Abstract
We compared secondary attack rates in households with B.1.1.7 variant of concern (VOC) versus non-VOC index cases in a matched cohort in Ontario, Canada. The secondary attack rate for VOC index cases was 1.31 times higher than non-VOC index cases. This increase was particularly accentuated for asymptomatic or presymptomatic index cases.
Keywords: COVID-19, SARS-CoV-2, variants of concern, household, transmission
The prevalence of variants having the N501Y mutation have rapidly increased globally, including in Ontario, Canada, where this prevalence increased dramatically in February 2021 [1]. These patterns of rapid strain replacement suggest increased transmissibility of variants with the N501Y mutation, which is present across variants of concern (VOC), including B.1.1.7, B.1.351, and P.1 lineages [2]. However, the exact degree of increased transmissibility, and specific settings when increased transmissibility occurs, remains unclear [3, 4]. Higher secondary attack rates related to VOC index cases have been reported [5], but have not been explored within households, which continue to be an important source of coronavirus disease 2019 (COVID-19) transmission [6]. The objective of this study was to compare secondary attack rates in households with VOC versus non-VOC index cases in Ontario, Canada.
METHODS
We identified individuals with laboratory-confirmed COVID-19 reported in the Public Health Case and Contact Management Solution (CCM), Ontario’s COVID-19 reporting system, and included households with index cases reported from February 7 to 27, 2021. VOC cases included either individuals confirmed as B.1.1.7 using whole-genome sequencing or those that screened positive for the N501Y mutation using real-time polymerase chain reaction (PCR). B.1.1.7 accounted for more than 90% of confirmed VOC cases reported in Ontario until February 27, 2021. All PCR-positive specimens in Ontario with cycle threshold ≤35 underwent screening for the N501Y mutation during the study period using a single-nucleotide polymorphism real-time PCR assay that screened for the N501Y mutation in the spike gene [7]. Non-VOC cases were those that screened negative for the N501Y mutation.
We grouped cases living in the same household based on residential address [8]. Index cases were defined as the first case in the household based on symptom onset date (or specimen collection date, if symptom-onset date was not available) and secondary cases were included if they occurred 1–14 days after the index case. We excluded cases in congregate settings, as well as households with one individual or with >1 case with the same earliest symptom onset date. We used reported household size to calculate secondary attack rates by dividing the number of secondary cases by the total number of household secondary contacts (ie, household size – 1). Cases with and without symptoms were classified based on symptom information and onset date reported in CCM.
Poisson regression was performed for the unadjusted and adjusted analyses, overall and by strata. The models were specified with the count of household secondary cases as the outcome, the logarithm of household size as the offset, and VOC status as a binary exposure covariate. Clustering was accounted for with a random intercept for household to account for known overdispersion.
Our unadjusted risk ratios (RR) included the entire cohort of households with a VOC or non-VOC index case in Ontario. Adjusted RRs were based on a propensity score-matched analysis. VOC index cases were 1:1 matched to non-VOC index cases based on sex and age group, and matched on the logit of the propensity score, using a caliper width of 0.2 times the standard deviation [9]. The propensity score was based on a logistic regression model of VOC status as a function of 5 covariates: reported date, time between symptom onset and testing, association with a reported outbreak, as well as the neighborhood proportion of visible minority residents (non-White and non-Indigenous population), and household crowding as determined using 2016 Canadian Census data [10]. Regression estimates were reported using RRs and 95% confidence intervals (CI). Statistical analysis was performed in R version 4.0.4 [11].
This study was approved by Public Health Ontario’s Research Ethics Board (file number 2020-029.03).
RESULTS
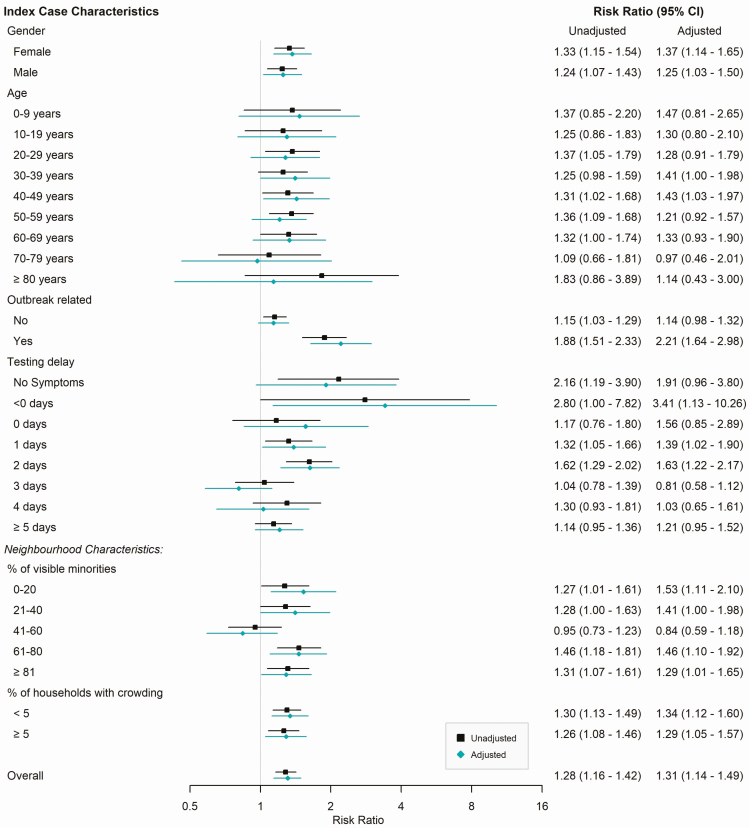
We identified 5617 index cases and 3397 secondary cases across the study period. Among index cases, 1318 were classified as VOC (151 B.1.1.7 and 1167 N501Y) and 4299 were classified as non-VOC. The overall secondary attack rate was higher for VOC index cases (25.9%) compared with non-VOC (20.5%, P < .01) with consistently higher secondary attack rates for VOCs across individual characteristics of the index cases (Supplementary Table S1). The secondary attack rate of VOC index cases was 1.28 times higher than that of non-VOC index cases (RR = 1.28; 95% CI, 1.16–1.42); a similar trend of increased secondary attack rate was observed across subgroups (Figure 1).
Figure 1.
Risk ratio comparing household secondary attack rate associated with VOC vs.
non-VOC index cases by characteristic of index case, unadjusted
( ) and
adjusted by 1:1 propensity-score matching (
) and
adjusted by 1:1 propensity-score matching ( ) estimates. Unadjusted
risk ratio accounts for household clustering and includes the full cohort.
Adjusted risk ratio refers to the propensity score-matched cohort.
Abbreviations: CI, confidence interval; VOC, variant of concern.
) estimates. Unadjusted
risk ratio accounts for household clustering and includes the full cohort.
Adjusted risk ratio refers to the propensity score-matched cohort.
Abbreviations: CI, confidence interval; VOC, variant of concern.
We included 1259 index VOC and non-VOC cases in the propensity score-matched analysis. The secondary attack rate for VOC index cases in this matched cohort was 1.31 times higher than non-VOC index cases (RR = 1.31; 95% CI, 1.14–1.49), similar to the unadjusted estimate. In stratified analyses, the higher secondary attack rate for VOC compared with non-VOC index cases was accentuated for asymptomatic index cases (RR = 1.91; 95% CI, 0.96–3.80) and presymptomatic cases (RR = 3.41; 95% CI, 1.13–10.26); however, CIs were wide.
DISCUSSION
In our cohort, we estimated that the household secondary attack rate of VOC index cases was 31% higher than non-VOC index cases, providing evidence of increased transmissibility. This is consistent with previous VOC (ie, B.1.1.7) secondary attack rate estimates from the United Kingdom (relative secondary attack rate [32%, 12.9% vs 9.7% among all contacts]) [6]; however, households are an important contributor to COVID-19 and provide a valuable setting in which to examine transmission [8]. Our estimates of increased transmission from asymptomatic and presymptomatic VOC index cases have not previously been reported and suggest an increased importance of prevention measures when individuals are not aware of their infection. The biological mechanism responsible for increased transmissibility has not been identified, but hypotheses include higher viral loads (ie, increased transmission potential per contact), and prolonged viral shedding (ie, longer infectious period) [3]. The N501Y mutation has also been associated with enhanced binding affinity of severe acute respiratory syndrome coronavirus-2 to angiotensin-converting enzyme 2 receptors [12].
Limitations of this study include potential misclassification of secondary cases as index cases and small sample sizes in some subgroups. We may have underestimated secondary attack rates because we only captured diagnosed secondary cases and lacked testing data on all household contacts; however, we do not believe this would be differential by VOC status of the index case. Ontario implemented more stringent measures for close contacts of all cases (not just VOC cases) in early February in response to VOC introductions, including increased frequency of testing during quarantine and outbreaks [13]. If household contacts were more likely to test once VOC status was known, a similar increase in the risk ratio would be expected across symptom status, suggesting an independent effect of asymptomatic and presymptomatic VOC cases on transmission. However, we cannot fully rule out the possibility that knowledge of the case’s VOC status (or association with a VOC outbreak) affected test-seeking behavior in household contacts.
This study provides strong evidence of increased transmissibility in households due to VOCs and suggests that asymptomatic and pre-symptomatic transmission may be of particular importance for VOCs. Our study suggests that more aggressive public health measures will be needed to control VOCs. Although measures effective for persons with unknown disease status such as physical distancing and masking may continue to be highly effective, measures focused on symptomatic individuals, such as public health contact tracing, may be increasingly ineffective unless extremely rapid. Ongoing research is needed to understand mechanisms of VOC transmissibility to curb their associated morbidity and mortality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. S. A. B. and K. A. B. conceptualized the study. S.T. performed the analysis. S.A.B. drafted the initial manuscript. S. A. B., N. D., K. A. B., S. T., and T. V. developed the methodology. S. A. B., K. A. B., and S. T. verified the underlying data. S. A. B., N. D., K. A. B., S. T., T. V., M. W., and M. M. reviewed the manuscript.
Acknowledgments. The authors thank James Johnson and Arezou Saedi for their contributions to the address-matching work and Lauren Paul for her contributions to developing the methodology for the household transmission analysis.
Financial support. This work did not have a direct funding source but was supported by Public Health Ontario.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Ontario Agency for Health Protection and Promotion (Public Health Ontario). Epidemiologic summary: COVID-19 in Ontario—January 15, 2020 to May 17, 2021.Toronto, ON: Queen’s Printer for Ontario. 2021. Available at: https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-daily-epi-summary-report.pdf?la=en. Accessed 18 May 2021. [Google Scholar]
- 2.Martin DP, Weaver S, Tegally H, et al. The emergence and ongoing convergent evolution of the N501Y lineages coincides with a major global shift in the SARS-CoV-2 selective landscape [published online March 10, 2021]. medRxiv. doi: 10.1101/2021.02.23.21252268. [Google Scholar]
- 3.Davies NG, Abbott S, Barnard SC, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England [published online March 3, 2021]. Science doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volz E, Mishra S, Chand M, et al. Transmission of SARS-CoV-2 lineage B.1.1.7 in England: insights from linking epidemiological and genetic data.2020. Available at: https://virological.org/t/transmission-of-sars-cov-2-lineage-b-1-1-7-in-england-insights-from-linking-epidemiological-and-genetic-data/576. Accessed 10 March 2021.
- 5.Public Health England. Investigation of novel SARS-CoV-2 variant. Variant of Concern 202012/01: technical briefing 5.2021. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/959426/Variant_of_Concern_VOC_202012_01_Technical_Briefing_5.pdf. Accessed 10 March 2021.
- 6.Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open 2020; 3:e2031756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ontario Agency for Health Protection and Promotion (Public Health Ontario). SARS-CoV-2 (COVID-19 virus) variant of concern (VoC) surveillance.Toronto, ON: Queen’s Printer for Ontario. 2021. Available at: https://www.publichealthontario.ca/en/laboratory-services/test-information-index/covid-19-voc. Accessed 28 March 2021. [Google Scholar]
- 8.Paul LA, Daneman N, Brown KA, et al. Characteristics associated with household transmission of SARS-CoV-2 in Ontario, Canada: a cohort study [published online March 5, 2021]. Clin Infect Dis doi: 10.1093/cid/ciab186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Statistics Canada. Dictionary, Census of Population, 2016.2017. Available at: https://www12.statcan.gc.ca/census-recensement/2016/ref/dict/az1-eng.cfm?topic=az1. Accessed 10 March 2021.
- 11.R Core Team. R: A language and environment for statistical computing. Version 4.0.4. 2021.R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/. Accessed 24 March 2021. [Google Scholar]
- 12.Gu H, Chen Q, Yang G, et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science 2020; 369:1603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health. COVID-19 Variant of Concern: Case, Contact and Outbreak Management Interim Guidance.2021. Available at: https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/VOC_guidance.pdf. Accessed 25 March 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.