Supplemental Digital Content is Available in the Text.
Intravitreal aflibercept administered in an early-start treat-and-extend regimen was noninferior to a late-start treat-and-extend regimen in patients with treatment-naïve nAMD. Improvements in functional (+4.3 and +7.9 letters) and anatomic (−162 µm and −159 µm) outcomes were observed at Week 104. Approximately half of patients had a last injection interval of ≥12 weeks.
Key words: neovascular age-related macular degeneration, AMD, antivascular endothelial growth factor, aflibercept, treat-and-extend
Abstract
Background/Purpose:
Treating neovascular age-related macular degeneration with intravitreal aflibercept treat-and-extend (T&E) can reduce treatment burden. ARIES assessed whether intravitreal aflibercept early-start T&E was noninferior to late-start T&E.
Methods:
A randomized, open-label, Phase 3b/4 study that included treatment-naïve patients aged ≥50 years with the best-corrected visual acuity 73–25 Early Treatment Diabetic Retinopathy Study letters and active choroidal neovascularization secondary to AMD. Patients received 2 mg intravitreal aflibercept at Week (W) 0, W4, W8, and W16. At W16, patients were randomized 1:1 to early-start (2W interval adjustments) or late-start T&E (8W intervals until W48 then 2W interval adjustments). Primary endpoint: the best-corrected visual acuity change from randomization to W104.
Results:
Two-hundred seventy-one patients were randomized. The mean (SD) best-corrected visual acuity at baseline was 60.2 (12.1; early-T&E) and 61.3 (10.8; late-T&E) letters. The mean (SD) best-corrected visual acuity change (W16–104) was −2.1 (11.4) versus −0.4 (8.4) letters (early-T&E vs. late-T&E; least-squares mean difference: −2.0; 95% confidence interval: −4.75 to 0.71; P = 0.0162 for noninferior); +4.3 (13.4) versus +7.9 (11.9) letters (W0–104). The mean (SD) number of injections was 12.0 (2.3) versus 13.0 (1.8). From baseline to W104, 93.4% and 96.2% maintained best-corrected visual acuity; the mean (SD) central retinal thickness change was −161.6 (135.6) µm and −158.6 (125.1) µm. The last injection interval (W104) was ≥12W for 47.2% and 51.9% of patients.
Conclusion:
Outcomes were similar between patients with neovascular age-related macular degeneration treated with an intravitreal aflibercept early-T&E or late-T&E regimen after initial dosing, with one injection difference over 2 years.
Trial Registration:
ClinicalTrials.gov Identifier: NCT02581891 https://clinicaltrials.gov/ct2/show/NCT02581891. Supplemental Digital Contents (files 1 http://links.lww.com/IAE/B419).
Antivascular endothelial growth factor (VEGF) agents are routinely used to manage neovascular age-related macular degeneration (nAMD).1,2 Evidence demonstrates rapid functional and anatomic improvements in nAMD with initial doses of anti-VEGF agents. However, these improvements are not always maintained in real-world clinical practice when reactive treatment regimens, such as pro-re-nata, are used.3–6 An analysis of the Comparison of Age-related Macular Degeneration Treatments Trials and Inhibition of VEGF in Age-related Choroidal Neovascularisation study found that fluctuation of central subfield thickness in eyes of patients with nAMD treated with anti-VEGF therapy was predictive of functional outcomes, where better disease control (less central subfield thickness fluctuation) was associated with better functional outcomes.3,7 Studies have shown that fluid fluctuations are minimized with proactive treat-and-extend (T&E), compared with reactive pro-re-nata dosing.8 T&E is a proactive, individualized dosing strategy whereby the injection interval can be gradually extended if functional and anatomic stability are maintained, and the interval shortened if deterioration is observed, to minimize the risk of disease recurrence rather than responding to it.4,5 At the time of the ARIES study design, intravitreal aflibercept (IVT-AFL) was registered to treat nAMD with bimonthly dosing (after three initial doses). The initiation of a T&E regimen, based on functional and anatomic outcomes, was possible only after 12 months of fixed dosing. Earlier initiation of the T&E regimen was subsequently approved in Europe and many other countries.
T&E regimens offer physicians the opportunity to consider flexible and individualized treatment of patients, with the aim of improving and maintaining functional and anatomic gains, while minimizing monitoring and treatment burden.4,6 The use of IVT-AFL T&E in the first year of nAMD treatment was initially investigated in the ALTAIR study.9 Findings demonstrated that an IVT-AFL T&E regimen, after 3 initial monthly doses, was efficacious in the first year of treatment and continuously efficacious in the second year, using 2-week (W) or 4W adjustments based on predefined extension, maintenance, or shortening criteria. Results showed improved functional and anatomic outcomes in patients with nAMD, and reductions in treatment burden.9 IVT-AFL was subsequently indicated to treat patients with nAMD starting the T&E regimen after 3 initial monthly doses and one dose after 8W, with the introduction of 2W or 4W adjustment steps, based on functional and anatomic outcomes.10
The aim of the ARIES (NCT02581891) study was to assess the efficacy and safety of 2-mg IVT-AFL in treatment-naïve patients with nAMD and determine if initiating a T&E regimen with IVT-AFL immediately after 3 initial monthly doses (early-start) was noninferior to initiating a T&E regimen after 1 year of fixed dosing every 8W (2q8; late-start).
Methods
ARIES was a 104W, randomized, open-label, Phase 3b/4 study that investigated the efficacy and safety of repeated doses of IVT-AFL with two different T&E approaches in patients with nAMD. The study was conducted in 39 centers in 8 countries (Australia, Canada, France, Germany, Hungary, Italy, Spain, and the United Kingdom) in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines E6: Good Clinical Practice. The protocol and any amendments were approved by the independent ethics committee/institutional review board at each site. All patients provided written informed consent.
Study Design
Patients received 2-mg IVT-AFL at W0, W4, W8, and W16 (Figure 1). If patients required injections more frequently than 2q8 (before or at W16), they were withdrawn from the study. At W16, patients were stratified based on functional outcomes (<8 or ≥8 letters gain in the best-corrected visual acuity [BCVA]) and randomized 1:1 to:
Early-start T&E arm (early-T&E)—from W16, patients received treatment in intervals adjusted by 2W (maximum of 16W), provided all anatomic criteria were met (functional criteria not assessed); patients who were completely dry at W16 could have their interval extended by 4W (next visit at W28) but from W28, 2W adjustments were implemented; or
Late-start T&E arm (late-T&E)—patients received treatment 2q8 to the end of Year 1; in Year 2 (starting from W48), treatment intervals were adjusted by 2W (maximum of 16W), provided all anatomic criteria were met (functional criteria not assessed).
Fig. 1.
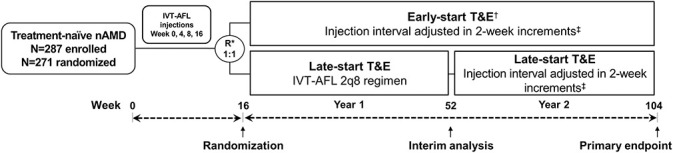
ARIES study design. *Patients were stratified based on visual outcomes from baseline to Week 16 (<8 letters or ≥8 letters gain in the BCVA). †If no IRF and no SRF at Week 16, treatment could be extended from 8 to 12 weeks. ‡Injection interval could be extended to a maximum of 16 weeks. 2q8, every 8 weeks; IRF, intraretinal fluid; R, randomization; SRF, subretinal fluid.
The anatomic criteria for extending the treatment intervals for the study eye, based on spectral-domain optical coherence tomography, were the absence of intraretinal fluid, absence of new neovascularization or hemorrhage, and subretinal fluid not exceeding 50 µm in thickness. If the extension criteria were not met, treatment intervals were reduced to the last effective interval.
Patients
Patients aged ≥50 years with the BCVA of 73–25 Early Treatment Diabetic Retinopathy Study (ETDRS) letters and active choroidal neovascularization lesions secondary to AMD with foveal involvement in the study eye were included. Further details are available in Supplemental Digital Content 2 (see file 2, http://links.lww.com/IAE/B419).
Endpoints
The primary endpoint was change in the BCVA (ETDRS letters) from randomization (W16) to W104. The key secondary endpoint was the proportion of patients who maintained vision (<15-letter loss) at W104 compared with baseline. Secondary endpoints included change in the BCVA from baseline to W52 and W104, and from W16 to W52; proportion of patients who maintained vision at W52 compared with baseline; proportion of patients with the BCVA gains of ≥15 letters from baseline to W52 and W104; change in central retinal thickness (CRT) from baseline to W52 and W104; the number of IVT-AFL injections from baseline to W52 and W104; and the duration of the last treatment interval. The absolute BCVA from baseline to W104 was also reported. Morphologic outcomes were assessed by the investigator and confirmed by the central reading center before entry into the database.
Adverse events were treatment-emergent adverse events (TEAEs) if they occurred or worsened after the first dose but ≤30 days after the last dose of the study drug. An adjudication of adverse events according to the Antiplatelet Trialists' Collaboration criteria was performed.
Statistical Analysis
A sample size of 108 evaluable patients per treatment arm was planned. Assuming an ETDRS letter score SD of 13 in the mean BCVA change from W16 to 104 and an equal mean BCVA change from W16 to 104 in the 2 treatment arms, this sample size provided a power of 80% for the one-sided noninferiority test with noninferiority margin of 5 letters using an alpha of 2.5%. All variables were summarized by descriptive statistics, and frequency tables were generated for categorical variables. The primary statistical analysis was performed on the per-protocol set (PPS). The early-T&E regimen was noninferior to the late-T&E regimen if the 2-sided 95% confidence interval (CI) of the letter score difference lay entirely above −5, where a positive difference favored the early-T&E regimen. If noninferiority of the early-T&E regimen was proven in the primary efficacy analysis, confirmatory testing was conducted on the PPS to assess the noninferiority of the early-T&E regimen regarding the key secondary efficacy variable. The early-T&E regimen was noninferior to the late-T&E regimen if the CI of the difference lay entirely above −7%, where a positive difference favored the early-T&E regimen. Other secondary functional endpoints were summarized descriptively. Full statistical analyses are available in Supplemental Digital Content 2 (see file 2, http://links.lww.com/IAE/B419).
Results
Patients
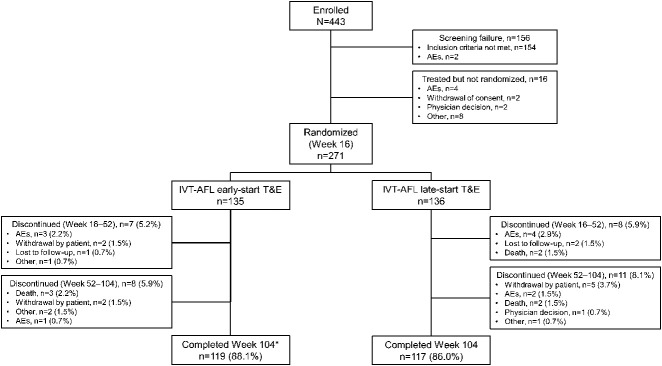
The patient disposition is provided in Figure 2. At W16, 271 patients were randomized; 134 and 135 patients were included in the early-T&E and late-T&E arms (full-analysis set). Overall, 88.1% (n = 119) and 86.0% (n = 117) of patients in the early-T&E and late-T&E arms completed the study; of which, 106 and 104 patients were included in the PPS. Reasons for discontinuation from W16 to W104 are provided in Figure 2. Baseline demographics were similar between the two arms (Table 1). Twenty-three patients (early-T&E, n = 9 and late-T&E, n = 14) were mis-stratified because of errors in data entry. This contributed to a slightly greater proportion of low gainers (<8-letter gain) being randomized to the early-T&E arm (58.2%; n = 78) than to the late-T&E arm (54.8%; n = 74) and probably led to the slight BCVA imbalance at W16.
Fig. 2.
Patient disposition. *One patient discontinued during the follow-up period after completion of treatment.
Table 1.
Patient Demographic and Characteristics (PPS)
| Characteristic | Early-T&E (n = 106) | Late-T&E (n = 104) |
| Baseline | ||
| Age, years | 75.5 (9.0) | 76.6 (8.7) |
| Age group, years, n (%) | ||
| ≤64 | 14 (13.2) | 8 (7.7) |
| 65–84 | 74 (69.8) | 80 (76.9) |
| ≥85 | 18 (17.0) | 16 (15.4) |
| Female, n (%) | 62 (58.5) | 58 (55.8) |
| BCVA, ETDRS letters | 60.2 (12.1)* | 61.3 (10.8)* |
| CRT, µm | 443.7 (120.0) | 448.3 (133.1) |
| CNV area, mm2 | 5.4 (4.4) | 5.4 (4.5) |
| Total lesion size, mm2 | 5.6 (4.7) | 5.9 (5.0) |
| PCV, n (%)† | 5 (4.7) | 3 (2.9) |
| RAP, n (%) | 8 (7.5) | 5 (4.8) |
| Duration of nAMD, years (min–max) | 0.18 (0.01–3.77) | 0.21 (0.01–6.00) |
| Week 16‡ | ||
| BCVA, ETDRS letters | 66.7 (13.0)§ | 69.6 (11.6)¶ |
| CRT, µm | 321.4 (93.4) | 322.5 (104.0) |
Mean (SD) unless otherwise stated.
Approximately 20/63 Snellen equivalent.
Indocyanine green angiography was not used to diagnose PCV.
From baseline to Week 16, there was one treatment group, and patients received 3 initial injections of IVT-AFL and were randomized to one of 2 treatment groups at Week 16.
Approximately 20/50 Snellen equivalent.
Approximately 20/40 Snellen equivalent.
CNV, choroidal neovascularization; PCV, polypoidal choroidal vasculopathy; RAP, retinal angiomatous proliferation.
Efficacy
Functional outcomes
Primary endpoint
In the PPS, the mean (SD) change in the BCVA (ETDRS letters) from randomization (W16) to W104 was −2.1 (11.4) versus −0.4 (8.4) in the early-T&E versus late-T&E arms (Figure 3A). At W104, the least squares mean difference (standard error) between the early-T&E and late-T&E arms was −2.0 (1.4); treatment effect 95% CI: −4.75 to 0.71; P = 0.0162 for the noninferiority test with a 5-letter noninferiority margin. A sensitivity analysis (full-analysis set population) showed a mean (SD) BCVA change from W16 to 104 of −2.1 (11.0) and −1.0 (10.0) in the early-T&E and late-T&E arms. At W104, the least squares means difference between the arms was −1.1 (1.3); 95% CI: −3.67 to 1.38.
Fig. 3.
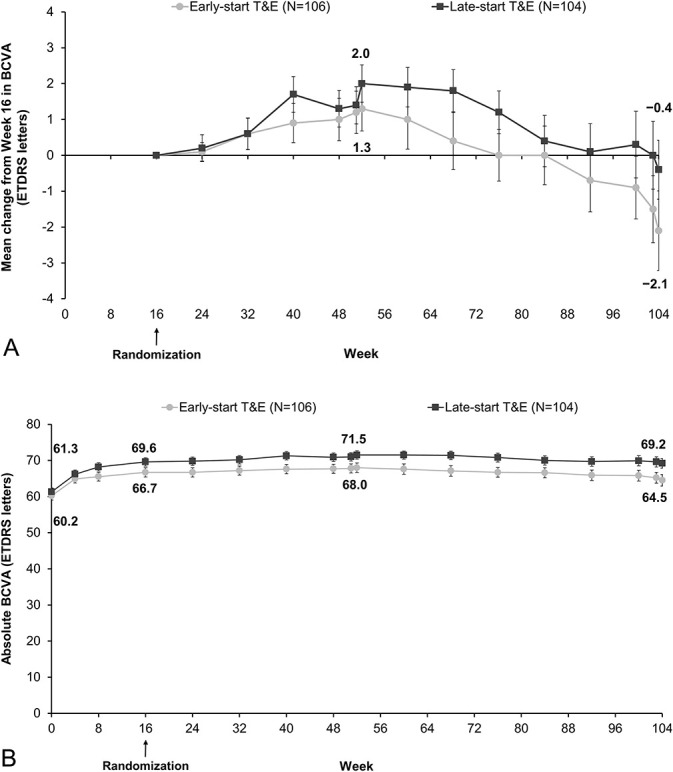
A. The mean BCVA change (Week 16 to Week 104). B. Absolute BCVA (baseline to Week 104; PPS). Error bars indicate standard error of the mean. Analyses were conducted as a last observation carried forward from the PPS. *Adjusted treatment difference (least squares means). †CI excludes −5 (prespecified noninferiority margin).
Secondary endpoints
All patients maintained baseline vision (<15-letter loss) at W52. At W104, 93.4% (early-T&E) and 96.2% (late-T&E) of patients maintained baseline vision (see eFigure 1, Supplemental Digital Content 2, http://links.lww.com/IAE/B419). The treatment effect difference was −2.5% (95% CI: −8.46 to 3.46). As the 95% CI of the difference did not lie entirely above −7%, it could not be concluded that early-T&E was noninferior to late-T&E for this secondary endpoint.
The mean (SD) BCVA change (ETDRS letters) from baseline to W52 and to W104 was 7.8 (9.4) and 4.3 (13.4) in the early-T&E arm and 10.2 (9.3) and 7.9 (11.9) in the late-T&E arm (see eFigure 2, Supplemental Digital Content 2, http://links.lww.com/IAE/B419). The mean BCVA change from W16 to W52 was 1.3 (6.4) and 2.0 (5.3) in the early-T&E and late-T&E arms. The mean absolute BCVA at baseline and W104 were 60.2 (12.1) and 64.5 (16.3) in the early-T&E arm and 61.3 (10.8) and 69.2 (13.7) in the late-T&E arm (Figure 3B). The proportion of patients who gained ≥15 letters from baseline to W52 and to W104 was 19.8% and 18.9% in the early-T&E arm and 27.9% and 22.1% in the late-T&E arm (see eFigure 1, Supplemental Digital Content 2, http://links.lww.com/IAE/B419).
Anatomic outcomes
The mean (SD) CRT change from baseline to W52 and to W104 was −164.9 (117.3) µm and −161.6 (135.6) µm in the early-T&E arm and −167.1 (117.1) µm and −158.6 (125.1) µm in the late-T&E arm (see eFigure 3, Supplemental Digital Content 2, http://links.lww.com/IAE/B419). The mean CRT change from W16 to W52 and to W104 was −28.5 (56.3) µm and −25.1 (68.9) µm in the early-T&E arm, and −28.7 (54.0) µm and −20.2 (70.0) µm in the late-T&E arm.
Treatment Exposure
In the PPS, the mean (SD) number of injections was 7.1 (0.8) and 8.0 (0.2) (up to W52), and 12.0 (2.3) and 13.0 (1.8) (up to W104) in the early-T&E and late-T&E arms. The median (min, max; Q1, Q3) number of injections up to W104 was 12.0 (7–20; 10.0, 14.0) and 13.0 (8–20; 12.0, 14.0) in the early-T&E and late-T&E arms. Overall, the mean (SD) duration of the last treatment interval up to W104 (the interval between the last two injections) was 11.5 (3.7) and 11.4 (3.7) W in the early-T&E and late-T&E arms. The proportion of patients according to the duration of their last treatment interval is presented in Figure 4.
Fig. 4.
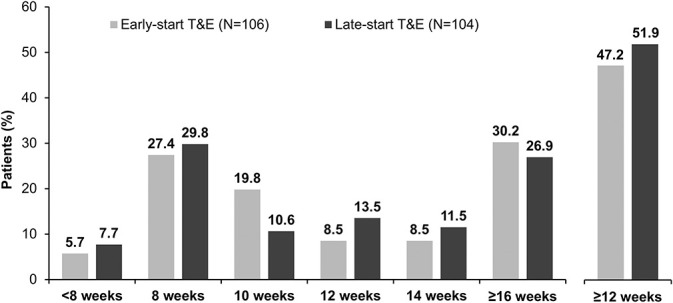
Duration of last treatment interval up to Week 104 (PPS). Analyses were conducted as a LOCF from the PPS. Intervals greater than 16 weeks were considered minor deviations and were included in the PPS. LOCF, last observation carried forward.
Safety
The main safety findings are shown in Table 2. The incidence of TEAEs and ocular TEAEs was comparable between the early-T&E and late-T&E arms (TEAEs: 84.4% and 82.4%; ocular TEAEs: 55.6% and 55.1%). Nonocular TEAEs were reported in fewer patients in the early-T&E (57.8%) than in the late-T&E arm (63.2%). The incidence of serious TEAEs was comparable between the two arms (21.5% and 25.7%); serious TEAEs related to IVT-AFL were reported in four patients (n = 2 before randomization and n = 2 in the late-T&E arm). No serious ocular TEAEs occurred in the early-T&E arm, and four (2.9%) events were reported in the late-T&E arm. The proportion of Antiplatelet Trialists' Collaboration events was similar between the treatment arms (1.5% and 3.7% in the early-T&E and late-T&E arms). There were seven deaths during the study; three in the early-T&E and four in the late-T&E arm. Three of the deaths were treatment-emergent, and none were considered related to IVT-AFL.
Table 2.
Safety Overview at Week 104 (SAS)
| No. of Patients (%) | Early-T&E (n = 135) | Late-T&E (n = 136) | Randomization Failure (n = 16) |
| Any TEAE | 114 (84.4) | 112 (82.4) | 12 (75.0) |
| Mild | 40 (29.6) | 29 (21.3) | 4 (25.0) |
| Moderate | 58 (43.0) | 58 (42.6) | 7 (43.8) |
| Severe | 16 (11.9) | 25 (18.4) | 1 (6.3) |
| Ocular TEAE (study eye) | 75 (55.6) | 75 (55.1) | 5 (31.3) |
| Any ocular TEAE related to the study drug (study eye) | 6 (4.4) | 4 (2.9) | 0 |
| Any TEAE related to the injection procedure | 41 (30.4) | 37 (27.2) | 2 (12.5) |
| Any TEAE related to procedures required by the protocol | 6 (4.4) | 7 (5.1) | 0 |
| Any serious TEAE | 29 (21.5) | 35 (25.7) | 3 (18.8) |
| Treatment-emergent deaths | 1 (0.7) | 2 (1.5) | 0 |
| Any serious TEAE related to the study drug | 0 | 2 (1.5)* | 2 (12.5)† |
| Any serious TEAE related to the injection procedure | 0 | 0 | 0 |
| Any serious TEAE related to other procedures required by the protocol | 0 | 0 | 0 |
| Any serious ocular TEAE (study eye) | 0 | 4 (2.9) | 0 |
| Discontinuation of the study drug because of TEAEs | 2 (1.5) | 5 (3.7) | 2 (12.5) |
| Discontinuation of the study drug because of serious TEAEs | 0 | 3 (2.2) | 1 (6.3) |
| APTC | 2 (1.5) | 5 (3.7) | 2 (12.5) |
| Any deaths‡ | 3 (2.2) | 4 (2.9) | 0 |
Gastrointestinal hemorrhage (n = 1) and pulmonary embolism (n = 1).
Cerebrovascular accident (n = 1) and acute myocardial infarction (n = 1).
Three deaths were treatment-emergent (acute cor pulmonale; acute hepatic failure, acute kidney injury, hypovolemic shock, ileus, and multiple organ dysfunction syndrome; and aortic dissection/pericardial hemorrhage), and none were considered related to IVT-AFL.
APTC, Antiplatelet Trialists' Collaboration; SAS, safety analysis set.
Discussion
The ARIES study showed that in treatment-naïve patients with nAMD, IVT-AFL administered using an early-T&E (immediately after the initial doses) or late-T&E (after Year 1) regimen improved functional and anatomic outcomes at W104. Regarding the primary endpoint, the mean BCVA change (ETDRS letters) from W16 to W104, the early-T&E regimen (−2.1) was statistically noninferior to the late-T&E regimen (−0.4; P = 0.0162 for noninferiority). Approximately half of patients (47.2% [early-T&E] and 51.9% [late-start]) had a last injection interval ≥12W. The CRT change from baseline to W104 was similar regardless of whether patients received IVT-AFL T&E in the first (−161.6 µm) or second year (−158.6 µm). Functional and anatomic improvements were achieved with a mean of 12 (early-T&E) versus 13 (late-T&E) injections. Early-T&E saved one injection in Year 1. In Year 2, the number of injections was the same in both treatment arms. The safety profile was consistent with prior studies of IVT-AFL.11,12
Regarding the key secondary endpoint, the proportion of patients who maintained baseline vision at W104, the treatment effect difference was −2.5% (95% CI: −8.46 to 3.46). As the 95% CI of the difference did not lie entirely above −7%, it could not be concluded that the early-T&E regimen was noninferior to the late-T&E regimen for this endpoint, suggesting that visual gains in the early-T&E group were lower. It should be noted that the study was not formally powered for this test, and this endpoint was based on the change from baseline and not from W16, when patients were randomized and treatment regimens started to differ. Although the statistical analysis of the key secondary endpoint was inconclusive (likely because of the lack of power for this analysis and the imbalance between treatment arms at W16), the proportion of patients who maintained vision was high (93.4% [early-T&E] and 96.2% [late-T&E]). Findings were similar to those observed in the IVT-AFL 2q8 arm of the VIEW study at W52 (95.1%) and W96 (92.4%).11,12
The predefined T&E regimen (in line with generally accepted T&E regimens at the time of study design) called for stepwise extensions before shortening by 2W when the treatment interval exceeded the patient's maximum tolerated interval. Therefore, 2W interval reductions were built into the T&E regimen. The most stringent possible definition of a relapse is the reduction of retreatment interval by at least 4W compared with the maximum achieved treatment interval for the patient. Under this definition, 21.9% (n = 46) of patients in the PPS had at least one relapse, although could be reextended based on meeting the extension criteria. Conversely, 78.1% of patients adhered to the T&E regimen without relapse. In the full-analysis set, which included patients who discontinued the study early or had protocol deviations, the proportion of patients with relapse was similar (n/N = 62/269; 23.0%).
There was a bimodal distribution of patients for the last treatment interval; between one-quarter and one-third of patients remained on an 8W treatment interval, and a similar proportion of patients were extended to a 16W treatment interval, consistent with what was observed in the ALTAIR study.9 There was a small proportion of patients (6–8%) who were relatively treatment intensive and required injections at intervals <8W. An advantage of T&E is that such patients can be identified.
Fixed dosing is a predictable regimen and therefore straightforward for the clinic and the patient. However, fixed dosing is usually associated with high clinic and patient burden and can lead to either overtreatment or undertreatment if the intervals between treatments are too short or too long.4 In real-world clinical practice, regimens such as T&E and pro-re-nata are often adopted to reduce treatment burden while maintaining functional outcomes. Utilization of proactive IVT-AFL T&E regimens allows for a pragmatic approach to treatment and offers benefits to physicians and patients. With proactive, individualized T&E dosing regimens, the need for interim monitoring is minimized, as is the risk of disease recurrence. Reducing the number of appointments per patient and minimizing the need for monitoring visits could ease clinic flow and patient burden. Furthermore, planning the next injection helps minimize the possibility of treatment delays and facilitates clinic management.4
The molecular attributes of aflibercept allow for extended treatment intervals. Previous studies of IVT-AFL in patients with nAMD have reported a median aqueous half-life of approximately 9 days13 and intraocular VEGF suppression times up to 16W.14,15 The results from ARIES indicate that, with IVT-AFL T&E, the treatment interval can be extended to ≥12W in approximately 50% of patients and up to 16W in approximately 30% of patients, consistent with the second-year data from the VIEW trial.16
The goal of anti-VEGF treatment for nAMD is to improve and maintain functional and anatomic improvements over and beyond the first year of treatment, while minimizing treatment burden on patients.4,6 In a retrospective study of IVT-AFL, vision gains achieved with fixed dosing in Year 1 were maintained to Year 3 with proactive T&E dosing.17 In a meta-analysis of 42 real-world observational studies, patients treated with a T&E regimen had better functional outcomes with more injections and fewer visits than those who received pro-re-nata dosing over 3 years.18
The functional outcomes in ARIES are comparable with those observed in the IVT-AFL 2q8 arm of the VIEW study and in other IVT-AFL T&E studies.11,12,19,20 The mean BCVA change from baseline to W52 was 9.0, 8.4, 7.2, and 5.2 letters in ARIES, VIEW, ATLAS, and RIVAL, respectively, and from baseline to the end of the study was 6.1 (W104), 7.6 (W96), and 2.4 (Year 2) letters in ARIES, VIEW, and ATLAS, respectively. The anatomic outcomes in ARIES are comparable with those observed for the IVT-AFL 2q8 arm in the VIEW study11,12; the mean CRT change from baseline to W52 was −166.0 µm and −139 µm, and to the end of the study was −160.1 µm (W104) and −133 µm (W96) in ARIES and VIEW, respectively. The results of ARIES also corroborate the results of ALTAIR, the study that supported the European Union label update to T&E in Year 1 of nAMD treatment.9
During the first year of ARIES, patients in the late-T&E arm were treated according to the recommended treatment posology indicated for IVT-AFL at the time of the study (fixed 2q8). To the best of our knowledge, ARIES is the first randomized clinical trial of IVT-AFL where treatment intervals could be <8W (reflective of real-world clinical practice) or extended to 16W. Extending the treatment interval beyond 12W and up to 16W offers potential advantages for both patients (reduced treatment burden) and health care providers (scheduling visits).4 The treatment interval criteria in ARIES can be easily implemented and are reflective of current clinical practice.
A limitation of the ARIES study is that there as a relatively small number of patients compared with pivotal trials. A sensitivity analysis addressed the influence of the mis-stratification and subsequent misrandomization of 23 patients on the change from baseline to W16. The mis-randomization of patients contributed to the slightly lower BCVA observed for the early-start arm at W16. No variables were identified that could account for the difference between the two arms at randomization (W16).
In conclusion, findings from the ARIES study demonstrate noninferiority of an IVT-AFL T&E regimen initiated immediately after the initial three doses, consistency of functional and anatomic outcomes with both early-T&E and late-T&E regimens for the treatment of nAMD, and a bimodal response for the individual treatment interval, with most patients requiring either 2q8 or ≥12W treatment intervals. This study supports the efficacy and safety of IVT-AFL in a T&E regimen as an alternative to fixed dosing in the first year. The results reinforce outcomes reported from previous studies of IVT-AFL T&E in treatment-naïve patients with nAMD.
Supplementary Material
Acknowledgments
The authors thank all the patients and investigators who participated in the study. Medical writing and editorial support for the preparation of this article, under the guidance of the authors, were provided by Mia Cahill, ApotheCom, UK, and were funded by Bayer Consumer Care AG, Switzerland. The authors thank Marvin Sperling, MD (former Head of Ophthalmology Medical Affairs at Bayer) and Daniel Janer, MD (former Senior Medical Affairs Ophthalmology Physician at Bayer) who provided input and expert medical guidance for the analysis and interpretation of the data and preparation of the article. All individuals have provided written permission to be acknowledged.
Access to data and data analysis: P. Mitchell had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ARIES study Steering Committee
Paul Mitchell, Sydney West Retina, Australia; Eric Souied, Hopital Intercommunal de Creteil, France; Edoardo Midena, University of Padova, Italy; Frank G. Holz, Universitaetsklinikum Bonn, Germany; Philip Hykin, Moorfields Eye Hospital, UK; and Giovanni Staurenghi, Ospedale Sacco, Università di Milano, Italy.
ARIES study investigators
Dr. Amelie Pielen, Medizinische Hochschule Hannover, Germany; Dr. Dahlke, University of Cologne, Germany; Dr. Sascha Fauser, University of Cologne, Germany; Dr. Karl Ulrich Bartz-Schmidt, Universitaetsklinik Tuebingen, Germany; Dr. Fanni Molnar, Universitaetsklinikum Freiburg, Germany; Dr. Kai Januschowski, Knappschaftskrankenhaus Sulzbach, Germany; Dr. Engelmann, Klinikum Chemnitz GmbH, Germany; Patrick Straßburger, Klinikum Chemnitz GmbH, Germany; Dr. Wachtlin, Sankt Gertrauden Krankenhaus Berlin, Germany; Dr. Michael Briggs, Royal Liverpool University Hospital, UK; Dr. Adam Ross, Bristol Eye Hospital, UK; Dr. Susan Downes, John Radcliffe Hospital, UK; Dr. Afrsar Jafree, East Kent Hospitals NHS University Foundations Trust, UK; Dr. Nishal Patel, East Kent Hospitals NHS University Foundations Trust, UK; Prof. Stéphanie Baillif, CHU de Nice, France; Dr. Monica Varano, IRCCS—Fondazione G.B. Bietti per lo studio e la ricercar, France; Prof. Francesco Bandello, Fondazione Centro San Raffaele del Monte Tabor, Italy; Dr. Alvaro Fernandez Vega, Instituto Oftalmologico Fernandez Vega, Spain; Dr. Pilar Calvo, Miguel Servet University Hospital, Spain; Dr. Alicia Valverde Megias, Hospital Clinico San Carlos, Spain; Dr. William Britton, The Retina Center of Ottawa, Canada; Dr. Laurent Lalonde, Institut De l'oeil des Laurentides, Canada; Dr. Varun Chaudhary, St. Josephs Healthcare Hamilton King Campus, Canada; Prof. Mark Gillies, Sydney Eye Hospital, Australia; Dr. Sukhpal Sandhu, Centre for Eye Research Australia, Australia; Dr. Brendan Vote, Thistle Street Medical Centre, Australia; Dr. Wong, Strathfield Retina Clinic, Australia; Dr. Balázs Varsányi, Ganglion Medical Center, Hungary; Dr. Andras Papp, Semmelweis Egyetem Szemészeti Klinika, Hungary; Dr. Attila Vajas, Debreceni Egyetem Klinikai Központ, Hungary; Dr. Andras Seres, Budapest Retina Associates, Hungary; Dr. Agnes Kerenyi, Bajcsy-Zsilinszky Kórház és Rendelőintézet, Hungary; Dr. Maria Eva Ferencz, Szent Imre Egyetemi Oktatókórház, Hungary; Dr. Gabor Vogt, MH Egészségügyi Központ, Hungary; Dr. Gyorgy Bator, Markusovszky Egyetemi Oktatókórház, Hungary; and Dr. Katalin Gombos, Szent János Kórház és Észak-budai Egyesített Kórházak, Hungary.
Footnotes
Medical writing and editorial support for the preparation of this article was funded by Bayer Consumer Care AG, Switzerland. This study was sponsored by Bayer HealthCare AG, Germany.
Prior presentation: European Society of Retina Specialists (EURETINA) Congress, Vienna, Austria, September 20–23, 2018; Asia-Pacific Academy of Ophthalmology (APAO) Congress, Bangkok, Thailand, March 6–9, 2019; Association for Research in Vision and Ophthalmology (ARVO) Congress, Vancouver, British Columbia, Canada, April 28–May 2, 2019; European Society of Retina Specialists (EURETINA) Congress, Paris, France, September 5–8, 2019; The Royal Australian and New Zealand College of Ophthalmologists (RANZCO) Congress, Adelaide, Australia, November 17–21, 2019; Asia-Pacific Vitreo-Retina Society (APVRS) Congress, Shanghai, China, November 22–24, 2019; Association for Research in Vision and Ophthalmology (ARVO) Congress, Baltimore, ML, USA, May 3–7, 2020.
P. Mitchell has received consulting fees from Bayer, Novartis, and Allergan and is an ARIES Steering Committee member. F. Holz has served as a consultant for Acucela, Apellis Pharmaceuticals, Bayer, Boehringer-Ingelheim, Bioeq/Formycon AG, Roche/Genentech, Geuder AG, Graybug Vision, Heidelberg Engineering, Chengdu Kanghong Pharmaceuticals, Lin Bioscience, Novartis, Pixium Vision, Oxurion, and Stealth BioTherapeutics; has received research support from Acucela, Apellis Pharmaceuticals, Bayer, Bioeq/Formycon AG, CenterVue, Roche/Genentech, Heidelberg Engineering, Chengdu Kanghong Pharmaceuticals, NightstaRx, Novartis, Optos, Pixium Vision, and Zeiss Pharma; has received honoraria or travel reimbursement from Acucela, Apellis Pharmaceuticals, Bayer, Ellex, Roche/Genentech, Graybug Vision, Heidelberg Engineering, Lin Bioscience, Novartis, Pixium Vision, Oxurion, Stealth BioTherapeutics, and Zeiss Pharma; and is an ARIES Steering Committee member. P. Hykin has received consultant fees from Bayer HealthCare, Novartis, and Allergan; has received travel support to participate in review activities for meetings from Bayer; has received support for provision of writing assistance, medicines, equipment, or administrative support from Bayer; has received payment for lectures and support for conference attendance from Allergan and Novartis; and is an ARIES Steering Committee member. S. Wolf has served as a consultant for Allergan, Bayer, Boehringer-Ingelheim, Chengdu Kanghong Biotech, Heidelberg Engineering, Novartis, Oxurion, Zeiss, and Roche; has received grant support from Heidelberg Engineering; and is an ARIES Steering Committee member. H. Allmeier, G. Lambrou, and T. Schmelter are employees of Bayer. T. Schmelter owns stocks of Bayer AG. E. Souied is an ARIES Steering Committee member. E. Midena is an ARIES Steering Committee member.
Bayer Consumer Care AG participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
Contributor Information
Frank G. Holz, Email: frank.holz@ukb.uni-bonn.de.
Philip Hykin, Email: philhykin@gmail.com.
Edoardo Midena, Email: edoardo.midena@unipd.it.
Eric Souied, Email: eric.souied@chicreteil.fr.
Helmut Allmeier, Email: helmut.allmeier@bayer.com.
George Lambrou, Email: george.lambrou@bayer.com.
Thomas Schmelter, Email: thomas.schmelter@bayer.com.
Sebastian Wolf, Email: sebastian.wolf@insel.ch.
References
- 1.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron 2012;75:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt-Erfurth U, Chong V, Loewenstein A, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol 2014;98:1144–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maguire MG, Martin DF, Ying GS, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology 2016;123:1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanzetta P, Loewenstein A; Vision Academy Steering Committee. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol 2017;255:1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freund KB, Korobelnik JF, Devenyi R, et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina 2015;35:1489–1506. [DOI] [PubMed] [Google Scholar]
- 6.Patel PJ, Devonport H, Sivaprasad S, et al. Aflibercept treatment for neovascular AMD beyond the first year: consensus recommendations by a UK expert roundtable panel, 2017 update. Clin Ophthalmol 2017;11:1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119:1399–1411. [DOI] [PubMed] [Google Scholar]
- 8.Giannakaki-Zimmermann H, Ebneter A, Munk MR, et al. Outcomes when switching from a pro re nata regimen to a treat and extend regimen using aflibercept in neovascular age-related macular degeneration. Ophthalmologica 2016;236:201–206. [DOI] [PubMed] [Google Scholar]
- 9.Ohji M, Takahashi K, Okada AA, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR. Adv Ther 2020;37:1173–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayer. Eylea (Aflibercept) Summary of Product Characteristics. 2018. Available at: https://www.ema.europa.eu/en/documents/product-information/eylea-epar-product-information_en.pdf. Accessed April 9, 2021. [Google Scholar]
- 11.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537–2548. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 2014;121:193–201. [DOI] [PubMed] [Google Scholar]
- 13.Do DV, Nguyen QD. Pharmacokinetics of free aflibercept in patients with neovascular age related macular degeneration. Invest Ophthalmol Vis Sci 2017;58:406. [Google Scholar]
- 14.Fauser S, Schwabecker V, Muether PS. Suppression of intraocular vascular endothelial growth factor during aflibercept treatment of age-related macular degeneration. Am J Ophthalmol 2014;158:532–536. [DOI] [PubMed] [Google Scholar]
- 15.Fauser S, Muether PS. Clinical correlation to differences in ranibizumab and aflibercept vascular endothelial growth factor suppression times. Br J Ophthalmol 2016;100:1494–1498. [DOI] [PubMed] [Google Scholar]
- 16.Khurana RN, Rahimy E, Joseph WA, et al. Extended (every 12 weeks or longer) dosing interval with intravitreal aflibercept and ranibizumab in neovascular age-related macular degeneration: post hoc analysis of VIEW trials. Am J Ophthalmol 2019;200:161–168. [DOI] [PubMed] [Google Scholar]
- 17.Eleftheriadou M, Gemenetzi M, Lukic M, et al. Three-year outcomes of aflibercept treatment for neovascular age-related macular degeneration: Evidence from a clinical setting. Ophthalmol Ther 2018;7:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim LN, Mehta H, Barthelmes D, et al. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina 2016;36:1418–1431. [DOI] [PubMed] [Google Scholar]
- 19.DeCroos FC, Reed D, Adam MK, et al. Treat-and-Extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial. Am J Ophthalmol 2017;180:142–150. [DOI] [PubMed] [Google Scholar]
- 20.Gillies MC, Hunyor AP, Arnold JJ, et al. Effect of Ranibizumab and Aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol 2019;137:372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.