Structured Abstract
Objective:
To understand the impact of Black race on breast cancer (BC) presentation, treatment, and survival among Hispanics.
Summary Background Data:
It is well-documented that non-Hispanic Blacks (NHB) present with late-stage disease, less likely to complete treatment, and have worse survival compared to their non-Hispanic White (NHW) counterparts. However, no data evaluates whether this disparity extends to Hispanic Blacks (HB) and Hispanic Whites (HW). Given our location in Miami, gateway to Latin America and the Caribbean, we have the diversity to evaluate BC outcomes in HB and HW.
Methods:
Retrospective cohort study of stage I-IV BC patients treated at our institution from 2005–2017. Kaplan-Meier survival curves were generated and compared using the log-rank test. Multivariable survival models were computed using Cox proportional hazards regression.
Results:
Race/ethnicity distribution of 5,951 patients: 28% NHW, 51% HW, 3% HB, and 18% NHB. HB were more economically disadvantaged, had more aggressive disease, and less treatment compliant compared to HW. 5-year OS by race/ethnicity was: 85% NHW, 84.8% HW, 79.4% HB, and 72.7% NHB (p<0.001). After adjusting for covariates, NHB was an independent predictor of worse OS [HR:1.25 (95% CI: 1.01–1.52), p< 0.041)].
Conclusions:
In this first comprehensive analysis of HB and HW, HB has worse OS compared to HW, suggesting that race/ethnicity is a complex variable acting as a proxy for tumor and host biology, as well as individual and neighborhood-level factors impacted by structural racism. This study identifies markers of vulnerability associated with Black race and markers of resiliency associated with Hispanic ethnicity to narrow a persistent BC survival gap.
Keywords: Breast cancer, disparities, safety-net hospitals, multidisciplinary care
Mini-Abstract:
Novel discovery of a distinct Hispanic Black population identifies more aggressive breast cancer characteristics and worse survival compared to Hispanic Whites; but less aggressive tumor characteristics and improved survival compared to non-Hispanic Blacks. This reflects biologic and social vulnerability with Black race, and markers of resiliency associated with Hispanic ethnicity.
Introduction:
Hispanics are the second largest and fastest growing ethnic group in the US and are projected to constitute 35% of the US population by 2050 [1]. Breast cancer is the most common cancer diagnosed and is the leading cause of cancer-related mortality among Hispanic women in the US [2,3,4]. Therefore, it is critical to analyze breast cancer outcomes in this population sub-group to inform future cancer control and targeted treatment interventions to attenuate the risk of cancer disparity.
Studies have established significant differences in sociodemographic factors, tumor and treatment characteristics, and outcomes by Black race between non-Hispanic Whites (NHW) and non-Hispanic Blacks (NHB). [5]. In general, NHB are usually of lower socioeconomic status (SES), have more advanced stage disease at presentation, more aggressive breast cancer subtypes [e.g., triple negative breast cancer (TNBC)], are less likely to receive treatment, and have worse survival outcomes than their NHW counterparts [5]. However, no data, to our knowledge, examines the impact of Black race on breast cancer presentation, treatment, and survival outcomes among those of Hispanic ethnicity [5, 6, 7, 8].
Given our location in Miami-Dade County, gateway to Latin America and the Caribbean, Hispanic Whites (HW) and Hispanic Blacks (HB) account for approximately 70% of the population, which makes us perfectly poised to address this knowledge gap and comprehensively examine breast cancer characteristics, treatment, and outcomes in HB compared to HW, NHW, and NHB. Thus, the overall objective of this study is to evaluate the impact of Black race in a diverse Hispanic population and also explore covariates including sociodemographic, tumor characteristics, and National Comprehensive Care Network (NCCN)-guideline based treatment.
Methods:
Study Population
The University of Miami-Sylvester Comprehensive Cancer Center (SCCC), an NCI-designated Cancer Center, and its affiliate Jackson Health System, provide health care services for Miami-Dade County and much of South Florida, allowing these health systems to care for one of the most ethnically, racially, and socioeconomically diverse catchment areas in the country. According to the 2018 American Community Survey population estimates, Miami-Date County is one of the few counties in the US where racial/ethnic minority groups comprise the majority of the population: 69.4% of County residents identify as Hispanic or Latino,17.7% as NHB, and 12.9% as NHW. Additionally, 16% of the county residents live below the federal poverty level and 19.5%, under the age of 65, were uninsured [10]. Uninsured patients who can provide proof of residence within the County are eligible to receive county tax-funded care within the Jackson Health System, which is the safety-net hospital (SNH) for County residents. [11]. Patients presenting either to our SNH or SCCC with breast cancer between 2005 and 2017 were identified from our local tumor registry. Patients with stages I-IV invasive ductal or lobular carcinoma were included in the analysis. We excluded women with carcinoma in situ and/or other malignancies of the breast (sarcoma, lymphoma, etc.) and those with incomplete diagnosis or follow-up times. This study was approved by our institutional review board.
Variables of Interest
Patient Characteristics
Patient, tumor, and treatment characteristics were collected from electronic medical records by two surgical oncologists. Data variables included patient demographics (age, sex, marital status, race, ethnicity, country of birth, insurance, median income, residence), clinical data [(body-mass index (BMI), comorbidities, smoking status, alcohol use], tumor characteristics (stage, histologic grade, receptor status), and treatments (surgery, radiation, chemotherapy, endocrine therapy). Patients were stratified by age: <50 years old, 50–69 years old, >70–79 years old, and ≥80 years old. Marital status was divided into three main groups: single, married, and no longer married (divorced, separated, or widowed). Race was grouped as White, Black and other. The other group accounted for 3.2% of the entire population of interest and included Asians, Pacific Islanders, and American Indians. Patients were classified as Hispanic or non-Hispanic and those with missing information (<1%) were included in the non-Hispanic group. Insurance was divided into: private, Medicare, Medicaid, none, and other (military, Veterans Affairs, Indian public health services, and other non-specified insurance). Median income was calculated based on the patient’s home zip code, as individual income was not available from the electronic medical records. This ranged from $14,476-$177,000 for the entire population. The population was divided into quartiles: <$36,572, $36,573–48,450, $48,451–64,599, and >$64,600.
Tumor Characteristics
Clinical stage at time of diagnosis and pathologic stage were determined using the American Joint Committee on Cancer (AJCC) 7th Edition. Patients were grouped as stage I-IV or unknown. Histologic tumor grading was stratified as well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated/anaplastic. Patients were grouped according to receptor status. Estrogen receptor (ER)+ or ER- and human epidermal growth factor receptor 2 positive (HER2)+ or HER2-.
Treatment Characteristics
Patients were classified according to the treatments they initiated: surgery, chemotherapy, radiation, and endocrine therapy. Guideline concordant treatment for each patient was then determined by two surgical oncologists using NCCN Breast Cancer Guidelines for each patient based on their receptor status and clinical and pathologic stage.
Statistical Analysis
Descriptive statistics were calculated for patient, tumor, and treatment characteristics using frequencies (percentage) for categorical data and mean (standard deviation) or median (interquartile rage Q1–Q3) for continuous data. Univariate analysis using Student’s t-tests and chi-square analysis as appropriate to the data were used to examine clinical treatment practices and guideline adherence at each hospital.
Survival Analysis
Our primary outcome was 5-year overall survival (OS). Five-year OS by race/ethnicity and breast cancer subtype specific survival by race/ethnicity was calculated using the date of diagnosis and date of death or last known follow-up. Kaplan-Meier survival curves were generated and compared using the log-rank test. Multivariable survival models were computed using Cox proportional hazards regression. Variables with a p-value <0.1 on log rank test were included in the Cox proportional hazards model. A final p-value <0.05 was considered statistically significant. All statistics were performed using SPSS version 25 (IBM Corp).
Results:
Patient Characteristics
Overall, 5,951 patients with breast cancer were treated during the study period, of which 1,647 (27.7%) were NHW, 3,127 (52.5%) were HW, 107 (1.8%) were HB, and 1,070 (18.0%) were NHB (Table 1). The median age at diagnosis was significantly lower (<50 years old) for racial/ethnic minorities (HW, HB, and NHB) compared to their NHW counterparts. The majority of Hispanics were born outside the United States compared to non-Hispanics. HB and NHB were more likely to be single and of the lowest income quartile. HB were more likely to be uninsured. A greater percentage of HB were current smokers compared to HW, while a greater percentage of NHW reported current alcohol use compared to HW and NHB. NHB were three times more likely to meet World-Health Organization Class III obesity (BMI 40+) criteria compared to NHW (7.9% vs 2.4%, p<0.001) and were significantly more likely to have comorbidities, specifically hypertension and diabetes compared to NHW, HW, and HB (Table 1).
Table 1:
Patient Sociodemographics and Risk Factors
| Factor | Non-Hispanic White N=1647 |
Hispanic White N=3127 |
Hispanic Black N=107 |
Non-Hispanic Black N=1070 |
All N=5951 |
p-value |
|---|---|---|---|---|---|---|
| Sociodemographics | ||||||
|
| ||||||
| Age at diagnosis | p<0.001 | |||||
| <50 years | 441 (26.8%) | 990 (31.7%) | 30 (28.0%) | 358 (33.5%) | 1819 (30.6%) | |
| 50–69 years | 898 (54.5%) | 1769 (56.6%) | 62 (57.9%) | 607 (56.7%) | 3336 (56.1%) | |
| 70–79 years | 205 (12.4%) | 279 (8.9%) | 14 (13.1%) | 76 (7.1%) | 574 (9.6%) | |
| 80+ years | 103 (6.3%) | 89 (2.8%) | 1 (0.9%) | 29 (2.7%) | 222 (3.7%) | |
|
| ||||||
| Birth Place | p<0.001 | |||||
| US-born | 778 (47.2%) | 139 (4.4%) | 4 (3.7%) | 482 (45.0%) | 1403 (23.6%) | |
| Foreign-born | 214 (13.0%) | 2307 (73.8%) | 92 (86.0%) | 430 (40.2%) | 3043 (51.1%) | |
| Unknown | 655 (39.8%) | 681 (21.8%) | 11 (10.3%) | 158 (14.8%) | 1505 (25.3%) | |
|
| ||||||
| Relationship | p<0.001 | |||||
| Married | 947 (57.5%) | 1462 (46.8%) | 34 (31.8%) | 355 (33.2%) | 2798 (47.0%) | |
| Single | 295 (17.9%) | 756 (24.2%) | 42 (39.3%) | 472 (44.1%) | 1565 (26.3%) | |
| Divorced/Separated/Widow | 346 (21.0%) | 832 (26.6%) | 28 (26.2%) | 218 (20.4%) | 1424 (23.9%) | |
| Other/Unknown | 59 (3.6%) | 77 (2.5%) | 3 (2.8%) | 25 (2.3%) | 164 (2.8%) | |
|
| ||||||
| Median Income Quartiles | p<0.001 | |||||
| <$36,572 | 130 (8.0%) | 909 (29.8%) | 40 (38.1%) | 405 (39.6%) | 1484 (25.6%) | |
| $36,573–48,450 | 317 (19.6%) | 787 (25.8%) | 32 (30.5%) | 384 (37.5%) | 1520 (26.2%) | |
| $48,451–64,599 | 521 (32.3%) | 700 (22.9%) | 26 (24.8%) | 143 (14.0%) | 1390 (24.0%) | |
| >$64,600 | 649 (40.1%) | 658 (21.5%) | 7 (6.7%) | 91 (8.9%) | 1405 (24.2%) | |
|
| ||||||
| Insurance | p<0.001 | |||||
| Private | 1054 (64.0%) | 1111 (35.5%) | 24 (22.4%) | 397 (37.1%) | 2586 (43.5%) | |
| Medicare | 320 (19.4%) | 343 (11.0%) | 16 (15.0%) | 117 (10.9%) | 796 (13.4%) | |
| Medicaid | 94 (5.7%) | 716 (22.9%) | 34 (31.8%) | 276 (25.8%) | 1120 (18.8%) | |
| Uninsured | 72 (4.4%) | 667 (21.3%) | 28 (26.2%) | 187 (17.5%) | 954 (16.0%) | |
| Other | 107 (6.5%) | 290 (9.3%) | 5 (4.7%) | 93 (8.7%) | 495 (8.3%) | |
|
| ||||||
| Risk Factors | ||||||
|
| ||||||
| Tobacco Use | N=1439 | N=2945 | N=102 | N=1006 | N=5492 | p<0.001 |
| Never Smoker | 853 (51.8%) | 2113 (67.6%) | 72 (67.3%) | 798 (74.6%) | 3836 (64.5%) | |
| Current Smoker | 110 (6.7%) | 248 (7.9%) | 15 (14.0%) | 64 (6.0%) | 437 (7.3%) | |
| Former Smoker | 476 (28.9%) | 584 (18.7%) | 15 (14.0%) | 144 (13.5%) | 1219 (20.5%) | |
|
| ||||||
| Alcohol Use | N=1435 | N=2939 | N=102 | N=1002 | N=5478 | p<0.001 |
| None | 670 (40.7%) | 2289 (73.2%) | 80 (74.8%) | 802 (75.0%) | 3841 (64.5%) | |
| Current use | 755 (45.8%) | 628 (20.1%) | 21 (19.6%) | 193 (18.0%) | 1597 (26.8%) | |
| Former use | 10 (0.6%) | 22 (0.7%) | 1 (0.9%) | 7 (0.7%) | 40 (0.7%) | |
|
| ||||||
| BMI Categories | N=1327 | N=2714 | N=94 | N=924 | N=5059 | p<0.001 |
| Underweight (<18.5) | 27 (1.6%) | 19 (0.6%) | 0 (0.0%) | 5 (0.5%) | 51 (0.9%) | |
| Normal weight (18.5–24.9) | 542 (32.9%) | 702 (22.4%) | 22 (20.6%) | 179 (16.7%) | 1445 (24.3%) | |
| Overweight (25–29.9) | 431 (26.2%) | 1031 (33.0%) | 33 (30.8%) | 290 (27.1%) | 1785 (30.0%) | |
| Class I Obesity (30–34.9) | 198 (12.0%) | 614 (19.6%) | 17 (15.9%) | 238 (22.2%) | 1067 (17.9%) | |
| Class II Obesity (35–39.9) | 90 (5.5%) | 226 (7.2%) | 17 (15.9%) | 128 (12.0%) | 461 (7.7%) | |
| Class III Obesity (40+) | 39 (2.4%) | 122 (3.9%) | 5 (4.7%) | 84 (7.9%) | 250 (4.2%) | |
|
| ||||||
| Comorbidities | ||||||
| Hypertension | 419 (25.4%) | 743 (23.8%) | 33 (30.8%) | 363 (33.9%) | 1558 (26.2%) | p<0.001 |
| Diabetes | 86 (5.2%) | 226 (7.2%) | 9 (8.4%) | 124 (11.6%) | 445 (7.5%) | p<0.001 |
| Coronary Artery Disease | 8 (0.5%) | 13 (0.4%) | 1 (0.9%) | 7 (0.7%) | 29 (0.5%) | p=0.709 |
| Hyperlipidemia | 145 (8.8%) | 179 (5.7%) | 6 (5.6%) | 50 (4.7%) | 380 (6.4%) | p<0.001 |
Tumor Characteristics
When comparing tumor characteristics, differences by race/ethnicity remained pronounced (Table 2). NHB were more likely to present with aggressive disease characteristics such as advanced stage (III or IV), higher grade tumors, and more aggressive disease subtypes [triple negative breast cancer (TNBC)] compared to HB, HW, and NHW. Specifically, HB and NHB were almost twice as likely to present with stage IV disease than NHW and HW. HB and NHB were also more likely to have more aggressive (high grade) tumors (34.6% and 42.1%) and were also more likely to have more aggressive tumor subtypes (TNBC and HER2+) compared to NHW and HW (25.2% and 30.7%), p<0.001.
Table 2:
Tumor and Treatment Characteristics
| Factor | Non-Hispanic White N=1647 |
Hispanic White N=3127 |
Hispanic Black N=107 |
Non-Hispanic Black N=1070 |
All N=5951 |
p-value |
|---|---|---|---|---|---|---|
| Clinical Stage | p<0.001 | |||||
| I | 765 (46.4%) | 1137 (36.4%) | 28 (26.2%) | 281 (26.3%) | 2211 (37.2%) | |
| II | 512 (31.1%) | 1120 (35.8%) | 38 (35.5%) | 386 (36.1%) | 2056 (34.5%) | |
| III | 211 (12.8%) | 563 (18.0%) | 24 (22.4%) | 221 (20.7%) | 1019 (17.1%) | |
| IV | 122 (7.4%) | 226 (7.2%) | 14 (13.1%) | 141 (13.2%) | 503 (8.5%) | |
| Unknown | 37 (2.2%) | 81 (2.6%) | 3 (2.8%) | 41 (3.8%) | 162 (2.7%) | |
|
| ||||||
| Tumor Grade | p<0.001 | |||||
| Low | 334 (20.3%) | 531 (17.0%) | 13 (12.1%) | 132 (12.3%) | 1010 (17.0%) | |
| Intermediate | 715 (43.4%) | 1341 (42.9%) | 46 (43.0%) | 370 (34.6%) | 2472 (41.5%) | |
| High | 415 (25.2%) | 959 (30.7%) | 37 (34.6%) | 450 (42.1%) | 1861 (31.3%) | |
| Anaplastic | 7 (0.4%) | 19 (0.6%) | 2 (1.9%) | 20 (1.9%) | 48 (0.8%) | |
| Unknown | 176 (10.7%) | 277 (8.9%) | 9 (8.4%) | 98 (9.2%) | 560 (9.4%) | |
|
| ||||||
| Receptor Status | p<0.001 | |||||
| ER+/HER2- | 1078 (65.5%) | 1983 (63.4%) | 60 (56.1%) | 525 (49.1%) | 3646 (61.3%) | |
| ER+/HER2+ | 170 (10.3%) | 336 (10.7%) | 18 (16.8%) | 109 (10.2%) | 633 (10.6%) | |
| ER-/HER2+ | 84 (5.1%) | 237 (7.6%) | 7 (6.5%) | 101 (9.4%) | 429 (7.2%) | |
| ER-/HER2- | 315 (19.1%) | 571 (18.3%) | 22 (20.6%) | 335 (31.3%) | 1243 (20.9%) | |
|
| ||||||
| Pathologic Stage | p<0.001 | |||||
| 0 | 12 (0.7%) | 20 (0.6%) | 1 (0.9%) | 7 (0.7%) | 40 (0.7%) | |
| I | 759 (46.2%) | 1086 (34.7%) | 31 (29.2%) | 281 (26.3%) | 2157 (36.3%) | |
| II | 406 (24.7%) | 859 (27.5%) | 26 (24.5%) | 268 (25.0%) | 1559 (26.2%) | |
| III | 146 (8.9%) | 340 (10.9%) | 12 (11.3%) | 106 (9.9%) | 604 (10.2%) | |
| IV | 44 (2.7%) | 81 (2.6%) | 9 (8.5%) | 37 (3.5%) | 171 (2.9%) | |
| Unknown | 277 (16.8%) | 740 (23.7%) | 27 (25.5%) | 371 (34.7%) | 1415 (23.8%) | |
|
| ||||||
| Treatments | ||||||
| Surgery | 1494 (90.7%) | 2782 (89.0%) | 88 (82.2%) | 856 (80.0%) | 5220 (87.7%) | p<0.001 |
| Chemotherapy | 854 (51.9%) | 1891 (60.5%) | 61 (57.0%) | 658 (61.5%) | 3464 (58.2%) | p<0.001 |
| Radiation | 848 (51.5%) | 1761 (56.3%) | 56 (52.3%) | 528 (49.3%) | 3193 (53.7%) | p<0.001 |
| Endocrine Therapy | 1121 (68.1%) | 1924 (61.5%) | 59 (55.1%) | 482 (45.0%) | 3586 (60.3%) | p<0.001 |
| NCCN Guideline-Appropriate Treatment | 1311 (79.6%) | 2366 (75.7%) | 77 (72.0%) | 745 (69.6%) | 4499 (75.6%) | p<0.001 |
| Treatment at Comprehensive Cancer Center | 1368 (83.1%) | 1445 (46.2%) | 37 (34.6%) | 432 (40.4%) | 3282 (55.2%) | p<0.001 |
Guideline Adherence and Treatment Receipt.
HB (69.6%) and NHB (72.0%) were less likely to receive NCCN guideline-based concordant therapy based on stage and receptor subtype compared to HW (75.7%) and NHW (79.6%), p<0.001. NHW were most likely to receive treatment at the Comprehensive Cancer Center. HW, HB, and NHB were more likely to receive care at the SNH.
Overall Survival
With a median follow-up time of 65 months, unadjusted 5-year OS by race and ethnicity was greater for NHW (85%) compared to HW (84.8%), HB (79.4%), or NHB (72.7%), p<0.001 (Figure 1). This OS pattern of NHW having improved survival compared to HW who had improved survival compared to HB who had improved survival compared to NHB remained consistent for each receptor subtype (Figure 2).
Figure 1: 5-year Overall Survival by Race and Ethnicity.
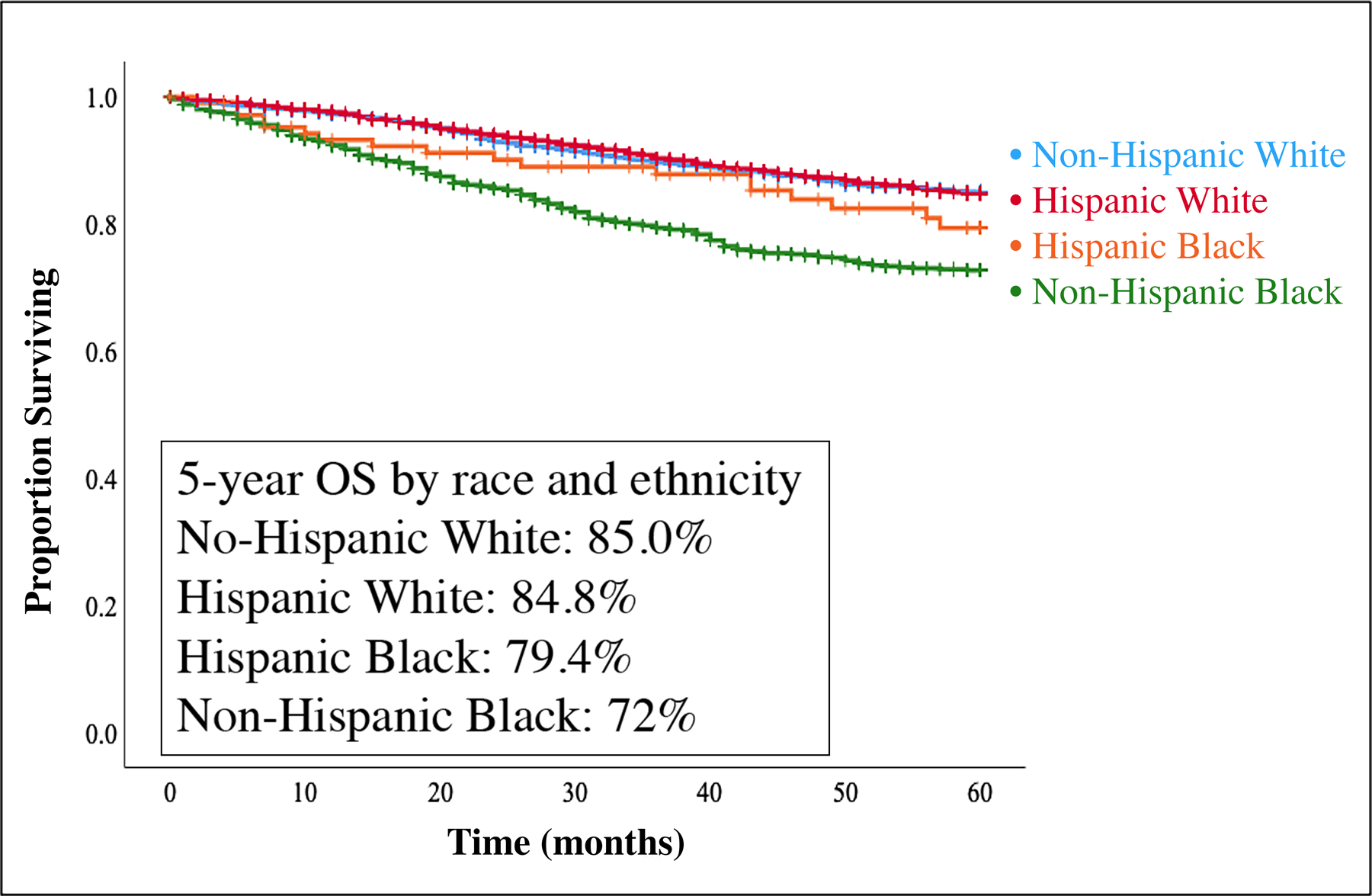
Kaplan-Meier overall survival (OS) curves by race/ethnicity (n=5,951). With a median follow-up time of 65 months, 5-year OS for the entire cohort was 82.5%. 5-year OS for non-Hispanic Whites, Hispanic Whites, Hispanic Blacks, and non-Hispanic Blacks were 85%, 84.8%, 79.4%, and 72.7%, p<0.001.
Figure 2: 5-year Overall Survival by Tumor Receptor Subtype and Race/Ethnicity.
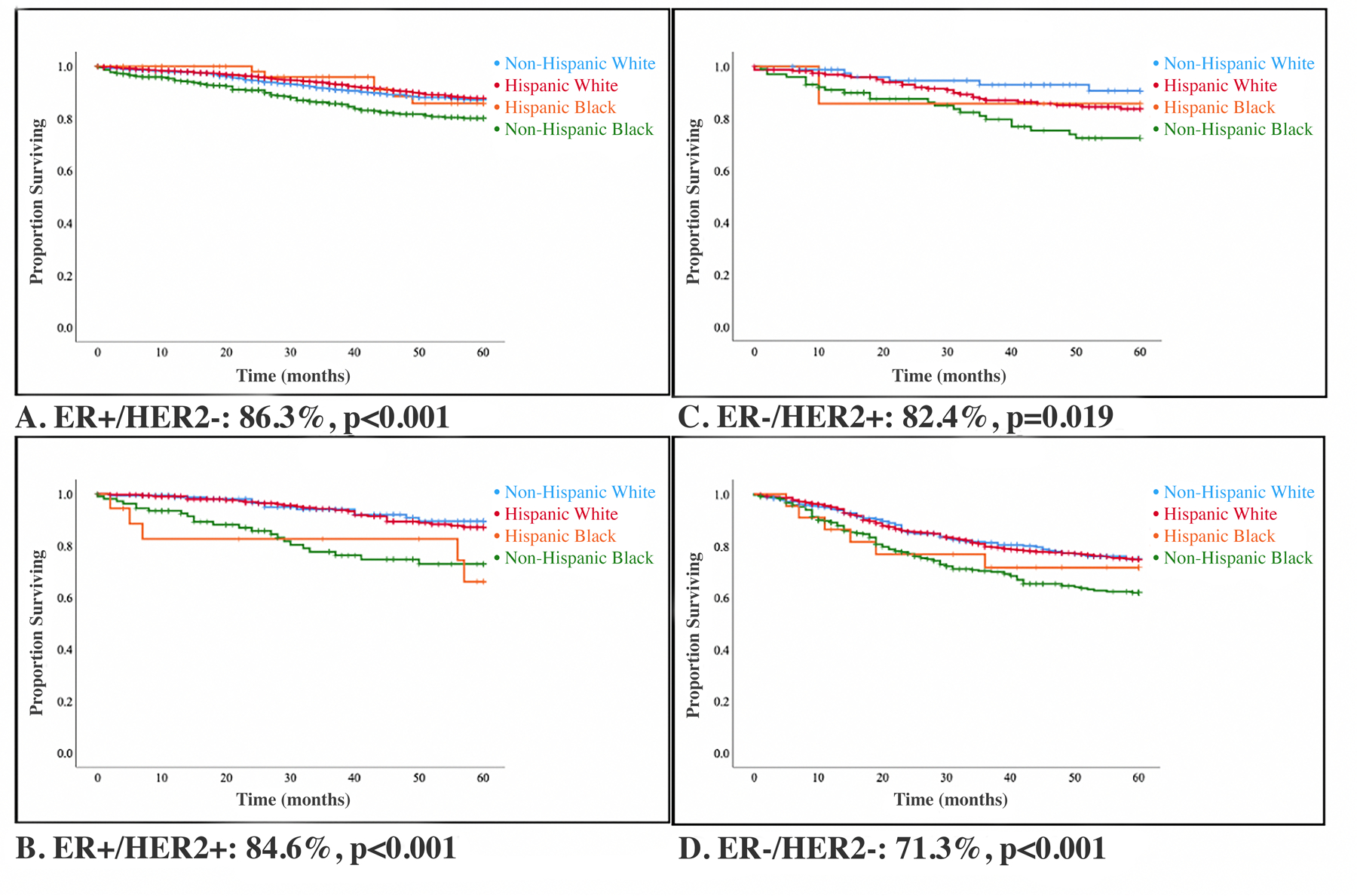
Kaplan-Meier overall survival (OS) curves by tumor receptor subtype and race/ethnicity (n=5,951). With a median follow-up time of 65 months, 5-year OS for ER+/HER2-, ER+/HER2+, ER-/HER2+, ER-/HER2- were 86.3%, 84.6%, 82.14%, and 71.3%, respectively.
On cox proportional hazards modeling adjusting for age, insurance status, marital status, income, smoking, alcohol intake, comorbidities, stage, tumor subtype, tumor grade, and treatment, NHB (HR: 1.25 (95% CI: 1.01–1.55), p<0.04) had a statistically significant increased hazard of death compared to NHW (Table 3). Hispanic ethnicity was associated with improved survival, although not statistically significant on the fully adjusted model (Table 3).
Table 3:
Cox proportional hazards adjusted survival analysis stratified by race/ethnicity
| HRa | Lower CI | Upper CI | p-value | |
|---|---|---|---|---|
| Non-Hispanic White (ref) | -- | -- | -- | -- |
| Hispanic White | 0.88 | 0.72 | 1.07 | 0.19 |
| Hispanic Black | 0.86 | 0.53 | 1.42 | 0.56 |
| Non-Hispanic Black | 1.25 | 1.01 | 1.55 | 0.04 |
Model adjusted for age, insurance, marital status, income, smoking, alcohol, body mass index, comorbidities, stage, tumor subtype, tumor grade, and treatment.
Discussion:
This novel study expands current understanding of breast cancer risk factors, tumor and treatment characteristics, and survival by defining HB as a distinct population with clinically significant breast cancer characteristics and survival outcomes. Given our large, predominantly Hispanic population from South and Central America, as well as the Caribbean, we had the racial/ethnic diversity to discover that HB, a population that has historically been grouped with HW, has worse OS compared to HW and NHW, but improved OS compared to NHB. This graded OS difference (NHW > HW > HB > NHB) reflects the complex interplay between more aggressive tumor biologic characteristics and lower socioeconomic status associated with Black race, along with potential unaccounted sociocultural characteristics associated with improved survival in those Hispanic ethnicity.
Tumor Biologic Differences by Race: Hispanic Whites and Hispanic Blacks
We found that among Hispanic women, survival differences were exaggerated by Black race with HB having worse OS than HW. HB presented with more advanced stage disease (III or IV), higher grade tumors, and more aggressive tumor subtypes such TNBC. This trifecta of more aggressive tumor characteristics suggests that tumor biologic differences associated with Black race are in part driving survival differences, even among women of shared Hispanic ethnicity. Although it is well-established that NHB present with later stage disease and have a higher incidence of TNBC compared to NHW, our findings are the first to suggest that this disparity extends to HB [12, 13, 14, 15].
To understand the biologic mechanisms driving these observed racial disparities, researchers have turned to genetic ancestry. A study of 154 Black patients of African ancestry and 776 White patients of European ancestry revealed that compared with White patients, Black patients had a worse breast cancer-free interval [HR: 1.67 (95% CI: 1.02–2.74), P = .043], a higher likelihood of TNBC [OR: 3.80 (95% CI: 2.46–5.87). P < .001], and more TP53 mutations than Whites [16]. They also identified molecular differences in DNA methylation probes, DNA copy number segments, a protein, and genes that were differentially expressed, leading to a gene-based signature to distinguish breast tumors from Black and White patients [16]. These findings underscore the biologic differences driven by race among predominantly NHB and NHW populations but remain to be validated in a diverse Hispanic population [16].
Non-Tumor Biologic Differences by Race: Hispanic Whites and Hispanic Blacks
Along with the aforementioned tumor biologic heterogeneity among HW and HB, we identified disparities in non-biologic factors. HB were more likely to live in neighborhoods with the lowest median household income quartiles (<$36,572), lack insurance, and not receive NCCN-guideline concordant treatment. Although socioeconomic and access to care disparities are well-documented in NHB and NHW breast cancer populations, our study brings to light these findings among HB and HW [17]. A potential explanation for these racial disparities is rooted in structural racism, in particular residential segregation. Black women living in racially segregated neighborhoods are less likely to have access to screening and treatment, contributing to advanced stage at diagnosis and worse OS, respectively [18, 19, 20].
Residential segregation also contributes to Black women living in socioeconomically disadvantaged neighborhoods which presents an added problem of obesity and diabetes due to limited exercise outlets (e.g., less walkable and safe neighborhoods) and less access to healthy food options (e.g., food deserts) [21]. Both obesity and diabetes have also been implicated in increased rates of TNBC [21]. Our data extend these findings by revealing that HB are more likely to be obese compared to HW. Additionally, Shariff-Marco et al identified that racism may deter utilization of health care because of patient mistrust and prior negative experiences, which in turn exacerbates disparities [22,23]. These health comorbidities compounded with limited screening and treatment adherence likely contribute to worse OS. Overall, our novel findings of improved outcomes with White race even amongst Hispanic populations underscores the importance of cancer control programming at the prevention (e.g., increase active lifestyle to decrease obesity), screening (e.g., reduce late-stage diagnosis), and treatment (e.g., reduce mistrust and barriers to treatment) levels in Black populations.
Tumor Biologic Differences by Ethnicity: Hispanic Blacks and Non-Hispanic Blacks
We also discovered that HB have improved OS compared to NHB, even after accounting for sociodemographic, tumor characteristics, and receipt of treatment. This suggests unaccounted factors associated with Hispanic ethnicity that may confer a survival advantage. Hispanics are an admixed population made of predominantly European, Native American, and West African ancestries. Recent studies analyzing differences in breast cancer mortality by genetic ancestry in Hispanics have shown mixed results. Fejerman et al. showed that 50% or more Native American ancestry in Hispanic women with breast cancer is associated with increased mortality, however this study did not account for guideline-concordant treatment [30]. In a follow-up study, they found that equal access to care eliminated the association between Native American ancestry and increased breast cancer mortality [31]. A limitation of both studies is a predominantly homogenous Mexican American cohort. Future studies need to evaluate the impact on genetic ancestry on tumor biology, and breast cancer survival in a diverse Hispanic population to understand potential molecular resilience associated with Hispanic ethnicity [24, 25, 26, 27, 28].
Non-Tumor Biologic Differences by Ethnicity: Hispanic Blacks and Non-Hispanic Blacks
Hispanic ethnicity may also serve as a proxy for nongenomic factors associated with improved survival in HB compared to NHB. Even though HB had many similar patient characteristics (e.g., socioeconomic status, rates of alcohol and tobacco use, and comorbidities), tumor characteristics (e.g., stage at presentation) and treatment characteristics (e.g., rates of guideline-appropriate treatment) to NHB, HB still had improved OS compared to NHB for each tumor subtype. This can potentially be explained due to a greater percentage of HB being foreign born. Studies have reported that even though foreign-born Hispanics are more likely to be diagnosed with more advanced stages, they have better survival than US-born Hispanics [32, 33, 34]. This phenomenon called “the Hispanic paradox” refers to the better health outcomes observed for Hispanic populations in the United States compared to non-Hispanic populations of similar socioeconomic background [35]. These survival advantages may in part be due to the lifestyles adopted in Latin enclaves, which may promote better health attitudes and healthier diets [32, 33, 34, 36, 37]. Specifically, we found that HB were more likely to have class 3 morbid obesity compared to NHB, a known breast cancer risk factor [38]. A criticism of the “Hispanic paradox” is that the improved survival of Hispanics, particularly foreign-born, could be an artifact of underestimating mortality due to women returning to their native countries, termed the “Salmon Bias” [33, 37]. However, a study examining this paradox in Cubans (a large proportion of our study population), who face barriers returning to Cuba, found that this did not explain the observed mortality paradox [39]. Future studies should focus on identifying factors associated with improved survival among Hispanics to uncover novel markers of resiliency.
Overall, this study has many strengths and potential limitations inherent to large retrospective studies. Our tumor registry database may not capture treatments received at other facilities, thus making guideline-appropriate care difficult to analyze in these cases. Despite this, each patient’s care was evaluated by two physicians to determine if the patient met strict, up to date, NCCN-guideline appropriate treatment. Our study is also novel in its comprehensive approach to studying breast cancer outcomes in South Florida where approximately 70% of the population is Hispanic, with representation from Central and South America, and the Caribbean. Being situated in this geographic location allows for this first comprehensive look comparing HB to HW and NHB. However, it is important to note that HB still comprised a relatively small percentage of the study population (<2%) and as a result larger national and international studies powered with more HB need to be conducted to validate our findings. Our medical campus provides the opportunity to serve women from many socioeconomic backgrounds allowing for novel insight on the compounding impact of socioeconomic status with race/ethnicity [40]. Given clear differences in patient characteristics, tumor and treatment characteristics, and survival among HW and HB, we believe that conventional reporting on the Hispanic population as an aggregate group masks clinically significant differences associated with survival outcomes.
Conclusion:
At our academic institution we treat a large number of patients with breast cancer from South Florida, Central and South America, and the Caribbean who self-report as Hispanic. Our study is the first to suggest more aggressive tumor characteristics, specifically higher rates of late-stage disease at presentation, higher-grade tumors, increased TNBC, and worse OS among HB compared to HW. Moreover, HB had improved OS compared to NHB, suggesting unaccounted factors associated with longer survival in those of Hispanic ethnicity. Race/ethnicity is a complex variable that is likely acting as a proxy for tumor and host biology, as well as individual and neighborhood-level factors impacted by structural racism. These findings underlie the importance of studying populations through the combined lens of precise genomic measures of race/ethnicity to accurately tailor prognosis and treatment based on potential ancestrally-driven molecular alterations associated with more aggressive disease and through the lens of social epidemiology to better understand the individual and neighborhood-level factors preventing early-stage detection and treatment adherence in these populations. This transdisciplinary approach is critical to unmasking clinically relevant differences to attain health equity in diverse populations.
Footnotes
Disclosures: The authors declare no disclosures or potential conflicts of interest.
Design: Retrospective cohort study utilizing local tumor registry data from 2005–2017.
References:
- 1.Parker K, Morin R, Menasce Horowitz J (2019, December 31). Views of America’s future in 2050 Retrieved September 10, 2020, from https://www.pewsocialtrends.org/2019/03/21/america-in-2050/ [Google Scholar]
- 2.Keegan TH, Quach T, Shema S et al. The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC Cancer 10, 603 (2010). 10.1186/1471-2407-10-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinheiro PS, Callahan KE, Koru-Sengul T, Ransdell J, Bouzoubaa L, Brown CP, & Kobetz E (2019). Risk of Cancer Death Among White, Black, and Hispanic Populations in South Florida. Preventing Chronic Disease, 16. doi: 10.5888/pcd16.180529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern MC, Fejerman L, Das R, Setiawan VW, Cruz-Correa MR, Perez-Stable EJ, & Figueiredo JC (2016). Variability in Cancer Risk and Outcomes Within US Latinos by National Origin and Genetic Ancestry. Current epidemiology reports, 3, 181–190. 10.1007/s40471-016-0083-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat 2011;127(3):729–38. 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015;313(2):165–73. 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 7.Pinheiro PS, Williams M, Miller EA, Easterday S, Moonie S, Trapido EJ. Cancer survival among Latinos and the Hispanic paradox. Cancer Causes Control 2011;22(4):553–61. 10.1007/s10552-011-9727-6. [DOI] [PubMed] [Google Scholar]
- 8.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med 2003;163(1):49–56. 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Census Bureau (2018). American Community Survey 1-year estimates Retrieved from Census Reporter Profile page for Miami-Dade County, FL http://censusreporter.org/profiles/05000US12086-miami-dade-county-fl/
- 10.Noe-Bustamante L (2020, May 31). Key facts about U.S. Hispanics and their diverse heritage Retrieved July 26, 2020, from https://www.pewresearch.org/fact-tank/2019/09/16/key-facts-about-u-s-hispanics/ [Google Scholar]
- 11.Allen VC Jr, Lachance C, Rios-Ellis B, Kaphingst KA. Issues in the Assessment of “Race” among Latinos: Implications for Research and Policy. Hisp J Behav Sci 2011;33(4):411–424. doi: 10.1177/0739986311422880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott LC, Mobley LR, Kuo T‐M and Il’yasova D (2019), Update on triple‐negative breast cancer disparities for the United States: A population‐based study from the United States Cancer Statistics database, 2010 through 2014. Cancer, 125: 3412–3417. 10.1002/cncr.32207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg 2017;152(5):485–93. [DOI] [PubMed] [Google Scholar]
- 14.Newman LA, Reis-Filho JS, Morrow M, Carey LA, King TA. The 2014 Society of Surgical Oncology Susan G. Komen for the Cure Symposium: triple-negative breast cancer. Ann Surg Oncol 2015;22(3):874–82. [DOI] [PubMed] [Google Scholar]
- 15.Dietze E, Sistrunk C, Miranda-Carboni G et al. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer 15, 248–254 (2015). 10.1038/nrc3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huo D, Hu H, Rhie SK, et al. Comparison of Breast Cancer Molecular Features and Survival by African and European Ancestry in The Cancer Genome Atlas. JAMA Oncol 2017;3(12):1654–1662. doi: 10.1001/jamaoncol.2017.0595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conroy SM, Shariff-Marco S, Koo J, Yang J, Keegan TH, Sangaramoorthy M, Hertz A, Nelson DO, Cockburn M, Satariano WA, Yen IH, Ponce NA, John EM, Gomez SL. Racial/Ethnic Differences in the Impact of Neighborhood Social and Built Environment on Breast Cancer Risk: The Neighborhoods and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev 2017April;26(4):541–552. doi: 10.1158/1055-9965.EPI-16-0935.Epub 2017 Feb 14. PMID: 28196846; PMCID: PMC5380527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet 2017;389:1453–1463. [DOI] [PubMed] [Google Scholar]
- 19.Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health 2019;40:105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep 2001;116:404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noonan AS, Velasco-Mondragon HE, & Wagner FA (2016). Improving the health of African Americans in the USA: an overdue opportunity for social justice. Public health reviews, 37, 12. 10.1186/s40985-016-0025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klassen AC, Smith KC, Shariff-Marco S, Juon HS. A healthy mistrust: how worldview relates to attitudes about breast cancer screening in a cross-sectional survey of low-income women. Int J Equity Health 2008January31;7:5. doi: 10.1186/1475-9276-7-5.PMID: 18237395; PMCID: PMC2267195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy BR, Mathis CC, Woods AK. African Americans and their distrust of the health care system: healthcare for diverse populations. J Cult Divers 2007Summer;14(2):56–60. PMID: 19175244. [PubMed] [Google Scholar]
- 24.Torres Á, Oliver J, Frecha C, Montealegre AL, Quezada-Urbán R, Díaz-Velásquez CE, et al. Cancer genomic resources and present needs in the Latin American region. Public Health Genomics 2017;20(3):194–201. [DOI] [PubMed] [Google Scholar]
- 25.Spratt DE, Chan T, Waldron L, Speers C, Feng FY, Ogunwobi OO, et al. Racial/ethnic disparities in genomic sequencing. JAMA Oncol 2016;2(8):1070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature 2016;538(7624):161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan J, Hu Z, Mahal BA, Zhao SD, Kensler KH, Pi J, Hu X, Zhang Y, Wang Y, Jiang J, Li C, Zhong X, Montone KT, Guan G, Tanyi JL, Fan Y, Xu X, Morgan MA, Long M, Zhang Y, Zhang R, Sood AK, Rebbeck TR, Dang CV, Zhang L. Integrated Analysis of Genetic Ancestry and Genomic Alterations across Cancers. Cancer Cell 2018October8;34(4):549–560.e9. doi: 10.1016/j.ccell.2018.08.019. PMID: 30300578; PMCID: PMC6348897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fejerman L, John EM, Huntsman S, Beckman K, Choudhry S, Perez-Stable E, et al. Genetic ancestry and risk of breast cancer among U.S. Latinas. Cancer Res 2008;68(23):9723–8. 10.1158/0008-5472.CAN-08-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fejerman L, Romieu I, John EM, Lazcano-Ponce E, Huntsman S, Beckman KB, et al. European ancestry is positively associated with breast cancer risk in Mexican women. Cancer Epidemiol Biomark Prev 2010;19(4):1074–82. 10.1158/1055-9965.EPI-09-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fejerman L, Hu D, Huntsman S, John EM, Stern MC, Haiman CA, et al. Genetic ancestry and risk of mortality among U.S. Latinas with breast cancer. Cancer Res 2013;73(24):7243–53. 10.1158/0008-5472.CAN-13-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engmann NJ, Ergas IJ, Yao S, Kwan ML, Roh JM, Ambrosone CB, et al. Genetic ancestry is not associated with breast cancer recurrence or survival in U.S. Latina women enrolled in the Kaiser Permanente Pathways study. Cancer Epidemiol Biomark Prev 2017;26(9):1466–9. 10.1158/1055-9965.EPI-17-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinheiro PS, Bungum TJ. Country of origin and breast cancer survival. Asia Pac J Clin Oncol 2014;10(3):279. [DOI] [PubMed] [Google Scholar]
- 33.US Census Bureau. Population: Hispanic origin. Census.gov. https://www.census.gov/topics/population/hispanic-origin/about.html. Accessed 17 Jan 2021.
- 34.Pinheiro PS, Callahan KE, Boscoe FP, Balise R, Cobb TR, Lee DJ, et al. Cancer-site-specific disparities in New York, including the 1945–1965 birth cohort’s impact on liver cancer patterns. Cancer Epidemiol Biomarkers Prev 2018;27(8):917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palloni A, Arias E. Paradox lost: explaining the Hispanic adult mortality advantage. Demography 2004;41(3):385–415. doi: 10.1353/dem.2004.0024 [DOI] [PubMed] [Google Scholar]
- 36.Cresce AR, Ramirez RR. Analysis of general Hispanic responses in census 2000. Population division working paper 2003;(72).https://scholar.google.com/scholar?q=Cresce%20AR%2C%20Ramirez%20RR.%20Analysis%20of%20general%20Hispanic%20responses%20in%20census%202000.%20Population%20division%20working%20paper%202003%3B%2872%29. [Google Scholar]
- 37.Pinheiro PS, Bungum TJ, Jin H. Limitations in the imputation strategy to handle missing nativity data in the Surveillance, Epidemiology, and End Results program. Cancer 2014;120(20):3261–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorincz AM, & Sukumar S (2006). Molecular links between obesity and breast cancer, Endocrine-Related Cancer Endocr Relat Cancer, 13(2), 279–292. Retrieved Jan 17, 2021, from https://erc.bioscientifica.com/view/journals/erc/13/2/0130279.xml [DOI] [PubMed] [Google Scholar]
- 39.Turra CM, Elo IT. The Impact of Salmon Bias on the Hispanic Mortality Advantage: New Evidence from Social Security Data. Popul Res Policy Rev 2008;27(5):515–530. doi: 10.1007/s11113-008-9087-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly KN, Hernandez A, Yadegarynia S, Ryon E, Franceschi D, Avisar E, Kobetz EN, Merchant N, Kesmodel S, Goel N. Overcoming disparities: Multidisciplinary breast cancer care at a public safety net hospital. Breast Cancer Res Treat 2021January25. doi: 10.1007/s10549-020-06044-z.Epub ahead of print. PMID: 33495917. [DOI] [PubMed] [Google Scholar]


