Abstract
Objective
To synthesize evidence on the prevalence and incidence of physical health conditions in people with intellectual disability (ID).
Methods
We searched Medline, PsycInfo, and Embase for eligible studies and extracted the prevalence, incidence, and risk of physical health conditions in people with ID.
Results
Of 131 eligible studies, we synthesized results from 77 moderate- to high-quality studies, which was mainly limited to high-income countries. The highest prevalence estimates were observed for epilepsy, ear and eye disorders, cerebral palsy, obesity, osteoporosis, congenital heart defects, and thyroid disorders. Some conditions were more common in people with a genetic syndrome. Compared with the general population, many health conditions occur more frequently among people with ID, including asthma and diabetes, while some conditions such as non-congenital circulatory diseases and solid cancers occur at the same or lower rate. The latter associations may reflect under-detection.
Conclusions
People with ID have a health profile more complex than previously known. There is a pressing need for targeted, evidence-informed population health initiatives including preventative programs for this population.
Introduction
People with intellectual disability (ID), defined by cognitive and adaptive-functioning impairments with onset during childhood, constitute about 1% of the global population [1]. They experience significant health inequalities and barriers to effective healthcare and are more likely to die prematurely and from potentially avoidable causes than the general population [2, 3]. The excess deaths amenable to healthcare interventions suggest inadequate recognition of health needs specific to people with ID in healthcare policy and systems [4, 5]. Effective and equitable healthcare requires a comprehensive understanding of the epidemiology of health conditions in this population.
The evidence on psychiatric conditions in people with ID is extensive [6–8], whereas that for physical health conditions is relatively sparse [9]. Compared to the general population, people with ID have a higher prevalence of physical conditions [10], especially neurological disorders, sensory impairments, obesity, constipation, and congenital malformation [11–15]. In contrast, they are less likely to have solid cancers [16, 17]. Low awareness of the disease epidemiology may expose this population to underdiagnosis, misdiagnosis, inappropriate pharmaceutical interventions, and missed opportunities for preventative healthcare.
Previous reviews mainly examined single health conditions [11, 18–32], such as epilepsy, or a specific sub-population (e.g., people with Down Syndrome (DS)) [15, 33]. Only one review included risk estimates relative to the general population [34]. Importantly, few reviews required representative populations [35], and none, to our knowledge, excluded studies potentially subject to bias.
This systematic review aimed to synthesise the population-based prevalence and incidence of physical health conditions in people with ID and compared with the general population. We sought to better inform approaches to recognition and management of physical health conditions and the inclusion of this vulnerable population in preventative health programs and policy.
Methods
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and is registered with PROSPERO (CRD42019132214) [36]. Throughout this paper, ID, if unspecified, refers to ID of any aetiology.
Search strategy
We searched MEDLINE, PsycINFO, and EMBASE. Search terms included ID, physical disorders, and prevalence/incidence or related terms. Each search term was translated into Medical Subject Headings where possible. Details are provided in S5 File. Initially, journal articles published in English on or before 28 February 2020 were included; we also searched reference lists of included articles. The search was updated on 11 May 2021.
Study selection
Two reviewers independently screened titles and abstracts to identify original peer-reviewed studies reporting prevalence or incidence of physical disorders in people with ID or genetic syndromes invariably related to ID, such as DS.
Two reviewers then screened the full texts independently. Studies were eligible if they were quantitative, observational studies reporting prevalence or incidence, or values that permitted their calculation. Samples were considered population-based if they were derived from a representative sampling frame such as multiple disability services, disability services combined with multiple healthcare organisations, birth cohorts/registries, national or regional medical registries of people with ID, or both mainstream and special schools. Purely hospital-based samples were excluded unless the authors justified representativeness or where health initiatives are known to prompt proactive identification and registration of people with ID (i.e., UK GP registries). Studies with voluntary/convenience participants or people with additional specific health conditions were excluded, as they were not likely to represent the whole ID population and were at risk of selection bias. To maximise comparability across studies and generalisability of findings, we excluded conditions that could not be mapped to the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10).
We only included the study with the highest quality rating if multiple studies had the same or largely overlapping study populations; when such studies received the same quality rating, only the most recent was included.
Data extraction and quality assessment
We developed a standardised data extraction form for study characteristics, participants (including people with ID and controls), and outcomes. Data were extracted by the primary author and cross-checked by the second reviewer. Disagreements were resolved through consensus and discussion with a third reviewer if necessary.
We adapted the Newcastle-Ottawa Scale (NOS), a validated and widely used tool, to assess the quality of included studies (S1 and S2 Tables in S1 File) [37]. The NOS framework was unchanged, the adaptations made the NOS fit-for-purpose for our study design. Two reviewers independently classified studies as low, medium, or high quality (S3 Table in S1 File).
Data synthesis
Low quality studies were excluded to maximise evidence validity. Disorders were grouped into ICD-10 disease chapters. We used a narrative analysis approach [38], as heterogeneous samples and methodologies prevented meta-analysis. Prevalence and incidence were summarized separately for people with ID of any aetiology and with genetic causes (e.g., DS). For comparative studies, only age-adjusted estimates (i.e., controlled for age or single age group) were included.
Patients and public involvement
No patients or the public were involved in any study stage as the research reviewed published data.
Results
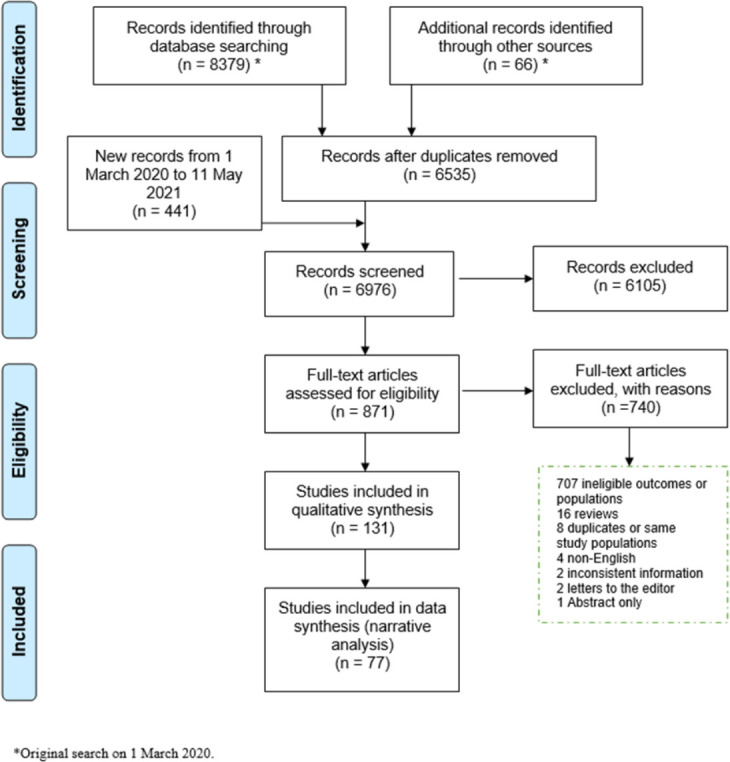
In total, we identified 6976 studies from the database and manual searches after removing duplicates (Fig 1). 871 full texts were assessed for eligibility, and 131 studies published 1970–2021 met the inclusion criteria.
Fig 1. PRISMA flowchart diagram for study selection.
Study populations
S4 and S5 Tables in S2 File show the study characteristics. Over half were conducted in Europe (n = 77), predominantly the UK (n = 30) and Scandinavia (n = 26). The remaining were mainly from North America and Australasia. No studies were from low-income countries.
The sample size ranged from 24 to 64 008. Sixty-eight studies did not distinguish ID aetiology, while 63 included people only with DS (n = 51), Prader-Willi syndrome (PWS; n = 3), Fragile X syndrome (FXS; n = 3), Rett syndrome (RS; n = 3), or others (n = 3).
Forty-eight studies reported ID severity; most were mild to profound (n = 38). All except 3 studies reported participant age: ≥14 years (n = 56), children and young adults (£29 years) (n = 49), and children and adults (n = 23).
Forty-two studies included comparison populations, either individuals without ID or chromosomal abnormalities, or the general population (S6 and S7 Tables in S2 File). The comparison group(s) ranged in size from 23 to 16 813 290.
Study quality and methods
The score for each quality assessment item can be found in S8 Table in S3 File; the majority of studies were judged to be of moderate or high quality. Seventy-six studies identified people with ID from national or regional registries including registries linked to birth defect surveillance or primary health care (n = 61), national or regional surveys (n = 7), or birth cohorts (n = 8). Twelve studies diversified their sample sources to make their study populations as representative as possible, with disability and health service users being the most frequent combination. Forty-three studies involved a single source of participants such as disability service users.
ID was ascertained by clinical examination (7.6%), medical records/registry (58.8%), non-health registries such as schools (2.3%), and self- or informant-reports (5.3%). Diagnostic classification systems for ID varied across studies; ICD (n = 25) [13, 16, 39–61], American Association on Intellectual and Developmental Disabilities (n = 5) [62–66], other (n = 16) [10, 67–80], and the remainder did not report the diagnostic guideline used (n = 85). The methods to ascertain the health conditions included clinical examination (11.1%), medical records/registry (54.3%), and self- or informant-reports (29.7%).
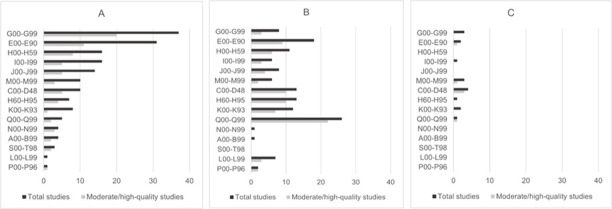
Seventy-seven studies were of moderate to high quality and entered the final evidence synthesis. Over half of these studies were conducted in Scandinavia and the UK. Study populations included children and adults with ID of any aetiology (n = 31) and with a specific genetic syndrome (n = 46). Twenty-two studies reported ID severity, and most covered all severities, from mild to profound. Study populations were mostly derived from multiple sources or comprehensive healthcare registries (i.e., UK GP registries and hospital records to form a birth cohort). Only a few studies were solely based on disability service records. ID diagnosis was ascertained from medical records (n = 61), clinical assessments (n = 7), or was not reported (n = 9). Few studies reported the diagnostic classification system; only 19 used the ICD. Most studies (n = 58) ascertained at least one health outcome from medical records, and 14 studies performed physical examinations. Fig 2 shows the distribution of physical disorders before and after exclusion based on study quality.
Fig 2.
Number of studies by ICD-10 disease chapter before and after quality assessment for (A) people with ID of any aetiology, (B) people with Down syndrome, and (C) people with other genetic syndromes. G00-G99: Neurological disorders; E00-E90: Endocrine, nutritional, and metabolic disorders; H00-H59: Eye disorders; I00-I99: Cardiovascular disorders; J00-J99: Respiratory disorders; M00-M99: Musculoskeletal disorders; C00-D48: Neoplasms; H60-H95: Ear disorders; K00-K93: Gastrointestinal disorders; Q00-Q99: Congenital abnormalities; N00-N99: Genitourinary disorders; A00-B99: Infectious disorders; S00-T98: Injuries; L00-L99: Skin disorders; P00-P96: Perinatal-oriented disorders.
Nineteen studies with comparison groups were rated as moderate to high quality. These studies reported odds/prevalence/incidence/standardised incidence ratios (OR/PR/IR/SIR). Comparison groups were mainly from the same registries or surveys. All but one study adjusted for demographic factors or included only children. This study was classified as moderate quality because other methodological features were robust [81], however, the PRs from this study were not included in the evidence synthesis.
Summary of prevalence and incidence of physical health conditions
S9–S12 Tables in S4 File display results from the 77 moderate- to high-quality studies by ICD-10 disease chapter and measure type. A wide range of physical health conditions was identified, spanning 15 ICD-10 disease chapters. The number of studies varied strikingly across ICD-10 disease chapters (Fig 2).
The most prevalent physical health conditions in people with ID of any aetiology
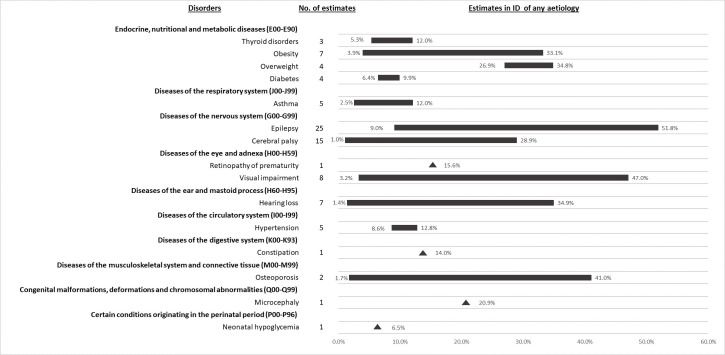
Epilepsy (9.0%-51.8%) [10, 44, 45, 51–53, 58, 65–69, 72, 81–87], visual impairment (3.2%-47.0%) [10, 44, 52, 65, 66, 82, 83], hearing loss (1.4%-34.9%) [10, 65, 66, 82, 88], osteoporosis (1.7%-41.0%) [68, 89], obesity/overweight (3.9%-34.8%) [46, 52, 66, 83, 90–92], cerebral palsy (1.0%-28.9%) [65, 67, 69, 72, 82, 84, 86], and microcephaly (20.9%) [69] were the most prevalent disorders in people with ID (Fig 3). Prevalence estimates varied markedly across studies for several disorders, likely due to variation in study population age and ID severity. For example, lower prevalence estimates for obesity were observed in adolescents and relatively young adults than in older populations [66, 91, 92]. For epilepsy and cerebral palsy, the lowest estimates were for older people [58, 84], and the highest estimates for people of all ages and for children with severe to profound ID [67, 82]. Two epilepsy studies observed trends by level of ID severity (12.2%-12.4%-22.8%-59% [45] and 11.3%-12.5%-15.3%-26.3% [65] for mild-moderate-severe-profound respectively). The corresponding prevalence estimates for a cerebral palsy study were 12.2%-14.3%-33.7%-74.6% [65].
Fig 3. Prevalence range (%) of 15 most common physical health conditions in people with ID of any aetiology.
The most prevalent physical health conditions in people with genetic syndromes
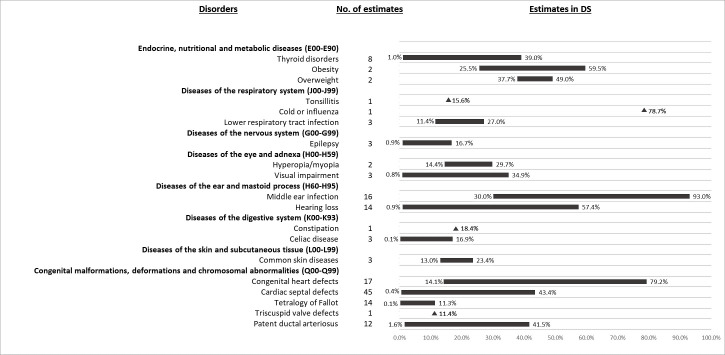
The disease pattern in the whole ID population was similarly in people with DS (Fig 4). Obesity/overweight (25.5%-59.5%) [71, 93, 94], visual impairment including blindness (0.8%-34.9%) [94–96], hearing loss (0.9%-57.4%) [88, 97–103], middle ear infection (30.0%-93.0%) [97, 99, 102, 104, 105], congenital heart defects (unspecified) (14.1%-79.2%) [40, 43, 47, 57, 59, 76, 78, 96, 97, 101, 105–112], cold or influenza (78.7%) [99], thyroid disorders (1.0%-39.0%) [95, 97, 96, 99, 105, 107, 112, 113], common skin diseases (13.0%-23.4%) [96, 113], refractive error (14.4%-29.7%) [99], and lower respiratory tract infection (11.4%-27.0%)[97, 99] were common in people with DS (Fig 4). For hearing and visual impairments, the lower prevalence estimates were found for specific hearing [98, 100, 102, 104] or visual impairments [94, 96], including deafness (0.9%) [101] and blindness (0.8%) [94], and higher prevalence estimates for unspecified impairments. The lowest prevalence of unspecified hearing loss in children with DS was based on parent-reported data [99]. The age of the study populations may partly explain these variations. For example, the highest prevalence of middle ear infection in people with DS was in children aged one (93.0%) and it decreased with increasing age [105]. Thyroid disorders also appeared to be age sensitive, with lower prevalence estimates for children or adolescents [99, 107, 112]. One study reported a higher prevalence of thyroid disorder for participants aged 65–74 (45.5%) than those aged 45–64 (33.6%) [95].
Fig 4. Prevalence range (%) of 15 most common physical health conditions in people with Down syndrome.
Prevalence estimates in people with other genetic syndromes are limited. Hypothyroidism, hypogonadism and cryptorchidism were prevalent in children and adolescents with PWS at 24.4%, 93.1% and 87.8%, respectively [70]. Additionally, scoliosis was found in 33.0% of people with Angelman syndrome [114].
The incidence of physical health conditions in people with ID
Most studies reporting incidence examined cancer (S10 Table in S4 File). Crude incidence rates for all cancers ranged from 12.8 to 33.1 per 10 000 person-years in people with ID of any aetiology [16, 77], from 6.4 to 12.6 per 10 000 person-years in people with DS [54, 115–117] and 29 per 10 000 person-years in people with PWS [55]. The incidence of solid tumours was low, regardless of ID aetiology, with the highest incidence observed for gastrointestinal cancer in people with mild to moderate ID [16]. There was a high incidence of leukemia in children with DS [115]. The cancer incidence rates were not age-standardised, thus differences in age distributions between ID subgroups may partly explain the observed variation.
Two studies reported the incidence of other conditions. A British study examined injury incidence and found that on average 20 per 100 adults with ID experienced accidental injuries within one year [44]. Another study reported an incidence of 32.5 per 10 000 person-years for celiac disease in people with DS [61].
Comparisons of physical health conditions between people with ID and the general population
Risk estimates for physical health conditions from 14 ICD-10 disease chapters were identified (S11 and S12 Tables in S4 File).
People with ID of any aetiology
People with ID of any aetiology had a significantly elevated risk of 16 diseases. Diabetes (undefined or both type I and II), epilepsy and asthma were consistently reported to be more prevalent [10, 53, 68, 95, 118]. Epilepsy had the highest effect size, ranging from 23.73 to 31.03. Thyroid disorders (OR = 2.36; 95% CI = 2.17, 2.58) [10], constipation (OR = 11.19; 95% CI = 10.97, 12.68) [10], hearing loss (OR = 2.81; 95% CI = 2.59, 3.06) [10], visual impairment (OR = 7.81; 95% CI = 6.86, 8.89) [10], retinopathy of prematurity (PR = 2.79; p < 0.05) [39], injuries (SIR = 1.78; 95% CI = 1.44, 2.17) [44], osteoporosis (PR = 1.84; 95% CI = 1.60, 2.12) [68], migraine (OR = 1.32; 95% CI = 1.02, 1.71) [10], Parkinson’s disease (OR = 2.82; 95% CI = 1.95, 4.13) [10], bronchiectasis (OR = 1.68; 95% CI = 1.08, 2.61) [10], and gallbladder cancer (SIR = 2.8; 95% CI = 1.1, 5.8)[16] were reported to occur more frequently in people with ID.
People with ID had a significantly lower risk of some conditions, including diverticular disease (OR = 0.49; 95% CI = 0.25, 0.96) [10], prostate disease (OR = 0.60; 95% CI = 0.44, 0.82) [10], inflammatory arthritis (OR = 0.57; 95% CI = 0.48, 0.67) [10], multiple sclerosis (OR = 0.49; 95% CI = 0.39, 0.63) [10], chronic sinusitis (OR = 0.44; 95% CI = 0.26, 0.62) [10], and cancer (SIR = 0.9; 95% CI = 0.8, 1.0) [16]. Specifically, cancers of the male genital organs (SIR = 0.4; 95% CI = 0.1, 0.8) and urinary tract (SIR = 0.3; 95% CI = 0.1, 0.7) were observed less frequently in people with ID in one study [16].
Risk of coronary heart disease was reduced by 35–56% among adults with ID in the UK (PR = 0.65, 95% CI = 0.57, 0.74; OR = 0.43, 95% CI = 0.37, 0.51), while risk of hypertension (PR = 0.93, 95% CI = 0.89, 0.98; OR = 0.72, 95% CI = 0.66, 0.78), chronic obstructive pulmonary disease (PR = 0.84, 95% CI = 0.71, 0.99; OR = 0.84, 95% CI = 0.73, 0.97) and atrial fibrillation (PR = 0.91, 95% CI = 0.75, 1.09; OR = 0.83, 95% CI = 0.61, 0.98) were modestly decreased [10, 68]. The evidence is mixed for peripheral vascular disease, (OR = 0.44, 95% CI = 0.33, 0.60 [10]; PR = 0.90, 95% CI = 0.69, 1.17) [68], heart failure (PR = 2.26; 95% CI = 1.84, 2.78 [68]; PR = 1.11; 95% CI = 0.89, 1.43 [10]) and chronic kidney disease (PR = 1.64; 95% CI = 1.49, 1.82 [68]; OR = 1.11; 95% CI = 0.93, 1.32 [10]). These risk estimates are from two UK GP practice studies with similar population characteristics and similar methods [10, 68]. One study randomly selected non-ID controls from the same GP practice as each case, matched on age and sex, and thus may have controlled for any practice variation in diagnostic care and the quality and completeness of recording for individuals with and without ID [68].
The published evidence indicated similar risks in people with ID and the general population for HIV, viral hepatitis [10, 119], gastrointestinal diseases (inflammatory bowel disease, irritable bowel syndrome) [10], glaucoma [10], and many types of cancer (mainly solid cancers such as breast cancer) [16]. All of these studies were based on existing medical or administrative records.
People with genetic syndromes
Fewer physical conditions were compared in the DS population than in the whole ID population. People with DS showed a higher risk of congenital malformations, hidradenitis suppurativa, and certain cancers. The well-established link between DS and congenital malformations was confirmed, especially for congenital heart defects (unspecified) (PR = 47, 95% CI = 44, 49; PR = 108, P<10-6) [43, 78]. For specific heart defects, the risk ratios were very high, for example: 1009 (P<10-6) for atrioventricular septal defect in a US infant population [78]; 850 (95% CI = 171, 1008) in a Norwegian population adjusted for maternal age and birth year [43]; and 510 (95% CI = 126.7, 999) in a South Korean population adjusted for age and sex [59]. There was also a significant increased risk of other malformations, such as gastrointestinal defects and congenital cataracts [78].
Regarding non-congenital disorders, one study reported a 5-times higher likelihood of hidradenitis suppurativa (OR = 5.24; 95% CI = 4.62, 5.94) [71]. All studies reported an elevated risk of leukemia in people with DS (SIR range of statistically significant estimates = 25.18 to 36). The highest risk was observed for acute myeloid leukemia (SIR range = 60 to 141) [42]. The high prevalence of leukemia in DS, especially in children, may account for the increased risk of cancer overall in children and adolescents in one study (SIR = 4.67; 95% CI = 1.9, 9.6) [116]. Otherwise, a non-significant decrease in overall cancer risk was reported in another study for people of all ages [117]. Lower risk of solid tumours has been observed in people with DS. One study reported a decreased risk of all solid cancers (SIR = 0.45; 95% CI = 0.34, 0.59) [117]. However, the evidence for individual solid cancers is inconclusive due to small case numbers [117].
For other genetic syndromes, evidence suggests people with Bardet-Biedl syndrome [120] or FXS [75] have a similar cancer risk to the general population.
Discussion
This is the first systematic review to present a comprehensive summary of physical morbidity in people with ID from moderate to high quality population-based studies. We identified hitherto unknown health needs for this population, such as diabetes and asthma, and identified potential under-diagnosis of multiple disorders. Our findings strongly emphasize the importance of preventative health care, early detection, and close management of complex health care needs in people with ID. The substantial knowledge gap for many conditions and countries and the methodological limitations identified in the review offer guidance for future research directions.
Main findings and comparison with other studies
This review identified high morbidity in people with ID. Epilepsy, cerebral palsy, sensory disorders, and metabolic and nutritional disorders were the most common conditions. Constipation, previously shown to be a leading health problem in this population [121], had only a moderately high estimated prevalence. The highest prevalence estimates in people with genetic syndromes were reported for congenital heart defects and otitis media in children with DS and endocrine abnormalities in PWS. Unsurprisingly, most of the highly prevalent health conditions are associated with the disability or genetic syndrome [122]. Whilst our findings are primarily in keeping with the existing knowledge from previous reviews and clinical experience [11, 23, 33, 123–128], this review indicates their relevance in representative populations with ID.
Regarding comparisons to the general population, people with ID have increased risk of health conditions across 12 different ICD-10 disease chapters. While elevated risk has been previously documented for some conditions, such as epilepsy and sensory impairments [34, 23], our findings of associations between ID and diabetes, asthma, migraine, and hidradenitis suppurativa, are less well known [19, 20, 129, 130]. For people with DS, genetic susceptibility, along with associated obesity and abnormal immune system function, may predispose them to hidradenitis suppurativa [131, 132]. The other conditions are likely to occur more commonly among people with ID due to overrepresented risk factors [20, 133, 134].
The review also observed lower risks of some conditions in people with ID compared to the general population, including diverticular disorders, some circulatory diseases, and certain solid cancers. A lower risk of cancer and non-congenital circulatory diseases in this population is in line with mortality data [2, 3], possibly partly explained by low smoking rates [3], however, an alternative explanation is that many such diseases require proactive help-seeking behaviour and/or specific investigations. Thus, timely detection may be less likely in a person with ID [66, 135], for whom there are known barriers to seeking healthcare, especially to accessing preventative healthcare crucial to early disease detection [136]. All studies reporting lower disease risk relied on existing health records and thus may reflect an underestimation of morbidity, rather than a real reduction in risk.
Overall, the results of the current review need to be interpreted with caution for several reasons in addition to potential under-detection. First is the selection of the study population. Most samples were from a combination of disability and health services or primary health care registries in the UK, which may underrepresent people with mild ID, and may not be generalisable to other settings or populations. Relatedly, morbidity estimates of conditions linked to ID severity, such as epilepsy, may be impacted. Furthermore, few studies reported ID severity, limiting the clinical translation. Despite being robust, the estimated increased risk of several conditions was limited to adults with ID of any aetiology or DS only (for hidradenitis suppurativa), thus the generalisability to other age groups or other genetic syndromes is unknown.
Implications for clinical and community practice
Our findings highlight the health needs specific to people with ID that require recognition and response. First is the high prevalence of several chronic health conditions, some of which may not be preventable but contribute to overall health burden and may compound management or outcomes associated with other diseases if left poorly managed. For example, epilepsy in people with ID is associated with more acute hospital visits, more comorbidities, and premature death [53, 137, 138]. Despite the importance of improving management for such conditions, current practice is generally suboptimal [139]. Second, this population appears to be more vulnerable to physical conditions which present risk for subsequent potentially avoidable deaths. Examples include diabetes, asthma, and injuries. This finding further emphasises the need for better prevention and tailored management of health conditions to reduce the risk. Last, optimal disease management cannot be achieved without early detection. The under-detection implied by this review suggests inadequate inclusion of people with ID in screening and preventative health programs and highlights the urgent need for this to be remedied. For example, evidence-based tools such as the Comprehensive Health Assessment Program can be introduced to help clinical decision making when attending to this group [140, 141]. Additionally, adjustments to services are needed to cater for needs related to disability features, such as immobility, and those related to other common concomitant disabilities.
Implications for future research
We observed two major knowledge gaps. The first is the lack of research on some age- and lifestyle-related health conditions in people with ID. This is concerning as the morbidity rates and related burdens are likely to increase as a result of increasing longevity and generally unhealthier lifestyles associated with transitions to living in the community [142–145]. Most evidence for cardiovascular disorders and risk factors stems from the UK [10, 68, 81, 97, 112], thus the generalisability to other countries is unclear. Future research should aim to provide representative data from different countries for health conditions related to aging and lifestyle factors, especially from low- and middle-income settings, where people with ID may be less likely to have their health needs identified and met [146, 147].
As discussed previously, despite being relatively robust, the current evidence may be subject to selection bias. It is important to maximise the representativeness of future study populations. Linkage of multiple administrative and clinical datasets, including multiple levels of healthcare and disability services, may be one solution [148], as illustrated in recent high-quality studies [40, 118]. Data linkage studies should also capture disability severity to enhance the clinical utility of their results. One intrinsic limitation of this approach is that detection bias cannot be avoided without the inclusion of health screening programs data.
Finally, most studies reported only prevalence estimates of health problems in people with ID. More longitudinal studies are needed to estimate incidence rates to identify causal links more robustly between ID and health conditions.
Strengths and limitations of the current review
A major strength of this review is that we extracted individual health conditions rather than broad categories (e.g., cardiovascular disorders). Given that disorders belonging to one broad disease group may have different morbidity patterns in people with ID (e.g., heart failure and hypertension), information based on broad disease groups can be misleading. Another strength is our approach to include population-based cohorts, so samples identified solely from healthcare services, except the UK GP sample or birth cohorts, were not included. Although we risked excluding high-quality clinical studies, we avoided including potentially biased samples which presented due to ill-health. Finally, we implemented a rigorous quality appraisal and excluded low-quality studies from the evidence synthesis.
There are some limitations of our review. First, excluding studies with voluntary and hospital-based samples reduced the representation of people with rare genetic syndromes. Second, we could not consider the diagnostic classification systems in the evidence synthesis, as this information was unavailable in many studies. Third, the identification of risk estimates of physical health conditions may be incomplete, as risk was not our primary outcome and was not included in the search strategy. Finally, although the adapted NOS we used for quality assessment has not been validated, our adaptations only optimised the wording and categories to incorporate ID and match our study design. The NOS has been found to have poor agreement between reviewers, and its scores depended on the extent to which the research methods were reported [149, 150]. However, any disagreements between reviewers were resolved through discussion.
Conclusions
The wide range of physical health conditions associated with ID indicates the complexity of health needs of this disadvantaged group. A comprehensive picture of morbidities can assist people with disability and their health professionals, disability professionals, and families to pursue timely diagnosis and better disease management. It will also assist services and governments to develop responsive health policy and health promotion programs. Age- and lifestyle-related physical conditions are worthy of more attention in future research. Globally, increased awareness and targeted preventative health care initiatives may improve the health outcomes for this vulnerable population.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOC)
Acknowledgments
We thank Dr. Rachael Cvejic (Department of Developmental Disability Neuropsychiatry (3DN, UNSW) for her input in designing how the diseases should be grouped and mapped to the ICD-10 disease chapters.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by National Health and Medical Research Council (NHMRC; Grant name: Partnership Project APP1056128, Project Grant APP1123033); and Scientia PhD Scholarship. Authors who received each grant: Scientia PhD scholarship: PL (URL to sponsor’s websites: https://www.scientia.unsw.edu.au/) NHMRC grant APP 1056128: JT; NHMRC grant APP 1123033: JT, CV, SR (URL to sponsor’s websites: https://www.nhmrc.gov.au/funding) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil. 2011;32(2):419–36. doi: 10.1016/j.ridd.2010.12.018 [DOI] [PubMed] [Google Scholar]
- 2.Trollor J, Srasuebkul P, Xu H, Howlett S. Cause of death and potentially avoidable deaths in Australian adults with intellectual disability using retrospective linked data. BMJ Open. 2017;7(2):e013489. doi: 10.1136/bmjopen-2016-013489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Leary L, Cooper SA, Hughes-McCormack L. Early death and causes of death of people with intellectual disabilities: A systematic review. J Appl Res Intellect Disabil. 2018;31(3):325–42. doi: 10.1111/jar.12417 [DOI] [PubMed] [Google Scholar]
- 4.Hosking FJ, Carey IM, Shah SM, Harris T, DeWilde S, Beighton C, et al. Mortality among adults with intellectual disability in England: comparisons with the general population. Am J Public Health. 2016;106(8):1483–90. doi: 10.2105/AJPH.2016.303240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heslop P, Blair PS, Fleming P, Hoghton M, Marriott A, Russ L. The Confidential Inquiry into premature deaths of people with intellectual disabilities in the UK: a population-based study. Lancet. 2014;383(9920):889–95. doi: 10.1016/S0140-6736(13)62026-7 [DOI] [PubMed] [Google Scholar]
- 6.Buckles J, Luckasson R, Keefe E. A systematic review of the prevalence of psychiatric disorders in adults with intellectual disability, 2003–2010. J Ment Health Res Intellect Disabil. 2013;6(3):181–207. doi: 10.1080/19315864.2011.651682 [DOI] [Google Scholar]
- 7.Einfeld SL, Ellis LA, Emerson E. Comorbidity of intellectual disability and mental disorder in children and adolescents: A systematic review. J Intellect Dev Disabil. 2011;36(2):137–43. doi: 10.1080/13668250.2011.572548 [DOI] [PubMed] [Google Scholar]
- 8.Whitaker S, Read S. The prevalence of psychiatric disorders among people with intellectual disabilities: An analysis of the literature. J Appl Res Intellect Disabil. 2006;19(4):330–45. [Google Scholar]
- 9.Robertson J, Hatton C, Baines S, Emerson E. Systematic Reviews of the Health or Health care of People with Intellectual Disabilities: A Systematic Review to Identify Gaps in the Evidence Base. J Appl Res Intellect Disabil. 2015;28(6):455–523. doi: 10.1111/jar.12149 [DOI] [PubMed] [Google Scholar]
- 10.Cooper S-A, McLean G, Guthrie B, McConnachie A, Mercer S, Sullivan F, et al. Multiple physical and mental health comorbidity in adults with intellectual disabilities: population-based cross-sectional analysis. BMC Fam. 2015;16(1):110. doi: 10.1186/s12875-015-0329-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson J, Hatton C, Emerson E, Baines S. Prevalence of epilepsy among people with intellectual disabilities: A systematic review. Seizure. 2015;29:46–62. doi: 10.1016/j.seizure.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 12.Cocks E, Thomson A, Thoresen S, Parsons R, Rosenwax L. Health status and use of medications by adults with intellectual disability in Western Australia. J Intellect Dev Disabil. 2016;41(2):87–96. doi: 10.3109/13668250.2015.1125456 [DOI] [Google Scholar]
- 13.Kinnear D, Morrison J, Allan L, Henderson A, Smiley E, Cooper SA. Prevalence of physical conditions and multimorbidity in a cohort of adults with intellectual disabilities with and without Down syndrome: cross-sectional study. BMJ Open. 2018;8(2):e018292. 10.1136/bmjopen-2017-018292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goddard L, Davidson PM, Daly J, Mackey S. People with an intellectual disability in the discourse of chronic and complex conditions: an invisible group? Aust Health Rev. 2008;32(3):405–14. doi: 10.1071/ah080405 [DOI] [PubMed] [Google Scholar]
- 15.Capone GT, Chicoine B, Bulova P, Stephens M, Hart S, Crissman B, et al. Co-occurring medical conditions in adults with Down syndrome: A systematic review toward the development of health care guidelines. Am J Med Genet. 2018;176(A):116–33. doi: 10.1002/ajmg.a.38512 [DOI] [PubMed] [Google Scholar]
- 16.Patja K, Eero P, Iivanainen M. Cancer incidence among people with intellectual disability. J Intellect Disabil Res. 2001;45(Pt 4):300–7. doi: 10.1046/j.1365-2788.2001.00322.x [DOI] [PubMed] [Google Scholar]
- 17.Sullivan SG, Glasson EJ, Hussain R, Petterson BA, Slack-Smith LM, Montgomery PD, et al. Breast cancer and the uptake of mammography screening services by women with intellectual disabilities. Prev Med. 2003;37(5):507–12. doi: 10.1016/s0091-7435(03)00177-4 [DOI] [PubMed] [Google Scholar]
- 18.Srikanth R, Cassidy G, Joiner C, Teeluckdharry S. Osteoporosis in people with intellectual disabilities: a review and a brief study of risk factors for osteoporosis in a community sample of people with intellectual disabilities. J Intellect Disabil Res. 2011;55(1):53–62. doi: 10.1111/j.1365-2788.2010.01346.x [DOI] [PubMed] [Google Scholar]
- 19.McVilly K, McGillivray J, Curtis A, Lehmann J, Morrish L, Speight J. Diabetes in people with an intellectual disability: a systematic review of prevalence, incidence and impact. Diabet Med. 2014;31(8):897–904. doi: 10.1111/dme.12494 [DOI] [PubMed] [Google Scholar]
- 20.MacRae S, Brown M, Karatzias T, Taggart L, Truesdale-Kennedy M, Walley R, et al. Diabetes in people with intellectual disabilities: A systematic review of the literature. Res Dev Disabil. 2015;47:352–74. doi: 10.1016/j.ridd.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 21.Ranjan S, Nasser JA, Fisher K. Prevalence and potential factors associated with overweight and obesity status in adults with intellectual developmental disorders. J Appl Res Intellect Disabil. 2018;31Suppl 1:29–38. doi: 10.1111/jar.12370 [DOI] [PubMed] [Google Scholar]
- 22.Maiano C, Hue O, Morin AJ, Moullec G. Prevalence of overweight and obesity among children and adolescents with intellectual disabilities: a systematic review and meta-analysis. Obes Rev. 2016;17(7):599–611. doi: 10.1111/obr.12408 [DOI] [PubMed] [Google Scholar]
- 23.Carvill S. Sensory impairments, intellectual disability and psychiatry. J Intellect Disabil Res. 2001;45(Pt 6):467–83. doi: 10.1046/j.1365-2788.2001.00366.x [DOI] [PubMed] [Google Scholar]
- 24.Prasher VP. Down syndrome and thyroid disorders: a review. Downs Syndr Res Pract. 1999;6(1):25–42. doi: 10.3104/reviews.95 [DOI] [PubMed] [Google Scholar]
- 25.Vellinga A, Van Damme P, Meheus A. Hepatitis B and C in institutions for individuals with intellectual disability. J Intellect Disabil Res. 1999;43(Pt 6):445–53. [DOI] [PubMed] [Google Scholar]
- 26.Arya R, Kabra M, Gulati S. Epilepsy in children with Down syndrome. Epileptic Disord. 2011;13(1):1–7. doi: 10.1684/epd.2011.0415 [DOI] [PubMed] [Google Scholar]
- 27.Bertapelli F, Pitetti K, Agiovlasitis S, Guerra-Junior G. Overweight and obesity in children and adolescents with Down syndrome-prevalence, determinants, consequences, and interventions: A literature review. Res Dev Disabil. 2016;57:181–92. doi: 10.1016/j.ridd.2016.06.018 [DOI] [PubMed] [Google Scholar]
- 28.Palaska PK, Antonarakis GS. Prevalence and patterns of permanent tooth agenesis in individuals with Down syndrome: a meta-analysis. Eur J Oral Sci. 2016;124(4):317–28. doi: 10.1111/eos.12282 [DOI] [PubMed] [Google Scholar]
- 29.Scalioni FAR, Carrada CF, Martins CC, Ribeiro RA, Paiva SM. Periodontal disease in patients with Down syndrome: A systematic review. J Am Dent Assoc. 2018;149(7):628-39.e11. doi: 10.1016/j.adaj.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 30.Lee CF, Lee CH, Hsueh WY, Lin MT, Kang KT. Prevalence of Obstructive Sleep Apnea in Children With Down Syndrome: A Meta-Analysis. J Clin Sleep Med. 2018;14(5):867–75. doi: 10.5664/jcsm.7126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creavin AL, Brown RD. Ophthalmic abnormalities in children with Down syndrome. J Pediatr Ophthalmol Strabismus. 2009;46(2):76–82. doi: 10.3928/01913913-20090301-06 [DOI] [PubMed] [Google Scholar]
- 32.Watt T, Robertson K, Jacobs RJ. Refractive error, binocular vision and accommodation of children with Down syndrome. Clin Exp Optom. 2015;98(1):3–11. doi: 10.1111/cxo.12232 [DOI] [PubMed] [Google Scholar]
- 33.Oeseburg B, Dijkstra GJ, Groothoff JW, Reijneveld SA, Jansen DEMC. Prevalence of Chronic Health Conditions in Children With Intellectual Disability: A Systematic Literature Review. Intellect Dev Disabil. 2011;49(2):59–85. doi: 10.1352/1934-9556-49.2.59 [DOI] [PubMed] [Google Scholar]
- 34.Jansen DEMC, Krol B, Groothoff JW, Post D. People with intellectual disability and their health problems: a review of comparative studies. J Intellect Disabil Res. 2004;48(2):93–102. doi: 10.1111/j.1365-2788.2004.00483.x [DOI] [PubMed] [Google Scholar]
- 35.Chan M, Park JJ, Shi T, Martinon-Torres F, Bont L, Nair H, et al. The burden of respiratory syncytial virus (RSV) associated acute lower respiratory infections in children with Down syndrome: A systematic review and meta-analysis. J Glob Health. 2017;7(2):020413. doi: 10.7189/jogh.07.020413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.GA Wells BS, D O’Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available rom: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 38.Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atladottir HO, Schendel DE, Parner ET, Henriksen TB. A Descriptive Study on the Neonatal Morbidity Profile of Autism Spectrum Disorders, Including a Comparison with Other Neurodevelopmental Disorders. J Autism Dev Disord. 2015;45(8):2429–42. 10.1007/s10803-015-2408-7 [DOI] [PubMed] [Google Scholar]
- 40.Bergström S, Carr H, Petersson G, Stephansson O, Bonamy A-KE, Dahlström A, et al. Trends in Congenital Heart Defects in Infants With Down Syndrome. Pediatrics. 2016;138(1). doi: 10.1542/peds.2016-0123 [DOI] [PubMed] [Google Scholar]
- 41.Bhaumik S, Watson JM, Thorp CF, Tyrer F, McGrother CW. Body mass index in adults with intellectual disability: distribution, associations and service implications: a population-based prevalence study. J Intellect Disabil Res. 2008;52(Pt 4):287–98. 10.1111/j.1365-2788.2007.01018.x [DOI] [PubMed] [Google Scholar]
- 42.Bjorge T, Cnattingius S, Lie RT, Tretli S, Engeland A. Cancer risk in children with birth defects and in their families: A population based cohort study of 5.2 million children from Norway and Sweden. Cancer Epidemiol Biomarkers Prev. 2008;17(3):500–6. 10.1158/1055-9965.EPI-07-2630 [DOI] [PubMed] [Google Scholar]
- 43.Brodwall K, Greve G, Leirgul E, Klungsoyr K, Holmstrom H, Vollset SE, et al. The five-year survival of children with Down syndrome in Norway 1994–2009 differed by associated congenital heart defects and extracardiac malformations. Acta Paediatr. 2018;107(5):845–53. 10.1111/apa.14223 [DOI] [PubMed] [Google Scholar]
- 44.Finlayson J, Morrison J, Jackson A, Mantry D, Cooper SA. Injuries, falls and accidents among adults with intellectual disabilities. Prospective cohort study. J Intellect Disabil Res. 2010;54(11):966–80. 10.1111/j.1365-2788.2010.01319.x [DOI] [PubMed] [Google Scholar]
- 45.Forsgren L, Edvinsson SO, Blomquist HK, Heijbel J, Sidenvall R. Epilepsy in a population of mentally retarded children and adults. Epilepsy Res. 1990;6(3):234–48. doi: 10.1016/0920-1211(90)90079-b [DOI] [PubMed] [Google Scholar]
- 46.Hove O. Weight survey on adult persons with mental retardation living in the community. Res Dev Disabil. 2004;25(1):9–17. doi: 10.1016/j.ridd.2003.04.004 [DOI] [PubMed] [Google Scholar]
- 47.Kim MA, Lee YS, Yee NH, Choi JS, Choi JY, Seo K. Prevalence of congenital heart defects associated with Down syndrome in Korea. J Korean Med Sci. 2014;29(11):1544–9. 10.3346/jkms.2014.29.11.1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin J-D, Yen C-F, Li C-W, Wu J-L, Chwo M-J, Loh C-H, et al. Epilepsy and health care utilization among people with intellectual disability. Journal of Disability Research (Taiwan). 2003;1:65–77. [Google Scholar]
- 49.Lund J. Epilepsy and psychiatric disorder in the mentally retarded adult. Acta Psychiatr Scand. 1985;72(6):557–62. 10.1111/j.1600-0447.1985.tb02654.x [DOI] [PubMed] [Google Scholar]
- 50.McGrother C, Hauck A, Bhaumik S, Thorp C, Taub N. Community care for adults with learning disability and their carers: Needs and outcomes from the Leicestershire register. J Intellect Disabil Res. 1996;40(2):183–90. 10.1111/j.1365-2788.1996.tb00621.x [DOI] [PubMed] [Google Scholar]
- 51.McGrother CW, Bhaumik S, Thorp CF, Hauck A, Branford D, Watson JM. Epilepsy in adults with intellectual disabilities: prevalence, associations and service implications. Seizure. 2006;15(6):376–86. doi: 10.1016/j.seizure.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 52.Melville C, Cooper S, Morrison J, Allan L, Smiley E, Williamson A. The prevalence and determinants of obesity in adults with intellectual disabilities. J Appl Res Intellect Disabil. 2008;21(5):425–37. 10.1111/j.1468-3148.2007.00412.x. [DOI] [Google Scholar]
- 53.Morgan CL, Baxter H, Kerr MP. Prevalence of epilepsy and associated health service utilization and mortality among patients with intellectual disability. Am J Ment Retard. 2003;108(5):293–300. doi: [DOI] [PubMed] [Google Scholar]
- 54.Patja K, Pukkala E, Sund R, Iivanainen M, Kaski M. Cancer incidence of persons with Down syndrome in Finland: a population-based study. Int J Cancer. 2006;118(7):1769–72. doi: 10.1002/ijc.21518 [DOI] [PubMed] [Google Scholar]
- 55.Patja K, Sund R, Kaski M, Pukkala E. Cancer incidence among persons with Prader-Willi syndrome in Finland. Int J Disabil Hum Dev. 2008;7(1):69–72. 10.1515/IJDHD.2008.7.1.69. [DOI] [Google Scholar]
- 56.Prasher VP. Overweight and obesity amongst Down’s syndrome adults. J Intellect Disabil Res. 1995;39(5):437–41. doi: 10.1111/j.1365-2788.1995.tb00548.x [DOI] [PubMed] [Google Scholar]
- 57.So SA, Urbano RC, Hodapp RM. Hospitalizations of infants and young children with Down syndrome: evidence from inpatient person-records from a statewide administrative database. J Intellect Disabil Res. 2007;51(Pt 12):1030–8. doi: 10.1111/j.1365-2788.2007.01013.x [DOI] [PubMed] [Google Scholar]
- 58.Bishop L, McLean KJ, Rubenstein E. Epilepsy in adulthood: Prevalence, incidence, and associated antiepileptic drug use in autistic adults in a state Medicaid system. Autism. 2020. 10.1177/1362361320942982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho WK, Lee NY, Han K, Suh BK, Park YG. The population prevalence, associations of congenital heart defect and mortality risk for down’s syndrome in South Korea based on national health insurance service (NHIS) data. Clin Epidemiol. 2020;12:519–25. 10.2147/CLEP.S251637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kristianslund O, Drolsum L. Prevalence of Keratoconus in Persons with down Syndrome in a National Registry in Norway. JAMA Network Open. 2021;4(3):e210814. 10.1001/jamanetworkopen.2021.0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ostermaier KK, Weaver AL, Myers SM, Stoeckel RE, Katusic SK, Voigt RG. Incidence of Celiac Disease in Down Syndrome: A Longitudinal, Population-Based Birth Cohort Study. Clin Pediatr. 2020;59(12):1086–91. 10.1177/0009922820941247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashman AF, Suttie J. The medical and health status of older people with mental retardation in Australia. J Appl Gerontol. 1996;15(1):57–72. 10.1177/073346489601500104. [DOI] [Google Scholar]
- 63.Morin D, Merineau-Cote J, Ouellette-Kuntz H, Tasse MJ, Kerr M. A comparison of the prevalence of chronic disease among people with and without intellectual disability. Am J Intellect Dev Disabil. 2012;117(6):455–63. 10.1352/1944-7558-117.6.455 [DOI] [PubMed] [Google Scholar]
- 64.van Splunder J, Stilma JS, Bernsen RM, Arentz TG, Evenhuis HM. Refractive errors and visual impairment in 900 adults with intellectual disabilities in the Netherlands. Acta Ophthalmol Scand. 2003;81(2):123–9. doi: 10.1034/j.1600-0420.2003.00035.x [DOI] [PubMed] [Google Scholar]
- 65.Wellesley DG, Hockey KA, Montgomery PD, Stanley FJ. Prevalence of intellectual handicap in Western Australia: a community study. Med J Aust. 1992;156(2):94–6, 100, 2. [PubMed] [Google Scholar]
- 66.Beange H, McElduff A, Baker W. Medical disorders of adults with mental retardation: a population study. Am J Ment Retard. 1995;99(6):595–604. [PubMed] [Google Scholar]
- 67.Benassi G, Guarino M, Cammarata S, Cristoni P, Fantini MP, Ancona A, et al. An epidemiological study on severe mental retardation among schoolchildren in Bologna, Italy. Dev Med Child Neurol. 1990;32(10):895–901. doi: 10.1111/j.1469-8749.1990.tb08102.x [DOI] [PubMed] [Google Scholar]
- 68.Carey IM, Shah SM, Hosking FJ, DeWilde S, Harris T, Beighton C, et al. Health characteristics and consultation patterns of people with intellectual disability: a cross-sectional database study in English general practice. Br J Gen Pract. 2016;66(645):e264–70. 10.3399/bjgp16X684301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christianson AL, Zwane ME, Manga P, Rosen E, Venter A, Downs D, et al. Children with intellectual disability in rural South Africa: prevalence and associated disability. J Intellect Disabil Res. 2002;46(Pt 2):179–86. doi: 10.1046/j.1365-2788.2002.00390.x [DOI] [PubMed] [Google Scholar]
- 70.Diene G, Mimoun E, Feigerlova E, Caula S, Molinas C, Grandjean H, et al. Endocrine disorders in children with Prader-Willi syndrome—data from 142 children of the French database. Horm Res Paediatr. 2010;74(2):121–8. 10.1159/000313377 [DOI] [PubMed] [Google Scholar]
- 71.Garg A, Strunk A, Midura M, Papagermanos V, Pomerantz H. Prevalence of hidradenitis suppurativa among patients with Down syndrome: a population-based cross-sectional analysis. Br J Dermatol. 2018;178(3):697–703. 10.1111/bjd.15770 [DOI] [PubMed] [Google Scholar]
- 72.Gustavson KH, Hagberg B, Hagberg G, Sars K. Severe mental retardation in a Swedish county I. Epidemiology, gestational age, birth weight and associated CNS handicaps in children born 1959–70. Acta Pædiatrica. 1977;66(3):373–9. doi: 10.1111/j.1651-2227.1977.tb07910.x [DOI] [PubMed] [Google Scholar]
- 73.Lin JD, Yen CF, Loh CH, Hsu SW, Huang HC, Tang CC, et al. A cross-sectional study of the characteristics and determinants of emergency care utilization among people with intellectual disabilities in Taiwan. Res Dev Disabil. 2006;27(6):657–67. doi: 10.1016/j.ridd.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 74.Lin J-D, Yen C-F, Li C-W, Wu J-L. Patterns of Obesity among Children and Adolescents with Intellectual Disabilities in Taiwan. J Appl Res Intellect Disabil. 2005;18(2):123–9. 10.1111/j.1468-3148.2005.00241.x. [DOI] [Google Scholar]
- 75.Schultz-Pedersen S, Hasle H, Olsen JH, Friedrich U. Evidence of decreased risk of cancer in individuals with fragile X. Am J Med Genet. 2001;103(3):226–30. [PubMed] [Google Scholar]
- 76.Scott C, Thame M. The incidence of cardiac lesions among children with Down’s syndrome in Jamaica—A prospective study. West Indian Med J. 2014;63(7):693–7. 10.7727/wimj.2013.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sullivan SG, Hussain R, Threlfall T, Bittles AH. The incidence of cancer in people with intellectual disabilities. Cancer Causes Control. 2004;15(10):1021–5. doi: 10.1007/s10552-004-1256-0 [DOI] [PubMed] [Google Scholar]
- 78.Torfs CP, Christianson RE. Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet. 1998;77(5):431–8. . [DOI] [PubMed] [Google Scholar]
- 79.Yen CF, Lin JD, Li CW, Wu JL, Lee JT. Body mass index for adults with intellectual disabilities: A survey of caregivers in Taiwan. J Med Sci. 2005;25(3):131–7. [Google Scholar]
- 80.Yen CF, Lin JD, Loh CH, Shi L, Hsu SW. Determinants of prescription drug use by adolescents with intellectual disabilities in Taiwan. Res Dev Disabil. 2009;30(6):1354–66. 10.1016/j.ridd.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 81.Cooper SA, Hughes-McCormack L, Greenlaw N, McConnachie A, Allan L, Baltzer M, et al. Management and prevalence of long-term conditions in primary health care for adults with intellectual disabilities compared with the general population: A population-based cohort study. J Appl Res Intellect Disabil. 2018;31Suppl 1:68–81. 10.1111/jar.12386 [DOI] [PubMed] [Google Scholar]
- 82.Arvio M, Sillanpaa M. Prevalence, aetiology and comorbidity of severe and profound intellectual disability in Finland. J Intellect Disabil Res. 2003;47(Pt 2):108–12. doi: 10.1046/j.1365-2788.2003.00447.x [DOI] [PubMed] [Google Scholar]
- 83.Boyle A, Melville CA, Morrison J, Allan L, Smiley E, Espie CA, et al. A cohort study of the prevalence of sleep problems in adults with intellectual disabilities. J Sleep Res. 2010;19(1 Pt 1):42–53. 10.1111/j.1365-2869.2009.00788.x [DOI] [PubMed] [Google Scholar]
- 84.Janicki MP, Maceachron AE. Residential, health, and social service needs of elderly developmentally disabled persons. The Gerontologist. 1984;24(2):128. doi: 10.1093/geront/24.2.128 [DOI] [PubMed] [Google Scholar]
- 85.Matthews T, Weston N, Baxter H, Felce D, Kerr M. A general practice-based prevalence study of epilepsy among adults with intellectual disabilities and of its association with psychiatric disorder, behaviour disturbance and carer stress. J Intellect Disabil Res. 2008;52(Pt 2):163–73. 10.1111/j.1365-2788.2007.01025.x [DOI] [PubMed] [Google Scholar]
- 86.McQueen PC, Spence MW, Garner JB, Pereira LH, Winsor EJ. Prevalence of major mental retardation and associated disabilities in the Canadian Maritime Provinces. Am J Ment Defic. 1987;91(5):460–6. [PubMed] [Google Scholar]
- 87.Shepherd C, Hosking G. Epilepsy in school children with intellectual impairments in Sheffield: the size and nature of the problem and the implications for service provision. J Ment Defic Res. 1989;33(Pt 6):511–4. [DOI] [PubMed] [Google Scholar]
- 88.Meuwese-Jongejeugd A, Vink M, van Zanten B, Verschuure H, Eichhorn E, Koopman D, et al. Prevalence of hearing loss in 1598 adults with an intellectual disability: cross-sectional population based study. Int J Audiol. 2006;45(11):660–9. doi: 10.1080/14992020600920812 [DOI] [PubMed] [Google Scholar]
- 89.Burke É, Carroll R, O’Dwyer M, Walsh JB, McCallion P, McCarron M. Quantitative examination of the bone health status of older adults with intellectual and developmental disability in Ireland: a cross-sectional nationwide study. BMJ Open. 2019;9(4):e026939. doi: 10.1136/bmjopen-2018-026939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gale L, Naqvi H, Russ L. Asthma, smoking and BMI in adults with intellectual disabilities: a community-based survey. J Intellect Disabil Res. 2009;53(9):787–96. 10.1111/j.1365-2788.2009.01192.x [DOI] [PubMed] [Google Scholar]
- 91.Mikulovic J, Marcellini A, Compte R, Duchateau G, Vanhelst J, Fardy PS, et al. Prevalence of overweight in adolescents with intellectual deficiency. Differences in socio-educative context, physical activity and dietary habits. Appetite. 2011;56(2):403–7. 10.1016/j.appet.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 92.Simila S, Niskanen P. Underweight and overweight cases among the mentally retarded. J Ment Defic Res. 1991;35(Pt 2):160–4. doi: 10.1111/j.1365-2788.1991.tb01046.x [DOI] [PubMed] [Google Scholar]
- 93.Melville CA, Cooper SA, McGrother CW, Thorp CF, Collacott R. Obesity in adults with Down syndrome: a case-control study. J Intellect Disabil Res. 2005;49(Pt 2):125–33. doi: 10.1111/j.1365-2788.2004.00616.x [DOI] [PubMed] [Google Scholar]
- 94.Prasher VP, Glenn S, Cunningham C, Arshad H, Glenholmes P, Kirby A. Health morbidity and access to services by young adults with Down syndrome. Int J Dev Disabil. 2014;60(1):26–34. doi: 10.1179/204738713X.13673354444083 [DOI] [Google Scholar]
- 95.Kapell D, Nightingale B, Rodriguez A, Lee JH, Zigman WB, Schupf N. Prevalence of chronic medical conditions in adults with mental retardation: comparison with the general population. Ment Retard. 1998;36(4):269–79. doi: [DOI] [PubMed] [Google Scholar]
- 96.Henderson A, Lynch SA, Wilkinson S, Hunter M. Adults with Down’s sydrome: The prevalence of complications and health care in the community. Br J Gen Pract. 2007;57(534):50–5. [PMC free article] [PubMed] [Google Scholar]
- 97.Määttä T, Määttä J, Tervo-Määttä T, Taanila A, Kaski M, Iivanainen M. Healthcare and guidelines: A population-based survey of recorded medical problems and health surveillance for people with Down syndrome. J Intellect Dev Disabil. 2011;36(2):118–26. doi: 10.1080/13668250.2011.570253 [DOI] [PubMed] [Google Scholar]
- 98.Austeng ME, Akre H, Falkenberg E-S, Overland B, Abdelnoor M, Kvaerner KJ. Hearing level in children with Down syndrome at the age of eight. Res Dev Disabil. 2013;34(7):2251–6. 10.1016/j.ridd.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 99.Leonard S, Bower C, Petterson B, Leonard H. Medical aspects of school-aged children with Down syndrome. Dev Med Child Neurol. 1999;41(10):683–8. doi: 10.1017/s0012162299001401 [DOI] [PubMed] [Google Scholar]
- 100.Park AH, Wilson MA, Stevens PT, Harward R, Hohler N. Identification of hearing loss in pediatric patients with Down syndrome. Otolaryngol Head Neck Surg. 2012;146(1):135–40. 10.1177/0194599811425156 [DOI] [PubMed] [Google Scholar]
- 101.McGrother CW, Marshall B. Recent trends in incidence, morbidity and survival in Down’s syndrome. J Ment Defic Res. 1990;34(Pt 1):49–57. doi: 10.1111/j.1365-2788.1990.tb01514.x [DOI] [PubMed] [Google Scholar]
- 102.Yaneza MM, Hunter K, Irwin S, Kubba H. Hearing in school-aged children with trisomy 21—results of a longitudinal cohort study in children identified at birth. Clin Otolaryngol. 2016;41(6):711–7. 10.1111/coa.12606 [DOI] [PubMed] [Google Scholar]
- 103.Tedeschi AS, Roizen NJ, Taylor HG, Murray G, Curtis CA, Parikh AS. The prevalence of congenital hearing loss in neonates with Down syndrome. J Pediatr. 2015;166(1):168–71. 10.1016/j.jpeds.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 104.Austeng ME, Akre H, Overland B, Abdelnoor M, Falkenberg ES, Kvaerner KJ. Otitis media with effusion in children with in Down syndrome. Int J Pediatr Otorhinolaryngol. 2013;77(8):1329–32. 10.1016/j.ijporl.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 105.Barr E, Dungworth J, Hunter K, McFarlane M, Kubba H. The prevalence of ear, nose and throat disorders in preschool children with Down’s syndrome in Glasgow. Scott Med J. 2011;56(2):98–103. 10.1258/smj.2011.011036 [DOI] [PubMed] [Google Scholar]
- 106.Irving CA, Chaudhari MP. Cardiovascular abnormalities in Down’s syndrome: spectrum, management and survival over 22 years. Arch Dis Child. 2012;97(4):326–30. 10.1136/adc.2010.210534 [DOI] [PubMed] [Google Scholar]
- 107.Jaruratanasirikul S, Limpitikul W, Dissaneevate P, Booncharoen P, Tantichantakarun P. Comorbidities in Down syndrome livebirths and health care intervention: an initial experience from the birth defects registry in Southern Thailand. World J Pediatr. 2017;13(2):152–7. 10.1007/s12519-016-0093-z [DOI] [PubMed] [Google Scholar]
- 108.Weijerman ME, van Furth AM, van der Mooren MD, van Weissenbruch MM, Rammeloo L, Broers CJ, et al. Prevalence of congenital heart defects and persistent pulmonary hypertension of the neonate with Down syndrome. Eur J Pediatr. 2010;169(10):1195–9. 10.1007/s00431-010-1200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fabia J, Drolette M. Malformations and leukemia in children with Down’s syndrome. Pediatrics. 1970;45(1):60. [PubMed] [Google Scholar]
- 110.Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, Hassold TJ, et al. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998;80(3):213. doi: 2–8. [PubMed] [Google Scholar]
- 111.Santoro M, Coi A, Spadoni I, Bianchi F, Pierini A. Sex differences for major congenital heart defects in Down Syndrome: A population based study. Eur J Med Genet. 2018;61(9):546–50. 10.1016/j.ejmg.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 112.Arnell H, Fischler B. Population-based study of incidence and clinical outcome of neonatal cholestasis in patients with Down syndrome. J Pediatr. 2012;161(5):899–902. 10.1016/j.jpeds.2012.04.037 [DOI] [PubMed] [Google Scholar]
- 113.Prasher VP, Glenn S, Cunningham C, Arshad H, Glenholmes P, Kirby A. Health morbidity and access to services by young adults with Down syndrome. Int J Dev Disabil. 2014;60(1):26–34. 10.1179/204738713X.13673354444083. [DOI] [Google Scholar]
- 114.Thomson AK, Glasson EJ, Bittles AH. A long-term population-based clinical and morbidity profile of Angelman syndrome in Western Australia: 1953–2003. Disabil Rehabil. 2006;28(5):299–305. 10.1080/09638280500190631 [DOI] [PubMed] [Google Scholar]
- 115.Sullivan SG, Hussain R, Glasson EJ, Bittles AH. The profile and incidence of cancer in Down syndrome. J Intellect Disabil Res. 2007;51(Pt 3):228–31. doi: 10.1111/j.1365-2788.2006.00862.x [DOI] [PubMed] [Google Scholar]
- 116.Boker LK, Blumstein T, Sadetzki S, Luxenburg O, Litvak I, Akstein E, et al. Incidence of leukemia and other cancers in Down syndrome subjects in Israel. Int J Cancer. 2001;93(5):741–4. doi: 10.1002/ijc.1383 [DOI] [PubMed] [Google Scholar]
- 117.Hasle H, Friedman JM, Olsen Jo H, Rasmussen SA. Low risk of solid tumors in persons with Down syndrome. Genet Med. 2016;18(11):1151–7. 10.1038/gim.2016.23 [DOI] [PubMed] [Google Scholar]
- 118.Cuypers M, Leijssen M, Bakker-van Gijssel EJ, Pouls KPM, Mastebroek MM, Naaldenberg J, et al. Patterns in the prevalence of diabetes and incidence of diabetic complications in people with and without an intellectual disability in Dutch primary care: Insights from a population-based data-linkage study. Prim Care Diabetes. 2021;15(2):372–7. 10.1016/j.pcd.2020.11.012 [DOI] [PubMed] [Google Scholar]
- 119.Lunsky Y, Durbin A, Brown HK, Bansal S, Heifetz M, Antoniou T. Health profiles and associated service use among adults with HIV and intellectual and developmental disabilities. Aids. 2017;31(5):697–705. 10.1097/QAD.0000000000001361 [DOI] [PubMed] [Google Scholar]
- 120.Hjortshoj TD, Gronskov K, Rosenberg T, Brondum-Nielsen K, Olsen JH. Risk for cancer in patients with Bardet-Biedl syndrome and their relatives. Am J Med Genet A. 2007;143A(15):1699–702. doi: 10.1002/ajmg.a.31805 [DOI] [PubMed] [Google Scholar]
- 121.Robertson J, Baines S, Emerson E, Hatton C. Prevalence of constipation in people with intellectual disability: A systematic review. J Intellect Dev Disabil. 2017;43(4):392–406. doi: 10.3109/13668250.2017.1310829 [DOI] [PubMed] [Google Scholar]
- 122.van Schrojenstein Lantman-de Valk HM, Walsh PN. Managing health problems in people with intellectual disabilities. BMJ. 2008;337:a2507. doi: 10.1136/bmj.a2507 [DOI] [PubMed] [Google Scholar]
- 123.Maiano C, Hue O, Morin AJ, Moullec G. Prevalence of overweight and obesity among children and adolescents with intellectual disabilities: a systematic review and meta-analysis. Obes Rev. 2016;17(7):599–611. doi: 10.1111/obr.12408 [DOI] [PubMed] [Google Scholar]
- 124.Warburg M. Visual impairment in adult people with intellectual disability: literature review. J Intellect Disabil Res. 2001;45(5):424–38. doi: 10.1046/j.1365-2788.2001.00348.x [DOI] [PubMed] [Google Scholar]
- 125.Jasien J, Daimon CM, Maudsley S, Shapiro BK, Martin B. Aging and bone health in individuals with developmental disabilities. Int J Endocrinol. 2012;2012:469235. doi: 10.1155/2012/469235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heksch R, Kamboj M, Anglin K, Obrynba K. Review of Prader-Willi syndrome: the endocrine approach. Transl Pediatr. 2017;6(4):274–85. doi: 10.21037/tp.2017.09.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sacks B, Wood A. Hearing disorders in children with Down syndrome. DSNU. 2003;3(2):38–41. doi: 10.3104/dsupdate.222 [DOI] [Google Scholar]
- 128.Morrison ML, McMahon CJ. Congenital Heart Disease in Down Syndrome. Advances in research on Down syndrome.2018. pp. 95. [Google Scholar]
- 129.Hamadah I, Haider M, Chisti M, Binamer Y. Hidradenitis suppurativa in Down syndrome: a case series. Pediatr Dermatol. 2017;34(4):461–4. 10.1111/pde.13188 [DOI] [PubMed] [Google Scholar]
- 130.Davis S. Asthma in intellectual disability: are we managing our patients appropriately? Breathe. 2016;12(4):310–7. doi: 10.1183/20734735.014716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blok J, Jonkman M, Horvath B. The possible association of hidradenitis suppurativa and Down syndrome: is increased amyloid precursor protein expression resulting in impaired Notch signalling the missing link? Br J Dermatol. 2014;170(6):1375–7. 10.1111/bjd.12887 [DOI] [PubMed] [Google Scholar]
- 132.Poizeau F, Sbidian E, Mircher C, Rebillat AS, Chosidow O, Wolkenstein P, et al. Prevalence and description of hidradenitis suppurativa in Down syndrome: a cross-sectional study of 783 subjects. Acta Derm Venereol. 2019;99(3):351–2. 10.2340/00015555-3095 [DOI] [PubMed] [Google Scholar]
- 133.Ali Z, Ulrik CS. Obesity and asthma: a coincidence or a causal relationship? A systematic review. Respir Med. 2013;107(9):1287–300. doi: 10.1016/j.rmed.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 134.Jette N, Patten S, Williams J, Becker W, Wiebe S. Comorbidity of migraine and psychiatric disorders—a national population-based study. Headache. 2008;48(4):501–16. doi: 10.1111/j.1526-4610.2007.00993.x [DOI] [PubMed] [Google Scholar]
- 135.Baxter H, Lowe K, Houston H, Jones G, Felce D, Kerr M. Previously unidentified morbidity in patients with intellectual disability. Br J Gen Pract. 2006;56(523):93–8. [PMC free article] [PubMed] [Google Scholar]
- 136.Weise J, Pollack A, Britt H, Trollor JN. Primary health care for people with an intellectual disability: an exploration of consultations, problems identified, and their management in Australia. J Intellect Disabil Res. 2017;61(5):399–410. doi: 10.1111/jir.12352 [DOI] [PubMed] [Google Scholar]
- 137.Robertson J, Hatton C, Emerson E, Baines S. Mortality in people with intellectual disabilities and epilepsy: A systematic review. Seizure. 2015;29:123–33. doi: 10.1016/j.seizure.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 138.Devinsky O, Asato M, Camfield P, Geller E, Kanner AM, Keller S, et al. Delivery of epilepsy care to adults with intellectual and developmental disabilities. Neurology. 2015;85(17):1512–21. doi: 10.1212/WNL.0000000000002060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hanlon P, MacDonald S, Wood K, Allan L, Cooper SA. Long-term condition management in adults with intellectual disability in primary care: a systematic review. BJGP Open. 2018;2(1):bjgpopen18X101445. doi: 10.3399/bjgpopen18X101445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lennox N, Bain C, Rey-Conde T, Purdie D, Bush R, Pandeya N. Effects of a comprehensive health assessment programme for Australian adults with intellectual disability: a cluster randomized trial. Int J Epidemiol. 2007;36(1):139–46. doi: 10.1093/ije/dyl254 [DOI] [PubMed] [Google Scholar]
- 141.Lennox N, Bain C, Rey-Conde T, Taylor M, Boyle FM, Purdie DM, et al. Cluster Randomized-Controlled Trial of Interventions to Improve Health for Adults with Intellectual Disability Who Live in Private Dwellings. J Appl Res Intellect Disabil. 2010;23(4):303–11. 10.1111/j.1468-3148.2009.00533.x. [DOI] [Google Scholar]
- 142.Patja K, Iivanainen M, Vesala H, Oksanen H, Ruoppila I. Life expectancy of people with intellectual disability: a 35-year follow-up study. J Intellect Disabil Res. 2000;44:591. doi: 10.1046/j.1365-2788.2000.00280.x [DOI] [PubMed] [Google Scholar]
- 143.Emerson E, Glover G, Hatton C, Wolstenholme J. Trends in age-standardised mortality rates and life expectancy of people with learning disabilities in Sheffield over a 33-year period. Tizard Learn Disabil Rev. 2014;19(2):90–5. doi: 10.1108/TLDR-01-2014-0003 [DOI] [Google Scholar]
- 144.Coppus AMW. People with intellectual disability: What do we know about adulthood and life expectancy? Dev Disabil Res Rev. 2013;18(1):6–16. doi: 10.1002/ddrr.1123 [DOI] [PubMed] [Google Scholar]
- 145.Adolfsson P, Sydner YM, Fjellström C, Lewin B, Andersson A. Observed dietary intake in adults with intellectual disability living in the community. Food Nutr Res. 2008;52. doi: 10.3402/fnr.v52i0.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Njenga F. Perspectives of intellectual disability in Africa: epidemiology and policy services for children and adults. Curr Opin Psychiatry. 2009;22(5):457–61. doi: 10.1097/YCO.0b013e32832e63a1 [DOI] [PubMed] [Google Scholar]
- 147.Jeevanandam L. Perspectives of intellectual disability in Asia: epidemiology, policy, and services for children and adults. Curr Opin Psychiatry. 2009;22(5):462–8. doi: 10.1097/YCO.0b013e32832ec056 [DOI] [PubMed] [Google Scholar]
- 148.Balogh R, Leonard H, Bourke J, Brameld K, Downs J, Hansen M, et al. Data linkage: Canadian and Australian perspectives on a valuable methodology for intellectual and developmental disability research. Intellect Dev Disabil. 2019;57(5):439–62. doi: 10.1352/1934-9556-57.5.439 [DOI] [PubMed] [Google Scholar]
- 149.Luchini C, Stubbs B, Solmi M, Veronese NJWJM-A. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. 2017;5(4):80–4. [Google Scholar]
- 150.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]