Abstract
Recent findings suggest that COVID-19 causes vascular dysfunction during the acute phase of the illness in otherwise healthy young adults. To date, to our knowledge, no studies have investigated the longer-term effects of COVID-19 on vascular function. Herein, we hypothesized that young, otherwise healthy adults who are past the acute phase of COVID-19 would exhibit blunted peripheral [brachial artery flow-mediated dilation (FMD) and reactive hyperemia] and cerebral vasodilator function (cerebral vasomotor reactivity to hypercapnia; CVMR) and increased central arterial stiffness. Sixteen young adults who were at least 4 wk past a COVID-19 diagnosis and 12 controls who never had COVID-19 were studied. Eight subjects with COVID-19 were symptomatic (SYM) and eight were asymptomatic (ASYM) at the time of testing. FMD and reactive hyperemia were not different between COVID and control groups. However, FMD was lower in SYM (3.8 ± 0.6%) compared with ASYM (6.8 ± 0.9%; P = 0.007) and control (6.8 ± 0.6%; P = 0.003) with no difference between ASYM and control. Similarly, peak blood velocity following cuff release was lower in SYM (47 ± 8 cm/s) compared with ASYM (64 ± 19 cm/s; P = 0.025) and control (61 ± 14 cm/s; P = 0.036). CVMR and arterial stiffness were not different between any groups. In summary, peripheral macrovascular and microvascular function, but not cerebral vascular function or central arterial stiffness were blunted in young adults symptomatic beyond the acute phase of COVID-19. In contrast, those who were asymptomatic had similar vascular function compared with controls who never had COVID-19.
NEW & NOTEWORTHY This study was the first to investigate the persistent effects of COVID-19 on vascular function in otherwise healthy young adults. We demonstrated that peripheral macrovascular and microvascular vasodilation was significantly blunted in young adults still symptomatic from COVID-19 beyond the acute phase (>4 wk from diagnosis), whereas those who become asymptomatic have similar vascular function compared with controls who never had COVID-19. In contrast, cerebral vascular function and central arterial stiffness were unaffected irrespective of COVID-19 symptomology.
Keywords: arterial stiffness, cerebral vasomotor reactivity, flow-mediated dilation, SARS-CoV-2, vascular dysfunction
INTRODUCTION
The novel coronavirus disease-2019 (COVID-19) has affected over 30 million individuals in the United States alone. To date, the prevalence of COVID-19 has been highest in young adults, with almost one in four cases being diagnosed in those between 18 and 29 yr of age (1). Notably, early investigations revealed that SARS-CoV-2, the virus causing COVID-19, infects and damages vascular endothelial cells (2). However, limited studies have directly investigated the effects of COVID-19 on vascular function.
Notably, recent work reported that young adults who had COVID-19 and were within 4 wk from diagnosis, i.e., within the acute phase of the illness (3), at the time of testing exhibited attenuated peripheral vascular function (4) and elevated arterial stiffness (4, 5) compared with those who never had COVID-19. However, whether these detrimental effects on the vasculature persist or resolve beyond the acute phase of the illness remains unknown. This becomes quite important because recent evidence showed that up to one in four young adults, including those with mild symptoms during the acute phase, report persisting symptoms for up to 6 mo from diagnosis (6). Also, individuals who have had COVID-19 frequently experience lingering headaches and cognitive deficits (6, 7) suggestive of cerebral vascular impairments. However, despite these observations, to our knowledge no studies have investigated whether cerebral vascular function is altered following COVID-19.
With this background in mind, we sought to comprehensively assess the potential persisting effects of COVID-19 on vascular function in young adults. We hypothesized that young, otherwise healthy adults who are beyond 4 wk from a COVID-19 diagnosis would exhibit blunted peripheral and cerebral vasodilator function and increased central arterial stiffness compared with those who did not have COVID-19.
METHODS
Study Population
Sixteen young otherwise healthy adults who had a laboratory-confirmed diagnosis of COVID-19 (COVID; SARS-CoV-2 RT-PCR or antigen test) were studied. All subjects with COVID-19 were a minimum of 4 wk from their diagnosis and reported being symptomatic at the time of diagnosis. Severity of each symptom was ranked on a scale of 1–10 and quantified both for the time of COVID-19 diagnosis and on the date of the study visit. At the time of testing, eight were asymptomatic [ASYM; age = 22 ± 4 yr; body mass index (BMI) = 22 ± 4 kg/m2; 12 ± 5 wk (range = 4 to 21 wk) from diagnosis; male/female = 5/3] (means ± SD), whereas eight remained symptomatic [SYM; age = 24 ± 3 yr BMI = 26 ± 3 kg/m2; 14 ± 4 wk (range = 7 to 20 wk) from diagnosis; male/female: 1/7]. Symptoms reported at the time of testing by SYM subjects were loss of smell and/or taste (n = 7), fatigue (n = 1), and severe muscle pain after exercise (n = 1), and the average symptom severity was 4 ± 1. All ASYM subjects reported that symptoms resolved within 1 mo of their COVID-19 diagnosis. Twelve adults who did not have COVID-19 were also studied and served as controls (age = 23 ± 3 yr; BMI = 23 ± 3 kg/m2; male/female = 6/6).
All subjects were nonsmokers and were not on any prescription medication. Subjects reported being recreationally active (control: 280 ± 230 exercise min/wk, ASYM: 259 ± 193 min/wk, SYM: 227 ± 98 min/wk; no difference between groups), and free from any known cardiovascular, cerebrovascular, metabolic, or neurological diseases. All experiments were carried out following an overnight fast in a temperature-controlled (20°C–22°C) dimly lit room. Subjects were instructed to abstain from caffeine and any over-the-counter medication for at least 12 h and alcohol and exercise for at least 24 h before the study. After receiving a detailed verbal and written explanation of the experimental protocol, subjects provided informed written consent. All experimental procedures conformed to the Declaration of Helsinki and were approved by the Institutional Review Board at the University of Texas at Arlington (2021-0197).
Experimental Measurements
Subjects were instrumented with a standard lead II electrocardiogram (model Q710, Quinton, Bothell, WA) to continuously measure heart rate (HR) and a pneumobelt (Pneumotrace II 1132, UFI, Morro Bay, CA) to monitor respiration. Beat-to-beat arterial blood pressure (BP) was measured via finger photoplethysmography (Finometer PRO, Finapres Medical Systems, Amsterdam, The Netherlands). Automated sphygmomanometer BPs (Welch Allyn, Skaneateles Falls, NY) were obtained for subject characterization and to confirm the Finometer blood pressures.
Experimental Protocol
Subjects rested supine for 20 min before data collection. For assessment of peripheral vascular function, the brachial artery flow-mediated dilation (FMD) technique was performed using current guidelines and as previously described (8, 9). Briefly, the brachial artery was imaged using duplex Doppler ultrasound (GE Logiq P5, Milwaukee, WI) and an 11-MHz linear array transducer. Continuous blood velocity and diameter measures were simultaneously obtained with the sample volume encompassing the entire lumen but not extending beyond the arterial wall edges. Baseline data were recorded for 2 min, following which a rapidly inflating cuff (Hokanson, Bellevue, WA) placed ∼2 cm distal to the antecubital fossa was inflated to a suprasystolic pressure (220 mmHg) for 5 min. Blood velocity and diameter data were recorded for 30 s before and for 3 min following cuff release.
Following FMD, central arterial stiffness was assessed by carotid-femoral pulse wave velocity (PWV) and pulse wave analysis (PWA) (SphygmoCor, Atcor Medical, Sydney, Australia) as described previously (9). For PWV, a cuff was placed on the thigh, and carotid and femoral pulses were palpated at the strongest points. Measurements were made between three sites (carotid artery to sternal notch, sternal notch to thigh cuff, and femoral artery to thigh cuff). An arterial BP waveform was detected using a handheld tonometer placed over the carotid artery while the thigh cuff was inflated. PWV was calculated (XCEL 1.3, Atcor Medical, Sydney, Australia) as the carotid-femoral artery distance divided by the pulse transit time. For PWA, a brachial cuff around the left arm measured peripheral pressure waveforms and generated a corresponding aortic waveform from which augmentation index normalized to an HR of 75 beats/min (AIx75) was derived. PWV and PWA measurements were not obtained in two control subjects.
Next, HR and automated brachial artery sphygmomanometer BPs were obtained during a 5-min quiet resting period. Subjects were then instrumented with a 2-MHz transcranial Doppler (Multigon Industries, Inc., Yonkers, NY) ultrasound probe placed over the left temporal window to obtain middle cerebral artery blood velocity (MCAv) measurements. The probe was held in place using a headband. A rebreathing protocol was carried out for assessment of cerebral vascular function (10). Briefly, subjects were fitted with a nose clip and breathed through a mouthpiece attached to a 3-way valve (Hans Rudolph Inc., Kansas City, KS). Oxygen (O2) was bled into the 5-L rebreathing bag throughout the protocol to ensure normal arterial O2 saturation () based on the subject’s basal metabolic rate as estimated using the Harris–Benedict formula (11). End-tidal carbon dioxide () and were measured through a sampling line connected to the mouthpiece, and pulse oximeter on their index finger, respectively, which were connected to a capnograph (Capnocheck Plus, Smiths Medical, Dublin, OH). Before the start of the protocol, the rebreathing bag was filled with the subject’s own air. A 3-min baseline was then performed while subjects breathed room air. The valve was then switched, and subjects rebreathed from the bag for 3 min or until they reached a Δ of at least 15 mmHg from baseline. The valve was then switched back to room air for 3 min of recovery. Two control subjects and one subject with COVID-19 (ASYM) did not perform the rebreathing protocol.
Data Analysis
HR, BP, respiration, MCAv, , and were recorded continuously at 1,000 Hz using PowerLab (ADInstruments, Bella Vista, Australia). For baseline subject characterization, HR was averaged over the 5-min resting period, and mean systolic (SBP) and diastolic BP (DBP) was calculated from three automated sphygmomanometer readings. A customized offline wall tracking and edge detection software (LabView, National Instruments, Austin, TX) were used to analyze brachial artery diameter and weighted mean blood velocity. For assessment of peripheral artery macrovascular function, brachial artery FMD was calculated as FMD% = (3-beat average peak diameter − baseline diameter)/baseline diameter × 100. Shear rate was calculated as 8 × mean blood velocity/diameter. The shear stimulus for FMD was calculated as the hyperemic shear rate area under the curve (AUC) via the sum of trapezoids method to peak brachial artery dilatation. Microvascular function was quantified as the three-beat average peak blood velocity following cuff release. Central arterial stiffness was quantified using the average of two measures of PWV and AIx75. For quantification of cerebral vasomotor reactivity (CVMR), average values for , MCAv, MAP, and cerebral vascular conductance index (CVCi = MCAv/MAP) were calculated over the last 1 min of baseline. During the rebreathe, three breath averages of MCAv and CVCi at Δ of 3, 6, 9, 12, and 15 mmHg were calculated. CVMR was quantified as a percent increase in MCAv (ΔMCAv%) and CVCi (ΔCVCi%) at each Δ.
Statistical Analysis
Resting cardiorespiratory and hemodynamic parameters, FMD, reactive hyperemia, PWV, and AIx75 between control and COVID group were analyzed using Student’s t test for independent samples (SPSS, v. 25). Further comparisons between control, ASYM, and SYM were made using one-way ANOVA. When significant group differences were observed, pairwise comparisons were made using Fisher’s least significant difference post hoc test. CVMR was analyzed using two-way repeated-measures ANOVA for effects of group (control vs. COVID and control vs. ASYM vs. SYM) and time (Δ level). Because of the observation of differences in the shear stimulus for FMD between control, ASYM, and SYM, ANCOVA was performed to covary statistically for the impact of hyperemic shear rate on FMD values. All data are presented as means ± SD, and the significance level was set at α < 0.05.
RESULTS
Control and COVID
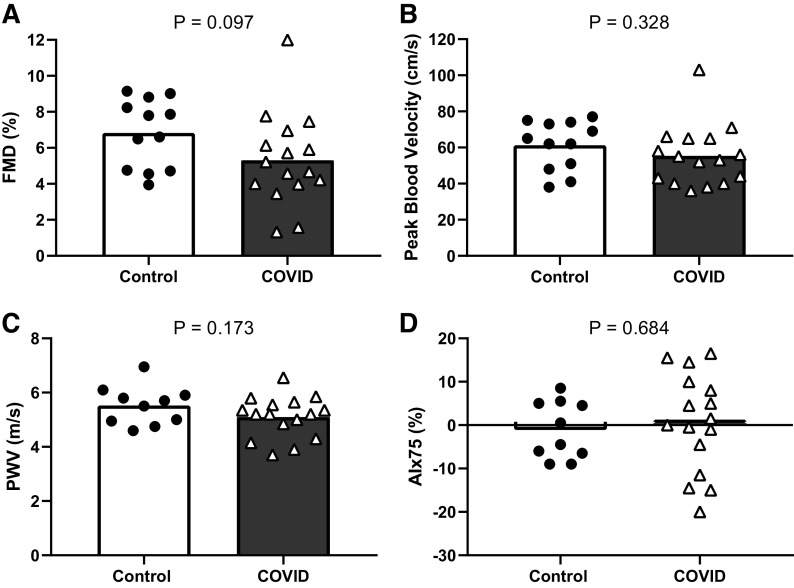
Resting brachial artery diameter (control: 0.34 ± 0.04 cm vs. COVID: 0.34 ± 0.06 cm) and mean blood velocity (control: 7.3 ± 3.3 cm/s vs. COVID: 6.9 ± 2.3 cm/s) were similar between control and COVID (P > 0.05 for both). FMD (Fig. 1A) and peak blood velocity following cuff release (Fig. 1B) were not different between groups. Similar results were observed for hyperemic blood velocity AUC to 30, 60, and 120 s after cuff release (all P > 0.05). Likewise, central arterial stiffness, assessed as PWV and AIx75 were also not different between groups (Fig. 1, C and D respectively). Resting MCAv (control: 86 ± 5 cm/s vs. COVID: 76 ± 4 cm/s) and CVCi (control: 1.04 ± 0.06 cm/s/mmHg vs. COVID: 0.92 ± 0.05 cm/s/mmHg) were similar between groups (P > 0.05 for both). For CVMR, there was no interaction between group and time (P = 0.503) or main effect of group (P = 0.807) for ΔMCAv% indicating no difference between groups at any level of Δ. Similar results were observed for ΔCVCi% (interaction: P = 0.505, main effect of group: P = 0.431).
Figure 1.
Group means and individual data for flow-mediated dilation (FMD, A), peak blood velocity following cuff release (B) (n = 12 control and n = 16 COVID for both), pulse wave velocity (PWV, C), and augmentation index normalized to heart rate of 75 beats/min (AIx75, D) (n = 10 control and n = 16 COVID for both) between control and COVID groups.
Control, ASYM, and SYM
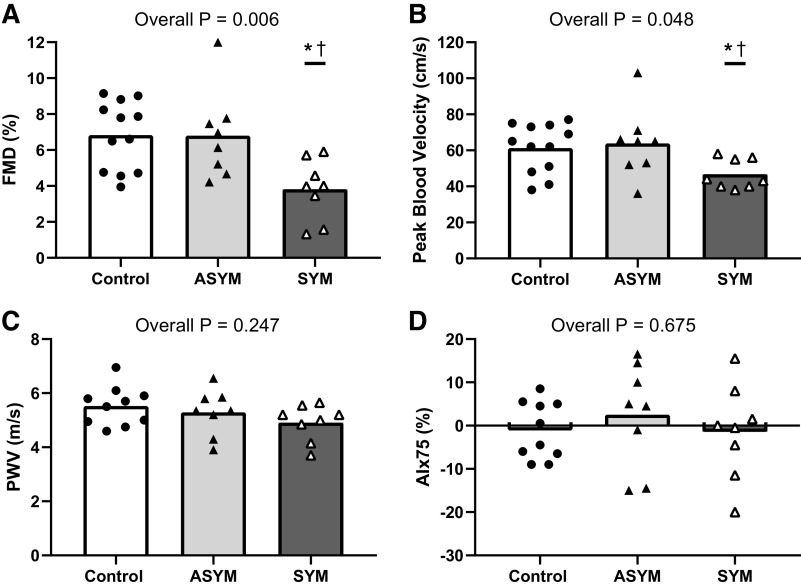
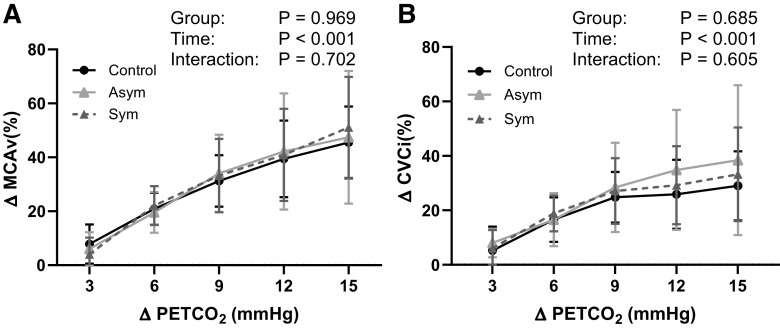
Resting brachial artery diameter and mean blood velocity were not different between control, ASYM, and SYM groups (Table 1). However, significant group differences for FMD were observed. Post hoc testing revealed that FMD was lower in SYM compared with both ASYM and control groups with no difference between control and ASYM (Fig. 2A). Shear rate AUC to peak diameter was lower in SYM [22,501 ± 6,633 arbitrary units (AU)] compared with ASYM (34,956 ± 15,589 AU, P = 0.034) and control (34,230 ± 9,799 AU, P = 0.029); however, group differences in FMD remained (P = 0.006) after statistically covarying for differences in shear stimulus (SYM = 4.3 ± 0.8%, ASYM = 6.6 ± 0.7%, control = 6.6 ± 0.6%; P < 0.05 for both comparisons). Likewise, peak blood velocity after cuff release was lower in SYM compared with both ASYM and control, with no differences between control and ASYM (Fig. 2B). Similar results were obtained for hyperemic blood velocity AUC to 30, 60, and 120 s after cuff release. PWV and AIx75 were not different between any groups (Fig. 2, C and D). Resting MCAv was lower in ASYM compared with SYM and control. CVCi was lower in ASYM compared with control (Table 1). However, for CVMR, there was no significant interaction or main effect of group for ΔMCAv% or ΔCVCi% (Fig. 3). Resting cardiorespiratory and hemodynamic measures for all groups are presented in Table 1.
Table 1.
Resting cardiorespiratory and hemodynamic measures
| Control | ASYM | SYM | P Value | |
|---|---|---|---|---|
| n | 12 | 8 | 8 | – |
| Heart rate, beats/min | 58 ± 8 | 61 ± 10 | 65 ± 10 | 0.259 |
| Systolic blood pressure, mmHg | 112 ± 9 | 110 ± 9 | 111 ± 6 | 0.923 |
| Diastolic blood pressure, mmHg | 66 ± 4 | 68 ± 5 | 70 ± 4 | 0.188 |
| Brachial artery diameter, cm | 0.34 ± 0.04 | 0.34 ± 0.05 | 0.34 ± 0.07 | 0.971 |
| Brachial artery mean blood velocity, cm/s | 7.4 ± 3.3 | 7.8 ± 2.7 | 6.0 ± 1.4 | 0.368 |
| Middle cerebral artery blood velocity, cm/s | 86 ± 16 | 67 ± 17*,† | 84 ± 11 | 0.042 |
| Cerebral vascular conductance index, cm/s/mmHg | 1.04 ± 0.20 | 0.82 ± 0.21* | 1.01 ± 0.10 | 0.048 |
| End-tidal carbon dioxide, mmHg | 47 ± 2 | 45 ± 7 | 44 ± 5 | 0.297 |
| Respiratory rate, breaths/min | 14 ± 3 | 13 ± 3 | 15 ± 4 | 0.293 |
Values are means ± SD. Middle cerebral artery blood velocity, cerebral vascular conductance index, end-tidal carbon dioxide, and respiratory rate data are for n = 10 controls and n = 7 asymptomatic (ASYM). One-way ANOVA followed by post hoc testing was performed between control, COVID-19 ASYM, and symptomatic (SYM) groups. *P < 0.05 between ASYM and control. †P < 0.05 between ASYM and SYM.
Figure 2.
Group means and individual data for flow-mediated dilation (FMD, A), peak blood velocity following cuff release (B) (n = 12 control, n = 8 ASYM, and n = 8 SYM), pulse wave velocity (PWV, C), and augmentation index normalized to heart rate of 75 beats/min (AIx75, D) (n = 10 control, n = 8 ASYM, and n = 8 SYM) between control, asymptomatic (ASYM), and symptomatic (SYM) groups. *P < 0.05 between SYM and ASYM. †P < 0.05 between SYM and control.
Figure 3.
Group means (±SD) data for percent change in middle cerebral artery blood velocity (ΔMCAv%, A) and cerebral vascular conductance index (ΔCVCi%, B) at increases in end-tidal carbon dioxide (Δ) of 3, 6, 9, 12, and 15 mmHg from baseline between control (n = 10), asymptomatic (ASYM; n = 7) and symptomatic (SYM; n = 8) groups.
DISCUSSION
This study was the first to investigate the persistent effects of COVID-19 on peripheral vascular function, central arterial stiffness, and cerebral vascular function in young otherwise healthy adults. The major novel findings are threefold. First, we demonstrate that in contrast to our hypothesis, vascular function was not different between young adults who are beyond 4 wk from a COVID-19 diagnosis and controls. The second major finding is that peripheral vascular function assessed via brachial artery FMD (i.e., macrovascular function) and reactive hyperemia (i.e., microvascular function) were blunted in those who had COVID-19 and were still symptomatic beyond the acute phase compared with controls. Conversely, FMD and reactive hyperemia were not different in asymptomatic subjects with COVID-19 compared with controls. Third, in contrast to peripheral vascular function, we found no differences in markers of central arterial stiffness (i.e., carotid-femoral PWV and AIx75), and cerebral vascular function (i.e., CVMR) between any groups. Collectively, these findings indicate that peripheral vascular function, but not cerebral vascular function, and central arterial stiffness is impaired in young adults who had mild COVID-19 illness but are still symptomatic past the acute phase. This impairment appears to be resolved in those who are no longer symptomatic.
A recent study by Ratchford et al. (4) reported that brachial artery FMD was blunted in young adults when tested during the acute phase of COVID-19 illness. The current study extends these findings by demonstrating that the impairment in brachial artery FMD persists in those who continue to be symptomatic beyond the acute phase. However, this impairment appears to be completely resolved in those who become asymptomatic. Notably, all individuals who had COVID-19 in the current study would be classified to have had mild to moderate illness (12) and were on average at 3 mo from diagnosis. We also found that reactive hyperemia was blunted in symptomatic individuals. Interestingly, Ratchford et al. (4) reported no impairment in reactive hyperemia during the acute phase suggesting that persistent, but not acute COVID-19 illness may be a primary mediator of microvascular dysfunction. In contrast, we found central arterial stiffness to be unaffected in those who had COVID-19 irrespective of their symptomology, whereas previous work reported that arterial stiffness was elevated in young adults during the acute phase of COVID-19 illness (4, 5). These findings may suggest that the detrimental impact of COVID-19 on central large arteries is an acute and transient phenomenon that resolves over time. Nonetheless, additional studies are warranted.
Despite the consistent impairments in peripheral vasodilation observed, we found that cerebral vascular function was not impaired following COVID-19 in young adults even in those with persistent symptoms. However, it should be noted that only two individuals reported experiencing cognitive symptoms (i.e., “brain fog”) at the time of diagnosis and these symptoms resolved within 2 wk. Thus, it would be important to perform additional studies to assess cerebral vascular function in those who report persistent cognitive impairments following COVID-19.
It is intriguing that those with persistent symptoms exhibited peripheral vascular dysfunction, whereas those who were asymptomatic at the time of testing had similar macrovascular and microvascular vasodilation to controls. Moreover, the symptomatic group only reported one or two symptoms of mild to moderate severity. Nevertheless, although the impairments in peripheral vascular function in those with persisting symptoms were clear, the mechanism(s) responsible remain unclear. Although beyond the scope of this rapid report, some discussion is warranted. One proposed mechanism contributing to persistent COVID-19 symptoms is ongoing and/or a dysregulated immune response to the acute infection (3). In support of this hypothesis, de Melo et al. (13) demonstrated the presence of local inflammation in the olfactory epithelium in those with persistent loss of smell following COVID-19. Moreover, despite the lack of direct evidence of a link between COVID-19 symptomology, vascular dysfunction, and inflammation, previous investigations have demonstrated a clear link between acute inflammation and impaired endothelial function (14, 15). Notably, improvement in endothelial function following acute viral infection has been shown to be accompanied by a reduction in blood inflammatory markers (14). Given these observations, it is plausible that ongoing inflammation contributes to both the persistent vascular dysfunction and lingering symptoms following COVID-19. Nonetheless, further studies are warranted to directly investigate the link between COVID-19 symptomology and vascular function.
Conclusion
The findings from the current study demonstrate that young otherwise healthy adults who continue to experience symptoms from COVID-19 beyond the acute phase of the illness exhibited peripheral vascular dysfunction. In contrast, vascular function appears to be restored in those who are no longer symptomatic. Furthermore, central arterial stiffness and cerebral vascular function were unaffected in subjects with COVID-19 beyond the acute phase irrespective of symptomology. Collectively, these findings highlight that the persistence of symptoms following COVID-19 is associated with peripheral vascular dysfunction in otherwise healthy young adults.
GRANTS
This work was supported by The University of Texas at Arlington College of Nursing and Health Innovation. Both D.N. (827597) and B.Y.S. (20PRE34990010) are supported by American Heart Association Pre-Doctoral Fellowships.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.N. and P.J.F. conceived and designed research; D.N., B.E.Y., B.Y.S., A.-K.G., and R.J.S. performed experiments; D.N. analyzed data; D.N., B.E.Y., B.Y.S., A.-K.G., R.J.S., and P.J.F. interpreted results of experiments; D.N. prepared figures; D.N. drafted manuscript; D.N., B.E.Y., B.Y.S., A.-K.G., R.J.S., and P.J.F. edited and revised manuscript; D.N., B.E.Y., B.Y.S., A.-K.G., R.J.S., A.J.M., F.P.H., and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects for time and participation in the study and acknowledge the subject recruitment efforts of Drs. Angela Middleton and Florence Haseltine.
REFERENCES
- 1.CDC. COVID Data Tracker [Online]. 2020. https://covid.cdc.gov/covid-data-tracker/#datatracker-home[2021 Jun 25].
- 2.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395: 1417–1418, 2020. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med 27: 601–615, 2021. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratchford SM, Stickford JL, Province VM, Stute N, Augenreich MA, Koontz LK, Bobo LK, Stickford ASL. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol 320: H404–H410, 2021. doi: 10.1152/ajpheart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szeghy RE, Province VM, Stute NL, Augenreich MA, Koontz LK, Stickford JL, Stickford ASL, Ratchford SM. Carotid stiffness, intima–media thickness and aortic augmentation index among adults with SARS‐CoV‐2. Exp Physiol, 2021. doi: 10.1113/EP089481. [33904234] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, Chu HY. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 4: e210830, 2021[Erratum inJAMA Netw Open4: e214572, 2021]. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, DiBiase RM, Jia DT, Balabanov R, Ho SU, Batra A, Liotta EM, Koralnik IJ. Persistent neurologic symptoms and cognitive dysfunction in non‐hospitalized Covid‐19 “long haulers. Ann Clin Transl Neurol 8: 1073–1085, 2021. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thijssen DHJ, Bruno RM, Van Mil ACCM, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ, Ghiadoni L. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40: 2534–2547, 2019. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 9.Stephens BY, Kaur J, Vranish JR, Barbosa TC, Blankenship JK, Smith SA, Fadel PJ. Effect of acute high-phosphate intake on muscle metaboreflex activation and vascular function. Am J Physiol Heart Circ Physiol 317: H308–H314, 2019. doi: 10.1152/ajpheart.00082.2019. [DOI] [PubMed] [Google Scholar]
- 10.Hurr C, Patik JC, Kim KY, Brothers RM. Blunted cerebral vascular responsiveness to hypercapnia in obese individuals. Exp Physiol 102: 1300–1308, 2017. doi: 10.1113/EP086446. [DOI] [PubMed] [Google Scholar]
- 11.Claassen JAHR, Zhang R, Fu Q, Witkowski S, Levine BD. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J Appl Physiol (1985) 102: 870–877, 2007. doi: 10.1152/japplphysiol.00906.2006. [DOI] [PubMed] [Google Scholar]
- 12.NIH. Clinical Spectrum | COVID-19 Treatment Guidelines [Online]. 2021. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ [2021 Jul 1].
- 13.de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, Verillaud B, Aparicio C, Wagner S, Gheusi G, Kergoat L, Kornobis E, Donati F, Cokelaer T, Hervochon R, Madec Y, Roze E, Salmon D, Bourhy H, Lecuit M, Lledo P-M. COVID-19–related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 13: eabf8396, 2021. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchesi S, Lupattelli G, Lombardini R, Sensini A, Siepi D, Mannarino M, Vaudo G, Mannarino E. Acute inflammatory state during influenza infection and endothelial function. Atherosclerosis 178: 345–350, 2005. doi: 10.1016/j.atherosclerosis.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, Donald AE, Palacios M, Griffin GE, Deanfield JE, MacAllister RJ, Vallance P. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation 102: 994–999, 2000. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]