Abstract
Childhood internalizing disorders, like anxiety and depression, are common, impairing, and difficult to detect. Universal childhood mental health screening has been recommended, but new technologies are needed to provide objective detection. Instrumented mood induction tasks, designed to press children for specific behavioral responses, have emerged as means for detecting childhood internalizing psychopathology. In our previous work, we leveraged machine learning to identify digital phenotypes of childhood internalizing psychopathology from movement and voice data collected during negative valence tasks (pressing for anxiety and fear). In this work, we develop a digital phenotype for childhood internalizing disorders based on wearable inertial sensor data recorded from a Positive Valence task during which a child plays with bubbles. We find that a phenotype derived from features that capture reward responsiveness is able to accurately detect children with underlying internalizing psychopathology (AUC=0.81). In so doing, we explore the impact of a variety of feature sets computed from wearable sensors deployed to two body locations on phenotype performance across two phases of the task. We further consider this novel digital phenotype in the context of our previous Negative Valence digital phenotypes and find that each task brings unique information to the problem of detecting childhood internalizing psychopathology, capturing different problems and disorder subtypes. Collectively, these results provide preliminary evidence for a mood induction task battery to develop a novel diagnostic for childhood internalizing disorders.
Keywords: Machine Learning, Digital Health, Wearables, Anxiety, Depression, Positivity
I. Introduction
NEARLY 1 out of every 5 children experience an internalizing disorder (19.6% anxiety, 2.1% depression) during childhood [1], [2]. Anxiety and depression (collectively internalizing disorders) are chronic conditions that start as early as the preschool years [3], [4] and impair a child’s relationships, development, and functioning [5]–[9]. If left untreated, childhood internalizing disorders predict later health problems including substance abuse [10], [11], development of comorbid psychopathology, increased risk for suicide [12]–[15], and substantial functional impairment [16], [17]. These negative long-term outcomes highlight the individual and societal burden of internalizing disorders [18] and make clear the need for effective early identification.
The American Academy of Pediatrics and the US Preventative Services Task Force have recommended universal screening for behavioral health disorders in pediatric primary care to detect more children with mental health concerns [19]. Children under 6 have the highest rate of unmet needs [20], [21], likely due to difficulties understanding and expressing their emotions [22], [23]. Current screeners - typically parent-report questionnaires - rely on observable child behaviors. Anxiety and depressive symptoms, characterized by trouble with abstract emotions, however, are often unobservable and thus are overlooked or misinterpreted [24], [25]. Given these barriers and limited access to expert clinical assessment in most communities [19], pediatricians urgently need objective, accurate low-burden tools for detecting anxiety and depression in young children.
Several dimensional frameworks of psychopathology are particularly salient for translational childhood research. Some of these frameworks aid research in studying heterogeneity of psychopathology by focusing on broad-scale problems of internalizing disorders (e.g., Hierarchical Taxonomy Of Psychopathology - HiTOP [26], [27]; Child Behavior Checklist - CBCL [28]). In early childhood, anxiety and depressive disorders are less distinctive [2], [12], [13], [31] with complex presentations [3], indicating that research on broad internalizing disorders, rather than anxiety or depression specifically, is appropriate. Other frameworks aid research into the mechanisms underlying these broad scale problems via objective markers of related constructs (e.g., Research Domain Criteria - RDoC [29], [30]). The RDoC highlights five constructs as core building blocks for research, including such Negative and Positive valence, focusing on their interrelationships [29], [30] with specific recommendations for assessing each [32]. For young children, mood induction tasks have been recommended to “press” for specific valence constructs [33]–[35]. RDoC Negative and Positive Valence constructs have been consistently linked to internalizing disorders using mood induction paradigms to expose extreme affect associated with psychopathology [36].
Typically, researchers watch videos of children completing mood induction paradigms and are trained to behaviorally code negative and positive verbal and non-verbal behaviors [37], [38]. Specifically, studies have linked Negative Valence paradigm behavioral codes [33], [37], [39]–[42] to “withdrawn/depressed”, “anxious/depressed” [38], and internalizing problems [42]. However, it takes months to train behavioral coders and to reliably code all observations, thus our work has sought new, rapid methods of objectively assessing verbal and non-verbal child behaviors during mood induction paradigms. In our prior work, we developed non-verbal and verbal Negative Valence digital phenotypes from motion and voice data recorded during RDoC Negative Valence subconstructs of Potential Threat (Anxiety) and Acute Threat (Fear) paradigms that detected child internalizing disorder with 80-81% accuracy (54-67% sensitivity; 88-93% specificity) [43]–[46].
Positive Valence paradigm behaviorally codes from the subconstruct Reward Responsiveness have also been related to depression [47]–[50], anxiety [51]–[54] and internalizing behaviors [55]–[57]. Specifically, individuals with internalizing disorders often have impaired regulation of positive emotions and response to positive stimuli [58], [59]. Anhedonia, or the lack of positive valence, has been a significant negative prognostic indicator for older children and adolescents with depression [60], [61]. These findings have also been replicated in younger children—depressed preschoolers with anhedonia are characterized by significantly increased symptomology and changes in stress cortisol reactivity when compared to depressed children without reported anhedonia [62]. Low positivity puts children at increased risk for later psychopathology [63], [64] and internalizing problems [65].
Thus, the literature indicates that a multi-construct consideration of internalizing disorder phenotypes, inclusive of data derived from both Positive and Negative Valence paradigms, may lead to a more comprehensive, dimensional understanding of internalizing disorders [66]. Although we have previously published on Negative Valence paradigms and their accuracy in detecting internalizing disorders using rapid verbal and non-verbal methods, we have not yet evaluated Positive Valence paradigms in this way. Herein, we focus on a paradigm of one subconstruct of Positive Valence, Reward Responsiveness, defined as “processes that govern an organism’s hedonic response to impending reward, the receipt of reward, and following repeated receipt of reward” because it has been most widely used in young children. We use preliminary results to compare to findings from our prior research and examine if a collective task battery is indicated.
Therefore, the primary aim of this paper is to examine whether a Positive Valence paradigm-based digital phenotype can accurately detect childhood internalizing disorders. In so doing, we explore the relative clinical utility of motion feature sets derived from wearable sensors comparing timing (initial vs. end of the task) and position (lower back vs. head). We further aim to compare signal features and digital phenotypes from this Positive Valence task to our previous published results on only Negative Valence tasks. Collectively, we aim to examine whether consideration of a battery inclusive of Positive and Negative mood induction tasks for developing digital phenotypes of childhood internalizing disorders is indicated (see Fig. 1).
Fig. 1.
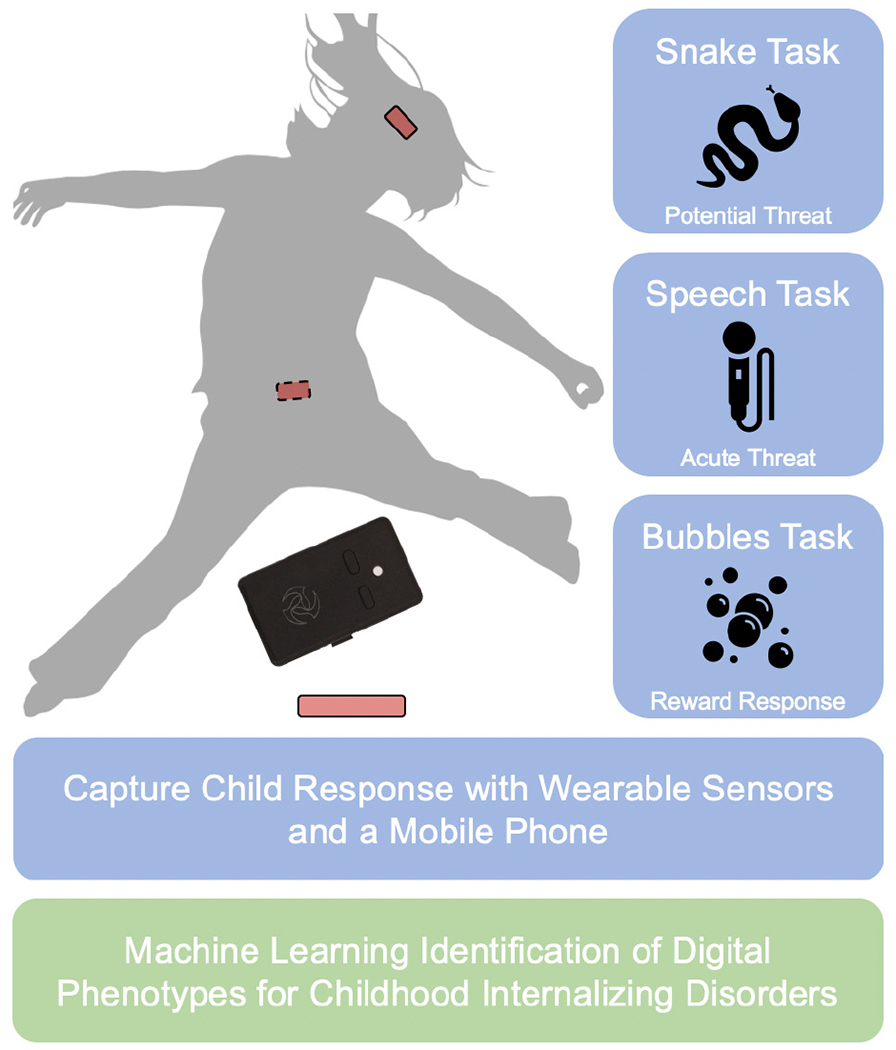
Instrumented mood induction task battery for detecting childhood internalizing disorders from wearable sensor and speech data collected during RDoC Positive and Negative Valence tasks. Each task requires less than three minutes to complete (with some requiring less than a minute). Machine learning analysis of the wearable sensor and speech data collected during each task can provide objective detection of children with underlying internalizing psychopathology.
II. Methods
A. Subjects
Data were collected from children 4 to 8 years old (M: 5.65 years; SD: 0.87) and their primary caregivers (95.2% mothers) in clinical research space at the University of Michigan. To be eligible for the study, participants had to speak fluent English and be between the ages of 3 and 8 with primary caregivers over the age of 18 years recruited from an early childhood psychiatric clinic, past participants from an observational study of peripartum anxiety, and the community. Exclusion criteria included children suspected or diagnosed with a pervasive developmental disorder (e.g., autism), having a serious medical condition, or taking medications that affect the central nervous system. Data are presented for the first time from the Bubbles Task (n=63; 57% female; 75% White, Non-Latinx, 11% Asian or Pacific Islander, 11% African American, 3% Bi-Racial). Data are also presented from two tasks in which we previously published features and accuracy sensitivity and specificity of digital phenotypes for internalizing disorders- the Snake [43] and Speech [44] tasks. Although these three tasks were originally a part of the same overarching study, due to procedure and logistical changes, there was little overlap among participants across tasks (see Fig. 2).
Fig. 2.
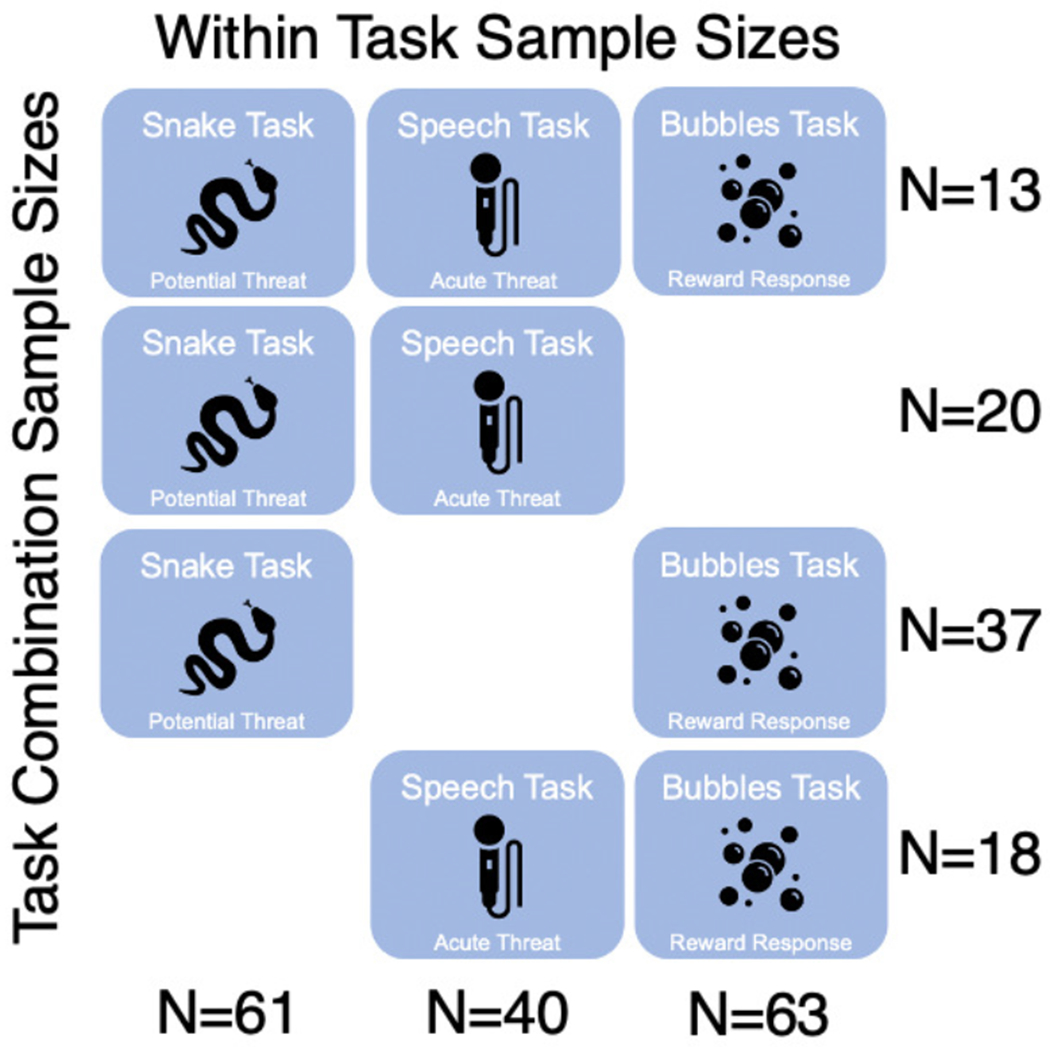
Overlap in subjects between three tasks in this study. Note that within task sample sizes ranged from 40 to 63, but data from only 13 participants were available from all three mood induction tasks.
B. Data Collection Procedure
Subjects and their primary caregivers were brought into the university-based laboratory at the University of Michigan and provided written consent to complete an array of mood induction tasks. Children were instrumented with two belt-worn Inertial Measurement Units (IMUs; 3-Space Sensor, YEI Technology, Portsmouth, OH, USA): one around the head and the other around the waist to characterize movement (Fig. 1), and audio- and video-recorded. Here we consider data from the ‘Bubbles Task’, ‘Snake Task’, and ‘Speech Task’.
The ‘Bubbles Task’, was intended to induce positivity, adapted from the Laboratory Temperament Assessment Battery (Lab-TAB) [39], [67]–[69] and the Autism Diagnostic Observation Schedule [35], [70], [71]. The duration of this task was approximately 180 seconds. During the task, administrators led the subjects into a room with a bubble machine on the table. The administrator turned the bubble machine on and gave scripted statements to encourage positive emotions and behavior such as “Try to pop the bubbles” and “Look at how fun this is”. We conceptualized this task as two distinct phases: 1) the first 30 seconds of the child playing with bubbles (herein referred to as “Initial”); 2) the last 30 seconds of the task (herein referred to as “End”).
In previous published works, we describe two Negative Valence paradigms from which we derived features and developed digital phenotypes to detect internalizing disorders. The first digital phenotype was derived from a task designed to evoke “Potential Threat” – the Snake Task [72], [73]. The ‘Snake Task’ has been shown to induce anxiety in young children [72]–[75], which we conceptualized as the RDoC subconstruct of Potential Threat. The child is led into a novel, dimly lit room while the administrator gives scripted statements to build anticipation and leads the child to the back of the room to an unknown object covered with a blanket. Children wore an inertial measurement unit (IMU) on a belt around their waist to measure their movement. Ten signal features (e.g., range of yaw angle) extracted from the data of 62 participants demonstrated that children with internalizing disorders turned further away and more quickly from the potentially threatening stimulus. A statistical classification model based on these movement data was able to identify children with an internalizing diagnosis with 81% accuracy, 67% sensitivity, 88% specificity, and 0.85 AUC (see Table I; row 9).
TABLE I.
Bubbles Task Decision Tree Performance by Location and Feature Set with Snake and Speech Task Performance on internalizing diagnostic status
| Task | Feature Set | Data | ACC | SEN | SPE | AUC |
|---|---|---|---|---|---|---|
| B | Initial | Head N=48 | 0.58 | 0.43 | 0.65 | 0.47 |
| End | 0.71 | 0.57 | 0.76 | 0.77 | ||
| Initial+Motion | 0.52 | 0.36 | 0.59 | 0.39 | ||
| End+Motion | 0.75 | 0.64 | 0.79 | 0.81 | ||
|
| ||||||
| B | Initial | Waist N=54 | 0.56 | 0.39 | 0.64 | 0.70 |
| End | 0.46 | 0.22 | 0.58 | 0.29 | ||
| Initial+Motion | 0.46 | 0.33 | 0.53 | 0.36 | ||
| End+Motion | 0.52 | 0.11 | 0.72 | 0.29 | ||
|
| ||||||
| Sn | - | Waist N=62 | 0.81 | 0.67 | 0.88 | 0.85 |
| Sp | - | Audio N=42 | 0.80 | 0.54 | 0.93 | 0.75 |
ACC=Accuracy, SEN=Sensitivity, SPE=Specificity, AUC=Area under the receiver operating characteristic (ROC) curve, B=Bubble Task, Sn=Snake Task, Sp=Speech Task. Head and Waist refer to IMU data sampled from those locations. Performance characteristics of models that outperform random chance in permutation testing are bolded.
The next digital phenotype was derived from a task designed to evoke “Acute Threat” – the Speech Task [44]. The ‘Speech Task’ is an adapted version of the Trier Social Stress Task for children (TSST-C), which has been shown to induce fear in children [44], [75, p.], [76] and which we conceptualized as the RDoC subconstruct of Acute Threat. Participants are instructed to tell a three-minute story in which they will be judged based on how interesting it is. A buzzer is used to interrupt the participant throughout the task. Following the task, children are given positive feedback. Eight audio features (e.g., perceptual spectral centroid, spectral flatness) extracted from the data of 42 participants indicate that children with internalizing disorders exhibit low-pitch voices with repeatable speech inflections and content and high-pitched response to surprising stimuli. A statistical classification model based on these audio data was able to identify children with an internalizing diagnosis with 80% accuracy, 54% sensitivity, 93% specificity, and 0.75 AUC (see Table I, row 10).
While at the lab, caregivers also completed parent-report questionnaires and a diagnostic interview. The questionnaire filled out was the Child Behavior Checklist (CBCL) which is widely used and designed to assess child problem behaviors [1]. It is normed by age and gender (T scores), with slightly different versions needed to capture internalizing and externalizing behaviors at different ages (one for ages 1.5 to 5 and another for ages 6 to 18). Items are summed to provide scores in five “problem syndrome scales” (Anxious/Depressed, Somatic Complaints, Withdrawn, Attention Problems, and Aggressive Behavior) four “DSM-oriented problem scales” (Affective, Anxiety, Attention Deficit Hyperactive, and Oppositional Defiant) as well as two broad-band problem scales (Internalizing and Externalizing) that are common across the two versions. Cronbach’s alphas for internalizing and externalizing scales were .88 and .87 for children younger than 6 years and .86 and .85 for children older than 6 years, respectively. The diagnostic interview (Kiddie-Schedule for Affective Disorders and Schizophrenia for School-Aged Children- Preschool Version [77]) was conducted by trained clinical psychology doctoral students or postdoctoral fellows and final diagnostic discussions were supervised by a team of licensed psychiatrists and psychologists. Internalizing diagnoses included depressive disorders, Separation Anxiety Disorder, Specific Phobia, Anxiety (Generalized Anxiety Disorder, and Not Otherwise Specified) and Post-Traumatic Stress Disorder). DSM-4 diagnostic status was determined by clinical consensus across diagnostic team, using all standardized data available.
C. Wearable Sensor Feature Extraction
During the ‘Bubbles Task’, child motion was characterized in terms of IMU-measured acceleration (m/s2) and angular velocity (radians/s) from each of the IMUs. These data were sampled at approximately 300 Hz, down-sampled to 100 Hz, and low-pass filtered using a fourth-order Butterworth IIR filter (cutoff of 20 Hz) in software prior to use. Of the N=63 subjects, N=48 had usable headband data and N=54 had usable waistband data during the bubbles task. Data were unusable if the child removed or turned off the device during the activity, or if the data were not labeled properly once downloaded. While the task was initially intended to be 180 seconds, only 150 seconds of the data were considered to maximize the number of subjects with usable data.
IMU data from the head and waist were separated into two phases during the task: Initial (first 30 seconds) and End (last 30 seconds) and signal features were extracted from the vector magnitude of acceleration and angular velocity. Signal features included mean, root mean square (RMS), skew, kurtosis, range, maximum, minimum, standard deviation, peak to RMS amplitude, signal power within specific frequency bands, and the location and height of peaks in the power spectrum and autocorrelation of the signal. This yielded 29 features for each signal (i.e., acceleration and angular velocity), resulting in a total of 58 features from each of the two phases.
In addition, the data from each sensor were classified into segments of motion (when the child was actively playing with the bubbles) and no motion (when the child stopped for a period of time). Child motion was determined by creating a single envelope of the data by high-pass filtering, full-wave rectifying, and low-pass filtering. Motion periods were then identified as instances where the envelope exceeded a minimum threshold. An additional set of features were extracted over all of the periods of motion for each subject. These features include total duration of motion as well as the average, maximum, minimum, median, standard deviation, skewness and kurtosis of the motion segment durations. This yielded 8 additional features from each signal. When combined with the features extracted from the Initial (Initial+Motion) and End (End+Motion) phases, this yielded a total of 74 features for developing digital phenotypes of childhood internalizing disorders as described next.
D. Digital Phenotype for Internalizing Disorders
A supervised learning approach was used to train binary statistical classification models relating IMU-derived features, from the Initial and End phases, and from the head and waist sensors, to internalizing diagnosis as established via the K-SADS-PL with clinical consensus. Performance of the classifiers was established using a leave-one-subject-out (LOSO) cross validation. This process partitions features from all but one subject (e.g., 47 of 48 for data from the headband IMU) into a training set before converting to z-scores and performing Davies-Bouldin Index [78] based feature selection to yield the 10 features with zero mean and unit variance that best discriminate between diagnostic groups. These features were used to train a decision tree (selected based on visualization of the feature set) for classifying the one remaining test subject as having an internalizing diagnosis or not. The decision threshold used to make this classification was determined from training data using Youden’s J statistic [79] within each iteration of the LOSO-CV. This process was repeated until the diagnosis of each subject had been predicted. This same process was used to establish the performance of classification models trained on the Initial + Motion and End + Motion feature sets.
We leveraged permutation testing to determine if machine learning approaches can be leveraged to develop a digital phenotype for childhood internalizing disorders based on wearable sensor measurements during a positive mood induction task (Aim 1). Specifically, permutation testing identifies which of the models is effectively capturing the relationship between the IMU-derived feature sets and child internalizing psychopathology. We leverage the approach that we have used previously [43], [44] for conducting these tests. Mann-Whitney U-test were used to identify the feature sets (Initial, Sustained, Initial+Motion, End+Motion) and device locations (head, waist) that yield classification models with error rates significantly different from those expected by chance from this dataset.
For models that demonstrated a statistically significant improvement in error rate above random chance, we further characterize model performance. The predicted diagnoses and decision scores from the LOSO cross validation process were used to compute accuracy, sensitivity, and specificity of the models as well as the area under the receiver operating characteristic (ROC) curve (AUC).
E. Comparisons Between Mood Induction Tasks
To determine if a battery of mood induction tasks for developing digital phenotypes of childhood internalizing disorders is indicated (Aim 2), we consider several comparisons between mood induction tasks. Comparisons are designed to examine the overlap between tasks in terms of both the features used as input to the statistical classification models as well as the output of those models. We also place model outputs in the context of patient symptoms and specific internalizing diagnosis. The ultimate goal of this analysis is to determine if each task adds unique information to the problem of detecting children with internalizing psychopathology and should thus be considered collectively.
To examine the overlap between the features extracted from each mood induction task, we consider spearman correlations between the wearable sensor or speech features extracted from each task for overlapping subjects (i.e., Fig. 2). If the tasks are each providing unique information, we would anticipate that there is little correlation between features across tasks (e.g., speech features do not correlate with bubbles features).
To examine the relationship between model outputs and patient symptoms, we consider spearman correlations between model classification scores and CBCL Problem T-Scores (Anxious/Depressive, Somatic, Withdrawn, Attention, Aggression, Affective, Anxiety, ADH, Oppositional, Internalizing, Externalizing). These associations reveal which symptoms could be driving the movement/speech patterns detected by the machine learning derived digital phenotypes. To provide additional context, we also examine the correlation between the CBCL scores and having an internalizing disorder (derived from the clinical interview: yes or no).
Finally, we explore qualitatively which internalizing disorders (e.g., Depressive Disorders, Specific Phobia) are best predicted by each mood induction task. This analysis helps to better understand which subsets of the population of children with internalizing disorders are best served by each of the mood induction tasks. Specific internalizing disorders and their rates of being accurately categorized as true positives are noted for each task.
III. Results
A. Bubbles Task Phenotype Performance
The novel model developed from headband data sampled during the End phase of the bubbles task outperforms the model from the Initial phase in detecting the presence of an internalizing diagnosis (Accuracy: 0.71 vs. 0.58; Sensitivity: 0.57 vs. 0.43; Specificity: 0.76 vs. 0.65; AUC: 0.77 vs. 0.47; see Table I, rows 1 & 2).
As illustrated in Fig. 3, the model developed from the data sampled during the End phase and all motion segments still outperforms the model from the Initial phase with the motion segments (Accuracy: 0.75 vs. 0.52; Sensitivity: 0.64 vs. 0.36; Specificity: 0.79 vs. 0.59; AUC: 0.81 vs 0.39; see Table I, rows 3 & 4). Moreover, the addition of the motion features improves classification performance relative to models trained on the End features alone (Accuracy: 0.75 vs. 0.71; Sensitivity: 0.64 vs. 0.57; Specificity: 0.79 vs. 0.76; AUC: 0.81 vs 0.77; see Table I, rows 2 & 4). In all cases, models based on the head data outperformed those based on waist data for this task (see Table I, rows 5-8). The End+Motion headband model accurately classified 6 of the 14 children with internalizing disorders, and accurately classified 27 of the 34 children without internalizing disorders.
Fig. 3.

Receiver operating characteristic (ROC) curves for the Initial+Motion, End+Motion, Initial, and End feature sets. Notice the significantly larger AUC for models that consider End features, and the slight improvement afforded by also including the Motion features.
B. Comparison Across Mood Induction Tasks
In previous work, we developed digital phenotypes using Negative Valence paradigms, the Snake Task and the Speech Task (see descriptions in Method) and [44], [73]. As demonstrated in Table I, the novel digital phenotype of childhood internalizing disorders derived from the Bubbles task, which presses for the RDoC Positive Valence construct, achieves 75% accuracy, 64% sensitivity, 79% specificity, and 0.81 AUC (row 4). These results are on par with our previously published phenotypes, demonstrating slightly higher AUC and sensitivity than the Speech Task, but lower specificity and accuracy than the Speech or Snake tasks. Notably, these findings were also on par with previously published [73] performance metrics for the standardized and widely used CBCL questionnaire (using both research and clinical cut-offs, T= 55 and 70, respectively) in our Snake Task sample, which exhibited slightly lower classification accuracy (.68-.75), slightly higher specificity (.88–1.00), lower sensitivity (.00-.42), and slightly lower AUCs (.75-.79) .
While task subsamples do not allow effective cross-task comparison of performance characteristics of a task battery (see Fig. 2), they do enable a preliminary examination of the relationship between features extracted from each task. To this end, the pairwise correlation plots of Fig. 4 demonstrate that there is low correlation between features extracted from each task indicating that each task may be identifying unique information not picked up by other tasks (generally, cross task correlations of 0.4 or lower).
Fig. 4.

Feature correlations within (snake: n=62, bubble: n=48, speech n=40) and across the snake and bubble (top left, n=37), snake and speech (top right, n=20), and speech and bubble (bottom left, n=18) tasks. Colors correspond to correlation strength (correlation key in bottom right), black lines denote task feature set boundaries. Note the lack of association between features across tasks.
To further examine the utility of each mood induction task, we extracted posterior probabilities of diagnosis from each statistical classification model. Models developed for the Speech and Snake tasks were logistic regression, allowing easy extraction of these posterior probabilities. For the Bubbles task, which leverages a decision tree, posterior probabilities were computed using Matlab’s embedded approach which is defined as the number of training observations that lead to that node with a diagnosis, divided by the total number of training observations that lead to that node. The posterior probabilities from each task were independently correlated with CBCL T scores from the problem subscales and DSM derived subscales (see Table II). The Bubbles digital phenotype posterior probabilities had by far the most statistically significant associations when compared to the Snake Task or Speech Task phenotypes (9 vs. 4 and 3, respectively, were at least trend-level significant). The Bubbles Task digital phenotype was significantly correlated with all of the CBCL subscales with the exception of Somatic and Withdrawn. The Snake Task digital phenotype was significantly associated with Anxious/Depressive, Withdrawn, Affective, and Oppositional subscales. Similarly, the Speech Task digital phenotype was associated with the Withdrawn, Affective, and Anxiety subscales. Notably, the overlap in associations across tasks seems to be complementary. For example, the Bubbles is not associated with Withdrawn subscale, but the Snake and Speech phenotypes are, and Snake is not associated with the DSM Anxiety score, but Bubbles and Speech are. The Bubbles task in particular also provides several unique associations including with the Externalizing, Internalizing, ADH, Aggression, and Attention subscales. Association between each digital phenotype and the CBCL subscales is important because internalizing disorder (diagnosed with an internalizing disorder or not) derived from the clinical interview is significantly associated with each subscale (Table II).
TABLE II.
Association Between CBCL Problem Scales, Instrumented Mood Induction Task Classification Scores, and Internalizing Diagnosis
| CBCL Scores | Bubbles N=47 | Snake N=58 | Speech N=39 | Diagnosis N=115 |
|---|---|---|---|---|
| Anxious/Depressive | 0.29 * | 0.25 t | 0.17 | 0.40 ** |
| Somatic | 0.22 | 0.02 | 0.11 | 0.27 ** |
| Withdrawn | 0.07 | 0.29 * | 0.31 t | 0.22 * |
| Attention | 0.30 * | −0.03 | 0.02 | 0.22 ** |
| Aggression | 0.33 * | 0.11 | 0.18 | 0.34 ** |
| Affective | 0.36 * | 0.29 * | 0.28 t | 0.40 ** |
| Anxiety | 0.26 t | 0.19 | 0.29 t | 0.40 ** |
| ADH | 0.32 * | 0.03 | 0.03 | 0.29 ** |
| Oppositional | 0.35 * | 0.23 t | 0.15 | 0.35 ** |
| Internalizing | 0.29 * | 0.08 | 0.19 | 0.42 ** |
| Externalizing | 0.37 ** | 0.05 | 0.21 | 0.34 ** |
Significant associations in bold,
p < 0.05,
p < 0.01,
p < 0.1. Ns are slightly lower than in Table I due to a few missing CBCL scores.
Finally, each task phenotype appeared to accurately identify different subtypes of internalizing disorders. The Bubbles Task phenotype accurately identified 3 of 3 children with Anxiety-NOS/GAD, 3 of 3 with PTSD and 2 of 2 with depressive disorders. The Snake Task phenotype accurately identified 5 of 6 children with a Specific Phobia. The Speech Task phenotype accurately identified 3 of 3 children with depressive disorders. No task was able to effectively identify children with Separation Anxiety Disorder (SAD).
IV. Discussion
Here we explore the use of motion data from a three-minute positive mood induction task (playing with bubbles) and machine learning in developing a digital phenotype to identify young children with internalizing disorders. We examine the performance of data derived from the initial and end phases of the task, as well as data derived from the head vs waist-worn sensors. Finally, we conduct a preliminary examination of the relative accuracy of this Positive Valence digital phenotype compared to our previously published Negative Valence digital phenotypes and discuss the promising complementary nature of the tasks in a cumulative assessment battery.
We developed a Positive Valence digital phenotype identifying young children with an internalizing disorder with 75% accuracy (64% sensitivity, 79% specificity). Motion data derived from a head-worn sensor during the last 30 seconds of the Bubbles task outperformed data from the waist-worn sensor and data collected from the initial 30 seconds of the task. Findings support previous literature demonstrating that the RDoC Positive Valence subconstruct “Reward Responsiveness” negatively relates to internalizing disorders in young children (e.g.,[53]–[55]). Specifically, our findings indicate that children with internalizing disorders end the task with less head motion in terms of frequency and intensity when engaging with bubbles than healthy children after 2.5 minutes of continuous play, possibly interpreted as a quicker ‘burn out’ of engagement with the bubbles.
In comparing this Positive Valence Reward Responsiveness task with our previously published works on Negative Valence Potential and Acute Threat tasks, we find several elements suggesting data from each task may complement one another and cumulatively may improve diagnostic accuracy. First, features from each task are weakly correlated, suggesting that each task provides unique data. Second, correlations of task posterior probabilities and CBCL T scores demonstrated each task to identify a different profile of emotional and behavioral problems. Thirdly, task digital phenotypes appear to differentially detect specific internalizing disorders.
Positive Valance Bubble Task digital phenotype was robust in its associations with internalizing and externalizing child problems, and accurately identified depressive, anxious, and trauma-related disorders. These findings suggest the Bubbles phenotype may be characterizing a profile of broad emotional-and behavioral- dysregulation (e.g.,[80]). Associations with heterogenous risk are 1) similar to the broad range of risk associated with the having an internalizing diagnosis derived from a gold-standard clinical interview (see Table II comparing columns “Bubbles” and “Diagnosis) and 2) common in children with internalizing disorders, with heterotypic comorbidity existing in up to 41% of children with an anxiety disorder [81] up to 83% of children with depressive disorders [82] and up to 86% of preschool children screening positive for mood disorders [77]. The Negative Valence Snake Task digital phenotype was correlated with child anxious depressive, withdrawn, and oppositional problems and provided accurate identification of Specific Phobia disorders, suggesting the Snake may be characterizing children with an emotionally reactive threat response (e.g., [83]). The Negative Valence Speech Task digital phenotype was correlated with child withdrawn, anxious and depressive problems and provided accurate identification of children with a depressive diagnosis. These findings suggest the Speech task may be characterizing children with an anhedonic, depressive profile (e.g., [62]).
Finally, tasks appear to have complementary performance characteristics. For instance, despite the Bubbles phenotype’s ability to detect a wider variety of child problems (i.e., CBCL subtypes and Anxiety, PTSD, and depressive diagnoses), it has the least overall accuracy (75% vs 80-81%), likely due to poorer specificity. The Bubbles digital phenotype may represent a common underlying psychological marker of internalizing disorders, whereas the Snake and Speech tasks may add specificity (88-93% vs 79%). Thus, collectively, unique features and differentiated child profiles may provide a more wholistic, accurate detection of internalizing disorders in young children. Notably, phenotype accuracies were on par with previously published performance metrics of the CBCL questionnaire [73] and previously published convergent validity and inter-rater kappas of standardized diagnostic interviews (.80-.90), diagnostic interview screeners (>.70) [77] and significantly better than mean kappas for children according to clinician diagnoses made without standardized diagnostic interviews (.39) [84].
Our small sample size limits statistical power and generalizability of our findings and thus results can only be evaluated as promising evidence to examine in future larger samples. Specifically, our cross-task sample sizes are small (ns=18-37) and literature suggests correlations only stabilize with much larger samples [85], thus this data must be replicated in future studies. Similarly, validation strategies that leverage held-out test data for establishing model performance should be leveraged in future work with larger samples to better establish expected accuracy, sensitivity, and specificity once deployed. Challenges for the future over and above validating this preliminary work in more generalizable samples will be to make the assessment battery more feasible and more sensitive. For instance, using sensors within a smartphone, smart watches, and accompanying app in lieu of external sensors and belts would make this screener more accessible, and potentially inclusive of additional physiological markers (e.g., heart rate, heart rate variability). Additionally, we found none of these three tasks account for identification of SAD, thus the addition of a separation task may enhance internalizing diagnostics given the higher prevalence of SAD in this age group. Taking these limitations and challenges into account, our current data can serve as evidence suggesting the need for future studies that combine the three or more mood induction tasks into an assessment battery which may yield a digital phenotype inheriting the best qualities from each.
V. Conclusion
Overall, preliminary results suggest the promise of combining separate RDoC domain-based paradigms to form a brief assessment battery for providing accurate and objective screening for early childhood internalizing disorders. Our next steps are to validate this assessment battery in a larger, representative sample and to develop a smartphone app to assess the necessary motion and speech recordings in order to attain the digital phenotype more feasibly.
Acknowledgments
This work was supported in part by NIMH under Grant K23-MH080147 (PI: Muzik), in part by the Michigan Institute for Clinical and Health Research (UL1TR000433, PI: Muzik; Biomedical and Social Sciences Scholar Program PI: E.W. McGinnis), in part by the Blue Cross Blue Shield of Michigan Foundation Grant (1982.SAP, PI: E.W. McGinnis), in part by the Brain Behavior Research Foundation (PI: Fitzgerald), and in part by NIMH under Grant R03MH102648 (PI: Fitzgerald; Rosenblum). (E.W. McGinnis and J. Scism are collaborative first authors on this work).
Contributor Information
Ellen W. McGinnis, Department of Child Psychiatry, University of Vermont, Burlington, VT 05405 USA
Jordyn Scism, Department of Electrical and Biomedical Engineering, University of Vermont, Burlington, VT 05405 USA.
Jessica Hruschak, Department of Psychiatry, University of Michigan, Ann Arbor, MI 48109 USA.
Maria Muzik, Department of Psychiatry, University of Michigan, Ann Arbor, MI 48109 USA.
Katherine L. Rosenblum, Department of Psychiatry, University of Michigan, Ann Arbor, MI 48109 USA
Kate Fitzgerald, Department of Psychiatry, University of Michigan, Ann Arbor, MI 48109 USA.
William Copeland, Department of Child Psychiatry, University of Vermont, Burlington, VT 05405 USA.
Ryan S. McGinnis, Department of Electrical and Biomedical Engineering, University of Vermont, Burlington, VT 05405 USA.
References
- [1].Egger HL and Angold A, “Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology,” J. Child Psychol. Psychiatry, vol. 47, no. 3–4, pp. 313–337, April. 2006, doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- [2].Bufferd SJ, Dougherty LR, Carlson GA, and Klein DN, “Parent-reported mental health in preschoolers: findings using a diagnostic interview,” Compr. Psychiatry, vol. 52, no. 4, pp. 359–369, August. 2011, doi: 10.1016/j.comppsych.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Luby JL, Si X, Belden AC, Tandon M, and Spitznagel E, “Preschool depression: Homotypic continuity and course over 24 months,” Arch. Gen. Psychiatry, vol. 66, no. 8, pp. 897–905, 2009, doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].“Internalizing Disorders in Early Childhood: A Review of Depressive and Anxiety Disorders - Child and Adolescent Psychiatric Clinics.” https://www.childpsych.theclinics.com/article/S1056-4993(09)00027-3/abstract (accessed May 28, 2019). [DOI] [PMC free article] [PubMed]
- [5].Luby JL, Belden AC, Pautsch J, Si X, and Spitznagel E, “The clinical significance of preschool depression: Impairment in functioning and clinical markers of the disorder,” J. Affect. Disord, vol. 112, no. 1–3, pp. 111–119, January. 2009, doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Towe-Goodman NR, Franz L, Copeland W, Angold A, and Egger H, “Perceived family impact of preschool anxiety disorders,” J. Am. Acad. Child Adolesc. Psychiatry, vol. 53, no. 4, pp. 437–446, April. 2014, doi: 10.1016/j.jaac.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bufferd SJ, Dougherty LR, Carlson GA, Rose S, and Klein DN, “Psychiatric disorders in preschoolers: continuity from ages 3 to 6,” Am. J. Psychiatry, vol. 169, no. 11, pp. 1157–1164, November. 2012, doi: 10.1176/appi.ajp.2012.12020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Belden AC, Gaffrey MS, and Luby JL, “Relational Aggression in Children With Preschool-Onset Psychiatric Disorders,” J. Am. Acad. Child Adolesc. Psychiatry, vol. 51, no. 9, pp. 889–901, September. 2012, doi: 10.1016/j.jaac.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Whalen DJ, Sylvester CM, and Luby JL, “Depression and Anxiety in Preschoolers: A Review of the Past 7 Years,” Child Adolesc. Psychiatr. Clin. N. Am, vol. 26, no. 3, pp. 503–522, July. 2017, doi: 10.1016/j.chc.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wittchen H-U and Sonntag H, “Nicotine consumption in mental disorders: a clinical epidemiological perspective,” Eur. Neuropsychopharmacol, no. 10, p. 119, 2000.10706993 [Google Scholar]
- [11].Compton SN, Burns BJ, Helen LE, and Robertson E, “Review of the evidence base for treatment of childhood psychopathology: internalizing disorders,” J. Consult. Clin. Psychol, vol. 70, no. 6, pp. 1240–1266, December. 2002. [DOI] [PubMed] [Google Scholar]
- [12].Bittner A, Egger HL, Erkanli A, Jane Costello E, Foley DL, and Angold A, “What do childhood anxiety disorders predict?,” J. Child Psychol. Psychiatry, vol. 48, no. 12, pp. 1174–1183, December. 2007, doi: 10.1111/j.1469-7610.2007.01812.x. [DOI] [PubMed] [Google Scholar]
- [13].Beesdo K et al. , “Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life,” Arch. Gen. Psychiatry, vol. 64, no. 8, pp. 903–912, August. 2007, doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- [14].Cole DA, Peeke LG, Martin JM, Truglio R, and D A, “A longitudinal look at the relation between depression and anxiety in children and adolescents,” J. Consult. Clin. Psychol, vol. 66, no. 3, pp. 451–460, 1998, doi: 10.1037/0022-006X.66.3.451. [DOI] [PubMed] [Google Scholar]
- [15].Gould MS et al. , “Psychopathology Associated With Suicidal Ideation and Attempts Among Children and Adolescents,” J. Am. Acad. Child Adolesc. Psychiatry, vol. 37, no. 9, pp. 915–923, September. 1998, doi: 10.1097/00004583-199809000-00011. [DOI] [PubMed] [Google Scholar]
- [16].Craske MG and Stein MB, “Anxiety,” The Lancet, vol. 388, no. 10063, pp. 3048–3059, December. 2016, doi: 10.1016/S0140-6736(16)30381-6. [DOI] [PubMed] [Google Scholar]
- [17].Rapee RM, Schniering CA, and Hudson JL, “Anxiety Disorders During Childhood and Adolescence: Origins and Treatment,” Annu. Rev. Clin. Psychol, vol. 5, no. 1, pp. 311–341, 2009, doi: 10.1146/annurev.clinpsy.032408.153628. [DOI] [PubMed] [Google Scholar]
- [18].Konnopka A, Leichsenring F, Leibing E, and König H-H, “Cost-of-illness studies and cost-effectiveness analyses in anxiety disorders: A systematic review,” J. Affect. Disord, vol. 114, no. 1, pp. 14–31, April. 2009, doi: 10.1016/j.jad.2008.07.014. [DOI] [PubMed] [Google Scholar]
- [19].Weitzman C, Wegner L, and C. on P. A. of C. and F. H.the SECTION ON DEVELOPMENTAL AND BEHAVIORAL PEDIATRICS, “Promoting Optimal Development: Screening for Behavioral and Emotional Problems,” Pediatrics, vol. 135, no. 2, pp. 384–395, February. 2015, doi: 10.1542/peds.2014-3716. [DOI] [PubMed] [Google Scholar]
- [20].Kataoka SH, Zhang L, and Wells KB, “Unmet Need for Mental Health Care Among U.S. Children: Variation by Ethnicity and Insurance Status,” Am. J. Psychiatry, vol. 159, no. 9, pp. 1548–1555, September. 2002, doi: 10.1176/appi.ajp.159.9.1548. [DOI] [PubMed] [Google Scholar]
- [21].Lavigne JV et al. , “Psychopathology and health care use among preschool children: a retrospective analysis,” J. Am. Acad. Child Adolesc. Psychiatry, vol. 37, no. 3, pp. 262–270, March. 1998, doi: 10.1097/00004583-199803000-00010. [DOI] [PubMed] [Google Scholar]
- [22].Kaminer Y, Feinstein C, and Seifer R, “Is there a need for observationally based assessment of affective symptomatology in child and adolescent psychiatry?,” Adolescence, vol. 30, no. 118, pp. 483–489, 1995. [PubMed] [Google Scholar]
- [23].Chansky TE and Kendall PC, “Social expectancies and self-perceptions in anxiety-disordered children,” J. Anxiety Disord, vol. 11, no. 4, pp. 347–363, August. 1997. [DOI] [PubMed] [Google Scholar]
- [24].Kolko DJ and Kazdin AE, “Emotional/behavioral problems in clinic and nonclinic children: Correspondence among child, parent and teacher reports,” J. Child Psychol. Psychiatry, vol. 34, no. 6, pp. 991–1006, 1993, doi: 10.1111/j.1469-7610.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- [25].Renouf AG and Kovacs M, “Concordance between mothers’ reports and children’s self-reports of depressive symptoms: A longitudinal study,” J. Am. Acad. Child Adolesc. Psychiatry, vol. 33, no. 2, pp. 208–216, February. 1994, doi: 10.1097/00004583-199402000-00008. [DOI] [PubMed] [Google Scholar]
- [26].Conway CC et al. , “A Hierarchical Taxonomy of Psychopathology Can Transform Mental Health Research,” Perspect. Psychol. Sci. J. Assoc. Psychol. Sci, vol. 14, no. 3, pp. 419–436, 2019, doi: 10.1177/1745691618810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ruggero CJ et al. , “Integrating the Hierarchical Taxonomy of Psychopathology (HiTOP) into Clinical Practice,” J. Consult. Clin. Psychol, vol. 87, no. 12, pp. 1069–1084, December. 2019, doi: 10.1037/ccp0000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Achenbach TM and Rescorla LA, “ASEBA School Age Forms and Profiles,” Burlingt. Vt ASEBA, 2001, Accessed: Jan. 21, 2013. [Online]. Available: http://www.childbehaviorchecklist.com/ordering/ASEBA%20Reliability%20and%20Validity-School%20Age.pdf.
- [29].Cuthbert BN and Insel TR, “Toward the future of psychiatric diagnosis: the seven pillars of RDoC,” BMC Med, vol. 11, p. 126, 2013, doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fernandez KC, Jazaieri H, and Gross JJ, “Emotion Regulation: A Transdiagnostic Perspective on a New RDoC Domain,” Cogn. Ther. Res, pp. 1–15, March. 2016, doi: 10.1007/s10608-016-9772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Biederman J et al. , “Developmental trajectories of anxiety disorders in offspring at high risk for panic disorder and major depression,” Psychiatry Res, vol. 153, no. 3, pp. 245–252, December. 2007, doi: 10.1016/j.psychres.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Torous J, Rodriguez J, and Powell A, “The New Digital Divide For Digital BioMarkers,” Digit. Biomark, vol. 1, no. 1, pp. 87–91, September. 2017, doi: 10.1159/000477382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vasey MW and Lonigan CJ, “Considering the clinical utility of performance-based measures of childhood anxiety,” J. Clin. Child Psychol, vol. 29, no. 4, pp. 493–508, 2000. [DOI] [PubMed] [Google Scholar]
- [34].Mash EJ and Foster SL, “Exporting analogue behavioral observation from research to clinical practice: useful or cost-defective?,” Psychol. Assess, vol. 13, no. 1, pp. 86–98, March. 2001. [DOI] [PubMed] [Google Scholar]
- [35].Lord C et al. , “The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism,” J. Autism Dev. Disord, vol. 30, no. 3, pp. 205–223, June. 2000. [PubMed] [Google Scholar]
- [36].Simpson HB, “The RDoC Project: A New Paradigm for Investigating The Pathophysiology of Anxiety,” Depress. Anxiety, vol. 29, no. 4, pp. 251–252, April. 2012, doi: 10.1002/da.21935. [DOI] [PubMed] [Google Scholar]
- [37].Durbin CE, Hayden EP, Klein DN, and Olino TM, “Stability of laboratory-assessed temperamental emotionality traits from ages 3 to 7,” Emot. Wash. DC, vol. 7, no. 2, pp. 388–399, May 2007, doi: 10.1037/1528-3542.7.2.388. [DOI] [PubMed] [Google Scholar]
- [38].Moser JS, Durbin CE, Patrick CJ, and Schmidt NB, “Combining Neural and Behavioral Indicators in the Assessment of Internalizing Psychopathology in Children and Adolescents,” J. Clin. Child Adolesc. Psychol. Off. J. Soc. Clin. Child Adolesc. Psychol. Am. Psychol. Assoc. Div 53, January. 2014, doi: 10.1080/15374416.2013.865191. [DOI] [PubMed] [Google Scholar]
- [39].Gagne JR, Van Hulle CA, Aksan N, Essex MJ, and Goldsmith HH, “Deriving childhood temperament measures from emotion-eliciting behavioral episodes: scale construction and initial validation,” Psychol. Assess, vol. 23, no. 2, pp. 337–353, June. 2011, doi: 10.1037/a0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Durbin CE, “Validity of young children’s self-reports of their emotion in response to structured laboratory tasks,” Emot. Wash. DC, vol. 10, no. 4, pp. 519–535, August. 2010, doi: 10.1037/a0019008. [DOI] [PubMed] [Google Scholar]
- [41].Durbin CE, Klein DN, Hayden EP, Buckley ME, and Moerk KC, “Temperamental emotionality in preschoolers and parental mood disorders,” J. Abnorm. Psychol, vol. 114, no. 1, pp. 28–37, February. 2005, doi: 10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- [42].Olino TM, Klein DN, Dyson MW, Rose SA, and Durbin CE, “Temperamental emotionality in preschool-aged children and depressive disorders in parents: associations in a large community sample,” J. Abnorm. Psychol, vol. 119, no. 3, pp. 468–478, August. 2010, doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].McGinnis RS et al. , “Rapid detection of internalizing diagnosis in young children enabled by wearable sensors and machine learning,” PLOS ONE, vol. 14, no. 1, p. e0210267, January. 2019, doi: 10.1371/journal.pone.0210267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McGinnis EW et al. , “Giving Voice to Vulnerable Children: Machine Learning Analysis of Speech Detects Anxiety and Depression in Early Childhood,” IEEE J. Biomed. Health Inform, pp. 1–1, 2019, doi: 10.1109/JBHI.2019.2913590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McGinnis RS et al. , “Wearable Sensors and Machine Learning Diagnose Anxiety and Depression in Young Children,” presented at the 2018 IEEE International Conference on Biomedical and Health Informatics (BHI), Las Vegas, NV, Mar. 2018. [Google Scholar]
- [46].McGinnis RS et al. , “Rapid Anxiety and Depression Diagnosis in Young Children Enabled by Wearable Sensors and Machine Learning,” in 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jul. 2018, pp. 3983–3986, doi: 10.1109/EMBC.2018.8513327. [DOI] [PubMed] [Google Scholar]
- [47].Sloan DM, Strauss ME, and Wisner KL, “Diminished response to pleasant stimuli by depressed women,” J. Abnorm. Psychol, vol. 110, no. 3, pp. 488–493, 2001, doi: 10.1037/0021-843X.110.3.488. [DOI] [PubMed] [Google Scholar]
- [48].Suslow T, Dannlowski U, Lalee-Mentzel J, Donges U-S, Arolt V, and Kersting A, “Spatial processing of facial emotion in patients with unipolar depression: a longitudinal study,” J. Affect. Disord, vol. 83, no. 1, pp. 59–63, November. 2004, doi: 10.1016/j.jad.2004.03.003. [DOI] [PubMed] [Google Scholar]
- [49].Henriques JB, Glowacki JM, and Davidson RJ, “Reward fails to alter response bias in depression,” J. Abnorm. Psychol, vol. 103, no. 3, pp. 460–466, 1994, doi: 10.1037/0021-843X.103.3.460. [DOI] [PubMed] [Google Scholar]
- [50].Hayden EP, Klein DN, Durbin CE, and Olino TM, “Positive emotionality at age 3 predicts cognitive styles in 7-year-old children,” Dev. Psychopathol, vol. 18, no. 2, pp. 409–423, 2006, doi: 10.1017/S0954579406060226. [DOI] [PubMed] [Google Scholar]
- [51].“Striatal Functional Alteration During Incentive Anticipation in Pediatric Anxiety Disorders | American Journal of Psychiatry.” https://ajp.psychiatryonline.org/doi/full/10.1176/appi.ajp.2011.11010006 (accessed May 27, 2020). [DOI] [PMC free article] [PubMed]
- [52].Sm H et al. , “Striatal Responses to Negative Monetary Outcomes Differ Between Temperamentally Inhibited and Non-Inhibited Adolescents,” Neuropsychologia, February. 2011. https://pubmed.ncbi.nlm.nih.gov/21167189/?dopt=Abstract (accessed May 27, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Forbes EE et al. , “Healthy Adolescents’ Neural Response to Reward: Associations With Puberty, Positive Affect, and Depressive Symptoms,” J. Am. Acad. Child Adolesc. Psychiatry, vol. 49, no. 2, pp. 162–172.e5, February. 2010, doi: 10.1016/j.jaac.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].“Neural reactivity to monetary rewards and losses differentiates social from generalized anxiety in children - Kessel - 2015 - Journal of Child Psychology and Psychiatry - Wiley Online Library.” https://acamh.onlinelibrary.wiley.com/doi/full/10.1111/jcpp.12355?casa_token=IqWHG8WRalQAAAAA%3Ajq7zXw6zooZA7kQ6X_cppasYD8jCzBgoKg6c0gCfOEHtnMxqFcKUCwVwRuYwuek6MJBC0LgWhbBRiIQa (accessed May 27, 2020). [DOI] [PMC free article] [PubMed]
- [55].Silk JS, Shaw DS, Forbes EE, Lane TL, and Kovacs M, “Maternal Depression and Child Internalizing: The Moderating Role of Child Emotion Regulation,” J. Clin. Child Adolesc. Psychol, vol. 35, no. 1, pp. 116–126, February. 2006, doi: 10.1207/s15374424jccp3501_10. [DOI] [PubMed] [Google Scholar]
- [56].Silk JS, Shaw DS, Skuban EM, Oland AA, and Kovacs M, “Emotion regulation strategies in offspring of childhood-onset depressed mothers,” J. Child Psychol. Psychiatry, vol. 47, no. 1, pp. 69–78, 2006, doi: 10.1111/j.1469-7610.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- [57].“NIMH » RDoC Matrix.” https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/constructs/rdoc-matrix.shtml (accessed Sep. 17, 2020).
- [58].Weiss NH, Gratz KL, and Lavender JM, “Factor Structure and Initial Validation of a Multidimensional Measure of Difficulties in the Regulation of Positive Emotions: The DERS-Positive,” Behav. Modif, vol. 39, no. 3, pp. 431–453, May 2015, doi: 10.1177/0145445514566504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, and Carter CS, “Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals,” Biol. Psychiatry, vol. 51, no. 9, pp. 693–707, May 2002, doi: 10.1016/S0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- [60].Baji I et al. , “[Symptoms of depression in children and adolescents in relation to psychiatric comorbidities],” Psychiatr. Hung. Magy. Pszichiátriai Társ. Tudományos Folyóirata, vol. 27, no. 2, pp. 115–126, 2012. [PubMed] [Google Scholar]
- [61].McMakin DL et al. , “Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression,” J. Am. Acad. Child Adolesc. Psychiatry, vol. 51, no. 4, pp. 404–411, April. 2012, doi: 10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Luby JL, Mrakotsky C, Heffelfinger A, Brown K, and Spitznagel E, “Characteristics of Depressed Preschoolers With and Without Anhedonia: Evidence for a Melancholic Depressive Subtype in Young Children,” Am. J. Psychiatry, vol. 161, no. 11, pp. 1998–2004, November. 2004, doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- [63].Shankman SA, Tenke CE, Bruder GE, Durbin CE, Hayden EP, and Klein DN, “Low positive emotionality in young children: association with EEG asymmetry,” Dev. Psychopathol, vol. 17, no. 1, pp. 85–98, 2005, doi: 10.1017/s0954579405050054. [DOI] [PubMed] [Google Scholar]
- [64].Vogel AC, Jackson JJ, Barch DM, Tillman R, and Luby JL, “EXCITABILITY AND IRRITABILITY IN PRESCHOOLERS PREDICTS LATER PSYCHOPATHOLOGY: THE IMPORTANCE OF POSITIVE AND NEGATIVE EMOTION DYSREGULATION,” Dev. Psychopathol, vol. 31, no. 3, pp. 1067–1083, August. 2019, doi: 10.1017/S0954579419000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang M and Saudino KJ, “Positive affect: phenotypic and etiologic associations with prosocial behaviors and internalizing problems in toddlers,” Front. Psychol, vol. 6, p. 416, 2015, doi: 10.3389/fpsyg.2015.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zahn-Waxler C, Klimes-Dougan B, and Slattery MJ, “Internalizing problems of childhood and adolescence: prospects, pitfalls, and progress in understanding the development of anxiety and depression,” Dev. Psychopathol, vol. 12, no. 3, pp. 443–466, 2000. [PubMed] [Google Scholar]
- [67].Burnson C, Poehlmann J, and Schwichtenberg AJ, “Effortful control, positive emotional expression, and behavior problems in children born preterm,” Infant Behav. Dev, vol. 36, no. 4, pp. 564–574, December. 2013, doi: 10.1016/j.infbeh.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Goldsmith HH and Lemery KS, “Linking temperamental fearfulness and anxiety symptoms: A behavior–genetic perspective,” Biol. Psychiatry, vol. 48, no. 12, pp. 1199–1209, December. 2000, doi: 10.1016/S0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- [69].Louie JY, Wang S, Fung J, and Lau A, “Children’s emotional expressivity and teacher perceptions of social competence: A cross-cultural comparison,” Int. J. Behav. Dev, vol. 39, no. 6, pp. 497–507, 2015. [Google Scholar]
- [70].Hurwitz S and Yirmiya N, “Autism diagnostic observation schedule (ADOS) and its uses in research and practice,” Compr. Guide Autism, pp. 345–353, 2014. [Google Scholar]
- [71].Luyster R et al. , “The Autism Diagnostic Observation Schedule—Toddler Module: A New Module of a Standardized Diagnostic Measure for Autism Spectrum Disorders,” J. Autism Dev. Disord, vol. 39, no. 9, pp. 1305–1320, September. 2009, doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McGinnis EW et al. , “Wearable sensors detect childhood internalizing disorders during mood induction task,” PloS One, vol. 13, no. 4, p. e0195598, 2018, doi: 10.1371/journal.pone.0195598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].McGinnis RS et al. , “Rapid detection of internalizing diagnosis in young children enabled by wearable sensors and machine learning,” PLOS ONE, vol. 14, no. 1, p. e0210267, January. 2019, doi: 10.1371/journal.pone.0210267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Calkins SD, Graziano PA, Berdan LE, Keane SP, and Degnan KA, “Predicting cardiac vagal regulation in early childhood from maternal-child relationship quality during toddlerhood,” Dev. Psychobiol, vol. 50, no. 8, pp. 751–766, December. 2008, doi: 10.1002/dev.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lopez-Duran NL, Hajal NJ, Olson SL, Felt BT, and Vazquez DM, “Individual differences in cortisol responses to fear and frustration during middle childhood,” J. Exp. Child Psychol, vol. 103, no. 3, pp. 285–295, July. 2009, doi: 10.1016/j.jecp.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, and Hellhammer D, “Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis,” Psychosom. Med, vol. 59, no. 4, pp. 419–426, August. 1997. [DOI] [PubMed] [Google Scholar]
- [77].Birmaher B et al. , “Schedule for affective disorders and schizophrenia for school-age children (K-SADS-PL) for the assessment of preschool children--a preliminary psychometric study,” J. Psychiatr. Res, vol. 43, no. 7, pp. 680–686, April. 2009, doi: 10.1016/j.jpsychires.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Davies DL and Bouldin DW, “A Cluster Separation Measure,” IEEE Trans. Pattern Anal. Mach. Intell, vol. PAMI-1, no. 2, pp. 224–227, April. 1979, doi: 10.1109/TPAMI.1979.4766909. [DOI] [PubMed] [Google Scholar]
- [79].Youden WJ, “Index for rating diagnostic tests,” Cancer, vol. 3, no. 1, pp. 32–35, January. 1950, doi: . [DOI] [PubMed] [Google Scholar]
- [80].Althoff RR, Verhulst FC, Rettew DC, Hudziak JJ, and van der Ende J, “Adult outcomes of childhood dysregulation: a 14-year follow-up study,” J. Am. Acad. Child Adolesc. Psychiatry, vol. 49, no. 11, pp. 1105–1116, November. 2010, doi: 10.1016/j.jaac.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Canals J, Voltas N, Hernández-Martínez C, Cosi S, and Arija V, “Prevalence of DSM-5 anxiety disorders, comorbidity, and persistence of symptoms in Spanish early adolescents,” Eur. Child Adolesc. Psychiatry, vol. 28, no. 1, pp. 131–143, January. 2019, doi: 10.1007/s00787-018-1207-z. [DOI] [PubMed] [Google Scholar]
- [82].Angold A and Costello EJ, “Depressive comorbidity in children and adolescents: Empirical, theoretical, and methodological issues,” Am. J. Psychiatry, vol. 150, no. 12, pp. 1779–1791, December. 1993. [DOI] [PubMed] [Google Scholar]
- [83].Kashani JH, Deuser W, and Reid JC, “Aggression and Anxiety: A New Look at an Old Notion,” J. Am. Acad. Child Adolesc. Psychiatry, vol. 30, no. 2, pp. 218–223, March. 1991, doi: 10.1097/00004583-199103000-00009. [DOI] [PubMed] [Google Scholar]
- [84].Rettew DC, Lynch AD, Achenbach TM, Dumenci L, and Ivanova MY, “Meta-analyses of agreement between diagnoses made from clinical evaluations and standardized diagnostic interviews,” Int. J. Methods Psychiatr. Res, vol. 18, no. 3, pp. 169–184, September. 2009, doi: 10.1002/mpr.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Schönbrodt FD and Perugini M, “At what sample size do correlations stabilize?,” J. Res. Personal, vol. 47, pp. 609–612, 2013. [Google Scholar]


