Abstract
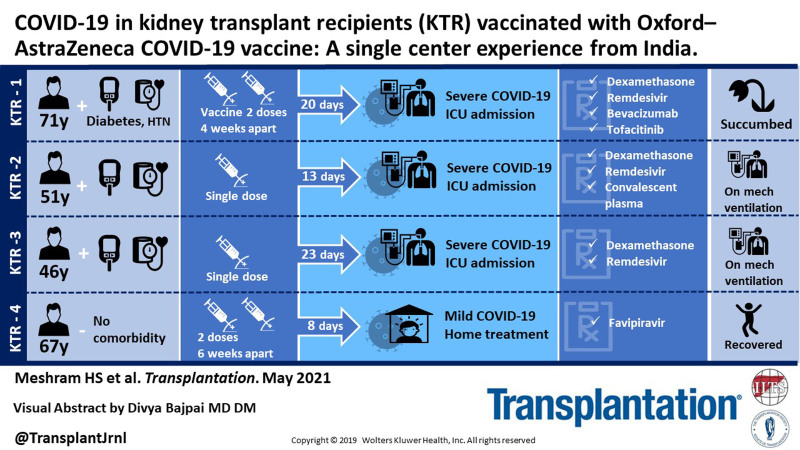
Severe acute respiratory syndrome coronavirus (SARS-CoV2) has been reporting global peaks in India in April 2021. The enormous task of vaccinating such a bulk population has become more difficult in the state where health resources for managing coronavirus disease 2019 (COVID-19) are rapidly emptying amid the COVID-19 surge. Indian advisories have approved 2 vaccines: Oxford–AstraZeneca COVID-19 (Covishield) and BBV152 (Covaxin) as of April 2021. The efficacy of these vaccines has been reported lesser compared with the mRNA vaccines in general population.1,2 Preliminary reports with mRNA vaccine in organ transplant patients have shown that around 75% of transplant patients have a suboptimal response to mRNA COVID-19 vaccine.3 Such data with Covishield vaccine are lacking. We report 4 cases of COVID-19 in kidney transplant recipients (KTRs) 2 of them after 2 doses of Covishield vaccine and another 2 after a single dose. The study is approved by the Institutional Ethics committee. We also abided by the Declaration of Helsinki and Declaration of Istanbul principles. None of them reported any minor or major side effects postvaccination. The probable exposure was community acquired in all the 4 cases. Three patients were on triple immunosuppression. Three cases required critical care admission and received steroids, remdesivir, anticoagulation, and investigational therapies (patient 1: tofacitinib and bevacizumab; patient 2: COVID-19 convalescent plasma) as anti-COVID-19 therapy during the stay. Patient 1 died and patients 2 and 3 are on mechanical ventilation with a poor prognosis (Table 1). Patient 4 had a mild COVID-19 course being managed at home. Time from the second dose of vaccine to onset of symptoms was 20 and 8 d for patients 1 and 4, respectively. Patients 2 and 3 developed symptoms after 13 and 23 d of the first dose of vaccine, respectively. SARS-CoV2 IgG antibody response was suboptimal in 3 cases, while seroconversion developed in patient 1. To the best of our knowledge, this is the first report of COVID-19 postvaccination after Covishield vaccine in KTRs. The documentation of attenuated response in our report bolsters our speculation that antibody response after Covishield might be suboptimal in KTRs. The recent reports3 of insufficient antibody response from mRNA vaccine whose efficacy is around 95% in general population compared with the lower efficacy vaccine used in Indian settings is alarming for the immunosuppressed group as they will have further attenuated response. The outcome of vaccinated patients acquiring COVID-19 is sparsely reported. There have been a few reports of COVID-19 infection postvaccination in organ transplants after mRNA vaccines.4,5 In conclusion, critical COVID-19 despite vaccination in our report is concerning and it emphasizes the fact that KTRs are more prone to COVID-19 even after vaccination. Hence, safety measures to prevent disease transmission should be continued. There is a special need to find a definitive therapy for COVID-19, as reports of vaccine efficacy in transplants are worrisome. The future implications of our report highlight the need for further research with reporting of outcome of vaccinated patients, efficacy of different vaccines, dosages, schedules, seroprotection levels, and antibody durability in KTRs.
TABLE 1.
Summary of the case developing COVID-19 after 2 doses of Oxford–AstraZeneca COVID-19 vaccine
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (y) | 71 | 51 | 46 | 67 |
| Sex | Male | Male | Male | Male |
| Comorbidities | Hypertension, diabetes | Hypertension, diabetes | Hypertension, diabetes | None |
| Basic disease | Diabetic nephropathy | ADPKD | Hypertension | ADPKD |
| Transplant | Living-related transplant | Deceased donor | Living-related transplant | Deceased donor |
| Induction | No induction | Thymoglobulin | No induction | Thymoglobulin |
| Ant-rejection therapy | No | Yes (AMR [ag1 PTC score 1 C4d2] in immediate transplant) | Yes (chronic rejection) | No |
| Time from transplant to COVID-19 | 16 y | 1.5 y | 9 y | 6 y |
| Baseline serum creatinine(mg/dL) | 1.4 | 1.8 | 4.32 | 1.5 |
| Oxford–AstraZeneca vaccination schedule | 4 wk apart; 2 doses | 1 dose | 1 dose | 6 wk apart; 2 doses |
| Onset of symptom after vaccine | 20 d | 13 | 23 | 8 d |
| COVID-19 exposure | Community | Community | Community | Community |
| Molecular analysis of SARS-CoV2 RT-PCR test | Positive | Positive | Positive | Positive |
| ORF fab, CT value | 29 | 16 | – | 15 |
| E gene, CT value | 22 | – | 22 | – |
| N gene, CT value | 24 | 16 | 24 | – |
| SARS-CoV2 seroconversion (Yes/No) | Yes | No | No | No |
| Clinical symptoms on admission | ||||
| Fever | 8 d | 1 d | 1 d | – |
| Cough | 8 d | 1 d | 1 d | 1 d |
| Difficulty of breathing | 5 d | – | 1 d | – |
| Weakness | – | – | 1 d | – |
| Diarrhea | – | 1 d | – | – |
| Vitals on presentation | ||||
| Pulse (per min) | 110 | 98 | 102 | 84 |
| Respiratory rate (per min) | 16 | 18 | 18 | 16 |
| Temperature (Fahrenheit) | 98.6 | 100.2 F | 98.6 | 98.6 |
| Systolic blood pressure (mm Hg) | 152 | 130 | 142 | 130 |
| Diastolic blood pressure (mm Hg) | 104 | 90 | 86 | 80 |
| Oxygen requirement on admission | Nonrebreather mask | Low-flow oxygen | Nonrebreather mask | Ambient air |
| Eastern Cooperative Oncology Group score on admission | Capable of only limited self-care | Capable of only limited self-care | Capable of only limited self-care | Fully active, able to carry on predisease performances without restriction |
| Acid–base gas analysis | Not done | |||
| pH | 7.23 | 7.23 | 7.2 | – |
| Pco2 (mm Hg) | 53 | 30 | 34 | – |
| Po2 (mm Hg) | 64 | 74 | 72 | – |
| HCO3– (mEq/L) | 19 | 12.5 | 12 | – |
| Base deficit | 5.8 | 13 | 14 | – |
| Laboratory abnormalities on admission (normal range) | ||||
| Hemoglobin (13.6–16 g/dL) | 12 | 11 | 7.9 | 10.9 |
| RBC count (4.6–6.2 million/mm3) | 4.6 | 3.6 | 4 | 4.13 |
| TLC (4–11 × 1000/mm3) | 15.45 | 4.5 | 15.9 | 4.8 |
| Platelet count (150–400 × 1000/mm3) | 340 | 177 | 216 | 214 |
| Neutrophils (60%–70%) | 90 | 88 | 93 | 88 |
| Lymphocytes (25%–33%) | 8 | 10 | 5 | 9 |
| Eosinophils (2%–6%) | 1 | 1 | 1 | 1 |
| Monocytes (4%–7%) | 1 | 1 | 1 | 2 |
| PCV (42%–52%) | 40 | 33.9 | 26.2 | 33.4 |
| MCV (82–92 FL) | 88 | 92 | 81.9 | 80.9 |
| MCH (27–32 pg) | 27 | 29 | 24.7 | 26.4 |
| MCHC (32–36 g/dL) | 31 | 32 | 30.2 | 32.6 |
| RDW (10.6%–15.7%) | 14 | 13.5 | 15.3 | 15.5 |
| aPTT (28–35 s) | 64.2 | – | – | 32 |
| PT-INR (0.64–1.8) | 1.7 | – | – | 1.03 |
| D-dimer (200–500 ng/mL) | 370 | 1010 | 1230 | 330 |
| ALP (64–306 IU/L) | 58 | 54 | 56 | 40 |
| Total bilirubin (0.3–1.2 mg/dL) | 0.8 | 0.6 | 0.4 | 0.6 |
| AST (0–40 IU/L) | 52 | 17 | 25 | 22 |
| ALT (0–40 IU/L) | 46 | 24 | 58 | 20 |
| Total protein (6–8.3 g/dL) | 6.4 | 5.2 | 6.5 | 6.5 |
| Albumin (3.2–5 g/dL) | 6.43.5 | 3.2 | 3.4 | 3.5 |
| Globulin (2.5–3.5 g/dL) | 2.9 | 2 | 3.10 | 2 |
| Blood urea | 45 | 90 | 90 | 42 |
| Serum creatinine (0.5–1.4 mg/dL) | 1.45 | 2.75 | Hemodialysis dependent | 1.52 |
| Serum chloride (96–108 mEq/L) | 100 | 98 | 102 | 101 |
| Serum sodium (135–145 mEq/L) | 139 | 131 | 141 | 136 |
| Serum potassium (3.5–4.5 mEq/L) | 4.4 | 3.7 | 4.2 | 4.12 |
| IL-6 (<7 pg/mL) | 119 | 179 | 24 | – |
| hs-CRP (0–10 mg/L) | 198 | 51.2 | 46 | 2.0 |
| PCT (<0.5 ng/mL) | 0.09 | 0.44 | 0.69 | |
| HCV ELISA | Nonreactive | Nonreactive | Nonreactive | Nonreactive |
| HIV ELISA | Nonreactive | Nonreactive | Nonreactive | Nonreactive |
| HBsAg ELISA | Nonreactive | Nonreactive | Nonreactive | Nonreactive |
| Serum ferritin (13–400 ng/mL) | 364 | 1000 | – | 124 |
| Baseline medicines | ||||
| Immunosuppression | ||||
| Tablet prednisolone | 5 mg OD | 10 mg OD | 7.5 mg OD | 10 mg OD |
| Tablet cyclosporine | 50 mg BD | – | – | |
| Tablet azathioprine | – | – | – | 75 mg OD |
| Capsule tacrolimus | – | 1.5 mg -1 mg | – | 1.25 mg -1 mg |
| Tablet mycophenolate | 750 mg BD | 360 mg TDS | 360 mg TDS | – |
| Antihypertensive | ||||
| Tablet clonidine | 0.1 mg TDS | |||
| Tablet nifedipine | 20 mg TDS | |||
| Tablet metaprolol | – | 50 mg OD | – | 25 mg OD |
| Tablet diltiazem | – | – | – | 30 mg BD |
| Tablet cilnidipine | 5 mg OD | – | – | – |
| Tablet telmisartan | – | – | 40 mg OD | – |
| Tablet losartan | 50 mg BD | – | – | – |
| Antidiabetic | – | – | – | |
| Injection human Mixtard (50/50) |
32 IU morning | – | – | – |
| Injection human Mixtard (30/70) | 16 IU night | – | – | – |
| Injection basalog | – | 6 IU | – | – |
| Tab vildagliptin | 50 mg BD | – | ||
| Tablet gliclazide | 30 mg OD | – | - | – |
| Tablet metformin | 1000 mg BD | – | - | – |
| Others | ||||
| Tablet clopidogrel | 75 mg OD | – | – | – |
| Tablet atorvastatin | 10 mg OD | – | – | – |
| Tablet tamsulosin | 0.4 mg OD | 0.4 mg OD | – | – |
| Treatment regimen for COVID-19 received during stay | Critical care admission | Critical care admission | Critical care admission | Home-based therapy |
| Immunosuppression | CNI + antimetabolite stopped | CNI + antimetabolite stopped | CNI + antimetabolite stopped | No change |
| Tablet azithromycin | – | 500 mg OD | 500 mg OD | 500 mg OD |
| Tab favipiravir | – | – | – | 1600 mg BD; 800 mg BD for 5 d |
| Tablet tofacitinib | 10 mg BD | – | – | |
| Injection dexamethasone, 6 mg OD since admission | Yes | Yes | Yes | – |
| Remdesivir 200 mg on d 1, followed by 100 mg for 5 d | Yes | Yes | Yes | – |
| Injection bevacizumab | 400 mg on d 4 of admission | – | – | – |
| COVID-19 convalescent plasma component | – | 2 doses | – | – |
| Injection LMWH (0.6 S/C OD) | Yes | Yes | Yes | – |
| BiPAP | Since 12 h of admission | On d 7 of admission | Since d 2 of admission | – |
| Mechanical ventilation | On d 4 of admission | On d 14 of admission | – | – |
| Outcome | Died on d 8 of admission | Admitted on ventilator, d 16 of admission | Admitted on ventilator, post-CPR on d 4 of admission, dialysis dependent | At home, stable and recovered |
ADPKD, autosomal polycystic kidney disease; ALP, alkaline phosphatase; ALT, alanine transaminase; aPTT, activated partial thromboplastin time; AST, alanine transferase; BiPAP, bi-level positive pressure ventilation; CNI, calcineurin inhibitors; COVID-19, coronavirus disease; ELISA, enzyme-linked immunosorbent assay; HBsAg, hepatitis B virus; HCO3-, bicarbonate; HCV, hepatitis C virus; hs-CRP, high sensitive C-reactive protein; IL-6, interleukin-6; LMWH, low-molecular-weight heparin; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; PCT, procalcitonin; PCV, packed cell volume; PT-INR, prothrombin time – international normalized ratio; RBC, red blood cell count; RDW, red cell distribution width; SARS-CoV2 RT-PCR, severe acute respiratory syndrome coronavirus real rime polymerase chain test; TLC, total leukocyte count.
ACKNOWLEDGMENTS
The authors express our sincere gratitude to all the resident doctors and healthcare staffs who are tirelessly doing a mammoth job of managing the COVID-19 cases in India, despite facing resource crisis.
Footnotes
The authors declare no funding or conflicts of interest.
Data will be available from the corresponding author on request.
All authors contributed equally to the conception and design of the work; acquisition, analysis, interpretation of data; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work.
REFERENCES
- 1.Voysey M, Clemens SAC, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basic-Jukic N, Ivo J. SARS-CoV-2 infection after two doses of mRNA vaccine in renal transplant recipients. Transpl Infect Dis. 2021;00:e13628. [DOI] [PubMed] [Google Scholar]
- 5.Wadei HM, Gonwa TA, Leoni JC, et al. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant. [Epub ahead of print. April 23, 2021]. doi:10.1111/ajt.16618 [DOI] [PMC free article] [PubMed] [Google Scholar]


