Abstract
Objective:
To examine the role of cadherin-11, an integral membrane adhesion molecule, in periodontal ligament cells (PDLCs) under mechanical stimulation.
Materials and Methods:
Human PDLCs were cultured and subjected to mechanical stress. Cadherin-11 expression and cell morphology of PDLCs were investigated via immunofluorescence staining. The mRNA and protein expressions of cadherin-11 and type I collagen (Col-I) of PDLCs were evaluated by quantitative real-time polymerase chain reaction and Western blot, respectively. Small interfering RNA was used to knock down cadherin-11 expression in PDLCs. The collagen matrix of PDLCs was examined using toluidine blue staining.
Results:
Cadherin-11 was expressed in PDLCs. Mechanical stress suppressed cadherin-11 expression in PDLCs with prolonged force treatment time and increased force intensity, accompanied by suppressed β-catenin expression. Simultaneously, mechanical stress altered cell morphology and repressed Col-I expression in a time- and dose-dependent manner in PDLCs. Moreover, knockdown of cadherin-11 with suppressed β-catenin expression resulted in altered PDLC morphology and repressed collagen expression, which were consistent with the changes observed under mechanical stress.
Conclusions:
Results of this study suggest that cadherin-11 is expressed in PDLCs and modulates PDLC morphology and collagen synthesis in response to mechanical stress, which may play an important role in the homeostasis and remodeling of the PDL under mechanical stimulation.
Keywords: Cadherin-11, β-catenin, Mechanical stress, Periodontal ligament cells, Type I collagen
INTRODUCTION
The periodontal ligament (PDL) is a specialized connective tissue that attaches a tooth to the alveolar bone. Periodontal ligament cells (PDLCs) are the predominant cell type in the PDL and play an important role in PDL homeostasis and remodeling.1 PDLCs are subjected to mechanical stimulation generated by mastication, speech, and orthodontic treatment and can sense and respond to mechanical stress.2 Although mechanical stimulation alters biological activities in PDLCs,3,4 how PDLCs respond to mechanical stimulation has not been fully elucidated.
Cadherins mediate calcium-dependent homophilic cell-cell interaction and play critical roles in tissue formation and cell sorting during development.5 Cadherin-11 (encoded by the CDH11 gene) is a type II classic cadherin that mediates intercellular adhesion between cells of the same type, which is crucial for tissue morphogenesis and architecture.6 Cadherin-11 is mostly expressed in mesenchymal-type cells such as fibroblasts and osteoblasts,7,8 which suggests that cadherin-11 may also be expressed in PDLCs. Mechanical stimulation can regulate cadherin-11 expression in synovial fibroblasts.9 However, the response and role of cadherin-11 in PDLCs under mechanical stimulation remain unknown.
Collagen is the main component of the extracellular matrix (ECM) in the PDL.10 PDLCs secrete ECM components, such as collagen, building up the PDL collagen fibers and having significant function in the remodeling of PDL collagen.11 Collagen expression in cultured PDLCs can be regulated by mechanical stimulation.12,13 During orthodontic tooth movement, PDL collagen is degraded in the compression region, with altered expression of type I collagen (Col-I) and cytoskeleton of cells.14,15 Hence, the present study aimed to test the hypothesis that cadherin-11 is expressed in PDLCs and modulates cell morphology and collagen synthesis in PDLCs in response to mechanical stress.
MATERIALS AND METHODS
Cell Culture
The protocol used to obtain human tissue samples in this study was approved by the Ethical Guidelines of Peking University (PKUSSIRB-201311103) and was performed with appropriate informed consent. Human PDLCs were isolated from PDLs of normal, orthodontically extracted bicuspids as previously described.16 The harvested PDLCs were cultured with alpha modification of Eagle's medium containing 15% fetal calf serum (GIBCO, Carlsbad, Calif), 100 U/mL penicillin, and 100 g/mL streptomycin (Biofluids, Rockville, Md) and were used at passage 4. The alignment of cultured PDLCs was observed using a light inverted microscope.
Mechanical stress was applied on cultured PDLCs using the uniform method.17,18 In brief, a glass cover and additional metal weights on top were placed over an 80% confluent cell layer in six-well plates. The PDLCs were subjected to different intensities of mechanical stress ranging from 0 to 2 g/cm2 for 24 hours or at 1 g/cm2 for different time ranging from 0 to 24 hours.
Small Interfering RNA Transfection
Cadheirn-11 small interfering RNA (siRNA) and negative control siRNA were purchased from GenePharma (Suzhou, China). The siRNA sequences of CDH11 were as follows: siCDH11 number 1: 5′-AGGAAGUAGGAAGAGUGAAAGCUAA-3′; siCDH11 number 2: 5′-CAUCGUCAUUCUCCUGGUCAUUGUA-3′. The PDLCs were transfected with siRNA by lipofectamine RNAiMax reagent (Invitrogen, Carlsbad, Calif) according to the manufacturer's protocol.
Immunofluorescence Staining
Immunofluorescence staining was performed as previously described.19 Briefly, cultured PDLCs were washed with phosphate-buffered saline, fixed using 4% paraformaldehyde fixative, and incubated with cadherin-11 (1:200; Cell Signaling Technology, Danvers, Mass), β-catenin (1:200; Cell Signaling Technology), and phalloidin (1:200; Sigma, Danvers, Mass). The nuclei were counterstained with 4′, 6-diamidino-2-phenylindole. Confocal microscopic images were acquired using a Zeiss laser scanning microscope (LSM 510; Jena, Germany), and the images were processed using LSM 5 Release 4.2 software.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from cells with Trizol reagent (Invitrogen). Reverse transcription and real-time PCR were performed as previously described.19 The primers designed by Primer Premier 5.0 software and commercially synthesized were as follows: human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) sense/antisense, 5′-ATGGGGAAGGTGAAGGTCG-3′/5′-GGGGTCATTGATGGCAACAATA-3′; human cadherin-11 sense/antisense, 5′-AGAGGTCCAATGTGGGAACG-3′/5′-GGTTGTCCTTCGAGGATACTGT-3′; and human Col-I sense/antisense, 5′-CCAGAAGAACTGGTACATCAGCAA-3′/5′-CGCCATACTCGAACTGGAATC-3′. The efficiency of the newly designed primers was confirmed by sequencing. Relative quantification of gene expression was assessed with the comparative cycle threshold method, using GAPDH as internal control.
Western Blot
Western blot was performed as previously described in detail.19 Antibodies of cadherin-11 (1:1000; Cell Signaling Technology), β-catenin (1:1000; Cell Signaling Technology), Col-I (1:1000; Proteintech, Wuhan, China), and GAPDH (1:1000; Santa Cruz Biotechnology Inc, Santa Cruz, Calif) were used. Each experiment was repeated three times to obtain comparable results.
Toluidine Blue Staining
Cells were fixed in 4% paraformaldehyde and toluidine blue (3%) stain, and were incubated overnight at 4°C. Ethyl alcohol (95%) was subsequently added, excess dye was washed out, then the cells in plates were observed by optical microscope.
Statistical Analysis
Statistical analysis was performed with SPSS 13.0 (IBM Corp, Armonk, NY). Comparisons among groups were statistically analyzed by one-way analysis of variance, followed by the least significant, difference multiple-comparison test. All data were presented as mean ± SD. Statistical significance was considered at P < .05.
RESULTS
Cadherin-11 and β-Catenin Expressions in PDLCs Were Suppressed Under Mechanical Stress
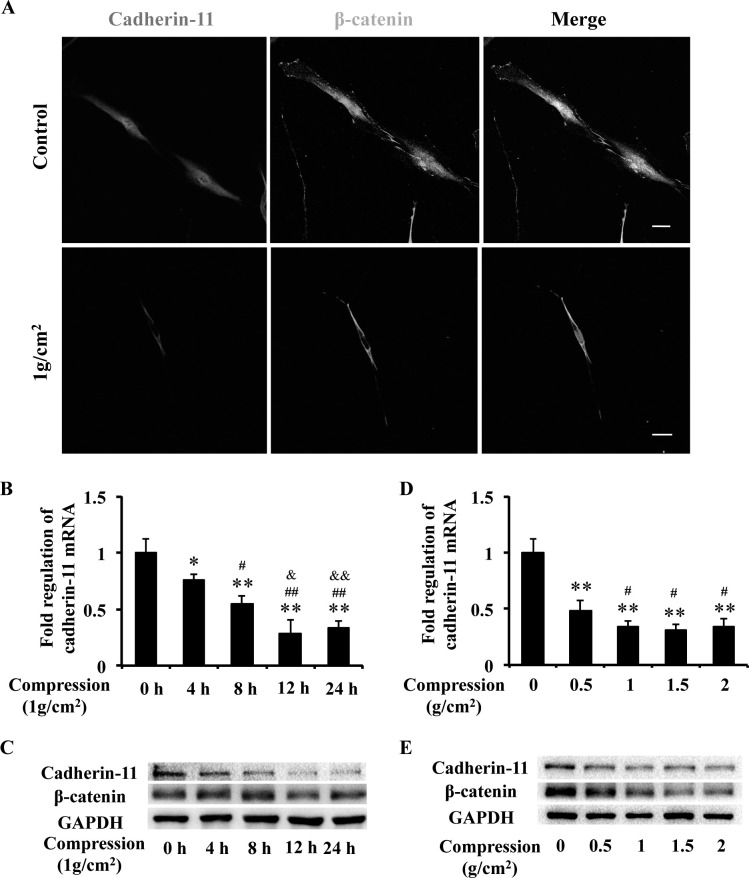
Cadherin-11 plays an important role in sensing and transmitting force into the cell interior and interacts with β-catenin to regulate cell functions.20 Immunofluorescence showed that cadherin-11 was expressed in PDLCs and β-catenin located in both cytoplasm and nucleus. Under mechanical stress (1 g/cm2) for 24 hours, the expressions of cadherin-11 and β-catenin were suppressed, and β-catenin activity in the nucleus was prominently reduced (Figure 1A).
Figure 1.
Cadherin-11 and β-catenin expressions in PDLCs were suppressed under mechanical stress. (A) Immunofluorescence staining showed that cadherin-11 (red) was expressed in PDLCs and β-catenin (green) located in both cytoplasm and nucleus. Under mechanical stress, the expression of cadherin-11 (red) and β-catenin (green) was suppressed, and β-catenin (green) activity in the nucleus prominently reduced. Scale bars: 20 μm. (B, C) The mRNA and protein expressions of cadherin-11 and protein expression of β-catenin in PDLCs were suppressed with prolonged stress treatment time, examined by real-time PCR (B) and Western blot (C). * P = .05; ** P = .01 versus 0-hour group; # P = .05 ## P = .01 vs 4-hour group; & P = .05, && P = .01 vs 8-hour group. (D, E) The mRNA and protein expressions of cadherin-11 and protein expression of β-catenin in PDLCs were suppressed under mechanical stress with variable intensities, examined by real-time PCR (D) and Western blot (E). ** P = .01 vs 0 g group; * P = .05 vs 0.5-g group.
The mRNA and protein expressions of cadherin-11 in PDLCs were suppressed with prolonged stress treatment time (4, 8, 12, and 24 hours) at an intensity of 1 g/cm2, accompanied by suppressed β-catenin protein expression (Figure 1B,C). In addition, the suppressed mRNA and protein expressions of cadherin-11 expression were observed at an intensity of 0.5 g/cm2 and further decreased at intensities of 1, 1.5, and 2 g/cm2 of mechanical stress, accompanied by suppressed β-catenin protein expression (Figure 1D,E). These results suggest that cadherin-11 is expressed in PDLCs and that PDLCs respond to mechanical stress with a suppressed cadherin-11/β-catenin pathway.
Mechanical Stress Altered PDLC Morphology and Repressed Col-I Expression
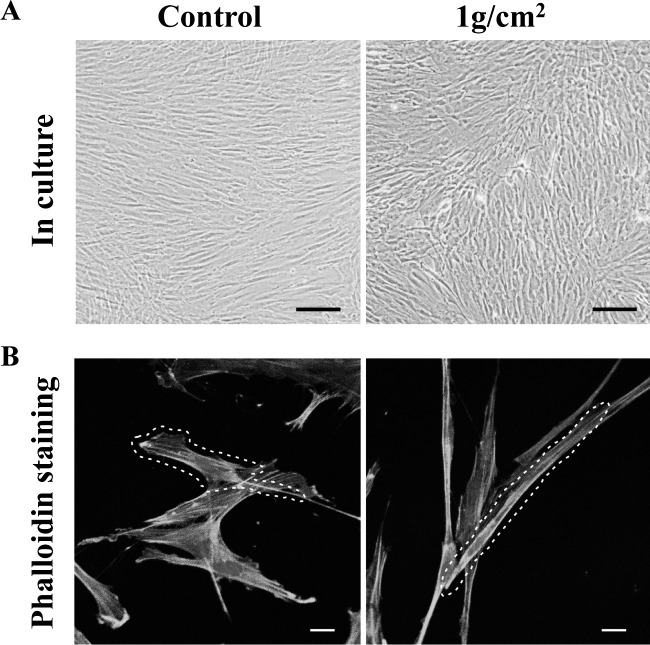
To investigate the function of mechanical stress-suppressed cadherin-11 on PDLCs, we observed the morphology and cytoskeleton organization of PDLCs under mechanical stress. Under mechanical stress (1 g/cm2) for 24 hours, PDLCs displayed less aligned and loose cell-cell conjunction (Figure 2A) and appeared significantly elongated morphologically with denser F-actin distribution (Figure 2B), compared with PDLCs from the control group.
Figure 2.
Mechanical stress altered cell alignment (A) and morphology (B) in PDLCs. (A) Scale bars: 200 μm. (B) Scale bars: 20 μm.
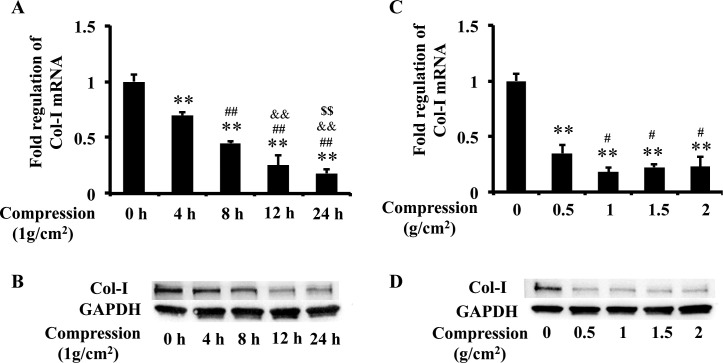
Apart from morphological changes, application of mechanical stimulation also affected a variety of cellular processes in PDLCs.21 Col-I is the major component of the PDL.22 We found that mRNA and protein expressions of Col-I in PDLCs were repressed by mechanical stress with prolonged stress treatment time (4, 8, 12, and 24 hours) and various stress intensities (0.5 g/cm2, 1 g/cm2, 1.5 g/cm2, and 2 g/cm2) (Figure 3), which were consistent with the suppressed expression of cadherin-11 in response to mechanical stress. These data indicate that mechanical stress–suppressed cadherin-11 could alter cell morphology and repress collagen synthesis in PDLCs.
Figure 3.
Mechanical stress repressed Col-I expression in PDLCs. (A, B) The mRNA and protein expressions of Col-I in PDLCs were repressed with prolonged stress treatment time, examined by real-time PCR (A) and Western blot (B). ** P = .01 vs 0-hour group; ## P = .01 vs 4-hour group; && P = .0 1 vs 8-hour group; $$ P = .01 vs 12-hour group. (C, D) The mRNA and protein expressions of Col-I in PDLCs were repressed under mechanical stress with variable intensities, examined by real-time PCR (C) and western blot (D). ** P = .01 vs 0-g group; # P = .05 vs 0.5-g group.
Knockdown of CDH11 Altered Cell Morphology and Repressed Col-I Expression in PDLCs
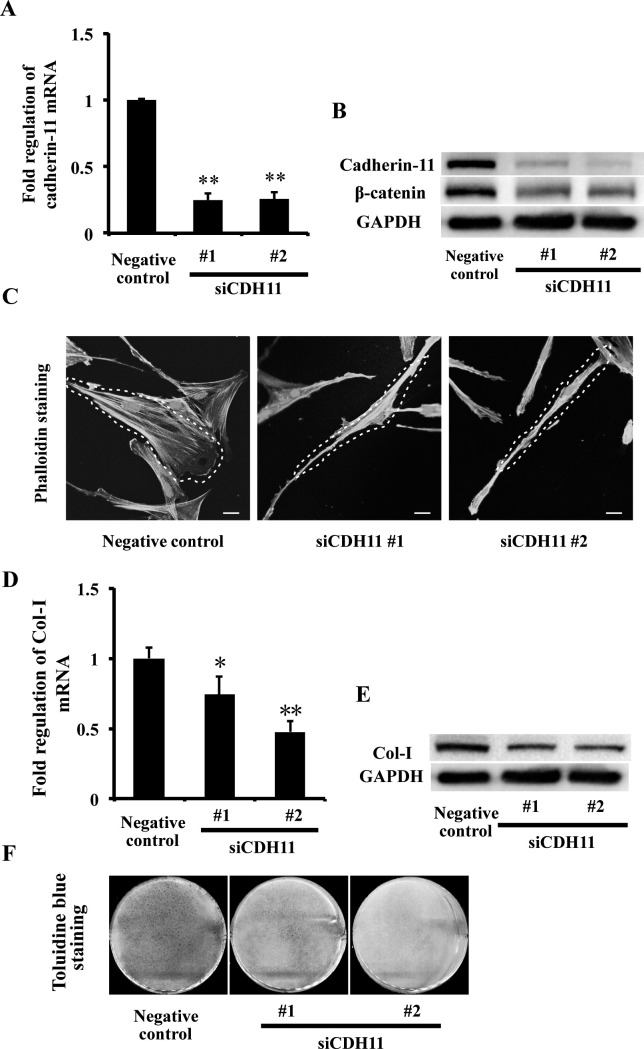
To confirm whether the suppressed cadherin-11 contributed to the altered PDLC morphology and repressed collagen expression under mechanical stress, cadherin-11 expression in PDLCs was knocked down using CDH11-targeting siRNA. Two siCDH11 sequences were used to confirm the efficacy of siCDH11 transfection. Compared with negative control, PDLCs transfected with siCDH11 sequences resulted in significant suppression of cadherin-11 at both the mRNA (Figure 4A) and protein levels (Figure 4B). β-Catenin protein expression was also suppressed (Figure 4B).
Figure 4.
Knockdown of CDH11 altered PDLC morphology and repressed Col-I expression. (A, B) The mRNA and protein expressions of cadherin-11 and protein expression of β-catenin in PDLCs were suppressed in the siCDH11 No.1 and siCDH11 No. 2 groups, examined by real-time PCR (A) and Western blot (B). ** P = .01 vs negative control group. (C) Phalloidin staining showed morphology of PDLCs in negative control, siCDH11 No.1, and siCDH11 No. 2 groups. Scale bars: 20 μm. (D, E) The mRNA and protein expressions of Col-I in PDLCs were repressed in the siCDH11 No.1 and siCDH11 No. 2 groups, examined by real-time PCR (D) and Western blot (E). * P = .05, ** P = .01 vs negative control group. (F) Toluidine blue staining showed that collagen matrix of PDLCs was repressed in the siCDH11 No.1 and siCDH11 No. 2 groups compared with the negative control group.
Knockdown of cadherin-11 expression in PDLCs resulted in elongated cell morphology and denser F-actin distribution, which were consistent with the changes produced by mechanical stress (Figure 4C). Furthermore, Col-I expression in PDLCs was repressed after depletion of cadherin-11 at the mRNA (Figure 4D) and protein levels (Figure 4E) and at the extracellular collagen matrix (Figure 4F), which were also consistent with the effect of mechanical stress. These data suggest that cadherin-11 could modulate cell morphology and collagen synthesis in PDLCs.
DISCUSSION
In the present study, we were the first to demonstrate that cadherin-11 modulates cell morphology and collagen synthesis of PDLCs in response to mechanical stress. First, cadherin-11 was expressed in PDLCs, and the expressions of cadherin-11 and β-catenin were suppressed by mechanical stress with prolonged stress treatment time and increased stress intensity. Second, mechanical stress altered PDLC morphology and repressed collagen expression in time- and dose-dependent manners. Moreover, knockdown of cadherin-11 expression in PDLCs resulted in altered cell morphology and repressed collagen expression, which were consistent with the changes produced by mechanical stress in PDLCs. These data indicate that mechanical stress suppresses the cadherin-11/β-catenin pathway in PDLCs, which may contribute to altered PDLC morphology and repressed collagen synthesis.
Cadherins are a large superfamily of calcium-dependent adhesion proteins mediating cell-cell cohesion in soft tissues.5 Moreover, cadherin complexes are force sensors that transduce fluctuations in intercellular tension into intracellular signals to regulate tissue remodeling.20 A previous study has shown that hydrostatic pressure increased cadherin-11 expression in synovial fibroblasts.9 In the present study, the PDLCs were subjected to mechanical stress, and cadherin-11 expression was significantly suppressed, accompanied by suppressed β-catenin expression. β-Catenin is recruited directly to the cadherin cytoplasmic tail, functioning as a signal transducer in the Wnt signaling pathway.23,24 Wnt/β-catenin signaling is required for mechanotransduction in bone.25 Mechanical strain and fluid shear stress induce nuclear translocation of β-catenin in calvarial osteoblasts and osteocytes.26,27 Our findings, for the first time, reported that cadherin-11 was expressed in PDLCs and suggest that cadherin-11/β-catenin axis might play an important role in the signal transduction of mechanical stimulation to biological function in PDLCs.
Cadherin-11 regulated PDLC morphology under mechanical stress. Actin cytoskeleton can be coupled to sites of adherens junctions, and they produce force to generate changes in cell shape and organization.5 In this study, mechanical stress resulted in loose cell-cell conjunctions, elongated morphology, and denser F-actin distribution in PDLCs, accompanied by the suppressed expressions of cadherin-11 and β-catenin. A previous study has shown that compressive force reduced the F-actin level, leading to PDLC spreading,28 which is consistent with our study. The cadherin-catenin complex plays important roles in regulating the rearrangement of actin cytoskeleton and controlling tissue morphogenesis.29 Our results further showed that knockdown of cadherin-11 expression resulted in altered PDLC morphology, which was consistent with the changes produced by the application of mechanical stress. These findings suggest that the suppressed cadherin-11/β-catenin pathway under mechanical stress is not only a concomitant phenomenon but also may be the cause of altered morphology of the PDLCs, and indicate that cadherin-11 might be involved in the architecture of PDL by regulating the morphology of PDLCs.
Changes in the cellular environment can affect adhesion complexes, modify the actin organization, and then initiate downstream biosynthetic responses. The primary function of PDLCs is elaboration of collagen-predominant ECM. Our study showed that mechanical stress suppressed cadherin-11 and Col-I expression in PDLCs. Moreover, knockdown of cadherin-11 expression resulted in repressed PDLC collagen expression. A previous study has shown that cadherin-11 was necessary for cell-cell adhesion and matrix production of the synovial lining.30 Additionally, knockdown of cadherin-11 in tendons results in cell separation and misalignment of the collagen fibrils in the tendon ECM.31 Our results are consistent with these previous studies. A previous study has shown that PDL undergoes degradation and PDLCs lose their regular arrangement on the compression side under orthodontic force.32 Our study might reveal a novel mechanism by which cadherin-11 modulates PDLC morphology and collagen synthesis under mechanical stimulation.
CONCLUSIONS
For the first time, our results demonstrate that cadherin-11 is expressed in PDLCs and modulates PDLC morphology and collagen synthesis in response to mechanical stress, which provides a new understanding of how PDLCs respond to mechanical stimulation during physiological activity.
ACKNOWLEDGMENTS
This study was supported by grants from the International S&T cooperation program of China (Grant 2013DFB30360), National Natural Science Foundation of China (Grants 81470717, 81300897, and 81571815), and Beijing Municipal Natural Science Foundation (7152156).
REFERENCES
- 1.Jonsson D, Nebel D, Bratthall G, Nilsson BO. The human periodontal ligament cell: a fibroblast-like cell acting as an immune cell. J Periodontal Res. 2011;46:153–157. doi: 10.1111/j.1600-0765.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 2.Myokai F, Oyama M, Nishimura F, et al. Unique genes induced by mechanical stress in periodontal ligament cells. J Periodontal Res. 2003;38:255–261. doi: 10.1034/j.1600-0765.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- 3.Sen S, Diercke K, Zingler S, Lux CJ, Erber R. Compression induces Ephrin-A2 in PDL fibroblasts via c-fos. J Dent Res. 2015;94:464–472. doi: 10.1177/0022034514567197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Yang Y, Li X, et al. Functional analysis of core binding factor a1 and its relationship with related genes expressed by human periodontal ligament cells exposed to mechanical stress. Eur J Orthod. 2010;32:698–705. doi: 10.1093/ejo/cjq010. [DOI] [PubMed] [Google Scholar]
- 5.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 6.Kiener HP, Brenner MB. Building the synovium: cadherin-11 mediates fibroblast-like synoviocyte cell-to-cell adhesion. Arthritis Res Ther. 2005;7:49–54. doi: 10.1186/ar1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SK, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol Rev. 2010;233:256–266. doi: 10.1111/j.0105-2896.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki M, Takeshita S, Kawai S, et al. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem. 1994;269:12092–12098. [PubMed] [Google Scholar]
- 9.Wu M, Xu T, Zhou Y, Lu H, Gu Z. Pressure and inflammatory stimulation induced increase of cadherin-11 is mediated by PI3K/Akt pathway in synovial fibroblasts from temporomandibular joint. Osteoarthritis Cartilage. 2013;21:1605–1612. doi: 10.1016/j.joca.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Butler WT, Birkedal-Hansen H, Beegle WF, Taylor RE, Chung E. Proteins of the periodontium. Identification of collagens with the [alpha1(I)]2alpha2 and [alpha1(III)]3 structures in bovine periodontal ligament. J Biol Chem. 1975;250:8907–8912. [PubMed] [Google Scholar]
- 11.Lekic P, McCulloch CA. Periodontal ligament cell population: the central role of fibroblasts in creating a unique tissue. Anat Rec. 1996;245:327–341. doi: 10.1002/(SICI)1097-0185(199606)245:2<327::AID-AR15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Zhao D, Wu Y, Xu C, Zhang F. Cyclic stretch induced gene expression of extracellular matrix and adhesion molecules in human periodontal ligament cells. Arch Oral Biol. 2015;60:447–455. doi: 10.1016/j.archoralbio.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Howard PS, Kucich U, Taliwal R, Korostoff JM. Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J Periodontal Res. 1998;33:500–508. doi: 10.1111/j.1600-0765.1998.tb02350.x. [DOI] [PubMed] [Google Scholar]
- 14.Bumann A, Carvalho RS, Schwarzer CL, Yen EH. Collagen synthesis from human PDL cells following orthodontic tooth movement. Eur J Orthod. 1997;19:29–37. doi: 10.1093/ejo/19.1.29. [DOI] [PubMed] [Google Scholar]
- 15.Rygh P. Ultrastructural changes of the periodontal fibers and their attachment in rat molar periodontium incident to orthodontic tooth movement. Scand J Dent Res. 1973;81:467–480. doi: 10.1111/j.1600-0722.1973.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 16.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 17.Cao H, Kou X, Yang R, et al. Force-induced Adrb2 in periodontal ligament cells promotes tooth movement. J Dent Res. 2014;93;:1163–1169. doi: 10.1177/0022034514551769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He D, Kou X, Yang R, et al. M1-like macrophage polarization promotes orthodontic tooth movement. J Dent Res. 2015;94:1286–1294. doi: 10.1177/0022034515589714. [DOI] [PubMed] [Google Scholar]
- 19.Kou XX, Wu YW, Ding Y, et al. 17beta-estradiol aggravates temporomandibular joint inflammation through the NF-kappaB pathway in ovariectomized rats. Arthritis Rheum. 2011;63:1888–1897. doi: 10.1002/art.30334. [DOI] [PubMed] [Google Scholar]
- 20.Huveneers S, de Rooij J. Mechanosensitive systems at the cadherin-F-actin interface. J Cell Sci. 2013;126(Pt 2):403–413. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- 21.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nature reviews. Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 22.Choi JW, Arai C, Ishikawa M, Shimoda S, Nakamura Y. Fiber system degradation, and periostin and connective tissue growth factor level reduction, in the periodontal ligament of teeth in the absence of masticatory load. J Periodontal Res. 2011;46:513–521. doi: 10.1111/j.1600-0765.2011.01351.x. [DOI] [PubMed] [Google Scholar]
- 23.Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 26.Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif Tissue Int. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 28.de Araujo RM, Oba Y, Kuroda S, Tanaka E, Moriyama K. RhoE regulates actin cytoskeleton organization in human periodontal ligament cells under mechanical stress. Arch Oral Biol. 2014;59:187–192. doi: 10.1016/j.archoralbio.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 30.Lee DM, Kiener HP, Agarwal SK, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 31.Richardson SH, Starborg T, Lu Y, Humphries SM, Meadows RS, Kadler KE. Tendon development requires regulation of cell condensation and cell shape via cadherin-11-mediated cell-cell junctions. Mol Cell Biol. 2007;27:6218–6228. doi: 10.1128/MCB.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida Y, Sasaki T, Yokoya K, Hiraide T, Shibasaki Y. Cellular roles in relapse processes of experimentally-moved rat molars. J Electron Microsc. 1999;48:147–157. doi: 10.1093/oxfordjournals.jmicro.a023661. [DOI] [PubMed] [Google Scholar]