Abstract
Purpose
Advanced breast cancer is a heterogeneous disease with several well-defined subtypes, among which, hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2–) is most prevalent. Determination of HR and HER2 status influences prognosis and, thus, disease management. Although literature on these prognostic factors exist, especially in the early breast cancer setting, it remains unclear to what extent these factors can guide clinical decision-making in the advanced disease setting. Therefore, we sought to identify the strength and consistency of evidence for prognostic factors in patients with HR+/HER2– advanced breast cancer.
Methods
A systematic literature review (SLR) of the major electronic databases was conducted in November 2018 for primary research studies published since 2010. Endpoints of interest were tumor response, progression-free survival (PFS), overall survival (OS), and breast cancer-specific survival (BCSS).
Results
Seventy-nine studies were included wherein all patients were diagnosed with advanced breast cancer and ≥50% of the population were HR+/HER2–. OS was the most commonly assessed endpoint (n=67) followed by PFS (n=33), BCSS (n=5) and tumor response (n=3). The prognostic factors with strongest evidence of association with worse OS were negative progesterone receptor status, higher tumor grade, higher circulating tumor cell (CTC) count and higher Ki67 level, number of metastatic sites (eg multiple vs single) and sites of metastases (eg presence of liver metastases vs absence), shorter time to recurrence or progression to advanced breast cancer, poor performance status, prior therapy attributes in the early or metastatic setting (type of therapy, treatment line, response of prior therapy), and race (black vs white). The prognostic factors that had strongest evidence of association with PFS included CTC count, number and sites of metastases, and absence of prior therapy or higher lines of therapy in the early or metastatic setting. The directionality of association was consistent for all prognostic factors except between lymph node and OS, and de novo metastatic breast cancer and PFS.
Conclusion
Multiple disease, treatment, and patient-related prognostic factors impact survival, particularly OS, in patients with HR+/HER2– advanced breast cancer. Treatment outcomes can vary considerably due to these factors. Understanding poorer prognostic factors for patients can result in improved clinical decision-making.
Keywords: advanced breast cancer, prognostic factors, survival
Introduction
Advances in screening and treatment paradigms for breast cancer has led to an overall decline in mortality rate in the past decade.1 The survival rate depends on stage of breast cancer at diagnosis, among other factors.2 The five-year survival rate for patients diagnosed with Stage IV breast cancer is 22%, for Stage III is 72% and Stage II is >90%.3 Clinical decision-making in breast cancer management relies on determination of receptor status, as therapies have been developed that specifically benefit patients depending on hormone receptor (HR) and human epidermal growth factor (HER2) receptor status.4–6 HR+/HER2– status is the most common molecular subtype, accounting for two-thirds of US female breast cancer cases.7–9
In addition to advancements in treatment options over time, prognosis of breast cancer is influenced by factors that indicate growth, invasion, and metastatic potential of disease, thereby informing disease course and clinical outcome.4 The HR+/HER2– subtype has been associated with improved survival compared with other subtypes in the metastatic setting, also indicating some prognostic relationship between survival and receptor status.4,10 Amongst HR+/HER2– subtype, survival is influenced by other disease-related factors such as tumor grade, site of the metastasis (eg bone, liver, lung, or brain), prior therapy, as well as patient-related factors (eg age, race).11,12
Although several studies have identified prognostic factors associated with survival, especially in the early breast cancer setting,13–15 it remains unclear to what extent these factors impact prognosis in advanced breast cancer. Currently, there is no comprehensive summary assessing the collective available evidence and the strength of evidence for these prognostic factors among patients with HR+/HER2– advanced breast cancer that can aid clinical decision-making. Therefore, we conducted a systematic literature review (SLR) based on a pre-specified protocol to identify the prognostic factors associated with survival endpoints in patients with HR+/HER2– advanced breast cancer and qualitatively assess the evidence and its strength and consistency.
Method
Data Sources and Search Strategies
A SLR was conducted and reported in accordance with guidelines established by the Centre for Reviews and Dissemination (CRD),16 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,17 and Cochrane guidebook.18 Comprehensive searches were conducted in major electronic databases (MEDLINE, EMBASE, and Cochrane Controlled Register of Trials) to identify primary research studies published between January 1, 2010 and November 15, 2018. These were supplemented by searches of relevant conference proceedings (American Society of Clinical Oncology, European Society for Medical Oncology, European Cancer Organization, European Cancer Summit, Improving Care and Knowledge through Translational Research Breast Cancer Conference, The International Consensus Conference for Advanced Breast Cancer, San Antonio Breast Cancer Symposium, and American Association for Cancer Research) held in the two prior years to identify abstracts of interest. The primary publications related to the conference abstracts were searched. Relevant SLRs published recently were cross-checked to find additional studies. The search strategy was designed to include an extensive list of search terms (including MeSH/Emtree terms and natural language terms) which were broadly grouped into: 1) HR+/HER2- breast cancer, 2) advanced disease stage, 3) prognostic factors, 4) outcomes—including tumor response, also referred to as objective response or clinical benefit, progression-free survival (PFS), overall survival (OS), and breast cancer-specific survival (BCSS). Disease terms included a combination of terms to identify “advanced stage” breast cancer in combination with terms specific to “HR+/HER2-” status.
Study Selection and Data Extraction
Patients with HR+/HER2- advanced breast cancer were the population of interest for this SLR. However, there were limited studies that included this patient population exclusively. Besides, the proportion of patients with HR+/HER2- subtype widely varied across studies. Hence we decided to exclude studies where <50% of patients were either HR+ or HER2–. Since the proportion of patients with advanced/metastatic breast cancer also varied across studies, we included studies where ≥80% of patients were diagnosed with advanced breast cancer. These eligibility criteria allowed for inclusion of studies with the population of interest, thus striking a balance between validity and generalizability of the review. Observational studies with sample size of ≥300 patients and RCTs with sample size of ≥300 patients were eligible for inclusion. Editorials, letters, commentaries, reviews, invitro-studies, and non-English publications were excluded. Since “prognostic” and “predictive” terms are used, sometimes incorrectly as interchangeable in literature,19 we excluded studies that reported the interaction p-value between a factor and treatment – indicative of predictive association.
After removing duplicates, two reviewers independently screened abstracts and full-texts for eligibility. Disagreements were resolved by consensus or by a third reviewer. A single reviewer extracted all data, and a separate reviewer independently validated extracted data.
Evidence Assessment
Strength of evidence was determined in terms of consistency of evidence, directionality of association, use of multivariable analyses, and strength of association based on effect size. If >50% of studies that assessed an association found it to be significant, then evidence was considered consistent. Similarly, if the direction of association was the same in >50% of studies that demonstrated a significant association, then directionality of association was deemed consistent. For example, negative progesterone receptor status was associated with worse survival in 100% of studies that reported a significant relationship. Based on hazard ratios (HR) calculated in univariate and multivariate analyses, the strength of associations was categorized as strong (HR≥3), moderate (HR=1.5–2.9), or weak (HR<1.5).20
Prognostic factors satisfying all the following criteria were deemed to have the strongest evidence of association with OS or PFS: i) consistency of evidence; ii) consistency in the direction of association; iii) at least >5 studies demonstrating a significant association. For example, circulating tumor cell (CTC) count showed the strongest evidence of association with OS in nine out of 10 studies (ie, achieved consistency based on >50% studies with a significant association) and showed consistency in direction of association as well as strength of association based on effect size. The Quality In Prognosis Studies (QUIPS) risk of bias assessment tool was used to assess study quality.21 Based on our understanding of the literature base and variability expected in the patient population and study design, we did not plan to conduct a meta-analysis of the relationship between prognostic factors and survival endpoints.
Results
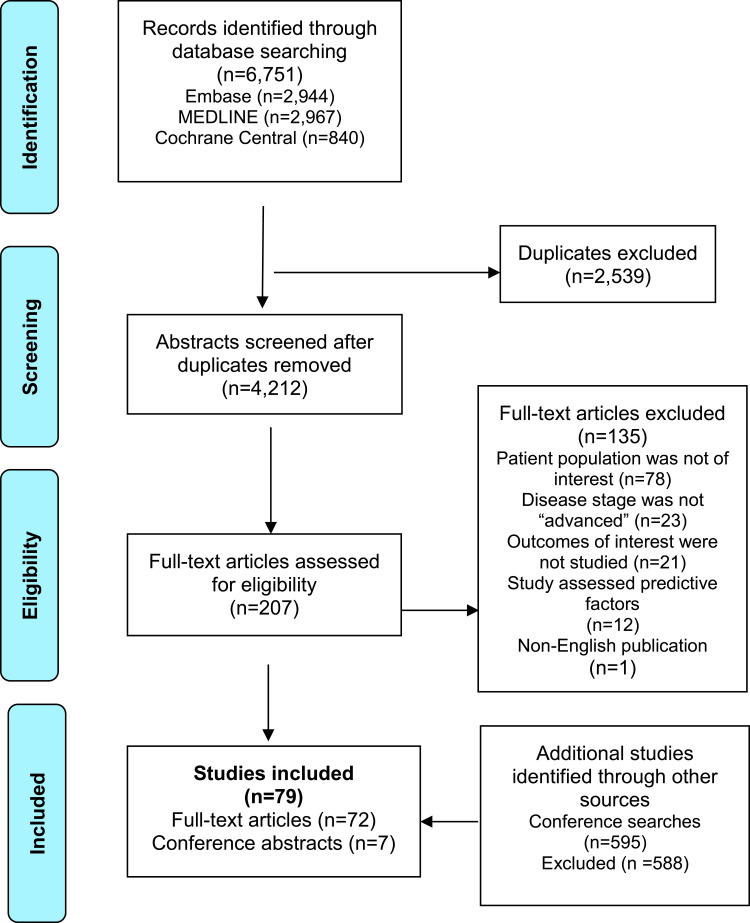
The PRISMA flow diagram summarizes the review (Figure 1). Overall, the SLR included 72 full-text articles and seven conference abstracts (Table 1).10,22–98 The studies identified included retrospective data analyses (71%), prospective cohort studies (16.5%), studies with both retrospective and prospective data collections (2.5%), randomized controlled trials (RCTs) or clinical trials (7.5%), and post-hoc analyses of RCTs (2.5%). OS was the most commonly assessed endpoint (n=67), followed by PFS (n=33), while BCSS (n=5) and tumor response (n=3) were assessed less frequently. The majority of studies were conducted in Europe (n=38), followed by North America (n=15), Asia (n=18), Northern Africa (n=1), the Middle East (n=1), and five studies were multinational. One study did not report study location.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for study selection.
Table 1.
Studies Included in the Systematic Literature Review
| Study [Ref] | Population in Prognostic Analysis | Sample Size (N) | Age (in Years) | HR + (%) |
ER + (%) |
PR + (%) |
HER2– (%) |
Category by HR+/HER2– Status (%) |
Description of BC |
|---|---|---|---|---|---|---|---|---|---|
| Arciero et al (2017)22 |
HR+/HER2– subgroup | 106,036 | Age at diagnosis, Mean (SD), White: 62.4 (13.6); and African American:58.9 (13.5) | 100 | NR | NR | 100 | ≥80 | Stage III and IV |
| Aversa et al (2014)61 |
Overall sample=488 Analysis conducted in subgroup of patients with CNS metastases (n=115) |
488 | Age at first BC diagnosis, Median (range): 51 (22–83) Age at first metastatic recurrence, median (range): 55 (24–85) |
67.8 | 66.2 | 49.7 | 68 | 50–79 | mBC with patients starting first-line chemotherapy |
| Ayala de la Pena et al (2017) (CA)62 |
Overall study population | 265 | Median (range): 59 (19–95) | 76 | NR | NR | 73 | 50–79 | mBC |
| Beije et al (2016)77 |
Overall study population | 154 | Age at inclusion, Median (range) ET: 67 (37–88); and CT: 61 (33–85) | NR | 83.7 | NR | 100 | ≥80 | mBC |
| Bertaut et al (2015)88 |
Overall study population | 232 | Age at diagnosis, Mean (SD): 64.7 (14.7) | 84 | NR | NR | 81 | ≥80 | Stage IV mBC |
| Bonechi et al (2018)64 |
Overall study population | 31 | Median (range): 64 (37–83) | 100 | 93.7 | 81.2 | 100 | ≥80 | mBC |
| Bonotto et al (2017)30 |
Overall study population | 446 | Age of women treated with first-line, Median (range) ET: 68 (39–92); and CT:58 (30–81) | 100 | NR | NR | 100 | ≥80 | mBC |
| Boudin et al (2016)52 | Overall study population | 235 | Median (range):46 (21–62) | 62.1 | NR | NR | 82.5 | 50–79 | mBC following treatment with high- dose chemotherapy (HDC) and autologous hematopoietic progenitor cell transplantation (AHSCT) |
| Carbognin et al. (2016)80 | Overall study population | 335 | Age at diagnosis of metastasis, Median (range): 61 (30–91) | 100 | 92.8 | 78.8 | 100 | ≥80 | advanced BC |
| Chandarlapaty et al. (2016)68 | Overall study population | 541 | Median (range), Everolimus and exemestane: 62 (34–93); Placebo and exemestane: 61 (28–90) | 100 | 100 | NR | 100 | ≥80 | mBC |
| Chen et al (2018)41 |
Overall sample= 390 analyses conducted in late recurrence group (n=109) |
390 | Age at diagnosis: Early recurrence: Age group ≤35: 39 (13.9%); Age group >35: 242 (86.1%). Late recurrence: Age group ≤35: 19 (17.4%); Age group >35: 90 (82.6%) |
100 | NR | NR | 100 | ≥80 | Patients with BC who relapsed after surgery |
| Cinausero et al (2018)37 |
Overall study population | 410 | Age at last line treatment, Median (range): 67.2 (31–92) |
71.0 | 75.9 | 60.7 | 82.2 | 50–79 | mBC |
| Delpech et al (2012)57 |
Overall study population | 241 | Median (range): 55 (29–93) | NR | 100 | 75 | 71 | 50–79 | mBC |
| Demian et al (2011)86 | Metastatic subgroup=20 | 20 | NR | NR | 80 | 80 | 65 | 50–79 | mBC |
| den Brok et al (2017)91 |
Subgroup (HR+/HER2–) | Relapse=1,174 De novo: n=406 |
Relapsed: Age group ≤50 years: 278 (23.7%) Age group >50 years: 896 (76.3%) De novo: Age group ≤50 years: 99 (24.4%) Age group >50 years: 307 (75.6%) |
100 | 100 | NR | 100 | ≥80 | mBC |
| Desille- Gbaguidi et al (2018)94 |
Overall study population | 139 | Mean (SD, range): 62.7 (15.4, 25–94) | 80 | 77.7 | 65.5 | 79.9 | 50–79 | mBC |
| Dominici et al (2011)89 |
Overall study population | 290 | Mean (SD): Non-surgery: 52.2 (13.6); Surgery: 53.4 (14.0) |
NR | 74.1 | NR | 67.2 | 50–79 | Stage IV mBC |
| Elsamany et al (2014)84 |
Overall study population | 60 | NR | 63.3 | NR | NR | 63.3 | 50–79 | mBC |
| Ercolani et al (2018)92 |
Overall study population | 51 | Mean (SD, range): 56 (11.4, 37–76) | NR | 69 | 47 | 61 | 50–79 | mBC |
| Fountzilas et al (2013)56 |
Overall study population | 64 | Median (range), Group A: 53.4 (32–75) and Group B: 60.65 (31–74) |
NR | 85.4 | 68.3 | 88.5 | ≥80 | mBC |
| Gampenrieder et al (2018)42 |
Overall study population | 116 | Median (range), Optimization set: 60 (29–86), Learning set: 61 (34–81) | 69 | NR | NR | 100 | 50–79 | mBC |
| Gamucci et al (2017)38 |
Overall study population | 314 | Median (range): 55 (27–82) | 80.9 | NR | NR | 100 | ≥80 | mBC |
| Gerratana et al (2015)63 | Overall study population | 544 | Median (range): 63 (53–73) | NR | 79.4 | 63.7 | 78.9 | 50–79 | mBC |
| Gilabert et al (2011)29 |
Overall study population | 75 | Age before starting capecitabine, Median (range): 53 (36–78) |
79 | NR | NR | 100 | 50–79 | mBC |
| Giordano et al (2011)24 |
Overall study population | 517 | Median (range): 49.3 (23.3–82) | NR | 64.2 | 43.9 | 80.5 | 50–79 | mBC |
| Goetz et al (2017) (CA)26 |
MONARCH 2, overall study population | 669 | Mean (SD): 59.9 (11.4) | 100 | NR | NR | 100 | ≥80 | mBC |
| Goetz et al (2017) (CA)26 |
MONARCH 3, overall study population | 493 | Mean (SD): 63.1 (9.92) | 100 | NR | NR | 100 | ≥80 | mBC |
| Gong et al (2018)55 |
Overall study population | 7,482 | NR | 74.4 | NR | NR | 67.7 | 50–79 | mBC |
| Griguolo et al (2017) (CA)34 |
Overall study population | 104 | Median (range): 56 (26–75) | 67.3 | NR | NR | 69.2 | 50–79 | BC patients with leptomeningeal metastasis |
| Guo et al (2017)35 |
Overall study population | 176 | Median (range): 49.5 (28–80) | NR | 56.3 | 47.7 | 63.6 | 50–79 | mBC |
| Inari et al (2017)45 |
Overall study population | 96 | Mean (SD): 51 (7.5) | 60.4 | 42.7 | 40.6 | 85.4 | 50–79 | mBC (metastatic lesions) |
| Jacot et al (2018) (CA)47 |
Overall study population | 72 | Median (range): 65 (35–87) | NR | 69.4 | NR | 81.9 | 50–79 | mBC |
| Jansson et al (2016)74 |
Overall study population | 52 | Age at mBC diagnosis, Median (range): 60 (40–83) | 75 | 88.4 | 88.4 | 82.7 | 50–79 | mBC |
| Jung et al (2011)43 |
Overall study population | 553 | Median (range): 55 (26–88) | 73.1 | NR | NR | 65.5 | 50–79 | mBC |
| Jung et al (2012)48 |
Overall study population | 557 | Median (range): 55 (26–88) | 73.2 | NR | NR | 65.5 | 50–79 | mBC |
| Kawano et al (2013)72 |
Overall study population | 69 | Median (range): 53 (27–86) | 100 | 92 | 81 | 87 | ≥80 | Stage IV, mBC |
| King et al (2016)95 |
ER-positive/HER2– negative subgroup |
92 | Median (range): 52 (21–79) | 100 | 100 | NR | 100 | ≥80 | Stage IV BC |
| Kobayashi et al (2016)53 |
Overall study population | 527 | Age starting treatment for mBC, Mean: 55 | 65.3 | NR | NR | 71.2 | 50–79 | mBC |
| Kono et al (2018)87 |
Overall study population | 389 | NR | 68.8 | NR | NR | 91 | 50–79 | Untreated primary BC and distant metastasis at diagnosis |
| Kontani et al (2014)49 |
Overall study population | 70 | Median (range): 60 (32–81) | 60 | NR | NR | 81.8 | 50–79 | mBC |
| Korantzis et al (2012)44 |
Overall study population | 159 | Median (range): 60.7 (27.3–86.7) | 70 | NR | NR | 62 | 50–79 | mBC |
| Lau et al (2017) (CA)97 | Overall study population | 82 | NR | 100 | NR | NR | 100 | ≥80 | mBC |
| Lawrie et al (2018)96 |
Overall study population | 103 | Median (range): 58 (27–86) | 100 | 100 | 91 | HER2– (Score 0 +1) =86.4 |
≥80 | mBC |
| Leone et al (2017)78 |
Overall study population | 9,143 | Median (range): 61 (19–102) | 65.9 | NR | NR | 51.3 | 50–79 | Stage IV BC at initial diagnosis |
| Li 201738 | Overall study population Overall study population |
7,199 | Median: 58 (IQR 49–67) | NR | 73.4 | 60.6 | 74 | 50–79 | Stage IV BC |
| Llombart- Cussac et al. (2014)71 |
Overall study population | 2,203 | Median (range): 53 (22–93) | 69 | 65 | 54 | 100 | 50–79 | Advanced locally recurrent or mBC |
| Lobbezoo et al (2013)10 |
Overall study population | 798 | Median (range): 59 (25–93) | 76.5 | NR | NR | 81.4 | 50–79 | mBC |
| Lobbezoo et al (2015)69 |
Overall study population | 815 | Median (range), De novo: 61 (25–89); Recurrent: 64 (25–93) | 75 | NR | NR | 78 | 50–79 | mBC |
| Lobbezoo et al (2016)54,99 |
Overall study population | 482 | Median (range), Initial CT: 52 (25–80), Initial ET: 61 (30–91) | 100 | NR | NR | 100 | ≥80 | mBC |
| Manuel et al (2012)59 |
Overall study population and subgroups | 133 | Median (range), Cohort A: 59.9 (36.5–84.1), Cohort B: 56.9 (34.7–79.9) | NR | 72.1 | 58.6 | 73.7 | 50–79 | mBC |
| Miyoshi, 201638 |
Overall study population | 318 | NR | 100 | 100 | 100 | 100 | ≥80 | Early and late recurrent BC |
| Motomura et al (2010)46 |
Overall study population | 41 | Median (range): 57 (42–79) | 100 | 97.6 | 97.5 | 92.7 | ≥80 | mBC |
| Muller et al (2012)75 |
Overall study population | 245 | Median: 57 | NR | 69.4 | 59.2 | 56.7 | 50–79 | mBC |
| Munzone et al (2012)83 |
Overall study population | 203 | Median (range): 57 (31–78) | 79.3 | NR | NR | 73.8 | 50–79 | mBC |
| Nieder et al (2017)79 |
Overall study population | 118 | Median (range): 61 (33–87) | NR | 77 | NR | 82 | 50–79 | mBC |
| Ogiya et al (2017)66,67 |
Overall study population | 339 | NR | NR | 100 | NR | 100 | ≥80 | BC patients with the early and late distant recurrence |
| Okazaki et al (2018)67 |
Overall study population | 18 | Median (range): 51 (34–81) | 100 | NR | 83 | 100 | ≥80 | Locally advanced or mBC |
| Paoletti et al (2017) (CA)31 |
Overall study population | 121 | NR | NR | 100 | NR | 100 | ≥80 | mBC |
| Papadaki et al (2018)32 |
Overall sample = 130 Analysis conducted in HER2– subgroup |
130 | Median (range): 59 (23–82) | NR | 68.5 | 63.8 | 78.5 | 50–79 | mBC |
| Park et al (2017)85 |
Overall study population | 100 | Mean (range): 48.1 (48–75) | 65 | NR | NR | 75 | 50–79 | Invasive ductal BC with bone-only metastasis |
| Pascual et al (2017)65 |
Overall study population | 90 | Median (range): 62 (37–84) | NR | 100 | NR | 100 | ≥80 | mBC |
| Peeters et al (2014)36 |
Overall study population | 154 | Median (range): 62.1 (32.9–90.8) | 55.2 | NR | NR | 69.9 | 50–79 | mBC |
| Pierga et al (2012)51 | Overall study population | 267 | Median: 57 | 62 | NR | NR | 83 | 50–79 | mBC |
| Pistilli et al (2017) (CA)50 |
Overall study population | 6,265 | Median (range), Age group <45 years: 40.0 (23–44) Age group >45 years: 63 (45–95) |
100 | NR | NR | 100 | ≥80 | mBC |
| Redondo et al (2018)58 |
Overall study population | 148 | Median (range): 50 (29–81) | NR | 74.3 | 61.5 | 100 | 50–79 | mBC |
| Ren et al (2016)73 | Overall study population | 323 | Median: 49 | NR | 58 | 45 | 50 | 50–79 | advanced BC |
| Schiavon et al (2015)23 | Subgroup of ESRI wild and mutant type | 128 | Median, ESR1 wild type: 58, ESR1 mutant: 64 | 100 | 100 | NR | 84 | ≥80 | advanced BC |
| Staudigl et al (2015)81 | Overall study population | 114 | Age at first diagnosis, Mean: 61.0 | 76.3 | NR | NR | 82.5 | 50–79 | mBC |
| Suh et al (2017)27 | Overall study population | 66 | Age at the time of treatment, Median: 44 | 100 | NR | NR | 100 | ≥80 | mBC and recurrent BC (Luminal A, Luminal B (HER2–),Unknown) |
| Turker et al (2014)98 | Overall study population | 302 | Median (range): 50 (21–83) | NR | 72.2 | 60.6 | 59.6 | 50–79 | Stage IIIC with >10 positive axillary |
| Uyeturk et al (2014)25 | Overall study population | 466 | Median (range): 50 (18–90) | 73.2 | 68.5 | 60.5 | 65.5 | 50–79 | mBC |
| Vaz-Luis et al (2015)85 | HR+ HER2– subgroup | 3,151 | NR | 100 | NR | NR | 100 | ≥80 | Stage IV, mBC |
| Wallwiener et al (2013)76 | Overall study population | 486 | Median (range): 55 (23–91) | NR | 67.3 | 58.7 | 69.8 | 50–79 | mBC |
| Wu et al (2017)90 | Overall study population | 9,256 | NR | 76.6 | NR | NR | 74.2 | 50–79 | Stage IV BC |
| Xie et al (2015)33 |
Overall study population | 699 | Age at BC diagnosis, Median (range), patients with multiple metastases: 61.0 (35.0–83.0); patients with a single metastasis: 61.0 (33.0–90.0) | 100 | NR | NR | 100 | ≥80 | mBC |
| Xiong et al (2018)28 |
Overall study population | 8,901 | NR | 70.5 | NR | NR | 65.6 | 50–79 | BC with initial bone metastasis |
| Yamamura et al (2018)39 |
Overall study population | 172 | For stage IV: Age group <50 years: 12 (18%) Age group 50–70 years: 16 (25%) Age group ≥70 years: 37 (57%) For recurrent mBC: Age group <50 years: 20 (19%) Age group 50–70 years: 25 (23%) Age group ≥70 years: 62 (58%) |
100 | NR | NR | 100 | ≥80 | Stage IV and recurrent and mBC |
| Yerushalmi et al (2012)93 |
Subgroup Luminal A and Luminal B |
566 | NR | 100 | NR | NR | 100 | ≥80 | mBC |
| Zhang et al (2013)60 |
Overall study population | 134 | Median (range): 52 (28–74) | 100 | NR | NR | 56 | 50–79 | mBC |
| Zhao et al (2011)70 |
Overall study population | 60 | Median (range), Metonomic Arm: 46 (35–73); Conventional Arm: 51 (33–73) | NR | 66.6 | NR | 76.6 | 50–79 | Stage IV BC with bone metastases |
Abbreviations: BC, breast cancer; CT, chemotherapy; ET, endocrine therapy; IQR, interquartile range; mBC, metastatic breast cancer; NR, not reported; SD, standard deviation.
Baseline Characteristics
The median age of patients in the included studies ranged between 44–68 years; age was not reported in 12 studies.28,31,55,66,84–87,93,97 In 22 studies, the entire study population was HR+ and HER2–, while in eight studies the proportion of patients with HR+ and/or HER2– status was between 80–99%, and the remaining 49 studies included patients with HR+ and/or HER2– status ranging between 50–79%.
Prognostic Factors
Disease-Related Factors
Progesterone Receptor (PR) Expression
Patients with breast cancer positive for progesterone, estrogen, or both receptors were deemed HR positive. The relationship between PR status and OS (n=10), PFS (n=2), and tumor response (n=1) was evaluated, with a significant association reported in 80% (n=8), 50% (n=1), and 100% (n=1) of studies, respectively.26,38,45,56,66,72,73,92,98 The association of PR status with BCSS was assessed in one study, and it did not report any significant relationship.40 Patients with negative PR status compared with positive were moderately associated with worse OS. The evidence was insufficient to assess the strength of association between PFS/tumor response and PR status.
Tumor Grade
The type of tumor grading system used was reported in only four studies that assessed OS. Two studies used the Scarff Bloom Richardson grading,52,88 one utilized the modified Bloom–Richardson grading,96 and the other study employed the Elston-Ellis modification of Scarff-Bloom-Richardson grading system.82
The relationship between tumor grade and OS (n=21), PFS (n=4), BCSS (n=3), and tumor response (n=1) was evaluated, with a significant association reported in 62% (n=13), 75% (n=3), 100% (n=1), and 100% (n=1) of studies, respectively.26,28,29,38,42,55,66,73,78,81,82,85,88,90 Survival was worse in patients with poorly to moderately differentiated tumors compared with well-differentiated tumors. Consistency in evidence and directionality of association was observed for all survival endpoints. Overall, the effect size of the association between tumor grade and survival endpoints was moderate.
Tumor Size
Relationship between tumor size and OS (n=12) and BCSS (n=2) was evaluated, with a significant association reported in 42% (n=5) and 50% (n=1) of studies, respectively.28,38,81,90,92 No included study assessed the association between tumor size and PFS or tumor response. In four studies, large tumors (>5 cm diameter) were associated with worse survival,28,38,90,92 while one study showed improved OS in patients with T2 tumors (>2 cm and <5 cm) compared with T1 tumors (≤2 cm).81 Less than 50% of studies that assessed the association between tumor size and OS reported a significant association, although among those, directionality of evidence was consistent in the five studies. Overall, the effect size of the association between tumor size and survival endpoints ranged from weak-to-moderate.
Lymph Node Involvement
The relationship between lymph node involvement and OS (n=11), PFS (n=1), and BCSS (n=2) was evaluated, with a significant association reported in 36% (n=4), 100% (n=1), and 100% (n=2) of studies, respectively.28,38,40,66,70,90
In two of four studies demonstrating a relationship with OS, N1, N2, and N3 categories were associated with better OS than patients with no lymph node involvement (N0);28,90 these studies involved stage IV de novo metastatic patients from the Surveillance, Epidemiology, and End Results (SEER) registry. The trend, however, was converse in the other two studies, among patients with metastatic disease with no prior diagnosis and another with recurrent disease after breast surgery or neoadjuvant chemotherapy, in which greater lymph node involvement was associated with greater risk of death.38,66 The two studies focusing on BCSS and the one study70 focusing on PFS also reported higher lymph node involvement was associated with greater risk of death.28,90 In summary, the directionality of association was inconsistent across studies assessing OS and lymph node involvement. Overall, the effect size of the association between lymph node involvement and survival endpoints was moderate.
Histological Type
In Gampenrieder et al,42 patients with lobular carcinoma (HR=3.44; 95% CI=1.07–11.11; P=0.039) or other type of carcinoma (HR=3.19; 95% CI=1.05–9.70; P=0.041) were associated with 3-fold greater risk of death compared with ductal carcinoma; similar results were observed for PFS. The effect size of the association between histological type (lobular vs ductal) and survival endpoints was strong. The evidence of association was insufficient as a significant association was reported in only one of five studies with OS and two studies with PFS.
Biomarkers
Relationship between CTC count and OS (n=10), PFS (n=10), and BCSS (n=1) was evaluated, with a significant association reported in 90% (n=9), 80% (n=8), and 0% (n=0) of studies, respectively.24,31,32,36,47,51,74–76,83 The presence of a higher CTC count (≥5/7.5 mL whole blood) was consistently associated with poor OS and PFS.
The relationship between Ki67 expression and OS (n=7), PFS (n=4), and tumor response (n=1) was evaluated, with a significant association reported in 86% (n=6), 100% (n=4), and 100% (n=1) of studies, respectively.27,30,45,57,60,66,67,80 Studies did not consistently report the source of the Ki67 (primary or metastatic tumor site). High Ki67 expression was associated with worse OS, PFS, and tumor response. The thresholds for the Ki67 was inconsistent across studies, with a Ki67 index of ≤14% vs >14% being the most common.
The association of both CTCs and Ki67 with OS and PFS was harmonious with respect to consistency of evidence and directionality of association. Overall, the effect size of the association between these biomarkers and survival endpoints were moderate.
De Novo Metastatic Breast Cancer (mBC)
The relationship between de novo mBC and OS (n=5), PFS (n=3), and BCSS (n=1) was evaluated, with a significant association reported in 100% (n=5), 67% (n=2), and 0% (n=0) of studies, respectively.30,33,39,57,62,91 Four studies demonstrated longer OS in patients with mBC at diagnosis compared with recurrent breast cancer;30,39,57,91 while one study reported shorter OS in patients with de novo mBC.62 Similarly, one study showed longer PFS associated with patients with de novo mBC,30 while another study showed a reverse relationship.33
The association of de novo mBC with OS and PFS was consistent with respect to evidence. The directionality of association was consistent with OS but not with PFS. The effect size of the association between de novo mBC and survival endpoints ranged between weak to moderate.
Number of Metastatic Sites
The relationship between number of metastatic sites and OS (n=27), PFS (n=11), and BCSS (n=1) was evaluated, with a significant association reported in 89% (n=24), 55% (n=6), and 100% (n=1) of studies, respectively.10,28–30,33,38,39,41,43,44,48,51–55,57,59,60,66,69,71,75,79,80,89 Multiple metastases were associated with significantly worse OS and PFS. There were variations in the way comparisons between the number of metastatic sites were made across studies (eg, ≤1 vs >1; ≤3 vs >3); however, the multiple vs single site of metastases (ie, >1 vs 1) comparison was the most common. Most studies compared either the number of metastatic sites (eg, >1 vs 1) or types of sites/location of metastasis (eg, lungs vs brain, visceral vs non-visceral) However, three studies10,28,54 compared multiple metastatic sites (visceral, brain, skin, lymph nodes) to bone metastasis and found significantly greater risk of death associated with the former. Consistency in evidence and directionality of association was observed for OS and PFS. The effect size of the association between number of metastatic sites and survival endpoints ranged from moderate to strong, depending on the comparison groups.
Sites of Metastasis
Twenty-two of the 34 studies found a significant association between sites of metastasis and OS.10,29,30,32,36,38,39,42–44,48,52,54,59,63,69,71,75,78–80,89 Sites of metastasis were compared heterogeneously (eg, visceral vs non-visceral, visceral vs bone, hepatic vs no hepatic, brain vs no brain). Liver involvement was the most widely studied (n=1230,32,38,39,43,48,59,63,71,78,79,89), followed by brain/CNS (n=1410,29,30,38,43,48,54,61,63,69,78–80,89), visceral (n=1310,30,36,42–44,52,54,63,69,75,78,99), bone (n=910,38,48,54,59,63,69,75,78), and lung (n=730,38,63,71,78,79,89). All these studies reported shorter OS associated with the presence of metastasis at these specific sites compared to lack of it (eg, visceral vs non-visceral). Bone metastasis was also often used as the reference category when comparing the effect of other metastatic sites on survival, and was associated with improved prognosis compared to these other sites.10,44,54,69,75,78
Ten of 13 studies reported a significant association with PFS.26,29,30,32,33,36,60,63,76,84 Bone was the most assessed site (n=533,36,63,76,84), followed by liver (n=426,30,32,63), and visceral (n=430,60,63,76). As with OS, the presence of metastasis compared with absence in bone, liver, and visceral sites was associated with worse PFS; visceral sites reported worse PFS when compared with bone.10,78 Only one study reported poor tumor response associated with liver metastases.26
The definition of visceral sites varied across studies, most commonly defined as lung, liver, pericardial/pleural/peritoneal, and brain. Consistency in evidence and directionality of association was observed for OS and PFS. The overall effect size of association with survival was: moderate for liver, brain, and visceral sites; weak for lung; and ranged from weak to moderate for bone.
Time to Recurrence or Progression to Advanced Breast Cancer
Time to recurrence or progression to advanced breast cancer was most often defined as the time between date of diagnosis of primary breast cancer, and date of diagnosis of first distant metastasis or recurrence. Disease-free interval (DFI), metastasis-free interval (MFI), and recurrence-free interval (RFI) are other terminology used to describe this. In Zhao et al,70 it was defined as the date from surgery to first recurrence. Eight studies did not report the definition.36,45,49,52–54,66,71
The relationship between time to recurrence or progression to advanced breast cancer and OS (n=18) and PFS (n=5) was evaluated, with a significant association reported in 78% (n=14) and 80% (n=4) of studies, respectively.10,29,36,39,45,48,49,52–54,60,66,70,71,91 In 13 studies, shorter time to recurrence or progression to advanced breast cancer was associated with worse survival relative to longer time, except in Jung et al,48 where the 1–5 years vs <1 year MFI was associated with worse OS (HR=1.30; 95% CI=1.02–1.65; P=0.032). The 2-year time interval was the most commonly studied cut-off point. Four studies showed a shorter time to recurrence or progression to advanced breast cancer (eg, <2 years) was associated with worse PFS.29,54,60,70 Consistency in evidence and directionality of association was observed for OS and PFS. The overall effect size of the association between time to recurrence or progression to advanced breast cancer and survival endpoints was moderate.
Prior Therapy
Given the patient population had advanced breast cancer, patients were likely to have received prior therapy (except those with de novo mBC) – such as surgery, chemotherapy, radiation therapy, hormone therapy – to treat early breast cancer. Type of prior therapy, line of prior therapy received in the metastatic setting, or clinical benefit to prior therapy were all grouped under the “prior therapy” category in this review.
Twenty-seven of 35 studies found a significant relationship between OS and prior therapy.10,25,28,30,33,34,38,44,49,52,54,55,57,58,60,66,71,72,76,79,80,88–91,94 Prior therapy was either adjuvant or neoadjuvant chemotherapy or hormonal therapy in 19 studies that assessed OS.10,29,30,32,33,38,41,42,44,45,54,57,58,60,66,71,80,91,92 Lack of 1st-line hormonal therapy in patients with advanced breast cancer was also associated with worse survival compared with receiving hormonal therapy.80 Furthermore, the absence of hormonal maintenance therapy in the advanced setting was associated with worse OS in three studies.38,58,80 Two studies reported that adjuvant hormonal therapy use was associated with shorter survival compared with lack of use.33,54 Lobbezoo et al54 reported shorter survival was associated with receipt of initial chemotherapy compared with initial hormonal therapy in the metastatic setting.
Surgery was the prior therapy in ten studies that assessed OS.25,28,38,41,55,58,88–90,94 Seven studies showed that receipt of surgery, compared with lack of surgery or best supportive care, resulted in significantly longer survival; five of these studies included de novo mBC patients28,55,89,90,94 and in the remaining two studies, surgery was conducted in early stage breast cancer.38,88
Prior radiotherapy was received in six studies that evaluated OS.38,41,66,79,82,89 First-line radiotherapy (yes vs no) was significantly associated with longer survival for mBC;38 however, the association was not uniform for 1st-line chemotherapy (multiagent vs none/single−agent); Li 2017 38 reported improved OS, while Xie et al33 reported worse OS.
Longer treatment durations in the advanced setting were associated with improved OS, while greater lines of treatment were associated with worse OS.66,76 Four studies demonstrated that the presence of clinical benefit or response to a specific treatment was associated with better OS.38,52,57,72
Fifteen studies assessed the association of PFS with line/type of prior therapy; 13 showed a significant relationship.23,27,29,30,32,33,38,47,54,58,60,70,76,84 Eleven of the 15 studies reported adjuvant or neoadjuvant chemotherapy or hormonal therapy,23,27,29,30,32,33,38,42,54,58,60 while four studies reported chemotherapy as prior treatment.47,70,76,84
Four studies compared multiple vs single lines of treatment and found that increasing treatment line in the metastatic setting correlated with worse prognosis.27,32,33,76 In two studies that included de novo patients, prognostic relevance was shown for surgery vs no surgery as prior therapy and found improved BCSS in patients undergoing breast-conserving surgery/mastectomy.55,90
There was substantial heterogeneity in reporting of type/class of therapy received. In general, patients receiving interventions (surgery/radiotherapy/systemic therapy), responding to treatments, or receiving fewer lines of treatment in the metastatic setting were likely to have better prognosis. Consistency in evidence and directionality of association was observed for OS, PFS, and BCSS. The effect size of the association between prior therapy attributes and survival endpoints was moderate.
Patient-Related Factors
Age
Relationship between age and OS (n=37), PFS (n=7), and BCSS (n=3) was evaluated, with significant association reported in 46% (n=17), 29% (n=2), and 67% (n=2) of studies, respectively.10,28,30,33,36,38,43,48,55,57,58,62,69,78,84,85,90–92 Among studies that found a significant association, increasing age was associated with worse OS, PFS, or BCSS. The time-point at which age data was collected in the study (whether age at diagnosis or at treatment initiation) was not reported in the majority of included studies. Among studies that did report, age at diagnosis was the most common. In three studies, increasing age was associated with worse OS.57,62,69 Age groups compared across studies varied widely (eg, >50 vs ≤50 years, >65, or 50–64 vs 18–49 years). Among the different age group comparisons, age ≥50 years was the most common cut-off point, reported in six studies.10,54,55,78,90,91 Less than 50% of studies found a significant association between age and OS as well as PFS, however, the directionality of association was consistent (ie, increasing age was associated with shorter survival). The effect size of the association between age and survival endpoints varied widely across studies, ranging from weak to strong.
Race
Relationship between race and OS (n=13) and BCSS (n=3) was evaluated, with a significant association reported in 54% (n=7) and 100% (n=3) of studies, respectively.22,28,38,55,73,78,90 Poorer OS or BCSS was observed in blacks compared with whites. One study reported that better OS was observed for patients of other races vs whites (HR=0.59; 95% CI=0.44–0.78; P<0.001).38 One study evaluated but did not report a significant association between race and PFS.33 Consistency in evidence and directionality of association was observed between race and OS as well as BCSS. The effect size of the association between race and survival endpoints was weak.
Performance Status
Relationship between performance status and OS (n=14) and PFS (n=8) was evaluated, with a significant association reported in 79% (n=11) and 50% (n=4) of studies, respectively. ECOG scale was used in all but two studies; one study employed the World Health Organization (WHO) performance status scale,51 and one did not define the performance status scale.59 Comparison of different ECOG statuses varied across studies; most studies compared ECOG levels ≥2 vs 0–1, three studies compared ≥1 vs 0, while one study compared ≥3 vs 0–2.33,34,37,42,51,63,71,80 All studies found poor performance status or limitations in daily activity to be significantly associated with worse OS or PFS. Consistency in evidence and directionality of association was observed between performance status and OS. Less than 50% of studies found a significant association between PFS and performance status, however, the directionality of association was consistent. The effect size of the association between performance status and survival endpoints was moderate.
Strength of Evidence
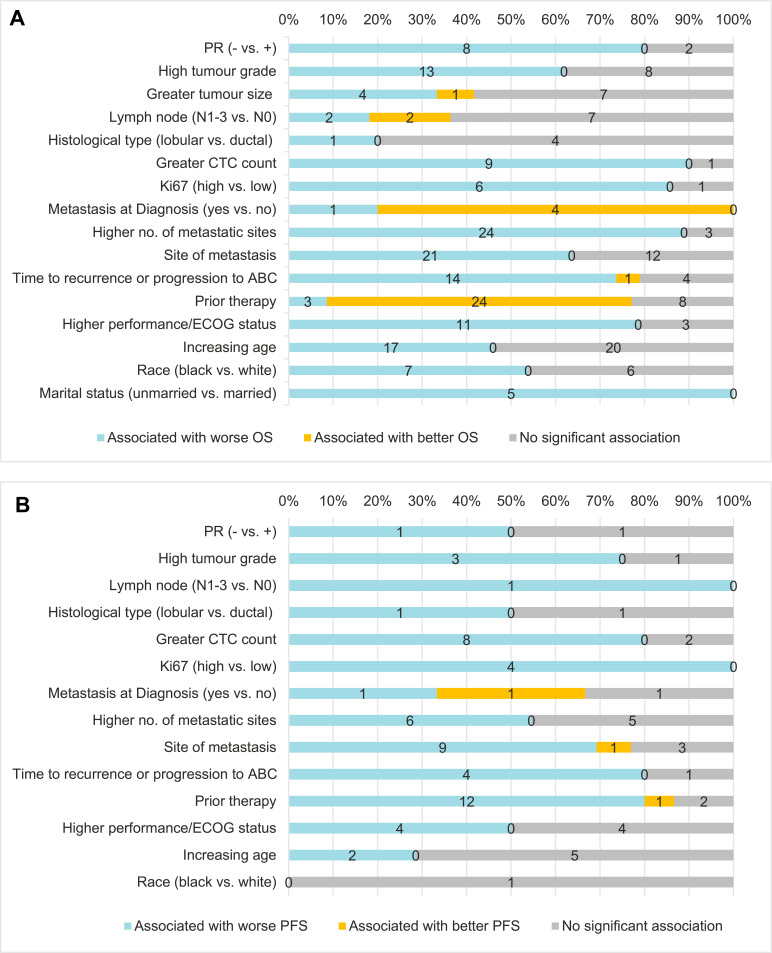
Table 2 summarizes the strength of evidence between prognostic factors and survival endpoints. Figure 2 shows the number of studies that reported better, worse, or no association between the prognostic factors and OS (Figure 2A) and PFS (Figure 2B).
Table 2.
Strength of Evidence Assessment
| Prognostic Factor |
Type of Outcome |
No. of Studies That Assessed Association |
No. of Studies Reporting Significant Association |
No. of Studies with Univariate Analysis |
No. of Studies with Significant Univariate Analysis |
No. of Studies with Multivariate Analysis |
No. of Studies with Significant Multivariate Analysis |
Consistency Based on >50% Studies with Significant Association |
Directionality of Relationship |
Strength of Association Based on Effect Size |
Studies with Low Sample Size (<100) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PR status | OS | 10 | 8 | 10 | 8 | 7 | 5 | Consistent | Consistent | Moderate | 4 |
| PFS | 2 | 1 | 1 | 0 | 0 | 0 | Inconsistent | NA (single study) | NA | 1 | |
| Tumor grade | OS | 21 | 13 | 15 | 7 | 15 | 11 | Consistent | Consistent | Moderate | 4 |
| PFS | 4 | 3 | 2 | 1 | 2 | 1 | Consistent | Consistent | Strong | 2 | |
| Tumor size | OS | 12 | 5 | 10 | 4 | 8 | 5 | Inconsistent | Consistent | Weak– moderate |
4 |
| Lymph node | OS | 11 | 4 | 10 | 4 | 5 | 1 | Inconsistent | Inconsistent | Moderate | 5 |
| PFS | 1 | 1 | 1 | 1 | 0 | 0 | NA (single study) | NA (single study) | NA | 1 | |
| Histological type | OS | 5 | 1 | 4 | 0 | 1 | 1 | Inconsistent | NA | Strong | 3 |
| PFS | 2 | 1 | 1 | 0 | 2 | 1 | Inconsistent | NA (single study) | Strong | 2 | |
| CTC count | OS | 10 | 9 | 9 | 8 | 7 | 7 | Consistent | Consistent | Strong | 3 |
| PFS | 10 | 8 | 8 | 5 | 6 | 6 | Consistent | Consistent | Moderate | 3 | |
| Ki67 | OS | 7 | 6 | 6 | 5 | 4 | 3 | Consistent | Consistent | Moderate | 2 |
| PFS | 4 | 4 | 3 | 3 | 3 | 3 | Consistent | Consistent | Moderate | 2 | |
| De novo metastatic BC | OS | 5 | 5 | 4 | 4 | 5 | 2 | Consistent | Consistent | Weak | 0 |
| PFS | 3 | 2 | 2 | 1 | 2 | 1 | Consistent | Inconsistent | Moderate | 1 | |
| No. of Metastatic sites |
OS | 27 | 24 | 21 | 19 | 20 | 14 | Consistent | Consistent | Moderate | 3 |
| PFS | 11 | 6 | 7 | 4 | 9 | 5 | Consistent | Consistent | Moderate | 2 | |
| Site of metastasis | OS | 34 | 22 | 21 | 14 | 25 | 19 | Consistent | Consistent | Moderate | 7 |
| PFS | 13 | 10 | 7 | 6 | 10 | 6 | Consistent | Consistent | Moderate | 4 | |
| Time to recurrence or progression to ABC | OS | 18 | 14 | 12 | 10 | 12 | 9 | Consistent | Consistent | Moderate | 4 |
| PFS | 5 | 4 | 3 | 3 | 4 | 3 | Consistent | Consistent | Moderate | 3 | |
| Prior therapy | OS | 35 | 27 | 24 | 18 | 25 | 22 | Consistent | Consistent | Moderate | 6 |
| PFS | 15 | 13 | 10 | 8 | 12 | 10 | Consistent | Consistent | Moderate | 6 | |
| Performance | OS | 14 | 11 | 7 | 6 | 11 | 8 | Consistent | Consistent | Moderate | 2 |
| PFS | 8 | 4 | 3 | 1 | 6 | 4 | Inconsistent | Consistent | Moderate | 1 | |
| Age | OS | 37 | 17 | 25 | 10 | 24 | 13 | Inconsistent | Consistent | Weak– Moderate |
5 |
| PFS | 7 | 2 | 5 | 0 | 3 | 2 | Inconsistent | Consistent | Moderate– Strong |
2 | |
| Race | OS | 13 | 7 | 10 | 6 | 10 | 6 | Consistent | Consistent | Weak | 1 |
| PFS | 1 | 0 | 0 | 0 | 1 | 0 | NA | NA | NA | 0 |
Abbreviations: BC, breast cancer; BCSS, breast cancer-specific survival; CTC, circulating tumor cell; NA, not applicable; OS, overall survival; PFS, progression-free survival; PR, progesterone receptor.
Figure 2.
Association between selected prognostic factors and OS (A), and PFS (B).
Abbreviations: BCSS, breast cancer-specific survival; CTC, circulating tumor cell; ECOG, Eastern Cooperative Oncology Group; OS, overall survival; PFS, progression-free survival; PR, progesterone receptor.
Associations between OS and PR status, tumor grade, CTC count, Ki67 level, de novo mBC, number and sites of metastases, time to recurrence or progression to advanced breast cancer, race, and prior therapy attributes were consistent (>50% of studies found a significant association). However, the evidence was limited (<50% of studies reported a significant association) for tumor size, histological type, lymph node involvement, and age. The direction of association was consistent for all the prognostic factors summarized in this study except for lymph node involvement. Based on effect size, strength of association with OS was moderate (HR=1.5–2.9) for PR status, tumor grade, Ki67 level, number and sites of metastases, time to recurrence or progression to advanced breast cancer, performance status, and prior therapy attributes, and weak (HR<1.5) for de novo metastatic breast cancer and race.
After applying the strongest evidence criteria, disease-related factors – such as PR status, tumor grade, CTC count, Ki67 level, number and sites of metastases, and time to recurrence or progression to advanced breast cancer, performance status, prior therapy attributes, and race – were found to have the strongest evidence of an association with OS.
Associations between PFS and tumor grade, CTC count, Ki67 level, number and sites of metastases, time to disease recurrence or progression to advanced breast cancer, and prior therapy attributes were consistent. However, the evidence was limited for PR status, lymph node involvement, histological type, performance status, age, and race; no data were reported for association between PFS and tumor size or marital status. The direction of association was consistent for all the prognostic factors, except for de novo metastatic breast cancer.
Since fewer studies assessed PFS than OS, evidence on prognostic factors related to PFS was limited. Thus, high CTC count, number and sites of metastases, and prior therapy attributes in the early or metastatic setting were the only four prognostic factors with the strongest evidence of an association with worse PFS. Similarly, there was limited information for the other endpoints.
Other Variables
There were many other variables assessed in the included studies. However, these were reported sparsely and we could not assess strength of evidence for them. They consisted of many genetic/biomarkers factors, for example, estrogen receptor gene (ESR1) mutation status,68 ligand binding domain (LBD) status,97 CA 15–3 level,51,70 alkaline phosphatase level,79 serum C-reactive protein level (CRP),79 lactic acid dehydrogenase (LDH) level,51,59,79 along with other demographic-related factors like marital status,55 income level,55 menopausal status,59 and education status.55 A high level summary can be found in Table 3.
Table 3.
Additional Variables Assessed in the Included Studies
| Prognostic Factor | Type of Outcome |
No. of Studies That Assessed Association | No. of Studies Reporting Significant Association | No. of Studies Reporting Worse Outcome | No. of Studies Reporting Better Outcomes | Summary of Direction of Association |
|---|---|---|---|---|---|---|
| Biomarkers or genetic markers | ||||||
| ALDH1 (1 vs >1%) | OS | 1 | 140 | 1 | – | ALDH1 1% was associated with worse OS than ALDH1 >1% |
| ALP (high vs normal) | OS | 2 | 179 | 1 | – | High ALP was associated with worse OS than normal ALP |
| ALP > upper limit of normal | PFS | 1 | 151 | 1 | – | High ALP was associated with worse PFS than normal ALP |
| Baseline SUVmax (intermediate and highest tertile vs lowest tertile) | OS | 1 | 160 | – | – | Baseline SUVmax intermediate and highest tertiles were associated with worse OS than the lowest tertile |
| PFS | 1 | 160 | 1 | – | Baseline SUVmax intermediate and highest tertiles were associated with worse PFS than the lowest tertile | |
| CA 15–3 (high vs normal) | OS | 2 | 179 | 1 | – | Higher levels of CA 15–3 was associated with worse OS compared to normal levels |
| CA 15–3 >upper limit of normal | PFS | 1 | 151 | 1 | – | CA 15–3 > upper limit of normal was associated with worse PFS |
| CEA > upper limit of normal | PFS | 1 | 151 | 1 | – | CEA > upper limit of normal was associated with worse PFS |
| Serum CRP levels (high, >60 vs normal) |
OS | 1 | 179 | 1 | – | Higher serum CRP levels compared to normal was associated with worse OS |
| ESR1 mutations (mutated vs wild type) | OS | 1 | 168 | 1 | – | Mutated ESR1 (D538G, Y537S) was associated with worse OS than wild type |
| PFS | 1 | 168 | 1 | – | Mutated ESR1 (D538G) was associated with worse PFS than wild type | |
| Gamma- glutamyltransferase (GGT) (high vs low risk groups) | OS | 1 | 181 | 1 | – | High risk groups C and D (elevated and highly elevated GGT) was associated with significantly decreased OS time compared to the low risk groups A and B (normal and normal high GGT) |
| GIT1 expression (no vs yes) | OS | 1 | 196 | 1 | – | Loss of GIT1 expression in lymph nodes (± pattern) was associated with worse OS |
| Hemoglobin (<11.5 vs ≥11.5 g/dL) | OS | 2 | 259,79 | 2 | – | Lower hemoglobin levels (<11.5 g/dL) was associated with worse OS compared to high levels (≥11.5 g/dL) |
| HER2 blood mRNA levels (high vs low) | OS | 1 | 144 | – | 1 | High blood HER2 mRNA levels were found to be associated with significantly improved OS compared to low levels |
| IHC, Tau protein (+ vs–) | PFS | 1 | 156 | – | 1 | Presence of Tau protein expression was associated with better PFS |
| IHC, Topo IIa (+ vs–) | PFS | 1 | 156 | 1 | – | Topo IIa protein positivity was associated with unfavorable PFS |
| IL-18 levels (high, ≥8 ng/ mL vs low) | OS | 1 | 186 | – | 1 | High IL-18 levels (≥8 ng/mL) at the time of diagnosis was associated with better OS than low IL-18 levels |
| IL-8 (high vs low) | OS | 1 | 144 | 1 | – | Higher IL-8 levels compared to lower levels was associated with worse OS |
| LBD mutations (yes vs no) | OS | 1 | 197 | 1 | – | Presence of LBD mutations were associated with worse OS |
| PFS | 1 | 197 | 1 | – | Presence of LBD mutations were associated with worse PFS | |
| LDH levels (high, ≥600 vs low <600 UI/mL) | OS | 3 | 351,59,79 | 3 | – | Higher LDH levels was associated with worse OS than lower LDH levels |
| LDH levels (high vs low) | PFS | 1 | 151 | 1 | – | LDH levels > upper limit of normal was associated with worse PFS |
| Lymphocytes (<1 vs ≥1 Giga/L) | OS | 1 | 159 | 1 | – | Lower lymphocyte count was associated with worse OS |
| MAPT RQ values, 50% cut- off (high vs low) | OS | 1 | 156 | – | 1 | High MAPT RQ values was associated with better OS |
| Metastatic EZH2 expression (high vs low) | OS | 1 | 145 | 1 | – | High metastatic EZH2 expression was associated with worse OS than low EZH2 expression |
| NDL (>1 vs ≤1 Giga/L) | OS | 1 | 159 | 1 | – | NDL >1 Giga/L was associated with worse OS than NDL ≤1 Giga/L |
| N-telopeptide of type I collagen (NTx) (<18 nM BCE vs > 18 nM BCE) | PFS | 1 | 170 | – | 1 | Baseline serum NTx of less than 18 nM was associated with significantly better PFS than baseline serum NTx of greater than 18 nM |
| PNN (<7.5 vs ≥7.5 Giga/L) | OS | 1 | 159 | – | 1 | PNN <7.5 Giga/L was associated with better OS than PNN ≥7.5 Giga/L |
| 21-gene recurrence score (low vs high risk group) |
OS | 1 | 195 | – | 1 | Low risk group (<18 score) was associated with better OS than high risk group (≥31 score) |
| Serum albumin (low vs normal) | OS | 1 | 179 | 1 | – | Low serum albumin compared to normal levels was associated with worse OS |
| Serum VEGF after 3 months (normal vs abnormal) | PFS | 1 | 170 | – | 1 | Serum VEGF of less than 500 pg/mL (normal) after 3 months of intervention was associated with better PFS compared to VEGF >500 pg/mL (abnormal) |
| SET(ER/PR) Index (high vs low) | OS | 1 | 197 | – | 1 | Higher SET(ER/PR) Index was associated with better OS |
| SET(ER/PR) Index (high vs low) | PFS | 1 | 197 | – | 1 | Higher SET(ER/PR) Index was associated with better PFS |
| SNP, ABCB1 1236C/T rs1128503 (C vs T or T/C) |
PFS | 1 | 156 | 1 | – | SNP, ABCB1 1236C/T rs1128503 (C) was associated with worse PFS than ABCB1 1236C/T rs1128503 (T or T/C) |
| SNP, ABCB1 2677G/A/T rs2032582 (G or G/A vs T or T/G) | OS | 1 | 156 | 1 | – | SNP, ABCB1 2677G/A/T rs2032582 (G or G/A) was associated with worse OS than ABCB1 2677G/A/T rs2032582 (T or T/G) |
| SNP, ABCB1 3435C/T rs1045642 (C vs T or T/C) |
OS | 1 | 156 | 1 | – | SNP, ABCB1 3435C/T rs1045642 (C) was associated with worse OS than SNP, ABCB1 3435C/T rs1045642 (T or T/C) |
| Surgical margin (negative, R0 vs positive, R1) | OS | 1 | 192 | – | 1 | Negative surgical margin was associated with better OS than positive surgical margin |
| T-cell receptor diversity (≤33 vs >33%) |
OS | 1 | 159 | 1 | – | T-cell receptor diversity of ≤33% compared with >33% was associated with worse OS |
| Demographic or Patient characteristics | ||||||
| BMI (<20 vs 20–24.9) | OS | 1 | 148 | – | 1 | Low BMI was associated with better OS than higher BMI |
| Surgical margin (negative, R0 vs positive, R1) | OS | 1 | 192 | – | 1 | Negative surgical margin was associated with better OS than positive surgical margin |
| T-cell receptor diversity (≤33 vs >33%) |
OS | 1 | 159 | 1 | – | T-cell receptor diversity of ≤33% compared with >33% was associated with worse OS |
| Demographic or Patient characteristics | ||||||
| BMI (<20 vs 20–24.9) | OS | 1 | 148 | – | 1 | Low BMI was associated with better OS than higher BMI |
| Education level (high vs low) | OS | 2 | 243,55 | 1 | 1 | Mixed result (worse and better OS) |
| BCSS | 1 | 155 | 1 | – | Higher education level was associated with worse BCSS | |
| Household income (per $10,000 annual increase) |
OS | 1 | 155 | – | 1 | Higher median household income was associated with better OS |
| BCSS | 1 | 155 | – | 1 | Higher median household income was associated with better BCSS | |
| Insurance status (uninsured vs insured) |
OS | 1 | 155 | 1 | – | Patients who were uninsured had worse OS than those who were insured |
| BCSS | 1 | 155 | 1 | – | Patients who were uninsured had worse BCSS than those who were insured | |
| Marital status (unmarried vs married) | OS | 5 | 528,55,78,85,90 | 5 | –- | Unmarried status was associated with worse OS than married |
| BCSS | 2 | 255,90 | 2 | – | Unmarried status was associated with worse BCSS than married | |
| Menopausal status (yes vs no; post vs pre) | OS | 4 | 259,98 | 2 | – | Presence of menopause was associated with worse OS than absence of menopause; Similarly, postmenopausal status was associated with worse OS compared to postmenopausal status |
| PFS | 3 | 184 | 1 | – | Postmenopausal status was associated with worse PFS compared to postmenopausal status | |
| Received blood transfusion before RT (yes vs no) | OS | 1 | 179 | – | 1 | Receiving blood transfusion before RT was associated with better OS as compared to not receiving |
| Residence type (rural vs urban) | OS | 1 | 155 | 1 | – | Patients from rural residence was associated with worse OS than urban residence |
| BCSS | 1 | 155 | 1 | – | Patients from rural residence was associated with worse BCSS than urban residence | |
| Comorbidities | ||||||
| History of hypertension (yes vs no) |
OS | 2 | 243,48 | 2 | – | History of hypertension was associated with worse OS |
| Jaundice (yes vs no) | OS | 1 | 137 | 1 | – | Presence of jaundice was associated with worse OS |
| Liver function impairment (yes vs no) |
OS | 1 | 137 | 1 | – | Impaired liver function was associated with worse OS |
| Charlson/Deyo score (1 vs 0, ≥2 vs 0) |
OS | 4 | 433,38,48,85 | 4 | – | Higher Charlson/Deyo scores compared to lower scores were associated with worse OS |
| Anorexia/weight loss/ cachexia (yes vs no) |
OS | 1 | 137 | 1 | -– | Presence of anorexia/weight loss/cachexia was associated with worse OS |
| Risk factors (≥2 vs 0/1) | OS | 1 | 171 | 1 | – | Presence of multiple risk factors was associated with worse OS than having a single, or no risk factor |
| Chronic Pulmonary disease (yes vs no) | OS | 2 | 148 | 1 | – | Presence of chronic pulmonary disease was associated with worse OS compared to absence of the condition |
| Miscellaneous | ||||||
| CNS-M (Isolated CNS progression vs CNS-M plus systemic progression) | OS | 1 | 161 | 1 | – | Having isolated CNS compared with CNS-M plus systemic progression was associated with reduced risk of OS |
| Density (high vs low) | PFS | 1 | 184 | 1 | – | High density compared to low was associated with worse PFS |
| Detection of PD-L1 (+)-CTCs (yes vs no) | PFS | 1 | 147 | 1 | – | Detection of PD-L1(+)-CTCs was associated with worse PFS |
| Follow-up interval after diagnosis (2–5 years vs 1 year) | OS | 1 | 188 | 1 | – | Longer follow-up interval after diagnosis compared to shorter was associated with worse OS |
| Laterality (unknown vs right) | OS | 1 | 128 | – | 1 | Unknown laterality was associated with better OS than right laterality |
| Lymph node ratio (>0.75 vs 0.75) | OS | 1 | 198 | 1 | – | Higher lymph node ratio was associated with worse OS |
| Neutrophil-lymphocyte ratio (NLR) | OS | 1 | 162 | 1 | – | A higher NLR at diagnosis was associated with worse OS than lower NLR |
| Prescribing physician (BC specialist vs others) |
OS | 1 | 137 | – | 1 | Evaluation of a BC specialist compared with others was associated with lower risk of death within 30 days |
| Response status based on PET (responder vs non- responder) | OS | 1 | 182 | –- | 1 | Responders were associated with better OS than non-responders based on PET imaging |
| SEER region (Detroit, Kentucky vs California) | OS | 1 | 185 | 1 | – | Patients from SEER regions including Detroit and Kentucky were associated with worse OS as compared to patients from California |
Abbreviations: ALDH1, aldehyde dehydrogenase 1; ALP, alkaline phosphatase; BC, breast cancer; BCE, bone collagen equivalents per liter; BMI, body mass index; CA 15–3, cancer antigen 15–3; CEA, carcinoembryonic antigen; CRP, C-reactive protein; ESR1, estrogen receptor 1; EZH2, enhancer of zeste homolog 2; GIT1, G-protein-coupled receptor kinase interacting protein 1; IHC, immunohistochemistry; IL-18, interleukin-18; LBD, ligand-binding domain; LDH, lactate dehydrogenase; MAPT RQ, microtubule-associated protein tau; NDL, numeration and diversity of lymphocytes or lympho-divpenic status; NLR, neutrophil-lymphocyte ratio; PET, positron emission tomography; PNN, poly nuclear neutrophils; SET(ER/PR), cumulative measure of gene expression for transcripts associated with estrogen and progesterone receptors; SNP, single nucleotide polymorphism; SUVmax, maximum standard unit value; VEGF, vascular endothelial growth factor.
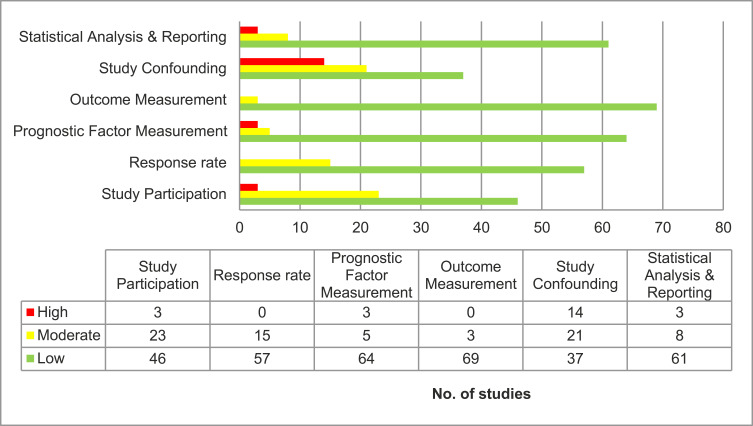
Quality of Evidence
The overall risk of bias was considered high for three studies, moderate for 22 studies, and low for the remaining 47 studies (Figure 3). Studies that failed to report exclusion criteria, definition of survival endpoints, or did not perform multivariate analysis to account for confounding were deemed “high” risk of bias.
Figure 3.
Risk of bias assessment for each domain of QUIPS tool.
Discussion
This comprehensive SLR was conducted to evaluate the strength and consistency of evidence of prognostic factors associated with survival in patients with HR+/HER2– advanced breast cancer. As commonly observed in oncology literature, OS was the most widely assessed survival endpoint, followed by PFS. The evidence was limited for tumor response (n=3) and BCSS (n=5). Hence, this review focused on prognostic factors associated with OS and PFS.
Higher CTC count, Ki67 level, number of metastases (multiple vs single), and sites of metastases (presence of liver metastases vs absence), prior therapy attributes, negative PR status, higher tumor grade, shorter time to recurrence or progression to advanced breast cancer, poor performance status and race (black vs white) were the prognostic factors with strongest evidence of association with OS and PFS. Previously published studies11−,12−,24−,100−,104 have also demonstrated the prognostic relationship between survival endpoints and disease-related factors – such as PR status, CTC count, Ki67 level, number and sites of metastasis – and treatment-related factors and performance status. Other studies in the literature103,105–107 have also reported older age, black race, and unmarried status to be associated with shorter survival rates. Future cohort studies exclusively in HR+/HER2- advanced breast cancer will be beneficial to further validate the collective set of prognostic factors with the strongest evidence.
In the advance disease setting, breast cancer is incurable and the treatment goal is mainly palliative, improving quality-of-life and prolonging survival. Many factors are generally considered in developing treatment plans including patient-related factors like patient preferences, age, menopausal status, co-morbidities, performance status, socioeconomic status, psychological factors, treatment availabilities, and disease-related factors like DFI, previous therapies, tumor burden (number and sites), and any need for rapid disease control.108
This comprehensive review substantiates the importance of these factors in clinical decision-making for HR+/HER2– advanced breast cancer. The directionality of relationship between the prognostic factors and OS and PFS was largely consistent, except for lymph node involvement with OS and de novo metastatic breast cancer with PFS. Another published study109 also reported the divergent association between lymph node involvement and OS. A retrospective cohort study109 reported patients with N1 Stage IV BC had better OS than did those without lymph node metastasis (HR=0.902, 95% CI=0.825–0.986, p-value=0.023). One potential explanation could be that the invasion of tumor cell into lymph nodes may have activated an antitumor immune response, which renders beneficial effect on patients with lymph node metastasis.110 Other studies106,111,112 have observed better OS in patients without lymph node metastasis compared to those with lymph node involvement. Similarly, the prognosis of de novo stage IV breast cancer was found to be better than those with recurrent tumors in several studies.4,113,114
Definitions of survival endpoints used across studies varied. The most common definition of OS was the time from diagnosis to death from any cause or last follow-up; many studies calculated the time interval from date of treatment initiation or patient selection. There was overlap in definitions of OS and BCSS. Gong et al55 defined BCSS as time from date of diagnosis to date of death attributed to breast cancer or date of last follow-up, while Yerushalmi et al93 defined BCSS as time from diagnosis of distant metastasis to death or censor date; two other studies did not define BCSS.22,90 It was observed that BCSS was not commonly assessed across included studies.
We observed heterogeneity in the comparison groups for certain prognostic factors – for example, different age groups being compared (eg, >50 vs ≤50, >65, 50–64 vs 18–49); different cut-off points for Ki67 levels (10%, 14%, 25%, 30%); different prior therapies were compared (initial chemotherapy vs initial endocrine therapy, adjuvant endocrine therapy vs absence of prior therapy); site of metastasis (eg, presence vs absence of liver metastasis, visceral sites vs bone). Due to the differences in categorizations of prognostic factors as well as other factors – such as differences in study design and patient population – it was not possible to perform meta-analysis or derive a single hazard ratio estimate representing the relationship between the prognostic factors and survival endpoints. Despite inconsistencies in comparators groups, we observed an overall trend in directionality of association for some prognostic factors. For example, tumor size >5 cm diameter, CTC count ≥5/7.5 mL whole blood, time to recurrence or progression to advanced breast cancer of <2 years, and multiple vs single site of metastases were associated with worse survival.
This review focused on patients with HR+/HER2– advanced breast cancer; however, in 62% of 79 included studies, the proportion of patients with HR+/HER2– breast cancer ranged between 50–79%. The results of such studies may not be reflective entirely of patients with HR+/HER2– advanced breast cancer. We observed a dearth of studies investigating the prognostic factors in exclusively patients with HR+/HER2– advanced breast cancer. For studies that included de novo metastatic patients, including in subgroups, baseline characteristics were captured in the metastatic setting. However, for the remaining studies, it was difficult to distinguish whether baseline characteristics were collected at initial diagnosis or when patients progressed to metastatic stage (as this information was not reported).
This review was subject to some limitations. An overall rating for risk of bias (low/moderate/high) was estimated for each study by taking into account the risk levels for the six domains of the QUIPS tool. The cut-off points chosen to derive the overall rating, though based on previously published SLRs, were essentially arbitrary.115–117 Other limitations may be the exclusion of non-English studies, though English language studies from across the globe were included, and that studies published before 2010 and after 2018 were not included. Since the studies included in this SLR were published between 2010–2018, there were no studies that assessed the association between the newer targeted therapies such as CDK4/6 inhibitors, mTOR inhibitor, PI3K inhibitor, or kinase inhibitors, and survival endpoints. The conference abstracts included in this SLR contained limited relevant data and full-text publications related to these abstracts were not available. We found limited evidence on the prognostic value of genetic or tumor biomarkers in patients specifically with HR+/HER2– advanced BC. Some of these studies showed the relationship between tumor markers such as LDH,51,59,79 ALP,79 CEA,51,70 CA,51,70 to be associated with survival. This review did not distinguish the nature of the outcomes assessed (ie, primary or secondary) and therefore findings must be interpreted cautiously. Additionally, there was uncertainty around the power of subgroup analyses data reported in both observational studies and trials.
Strengths of this review include that this is the first SLR, to our knowledge, to comprehensively assess prognostic factors associated with survival in patients with HR+/HER2– advanced breast cancer. This review presents a complete overview of a large number of studies published recently with multivariate robust results that would help account for confounding of other key variables in understanding the association. This review was performed based on best practice guidelines, included supplementary searches of key conference proceedings and cross-referencing of other SLRs, and incorporated a double-blind study selection process, all of which lend to the robustness of this review’s methodology.
Conclusion
The strongest evidence for prognostic factors associated with worse OS included negative PR status, higher tumor grade, higher CTC count (≥5 vs <5), higher Ki67 levels (>14%), number of metastatic sites (multiple vs single), specific sites of metastases (presence of liver metastases vs absence), shorter time to recurrence or progression to advanced breast cancer, absence of prior therapy-related attributes (type of therapy, treatment line, response of prior therapy) in early or metastatic setting, poor performance status, and race (black vs white). The strongest evidence for prognostic factors associated with worse PFS included higher CTC count, number and sites of metastases, and prior therapy-related attributes in early or metastatic settings.
Apart from the commonly used markers recommended for routine use (eg, ER, PR, HER2), evaluation of the aforementioned factors shed light on the history and pathophysiology of the breast cancer in a patient, thereby providing a comprehensive clinical picture that may enable clinicians to enhance personalized treatment approaches and supportive care to improve patient outcomes. Identification of these prognostic factors will also guide future research in the HR+/HER2– advanced breast cancer setting.
Acknowledgments
We thank Michael Friedman for his editorial inputs.
Funding Statement
ICON PLC. received funding from Eli Lilly and Company to conduct this review.
Disclosure
Keri Stenger, and Claudia Morato Guimarães are employees and shareholders of Eli Lilly and Company. Gebra Cuyún Carter was an employee of Eli Lilly and Company when the review was being conducted. She is a shareholder of Eli Lilly and Company. She reports personal fees from Exact Sciences. Maitreyee Mohanty, Shivaprasad Singuru, Vanita Tongbram are employees of ICON PLC. Pradeep Basa and Sheena Singh were employees of ICON PLC. when the review was being conducted. Dr. Kuemmel reports personal fees from Eli Lilly and Company, Roche, Genomic Health, Novartis, Amgen, Celgene, Daiichi Sankyo, Sonoscope, AstraZeneca, Somatex, MSD, Pfizer, Puma Biotechnology, PFM medical, non-financial support from Roche, Daiichi Sankyo outside the submitted work. Dr. Guarneri reports personal fees from Eli Lilly and Company, Novartis, Roche, MSD, outside the submitted work. Dr Tolaney reports grants and personal fees Eli Lilly and Company, AstraZeneca, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Exelixis, Bristol-Myers Squibb, Eisai, Nanostring, Puma, Cyclacel, sanofi, Celldex, Odonate, Seattle Genetics, Silverback Therapeutics, G1 Therapeutics, AbbVie, Anthenex, OncoPep, Kyowa Kirin Pharmaceuticals, Daiichi-Sankyo, Mersana Therapeutics, Certara, CytomX, Samsung Bioepsis Inc., Gilead, outside the submitted work.
References
- 1.NCI. Breast Cancer Treatment (PDQ®)—Health Professional Version - National Cancer Institute. General Information about Breast Cancer. Available from:https://www.cancer.gov/types/breast/hp/breast-treatment-pdq#section/_1. Accessed June12, 2018.
- 2.Caswell-Jin JL, Plevritis SK, Tian L, et al. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectrum. 2018;2(4):pky062. doi: 10.1093/jncics/pky062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss A, Chavez-MacGregor M, Lichtensztajn DY, et al. Validation study of the american joint committee on cancer eighth edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018;4(2):203–209. doi: 10.1001/jamaoncol.2017.4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21(11):2169–2174. doi: 10.1093/annonc/mdq220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balduzzi S, Mantarro S, Guarneri V, et al.Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;(6):CD006242. doi: 10.1002/14651858.CD006242.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliano M, Schettini F, Rognoni C, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360–1369. doi: 10.1016/S1470-2045(19)30420-6 [DOI] [PubMed] [Google Scholar]
- 7.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 8.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Molecular Oncology. 2011;5(1):5–23. doi: 10.1016/j.molonc.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): breast Cancer. Version 2.2017. Available athttps://www.nccn.org/professionals/physician_gls/default.aspx. Accessed August27, 2018.
- 10.Lobbezoo DJA, Van Kampen RJW, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Research and Treatment. 2013;141(3):507–514. doi: 10.1007/s10549-013-2711-y [DOI] [PubMed] [Google Scholar]
- 11.Chang E, Mougalian SS, Adelson KB, Young MR, Yu JB. Association between prolonged metastatic free interval and recurrent metastatic breast cancer survival: findings from the SEER database. Breast Cancer Research and Treatment. 2019;173(1):209–216. doi: 10.1007/s10549-018-4968-7 [DOI] [PubMed] [Google Scholar]
- 12.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–2019. doi: 10.1093/annonc/mdn424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wishart GC, Bajdik CD, Azzato EM, et al. A population-based validation of the prognostic model PREDICT for early breast cancer. European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2011;37(5):411–417. doi: 10.1016/j.ejso.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 14.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies–improving the management of early breast cancer: st Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi: 10.1093/annonc/mdv221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. The Oncologist. 2004;9(6):606–616. doi: 10.1634/theoncologist.9-6-606 [DOI] [PubMed] [Google Scholar]
- 16.Systematic Reviews. Centre for Reviews and Dissemination. University of York; 2009. Available from:https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. Accessed August 2019. [Google Scholar]
- 17.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Int Med. 2015;162(11):777–784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. 2019.
- 19.Ballman KV. Biomarker: predictive or Prognostic? J Clin Oncol. 2015;33(33):3968–3971. doi: 10.1200/JCO.2015.63.3651 [DOI] [PubMed] [Google Scholar]
- 20.Davis P, Hayden J, Springer J, Bailey J, Molinari M, Johnson P. Prognostic factors for morbidity and mortality in elderly patients undergoing acute gastrointestinal surgery: a systematic review. Can J Surg. 2014;57(2):E44–E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Internal Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 22.Arciero CA, Yang J, Peng L, et al. African American patients with breast cancer have worse prognosis than white patients in certain subtypes and stages. Breast Cancer Res Treatment. 2017;166(3):743–755. doi: 10.1007/s10549-017-4484-1 [DOI] [PubMed] [Google Scholar]
- 23.Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7(313):no pagination. doi: 10.1126/scitranslmed.aac7551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giordano A, Giuliano M, De Laurentiis M, et al. Artificial neural network analysis of circulating tumor cells in metastatic breast cancer patients. Breast Cancer Res Treatment. 2011;129(2):451–458. doi: 10.1007/s10549-011-1645-5 [DOI] [PubMed] [Google Scholar]
- 25.Uyeturk U, Oksuzoglu B, Akman T, et al. Assessment of tumor characteristics and factors affecting survival in patients with primary metastatic breast carcinoma: a Multicenter Study of the Anatolian Society of Medical Oncology. Med Oncol. 2014;31(4):929. doi: 10.1007/s12032-014-0929-0 [DOI] [PubMed] [Google Scholar]
- 26.Goetz MP, O’Shaughnessy J, Sledge GW, et al. The benefit of abemaciclib in prognostic subgroups: an exploratory analysis of combined data from the MONARCH 2 and 3 studies. Cancer Res Conf. 2017;78(4 Supplement):1. [Google Scholar]
- 27.Suh KJ, Kim SH, Lee KH, et al. Bilateral Salpingo-oophorectomy compared to gonadotropin-releasing hormone agonists in premenopausal hormone receptor-positive metastatic breast cancer patients treated with aromatase inhibitors. Cancer Res Treatment. 2017;49(4):1153–1163. doi: 10.4143/crt.2016.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Z, Deng G, Huang X, et al. Bone metastasis pattern in initial metastatic breast cancer: a population-based study. Cancer Manage Res. 2018;10:287–295. doi: 10.2147/CMAR.S155524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilabert M, Bertucci F, Esterni B, et al. Capecitabine after anthracycline and taxane exposure in HER2-negative metastatic breast cancer patients: response, survival and prognostic factors. Anticancer Res. 2011;31(3):1079–1086. [PubMed] [Google Scholar]
- 30.Bonotto M, Gerratana L, Di Maio M, et al. Chemotherapy versus endocrine therapy as first-line treatment in patients with luminal-like HER2-negative metastatic breast cancer: a propensity score analysis. Breast. 2017;31:114–120. doi: 10.1016/j.breast.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 31.Paoletti C, Regan MM, Liu MC, et al. Circulating tumor cell number and CTC-endocrine therapy index predict clinical outcomes in ER positive metastatic breast cancer patients: results of the COMETI Phase 2 trial. Cancer Res Conf. 2017;77(4Supplement 1). [Google Scholar]
- 32.Papadaki MA, Stoupis G, Theodoropoulos PA, Mavroudis D, Georgoulias V, Agelaki S. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol Cancer Ther. 2018;06:06. [DOI] [PubMed] [Google Scholar]
- 33.Xie J, Hao Y, Li N, et al. Clinical outcomes among HR+/HER2- metastatic breast cancer patients with multiple metastatic sites: a chart review study in the US. Exp Hematol Oncol. 2015;4(1):31. doi: 10.1186/s40164-015-0023-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griguolo G, Pouderoux S, Dieci MV, et al. Clinical presentation and outcome of leptomeningeal metastasis in patients with breast cancer in relation to histology and tumor subtypes. Cancer Res Conf. 2017;78(4 Supplement):1. [Google Scholar]
- 35.Guo X, Xu J, Ying E, Yu Z, Sun T. Correlation between hormone receptor status and depressive symptoms in patients with metastatic breast cancer. Oncotarget. 2017;8(31):50774–50781. doi: 10.18632/oncotarget.15037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peeters DJE, Van Dam PJ, Van Den Eynden GGM, et al. Detection and prognostic significance of circulating tumour cells in patients with metastatic breast cancer according to immunohistochemical subtypes. Br J Cancer. 2014;110(2):375–383. doi: 10.1038/bjc.2013.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cinausero M, Gerratana L, De Carlo E, et al. Determinants of last-line treatment in metastatic breast cancer. Clinical Breast Cancer. 2018;18(3):205–213. doi: 10.1016/j.clbc.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 38.Gamucci T, Mentuccia L, Natoli C, et al. A real-world multicentre retrospective study of paclitaxel-bevacizumab and maintenance therapy as first-line for HER2-negative metastatic breast cancer. J Cell Physiol. 2017;232(6):1571–1578. doi: 10.1002/jcp.25685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamura J, Kamigaki S, Fujita J, Osato H, Komoike Y. The difference in prognostic outcomes between de novo stage IV and recurrent metastatic patients with hormone receptor-positive, HER2-negative Breast Cancer. Vivo (Athens, Greece). 2018;32(2):353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyoshi Y, Shien T, Ogiya A, et al. Differences in expression of the cancer stem cell marker aldehyde dehydrogenase 1 among estrogen receptor-positive/human epidermal growth factor receptor type 2-negative breast cancer cases with early, late, and no recurrence. Breast Cancer Research. 2016;18(1):73. doi: 10.1186/s13058-016-0731-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Fan Y, Xu B. Distinct characteristics and metastatic behaviors of late recurrence in patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer: a single institute experience of more than 10 years. Clinical Breast Cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 42.Gampenrieder SP, Rinnerthaler G, Hackl H, et al. DNA methylation signatures predicting bevacizumab efficacy in metastatic breast cancer. Theranostics. 2018;8(8):2278–2288. doi: 10.7150/thno.23544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung SY, Sereika SM, Linkov F, Brufsky A, Weissfeld JL, Rosenzweig M. The effect of delays in treatment for breast cancer metastasis on survival. Breast Cancer Res Treatment. 2011;130(3):953–964. doi: 10.1007/s10549-011-1662-4 [DOI] [PubMed] [Google Scholar]
- 44.Korantzis I, Kalogeras KT, Papaxoinis G, et al. Expression of angiogenic markers in the peripheral blood of patients with advanced breast cancer treated with weekly docetaxel. Anticancer Res. 2012;32(10):4569–4580. [PubMed] [Google Scholar]
- 45.Inari H, Suganuma N, Kawachi K, et al. Expression of enhancer of zeste homolog 2 correlates with survival outcome in patients with metastatic breast cancer: exploratory study using primary and paired metastatic lesions. BMC Cancer. 2017;17(1):160. doi: 10.1186/s12885-017-3154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motomura K, Ishitobi M, Komoike Y, et al. Expression of estrogen receptor beta and phosphorylation of estrogen receptor alpha serine 167 correlate with progression-free survival in patients with metastatic breast cancer treated with aromatase inhibitors. Oncology. 2010;79(1–2):55–61. doi: 10.1159/000319540 [DOI] [PubMed] [Google Scholar]
- 47.Jacot W, Mazel M, Mollevi C, et al. Expression of PD-L1 on circulating breast cancer cells: correlation with clinicopathologic data and impact on prognosis. Cancer Res Conf. 2018;78(13 Supplement):1. [Google Scholar]
- 48.Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012;23(1):103–112. doi: 10.1007/s10552-011-9859-8 [DOI] [PubMed] [Google Scholar]
- 49.Kontani K, Hashimoto SI, Murazawa C, et al. Factors responsible for long-term survival in metastatic breast cancer. World J Surg oncol. 2014;12(1):344. doi: 10.1186/1477-7819-12-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pistilli B, Lardy-Cleaud A, Jacquet E, et al. FICHE-YOUNG: fIrst-line treatment CHoicE in hormone receptor positive (HR1)/HER2-negative metastatic breast cancer patients (MBC) 45 years old. A large observational multicenter cohort survival analysis. Ann Oncol. 2017;28(Supplement 5):v92–v93. doi: 10.1093/annonc/mdx365.043 [DOI] [Google Scholar]
- 51.Pierga JY, Hajage D, Bachelot T, et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol. 2012;23(3):618–624. doi: 10.1093/annonc/mdr263 [DOI] [PubMed] [Google Scholar]
- 52.Boudin L, Chabannon C, Sfumato P, et al. Immunohistochemical subtypes predict survival in metastatic breast cancer receiving high-dose chemotherapy with autologous haematopoietic stem cell transplantation. Eur J Cancer. 2016;57:118–126. doi: 10.1016/j.ejca.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi K, Ito Y, Matsuura M, et al. Impact of immunohistological subtypes on the long-term prognosis of patients with metastatic breast cancer. Surg Today. 2016;46(7):821–826. doi: 10.1007/s00595-015-1252-x [DOI] [PubMed] [Google Scholar]
- 54.Lobbezoo DJA, Van Kampen RJW, Voogd AC, et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the Southeast Netherlands breast cancer consortium. Ann Oncol. 2016;27(2):256–262. doi: 10.1093/annonc/mdv544 [DOI] [PubMed] [Google Scholar]
- 55.Gong Y, Zhang J, Ji P, Ling H, Hu X, Shao Z-M. Incidence proportions and prognosis of breast cancer patients with bone metastases at initial diagnosis. Cancer Med . 2018;7(8):4156–4169. doi: 10.1002/cam4.1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fountzilas G, Kotoula V, Pectasides D, et al. Ixabepilone administered weekly or every three weeks in HER2-negative metastatic breast cancer patients; a randomized non-comparative Phase II trial. PLoS One. 2013;8(7):e69256. doi: 10.1371/journal.pone.0069256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delpech Y, Wu Y, Hess KR, et al. Ki67 expression in the primary tumor predicts for clinical benefit and time to progression on first-line endocrine therapy in estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treatment. 2012;135(2):619–627. doi: 10.1007/s10549-012-2194-2 [DOI] [PubMed] [Google Scholar]
- 58.Redondo A, Ramos Vazquez M, Manso L, et al. Long-term response to first-line bevacizumab-based therapy in patients with metastatic breast cancer: results of the observational “LORENA” study. OncoTargets Ther. 2018;11:5845–5852. doi: 10.2147/OTT.S170303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manuel M, Tredan O, Bachelot T, et al. Lymphopenia combined with low TCR diversity (divpenia) predicts poor overall survival in metastatic breast cancer patients. OncoImmunology. 2012;1(4):432–440. doi: 10.4161/onci.19545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Jia Z, Ragaz J, et al. The maximum standardized uptake value of 18 F-FDG PET scan to determine prognosis of hormone-receptor positive metastatic breast cancer. BMC Cancer. 2013;13:42. doi: 10.1186/1471-2407-13-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aversa C, Rossi V, Geuna E, et al. Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014;23(5):623–628. doi: 10.1016/j.breast.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 62.Ayala de la Peña F, Yufera Soler JC, Ivars MA, et al. Neutrophil-lymphocyte ratio (NLR) as a prognostic factor in metastatic breast cancer. Ann Oncol. 2017;28(Supplement 5):v94. doi: 10.1093/annonc/mdx365.046 [DOI] [Google Scholar]
- 63.Gerratana L, Fanotto V, Bonotto M, et al. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis. 2015;32(2):125–133. doi: 10.1007/s10585-015-9697-2 [DOI] [PubMed] [Google Scholar]
- 64.Bonechi M, Galardi F, Biagioni C, et al. Plasma thymidine kinase-1 activity predicts outcome in patients with hormone receptor positive and HER2 negative metastatic breast cancer treated with endocrine therapy. Oncotarget. 2018;9(23):16389–16399. doi: 10.18632/oncotarget.24700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pascual T, Apellaniz-Ruiz M, Pernaut C, et al. Polymorphisms associated with everolimus pharmacokinetics, toxicity and survival in metastatic breast cancer. PLoS One. 2017;12(7):e0180192. doi: 10.1371/journal.pone.0180192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogiya A, Yamazaki K, Horii R, et al. Post-relapse survival in patients with the early and late distant recurrence in estrogen receptor-positive HER2-negative breast cancer. Breast Cancer. 2017;24(3):473–482. doi: 10.1007/s12282-016-0730-3 [DOI] [PubMed] [Google Scholar]
- 67.Okazaki M, Horimoto Y, Tanabe M, et al. Predictive markers for efficacy of everolimus plus exemestane in patients with luminal HER2-negative metastatic breast cancer. Med Oncol. 2018;35(4):48. doi: 10.1007/s12032-018-1112-9 [DOI] [PubMed] [Google Scholar]
- 68.Chandarlapaty S, Chen D, He W, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the bolero-2 Clinical Trial. JAMA Oncol. 2016;2(10):1310–1315. doi: 10.1001/jamaoncol.2016.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lobbezoo DJA, Van Kampen RJW, Voogd AC, et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? British Journal of Cancer. 2015;112(9):1445–1451. doi: 10.1038/bjc.2015.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao X, Xu X, Zhang Q, et al. Prognostic and predictive value of clinical and biochemical factors in breast cancer patients with bone metastases receiving “metronomic” zoledronic acid. BMC Cancer. 2011;11:403. doi: 10.1186/1471-2407-11-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Llombart-Cussac A, Pivot X, Biganzoli L, et al. A prognostic factor index for overall survival in patients receiving first-line chemotherapy for HER2-negative advanced breast cancer: an analysis of the ATHENA trial. Breast. 2014;23(5):656–662. doi: 10.1016/j.breast.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 72.Kawano A, Shimizu C, Hashimoto K, et al. Prognostic factors for stage IV hormone receptor-positive primary metastatic breast cancer. Breast Cancer. 2013;20(2):145–151. doi: 10.1007/s12282-011-0320-3 [DOI] [PubMed] [Google Scholar]
- 73.Ren Z, Li Y, Shen T, Hameed O, Siegal GP, Wei S. Prognostic factors in advanced breast cancer: race and receptor status are significant after development of metastasis. Pathology Research and Practice. 2016;212(1):24–30. doi: 10.1016/j.prp.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 74.Jansson S, Bendahl PO, Larsson AM, Aaltonen KE, Ryden L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer. 2016;16(1):433. doi: 10.1186/s12885-016-2406-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muller V, Riethdorf S, Rack B, et al. Prognostic impact of circulating tumor cells assessed with the CellSearch SystemTM and AdnaTest BreastTM in metastatic breast cancer patients: the DETECT study. Breast Cancer Research. 2012;14(4):R118. doi: 10.1186/bcr3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wallwiener M, Hartkopf AD, Baccelli I, et al. The prognostic impact of circulating tumor cells in subtypes of metastatic breast cancer. Breast Cancer Res Treatment. 2013;137(2):503–510. doi: 10.1007/s10549-012-2382-0 [DOI] [PubMed] [Google Scholar]
- 77.Beije N, Onstenk W, Kraan J, et al. Prognostic Impact of HER2 and ER status of circulating tumor cells in metastatic breast cancer patients with a HER2-negative primary tumor. Neoplasia (United States). 2016;18(11):647–653. doi: 10.1016/j.neo.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leone BA, Vallejo CT, Romero AO, et al. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res Treatment. 2017;161(3):537–548. doi: 10.1007/s10549-016-4066-7 [DOI] [PubMed] [Google Scholar]
- 79.Nieder C, Dalhaug A, Haukland E, Mannsaker B, Pawinski A. Prognostic Impact of the Tumor Marker CA 15-3 in##. J Clin Med Res. 2017;9(3):183–187. doi: 10.14740/jocmr2653w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carbognin L, Sperduti I, Ciccarese M, et al. Prognostic model for advanced breast carcinoma with luminal subtype and impact of hormonal maintenance: implications for post-progression and conditional survival. Breast. 2016;29:24–30. doi: 10.1016/j.breast.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 81.Staudigl C, Concin N, Grimm C, et al. Prognostic relevance of pretherapeutic gamma-glutamyltransferase in patients with primary metastatic breast cancer. PLoS One. 2015;10(4):e0125317. doi: 10.1371/journal.pone.0125317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park S, Yoon JK, Jin Lee S, Kang SY, Yim H, An YS. Prognostic utility of FDG PET/CT and bone scintigraphy in breast cancer patients with bone-only metastasis. Medicine (United States). 2017;96(50):e8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munzone E, Botteri E, Sandri MT, et al. Prognostic value of circulating tumor cells according to immunohistochemically defined molecular subtypes in advanced breast cancer. Clinical Breast Cancer. 2012;12(5):340–346. doi: 10.1016/j.clbc.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 84.Elsamany S, Alzahrani A, Elkhalik SA, et al. Prognostic value of mammographic breast density in patients with metastatic breast cancer. Medical Oncol. 2014;31(8):96. doi: 10.1007/s12032-014-0096-3 [DOI] [PubMed] [Google Scholar]
- 85.Vaz-Luis I, Lin NU, Keating NL, et al. Racial differences in outcomes for patients with metastatic breast cancer by disease subtype. Breast Cancer Res Treatment. 2015;151(3):697–707. doi: 10.1007/s10549-015-3432-1 [DOI] [PubMed] [Google Scholar]
- 86.Demian SR, Hamdy M, Ali IM. The regulatory effects of interleukin-12 on interleukin-18 and interferon-gamma production in Egyptian breast cancer patients. Alexandria J Med. 2011;47(4):283–290. doi: 10.1016/j.ajme.2011.07.016 [DOI] [Google Scholar]
- 87.Kono M, Fujii T, Matsuda N, et al. Somatic mutations, clinicopathologic characteristics, and survival in patients with untreated breast cancer with bone-only and non-bone sites of first metastasis. Journal of Cancer. 2018;9(19):3640–3646. doi: 10.7150/jca.26825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bertaut A, Mounier M, Desmoulins I, et al. Stage IV breast cancer: a population-based study about prognostic factors according to HER2 and HR status. European Journal of Cancer Care. 2015;24(6):920–928. doi: 10.1111/ecc.12306 [DOI] [PubMed] [Google Scholar]
- 89.Dominici L, Najita J, Hughes M, et al. Surgery of the primary tumor does not improve survival in stage IV breast cancer. Breast Cancer Research & Treatment. 2011;129(2):459–465. doi: 10.1007/s10549-011-1648-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu SG, Zhang WW, Sun JY, et al. The survival benefits of local surgery in stage IV breast cancer are not affected by breast cancer subtypes: a population-based analysis. Oncotarget. 2017;8(40):67851–67860. doi: 10.18632/oncotarget.18889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.den Brok WD, Speers CH, Gondara L, Baxter E, Tyldesley SK, Lohrisch CA. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Research and Treatment. 2017;161(3):549–556. doi: 10.1007/s10549-016-4080-9 [DOI] [PubMed] [Google Scholar]
- 92.Ercolani G, Zanello M, Serenari M, et al. Ten-year survival after liver resection for breast metastases: a single-center experience. Digestive Surgery. 2018;35(4):372–380. doi: 10.1159/000486523 [DOI] [PubMed] [Google Scholar]
- 93.Yerushalmi R, Tyldesley S, Kennecke H, et al. Tumor markers in metastatic breast cancer subtypes: frequency of elevation and correlation with outcome. Annals of Oncology. 2012;23(2):338–345. doi: 10.1093/annonc/mdr154 [DOI] [PubMed] [Google Scholar]
- 94.Desille-Gbaguidi H, Avigdor S, Body G, Ouldamer L. Survival impact of primary site surgery on metastatic breast cancer patients at diagnosis. Journal of Gynecology Obstetrics and Human Reproduction. 2018;21:21. [DOI] [PubMed] [Google Scholar]
- 95.King TA, Lyman JP, Gonen M, et al. Prognostic Impact of 21-Gene Recurrence Score in Patients With Stage IV Breast Cancer: TBCRC 013. Journal of Clinical Oncology. 2016;34(20):2359–2365. doi: 10.1200/JCO.2015.63.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lawrie CH, Goicoechea I, Rezola R, et al. Spatial intratumoural heterogeneity in the expression of GIT1 is associated with poor prognostic outcome in oestrogen receptor positive breast cancer patients with synchronous lymph node metastases. F1000Research. 2018;6:1606. doi: 10.12688/f1000research.12393.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lau R, Fu L, Samuels M, et al. A targeted RNA-seq assay to measure activating ER mutations and ER/PR-associated gene expression predicts sensitivity to endocrine therapy for metastatic breast cancer. Cancer Res Conf. 2017;77(13 Supplement):1. [Google Scholar]
- 98.Turker I, Arslan UY, Yazici O, et al. Prognostic factors in operated stage IIIC, pathological N3a breast cancer patients. Breast Care. 2014;9(6):421–427. doi: 10.1159/000366438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.PRISMA: transparent Reporting of Systematic Reviews and Meta-analyses. Available from::http://prisma-statement.org/Last. AccessedJune12, 2018.
- 100.Marshall EM, Bertaut A, Desmoulins I, et al. Prognostic factors of survival among women with metastatic breast cancer and impact of primary or secondary nature of disease on survival: a french population-based study. The Breast Journal. 2017;23(2):138–145. doi: 10.1111/tbj.12717 [DOI] [PubMed] [Google Scholar]
- 101.Geiger S, Cnossen JA, Horster S, DiGioia D, Heinemann V, Stemmler HJ. Long-term follow-up of patients with metastatic breast cancer: results of a retrospective, single-center analysis from 2000 to 2005. Anti-Cancer Drugs. 2011;22(9):933–939. doi: 10.1097/CAD.0b013e32834860af [DOI] [PubMed] [Google Scholar]
- 102.Khanfir A, Lahiani F, Bouzguenda R, Ayedi I, Daoud J, Frikha M. Prognostic factors and survival in metastatic breast cancer: a single institution experience. Rep Pract Oncol Radiother. 2013;18(3):127–132. doi: 10.1016/j.rpor.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jang RW, Caraiscos VB, Swami N, et al. Simple prognostic model for patients with advanced cancer based on performance status. Journal of Oncology Practice. 2014;10(5):e335–e341. doi: 10.1200/JOP.2014.001457 [DOI] [PubMed] [Google Scholar]
- 104.Di Leo A, O’Shaughnessy J, Sledge GW Jr, et al. Prognostic characteristics in hormone receptor-positive advanced breast cancer and characterization of abemaciclib efficacy. NPJ Breast Cancer. 2018;4:41. doi: 10.1038/s41523-018-0094-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gupta V, Haque I, Chakraborty J, Graff S, Banerjee S, Banerjee SK. Racial disparity in breast cancer: can it be mattered for prognosis and therapy. J Cell Commun Signal. 2018;12(1):119–132. doi: 10.1007/s12079-017-0416-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ganggayah MD, Taib NA, Har YC, Lio P, Dhillon SK. Predicting factors for survival of breast cancer patients using machine learning techniques. BMC Med Inform Decis Mak. 2019;19(1):48. doi: 10.1186/s12911-019-0801-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martínez ME, Unkart JT, Tao L, et al. Prognostic significance of marital status in breast cancer survival: a population-based study. PLoS One. 2017;12(5):e0175515. doi: 10.1371/journal.pone.0175515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)dagger. Ann Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang C, Wang P, Li X, et al. Lymph node status have a prognostic impact in breast cancer patients with distant metastasis. PLoS One. 2017;12(8):e0182953. doi: 10.1371/journal.pone.0182953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.da Cunha A, Antoniazi Michelin M, Cândido Murta EF. Phenotypic profile of dendritic and T cells in the lymph node of Balb/C mice with breast cancer submitted to dendritic cells immunotherapy. Immunology Letters. 2016;177:25–37. doi: 10.1016/j.imlet.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 111.Karihtala P, Jääskeläinen A, Roininen N, Jukkola A. Prognostic factors in metastatic breast cancer: a prospective single-centre cohort study in a Finnish University Hospital. BMJ Open. 2020;10(10):e038798. doi: 10.1136/bmjopen-2020-038798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giordano SH. Update on locally advanced breast cancer. The Oncologist. 2003;8(6):521–530. doi: 10.1634/theoncologist.8-6-521 [DOI] [PubMed] [Google Scholar]
- 113.Yardley DA, Kaufman PA, Brufsky A, et al. Treatment patterns and clinical outcomes for patients with de novo versus recurrent HER2-positive metastatic breast cancer. Breast Cancer Research and Treatment. 2014;145(3):725–734. doi: 10.1007/s10549-014-2916-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lambertini M, Ferreira AR, Di Meglio A, et al. Patterns of care and clinical outcomes of HER2-positive metastatic breast cancer patients with newly diagnosed stage IV or recurrent disease undergoing first-line trastuzumab-based therapy: a multicenter retrospective cohort study. Clinical Breast Cancer. 2017;17(8):601–610.e602. doi: 10.1016/j.clbc.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 115.den Bakker CM, Anema JR, Zaman A, et al. Prognostic factors for return to work and work disability among colorectal cancer survivors; a systematic review. PLoS One. 2018;13(8):e0200720. doi: 10.1371/journal.pone.0200720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thompson JY, Byrne C, Williams MA, Keene DJ, Schlussel MM, Lamb SE. Prognostic factors for recovery following acute lateral ankle ligament sprain: a systematic review. BMC Musculoskeletal Disorders. 2017;18(1):421. doi: 10.1186/s12891-017-1777-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rushton A, Zoulas K, Powell A, Staal JB. Physical prognostic factors predicting outcome following lumbar discectomy surgery: systematic review and narrative synthesis. BMC Musculoskeletal Disorders. 2018;19(1):326. doi: 10.1186/s12891-018-2240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]