/000479995. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Xu Y, Deng Y, Li H. 2018. MicroRNA-223 regulates cardiac fibrosis after myocardial infarction by targeting RASA1. Cell Physiol Biochem 46:1439–1454. 10.1159/000489185. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y. 2018. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med 22:5768–5775. 10.1111/jcmm.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tay Y, Rinn J, Pandolfi PP. 2014. The multilayered complexity of ceRNA crosstalk and competition. Nature 505:344–352. 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomson DW, Dinger ME. 2016. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet 17:272–283. 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Cheng LG, Li HG. 2019. LncRNA SNHG20 promoted the proliferation of glioma cells via sponging miR-4486 to regulate the MDM2-p53 pathway. Eur Rev Med Pharmacol Sci 23:5323–5331. 10.26355/eurrev_201906_18199. [DOI] [PubMed] [Google Scholar]
- 47.Gehlken C, Suthahar N, Meijers WC, de Boer RA. 2018. Galectin-3 in heart failure: an update of the last 3 years. Heart Fail Clin 14:75–92. 10.1016/j.hfc.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Barman SA, Li X, Haigh S, Kondrikov D, Mahboubi K, Bordan Z, Stepp DW, Zhou J, Wang Y, Weintraub DS, Traber P, Snider W, Jonigk D, Sullivan J, Crislip GR, Butcher JT, Thompson J, Su Y, Chen F, Fulton DJR. 2019. Galectin-3 is expressed in vascular smooth muscle cells and promotes pulmonary hypertension through changes in proliferation, apoptosis, and fibrosis. Am J Physiol Lung Cell Mol Physiol 316:L784–L797. 10.1152/ajplung.00186.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bieg D, Sypniewski D, Nowak E, Bednarek I. 2019. MiR-424-3p suppresses galectin-3 expression and sensitizes ovarian cancer cells to cisplatin. Arch Gynecol Obstet 299:1077–1087. 10.1007/s00404-018-4999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magnussen C, Blankenberg S. 2018. Biomarkers for heart failure: small molecules with high clinical relevance. J Intern Med 283:530–543. 10.1111/joim.12756. [DOI] [PubMed] [Google Scholar]
- 51.Strobel S, Encarnação JA, Becker NI, Trenczek TE. 2015. Histological and histochemical analysis of the gastrointestinal tract of the common pipistrelle bat (Pipistrellus pipistrellus). Eur J Histochem 59:2477. 10.4081/ejh.2015.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, Jin X, Liu B, Zhang P, Chen W, Li Q. 2019. CircRNA CBL.11 suppresses cell proliferation by sponging miR-6778-5p in colorectal cancer. BMC Cancer 19:826. 10.1186/s12885-019-6017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download MCB.00580-20-s0001.xlsx, XLSX file, 0.01 MB (9.4KB, xlsx)
Table S2. Download MCB.00580-20-s0002.xlsx, XLSX file, 0.02 MB (20.4KB, xlsx)
Captions to Tables S1 and S2. Download MCB.00580-20-s0003.pdf, PDF file, 0.1 MB (135.8KB, pdf)