Abstract
Although there is extensive literature around the biologic correlations of neurocognitive function in HIV/AIDS, less is known about the impact in everyday living. We conducted a systematic review of the association of neurocognitive impairment with everyday life functions in people with HIV on antiretroviral therapy. We specifically focused on attention, executive function, processing speed, and the central executive component of the working memory. We considered 3 domains of everyday functions: (1) autonomy, (2) decision making and adherence to treatment, and (3) quality of life and psychologic wellbeing. The relationship between neurocognitive impairment and mental health was examined, given its correlation with everyday life functions. Results indicate that people with HIV do experience problems with autonomy of daily living (especially if aged older than 50 years) and with decision making, and neurocognitive impairment plays a role in this regard. Psychologic wellbeing is associated with executive function and processing speed. These patients may also have a reduced quality of life, but the relationship between quality of life and cognition is uncertain or could be mediated by other factors. Neurocognitive impairment correlates with depression and anxiety; however, the relationship of cognitive performance with apathy is still controversial.
Keywords: chronic HIV disease, AIDS, antiretroviral therapy, everyday life, neurocognitive impairment, depression, anxiety
Introduction
HIV infection can directly affect the central nervous system,1 is associated with reduced brain volume,2 and can impact the structure and function of grey matter3,4 and white matter circuits.5–7 Consequently, roughly 30% to 55% of patients with chronic HIV infection and taking antiretroviral therapy (ART)8 present with HIV-associated neurocognitive disorder (HAND).9 This heterogeneous condition may involve the central executive component of working memory,10–12 attention,13 executive function,13 and processing speed.14–16 In this article, we specifically target the role of these functions.
Although other functions can be involved in HAND (eg, long-term memory, motor function), this review is limited to those functions strongly implicated in the supervision and qualitative control or realization of everyday life activities. The central executive component of working memory provides high-level attention regulation and activates the executive processes for tasks that cannot be automatically controlled by routine and implicit attentive resources (ie, focusing, dividing, shifting attention).17,18 The central executive component of working memory is not a discrete function but is a manifestation of other functions,19 such as executive control, attention, and processing speed. These functions should be studied in conjunction with one another. Currently, there is no consensus regarding their impact on everyday life outcomes20 or on the relationships between neurocognitive impairment and sex,21 age and aging,22 falls,23 frailty,24 body composition,25 sarcopenia,26 immunosuppression, inflammation,27 comorbidity28 and disease severity,29 timing of ART initiation or deintensification,30 and polypharmacy. Although neurocognition may be associated with these variables, the timing, strength of correlation, mediating factors, and relationships with everyday life activities are still to be ascertained.
In this article, we aimed to systematically review the literature in this field of HIV/AIDS research. We evaluated the putative association of neurocognitive impairment, as operationalized above, with everyday life outcomes in people with chronic HIV. We also studied the relationship between neurocognitive impairment and mental health, given its relevance to everyday life functions.
Methods
Eligibility Criteria
For this review, only descriptive or observational studies (cross-sectional, cohort, case-control, and hybrid designs; prospective or retrospective) were considered. Eligible studies included (1) those with adults with HIV (older than 18 years of age) who had been prescribed ART; (2) those with recorded information in terms of a raw or standardized score derived from formal neuropsychologic testing on the central executive, attention, executive function, and processing speed; and (3) those that considered or discussed everyday life outcomes. Animal and pediatric studies were not included. The review was reported following the recommendations set out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) working group.31
Information Sources and Search Strategy
We systematically searched the PubMed database for contributions published between January 1, 2000, and December 31, 2019. We also examined pertinent articles cited in the references of selected original research papers, book chapters, relevant reviews, and papers reporting the results of meta-analyses. Information retrieval used the same keywords in Google Scholar. Only original papers published in English were considered. A detailed description of the search strategy is provided in the protocol of this systematic review, which is available as Supplementary Information (together with the PRISMA checklist), and it was registered in the International Prospective Register of Systematic Reviews (PROSPERO).32
Outcomes
Given the multifaceted nature of the outcomes of this study, we considered a 3-dimension classification of everyday life function. The first dimension concerned independence and the possibility to conduct an autonomous life, as measured by autonomy in activities of daily living (ADL), instrumental activities of daily living (iADL), and employment status. ADL scores measure a person's ability to perform basic self-care tasks and iADL scores measure a person's ability to live independently. ADL and iADL are generally preserved in healthy aging; however, impairment has been described in people with HIV. The second dimension referred to a person's capacity to make decisions and adhere to treatment. For the third dimension, we examined quality of life and psychologic wellbeing in light of their theoretical relevance and practical importance.33,34 We considered mental health (which is highly correlated with everyday function) as a secondary outcome in terms of depression, apathy, and anxiety. These conditions have been assessed in the HIV literature in terms of the presence of symptoms, not necessarily diagnosis of a major disorder.
Data Management and Analysis
After the first screening of records identified in PubMed, selected items (data and metadata) were stored in a database and queried through structured query language (SQL). Two blinded reviewers (ER and MC) searched for papers eligible for inclusion by screening titles and abstracts. Information regarding the general setting, study design, sample size, follow-up duration, demographic characteristics of participants, and neuropsychologic testing was extracted from each paper. Selected baseline covariates included age, sex, duration of HIV infection, nadir and current CD4+ cell count, CD8+ cell count, viral load, duration of ART, presence of infections other than HIV (eg, hepatitis C virus [HCV]), and presence of other medical conditions. Results of the neuropsychologic evaluation (central executive, attention, executive function, and processing speed) presented as raw or standardized scores were extracted from each paper. Included studies were assessed for the possibility of selection bias and residual or unmeasured confounding. Given the high degree of heterogeneity and the methodologic differences observed across study designs and the potential bias that could be artificially created by meta-analyzing data under these conditions,35 we only summarized data qualitatively.
Results
Descriptive Findings
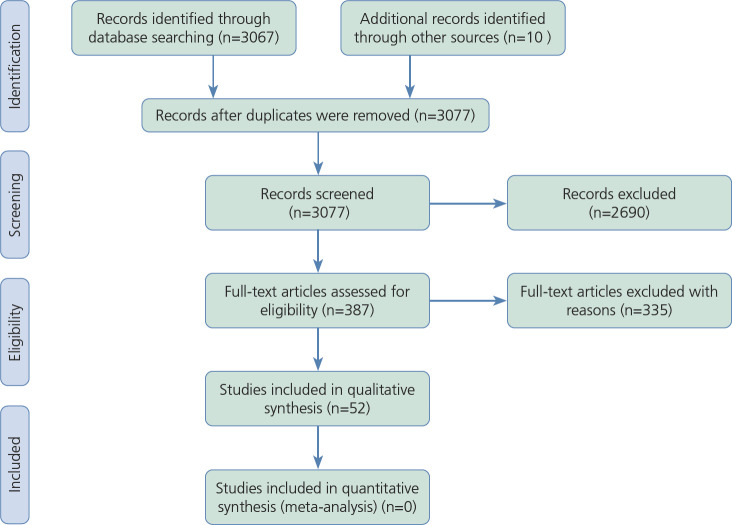
After removing duplicates, 3067 items were identified in PubMed and preliminarily screened. Of these, 377 were potentially eligible, and after exclusions, 42 studies were selected for the inclusion. Another 10 studies were added from a systematic search in books, book chapters, and bibliographies (Figure 1).
Figure.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow-chart of the studies included in the systematic review. From those screened, 377 items were initially retrieved from the PubMed database; 42 studies matched the inclusion criteria and were comprised in the present paper, and 10 studies were included by manual search for a total of 52 studies (see the Supplementary Tables). These 52 studies were published between 2000 and 2019, and had sample sizes ranging from 11 to 2863.
Clinical trials, letters, commentaries, and observational studies with no detailed assessment of neuropsychologic function or no consideration or discussion of everyday life outcomes were excluded, as was with testing of patients with acute HIV. In Supplemental Tables 1, 2, and 3, we show a summary of the main results obtained in each study.
The sample size of included studies ranged from 11 to 2863 (Supplemental Table 2). Most studies tested patients aged 30 to 49 years, except for 6 studies24,36–40 that selected patients aged 50 to 59. Outcomes were measured with different tools by different authors (Supplemental Table 3). Apathy was measured with a formal scale (the Apathy Evaluation Scale41,42), through an adaptation of subscales from the Neuropsychiatric Inventory,43 or by means of an ad hoc index.44
Only 27 of 52 studies tested a sample of matched controls, and several studies were affected by residual or unmeasured confounding. In addition, important medical variables and conditions potentially related to cognition, such as diabetes, hypertension, circulatory function, thyroid function, and HCV coinfection, were not included in the statistic models or the results of the analyses were not discussed in detail. Although 45 of 52 studies collected information on current CD4+ cell count, the nadir CD4+ cell count was reported in only 25 studies; current or nadir CD4+ cell count could independently correlate with neurocognition.45,46
The Autonomy in Everyday Life
Activities and Instrumental Activities of Daily Living
ADL or iADL were tested in 13 of 52 studies.24,38–40,44,47–53 Seven studies24, 38–40,47,48,52 documented association of neurocognitive impairment with ADL. In one study, executive function predicted self-reported iADL decline and unemployment status in people with HIV.47 Similar results were reported by Marquine and colleagues, who found that worse health conditions and presence of neurocognitive impairment are associated with ADL decline and unemployment.48 According to Watson and colleagues, trauma, economic hardship, and stress are expected to be associated with worse neurocognitive performance and functional decline in people with HIV.38 Two studies described correlation of neurocognitive impairment and iADL scores in middle- and older-aged participants.39,40 In Erlandson and colleagues' study, frailty and neurocognitive impairment were associated with greater risk of falls, disability, or death in people with HIV.24
Generally, the role of neurocognitive impairment in the ADL or iADL performance of people with HIV is controversial in the reviewed literature. Although asymptomatic people with HIV underperformed in tasks involving central executive activation and showed reduced information processing speed compared with those in the control group(s), this was not predictive of their ADL performance.50 A similar result was reported by Lawler and colleagues, who found that people with HIV were impaired in attention and central executive with no direct consequences in terms of ADL.51 In a study by Kamat and colleagues, no clear involvement of neurocognitive impairment in ADL was found, but people with HIV showed symptoms of the apathy spectrum that predicted poor ADL.44 In another study from the same authors, apathy, not executive function, was a predictor of iADL performance.49 Similar results were reported by Sadek and colleagues, who found that depressive symptoms were more sensitive in predicting a decline in instrumental iADL than neurocognitive impairment, including the central executive.53 Few studies reviewed herein controlled for relevant medical variables such as hypertension, occurrence of coronary artery disease, or cerebrovascular events in the analyses, which may have led to residual confounding (Supplemental Table 2).
Employment Status
Employment status was considered in 11 of 52 studies.47,48,52–60 Correlation of neurocognitive performance with employment status was found in 8 of these 11 studies.47,48,52–54,56,59,60 One study in particular showed that neurocognitive function (including central executive performance) together with ART status and presence of HCV infection were associated with the employment indicator.56 A specific contribution of the central executive in predicting employment status was also described.59 Similar results were found by Rabkin and colleagues, who reported that the executive function was a significant predictor of employment status,60 and by Heaton and colleagues, who found a correlation between employment status and neurocognitive performance, including testing of the central executive, attention, and executive function.52 Sadek and colleagues also reported that neurocognitive impairment was predictive of employment status, but the contributions of the central executive, attention, or executive control were not clear.53 However, from the preliminary findings of a longitudinal study conducted in the Netherlands, the TREVI project,55 a specific contribution of attention, central executive, or another neurocognitive function in predicting employment status in people with HIV did not emerge cross-sectionally. These patients aged 40 to 54 years had significantly lower employment rates than the general population in the Netherlands, and an association between employment status and depression did emerge. Similarly, Atkins and colleagues did not report an association between neurocognitive impairment and employment status.57 Moreover, Chernoff and colleagues did not find correlations between the central executive or executive function performance and employment status.58
Making Decisions and Adhering to Treatment
Decision Making
The ability to make decisions was discussed in 5 of 52 studies,61–65 which all documented an association with neurocognitive impairment. Gomez and colleagues tested people with HIV using a comprehensive neuropsychologic battery, including testing of the central executive. Participants were also assessed with a decision-making test (Game of Dice Task [GDT]). Neuropsychologic performance, presence of psychiatric comorbidities, and alcohol abuse were predictive of GDT performance.61 In a study by Fujiwara and colleagues, impairment in GDT performance was related to current immune status and was associated with executive function and processing speed.62 Thames and colleagues found that performance in a gambling task could be predicted by executive function and was partially mediated by presence of depression.64 An effect of central executive impairment and failure in inhibitory processes on decision making was found by Hardy and colleagues.63
Adherence to ART
Adherence to ART was considered in 6 of 52 studies.66–71 Association between neurocognitive impairment, as operationalized in this article, and adherence to treatment was documented in 2 studies.67,69 Ettenhofer and colleagues found that central executive performance was predictive of medical treatment adherence; however, the reverse association was also significant.67 Another study showed that the severity of neurocognitive impairment was associated with performance in everyday life function in terms of medication management.69 Childers and colleagues found that, during temporary treatment interruption, mood state and neurocognitive function remained relatively stable, despite worsened viral load and CD4+ cell count. Nevertheless, improved viral suppression and immune restoration when reinitiating ART led to an improvement in neurocognitive performance.66 By contrast, other authors did not describe a clear relationship between neurocognitive impairment and adherence to treatment. In a study by Fong and colleagues, partial drug adherence was associated with forgetfulness and missing medical appointments; this may be linked to long-term memory performance and not with impairment of the central executive or supervisory control.68 In another study, depression, not neurocognitive performance (which included a null effect of attention, executive function, and processing speed), emerged as a predictor of medication adherence, together with patients' satisfaction with their practitioners.70
Quality of Life and Psychologic Wellbeing
Quality of Life
Quality of life was considered in 8 of 52 studies.37,54,72–77 Association of neurocognitive impairment with reduced quality of life was documented in 5 studies.54,72,74,76,77 Harrison and colleagues measured central executive performance with an n-back task in a sample of people with HIV, and found that the scores were negatively correlated with quality of life and depression.72 Similar results were reported when considering attention and processing speed performance.72 In a study by Osowiecki and colleagues, executive control and processing speed were associated with quality of life in women with HIV, independent of emotional distress.74 Decreased neurocognitive performance, together with increased fatigue, determined a worse perceived quality of life in a sample of clade C-infected patients.76 In a study by Catalan and colleagues, everyday memory difficulties (with uncertain involvement of the central executive), anxiety, and depression were associated with self-reported measures of quality of life.37 Other authors did not describe association between neurocognitive performance and quality of life. In a study by Thein and colleagues, neither central executive nor attention performance correlated with quality of life.73 Apathy, not neurocognitive impairment or depression, has been reported in association with a reduced quality of life.75
Psychologic Wellbeing
Psychologic wellbeing was measured in 3 of 52 studies.78–80 Two studies reported association between this outcome and neurocognitive impairment.79,80 The performance in executive function and processing speed tasks correlated with reduced psychologic wellbeing defined by variables such as recreational drugs, irritability, and somatic complaints.79 Impairment of processing speed could be associated with high levels of anxiety and depression and with reduced psychologic wellbeing.80 However, in another study, the central executive, but not other cognitive functions, was significantly impaired in a sample of people with HIV, but this was not predictive of patients' psychologic wellbeing.78
Mental Health
Depression
Depression was targeted in 10 of 52 studies.36,57,72,80–86 An association between neurocognitive impairment and depression was reported in 7 studies.57,72,80,81,83,85,86 In Chartier and colleagues' study, attention and central executive performance emerged as predictors of depression.81 In another study, central executive and processing speed performance were associated with depression.72 Processing speed was also associated with depression in the investigation by Janssen and colleagues.80 Four other studies documented an association between depression and neurocognitive impairment, including testing functions responsible for supervisory control.57,83,85,86 However, one study did not find a correlation between impairment of attention or executive control and depression in a sample of people with HIV aged 50 to 59 years.36 Similar results were obtained by Kamat and colleagues, who reported that neurocognitive function was not associated with depression.44 Lifetime alcohol and marijuana consumption did not predict performance in tasks involving activation of the central executive, and there was no association with depression, but there was an association with adverse medical outcomes.82 Intellectual and central executive function, age, and somatic symptoms of depression (but not education) were significant predictors of HIV disease progression and survival, but the association between neurocognitive impairment and depression was unclear.84
Apathy
Apathy was considered by 4 of 52 studies41–44; association with neurocognitive impairment was found in 2 of the 4.42,43 Castellon and colleagues reported association of central executive and executive function performance with apathy,43 although in a study by Shapiro and colleagues, processing speed and attention, not central executive performance, were associated with apathy.42 However, 2 studies did not find an association between neurocognitive performance and apathy.41,44
Anxiety
Anxiety was considered in 3 of 52 studies80,85,86; all of these studies documented the association under investigation. A correlation between neurocognitive performance and anxiety, with no specific involvement of the central executive, was found in a follow-up study on the effects of efavirenz.86 According to Robertson and colleagues, neurocognitive status, including testing of functions involved in the supervisory control, was associated with anxiety.85 In another study, impairment of processing speed correlated with anxiety.80
Discussion
Neurocognitive impairment in people with HIV persists in the ART era.87,88 A large-sample multisite study89 demonstrated the presence of deficits in cognition in approximately 52% of people with HIV. About 33% of participants had asymptomatic cognitive disorders, 12% had mild neurocognitive impairment, and 2% had HIV-associated dementia. Although poor neuropsychologic function is a significant predictor of mortality in people with HIV,90 the picture is less clear concerning the sequelae of neurocognitive impairment in patients' everyday life functions. Central executive, attention, executive, and processing speed functions have often been described as impaired in people with HIV,78,91 but the consequences of such deficits, in terms of everyday life outcomes, are far from understood.
Autonomy in Everyday Life
From the current literature review, 7 studies24,38–40,47,48,52 documented an association between neurocognitive impairment and deficit in ADL. In one of these studies,52 although participants were young (mean age, 39.3 [standard deviation, 7.5]), they had relatively advanced stages of disease (34% had Centers for Disease Control and Prevention [CDC] stage C disease, 58% had AIDS, and the mean CD4+ count was 365.5 [standard deviation, 267]). Other studies described uncertain or nonsignificant relationship between neurocognitive impairment and ADL or iADL.44,49–51,53 Three of these studies44,49,53 reported an association between mental health and performance in ADL or iADL. Based on current evidence, we may assume that in patients treated with ART and with restored immunologic function, neurocognitive impairment may be associated with ADL or iADL. The relationship could be mediated by mental health or other unmeasured factors. For patients with relatively advanced disease or who are older, neurocognitive impairment could likely lead to a reduction of performance in ADL or iADL. Both aging and HIV disease progress may independently impact neurocognitive function, including a person's central executive, processing speed, and executive function.22
Aging is particularly important in contemporary ART research because currently more than 50% of people with HIV are 50 years or older. In the large MACS (Multicenter AIDS Cohort Study) investigation,22 age was described as a variable independently associated with episodic memory and motor function performance. The relationship between neurocognition and ADL or iADL in people with HIV in the transition phase (ie, 50–60 years of age) or older was examined by 3 studies reviewed herein.24,39,40 These studies described the association as under investigation.24
One study reported that advances in ART contributed to decreases in disability rates in people with HIV.92 Nevertheless, these rates remain higher than those reported in the general population. Thus, people with HIV are still potentially at risk for problems in ADL or iADL, especially at age 50 years and older, and should be prospectively monitored. Results of the neuropsychologic literature reported herein are in conflict in that no specific role of the central executive, attention, executive function, or processing speed has been demonstrated in determining a decline in ADL or iADL. One possible explanation was provided by Scott and colleagues, who showed that multitasking, not the activation of a single neuropsychologic function, is crucial in predicting iADL impairment in people with HIV.93
It has been suggested that health, not HIV status, could be related to performance at work among people with HIV.94 Contrary to this position, a national survey conducted in France concluded that even though the conditions among people with HIV in 2015 were far better than those observed in 2000 or before, unemployment is still an issue in this subpopulation.95 Further, financial hardship may be relevant for people with HIV, especially among ethnic minorities.96 Job security, not only employment, is an important predictor of mental health in people with HIV.54 In this regard, university graduation and being 40 years of age or older are protective factors for successful employment. A recent study highlighted the positive relationship between being employed and mood in people with HIV.97 By contrast, 3 risk factors for lower job security include frailty, HIV disease severity, and HCV coinfection98; the specific role of CD4+ cell count has not been determined.99
In the pre-ART era, people with HIV experienced several limitations restricting their ability to work and were frequently on work disability.97 In the ART era, many patients can continue working or return to work, or they are now transitioning to retirement.100 Unemployment is still associated with higher AIDS-related or non-AIDS-related mortality.101 Nevertheless, employment among people with HIV should be carefully considered, given that the proportion of people with HIV receiving disability benefit awards may affect the measurement. From the present review, it emerged that neurocognitive function, especially in terms of the central executive, attention, and executive function, is correlated with employment status.47,48,52–54,56,59,60 This result was not confirmed by data from the TREVI project,55 but such discrepancy could be explained by the fact that different studies tested patients of different age cohorts (eg, Kordovski et al56 and van Gorp et al59 considered patients in their 30s and early 40s years of age, while Wagener et al55 enrolled patients in their late 40s and 50s years of age). We also remark that the impact of the central executive, attention, and executive function in predicting the employment status of people with HIV should be studied in conjunction with the role of prospective memory, another predictor of employment status102 not targeted in this review. It has also been shown how less educated people with HIV could be at higher risk of unemployment,103 which might suggest a protective effect of cognitive reserve.
Capacity to Make Decisions and Adherence to Treatment
A noteworthy result of this review is that the central executive, processing speed, and executive function performance of a person are predictive of their decision-making ability.61–64 As such, it may provide a neurocognitive hypothesis of why some people with HIV are exposed to certain risk behaviors, such as substance use and gambling. Apart from mental health and social factors, there could be a purely cognitive component that explains why these patients are impaired in their risk behaviors and decision-making skills. This finding also highlights the importance of early psychologic intervention in people with HIV who show risky behaviors in everyday life.
In the literature, low CD4+ cell count, anxiety,104 and depression105,106 have already been associated with poor adherence to ART. An important question concerns whether or not neurocognitive impairment could directly impact treatment adherence. Results from this review are not converging. On the one hand, 2 studies documented a possible effect of neurocognitive performance, in particular the central executive.67,69 On the other hand, 3 studies found a null or uncertain effect of the central executive, attention, or supervisory control.66,68,70 We suggest that the interplay with other neuropsychologic functions, such as prospective memory, could be important in this regard. We also note that one study found that cognitive performance was associated with patients' abilities to navigate the Internet to complete important health-related tasks (eg, online pharmacy and health records navigation). These activities require central executive, attention, processing speed, and executive function integrity, which may be related to adherence to ART.107
Quality of Life and Psychologic Wellbeing
Recent research has focused on the roles of mental health108 and social support109 in determining quality of life in people with HIV. Important conditions such as the presence of depressive symptoms or pain have been associated with reduced quality of life.110 One study also reported that time since HIV diagnosis is more important than a person's age in defining quality of life in people with HIV.111 Biologic parameters, such as immune activation and viral load, also seem to be related to this outcome.112 However, the role of neurocognitive impairment, which was addressed in this review, seems uncertain.
Three studies reviewed herein showed the specific role of neurocognitive impairment on quality of life.72,74,76 In particular, Harrison and colleagues found an association between central executive, attention, and processing speed performance and quality of life,72 and Osowiecki and colleagues documented the contribution of executive control and processing speed.74 However, other studies failed to find a specific and independent role of these functions in quality of life.37,73,75 This discrepancy may be explained by the different tasks used by different authors, by the different strategies of controlling for confounding, and by the possibility of residual or unmeasured confounding.
Two studies reported an association between psychologic wellbeing and performance in executive function and processing speed.79,80 Although testing for neuropsychologic performance and psychologic wellbeing, one study did not describe an association.78 We may assume a mediating effect played by mental health (which was not explicitly tested by the authors). Indeed, absence of depressive or anxiety symptoms could protect the psychologic wellbeing of patients with mild or well-controlled neurocognitive problems.
Mental Health
Depression is a common comorbidity in people with HIV and is associated with the presence of other chronic diseases113 and anxiety.114 In the ART era, both depression and neurocognitive impairment remain prevalent and are associated conditions in people with HIV. This was recently confirmed by data from the European-Canadian network sCReen for Anxiety, depression, and Neurocognitive Impairment in HIV+ patients (CRANIum), where about 40% of screened participants tested positive for neurocognitive impairment and about 16% had depression.85 According to Todd and colleagues, depressive symptoms are commonly reported in about 30% of people with HIV and are associated with mortality.115 HAND can arise relatively independent of depression and is not a simply reactive disturbance.116 A longitudinal study found that lifetime major depressive disorders were associated with cognitive problems in everyday life, and difficulty increased at times of incident major depressive episodes. Individuals with incident major depressive episodes did not show consistent neuropsychologic decline.117
Results of this review indicate an association between neurocognitive impairment and depression.57,72,80,81,83,85,86 In particular, an association between attention, central executive performance,72,80 and processing speed was documented.72,80 Although negative results have been reported in the literature,36,44,82 most studies described a correlation. It is difficult to ascertain the prevalent direction of this association, for which more evidence coming from large-sample longitudinal studies is needed. With the current state of knowledge, we may assume that targeting neurocognitive function as a specific goal of an intervention with people with HIV could also lead to benefits in terms of mental health.
Presence of depressive symptoms and impairment in executive control emerged as 2 independent predictors of meta-memory performance,118 which may explain why people with HIV who report both neurocognitive impairment and depressive symptoms have difficulty managing several tasks of everyday life, such as keeping track of medical appointments and drug adherence. Though potentially meaningful, to our knowledge this possible explanation has not been explored in depth. It is also worth observing that from 1 study reviewed herein,82 important factors related to mental health, such as current and lifetime alcohol use and marijuana consumption, although associated with adverse medical outcomes, were not associated with performance in central executive and attention tasks.
The correlation between neurocognitive impairment and depression in people with HIV is well documented; however, the picture is much more fragmented in regards to apathy. Apathy was described in association with neurocognitive performance in 2 of the reviewed studies,42,43 but not in others.41,44 Apathy is related to the activity of the motivational system,119 which is relatively independent of the central executive, attention, or processing speed performance. This may explain the negative results (ie, no association of apathy with the neuropsychologic performance) reported in the literature.
All studies analyzed in this review described an association between neurocognitive impairment and anxiety.80, 85,86 This provides converging evidence with the early findings by Hestad and colleagues,120 who reported that central executive and processing speed difficulties were associated with anxiety in people with HIV. Even though the direction of the association is uncertain, the presence of anxiety symptoms should be specifically targeted by clinicians, which may lead to careful longitudinal monitoring for presence of cognitive decline. A factor related to anxiety that has not been specifically addressed in this review is stress and post-traumatic stress. A study conducted with the WIHS (Women's Inter-agency HIV Study) cohort121 showed that stress and post-traumatic stress correlate negatively with neurocognitive performance, even though the outcomes on everyday life function have not been explored. Early childhood trauma should be discussed in relation to cognitive impairment, mental health, and everyday life outcomes. Another WIHS investigation found that for women who have experienced early life trauma, resilience can be protective in terms of depression and quality of life.122 However, the relationship between early childhood trauma and neurocognitive impairment is still to be clarified.
Fatigue is another important variable, well known to many people with HIV and clinicians. One study documented the association of neurocognitive impairment with fatigue in people with HIV.123 Because fatigue has also been described as associated with depression and anxiety in people with HIV,124 all these variables should be studied together and inserted in a dynamic model to understand possible pathways toward personalized care. The role of HCV coinfection should also be explored more in depth,125 as well as its association with fatigue and ADL or iADL performance.126
Substance use is another relevant factor related to mental health that is connected with everyday life outcomes. A study conducted on the MACS cohort127 found that cocaine use in people with HIV led to a 3-fold–increased risk of depressive symptoms. Another MACS investigation128 showed that in the years 1984 to 2013, marijuana use decreased in people with HIV, but daily use among users increased; cigarette smoking and alcohol consumption were positively correlated with marijuana use. Together, these findings indicate the importance of substance use as a plausible mediating or moderating factor between neurocognition and everyday life outcomes.
Limitations
In the last 20 years, knowledge of everyday life outcomes in people with HIV has progressed considerably; nevertheless, there are still many issues open for future research. Limitations of the current literature include scarce consideration of disease stage, nadir CD4+ cell count, virologic suppression, illness consequent to opportunistic infection and inflammation, blood parameters such as dyslipidemia or insulin resistance,59 and brain measures such as axonal injury.129 The relationship and interplay of these parameters with neurocognitive impairment and everyday life outcomes are still unclear. Sex also appears to play a role in neurocognition in people with HIV; women seem more affected by neurocognitive impairment than men,21 but the relationship to everyday life function is not clear. Other important variables that should be assessed in conjunction with neurocognitive performance and everyday life outcomes are quality of sleep,130 pain,131 presence of cumulative stressful events,132 physical performance,40 social support, and quality of intimate relationships. The role of polypharmacy in relation to cognition and ADL or iADL should be assessed. A recent study found that people with HIV aged 50 years or older are at risk for increased and sustained polypharmacy. This could have a relevant impact on neurocognition and autonomy in daily living.133 Finally, most studies mainly enrolled patients aged 30 to 59 years, so everyday life outcomes of people with HIV who are 20 to 29 years old should be specifically targeted for future research.
Conclusion
In general, neurocognitive impairment in people with HIV is associated with everyday life outcomes, particularly with ADL or iADL, problems at work and employment status, decision-making ability, and psychologic wellbeing. The relationship between quality of life and adherence to treatment is debated because nonconverging results have emerged in the contemporary literature. Other intervening factors, such as mental health, could have an impact and mediate the relationship. Moreover, even though there is converging evidence suggesting correlation of neurocognitive impairment with everyday life outcomes in chronic HIV disease, the strength of this association, the relationship with specific biomarkers, and the effect of existing modifiers are still to be clarified. Together, these findings imply the need for policy makers to invest more resources into the investigation of the neuropsychology of HIV, especially considering progress in life expectancy of people with HIV. This should also be studied in light of the effects of SARS-CoV-2 infection in those with HIV134 The multidisciplinary intervention strategy for people with HIV may be remodulated, particularly in terms of longitudinally monitoring the neuropsychologic function and its interaction with mental health. Ecologic outcomes, such as quality of life and psychologic wellbeing, should be routinely assessed.
Footnotes
Financial affiliations in the past 24 months: Dr Ripamonti has no relevant financial affiliations to disclose. Dr Clerici has no relevant financial affiliations to disclose. (Updated: June 30, 2021)
Contributor Information
Enrico Ripamonti, Statistician at the Karolinska Institute in Stockholm, Sweden..
Mario Clerici, Head of the Department of Pathophysiology and Transplantation at the University of Milan in Italy..
References
- 1.Popov M, Molsberry SA, Lecci F, et al. Brain structural correlates of trajectories to cognitive impairment in men with and without HIV disease. Brain Imaging Behav. 2020;14(3):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortega M, Heaps JM, Joska J, et al. HIV clades B and C are associated with reduced brain volumetrics. J Neurovirol. 2013;19(5): 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang L, Tomasi D, Yakupov R, et al. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol. 2004;56(2):259–272. [DOI] [PubMed] [Google Scholar]
- 4.Becker KM, Heinrichs-Graham E, Fox HS, et al. Decreased MEG beta oscillations in HIV-infected older adults during the resting state. J Neurovirol. 2013;19(6):586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller-Oehring EM, Schulte T, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Callosal degradation in HIV-1 infection predicts hierarchical perception: a DTI study. Neuropsychologia. 2010;48(4):1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ernst T, Chang L, Arnold S. Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage. 2003;19(4):1686–1693. [DOI] [PubMed] [Google Scholar]
- 7.Watson C, Busovaca E, Foley JM, et al. White matter hyperintensities correlate to cognition and fiber tract integrity in older adults with HIV. J Neurovirol. 2017; 23(3):422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacktor N. Changing clinical phenotypes of HIV-associated neurocognitive disorders. J Neurovirol. 2018;24(2):141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan IL, Smith BR, Hammond E, et al. Older individuals with HIV infection have greater memory deficits than younger individuals. J Neurovirol. 2013;19(6):531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Failde-Garrido JM, Alvarez MR, Simon-Lopez MA. Neuropsychological impairment and gender differences in HIV-1 infection. Psychiatry Clin Neurosci. 2008;62(5): 494–502. [DOI] [PubMed] [Google Scholar]
- 12.Wilkie FL, Goodkin K, Khamis I, et al. Cognitive functioning in younger and older HIV-1–infected adults. JAIDS. 2003;33Suppl 2:S93–S105. [DOI] [PubMed] [Google Scholar]
- 13.Chang L, Yakupov R, Nakama H, Stokes B, Ernst T. Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J Neuroimmune Pharmacol. 2008; 3(2):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy DJ, Hinkin CH. Reaction time performance in adults with HIV/AIDS. J Clin Exp Neuropsychol. 2002;24(7):912–929. [DOI] [PubMed] [Google Scholar]
- 15.Hinkin CH, Castellon SA, Hardy DJ. Dual task performance in HIV-1 infection. J Clin Exp Neuropsychol. 2000;22(1):16–24. [DOI] [PubMed] [Google Scholar]
- 16.Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73(16):1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baddeley A, Hitch G. Working memory. In: Bower A, ed. The psychology of learning and motivation. Academic Press; 1974; 47–89. [Google Scholar]
- 18.Baddeley A. Human memory; theory and practice. Psychology Press, 1997. [Google Scholar]
- 19.Cohen RA. The neuropsychology of attention. 2nd ed.Springer, 2014. [Google Scholar]
- 20.Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev. 2009;19(2):186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin LH, Neigh GN, Sundermann EE, Xu Y, Scully EP, Maki PM. Sex differences in neurocognitive function in adults with HIV: patterns, predictors, and mechanisms. Curr Psychiatry Rep. 2019;21(10):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodkin K, Miller EN, Cox C, et al. Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV. 2017;4(9):e411-e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A, Vance DE, Hoover DR, et al. Impaired cognition predicts falls among women with and without HIV infection. JAIDS. 2020;83(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlandson KM, Perez J, Abdo M, et al. Frailty, neurocognitive impairment, or both in predicting poor health outcomes among adults living with human immunodeficiency virus. Clin Infect Dis. 2019;68(1): 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant PM, Kitch D, McComsey GA, et al. Long-term bone mineral density changes in antiretroviral-treated HIV-infected individuals. J Infect Dis. 2016;214(4):607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkins KL, Brown TT, Margolick JB, Erlandson KM. Geriatric syndromes: new frontiers in HIV and sarcopenia. AIDS. 2017;31(Suppl 2):S137–S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H, Surkan PJ, Irwin MR, et al. Inflammation and risk of depression in HIV: prospective findings from the Multicenter AIDS Cohort Study. Am J Epidemiol. 2019; 188(11):1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins LF, Sheth AN, Mehta CC, et al. The prevalence and burden of non-AIDS comorbidities among women living with or at-risk for HIV Infection in the United States. Clin Infect Dis. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basso MR, Bornstein RA. Effects of immunosuppression and disease severity upon neuropsychological function in HIV infection. J Clin Exp Neuropsychol. 2000; 22(1):104–114. [DOI] [PubMed] [Google Scholar]
- 30.Nichols SL, Bethel J, Kapogiannis BG, et al. Antiretroviral treatment initiation does not differentially alter neurocognitive functioning over time in youth with behaviorally acquired HIV. J Neurovirol. 2016;22(2): 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute for Health Research. PROSPERO. 2021. [Google Scholar]
- 33.World Health Organization. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41(10):1403–1409. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Initial steps to developing the World Health Organization's Quality of Life Instrument (WHO-QOL) module for international assessment in HIV/AIDS. AIDS Care. 2003;15(3): 347–357. [DOI] [PubMed] [Google Scholar]
- 35.Ripamonti E, Azoulay L, Abrahamowicz M, Platt RW, Suissa S. A systematic review of observational studies of the association between pioglitazone use and bladder cancer. Diabet Med. 2019;36(1):22–35. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong NM, Surkan PJ, Treisman GJ, et al. Association of long-term patterns of depressive symptoms and attention/ex-ecutive function among older men with and without human immunodeficiency virus. J Neurovirol. 2017;23(4):558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catalan J, Tuffrey V, Ridge D, Rosenfeld D. What influences quality of life in older people living with HIV? AIDS Res Ther. 2017;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson CW, Sundermann EE, Hussain MA, et al. Effects of trauma, economic hardship, and stress on neurocognition and everyday function in HIV. Health Psychol. 2019;38(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johs NA, Wu K, Tassiopoulos K, et al. Disability among middle-aged and older persons with human immunodeficiency virus infection. Clin Infect Dis. 2017;65(1): 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fazeli PL, Woods SP, Heaton RK, et al. An active lifestyle is associated with better neurocognitive functioning in adults living with HIV infection. J Neurovirol. 2014; 20(3):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapiro ME, Mahoney JR, Peyser D, Zingman BS, Verghese J. Cognitive reserve protects against apathy in individuals with human immunodeficiency virus. Arch Clin Neuropsychol. 2014;29(1):110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shapiro ME, Mahoney JR, Zingman BS, Pogge DL, Verghese J. Apathy correlates with cognitive performance, functional disability, and HIV RNA plasma levels in HIV-positive individuals. J Clin Exp Neuropsychol. 2013;35(9):934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castellon SA, Hinkin CH, Myers HF. Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. J Int Neuropsychol Soc. 2000;6(3):336–347. [DOI] [PubMed] [Google Scholar]
- 44.Kamat R, Morgan E, Marcotte TD, et al. Implications of apathy and depression for everyday functioning in HIV/AIDS in Brazil. J Affect Disord. 2013;150(3):1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ripamonti E, Clerici M. The association of memory disorders and chronic HIV disease in the antiretroviral therapy era: a systematic literature review. HIV Med. 2020;21(1):9–20. [DOI] [PubMed] [Google Scholar]
- 46.Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14): 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cattie JE, Doyle K, Weber E, Grant I, Woods SP. Planning deficits in HIV-associated neurocognitive disorders: component processes, cognitive correlates, and implications for everyday functioning. J Clin Exp Neuropsychol. 2012;34(9):906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marquine MJ, Flores I, Kamat R, et al. A composite of multisystem injury and neurocognitive impairment in HIV infection: association with everyday functioning. J Neurovirol. 2018;24(5):549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamat R, Woods SP, Marcotte TD, Ellis RJ, Grant I. Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Arch Clin Neuropsychol. 2012;27(5):520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez-Banfi M, Velez JI, Perea MV, et al. Neuropsychological performance in patients with asymptomatic HIV-1 infection. AIDS Care. 2018;30(5):623–633. [DOI] [PubMed] [Google Scholar]
- 51.Lawler K, Jeremiah K, Mosepele M, et al. Neurobehavioral effects in HIV-positive individuals receiving highly active antiretroviral therapy (HAART) in Gaborone, Botswana. PLoS One. 2011;6(2):e17233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. [DOI] [PubMed] [Google Scholar]
- 53.Sadek JR, Vigil O, Grant I, Heaton RK. The impact of neuropsychological functioning and depressed mood on functional complaints in HIV-1 infection and methamphetamine dependence. J Clin Exp Neuropsychol. 2007;29(3):266–276. [DOI] [PubMed] [Google Scholar]
- 54.Rueda S, Raboud J, Rourke SB, et al. Influence of employment and job security on physical and mental health in adults living with HIV: cross-sectional analysis. Open Med. 2012;6(4):e118-e126. [PMC free article] [PubMed] [Google Scholar]
- 55.Wagener MN, van den Dries L, Van EJ, Miedema HS, van Gorp ECM, Roelofs PDDM. Determinants of employment in people living with HIV in the Netherlands. J Occup Rehabil. 2018;28(1):45–56. [DOI] [PubMed] [Google Scholar]
- 56.Kordovski VM, Woods SP, Verduzco M, Beltran J. The effects of aging and HIV disease on employment status and functioning. Rehabil Psychol. 2017;62(4):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atkins JH, Rubenstein SL, Sota TL, et al. Impact of social support on cognitive symptom burden in HIV/AIDS. AIDS Care. 2010;22(7):793–802. [DOI] [PubMed] [Google Scholar]
- 58.Chernoff RA, Martin DJ, Schrock DA, Huy MP. Neuropsychological functioning as a predictor of employment activity in a longitudinal study of HIV-infected adults contemplating workforce reentry. J Int Neuropsychol Soc. 2010;16(1):38–48. [DOI] [PubMed] [Google Scholar]
- 59.van Gorp WG, Rabkin JG, Ferrando SJ, et al. Neuropsychiatric predictors of return to work in HIV/AIDS. J Int Neuropsychol Soc. 2007;13(1):80–89. [DOI] [PubMed] [Google Scholar]
- 60.Rabkin JG, McElhiney M, Ferrando SJ, Van GW, Lin SH. Predictors of employment of men with HIV/AIDS: a longitudinal study. Psychosom Med. 2004;66(1):72–78. [DOI] [PubMed] [Google Scholar]
- 61.Gomez D, Power C, Gill MJ, Fujiwara E. Determinants of risk-taking in HIV-associated neurocognitive disorders. Neuropsychology. 2017;31(7):798–810. [DOI] [PubMed] [Google Scholar]
- 62.Fujiwara E, Tomlinson SE, Purdon SE, Gill MJ, Power C. Decision making under explicit risk is impaired in individuals with human immunodeficiency virus (HIV). J Clin Exp Neuropsychol. 2015;37(7):733–750. [DOI] [PubMed] [Google Scholar]
- 63.Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20(3): 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thames AD, Streiff V, Patel SM, Panos SE, Castellon SA, Hinkin CH. The role of HIV infection, cognition, and depression in risky decision-making. J Neuropsychiatry Clin Neurosci. 2012;24(3):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin EM, DeHaan S, Vassileva J, Gonzalez R, Weller J, Bechara A. Decision making among HIV+ drug using men who have sex with men: a preliminary report from the Chicago Multicenter AIDS Cohort Study. J Clin Exp Neuropsychol. 2013;35(6):573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Childers ME, Woods SP, Letendre S, et al. Cognitive functioning during highly active antiretroviral therapy interruption in human immunodeficiency virus type 1 infection. J Neurovirol. 2008;14(6):550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ettenhofer ML, Foley J, Castellon SA, Hinkin CH. Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology. 2010;74(15):1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fong OW, Ho CF, Fung LY, et al. Determinants of adherence to highly active antiretroviral therapy (HAART) in Chinese HIV/AIDS patients. HIV Med. 2003;4(2): 133–138. [DOI] [PubMed] [Google Scholar]
- 69.Gandhi NS, Skolasky RL, Peters KB, et al. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. J Neurovirol. 2011; 17(2):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thames AD, Moizel J, Panos SE, et al. Differential predictors of medication adherence in HIV: findings from a sample of African American and Caucasian HIV-positive drug-using adults. AIDS Patient Care STDs. 2012;26(10):621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biswas B, Spitznagel E, Collier AC, et al. Characterizing HIV medication adherence for virologic success among individuals living with HIV/AIDS: Experience with the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) cohort. J HIV AIDS Soc Serv. 2014;13(1):8–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harrison JD, Dochney JA, Blazekovic S, et al. The nature and consequences of cognitive deficits among tobacco smokers with HIV: a comparison to tobacco smokers without HIV. J Neurovirol. 2017;23(4): 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thein H, Maruff P, Krahn M, et al. Cognitive function, mood and health-related quality of life in hepatitis C virus (HCV)-monoinfected and HIV/HCV-coinfected individuals commencing HCV treatment. HIV Med. 2007;8(3):192–202. [DOI] [PubMed] [Google Scholar]
- 74.Osowiecki DM, Cohen RA, Morrow KM, et al. Neurocognitive and psychological contributions to quality of life in HIV-1–infected women. AIDS. 2000;14(10):1327–1332. [DOI] [PubMed] [Google Scholar]
- 75.Kamat R, Woods SP, Cameron MV, Iudicello JE. Apathy is associated with lower mental and physical quality of life in persons infected with HIV. Psychol Health Med. 2016;21(7):890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cook R, Jones DL, Nehra R, et al. HIV clade-C infection and cognitive impairment, fatigue, depression, and quality of life in early-stage infection in Northern Indians. J Int Assoc Provid AIDS Care. 2016;15(4):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones JD, Kuhn T, Levine A, et al. Changes in cognition precede changes in HRQoL among HIV+ males: longitudinal analysis of the multicenter AIDS cohort study. Neuropsychology. 2019;33(3):370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janssen MA, Bertens D, Kessels L, Kessels RP, Koopmans PP. A case-control pilot study on cognitive functioning, symptom validity and psychological wellbeing in HIV-1–infected patients in the Netherlands. Int J STD AIDS. 2013;24(5):387–391. [DOI] [PubMed] [Google Scholar]
- 79.Janssen MAM, Koopmans PP, Kessels RPC. Cognitive decline in relation to psychological wellbeing and HIV disease- and treatment characteristics in HIV-infected patients on cART: a one-year follow-up study. AIDS Behav. 2017;21(6):1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janssen MA, Meulenbroek O, Steens SC, et al. Cognitive functioning, wellbeing and brain correlates in HIV-1 infected patients on long-term combination antiretroviral therapy. AIDS. 2015;29(16): 2139–2148. [DOI] [PubMed] [Google Scholar]
- 81.Chartier M, Crouch PC, Tullis V, et al. The Montreal cognitive assessment: a pilot study of a brief screening tool for mild and moderate cognitive impairment in HIV-positive veterans. J Int Assoc Provid AIDS Care. 2015;14(3):197–201. [DOI] [PubMed] [Google Scholar]
- 82.Lorkiewicz SA, Ventura AS, Heeren TC, et al. Lifetime marijuana and alcohol use, and cognitive dysfunction in people with human immunodeficiency virus infection. Subst Abus. 2018;39(1):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakasujja N, Skolasky RL, Musisi S, et al. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BMC Psychiatry. 2010;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farinpour R, Miller EN, Satz P, et al. Psychosocial risk factors of HIV morbidity and mortality: findings from the Multicenter AIDS Cohort Study (MACS). J Clin Exp Neuropsychol. 2003;25(5):654–670. [DOI] [PubMed] [Google Scholar]
- 85.Robertson K, Bayon C, Molina JM, et al. Screening for neurocognitive impairment, depression, and anxiety in HIV-infected patients in Western Europe and Canada. AIDS Care. 2014;26(12):1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clifford DB, Evans S, Yang Y, Acosta EP, Ribaudo H, Gulick RM. Long-term impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals (ACTG 5097s). HIV Clin Trials. 2009;10(6):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heaton RK, Franklin DR Jr., Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60(3):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heaton RK, Clifford DB, Franklin DRJ, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banerjee N, McIntosh RC, Ironson G. Impaired neurocognitive performance and mortality in HIV: assessing the prognostic value of the HIV-dementia scale. AIDS Behav. 2019;23(12):3482–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang L, Speck O, Miller EN, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57(6):1001–1007. [DOI] [PubMed] [Google Scholar]
- 92.Legarth R, Omland LH, Kronborg G et al. Employment status in persons with and without HIV infection in Denmark: 1996–2011. AIDS June 19, 2014;1489–1498. [DOI] [PubMed] [Google Scholar]
- 93.Scott JC, Woods SP, Vigil O, et al. A neuropsychological investigation of multitasking in HIV infection: implications for everyday functioning. Neuropsychology. 2011;25(4):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verbooy K, Wagener M, Kaddouri M, et al. Are people living with HIV less productive at work? AIDS Care. 2018;30(10): 1265–1272. [DOI] [PubMed] [Google Scholar]
- 95.Annequin M, Lert F, Spire B, Dray-Spira R. Has the employment status of people living with HIV changed since the early 2000s? AIDS. 2015;29(12):1537–1547. [DOI] [PubMed] [Google Scholar]
- 96.Ibrahim F, Anderson J, Bukutu C, Elford J. Social and economic hardship among people living with HIV in London. HIV Med. 2008;9(8):616–624. [DOI] [PubMed] [Google Scholar]
- 97.Ware D, Rueda S, Plankey M, et al. The longitudinal impact of employment, retirement and disability status on depressive symptoms among men living with HIV in the Multicenter AIDS Cohort Study. PLoS One. 2020;15(10):e0239291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gross M, Herr A, Hower M, Kuhlmann A, Mahlich J, Stoll M. Unemployment, health, and education of HIV-infected males in Germany. Int J Public Health. 2016;61(5):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodger AJ, Brecker N, Bhagani S, et al. Attitudes and barriers to employment in HIV-positive patients. Occup Med (Lond). 2010;60(6):423–429. [DOI] [PubMed] [Google Scholar]
- 100.Dray-Spira R, Legeai C, Le DM, et al. Burden of HIV disease and comorbidities on the chances of maintaining employment in the era of sustained combined antiretoviral [sic] therapies use. AIDS. 2012; 26(2):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wada N, Jacobson LP, Cohen M, French A, Phair J, Munoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013; 177(2):116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woods SP, Weber E, Weisz BM, Twamley EW, Grant I. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabil Psychol. 2011;56(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dray-Spira R, Gueguen A, Ravaud JF, Lert F. Socioeconomic differences in the impact of HIV infection on workforce participation in France in the era of highly active antiretroviral therapy. Am J Public Health. 2007;97(3):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adeoti AO, Dada M, Elebiyo T, Fadare J, Ojo O. Survey of antiretroviral therapy adherence and predictors of poor adherence among HIV patients in a tertiary institution in Nigeria. Pan Afr Med J. 2019; 33:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. JAIDS. 2008;47(3):384–390. [DOI] [PubMed] [Google Scholar]
- 106.Pokhrel KN, Pokhrel KG, Sharma VD, et al. Mental health disorders and substance use among people living with HIV in Nepal: their influence on non-adherence to anti-retroviral therapy. AIDS Care. 2019; 31(8):923–931. [DOI] [PubMed] [Google Scholar]
- 107.Woods SP, Iudicello JE, Morgan EE, et al. Health-related everyday functioning in the internet age: HIV-associated neurocognitive disorders disrupt online pharmacy and health chart navigation skills. Arch Clin Neuropsychol. 2016;31(2):176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Costa JO, Pearson SA, Acurcio FA, Bonolo PF, Silveira MR, Ceccato MDGB. Health-related quality of life among HIV-infected patients initiating treatment in Brazil in the single-tablet regimen era. AIDS Care. 2019;31(5):572–581. [DOI] [PubMed] [Google Scholar]
- 109.Xie F, Zheng H, Huang L, Yuan Z, Lu Y. Social capital associated with quality of life among people living with HIV/AIDS in Nanchang, China. Int J Environ Res Public Health. 2019;16(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pillay P, Wadley AL, Cherry CL, Karstaedt AS, Kamerman PR. Psychological factors associated with painful versus non-painful HIV-associated sensory neuropathy. AIDS Behav. 2018;22(5):1584–1595. [DOI] [PubMed] [Google Scholar]
- 111.McGowan JA, Sherr L, Rodger AJ, et al. Age, time living with diagnosed HIV infection, and self-rated health. HIV Med. 2017;18(2):89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schroecksnadel K, Sarcletti M, Winkler C, et al. Quality of life and immune activation in patients with HIV-infection. Brain Behav Immun. 2008;22(6):881–889. [DOI] [PubMed] [Google Scholar]
- 113.Abadiga M. Depression and its associated factors among HIV/AIDS patients attending ART clinics at Gimbi General hospital, West Ethiopia, 2018. BMC Res Notes. 2019;12(1):527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Familiar I, Sikorskii A, Murray S, et al. Depression symptom trajectories among mothers living with HIV in rural Uganda. AIDS Behav. 2019;23(12):3411–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Todd JV, Cole SR, Pence BW, et al. Effects of antiretroviral therapy and depressive symptoms on all-cause mortality among HIV-infected women. Am J Epidemiol. 2017;185(10):869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grant I, Olshen RA, Atkinson JH, et al. Depressed mood does not explain neuropsychological deficits in HIV-infected persons. Neuropsychology. 1993;7(1):53. [Google Scholar]
- 117.Cysique LA, Deutsch R, Atkinson JH, et al. Incident major depression does not affect neuropsychological functioning in HIV-infected men. J Int Neuropsychol Soc. 2007;13(1):1–11. [DOI] [PubMed] [Google Scholar]
- 118.Rourke SB, Halman MH, Bassel C. Neuropsychiatric correlates of memory-metamemory dissociations in HIV-infection. J Clin Exp Neuropsychol. 1999;21(6): 757–768. [DOI] [PubMed] [Google Scholar]
- 119.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16(7):916–928. [DOI] [PubMed] [Google Scholar]
- 120.Hestad K, Aukrust P, Ellertsen B, Klove H. Neuropsychological deficits in HIV-1 seropositive and seronegative intravenous drug users (IVDUs): a follow-up study. J Int Neuropsychol Soc. 1996;2(2):126–133. [DOI] [PubMed] [Google Scholar]
- 121.Rubin LH, Cook JA, Springer G, et al. Perceived and post-traumatic stress are associated with decreased learning, memory, and fluency in HIV-infected women. AIDS. 2017;31(17):2393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dale SK, Weber KM, Cohen MH, Kelso GA, Cruise RC, Brody LR. Resilience moderates the association between childhood sexual abuse and depressive symptoms among women with and at-risk for HIV. AIDS Behav. 2015;19(8):1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schifitto G, Deng L, Yeh TM, et al. Clinical, laboratory, and neuroimaging characteristics of fatigue in HIV-infected individuals. J Neurovirol. 2011;17(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aouizerat BE, Gay CL, Lerdal A, Portillo CJ, Lee KA. Lack of energy: an important and distinct component of HIV-related fatigue and daytime function. J Pain Symptom Manage. 2013;45(2):191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tavakkoli M, Ferrando SJ, Rabkin J, Marks K, Talal AH. Depression and fatigue in chronic hepatitis C patients with and without HIV co-infection. Psychosomatics. 2013;54(5):466–471. [DOI] [PubMed] [Google Scholar]
- 126.Barroso J, Harmon JL, Madison JL, Pence BW. Intensity, chronicity, circumstances, and consequences of HIV-related fatigue: a longitudinal study. Clin Nurs Res. 2014; 23(5):514–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mukerji S, Haghighat R, Misra V, et al. Longitudinal modeling of depressive trajectories among HIV-infected men using cocaine. AIDS Behav. 2017;21(7): 1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Okafor CN, Cook RL, Chen X, et al. Prevalence and correlates of marijuana use among HIV-seropositive and seronegative men in the Multicenter AIDS Cohort Study (MACS), 1984–2013. Am J Drug Alcohol Abuse. 2017;43(5):556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Woods SP, Morgan EE, Marquie-Beck J, Carey CL, Grant I, Letendre SL. Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cogn Behav Neurol. 2006; 19(4):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Low Y, Preud'homme X, Goforth HW, Omonuwa T, Krystal AD. The association of fatigue with depression and insomnia in HIV-seropositive patients: a pilot study. Sleep. 2011;34(12):1723–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsao JC, Stein JA, Ostrow D, Stall RD, Plankey MW. The mediating role of pain in substance use and depressive symptoms among Multicenter AIDS Cohort Study (MACS) participants. Pain. 2011;152(12):2757–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leserman J, Petitto JM, Golden RN, et al. Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. Am J Psychiatry. 2000;157(8):1221–1228. [DOI] [PubMed] [Google Scholar]
- 133.Ware D, Palella FJ, Chew KW, et al. Examination of polypharmacy trajectories among HIV-positive and HIV-negative men in an ongoing longitudinal cohort from 2004 to 2016. AIDS Patient Care STDs. 2019;33(8):354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.D'souza G, Springer G, Gustafson D, et al. COVID-19 symptoms and SARS-CoV-2 infection among people living with HIV in the US: the MACS/WIHS combined cohort study. HIV Res Clin Pract. 2020;21(5):130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]