Summary
This special issue of Molecular Microbiology marks the 25th anniversary of the discovery of the Extracytoplasmic Function (ECF) σ factors, proteins that subsequently emerged as the largest group of alternative σ factors and one of the three major pillars of signal transduction in bacteria, alongside one- and two-component systems. A single bacterial genome can encode >100 ECF σ factors, and combined with their cognate anti-σ factors, they represent a modular design that primarily functions in transmembrane signal transduction. Here, we first describe the immediate events that led to the 1994 publication in the Proceeding of the National Academy of Sciences USA, and then set them in the broader context of key events in the history of σ biology research.
Mark Buttner
Fortunately for me, in the summer of 1991, the major international Streptomyces meeting happened to be held in Madison, Wisconsin. While I was there, through a mutual friend, Simon Baumberg, I arranged to spend an afternoon in Carol Gross’s lab in ‘E. B. Fred Hall’ at the University. Carol organized a roundtable discussion that included my post-doc, Kelly Brown, who was also at the Streptomyces conference, and several members of Carol’s lab, including her graduate student Joan Mecsas. Our particular shared interest was that both labs were trying to purify enough of a minor alternative σ factor to obtain extensive N-terminal amino acid sequence, which we could then use to design degenerate oligonucleotides to clone the corresponding structural gene (this was before the advent of whole genome sequences). Confusingly (and arbitrarily), both σ factors had been named σE. In our case, the σE came from the filamentous Gram-positive bacteria Streptomyces, famous for their production of antibiotics, and in Joan and Carol’s case the σE came from Escherichia coli. Both σ factors had been identified biochemically, E. coli σE as the protein that directed transcription of the rpoH heat shock σ factor gene under extreme heat shock (Erickson and Gross, 1989), and Streptomyces σE as the protein that directed transcription from one of the promoters of a gene encoding a secreted agarase that allows Streptomyces coelicolor to use agar as a food source (Buttner et al., 1988; see also the article by Tran et al., in this issue). At the time, both proteins were seen as unusually small for σ factors, although the significance of that observation only became clear much later.
The lab of Tim Donohue was just down the hall from Carol’s lab. Tim was later to make ground-breaking contributions to the ECF sigma factor field (through the analysis of yet another protein named σE, this time from Rhodobacter. see the MicroReview by Donohue in this issue). Carol went looking for Tim, but he was out of town that day, and it was several more years before we met and became immediate friends.
Towards the end of the afternoon Carol introduced me to her graduate student, Mike Lonetto, who had not been at the roundtable discussion. Although Mike did benchwork, his heart lay in bioinformatics. Mike told me that he was writing a review article about evolutionary relationships and sequence conservation among σ factors and he showed me some of his preliminary alignments, which would eventually form part of his seminal Journal of Bacteriology Minireview published the following year (Lonetto et al., 1992).
At that point, neither my lab nor Carol’s lab had managed to purify our respective σE proteins in sufficient quantity to get useful amounts of N-terminal sequence, but in 1993 we succeeded in determining the sequence of the first 27 residues of Streptomyces σE and with that we were rapidly able to clone and sequence the sigE structural gene. With the full amino acid sequence in hand, we started searching the protein databases. It is hard to remember just how primitive things were back then. There was no internet and, in our case, we had to email our query sequences to the University of Kent to do database searches, sometimes waiting 24 hours to get the answers back. When we opened those emails containing the ranked hits, the answers were simultaneously exciting and alarming.
The sequence of Streptomyces σE did pull out known σ factors but the alignments were not convincing, having only limited amino acid sequence identity in domain 4 and worse alignment still in domain 2. On top of that, we had to put a large gap into the Streptomyces σE protein sequence to align domain 2 and domain 4 simultaneously. As we all now know, that’s because ECF σ factors lack domain 3 (accounting in part for their small size), but initially this large gap seemed improbable. More curiously still, the top hits that came back from Kent were not annotated as σ factors at all, but rather positive regulators encoded by genes that had been identified by classical mutant hunts and cloned by complementation. This implied either that we had discovered something very exciting, or that we had somehow screwed up. But since we had identified and purified Streptomyces σE based on its biochemical activity as a σ factor in in vitro run-off experiments, we were pretty confident that it was a σ factor. This raised the possibility that there were unrecognized, non-canonical σ factors floating in the databases.
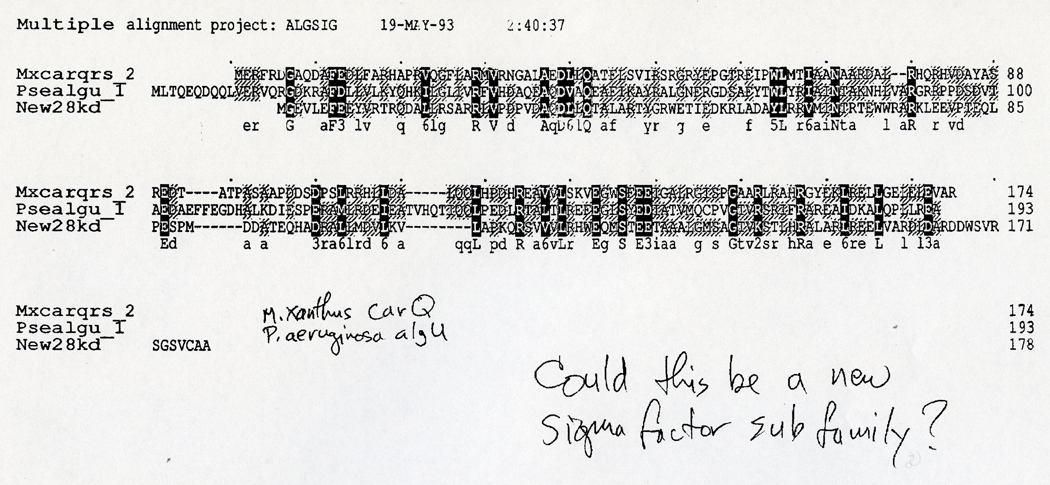
Having met him in Madison and then subsequently read and admired his 1992 Minireview when it came out in the Journal of Bacteriology, I thought “if anyone can make sense of this, it’s Mike Lonetto”, and so I emailed him the Streptomyces σE protein sequence and asked him if he would be interested in taking a look. A few days later, on 19th May 1993, Mike sent me a fax (you couldn’t add attachments to emails back then) showing an amino acid sequence alignment of Streptomyces σE with Myxococcus xanthus CarQ and Pseudomonas aeruginosa AlgU. On the fax, Mike had handwritten “Could this be a new sigma factor subfamily?” (Fig. 1).
Fig. 1.
Fax sent by Mike Lonetto to Mark Buttner, dated 19th May 1993, showing an alignment of the first 3 recognized ECF σ factors: Streptomyces σE (New28kd), Myxococcus xanthus CarQ (Mxcarqrs_2) and Pseudomonas aeruginosa AlgU (Psealgu_1). Mike’s handwriting can be seen on the fax, saying “Could this be a new sigma factor subfamily?”
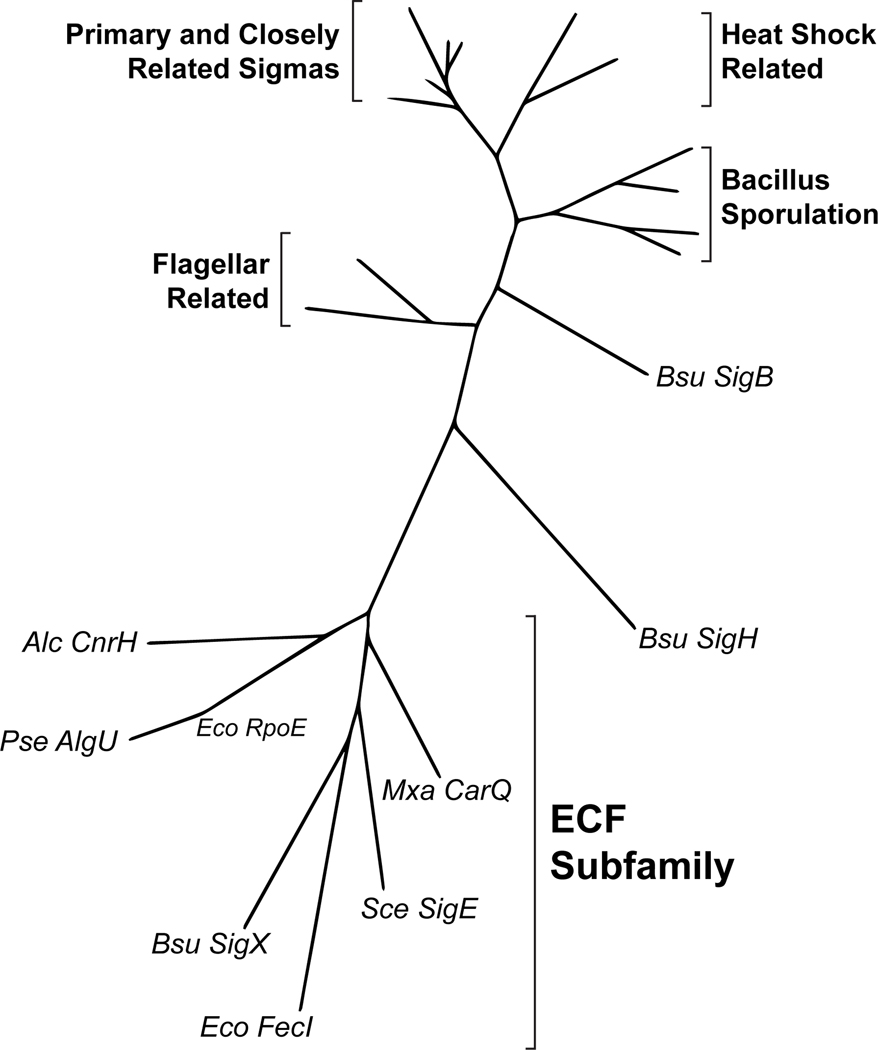
Through a lot of patient work, mostly by Mike, slowly the fog lifted, and it became clear that Streptomyces σE was indeed a founding member of a new subfamily of σ factors, but that these proteins were so diverged from previously known σ factors that many of them were not identified as σ factors using the standard similarity searching methods available at the time. Indeed, the sequence of one of these new σ factors, E. coli FecI, had been sitting in the databases for four years but nobody had suggested that it might be a σ factor. While we were writing up the work, additional unrecognized members of the new ECF subfamily kept being deposited in the databases. This included the E. coli σE structural gene, rpoE, which was sequenced by chance in Japan as part of a larger genomic fragment and recognized as a likely candidate for the σE structural gene by Ken Rudd, working in bioinformatics at NIH in Bethesda, a prediction confirmed by Joan Mecsas based on internal peptide sequence she had obtained from purified σE. When Mike finally created a new phylogenetic tree of the σ70 family incorporating the first members of the new ECF σ factor subfamily, it essentially doubled the diversity of the proteins in the tree (Fig. 2).
Fig. 2.
The phylogenetic tree of the σ70 family taken from Lonetto et al. (1994), incorporating 7 of the first 8 members of the newly identified ECF σ factor subfamily (Pseudomonas syringae HrpL was identified as an ECF σ factor in the paper but was not included in the phylogenetic analysis). The inclusion of the ECF σs essentially doubled the diversity of the σ70 family.
A word about Carol’s role in all this. In 1993–1994 I was tenure-track, and so the publication of the discovery of the ECF subfamily of σ factors was very important to me. Carol knew this, and for that reason, she never asked to have her name on the paper, even though Mike was her graduate student, and that by this time Carol had re-written sections of the manuscript to help us present the findings to the scientific community in an optimal way. That act speaks volumes for Carol as a person and I am hugely grateful to her to this day. Mike and I had written a complete first draft of the manuscript before Mike finally told Carol what we were up to. I will never forget receiving a phone call from Carol at home later that day, saying that she was very excited by the paper, but that she thought “it could be done better,” as Carol then amply demonstrated as she helped us pull our draft into shape. Eventually, in February 1994, we sent a copy of the final manuscript to Rich Losick, another pioneer in the σ factor field, who, as a recently elected member of the National Academy, generously offered to communicate the paper to the Proceedings (Lonetto et al., 1994).
A word also about the naming of these proteins. Based on the very limited amounts of information about the first eight members available at the time, we decided to call the new subfamily the Extracytoplasmic Function (ECF) σ factors, because they appeared to regulate and respond to extracytoplasmic conditions. This name proved not to be universally popular and several labs quite understandably prefer to give them the less loaded name of Group IV σ factors. Nevertheless, in 2009, by which time genome sequencing had revealed many hundreds of these σ factors and their cognate anti-σ factors, bioinformatic analysis showed that 81% of all ECF anti-σ factors are transmembrane proteins (Staroń et al., 2009), which implies that the inducing signal is coming from the cell envelope, consistent with the original ECF terminology. Of course, the remaining 19% of ECF anti-σ factors are cytoplasmic, including prominent, well-characterized examples like Rhodobacter ChrR (Campbell et al., 2007) and Streptomyces RsrA (Kang et al., 1999; Rajasekar et al., 2016), but I think our initial instincts have proved to be largely sound.
After Lonetto et al. (1994) came out, my new post-doc Mark Paget and I continued to work on Streptomyces σE and the cell envelope stress response that it controls (Paget et al., 1999a,b; Hong et al., 2002; Tran et al., 2019), but mostly shifted our attention to characterising another ECF sigma we named σR. We discovered that σR controls the principal oxidative stress response in Streptomyces, and found that its activity was controlled by RsrA, a cytoplasmic, zinc-dependent anti-σ that exploits a thiol-disulfide redox switch to sense and respond to oxidative stress (Paget et al., 1998; Kang et al., 1999; Paget et al., 2001a,b; Feeney et al., 2017). We found a conserved HxxxCxxC motif in RsrA that Tim Donohue also found in his anti-σ, ChrR. The two labs showed that this conserved motif was involved in zinc coordination and we named this family of anti-σs ‘ZAS proteins’, for zinc-dependent anti-sigma factors (Paget et al., 2001a; Anthony et al., 2001; Zdanowski et al., 2006). Most of our work on the σR-RsrA redox switch was conducted as part of very fruitful and enjoyable collaborations with Jung-Hye Roe and Colin Kleanthous, and Jung-Hye has written a review of current knowledge of the σR-RsrA system on pages XXX-XXX of this issue (Roe, 2019). In seemingly unrelated work, we were one of two labs that characterised the two families of proteins (the chaplins and the rodlins) required to form the external hydrophobic sheath that permits the reproductive aerial hyphae of Streptomyces to escape surface tension and grow into the air (Elliot et al., 2003; Claessen et al., 2003). Imaging our subsequent surprise when we discovered that transcription of the rodlin and chaplin genes was directed by a development-specific ECF σ we named σBldN and showed that its activity was in turn controlled by a novel transmembrane anti-σ factor, RsbN (Bibb et al., 2012; Schumacher et al., 2018).
Mike Lonetto
I was pleasantly surprised when Mark Buttner sent a brochure for the conference How Microbes View Their World and asked if I could attend the conference, to be held in September 2018 in Marburg, Germany. It had been many years since I had thought about the collaboration that had brought us together, and at the conference I was amazed at the size and variety of pathways and regulatory mechanisms that have been uncovered for the ECF σ factor family.
When I met Mark Buttner for the first time, I was working in collaboration with Michael Gribskov on a review of σ factor sequence conservation and evolution (Lonetto et al. 1992). Our motivation at the time was to use the protein sequence conservation and relationships between different σ factors to make predictions of which residues were important for its function, and to apply this to understanding the role of these structural motifs in σ-DNA interactions and transcription initiation. In order to understand this better we attempted to find as many σ sequences as possible. Both the data available for these searches and the power of available search methods were much more limited than today. There was no PsiBlast, and the first microbial whole genome sequences were work in progress. The analysis in the 1992 Minireview is based on an alignment of 31 sequences, essentially all that were known at the time. Our attempts at extending the family to include other related proteins, especially those with known structure, were frustrating and inconclusive. Using those sequence alignments and ProfileSearch, a position-weight-matrix search tool, there were some tantalizing hits below statistical significance. These included the multi-helix DNA-binding domains of several transcription factors, including the eukaryotic promoter recognition factor TFIID, as well as some other helical proteins with no known DNA binding activity. They also included E. coli FecI. FecI was identified as a transcriptional regulator, but not as a σ factor.
When Mark inquired about the S. coelicolor sigE gene in 1993, it opened up a new world of σs: first, Streptomyces σE was biochemically characterized as a sigma factor. Second, when it was aligned with the initial hits to M. xanthus CarQ and P. aeruginosa AlgU, they clearly formed a family, with matches throughout the length of all 3 proteins. Third, the consensus pattern was readily aligned with regions 2 and 4, the most conserved regions of the known σ70 family. Specifically, the pattern of conserved hydrophobic residues indicating the helical phase was conserved. Fourth, though clearly related, the initial 3 members of this group were highly divergent compared with the known σ factors. This last point enabled more sensitive database searching and improved alignments enabling the identification of additional family members, including FecI, as noted by Mark. With new sequences showing up as the analysis was underway, we eventually had 8 sequences from a range of Gram-positive and Gram-negative species and were able to show that the diversity of the σ70 family was much greater than had been shown based on previous data. Since then this group has grown into the thousands, with a diversity of roles and regulatory mechanisms we could not have guessed at the time.
While I soon left the study of bacterial sigma factors behind, the experience of finding and defining the conserved features of such a diverse family of proteins served me very well in helping to define the broad importance of common determinants in an applied setting, leading to swift progress on understanding a potential antimicrobial target (Smith et al., 2003).
Carol Gross
My love affair with sigmas (σs) started when I joined Dick Burgess’ laboratory towards the end of 1973, about 4 years after the discovery of the founding σ (σ70) factor of E. coli RNA polymerase. It is hard to believe that we are now at the 50th anniversary of that seminal discovery. Sigma was first described in a joint communication by Burgess and Travers (Harvard), and Dunn and Bautz (Rutgers), which documented that RNA polymerase required a dissociable factor to transcribe bacteriophage T4 DNA (Burgess et al, 1969). At that same time, Krakow et al. (1969) reported a dissociable factor from Azotobacter vinelandii. Later that year, Travers and Burgess (1969) showed that this factor was cyclically reused and affected initiation, and Bautz et al. (1969) showed that σ predominantly enhances transcription from authentic early bacteriophage promoters. Today, we know that all multi-subunit cellular RNA polymerases require initiation factor(s), not only to recognize the promoter, but also to help with subsequent steps of transcription initiation, but that fact was unknown in 1969. It is only in retrospect that we realize that the discovery of σ70 was the first step in establishing this paradigm.
Having completed my Ph.D. with Aaron Novick, one of the early pioneers of cutting-edge studies dissecting how the bacterial cell organizes its resources for maximal growth, I immediately noted that all of the seminal work on σ had been done in vitro. Given my background, perhaps it was not surprising that my question to Dick was: What does σ do in vivo? That one question not only set the direction of my postdoctoral studies, but also the direction of my own laboratory.
As a post-doctoral fellow, I identified the gene for σ70 (Gross et al., 1978), and then cloned and sequenced this gene (Gross et al., 1979; Burton et al., 1981), using the laborious technologies available at that time. Following the example of Cairns, who identified a mutant in DNA polymerase by in vitro assay, I developed a rapid purification scheme for σ activity and then used this assay to map the location of a σ70 variant. Unfortunately, this mutant had no phenotype in vivo (Gross et al., 1978), but a subsequent collaboration with Hope Liebke did identify a σts mutation, allowing us to show that almost, but not all, in vivo transcription stopped in mutant cells after shift to 42oC, as predicted by the in vitro studies (Liebke et al., 1980; Gross et al., 1984).
In my own laboratory, we took up the question of what cellular factors were responsible for the residual transcription observed in the σts mutant. This led Alan Grossman to find σ32, an alternative σ that specifically recognized heat shock promoters, rather than the canonical σ70 housekeeping promoters (Grossman et al., 1984; Cowing et al., 1985). Rich Losick and Jan Pero had earlier found that alternative σs were utilized during sporulation in B. subtilis, and by phage that infected B. subtilis (Losick and Pero, 2018). Our discovery of an E. coli alternative σ that controlled a stress response generalized their finding and suggested that alternative σs might be broadly utilized by bacteria.
Our broad-based study of the control of the σ32-mediated heat shock response eventually led us to a conundrum. We knew that σ32, in contrast to most E. coli proteins, was unstable, with a half-life of about 1 minute (Straus et al., 1987). Yet, at the lethal temperature of 50˚C, heat shock proteins are essentially the only proteins produced. This meant that σ32 must be continuously overexpressed at 50˚C, but we did not know how the σ32 transcript was made at that temperature.
Jim Erickson undertook a study of this issue. By mapping the transcription start sites of rpoH, the gene encoding σ32, both at the normal growth temperature and at 50˚C, Jim found that rpoH was transcribed from three promoters (p1, p3 and p4). Two of them (p1 and p4) were recognized by σ70. While present at high levels at normal growth temperatures, these transcripts were significantly less abundant at 50˚C. In contrast, the p3 promoter, a minor promoter during normal growth, was predominant at 50˚C. This promoter was recognized neither by σ70, nor by σ32 (Erickson et al., 1987). In subsequent work, Jim identified a new sigma, σE, which recognized p3, thereby identifying a second alternative σ in E. coli (Erickson et al., 1989). σE was considerably smaller than σ32, but we could not immediately study this protein because we lacked amino acid sequence to map the gene onto the E. coli chromosome. As you have heard, Mike Lonetto in my lab, working with Mark Buttner, identified a new clade of σs, based on Mark’s σ together with others in the database (Lonetto et al., 1994). It shortly became apparent that σE was a member of this family.
Having identified σE, the next question was to determine its role in the cell. In addition to rpoH, σE also transcribed the gene for the periplasmic protease DegP. Like σE, the DegP transcript was also more abundant at 42˚C (Erickson et al., 1989), leading us to suggest that the σE regulon was a second heat induced regulon, possibly related to thermotolerance (Erickson et al., 1989). Joan Mecas examined this question by conducting an unbiased genetic screen, looking for genes that when overexpressed or knocked out altered the activity of a σE promoter driving expression of an antibiotic cassette. Imagine our surprise when this screen fingered OMPs (outer membrane proteins/porins) as drivers of σE expression (Mecsas et al., 1993). I was both horrified and delighted at this information—my molecular biology roots had never considered any specific structures in bacteria, so I was initially at a loss for how to proceed. But, I embraced this challenge and since then have made the bacterial envelope a focus of my work.
Alejandro de las Peñas and Lynn Connolly tackled the question of how σE communicated with the outer membrane, identifying a protein bridge of negative regulators that extended to the periplasm (de las Peñas et al., 1987). Alejandro also showed that σE is essential (de las Peñas et al., 1997), unique among E. coli alternative σs. Subsequent students and postdoctoral fellows, along with our long-running collaborator Bob Sauer, and additional collaborations with Jörg Vogel and Gigi Storz, helped us to collectively tease out this amazing regulatory system. In essence, σE senses and integrates signals from every major constituent of the outer membrane—porins, lipopolysaccharide and lipoproteins, in a regulatory scheme that is faithful to the physiological connections among these machineries (Walsh et al., 2003; Papenfort et al., 2006; Gogol et al., 2011; Lima et al., 2013; Guo et al., 2014).
σE is conserved among γ-proteobacteria. Interestingly, the ability of σE to transcribe rpoH, the feature that led to the discovery of σE, is also conserved (Rodius et al., 2006). In retrospect, this is not surprising. σE monitors the outer membrane for external damage impacting proteostasis. In contrast, σ32 monitors the cytoplasm and inner membrane for proteostasis (Lim et al., 2013). By connecting these two circuits, σE serves as an early warning system to the cytoplasm—even in the absence of internal damage, severe damage to the environment-facing compartment of the cell upregulates σ32 to anticipate future issues.
Tim Donohue
While my lab has shed light on the function of ECF σ factors (Donohue, 2019; pages XXX-YYY of this issue), I was an outsider looking in during their discovery. As an Assistant Professor of Bacteriology at UW-Madison, I watched the field develop as a thesis committee member for James Erickson, who identified a high-temperature inducible transcript from the E. coli σ32 structural gene (rpoH), and isolated a protein from an SDS-PAGE gel that, when added to core RNA polymerase in vitro, produced a product from this promoter (Erickson and Gross, 1989). I also served on the thesis committee for Mike Lonetto, whose classification system for σ factors (Lonetto et al., 1992) has survived decades. As you have read, Mike and Mark Buttner went on to define ECF proteins as a distinct group of σ factors (Lonetto, et al., 1994). I also collaborated with Joan Mecsas, another of Carol Gross’ students who identified the σE gene and revealed its role in a newly identified response to misfolded outer membrane proteins (Mecsas et al., 1993).
Louis Pasteur is quoted as saying “In the field of observation, chance favours only the prepared mind”. As this work was developing elsewhere, my lab was beginning studies to define signals and proteins that control the expression of cycA, the structural gene for the periplasmic electron carrier, cytochrome c2, in the α-proteobacterium Rhodobacter sphaeroides. As “chance” would have it, Brenda Schilke, one of my graduate students, isolated Rb. sphaeroides mutants with increased cycA transcription (Schilke et al., 1992). Several years later we showed that the mutation which increased cycA expression inactivated ChrR, a member of a newly identified class of zinc-dependent anti-σ factors (ZAS proteins). ChrR inhibits an ECF σ we (unfortunately) named σE, due to the amino acid sequence identity to few known ECF subfamily proteins (Newman, et al., 1999; Newman et al., 2001). This led us to meet Mark Paget, Mark Buttner and Jung-Hye Roe, who characterized RsrA, another ZAS anti-σ that controls the principal oxidative stress response in Streptomyces (Kang et al., 1999; Paget et al., 2001a; Paget et al., 2001b; Roe, 2019). It also led to a collaboration to solve the three-dimensional structure of the Rb. sphaeroides σE-ChrR complex (with Liz Campbell and Seth Darst), work that provided a molecular view of how a ZAS protein blocks σ factor activity, and made the surprising finding that ChrR has a similar fold to RseA, the E. coli σE anti-σ factor, despite the lack of metal binding by RseA and the low degree of amino acid identity between these two inhibitors (Campbell et al., 2007).
With the advent of bacterial genomics, we (Jennifer Anthony and others in my lab) showed that the reactive oxygen species singlet oxygen (1O2) increased Rb. sphaeroides σE activity and identified genes in this newly-described stress response (Anthony et al., 2005). Using genomic and informatics techniques that were not available when Mike Lonetto and Mark Buttner defined the ECF σ factor subfamily, Yann Dufour and others identified genes that were directly transcribed by Rb. sphaeroides σE, and predicted the existence, function and evolution of the σE-ChrR regulon in other proteobacteria (Dufour, et al., 2008; Ziegelhoffer and Donohue, 2009).
I will close by saying that my foray into ECF σ factors has provided some of my most scientifically rewarding experiences. This story would not be told without the people in my lab and the openness of collaborators who posed the challenging questions, openly shared and developed tools to answer them, and made the advances.
REFERENCES
- Anthony J, Warczak KL and Donohue TJ 2005. A transcriptional response to singlet oxygen, a toxic byproduct of photosynthesis. Proceedings of the National Academy of Sciences of the United States of America, 102, 6502–6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautz EKF, Bautz FE and Dunn JJ (1969) E. coli σ factor: a positive control element in phage T4 development Nature, 223, 1022–1024. [DOI] [PubMed] [Google Scholar]
- Bibb MJ, Domonkos Á, Chandra G. and Buttner MJ (2012) Expression of the chaplin and rodlin hydrophobic sheath proteins in Streptomyces venezuelae is controlled by σBldN and a cognate anti-sigma factor, RsbN, Molecular Microbiology 84, 1033–1049. [DOI] [PubMed] [Google Scholar]
- Burgess RR, Travers AA, Dunn JJ and Bautz EKF Factor stimulating transcription by RNA polymerase (1969) Nature, 221, 43–46. [DOI] [PubMed] [Google Scholar]
- Burton Z, Burgess RR, Lin J, Moore D, Holder S. and Gross CA (1981) The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E. coli K12. Nucleic Acid Research, 9, 2889–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner MJ, Smith AM and Bibb MJ (1988) At least three different RNA polymerase holoenzymes direct transcription of the agarase gene (dagA) of Streptomyces coelicolor A3(2). Cell, 52, 599–607. [DOI] [PubMed] [Google Scholar]
- Campbell EA, Greenwell R, Anthony JR, Wang S, Lim L, Das K, Sofia HJ, Donohue TJ and Darst SA (2007) A conserved structural module regulates transcriptional responses to diverse stress signals in bacteria. Molecular Cell, 27, 793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessen D, Rink R, de Jong W, Siebring J, de Vreugd P, Boersma FG, Dijkhuizen L. and Wösten HAB (2003) A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes and Development, 17, 1714–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing DW, Bardwell JC, Craig EA, Woolford C, Hendrix RW and Gross CA (1985) Consensus sequence for Escherichia coli heat shock gene promoters. Proceedings of the National Academy of Sciences of the United States of America, 82, 2679–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Peñas A, Connolly L. and Gross CA (1997) The σE mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of σE. Molecular Microbiology, 24, 373–385. [DOI] [PubMed] [Google Scholar]
- De Las Peñas A, Connolly L. and Gross CA (1997) σE is an essential sigma factor in Escherichia coli. Journal of Bacteriology, 179, 6862–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue TJ (2019) Shedding light on a Group IV (ECF11) alternative σ factor. Molecular Microbiology, (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour YS, Landick R. and Donohue TJ (2008) Organization and evolution of the biological response to singlet oxygen stress. Journal of Molecular Biology, 383, 713–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot MA, Karoonuthaisiri N, Huang J, Bibb MJ, Cohen SN, Kao CM and Buttner MJ (2003) The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes and Development, 17, 1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J, Vaughn V, Walter WA, Neidhardt FC and Gross CA (1987) Regulation of the promoters and transcripts of rpoH, the Escherichia coli heat shock regulatory gene. Genes and Development, 1, 419–432. [DOI] [PubMed] [Google Scholar]
- Erickson JW and Gross CA (1989) Identification of the σE subunit of Escherichia coli RNA polymerase: a second alternate σ factor involved in high-temperature gene expression. Genes and Development 3, 1462–71. [DOI] [PubMed] [Google Scholar]
- Feeney MA, Chandra G, Findlay KC, Paget MSB and Buttner MJ (2017) Translational control of the SigR-directed oxidative stress response in Streptomyces via IF3-mediated repression of a non-canonical GTC start codon. mBio, 8, e00815–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogol EB, Rhodius VA, Papenfort K, Vogel J. and Gross CA (2011) Small RNAs endow a transcriptional activator with essential repressor functions for single-tier control of a global stress regulon. Proceedings of the National Academy of Sciences of the United States of America, 108, 12875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CA, Hoffman J, Ward C, Hager D, Burdick G, Berger H. and Burgess RR (1978) A mutation affecting thermostability of the sigma subunit of E. coli RNA polymerase lies near the dnaG locus at about 66 min on the E. coli genetic map. Proceedings of the National Academy of Sciences of the United States of America, 75, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CA, Blattner F, Taylor W, Lowe P. and Burgess RR (1979) Isolation and characterization of transducing phage coding for σ subunit of E. coli RNA polymerase. Proceedings of the National Academy of Sciences of the United States of America, 76, 5789–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CA, Grossman AD, Liebke H, Walter WA and Burgess RR (1984) Effects of the mutant sigma allele rpoD800 on the synthesis of specific macromolecular components of the E. coli K12 cell. Journal of Molecular Microbiology, 172, 283–300. [DOI] [PubMed] [Google Scholar]
- Grossman AD, Erickson JW and Gross CA (1984) The htpR gene product of E. coli is a sigma factor for heat shock promoters. Cell, 38, 383–390. [DOI] [PubMed] [Google Scholar]
- Guo MS, Updegrove TB, Gogol EB, Shabalina SA, Gross CA and Storz G. (2014) MicL, a new σE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes and Development 28, 1620–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HJ, Paget MSP and Buttner MJ (2002) A signal transduction system in Streptomyces coelicolor that activates the expression of a putative cell wall glycan operon in response to vancomycin and other cell wall-specific antibiotics. Molecular Microbiology, 44, 1199–1211. [DOI] [PubMed] [Google Scholar]
- Kang J-G, Paget MSB, Seok Y-J, Hahn M-Y, Bae J-B, Hahn J-S, Kleanthous C, Buttner MJ and Roe J-H (1999) RsrA, an anti-sigma factor regulated by redox change. EMBO Journal, 18, 4292–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow JS, Daley K. and Karstadt M. (1969) Azotobacter Vinelandii RNA Polymerase, VII. Enzyme transitions during unprimed r[I-C] Synthesis. Proceedings of the National Academy of Sciences of the United States of America, 62, 432–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebke H, Gross CA, Walter WA and Burgess. R.R. (1980) A new mutation rpoD800 affecting the sigma subunit is allelic to two other sigma mutants. Molecular and General Genetics, 177, 277–282. [DOI] [PubMed] [Google Scholar]
- Lim B, Miyazaki R, Neher S, Siegele DA, Ito K, Walter P, Akiyama Y, Yura T. and Gross CA (2013) Heat shock transcription factor σ32 co-opts the signal recognition particle to regulate protein homeostasis in E. coli. PLOS Biology, 11, e1001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S, Guo MS, Chaba R, Gross CA and Sauer RT (2013) Dual molecular signals mediate the bacterial response to outer-membrane stress. Science, 340, 837–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonetto M, Gribskov M. and Gross CA (1992) The σ70 family: sequence conservation and evolutionary relationships. Journal of Bacteriology 174, 3843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonetto MA, Brown KL, Rudd KE and Buttner MJ (1994) Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase σ factors involved in the regulation of extracytoplasmic functions. Proceedings of the National Academy of Sciences of the United States of America, 91, 7573–7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R. and Pero J. (2018) For want of a template. Cell 172, 1146–52. [DOI] [PubMed] [Google Scholar]
- Mecsas J, Rouviere PE, Erickson JW, Donohue TJ and Gross CA (1993) The activity of σE, an Escherichia coli heat-inducible sigma factor, is modulated by expression of outer membrane proteins. Genes and Development, 7, 2618–2628. [DOI] [PubMed] [Google Scholar]
- Newman J, Falkowski M, Schilke BA, Anthony L. and Donohue TJ (1999) The Rhodobacter sphaeroides ECF sigma factor, σE, and the target promoters cycA P3, and rpoE P1. Journal of Molecular Biology 294, 307–320. [DOI] [PubMed] [Google Scholar]
- Newman J, Anthony J. and Donohue TJ (2001) The importance of zinc coordination for ChrR function as an anti-sigma factor. Journal of Molecular Biology, 313, 485–499. [DOI] [PubMed] [Google Scholar]
- Paget MSB, Kang J-G, Roe J-H and Buttner MJ (1998) σR, an RNA polymerase sigma factor that modulates expression of the thioredoxin system in response to oxidative stress in Streptomyces coelicolor A3(2). EMBO Journal, 17, 5776–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget MS, Chamberlin L, Atrih A, Foster SJ and Buttner MJ (1999a) Evidence that the extracytoplasmic function sigma factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2), Journal of Bacteriology 181, 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget MS, Leibovitz E. and Buttner MJ (1999b) A putative two-component signal transduction system regulates σE, a sigma factor required for normal cell wall integrity in Streptomyces coelicolor A3(2). Molecular Microbiology 33, 97–107. [DOI] [PubMed] [Google Scholar]
- Paget MSB, Bae J-B, Hahn M-Y, Li W, Kleanthous C, Roe J-H and Buttner MJ (2001a) Mutational analysis of RsrA, a zinc-binding anti-sigma factor with a thiol-disulphide redox switch. Molecular Microbiology, 39, 1036–1047. [DOI] [PubMed] [Google Scholar]
- Paget MSB, Molle V, Cohen G, Aharonowitz Y. and Buttner MJ (2001b) Defining the disulphide stress response in Streptomyces coelicolor A3(2): identification of the σR regulon. Molecular Microbiology, 42, 1007–20. [DOI] [PubMed] [Google Scholar]
- Papenfort K, Pfeiffer V, Mika F, Lucchini S, Hinton JC and Vogel J. (2006) σE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Molecular Microbiology, 62, 1674–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekar KV, Zdanowski K, Yan J, Hopper JT, Francis ML, Seepersad C, Sharp C, Pecqueur L, Werner JM, Robinson CV, Mohammed S, Potts JR and Kleanthous C. (2016) The anti-sigma factor RsrA responds to oxidative stress by reburying its hydrophobic core. Nature Communications, 7, 12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe J-H (2019) MicroReview, this issue. Title unknown. [Google Scholar]
- Rhodius VA, Suh WC, Nonaka G, West J. and Gross CA (2006) Conserved and variable functions of the σE stress response in related genomes. PLOS Biology, 1, e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Bush MJ, Bibb MJ, Ramos-Léon F, Chandra G, Zeng W. and Buttner MJ (2018) The crystal structure of the RsbN-σBldN complex from Streptomyces venezuelae defines a new structural class of anti-σ factor. Nucleic Acids Research, 46, 7405–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilke BA and Donohue TJ. (1992) δ-Aminolevulinate couples transcription of cycA to changes in heme availability in Rhodobacter sphaeroides. Journal of Molecular Biology, 226, 101–115. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Petit CM, Aubart K, Smyth M, McManus E, Jones J, Fosberry A, Lewis C, Lonetto M. and Christensen SB. (2003) Structural variation and inhibitor binding in polypeptide deformylase from four different bacterial species. Protein Science, 12, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DB, Walter WA and Gross CA (1987) The heat shock response of E. coli is regulated by changes in the concentration of σ32. Nature, 329, 348–351. [DOI] [PubMed] [Google Scholar]
- Staroń A, Sofia HJ, Dietrich S, Ulrich LE, Liesegang H. and Mascher T. (2009) The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Molecular Microbiology, 74, 557–581. [DOI] [PubMed] [Google Scholar]
- Tran NT, Huang X, Hong H-J, Bush MJ, Chandra G, Pinto D, Bibb MJ, Hutchings MI, Mascher T. and Buttner MJ (2019) Defining the regulon of genes controlled by σE, a key regulator of the cell envelope stress response in Streptomyces coelicolor. Molecular Microbiology This issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers AA and Burgess RR (1969) Cyclic re-use of the RNA polymerase sigma factor. Nature, 222, 537–540. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Alba BM, Bose B, Gross CA and Sauer RT (2003) OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell, 113, 61–71. [DOI] [PubMed] [Google Scholar]
- Zdanowski K, Doughty P, Jakimowicz P, O’Hara L, Buttner MJ, Paget MSB and Kleanthous C. (2006) Assignment of the zinc ligands in RsrA, a redox-sensing ZAS protein from Streptomyces coelicolor. Biochemistry 45, 8294–8300. [DOI] [PubMed] [Google Scholar]
- Ziegelhoffer EC and Donohue TJ (2009) Bacterial responses to photooxidative stress. Nature Reviews Microbiology, 7, 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]