Chronic kidney disease is associated with a clustering of risk factors that greatly influence the development of cardiovascular complications in adults. In treatment of patients with the more advanced forms of kidney disease, dialysis is a necessary therapy.
In this current issue of Circulation Research1, the authors study a known problem with the peritoneal ‘form’ of dialysis; that the dialysis fluid can in itself contribute (or it is at least associate with) to disease. The authors discover that a degradation component of glucose in dialysis fluid can not only cause an inflammation of the peritoneum as has been known, but here they find it can also ‘cause’ pathological vascular remodeling in the arteriolar network within the omentum of the peritoneum. These observations come by way of Bartsova et. al from the University of Heidelberg and Vienna who analyzed samples and data from a multi-center international tissue and data repository from these children undergoing peritoneal dialysis for a duration of ~2 years.
While the risk factors for cardiovascular and kidney disease amplify with age in a generic sense, the current study comes from a sample population with chronic kidney disease absent of these variables: children. Children, in general, more commonly undergo peritoneal dialysis (as do patients with end stage renal disease1, 2), which requires less ‘in-patient’ time. The resultant findings inform of the potentially harmful components that can arise in dialysis fluids called glucose degradation products (also abbreviated to GDPs) and their effects on the peritoneal arteriolar network.
Some basics:
The kidney is our filtration system, and when it fails, physicians must ‘mechanically’ intervene to filter our blood. There are two basic types of intervention: peritoneal dialysis and hemodialysis. In peritoneal dialysis, the filtering of the kidneys is accomplished by the catheterization of the abdominal cavity (through the peritoneum), in which a solution is infused, which ‘absorbs’ waste in the abdomen which is then collected and discarded.
In patients with chronic kidney failure, water, electrolytes, and waste products accumulate in the body resulting in imbalances that can cause lethal toxicities to the patient. In peritoneal dialysis, the infusion of a concentrated pressure dialysis solution in the patients’ abdomen enables waste exchange and disposal across the peritoneal membrane. Glucose is generally used to maintain this high osmotic pressure to withdraw the waste and water from the bloodstream.
A key part of the article in this study is that those children calculated to have high levels of GDP in their peritoneal dialysis fluid exhibited pathological vascular remodeling in omental arterioles (peritoneal). To identify the novel mechanisms that could have been triggered by the GDPs to cause this remodeling, the authors implemented a profiling approach on the vessels, doing both microarray and proteome analyses on the children’s arterioles retrieved from the bio-bank. Numerous pathways were dysregulated in the high GDP-exposed arterioles, including cytoskeletal and junctional genes and proteins. Consistent with their prior observation,3 the profiling revealed the presence of increased advanced glycation end products (AGEs) in the high GDP group, which are surmised to be the culprits. This is consistent with mouse studies from another group, showing that the Receptor for AGE (RAGE) deficient mice treated with ‘lab-made’ peritoneal dialysis fluids that contained high GDP were protected from inflammation and peritoneal pathology.4 While more studies are needed, these observations support the idea that AGEs contribute to the pathology induced by the high GDP. From a more introspective perspective, while the thinking has been that the increased AGEs are a consequence of the high GDP, might it also be possible that factors/enzymes that increased AGEs could themselves be impacting the elevation or ‘toxicity’ of the high GDPs? Consistent with that possibility, children who have not undergone dialysis but suffer from chronic renal failure and type 1 diabetes may still have increased AGEs.5
This may be a little departure from the biology in the human body, but high levels of GDPs are mostly generated by the ‘manufacturing and heat sterilization’ of the dialysate solutions. Another aspect not related to biology is that the manner of dialysis delivery, can take high GDPs and turn them to lower GDPs using a ‘two chamber’ dialysis system versus ‘one chamber’. While glucose can ‘devolve’ into these potentially harmful GDPs, glucose does of course plays a key positive role in the peritoneal dialysis fluid, as it acts to produce the osmotic pressure gradient across the peritoneal membrane facilitating waste filtration. Dialysis solution generally contains a range of glucose from 1.36% glucose to 4.25%, while dextrose and other constituents can also be used to generate osmotic pressure.6–8 Whether the amount of glucose in the dialysis fluid can be titrated to minimize GDP formation is not fully clear, nor, if the degradation of the glucose itself (aside from the GDP formation), compromises its filtering ability.
So what exactly are these ‘glucose degradation products?’ For one, despite the similar name, they are chemically distinct from advanced glycation end-products, and also distinct in that the former arise in ‘bags’ and the latter in the body. When glucose is heat-sterilized for placement in the dialysis delivery bags, it is degraded to GDPs. The identity of those GDPs include formaldehyde, acetylaldehyde, and an important one, 3-deoxyglucosone (3-DG)9, [3-DG was also studied in an in vitro arm of this work by Bartsova et. al]. While 3-DG is also formed in the human body by the Maillard reaction, a characteristic of its dicarbonyl structure is reactivity, and when exiting the dialysis bags into the body it promotes AGE formation because of its reactive nature.10 Whether it is 3-DG or other GDPs, the consensus is that in dialysis solutions with low GDPs, the peritoneal membrane integrity is better preserved, and AGEs are reduced.11,12 An additional point of interest is that aside from glucose making high GDPs, high glucose itself (in peritoneal dialysis fluids) can induce glycosylation of substrate proteins. Importantly glucose levels were controlled for in the current study by Bartsova et al.
Bartsova et. al, make important findings, but also lay the groundwork for additional analyses and experimentation: In the current studies, GDPs are not directly identified or measured chemically in the patients’ plasma. While 3-DG may be a key component that arises from the catabolized glucose, one which was assessed by the authors in cultured human umbilical artery endothelial cells in the final article figure, determining the identity of the specific GDP that are elevated and are toxic to the vasculature would be important. Also, it would be interesting to assess vascular function in these high GDP, chronically exposed arterioles. However, attaining fresh human arterioles that are so-treated may make that difficult. Alternatively, vascular ‘ring’ studies in ‘naïve’ arterioles acutely exposed to different GDPs might be an equally important but more feasible study. Also, the omics studies here were performed in micro-dissected arterioles, but future studies could expand upon these findings with cell-specific analysis in not only endothelial cells, but also the impact in smooth muscle cells and adipose.
There were many key control points in this study. Dialytic glucose exposure was the same in patients with low or high GDP in dialysis fluid. This ruled out levels of glucose as a factor. The children studied in this work should have been largely absent of age-dependent development of vascular disease and other CVD risk factors, and yet they are developing vascular pathology after about two years of PD (high GDP group). Whether the observed remodeling is in fact pathological response, a protective adaptation, or somewhere in between is another story altogether. The transcriptomic and proteomic analysis of the arterioles exposed to high GDP points to endothelial-specific effects, but addition important information is likely therein. A final point of commentary: while high GDP exposure may extend beyond the peritoneal arterioles to impact the ‘major’ organ vasculature, the peritoneum itself can be an overlooked vascular organ/tissue, that is not only affected in dialysis but also important in metastasis,13 abdominal surgery,14 and gastrointestinal disease. More studies should look at the peritoneal vasculature in different disorders. Finally, the authors propose that targeting (or interfering) with high GDP overload in CKD should be an area of focus, so that new strategies to counteract high GDP formation are developed. Alternatively it may be necessary to administer and/or process peritoneal dialysis solutions which are less prone to degrading into harmful glucose metabolites.
The soul is healed by being with children.”
— Fyodor Dostoyevsky
Figure. High GDPs in dialysis solutions induce vascular remodeling in omental arterioles.
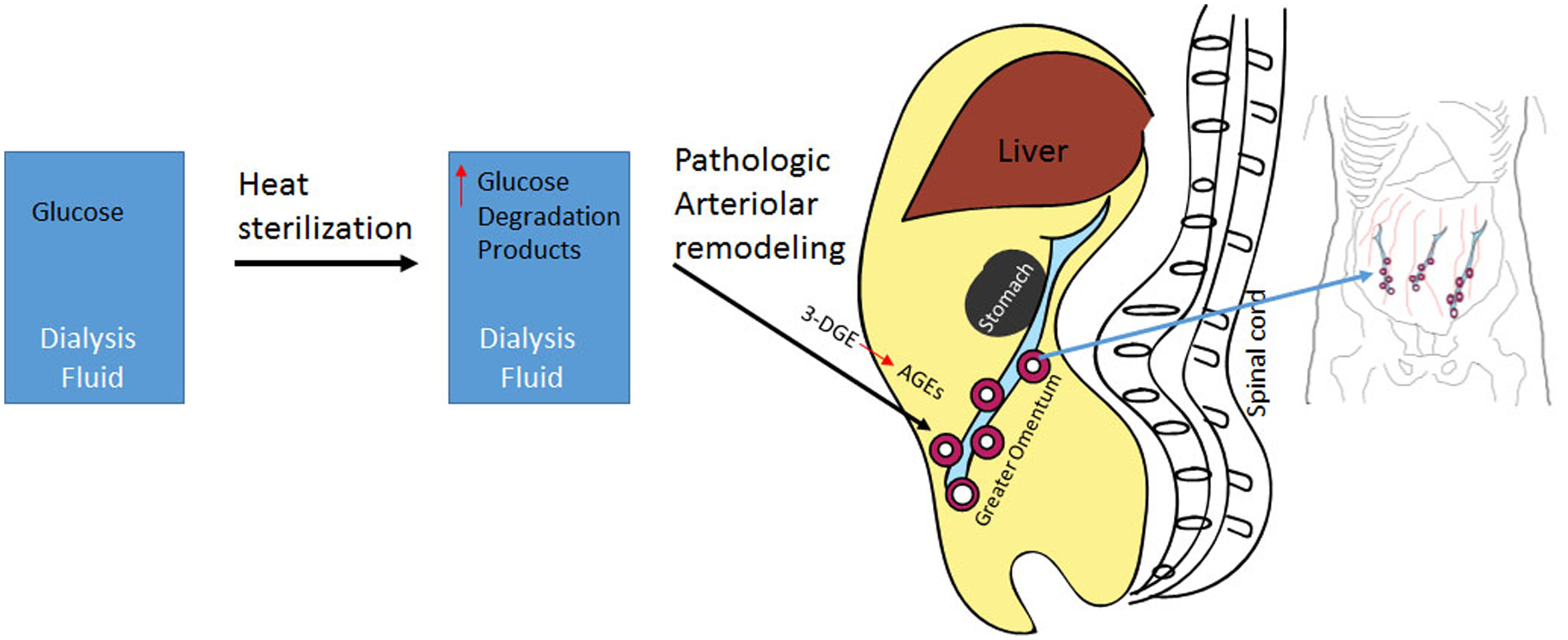
Heat sterilization of dialysis fluid can cause glucose to (at least) in part deteriorate into glucose degradation products. The current work finds that patients treated with dialysis fluid that are especially high in these glucose degradation products results in increased pathological remodeling in arterioles of the omentum (peritoneal membrane that hangs from the stomach). Further, the authors find advanced glycation products (distinct biochemically from glucose degradation products) are upregulated in the abdominal fluid from the children with high GDP, The study subjects in this article are children with chronic kidney disease who were treated by peritoneal dialysis.
Disclosures
R.D. Rudic and Z. Bagi share grants from the National Institutes of Health (R01 AG054651 and R01AG054651-04S1). The other authors report no conflicts.
References
- 1.Bartsova M, Zhang C, Schaefer B, Herzog R, Ridinger D, Damgov I, Levai E, Marinovic I, Eckert C, Romero P, et al. Glucose Derivative Induced Vasculopathy in Children on Chronic Peritoneal Dialysis. Circ Res: 2021; 129:xx–xxx. [DOI] [PubMed] [Google Scholar]
- 2.Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. Journal of the American Society of Nephrology : JASN. 2012;23:533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sloan CE, Coffman CJ, Sanders LL, Maciejewski ML, Lee S-YD, Hirth RA, Wang V. Trends in peritoneal dialysis use in the united states after medicare payment reform. Clinical Journal of the American Society of Nephrology. 2019;14:1763–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt CP, von Heyl D, Rieger S, Arbeiter K, Bonzel KE, Fischbach M, Misselwitz J, Pieper AK, Schaefer F. Reduced systemic advanced glycation end products in children receiving peritoneal dialysis with low glucose degradation product content. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22:2038–2044 [DOI] [PubMed] [Google Scholar]
- 5.Schwenger V, Morath C, Salava A, Amann K, Seregin Y, Deppisch R, Ritz E, Bierhaus A, Nawroth PP, Zeier M. Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end-products. Journal of the American Society of Nephrology. 2006;17:199–207 [DOI] [PubMed] [Google Scholar]
- 6.Misselwitz J, Franke S, Kauf E, John U, Stein G. Advanced glycation end products in children with chronic renal failure and type 1 diabetes. Pediatr. Nephrol 2002;17:316–321 [DOI] [PubMed] [Google Scholar]
- 7.Verrina EE, Perfumo F. Chapter 55 - pediatric peritoneal dialysis prescription. In: Geary DF, Schaefer F, eds. Comprehensive pediatric nephrology. Philadelphia: Mosby; 2008:835–853. [Google Scholar]
- 8.Becker BN, Schulman G. Chapter 77 - technical aspects of hemodialysis. In: Wilcox CS, ed. Therapy in nephrology & hypertension (third edition). Philadelphia: W.B. Saunders; 2008:845–858. [Google Scholar]
- 9.Muahmmad Alam K MW. Peritoneal dialysis solutions. In: Motwani S, ed. UpToDate; 2021. [Google Scholar]
- 10.Rippe B, Simonsen O, Heimbürger O, Christensson A, Haraldsson B, Stelin G, Weiss L, Nielsen FD, Bro S, Friedberg M, Wieslander A. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int. 2001;59:348–357 [DOI] [PubMed] [Google Scholar]
- 11.Schalkwijk CG, Posthuma N, ten Brink HJ, ter Wee PM, Teerlink T. Induction of 1,2-dicarbonyl compounds, intermediates in the formation of advanced glycation end-products, during heat-sterilization of glucose-based peritoneal dialysis fluids. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis. 1999;19:325–333 [PubMed] [Google Scholar]
- 12.Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, Passlick-Deetjen J, Euro Balance Trial G. The euro-balance trial: The effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int. 2004;66:408–418 [DOI] [PubMed] [Google Scholar]
- 13.Rippe B, Simonsen O, Heimburger O, Christensson A, Haraldsson B, Stelin G, Weiss L, Nielsen FD, Bro S, Friedberg M, Wieslander A. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int. 2001;59:348–357 [DOI] [PubMed] [Google Scholar]
- 14.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by mmp-2 cleavage of vitronectin and fibronectin. Journal of Clinical Investigation. 2008;118:1367–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito T, Shintani Y, Fields L, Shiraishi M, Podaru MN, Kainuma S, Yamashita K, Kobayashi K, Perretti M, Lewis-McDougall F, Suzuki K. Cell barrier function of resident peritoneal macrophages in post-operative adhesions. Nature Communications. 2021;12:2232. [DOI] [PMC free article] [PubMed] [Google Scholar]


