Abstract
Background:
Beyond yielding high blood ethanol concentrations (BECs), binge drinking models allow examination of drinking patterns which may be associated with ethanol’s (EtOH) rewarding effects, including front-loading and consummatory successive negative contrast (cSNC), a decrease in intake when only water is available to subjects expecting EtOH. The goals of the current study were to broaden our understanding of these reward-related behaviors during binge EtOH access in high-alcohol-preferring (HAP) replicate lines (HAP2 and 3) of mice selectively bred to prefer alcohol. We hypothesized that both lines would show evidence of front-loading during binge EtOH access and that we would find a cSNC effect in groups where EtOH was replaced with water, as these results have been shown previously in HAP1 mice.
Methods:
HAP replicate 2 and 3 female and male mice were given two hours of EtOH or water access in the home cage for 15 consecutive days using ‘Drinking in the Dark’ (DID) procedures. Mice received the same fluid (either 20% unsweetened EtOH or water) for the first 14 days. However, on the 15th day, half of the mice from these two groups were provided with the opposite assigned fluid (EtOH groups received water and vice versa). Intake was measured in one-minute bins using specialized sipper tubes, which allowed within-session analyses of binge drinking patterns.
Results:
EtOH front-loading was observed in both replicates. HAP3 mice displayed front-loading on the first day of EtOH access, whereas front-loading developed following alcohol experience in HAP2 mice, which may suggest differences in initial sensitivity to EtOH reward. Consummatory SNC, which manifests as lower water intake in mice expecting EtOH as compared to mice expecting water, was observed in both replicates.
Conclusions:
These findings increase confidence that defined changes in home-cage consummatory behavior are driven by the incentive value of EtOH. The presence of cSNC across HAP replicates indicates that this reaction to loss of reward is genetically mediated, which suggests that there is a biological mechanism that might be targeted.
Keywords: binge drinking, front-loading, consummatory successive negative contrast (cSNC), high-alcohol-preferring (HAP) mice, rate of alcohol/ethanol intake
Introduction
There is growing interest in how increases in the rate of alcohol (ethanol; EtOH) consumption may be a predictor of developing alcohol use disorder (AUD). Recent examples supporting this relationship include binge drinking rate predicting AUD symptoms in adolescents (Carpenter et al., 2019), as well as heavy drinkers and individuals considered at risk for AUD self-administering IV EtOH at a quicker rate than low risk ‘social drinkers’ (Sloan et al., 2019). Preclinical animal models of excessive EtOH consumption typically display ‘front-loading’ behavior, wherein the amount or proportion of EtOH consumed is highly skewed toward the onset of EtOH access. This phenomenon is often observed following many drinking experiences, and has therefore been suggested to reflect a progressive increase in the motivation to experience EtOH’s subjective rewarding effects (Darevsky et al., 2018, Linsenbardt and Boehm, 2014, Linsenbardt and Boehm, 2015, Rhodes et al., 2007, Salling et al., 2018, Wilcox et al., 2014).
Consummatory successive negative contrast (cSNC) is a phenomenon mainly assessed in preclinical models that may provide additional evidence supporting a relationship between front-loading and the positive motivational effects of consumed substances. Consummatory SNC effects are classically observed by measuring consumption after ‘downshifting’ a group of animals from a high value reward to a lower value reward. Typically, this is done by shifting animals unexpectedly from a high sucrose concentration reward to a lower concentration of sucrose. Animals experiencing this downshift drink less of the solution than animals expecting the lower value reward (Flaherty, 1996), and this suppression is evidence that the higher-value solution is more rewarding than the lower-value solution. The cognitive state induced by this downshift has classically been described as frustration (Amsel, 1962, Amsel, 1992). The relationship between EtOH and cSNC has primarily been studied within the context of assessing the ability of EtOH to relieve the frustrative effects associated with a sucrose downshift (Becker and Flaherty, 1982, Manzo et al., 2014, Manzo et al., 2015, Matson and Grahame, 2015). Interestingly, cSNC is greater in mice genetically predisposed to excessive alcohol consumption than their corresponding Low Alcohol Preferring (LAP) lines when using a shift from 32% sucrose to 4% sucrose (Matson and Grahame, 2015). Both front-loading and cSNC has also been observed in HAP1 mice following an unexpected switch from an EtOH solution to a water solution (Linsenbardt and Boehm, 2015). This reaction to an unexpected loss of reward has recently become of great interest as a potential behavioral endophenotype for AUD (Ortega et al., 2017), and may be evidence of an important but understudied cognitive construct amenable to intervention.
It is possible that these two phenotypic displays of motivation to experience reward (front-loading and cSNC) may be influenced by genetic factors. There is converging evidence from both rodent (Barkley-Levenson and Crabbe, 2014, Barkley-Levenson and Crabbe, 2015, Iancu et al., 2013, Linsenbardt and Boehm, 2015) and human literature (Bauer and Ceballos, 2014, Chassin et al., 2002, Gowin et al., 2017, Watson et al., 2013) that genetic background plays a role in an individual’s predisposition to engage in binge drinking behavior; and that binge drinking may be predictive of the development of an AUD (Addolorato et al., 2018, Kim et al., 2016). HAP mice are a rodent model of genetic risk of excessive EtOH consumption and are genetically predisposed to consuming EtOH excessively (Matson and Grahame, 2013). Phenotyping HAP replicates for binge intake patterns offers a unique tool to expand upon the current literature and gain a deeper understanding of how rate of EtOH consumption (presence of front-loading) and degree of cSNC may contribute to the development of problematic drinking patterns. If these effects are observed across replicate HAP lines, it would provide additional support that these behaviors are consistently related to genes increasing 24-hour, two-bottle choice intake, which is the selection phenotype. In turn, these phenotypic displays of motivation to experience the rewarding effects of EtOH would suggest a common biological mechanism underlying high DID drinking, front-loading, cSNC, and 24-hour, two-bottle choice drinking.
In the current study, HAP2 and HAP3 mice were subjected to almost identical procedures as previous studies using C57BL/6J (B6) (Linsenbardt and Boehm, 2014) and HAP1 (Linsenbardt and Boehm, 2015) mice in our laboratory. Our primary hypotheses were that both HAP replicates given daily access to EtOH would display front-loading consistently; additionally, that front-loading would develop only after alcohol drinking experience. We also hypothesized that cSNC would be observed when mice expecting EtOH were given water. Relatedly, we hypothesized that mice given EtOH access would reach binge levels of consumption each day. Secondarily, we hypothesized that when all groups of mice were injected with EtOH following repeated DID access, the mice with an EtOH-drinking history would display metabolic tolerance, but not locomotor sensitization. Evidence of metabolic tolerance, but not sensitization, has been found in HAP1 mice following a two-week history of DID drinking (Linsenbardt and Boehm, 2015). Additionally, metabolic tolerance has been shown in HAP1 mice consuming EtOH following chronic, two-bottle choice access (Matson et al., 2013). Assessing the development of metabolic tolerance and sensitization between fluid history groups allowed us to determine if the presence of front-loading and cSNC is related to changes in EtOH sensitivity. For a table of all hypotheses and whether or not they were supported, please see Table 1.
Table 1:
Hypotheses for the experiment and whether the hypothesis was supported, where a check mark indicates supported and an ⨯ mark indicates not supported.
| HAP2 | HAP3 | |
|---|---|---|
| The EtOH group will consume binge levels of intake each day (Fig 1) | ✓ | ✓ |
| EtOH front-loading will develop after alcohol drinking experience (Fig 2) | ✓ | ⨯ |
| The EtOH group will front-load most days (Fig 3) | ✓ | ✓ |
| cSNC will manifest in mice expecting EtOH and receiving water instead (Fig 5, 6) | ✓ | ✓ |
| Mice with an EtOH-drinking history will display metabolic tolerance (day 16 results) | ⨯ | ⨯ |
| Mice with an EtOH drinking history will not display locomotor sensitization (day 16 results) | ✓ | ✓ |
Methods
Subjects
Data were gathered from HAP2 female (n=32) and male (n=32) mice from the 63rd and 64th generation of selection ranging in age from 47–63 days at the beginning of the experiment, and HAP3 female (n=14) and male (n=47) mice from the 37th and 39th selection generation ranging in age from 95–110 days. See Oberlin and colleagues (2011) for details on the creation and response to selection of all replicate lines of HAP mice. Of note, both lines were selected for the same phenotype, 24-hour, two-bottle choice for 10% EtOH and water, from the same founder population, HS/Ibg. All mice were bred in the School of Science vivarium at Indiana University-Purdue University Indianapolis (IUPUI) and were single-housed in standard shoebox cages in a room with a 12-hour reverse light-dark cycle one week prior to the beginning of testing. All mice always had ad libitum access to standard laboratory rodent chow (LabDiet 5001), including during drinking-in-the-dark (DID) and injection experiments. Mice also had ad libitum access to water via standard home cage water bottles, except during two-hour DID sessions wherein normal water bottles were replaced with specialized sipper tubes containing either water or EtOH (see EtOH Solutions below). Standard home cage water bottles did not utilize the specialized sippers, but did contain sippers with identical sized drinking orifices. All procedures were approved by the IUPUI School of Science Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
EtOH Solutions
EtOH for drinking and injection experiments was prepared by diluting 190 proof EtOH from Pharmco, Inc. (Brookfield, CT) to 20% v/v in tap water or 0.9% sterile physiological saline, respectively. Drinking solution was prepared at the beginning of the experiment and stored in sealed fluid reservoirs connected to the volumetric drinking monitor (VDM) system (Columbus Instruments Inc., Columbus, OH), equipped with specialized sipper tubes that monitor fluid volume consumed with high temporal resolution. Fluids were topped-off halfway through the 15-day drinking testing period. Injectable solution was made immediately prior to use.
Drinking-in-the-Dark
DID procedures have been previously described (Linsenbardt and Boehm, 2014, Linsenbardt and Boehm, 2015). Briefly, 3 hours into the dark cycle, each mouse’s home cage water bottle was replaced with a volumetric sipper tube (Columbus Instruments Inc.) containing either tap water or 20% v/v EtOH. Mice were given two hours of access to their assigned fluid. All mice received either 20% EtOH or water for the first 14 days. However, on the 15th day, half of the mice from these two groups were provided with the opposite assigned fluid (EtOH groups received water (EW) and water groups received EtOH (WE)). The other half of the mice continued to receive their assigned fluid of EtOH (EE) or water (WW). See Table 2 for a breakdown of replicate line and sex for each fluid group. The volume of consumed fluid was measured in one-minute bins, allowing for within-session analyses of binge-drinking patterns.
Table 2:
DID: drinking-in-the-dark; HCL: home cage locomotion; EtOH: ethanol. Fluid intake data from 2 female HAP2 (WW) and 1 female HAP2 (EE) mice were removed from analyses on day 15 due to malfunctioning sipper tubes. Two HAP2 mice were removed from all day 16 analyses due to incomplete/unsuccessful injections. Two HAP2 and one HAP3 (all EW) mice were removed from all day 16 analyses due to faulty tracking. Fluctuation in numbers on days 1–14 (i.e. HAP2 male water; n = 14–16) was due to malfunction in individual sipper tubes on specific days which was resolved. Only days with malfunction were excluded. The remaining incoherence between HAP3 males from day 15 to day 16 is because tissue from the first cohort of this replicate of mice was used for a different experiment, thus this group of animals was unable to receive day 16 injections.
| Days 1–14 (2-hour DID + HCL) | Day 15 (2-hour DID + HCL) | Day 16 (Injection + HCL) | |
|---|---|---|---|
|
| |||
| HAP2 Female (n = 32) | EtOH (n = 16) | EtOH (EE; n = 7) | n = 8 |
| Water (EW; n = 8) | n = 7 | ||
| Water (n = 15–16) | Water (WW; n = 6) | n = 7 | |
| EtOH (WE; n = 8) | n = 8 | ||
| HAP2 Male (n = 32) | EtOH (n = 15–16) | EtOH (EE; n = 8) | n = 8 |
| Water (EW; n = 8) | n = 6 | ||
| Water (n = 14–16) | Water (WW; n = 8) | n = 8 | |
| EtOH (WE; n = 8) | n = 8 | ||
|
| |||
| HAP3 Female (n = 14) | EtOH (n = 6) | EtOH (EE; n = 3) | n = 3 |
| Water (EW; n = 3) | n = 2 | ||
| Water (n = 8) | Water (WW; n = 3) | n = 3 | |
| EtOH (WE; n = 5) | n = 4 | ||
| HAP3 Male (n = 47) | EtOH (n = 24) | EtOH (EE; n = 12) | n = 4 |
| Water (EW; n = 12) | n = 4 | ||
| Water (n = 23) | Water (WW; n = 12) | n = 4 | |
| EtOH (WE; n = 11) | n = 3 | ||
Injections
On day 16, instead of receiving sippers, all mice were given a 2 g/kg intraperitoneal (i.p.) injection of 20% v/v EtOH. Injections were given three hours into the dark cycle (the same time that DID had previously started for these mice on days 1–15). After injection, mice were placed back into their home cages and home cage locomotor activity was monitored for two hours.
Home Cage Locomotion
Home cage locomotor activity was monitored using AnyMaze software (Stoelting Co., Wood Dale, IL) and Logitech C920 cameras. Distance travelled was recorded for each mouse in centimeters for the duration of the two-hour DID session.
Blood EtOH Concentrations (BECs)
On test days 15 (the last day of DID) and 16 (injection), 50 μl of peri-orbital sinus blood were drawn from all mice immediately following the end of the two-hour session. Samples were centrifuged and plasma was withdrawn and stored at −20°C. BECs were determined using an Analox EtOH Analyzer (Analox Instruments, Lunenburg, MA).
Statistics: Mean Daily Intake, Front-loading and Home Cage Locomotor Activity
Mean total (two-hour) fluid intake, percentage of intake within the first-15 minutes, and home cage locomotor activity of the EtOH and water-consuming groups on days 1 to 14 were first analyzed using 2 (sex) × 2 (fluid group: EtOH or water) × 14 (day) mixed-methods 3-way analysis of variance (ANOVA)s, calculated separately for each replicate. To determine whether there were differences in mean total intake on days 1 to 14 between day 15 assignment groups, fluid history subdivisions were analyzed separately such that the WW and WE groups were compared and the EE and EW groups were compared using ANOVAs with intake on days 1 to 14 as the within subjects factor and the day 15 group assignment and sex as between subjects factors. D’Agostino-Pearson normality tests were calculated and determined that within the HAP2 replicate, both EtOH and water groups had non-normal distributions of intake. Further evaluation of Q-Q plots indicated that this was largely driven by differences in variance in intake within the first few days, likely while mice were acclimating to the VDM sippers. To account for this, Greenhouse-Geisser corrections were applied to analyses where appropriate (see results). In addition to the analyses described above, analyses of front-loading behavior first compared the percentage of fluid intake within the first 15 minutes of the DID session using 2 (sex) × 2 (fluid group) × 2 (day: 1 vs 14) 3-way ANOVAs calculated separately for each replicate. We note that 15 minute increments account for 12.5% of the total two-hour DID session, with mice needing to consume higher than 12.5% of their assigned fluid within the first 15 minutes to have been considered as having front-loaded on a given day. In addition to the comparison of first 15-minute intake between EtOH and water fluid groups, one-sample t-tests compared percentage of intake within the first 15-minutes of each fluid group to this 12.5% threshold to determine if front-loading was statistically significant. In both replicates, we observed that the EtOH group (except for HAP2 on day 1) both passed this threshold and often consumed more of their total fluid in the first 15 minutes than the water group. Interactions with sex were not detected in any of our analyses. Therefore, results and figures reported are from subsequent analyses conducted as reported above but collapsed on this factor. Total (two-hour) intakes for female versus male mice are provided as a supplemental table for researchers who may be interested in these data.
Statistics: Day 15 (cSNC) and Day 16 (metabolic tolerance and locomotor sensitization)
Given our a priori hypothesis that the EW group would consume less than the WW group on day 15 based upon previous findings from our laboratory showing this effect in the HAP1 replicate (Linsenbardt and Boehm, 2015), a t-test comparing total water intake from these two groups was performed to test for cSNC. Similarly, we assessed whether consummatory positive contrast had occurred using a t-test to discover whether the WE group consumed more than EE group within each replicate. Additionally, central moving averages (see below) on this day are presented to consider differences in intake patterns between groups (see details below). For day 16, we tested if there was evidence for metabolic tolerance by considering differences in BECs in groups by first using 2 (sex) × 2 (fluid history: EtOH or water) ANOVAs calculated separately for each replicate. Again, no sex differences were identified, so results are presented as a t-test considering BEC differences between fluid history divisions. We acknowledge that group sizes for HAP3 females on days 15 and 16 may have left us underpowered to sufficiently detect sex differences. Thus, while no sex differences were observed in the current study, we cannot completely rule out this possibility for these specific outcomes. The same analysis was conducted using distance traveled as the dependent variable to test for evidence of sensitization. Sidak’s post-hoc tests were utilized for all described analyses where appropriate and detailed within the results section. Data were considered significant at p < 0.05.
Statistics: Central Moving Averages.
To better characterize the most prominent within session pattern alterations, a central moving average was calculated on 15-minute bin increments as described in Linsenbardt and Boehm (2015). Briefly, data were binned into 15-minute averages and “moved” forward in time in 1-minute increments such that each subsequent bin included 1 additional minute into the future and excluded 1 minute furthest in time. Fifteen-minute increments were chosen because observations of intake data suggest that substantial changes occurred over DID sessions within the first 15 minutes of EtOH access; i.e. ‘front-loading’, and to allow for direct comparisons to previously published data (Linsenbardt and Boehm, 2015, Linsenbardt and Boehm, 2014).
Results
Total Intake and Assessment of Front-loading:
Days 1–14 HAP2:
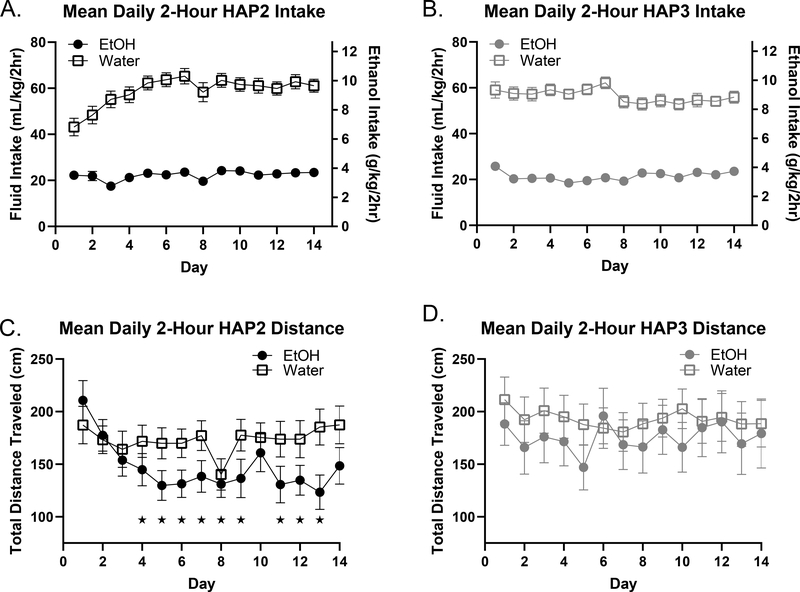
Analyses identified a main effect of day [F (6.055, 367.5) = 7.18, p < 0.0001], with consumption in both fluid groups shifting slightly on some days, and group [F (1, 62) = 207.1, p < 0.0001], where the water group drank more on all days than the EtOH group, and a day*group interaction, [F (13, 789) = 4.61, p < 0.0001)] (Fig 1A). Post-hoc analyses of within-replicate changes from the first day of access revealed that the HAP2 water group displayed significantly higher intake on days 3–14 (versus day 1). No differences from day 1 were noted in the EtOH group. Importantly, mean intakes of the EtOH group each day were always over 2.75 g/kg within the 2-hour period, which would likely result in blood alcohol levels over 80 mg/dl indicating binge intake (Allen et al., 1982).
Figure 1. Mean Daily Intake and Distance Travelled Over Two Weeks.
Intake: In both replicates, the water group consumed more total fluid each day during DID than the EtOH group, [post-hoc analysis following up on a main effect of group; p < 0.05]. We also note that EtOH intake in both replicates was reliable such that all animals readily consumed pharmacologically relevant (binge) levels of EtOH every day. Error bars are sometimes obscured by the plot symbols (A, B). Distance: In HAP2 mice, a day*group interaction is driven by initial greater locomotor activity in the EtOH group rapidly declining at a much faster rate than the water group whose activity stabilizes around day 3 (C). In HAP3, no significant effects were observed, indicating similar levels of activity between EtOH and water consuming animals in this replicate (D). Multiple comparison tests were conducted wherein distance travelled on each day within a given fluid group (EtOH or Water) was compared to day 1. Results indicate significant differences in the HAP2 replicate only, where days which differed from day 1 are indicated with *. These results indicate the potential of EtOH-induced locomotor sedation in HAP2 mice only.
Days 1–14 HAP3:
Similar to HAP2, there was a significant main effect of day [F (13, 754) = 2.53, p < 0.01], main effect of group [F (1, 58) = 224.7, p < 0.0001], with the water group drinking more on all days than the EtOH group, and a day*group interaction, [F (13, 754) = 3.24, p < 0.0001)] (Fig 1B). Like in HAP2, the EtOH group displayed binge-like drinking on each day. In both replicates, there were no significant differences in water or EtOH consumption over the first 14 days as a function of day 15 fluid assignment. In other words, EtOH (EE and EW) and water (WW and WE) groups had similar drinking histories prior to day 15 fluid access (data not shown; p > 0.05 for all main and interaction effects).
Mean Daily Distance Travelled Over Two Weeks:
In HAP2 mice, analyses considering distance travelled identified a main effect of day, [F (6.413, 389.2) = 3.62, p < 0.01], and a day*group interaction, [F (13, 789) = 1.20, p < 0.01], with locomotor activity in the EtOH group declining more quickly over days than the water group (Fig 1C). In HAP3, no significant effects were observed, indicating similar levels of activity between EtOH and water consuming animals in this replicate [p > 0.05] (Fig 1D). Multiple comparison tests were conducted wherein distance travelled on each day within a given fluid group (EtOH or Water) was compared to day 1. Results indicated significant differences in the HAP2 replicate only, where days which differed from day 1 are indicated with *. These results indicate EtOH-induced locomotor sedation in HAP2 mice only, notwithstanding similar EtOH intake between the lines.
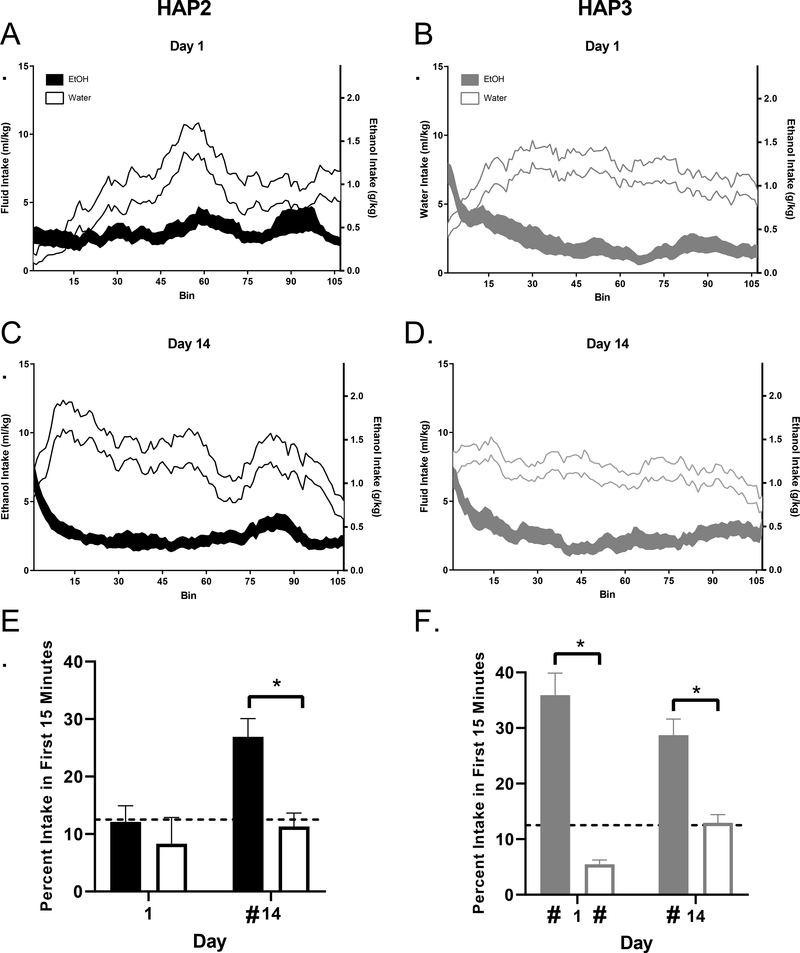
Days 1 and 14 Intake Patterns HAP2:
Intake patterns for days 1 (Fig 2A) and 14 (Fig 2C) identified one major time course difference between groups: EtOH-assigned mice displayed front-loading behavior on day 14, whereas intake patterns for the water drinking group indicated no differences in early water consumption on either day. Results considering differences in percentage of intake within the first 15 minutes between fluid groups indicate a main effect of day, [F (1, 120) = 7.270, p < 0.01], where EtOH front-loading significantly increased from day 1 to 14, and group [F (1, 120) = 8.64, p < 0.01], where percentage of EtOH intake in the first 15 minutes was greater than percentage of water intake in the first 15 minutes on day 14. There was no significant day * group interaction effect [p > 0.05]. (Fig 2E). Comparisons of percent intake within the first 15 minutes to the 12.5% threshold indicated that intake of the HAP2 EtOH group on day 14 was significantly higher than the threshold, [t (31) = 4.56, p < 0.0001], indicating frontloading (Fig 2E). However, intakes of the EtOH group on day 1 and water group on days 1 and 14 did not significantly differ from the threshold [p > 0.05].
Figure 2. Assessment of Front-Loading.
Intake patterns (displayed as means of central moving averages ± SEM) for each replicate were compared on day 1 (A and B) versus day 14 (C and D). Except for day 1 in HAP2 mice, the EtOH groups always had a significantly higher proportion of intake within the first 15 minutes (front-loading) than the water group (E and F). * indicates significant group differences as indicated by Sidak’s post-hoc testing which were conducted following a main effect of group in both replicates, see Results. # Indicates percentage of intake significantly differed from the 12.5% frontloading threshold, indicating front-loading in EtOH groups with the exception of HAP2 day 1.
Days 1 and 14 Intake Patterns HAP3.
Intake patterns of HAP3 for days 1 (Fig 2B) and 14 (Fig 2D) indicate that EtOH-assigned mice display front-loading behavior on both days 1 and 14, whereas early intake in the water drinking group was always lower than the EtOH group; main effect of group [F (1, 58) = 76.18, p < 0.0001]. However, there was an increase in early water intake from day 1 to 14, which drove a significant day*group interaction effect [F (1, 57) = 8.361, p < 0.01] (Fig 2F). Comparisons of percent intake within the first 15 minutes to the 12.5% threshold indicated that intake of the HAP3 EtOH group on days 1 and 14 were significantly higher than the threshold, [t (29) = 5.89, p < 0.0001 and t (28) = 5.60, p < 0.0001], respectively, indicating front-loading. Intake of the water group on day 1 was significantly lower than the threshold, [t (29) = 9.25, p < 0.0001]. There was no difference noted for the water group on day 14 [p > 0.05] (Fig 2F).
Days 1–14 First 15-minutes.
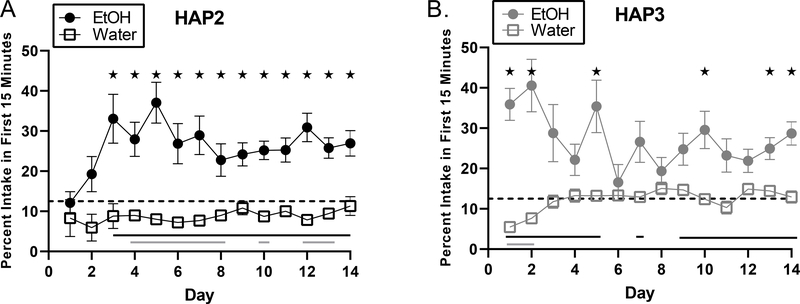
Next, we further tested the hypothesis that mice would consistently display front-loading and that front-loading would develop only after alcohol drinking experience. Given that the water group consumed more total fluid than the EtOH group (Fig 1), we compared the pattern between fluid groups of the percentage of intake which occurred within first 15 minutes over 14 days to assess front-loading. In both replicates, we observed that percent intake within the first 15 minutes of the EtOH group was almost always higher, and often significantly higher, than the 12.5% frontloading threshold. Additionally, the EtOH group often consumed more of their total fluid in the first 15 minutes than the water group (Fig. 3). In HAP2, analyses revealed a main effect of day, [F (7.729, 464.9) = 2.01, p < 0.05], where EtOH front-loading increases over days, and a main effect of group, [F (1, 62) = 92.61, p <0.0001], where EtOH-assigned mice front-loaded more than water-assigned mice, and a day*group interaction effect, [F (13, 782) = 1.94, p < 0.05], driven by the rapid increase in EtOH front-loading observed in the first few testing days coupled with fluctuations in percentage of first 15 water intake (Fig 3A). In HAP3, we also observed a main effect of group, [F (1, 58) = 59.82, p < 0.0001], with the EtOH group front-loading more than the water group. There was no main effect of day, [p > 0.05]. In HAP3, there was also a significant group*day interaction, [F (13, 767) = 6.30, p < 0.0001], driven by a high level of EtOH front-loading on early test days where the EtOH group consumed more than a third of their EtOH in the first eighth of the sessions on days 1 and 2. This pattern waned towards the middle of the testing period, and was coupled with an increase in early water intake at this time leading to the interaction effect (Fig 3B).
Figure 3. Assessment of Front-Loading Over Days.
Analyses considering the percentage of intake within the first 15-minutes of each two-hour DID session over the two-week period indicated a main effect of group in both replicates, where the EtOH group front-loaded more than the water group. * indicates days where percent intake within the first 15 minutes between groups significantly differed as determined by Sidak’s post-hoc testing, black lines indicate days where the EtOH group’s first 15 minute intake differed from the described 12.5% frontloading threshold (always significantly higher), gray lines indicate days where the water group’s first 15 minute intake significantly differed from this threshold (always significantly lower).
Assessment of cSNC:
Day 15 Total EtOH Intake and BECs.
No significant differences between day 15 intake or BEC were detected between the EE and WE group; thus, these fluid groups were collapsed within replicate for the following described regression analyses. Regression analyses indicate that EtOH intake predicted day 15 BEC in both replicates; HAP2 (R2 = .28, n = 31, p < 0.01, mean BEC: 99.29 ± 7.37 (SEM) mg/dl; mean EtOH intake: 3.53 ± 0.13 g/kg (22.32 ± 0.81 mL/kg); HAP3 (R2 = .14, n = 31, p < 0.05, mean BEC: 99.84 ± 9.73 mg/dl, mean EtOH intake: 3.72 ± 0.17 g/kg (23.55 ± 1.08 mL/kg).
Day 15 Intake Patterns: EtOH.
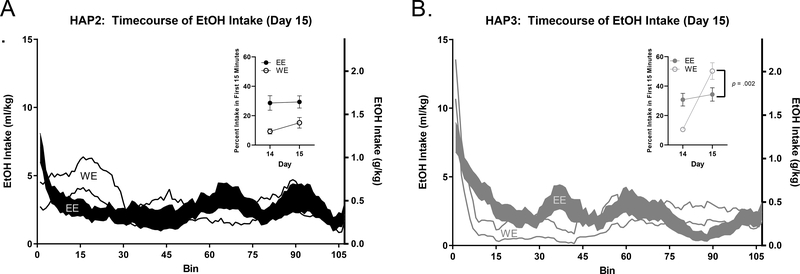
In mice lacking experience with EtOH (WE), we observed an inverse drinking pattern between the replicates. In HAP2, the WE group front-loaded EtOH less than mice who had been receiving EtOH throughout the duration of DID testing (EE) (Fig 4A + inset). However, in HAP3, the WE group consumed significantly more in the first 15 minutes than mice who had regularly been consuming EtOH. This replicates the different pattern of front-loading that we observed between HAP2 and HAP3 mice drinking EtOH on Day 1 (see Fig 2). Analyses considering change in percent of intake within the first 15 minutes from day 14 to day 15 indicated a main effect of group for HAP2 [F (1, 30) = 18.63, p < 0.001], where the EE group front-loads more than the WE group on both days. There was no main effect of day or day * group interaction, [p > 0.05] (Fig 4A inset). For HAP3, analyses indicated a significant main effect of day [F (1, 29) = 30.88, p < 0.0001] and a significant day*group interaction [F (1, 29) = 21.56, p < 0.0001; Sidak’s post-hoc comparing EE vs. WE on day 15: p < 0.05]. There was no main effect of group, [p > 0.05] (Fig 4B + inset).
Figure 4. Day 15 EtOH Intake Time course.
In mice experiencing EtOH for the first time (WE), we observed different drinking patterns between the replicates. In HAP2 (A), WE mice do not front-load EtOH at the same level as mice who have a 2-week drinking history (EE). In HAP3 (B), an opposite pattern was observed, where WE mice front-load significantly more than EE.
Day 15 cSNC in HAP2.
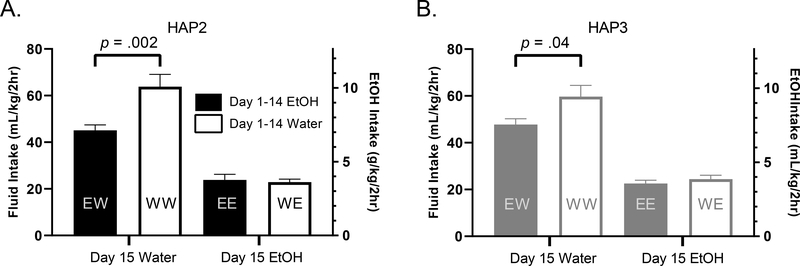
Analyses indicated a cSNC effect, where EW mice consumed significantly less water on day 15 than WW mice, [t (28) =3.38, p < 0.01]. Consummatory positive contrast effects (WE consumed more than EE) were not observed, as EtOH intake between these groups did not differ, [t (30) =0.36, p > 0.05] (Fig 5A).
Figure 5. Assessment of cSNC.
WW mice drink significantly more than EW mice on day 15, a cSNC effect that is seen across both HAP replicates. No significant differences in intake exist between EE and WE groups in either replicate (no evidence for postive contrast).
Day 15 cSNC in HAP3.
Like in the HAP2 replicate, analyses indicated a cSNC effect, where EW mice consumed significantly less water on day 15 than WW mice, [t (26) = 2.21, p < 0.05]. Consummatory positive contrast effects were not observed, as EtOH intake between WE and EE groups did not differ, [t (29) =0.85, p > 0.05] (Fig 5B).
Day 15 Intake Patterns: Water.
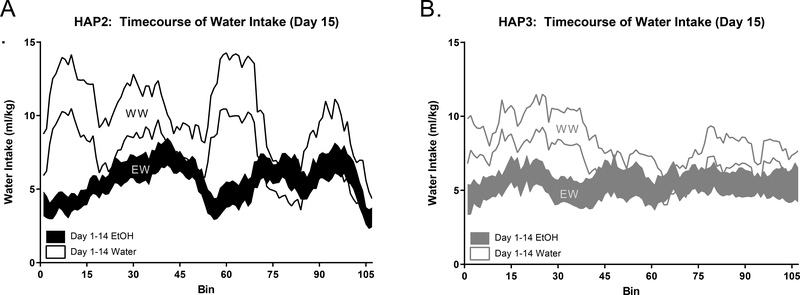
Our primary measure of cSNC was total session intake, which was statistically evaluated as described above and displayed in Fig 4. To further understand how the cSNC effect may have manifested, we considered a comparison of central moving averages to evaluate differences in within session intake patterns between EW and WW groups. We observed differences in patterns of water intake further demonstrating a cSNC effect, where mice of both replicates who had previously received EtOH (EW) change drinking patterns in response to a less desirable fluid (water) in a way that does not match the drinking pattern of mice who had been drinking water for the duration of DID testing (Fig 6).
Figure 6. Day 15 Water Intake Time course.
In both replicates we observe a cSNC effect which is further demonstrated by prolonged differences in water intake patterns, where mice with an EtOH history (EW) consumed approximately half as much water during the early portion of the session as mice without any previous EtOH experience (WW).
Assessment of Tolerance and Sensitization:
Day 16 Locomotion and BEC.
In both replicates, BECs measured following the 2 g/kg EtOH injection did not differ by fluid history group, indicating no evidence of metabolic tolerance [HAP2: t (60) = .57, p > 0.05; HAP3: t (26) = .36, p > 0.05]. Mean BEC values were as follows (all reported in mg/dL±SEM): HAP2 EtOH: 52.44±3.66; HAP2 water: 55.38±3.68; HAP3 EtOH: 33.53±6.31; HAP3 water: 32.46±5.79. A similar analysis of locomotor data comparing EtOH to water history groups revealed no evidence of alterations in locomotion following injected EtOH in either line [HAP2: t (58) = .09, p > 0.05; HAP3: t (24) = 1.87, p > 0.05]. Although previous studies have found evidence for behavioral tolerance to injected EtOH (Fritz et al., 2013, Linsenbardt and Boehm, 2015) and both metabolic and behavioral tolerance following 24-hour, two-bottle choice drinking (Matson et al., 2014), we observed neither here, presumably due to DID yielding shorter daily exposure to EtOH or lower BECs than were observed in that two-bottle choice study. Furthermore, although previous work has demonstrated sensitization following injected EtOH in HAP1 mice (Grahame et al., 2000) DID drinking did not lead to either metabolic tolerance or locomotor sensitization in the current study. Overall, these findings suggest that neither front-loading nor cSNC following two weeks of DID drinking are caused by changes in sensitivity to EtOH.
Discussion
These findings support our primary overarching hypothesis that repeated binge drinking experience alters the incentive motivational effects of EtOH. In both replicates, the EtOH groups consistently displayed front-loading following (or including) the first EtOH experience; mice consistently consumed a higher proportion of total intake during the early part of the session than would be expected given a flat distribution of intake (greater than 12.5% of total intake within the first 15 minutes), and also drank more fluid volume during this time than the water groups (Figs 2, 3). Although overall EtOH mice consistently front-loaded, the experience necessary to produce this effect varied between replicates. Indeed, HAP3 mice in the EtOH group show robust front-loading on the very first day of DID testing (Fig 2B). In contrast, front-loading behavior developed progressively over several sessions/days in the HAP2 EtOH group (Fig 2A/C), similar to what has been observed in previous studies in B6 and HAP1 mice. By day 14, both lines displayed similar levels of EtOH front-loading (Fig 3). This high level of front-loading behavior on the first day of testing in the HAP3 EtOH group may indicate a motivation to consume EtOH to experience its pre-absorptive effects (i.e. taste or smell), as mice had not yet had a chance to experience the rewarding post-absorptive effects (binge-level BEC). It has been shown previously that both male and female HAP3 mice drink more saccharin over a range of concentrations than male and female HAP2 mice (Oberlin et al., 2011). The fact that HAP3 mice drank more saccharin than HAP2 is consistent with the data presented in the current study. Specifically, the previous saccharin data and the current higher percent intake of EtOH within the first 15 minutes of DID on day 1 in HAP3 (Fig 2, 3), support the idea that HAP3 may initially be motivated by taste when consuming EtOH. Our front-loading finding is also consistent with previous studies of gene expression changes across generations as HAP3 mice were being selected, which implicated many olfactory genes (Hoffman et al., 2014), and suggests that HAP3 mice may have developed an innate, pre-ingestive preference for EtOH. Similar theories have been proposed for high EtOH-drinking B6 mice, who may initially consume EtOH because of a hedonic attraction to its taste (Belknap et al., 1977, Bachmanov et al., 1996). Alternatively or additionally, this observation may be influenced by the novelty of the EtOH solution. Indeed, a relationship between novelty seeking and AUD risk is well-described (Manzo et al., 2014, Flagel et al., 2014; for a review, see: Wingo et al., 2016), and is supported by our finding that HAP3 mice in the WE group front-load at higher levels during their first EtOH experience even than mice with extensive EtOH drinking experience (D15 EE group (Fig 4B)). In contrast, HAP2 mice may be more motivated to consume EtOH for the post-absorptive effects, as this replicate robustly increased front-loading behavior after many binge experiences. Differences in front-loading patterns between replicates coupled with our finding that early EtOH consumption rate on day 15 in the WE group(s) was found to differ between replicates, with HAP3 WE front-loading surpassing EE (Fig 4B inset), and HAP2 WE not showing this pattern (Fig 4A inset), at the very least points to the importance of novelty in the rate of EtOH consumption upon first EtOH encounter.
The most consistently observed phenomenon in this and the prior study of DID drinking in HAP1 mice (Linsenbardt and Boehm, 2015) was cSNC following an unexpected substitution of water for EtOH. That is, all groups of mice receiving water for the first time (EW) following two weeks of EtOH access displayed decreased total water intake (Fig 5A/B) as well as decreased rates of water consumption patterns compared to their two-week water drinking counterparts (WW; Fig 6A/B). This effect has previously been observed in the HAP1 replicate (Linsenbardt and Boehm, 2015), but interestingly was absent in B6 mice although BECs were similar across groups in both strains (Linsenbardt and Boehm, 2014). It is not clear whether the difference between HAP mice and B6 mice is specific to cSNC or whether this may indicate important differences between these populations in sensitivity to EtOH’s rewarding effects. In other strain comparisons of cSNC, previous research has demonstrated that in a sucrose downshift paradigm, HAP replicates display greater cSNC than LAPs (Matson and Grahame, 2015). LAP mice will not drink EtOH, and therefore we cannot assess whether EtOH cSNC would be found in them. However, the consistent presence of EtOH cSNC in three independently selected lines of HAP mice suggests that it is reliably associated with high EtOH intake in these lines.
The relationship between EtOH and cSNC has largely been studied within the context of assessing EtOH’s ability to relieve the frustrative effects associated with a sucrose downshift (Becker and Flaherty, 1982, Manzo et al., 2014, Manzo et al., 2015, Matson and Grahame, 2015). In the current study, we expand this literature by observing cSNC effects caused by a reaction to loss of alcohol. This is of interest because cSNC effects have shared neurochemical and neurobiological underpinnings with addiction (for a review, see: Ortega et al., 2017); for example, recent research has shown that inactivation of the central amygdala (CeA) prevents a cSNC effect following a sucrose downshift task (Guarino et al., 2020). These findings suggest that the CeA, a brain region also implicated in the development of alcohol use disorder (for a review, see: Roberto et al., 2020), is crucial for cSNC. Recent work has also found that rats exposed to binge levels of EtOH during adolescence show greater cSNC (lower sucrose consumption) following a downshift protocol than control (Lerma-Cabrera et al., 2019). Together, this body of literature indicates that cSNC effects may be an important but understudied cognitive construct related to alcohol use. Our findings of cSNC in three HAP replicate lines suggest that common genes underlie both response to reward loss and high alcohol intake.
Conclusions
The current study supports a growing body of literature establishing a relationship between genetic background and alterations in incentive motivational drive to consume EtOH: front-loading and cSNC. Both observations detected in the current study in HAP2 and HAP3 mice have been observed previously in HAP1 mice. As these results have been replicated across HAP lines, there is strong support that these behaviors are genetically mediated, which suggests that there is a biological mechanism that might be targeted. Future research should apply modern techniques to explore these biological mechanisms, with the goal of identifying treatments for experience-induced increases in motivation to consume alcohol excessively.
Supplementary Material
Acknowledgments
This work was supported in part by grant #s: AA022268 (DNL), AA025120 (DNL), Indiana Alcohol Research Center P60-AA007611, and the New Mexico Alcohol Research Center P50-AA022534.
References
- ADDOLORATO G, VASSALLO GA, ANTONELLI G, ANTONELLI M, TARLI C, MIRIJELLO A, AGYEI-NKANSAH A, MENTELLA MC, FERRARESE D, MORA V, BARBARA M, MAIDA M, CAMMA C, GASBARRINI A & ALCOHOL RELATED DISEASE, C. 2018. Binge Drinking among adolescents is related to the development of Alcohol Use Disorders: results from a Cross-Sectional Study. Sci Rep, 8, 12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLEN DL, LITTLE RG 2ND, THEOTOKATOS JE & PETERSEN DR 1982. Ethanol elimination rates in mice: effects of gender, nutrition, and chronic ethanol treatment. Pharmacol Biochem Behav, 16, 757–60. [DOI] [PubMed] [Google Scholar]
- AMSEL A 1962. Frustrative nonreward in partial reinforcement and discrimination learning: some recent history and a theoretical extension. Psychol Rev, 69, 306–28. [DOI] [PubMed] [Google Scholar]
- AMSEL A 1992. Frustration theory--many years later. Psychol Bull, 112, 396–9. [DOI] [PubMed] [Google Scholar]
- BACHMANOV AA, TORDOFF MG & BEAUCHAMP GK 1996. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res, 20, 201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKLEY-LEVENSON AM & CRABBE JC 2014. High drinking in the dark mice: a genetic model of drinking to intoxication. Alcohol, 48, 217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKLEY-LEVENSON AM & CRABBE JC 2015. Distinct ethanol drinking microstructures in two replicate lines of mice selected for drinking to intoxication. Genes Brain Behav, 14, 398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER LO & CEBALLOS NA 2014. Neural and genetic correlates of binge drinking among college women. Biol Psychol, 97, 43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER HC & FLAHERTY CF 1982. Influence of ethanol on contrast in consummatory behavior. Psychopharmacology (Berl), 77, 253–8. [DOI] [PubMed] [Google Scholar]
- BELKNAP JK, BELKNAP ND, BERG JH & COLEMAN R 1977. Preabsorptive vs. postabsorptive control of ethanol intake in C57BL/6J and DBA/2J mice. Behav Genet, 7, 413–25. [DOI] [PubMed] [Google Scholar]
- CARPENTER RW, PADOVANO HT, EMERY NN & MIRANDA R JR. 2019. Rate of alcohol consumption in the daily life of adolescents and emerging adults. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHASSIN L, PITTS SC & PROST J 2002. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: predictors and substance abuse outcomes. J Consult Clin Psychol, 70, 67–78. [PubMed] [Google Scholar]
- CUNNINGHAM CL 2014. Genetic relationship between ethanol-induced conditioned place preference and other ethanol phenotypes in 15 inbred mouse strains. Behav Neurosci, 128, 430–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAREVSKY D, GILL TM, VITALE KR, HU B, WEGNER SA & HOPF FW 2018. Drinking despite adversity: behavioral evidence for a head down and push strategy of conflict-resistant alcohol drinking in rats. Addict Biol. [DOI] [PubMed] [Google Scholar]
- FLAGEL SB, WASELUS M, CLINTON SM, WATSON SJ & AKIL H 2014. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology, 76 Pt B, 425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAHERTY C 1996. Incentive Relativity New York, NY, Cambridge University Press [Google Scholar]
- FRITZ BM, GRAHAME NJ & BOEHM SL 2ND 2013. Selection for high alcohol preference drinking in mice results in heightened sensitivity and rapid development of acute functional tolerance to alcohol’s ataxic effects. Genes Brain Behav, 12, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOWIN JL, SLOAN ME, STANGL BL, VATSALYA V & RAMCHANDANI VA 2017. Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. Am J Psychiatry, 174, 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUARINO S, CONRAD SE & PAPINI MR 2020. Frustrative nonreward: Chemogenetic inactivation of the central amygdala abolishes the effect of reward downshift without affecting alcohol intake. Neurobiol Learn Mem, 169, 107173. [DOI] [PubMed] [Google Scholar]
- GRAHAME NJ, RODD-HENRICKS K, LI TK & LUMENG L 2000. Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice. Psychopharmacology (Berl), 151, 252–60. [DOI] [PubMed] [Google Scholar]
- HOFFMAN PL, SABA LM, FLINK S, GRAHAME NJ, KECHRIS K & TABAKOFF B 2014. Genetics of gene expression characterizes response to selective breeding for alcohol preference. Genes Brain Behav, 13, 743–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IANCU OD, OBERBECK D, DARAKJIAN P, METTEN P, MCWEENEY S, CRABBE JC & HITZEMANN R 2013. Selection for drinking in the dark alters brain gene coexpression networks. Alcohol Clin Exp Res, 37, 1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM SG, KIM JS, PACK HJ & SUNG HN 2016. Usefulness of Heavy Drinking and Binge Drinking for the Diagnosis of Alcohol Use Disorder. Korean J Fam Med, 37, 214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMA-CABRERA JM, AREVALO-ROMERO CA, CORTES-TOLEDO GA, ADRIASOLA-CARRASCO AA & CARVAJAL F 2019. Emotional Reactivity to Incentive Downshift in Adult Rats Exposed to Binge-Like Ethanol Exposure During Adolescence. Front Psychol, 10, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINSENBARDT DN & BOEHM SL 2ND 2014. Alterations in the rate of binge ethanol consumption: implications for preclinical studies in mice. Addict Biol, 19, 812–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINSENBARDT DN & BOEHM SL 2ND 2015. Relative fluid novelty differentially alters the time course of limited-access ethanol and water intake in selectively bred high-alcohol-preferring mice. Alcohol Clin Exp Res, 39, 621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANZO L, DONAIRE R, SABARIEGO M, PAPINI MR & TORRES C 2015. Anti-anxiety self-medication in rats: oral consumption of chlordiazepoxide and ethanol after reward devaluation. Behav Brain Res, 278, 90–7. [DOI] [PubMed] [Google Scholar]
- MANZO L, GOMEZ MJ, CALLEJAS-AGUILERA JE, DONAIRE R, SABARIEGO M, FERNANDEZ-TERUEL A, CANETE A, BLAZQUEZ G, PAPINI MR & TORRES C 2014. Relationship between ethanol preference and sensation/novelty seeking. Physiol Behav, 133, 53–60. [DOI] [PubMed] [Google Scholar]
- MANZO L, GOMEZ MJ, CALLEJAS-AGUILERA JE, FERNANDEZ-TERUEL A, PAPINI MR & TORRES C 2014. Anti-anxiety self-medication induced by incentive loss in rats. Physiol Behav, 123, 86–92. [DOI] [PubMed] [Google Scholar]
- MATSON LM & GRAHAME NJ 2013. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol, 18, 921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSON LM & GRAHAME NJ 2015. Emotional reactivity to incentive downshift as a correlated response to selection of high and low alcohol preferring mice and an influencing factor on ethanol intake. Alcohol, 49, 657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSON LM, KASTEN CR, BOEHM SL 2ND & GRAHAME NJ 2014. Selectively bred crossed high-alcohol-preferring mice drink to intoxication and develop functional tolerance, but not locomotor sensitization during free-choice ethanol access. Alcohol Clin Exp Res, 38, 267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSON L, LIANGPUNSAKUL S, CRABB D, BUCKINGHAM A, ROSS RA, HALCOMB M & GRAHAME N 2013. Chronic free-choice drinking in crossed high alcohol preferring mice leads to sustained blood ethanol levels and metabolic tolerance without evidence of liver damage. Alcohol Clin Exp Res, 37, 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research (2003) Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academies Press; (US) Washington, DC. Available at: http://www.ncbi.nlm.nih.gov/books/NBK43327/. [PubMed] [Google Scholar]
- OBERLIN B, BEST C, MATSON L, HENDERSON A & GRAHAME N 2011. Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behav Genet, 41, 288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORTEGA LA, SOLANO JL, TORRES C & PAPINI MR 2017. Reward loss and addiction: Opportunities for cross-pollination. Pharmacol Biochem Behav, 154, 39–52. [DOI] [PubMed] [Google Scholar]
- RHODES JS, FORD MM, YU CH, BROWN LL, FINN DA, GARLAND T JR. & CRABBE JC 2007. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav, 6, 1–18. [DOI] [PubMed] [Google Scholar]
- ROBERTO M, KIRSON D & KHOM S 2020. The Role of the Central Amygdala in Alcohol Dependence. Cold Spring Harb Perspect Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALLING MC, SKELLY MJ, AVEGNO E, REGAN S, ZERIC T, NICHOLS E & HARRISON NL 2018. Alcohol Consumption during Adolescence in a Mouse Model of Binge Drinking Alters the Intrinsic Excitability and Function of the Prefrontal Cortex through a Reduction in the Hyperpolarization-Activated Cation Current. J Neurosci, 38, 6207–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLOAN ME, GOWIN JL, JANAKIRAMAN R, ESTER CD, STODDARD J, STANGL B & RAMCHANDANI VA 2019. High-risk social drinkers and heavy drinkers display similar rates of alcohol consumption. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON NF, BUCHWALD D & HARDEN KP 2013. A twin study of genetic influences on diurnal preference and risk for alcohol use outcomes. J Clin Sleep Med, 9, 1333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX MV, CUZON CARLSON VC, SHERAZEE N, SPROW GM, BOCK R, THIELE TE, LOVINGER DM & ALVAREZ VA 2014. Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology, 39, 579–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINGO T, NESIL T, CHOI JS & LI MD 2016. Novelty Seeking and Drug Addiction in Humans and Animals: From Behavior to Molecules. J Neuroimmune Pharmacol, 11, 456–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.