Abstract
Many patients who suffer from pulmonary diseases cannot inflate their lungs normally, as they need mechanical ventilation (MV) to assist them. The stress associated with MV can damage the delicate epithelium in small airways and alveoli, which can cause complications resulting in ventilation-induced lung injuries (VILIs) in many cases, especially in patients with acute respiratory distress syndrome (ARDS). Therefore, efforts were directed to develop safe modes for MV. In our work, we propose a different approach to decrease injuries of epithelial cells (EpCs) upon MV. We alter EpCs’ cytoskeletal structure to increase their survival rate during airway reopening conditions associated with MV. We tested two anti-inflammatory drugs dexamethasone (DEX) and transdehydroandrosterone (DHEA) to alter the cytoskeleton. Cultured rat L2 alveolar EpCs were exposed to airway reopening conditions using a parallel-plate perfusion chamber. Cells were exposed to a single bubble propagation to simulate stresses associated with mechanical ventilation in both control and study groups. Cellular injury and cytoskeleton reorganization were assessed via fluorescence microscopy, whereas cell topography was studied via atomic force microscopy (AFM). Our results indicate that culturing cells in media, DEX solution, or DHEA solution did not lead to cell death (static cultures). Bubble flows caused significant cell injury. Preexposure to DEX or DHEA decreased cell death significantly. The AFM verified alteration of cell mechanics due to actin fiber depolymerization. These results suggest potential beneficial effects of DEX and DHEA for ARDS treatment for patients with COVID-19. They are also critical for VILIs and applicable to future clinical studies.
NEW & NOTEWORTHY Preexposure of cultured cells to either dexamethasone or transdehydroandrosterone significantly decreases cellular injuries associated with mechanical ventilation due to their ability to alter the cell mechanics. This is an alternative protective method against VILIs instead of common methods that rely on modification of mechanical ventilator modes.
Keywords: airway reopening, ARDS, COVID-19, epithelial cells, VILI
INTRODUCTION
An efficient breathing mechanism requires successful contraction and relaxation of respiratory muscles around 30,000 times a day or a billion times over 90 years (1). The process of breathing requires a necessary pressure to inflate the lungs that comprises the pressure to overcome airway resistance and the pressure to overcome the elastic properties of the lung (2). In the lungs, gas exchanges occur naturally between the air we breathe and the pulmonary capillary blood through three processes, namely, ventilation, diffusion, and perfusion (3). This gas exchange occurs in the distal regions of the lungs through small airways and alveoli through the tracheobronchial tree and relies on mechanisms of bulk or convective transport and diffusive net transport (4). Many patients suffering from pulmonary diseases are subject to developing an inability to inflate their lungs normally and as a result would need an assisted ventilation therapy. These diseases include chronic obstructive pulmonary disease (COPD) (5), severe cystic fibrosis (CF) (6), severe asthma (7), hypercapnic respiratory failure resulting from restrictive thoracic diseases (RTD) (8), pulmonary emphysema (9), and acute respiratory distress syndrome (ARDS) (10).
The inability to inflate lungs can be overcome through assisted ventilation. The act of assisted ventilation has been known historically for thousands of years. However, the basis for lung inflations was not known but what was known is the necessity for breathing stimuli, and for this, various ineffective and sometimes brutal methods were applied, such as tickling of chest, throat, and mouth; dilating; fumigating; and blowing smoke into the rectum (11). These were accepted ideas for a long time. The physiological principles for the current ventilators were not established until the late 19th century when the first ventilation device was developed by Alfred Jones in 1864 where the patient sat inside a box that enclosed their body from the neck down. The box contained a plunger to decrease the pressure in the box and allowed inhalation; a converse produced exhalation (12). This mode of ventilation is known as negative-pressure ventilation where negative subatmospheric pressure is applied to the thorax during inspiration causing a thoracic expansion and a decrease in pleural and alveolar pressure, allowing the formation of a pressure gradient for the air to move from the airway opening into the alveoli (13).
The field of artificial ventilators continued to develop in the same direction of the negative-pressure ventilation until the Copenhagen polio epidemic in 1950s. The lack of equipment and the exhaustion of medical instruments due to the number of cases in the epidemic created a need for alternative solutions, where the patient was then ventilated by hand via an endotracheal tube, and from this point onward, positive-pressure ventilation was constantly used. Mechanical ventilation (MV) emerged from this point, as the ventilation cannot be done manually for many patients at a time due to the insufficiency of trained healthcare professionals. The past 66 years witnessed significant development for the mechanical ventilators in a variety of aspects and modes.
MV can cause complications, including lung injuries (14). Perhaps one of the earliest documentations on this is by the Father of MV John Fothergill in 1744 in his comment on William Tosach’s publication when he referred to a possible injury that could be caused when ventilating with bellows (12, 15). There were directions and general lines understood that negative pressure is not of any help to diseased lungs and cannot assist in gas exchange. The downside of positive pressure was discovered only upon attempts for improvement through applying larger forces to facilitate the gas exchange, but it was discovered that when this therapy was combined with a diseased lung that is already injured, it caused severe injuries known as barotrauma. This was only the beginning to the discovery of more injuries caused by MV. Perhaps one of the earliest observations on the pulmonary complications for patients on respirators came in 1956 in describing cases of patients with polio by d’Avingnon et al. (16). The concept of ventilation induced lung injury (VILI) was developed and modeled theoretically for the first time by Mead et al. (17) predicting lung hemorrhage in 1970. This model was developed only three years after the full description of acute respiratory distress syndrome (ARDS) by Ashsbuagh et al. in 1967 (18). They are often associated, but VILI is not only caused by ARDS; it can be caused under many other circumstances when MV is used.
In 1974, MV was demonstrated as an injury source for the lung for the first time in a study published by Webb and Tierney (19) on rats that developed pulmonary edema within 30 min and 60 min at inspiratory pressures of 30 and 45 cm H2O, respectively. The knowledge on VILI expanded and became a subject of interest due to its frequent occurrence and its inevitability since it is not limited to those patients with diseased lungs who use MV but can also occur in those with healthy lungs. The two main reasons behind VILI being caused are alveolar overdistension (barotrauma) and cyclic opening and closing of alveoli. There are several mechanisms that are known to be involved in the development of VILI and these include barotrauma (20), biotrauma (21, 22), volutrauma (23), and atelectrauma (24). Consequences of complications of VILI can be serious and life threatening, from a cytokine release storm (25) to multiple-organ dysfunction (26, 27).The VILI is almost inevitable, but many approaches have been experimented to find safer MV practices to prevent VILI and to decrease hydrodynamic stresses and hence lead to a reduction in cellular injuries upon MV. Despite the substantial efforts to prevent epithelium injury during MV, the majority of the VILI research is based on changing parameters to minimize magnitudes of injurious mechanical forces associated with MV while maintaining gas exchange and minimizing lung injuries associated with the ventilators through several techniques and protocols that rely on different modes and settings for the partial pressures (28) and tidal volume (29). Unfortunately, according to the outcomes of these investigations, the mortality rates did not significantly decrease (30).
Epidemics and pandemics impose new challenges on the healthcare sector (31). It is no longer the number of available ventilators that is the only dilemma for health care professionals, but also the methods of delivery and the complications that arise with VILI. The first reported case of the COVID-19 virus was on December 31, 2019. COVID-19 has been classified as a pandemic with reported daily cases of infection and deaths. A secondary outcome associated with the infection is mild to severe pneumonia classified as ARDS (32) according to the Berlin definition (33). The ARDS among patients with COVID-19 is known as CARDS (34). There is a constant need for MV in patients with ARDS and CARDS. As the numbers of newly positive cases and mortality cases are being updated daily, it is difficult to give an exact statistic of patients with COVID-19 who suffer from CARDS or die from it. However, there are broad lines that can be drawn, and estimations are based on previously published studies. A meta-analysis that analyzed seven clinical studies found that the rate of ARDS among COVID-19 patients is estimated to be 19.5% and the fatality rate was around 5.5% (35). The major reason for fatality among patients with COVID-19 treated in an intensive care unit (ICU) is ARDS/CARDS (32, 36, 37). Since ARDS is common in patients with COVID-19, associated injuries with MV treatment related to VILI are highly expected, and these could cause fatal complications(38). According to a hypothesis by Tremblay and Slutsky (39) in 1998, VILI can cause a biotrauma associated with cytokine storms that can lead to death. In COVID-19, cytokine storms are common and blocking them has been considered as a successful method for saving patients who suffer from severe COVID-19 symptoms (40). It is more urgent now than ever to consider the risk associated with VILI for its known role in EpC injuries and increased mortality rates among patients who would need to be mechanically ventilated, as well as the hypothesized fatal role along with cytokine storms in patients with COVID-19. Therefore, approaches are needed to minimize the damage that VILI can cause, as this can lead to a decrease in mortality rates among mechanically ventilated patients with VILI and patients with CARDS. An alternative approach to reduce mortality rates and prevent VILI upon MV is to alter the physical properties of EpCs to make them less prone to injury. In shear stress associated with airway reopening during MV, the EpCs experience deformation leading eventually to cell necrosis (41). The cells’ mechanical properties are viscoelastic and based on the cytoskeletal structure, they exhibit complex rheological properties (42–44). Two types of fibers shape the cytoskeleton, namely, solid-like elastic fibers (45) and fluid-like viscous fibers (46–48). For a given mechanical load, a compliant cell will undergo more deformation than a stiff cell, and more fluid-like cells will dampen the force and will deform slower compared with highly elastic solid-like cells (49).
A potential solution for the rescue of the EpCs by preventing the injury associated with VILI can be obtained based on the mechanical behavior of the cells. This hypothesis was first proposed in 2009 by our group (50). The theory states that the cell necrosis and detachment taking place upon the cyclic airway reopening relies on both the biostructural and the rheological properties of EpCs (51). To prevent cell injuries, alterations of the cytoskeleton structure can be done through the applications of drugs that depolymerize or destabilize actin filaments in the cytoskeleton. This has been a successful approach by our group when nitric oxide nanoparticles were applied pre-exposure to lung EpCs to airway reopening conditions mimicking those of MV (52). In the current work, we have selected dexamethasone (DEX) and dehydroepiandrosterone sulfate (DHEAS), which are anti-inflammatory steroidal agents that are known to affect the cytoskeletal structure of cultured cells (53, 54). Steroids have been proven to be beneficial in use during the treatment of COVID-19 (55). The first steroid has been verified to reduce mortality rate among ventilated patients with COVID-19, according to a recent published study (56). The cyclic airway reopening shear stress conditions can be generated through a model that was previously generated by our laboratory (57), as we use it to examine how the application of these two drugs on cultured EpCs affects cell susceptibility to injuries due to the propagating air-liquid interface.
MATERIALS AND METHODS
Cell Culture
CCL-149 (L2) rat lung cells were purchased from American Type Culture Collection (ATCC) [L2 (ATCC CCL-149)]. The cells were cultured at passage number 10–30 and were maintained in Ham’s F-12K medium with 10% fetal bovine serum and 1% antibiotic-antimycotic solution. Cells were harvested with 0.125% trypsin, counted and seeded onto 40-mm-diameter coverslips, and placed in 60-mm-diameter petri dish wells. The choice of coverslip size was selected to fit the bottom of the flow chamber. A concentration of 8 ×104 cells/mL and 2 mL of cell media were prepared for each well. Cells were grown until ∼100% confluency (mainly around 4 days), and the cell culture medium was changed every 2 days. Collagen solution (25 μL/mL) was used to coat the coverslips before cell seeding to prevent cellular detachment during bubble exposure.
Generation of Airway Reopening Conditions
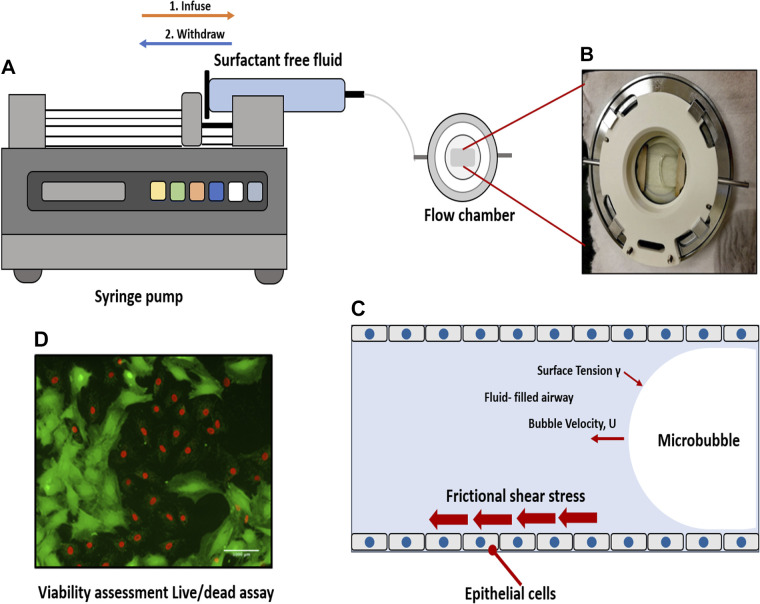
The EpCs were exposed to simulated shear stresses like those associated with airway reopening when mechanically ventilated in airway reopening conditions to test the effects of the selected anti-inflammatory steroidal agents in their efficacy to decrease cellular injuries during airway reopening. For this purpose, reopening conditions were generated using a parallel-plate microfluidic flow chamber system (Bioptechs-FCS2 chamber), as described in our previous work (57). The chamber consists of a rubber membrane sandwiched between an upper coverslip and a lower coverslip with EpCs. A silicone gasket with a thickness of 1 mm was used, and this specific height was selected in this study to represent the diameter of the airways in the flow in the distal lung (58). We have calculated the flow rate in the distal lungs and based on our calculations, we generated a bubble propagation of ∼3 mm/s over cell monolayers. PBS was used as the occlusion fluid since PBS represents a surfactant-deficient, high-surface tension airway-lining fluid, a characteristic of ARDS. Airway reopening was generated as follows: the flow chamber was first filled with PBS using the syringe pump (Touch P-400 syringe pump) at a rate of 3 mm/s, and control studies demonstrated that this initial filling of the chamber (PBS infusion) does not result in cell necrosis. The mimicking of airway reopening conditions was done through the withdrawal of the fluid from the chamber at a constant speed of 3 mm/s. A propagation of a single long air bubble over the surface of the EpCs occurs as a result. The chamber is refilled with a live/dead stain at 3 mm/s and incubated to prepare for visualization under the fluorescence microscope once the air bubble had displaced the occlusion fluid. During initial perfusion and bubble propagation (Fig. 1), cells are kept at 37°C. To maintain cells at body temperature during the cell injury experiments, the flow chamber was placed on a heating plate connected to a water circulation heater that maintains a constant temperature of 37°C within the chamber using the heater system supplied by Bioptechs.
Figure 1.
Experimental setup. A: a single bubble is propagated over cell monolayer by filling the chamber and then retracting fluid over the cells. B: a propagating bubble is seen. C: we exposed epithelial cells to frictional shear stress relevant to airway reopening in our setup. D: fluorescence live/dead stain method was used to assess viability. Here, green is calcein-stained live cells, and red is ethidium-stained dead cells.
Determination of Optimal Concentrations of Tested Agents
In this study, the aim was to increase the rate of EpC survival under shear stress conditions relevant to MV upon airway reopening conditions. In our approach, as stated previously, we alter the mechanical properties of the cells when anti-inflammatory steroidal drugs DEX and DHEA are applied. In this experiment, we also added latrunculin A (LAT) as a positive control to alter cell rheology. To find the optimum concentration of drugs for the bubble propagation experiment, the criterion of interest is a concentration that does not cause cell necrosis at static culture. To select the optimum concentration for each drug for cell treatments, several concentrations from each drug were selected, and cells were treated overnight followed by staining with a live/dead toxicity kit to test the viability.
Experimental Groups, Concentrations of Treatments, and Duration of Exposure
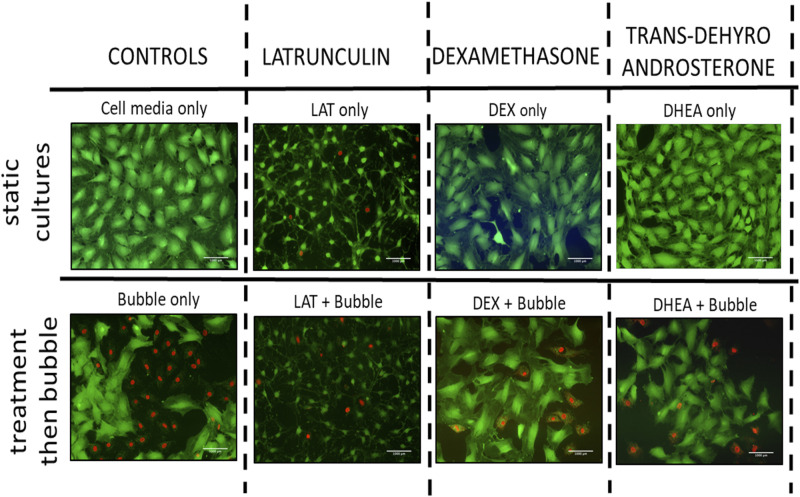
Experimental groups consisted of nine different groups that are prepared for viability assessment: 1) cell control group (cells were kept in media), 2) LAT control (i.e., cells were kept in 0.5 μM LAT solution for 1 h, LAT in Fig. 2B), 3) DEX control (i.e., cells were kept in 200 μg/mL DEX solution overnight, DEX in Fig. 2B), 4) DHEA control (i.e., cells were kept in 2.5 μg/mL DHEA solution overnight, DEX in Fig. 2B), 5) 1% DMSO control (i.e., cells were kept in 1% DMSO solution overnight, 1% DMSO in Fig. 2B), 6) bubble control (i.e., cells were exposed to bubble propagation only, C + b in Fig. 2B), 7) LAT + bubble (i.e., cells were treated with 0.5 μM LAT solution for 1 h before bubble propagation, LAT + b in Fig. 2B), 8) DEX + bubble (i.e., cells were treated with 200 μg/mL DEX solution overnight before bubble propagation, DEX + b in Fig. 2B), and 9) DHEA + bubble (i.e., cells were treated with 2.5 μg/mL DHEA solution overnight before bubble propagation, DHEA + b in Fig. 2B). LAT is an experimental agent known to alter cell cytoskeleton and cell mechanics and hence was included in the study as a control (50).
Figure 2.
Assessment of cell injuries for bubble experiments. Representative fluorescence live/dead assay pictures. Static cultures (top) did lead to minor cell injury. Bubble flow resulted in significant cell death, and DEX, DHEA, and LAT and then flow exposure decreased cell injury significantly (bottom). Scale bars = 1 mm. DEX, dexamethasone; DHEA, trans-dehydroandrosterone; LAT, latrunculin A.
Viability Assessment
A standard live/dead staining assay was used for cell viability after bubble propagation. The stain consists of 1 μM ethidium homodimer 1 and 1.2 μM calcein-AM in serum-free media. Cells are stained with 1 mL of live/dead stain solution and incubated in the dark for 10 min after the bubble propagation experiments. Cells were visualized later using a fluorescence microscope for counting and analysis. Ten pictures were taken for each field and analyzed through open-access software ImageJ, where green represents live cells and red represents dead cells (Fig. 1D). The injury percentage was calculated by dividing the number of dead cells by the total number of cells. The statistical analysis was performed with GraphPad Prism software v.8 for significance between groups. DEX and DHEA experiments were carried out in five independent trials. Also, LAT was carried out for two independent trials. The results were expressed as means ± standard error of the mean (SEM). They were analyzed using one-way analysis of variance (ANOVA test) and Tukey’s post hoc test. A P value of <0.01 was considered statistically significant.
Cytoskeletal Staining
Actin fibers are known as stress fibers, as they give mechanical strength to the cell. The actin fibers are usually stained with immunohistochemistry techniques. On coverslips that are placed in six-well plates, cell type CCL-149 L2 could grow with a concentration of 3 ×102 cells/mL and 2 mL of cell media. Cells were grown for ∼4 days (until confluency). The media was changed twice (every 2 days). The staining procedure starts by washing the cells twice with 1× PBS. The cells are then fixed with 4% paraformaldehyde for 15 min (2 mL in each well). Then, the cells are washed with PBS twice and permeabilized with 0.1% Triton X-100 for 5 min. Then, 1% bovine serum albumin (BSA) is added as a blocking agent for 15 min. The cells are then incubated with 2.5% Alexa-488-labeled phalloidin in the fridge (4°C) overnight. Then, the cells are labeled with DAPI stain (0.002 mg/mL) for 2 min and washed twice with distilled water. Following which, a drop of Gel Mount is placed on a glass slide, and the coverslip is placed over the Gel Mount with cells facing down. When all these steps were done, the cells are double-labeled and can be visualized with a fluorescence microscope. The blue filter is for Alexa-488-labeled phalloidin, and the UV filter is for DAPI. In this project, we expect that when we expose cell monolayers to tested agents (i.e., DEX and DHEAS), actin fibers will be reorganized. To confirm that we can assess changes in actin cytoskeletal structure, we expose cell monolayers to LAT as a positive control and then assess the cytoskeletal structure after exposure, by comparing with control cells. Fluorescence pictures are taken under identical exposure settings to be able to compare the fluorescence intensity that will represent actin fiber density. EpCs were grown on a circular coverslip (around 50% confluency) and then treated with tested agents (i.e., DEX overnight exposure, DHEAS overnight, 1 h latrunculin exposure, DMSO overnight, or no treatment as the control group).
Preparation of EpC Monolayers for Atomic Force Microscopy
EpCs were grown on coverslips until 50%–70% confluency and then treated with tested agents (i.e., DEX overnight exposure, DHEAS overnight exposure, 1 h latrunculin exposure, DMSO overnight, or no treatment as the control group). This was followed by washing the cell monolayers twice with PBS. Cells were then fixed with 4% paraformaldehyde for 15 min and washed with PBS and a last wash with distilled water to avoid the formation of crystallites (59).
Surface Topography Analysis with Atomic Force Microscopy
An atomic force microscope was used for the topography analyses to extract information about cell shape. The utilized atomic force microscopy (AFM) platform was MFP-3D (Asylum Research) equipped with a silicon probe (Al reflex coated Veeco model; OLTESPA, Olympus) under ambient conditions by using standard topography AC air (tapping mode in the air) (60).
RESULTS AND DISCUSSION
Viability Results following Bubble-Induced Shear Stress Exposure in the Flow Chamber
We designed nine experimental groups as stated earlier, and the experiments were repeated for six times. Figure 2 shows a fluorescence live/dead assay done via a fluorescence microscope for each group, and Fig. 3 shows a bar chart representation for death percentage. For DEX, LAT, or DHEA solutions, keeping the cell in culture media did not lead to cell death (static cultures). On the other hand, bubble propagation resulted in significant cell injury (54.7 ± 3.94%). LAT, DEX, or DHEA exposure and then bubble flows decreased cell death significantly to 20.9 ± 12%, 15.1 ± 3.43%, and 16.1 ± 2.9%, respectively (Fig. 3).
Figure 3.
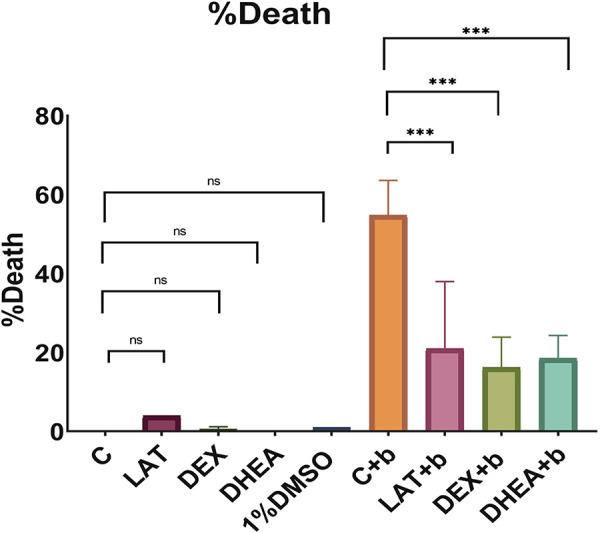
Bar chart representation of cell injuries for all experimental groups. DEX, dexamethasone; DHEA, trans-dehydroandrosterone; LAT, latrunculin A; C, control; b, bubble; ns, not significant. *Significant, **very significant, ***highly significant.
Effect of LAT, DEX, and DHEA Exposure on Cytoskeletal Structure
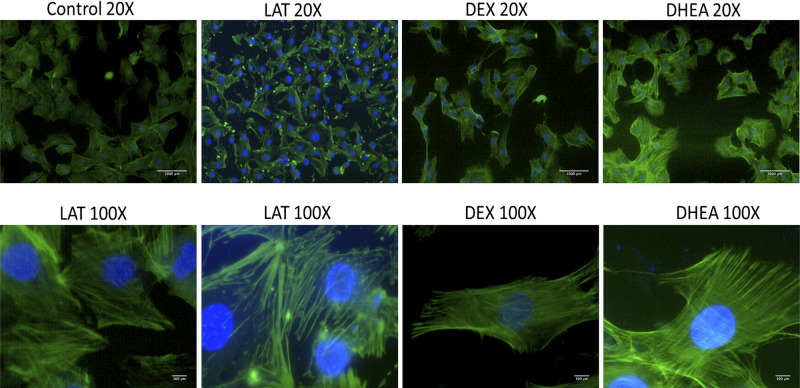
In this experiment, we examined the effect of LAT, DEX, or DHEA exposure on the cytoskeletal structure. Here, we immunostained actin fibers in confluent monolayers of control, LAT, DEX, and DHEA groups. LAT treatment cells were placed in 0.5 µM LAT solution for 1 h before immunostaining procedures. For DEX, we kept cells in 200 μg/mL DEX solution overnight before the immunostaining procedure. Also, the DHEA group was placed in 2.5 µg/mL DHEA solution overnight followed by immunostaining. Compared with the control, only the LAT group showed a significant difference in the intensity of green fluorescence signal, as shown in Fig. 3. This was anticipated due to the occurrence of severe depolymerization of actin fibers. Although we did not see a clear difference in fluorescence intensity for DEX and DHEA groups, we can see structural changes in these groups compared with the control. On the other hand, in the control group, actin fibers are evenly distributed in both longitudinal and circumferential directions, whereas in treated groups, we see more alignment longitudinally, which makes the cell also longitudinally elongated, as seen in Fig. 4.
Figure 4.
Immunostaining of actin cytoskeleton for nontreated and treated cells. In the slides, green is phalloidin-stained actin fibers, and blue is DAPI-stained cell nuclei. Compared with controls, all groups did not have any clear difference in the intensity of green, fluorescence signal, except LAL, where actin fibers are depolymerized. For both DEX- and DMSO-treated cells, actin fibers look longitudinally elongated. Scale bar in ×20 and ×100 pictures is 1 mm and 100 µm, respectively. DEX, dexamethasone.
Effect of LAT, DEX, and DHEA on Cell Morphology
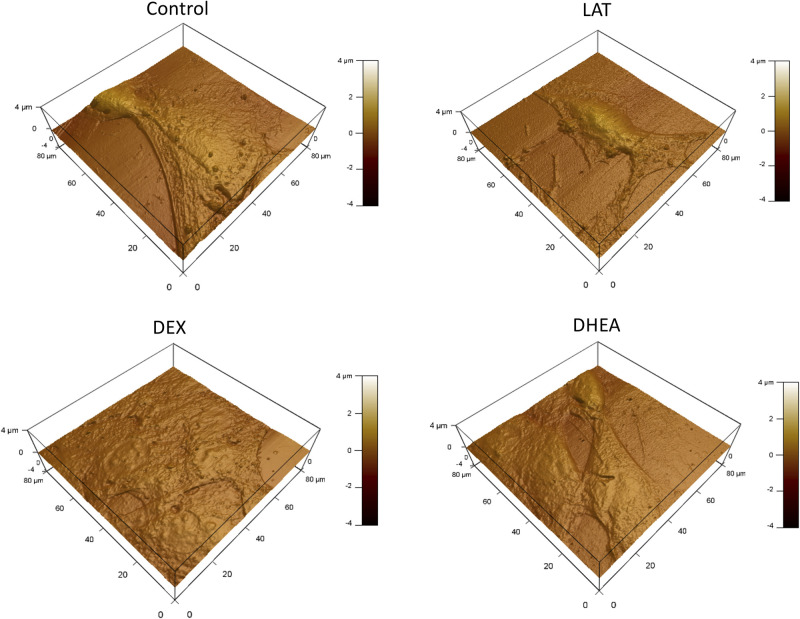
Surface morphology for control and treated cells was assessed by AFM. The results suggest a shrinkage in treated groups compared with the control group. The treated groups exhibit morphology like that of LAT (rounder and smaller), which suggests softening and liquid-like behavior (Fig. 5). This proposes that cytoskeleton remodeling and cell morphology change are the mechanisms by which the cell is protected against injury.
Figure 5.
Cell morphology for treated and nontreated cells via AFM. Although nontreated cells show a rounded morphology, all treated cells show a smaller more elongated morphology. AFM, atomic force microscopy; DEX, dexamethasone; DHEA, trans-dehydroandrosterone; LAT, latrunculin A.
Conclusions
To conclude, in this study, we aimed to enhance EpC survival in the distal lung during MV in airway reopening conditions. In this work, we studied how the alterations of the mechanical properties of EpCs can influence their survival from injurious cyclic airway reopening stresses associated with MV. We selected two anti-inflammatory steroid agents, dexamethasone (DEX) and transdehydroandrosterone (DHEA), because of their effects on the actin cytoskeleton in EpCs and, hence, cell mechanics holistically. This is expected to increase their survival upon exposure to shear stress. We used a previously developed in vitro model of flow system to simulate airway reopening shear stress conditions that are like those upon MV in patients who are exposed to airway reopening conditions. According to our results, we found that preexposure of cultured cells to either DEX or DHEA significantly decreased cellular injuries associated with VILI. We revealed dramatic actin polymer reorganization compared with controls (i.e., more elongated actin fibers) for both DEX- and DHEA-treated cells that was proven through cytoskeletal staining results. In addition, the mechanics of the cells were heavily altered for both treated cells, becoming smaller similar to LAT-treated cells. We showed that DEX- or DHEA-treated cells have become more fluid-like LAT-treated cells, resulting in less susceptibility to stresses. These results provide evidence for potential beneficial effects of anti-inflammatory agents such as DEX or DHEA against VILI for many cases where airway reopening conditions are needed. The results from this study are critical for VILI and can be readily applicable to future clinical studies. Our results agree with a recent clinical work showing that DEX treatment significantly decreased mortalities in patients with COVID-19 and ARDS receiving mechanical ventilation (56). Our study shows a clear advantage of using DEX and DHEA against VILI. These results are important and could be beneficial for patients who are exposed to airway reopening conditions, such as patients with CARDS and ARDS and patients with VILI in general. More studies need to be conducted to understand the precise mechanism of action for the selected drugs.
GRANTS
This study was made possible by an Undergraduate Research Experience Program Award [UREP21-050-3-010] from the Qatar National Research Fund (a member of The Qatar Foundation). The publication of this article was funded by the Qatar National Library.
DISCLAIMERS
The statements made herein are solely the responsibility of the authors.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.C.Y. conceived and designed research; M.K.A., F.H.A., S.S., and A.P. performed experiments; M.K.A., F.H.A., and S.S. analyzed data; M.K.A., S.S., and H.C.Y. interpreted results of experiments; M.K.A. and S.S. prepared figures; M.K.A. drafted manuscript; H.C.Y. edited and revised manuscript; H.C.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Professor Ala-Eddin Al Moustafa and students Mohamed Badie Ahmed, Maha Hussien, and Salma Ahmed.
REFERENCES
- 1.Macklem PT. The Act of Breathing BT - Mechanics of Breathing, edited byAliverti A, Brusasco V, Macklem PT, Pedotti A. Milan: Springer, 2002, p. 3–10. [Google Scholar]
- 2.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 369: 2126–2136, 2013[Erratum inN Engl J Med370: 1668–1669, 2014]. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 3.Wagner PD. The physiological basis of pulmonary gas exchange: implications for clinical interpretation of arterial blood gases. Eur Respir J 45: 227–243, 2015. doi: 10.1183/09031936.00039214. [DOI] [PubMed] [Google Scholar]
- 4.Butler JP, Tsuda A. Transport of gases between the environment and alveoli—theoretical foundations. Compr Physiol 1: 1301–1316, 2011. doi: 10.1002/cphy.c090016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed SM, Athar M. Mechanical ventilation in patients with chronic obstructive pulmonary disease and bronchial asthma. Indian J Anaesth 59: 589–598, 2015. doi: 10.4103/0019-5049.165856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis PB, di Sant’Agnese PA. Assisted ventilation for patients with cystic fibrosis. JAMA 239: 1851–1854, 1978. doi: 10.1001/jama.1978.03280450023017. [DOI] [PubMed] [Google Scholar]
- 7.Mansel JK, Stogner SW, Petrini MF, Norman JR. Mechanical ventilation in patients with acute severe asthma. Am J Med 89: 42–48, 1990. doi: 10.1016/0002-9343(90)90096-v. [DOI] [PubMed] [Google Scholar]
- 8.Windisch W, Walterspacher S, Siemon K, Geiseler J, Sitter H; German Society for Pneumology. Guidelines for non-invasive and invasive mechanical ventilation for treatment of chronic respiratory failure. Pneumologie 64: 640–652, 2010. doi: 10.1055/s-0030-1255558. [DOI] [PubMed] [Google Scholar]
- 9.Gammo RB, Shin MS, Buchalter SE. Pulmonary barotrauma in mechanical ventilation: patterns and risk factors. Chest 102: 568–572, 1992. doi: 10.1378/chest.102.2.568. [DOI] [PubMed] [Google Scholar]
- 10.Bouferrache K, Vieillard-Baron A. Acute respiratory distress syndrome, mechanical ventilation, and right ventricular function. Curr Opin Crit Care 17: 30–35, 2011. doi: 10.1097/MCC.0b013e328342722b. [DOI] [PubMed] [Google Scholar]
- 11.Raju TN. History of neonatal resuscitation: tales of heroism and desperation. Clin Perinatol 26: 629–640, 1999. doi: 10.1016/S0095-5108(18)30041-1. [DOI] [PubMed] [Google Scholar]
- 12.Slutsky AS. History of mechanical ventilation. From vesalius to ventilator-induced lung injury. Am J Respir Crit Care Med 191: 1106–1115, 2015. doi: 10.1164/rccm.201503-0421PP. [DOI] [PubMed] [Google Scholar]
- 13.Corrado A, Gorini M. Negative-Pressure Ventilation. In: Principles and practice of mechanical ventilation, edited by Tobin Martin J.. New York: McGraw-Hill Medical Pub. Division, 2012, p. 417–433. [Google Scholar]
- 14.Pingleton SK. Complications of acute respiratory failure. Am Rev Respir Dis 137: 1463–1493, 1988. doi: 10.1164/ajrccm/137.6.1463. [DOI] [PubMed] [Google Scholar]
- 15.Fothergill J. XI. Observations on a case published in the last volume of the medical essays, &c. of recovering a man dead in appearance, by distending the lungs with air. Printed at Edinburgh, 1744; by John Fothergill, Licent. Coll. Med. Lond. Philos Trans R Soc London 43: 275–281, 1744. doi: 10.1098/rstl.1744.0061. [DOI] [Google Scholar]
- 16.D’Avignon P, Hedenström G, Hedman C. XIII. Pulmonary complications in respirator patients. Acta Med Scand 154: 86–90, 2009. doi: 10.1111/j.0954-6820.1956.tb06263.x. [DOI] [PubMed] [Google Scholar]
- 17.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28: 596–608, 1970. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 18.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 290: 319–323, 1967. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [Google Scholar]
- 19.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis 110: 556–565, 1974. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 20.Meade MO, Cook DJ. The aetiology, consequences and prevention of barotrauma: a critical review of the literature. Clin Intensive Care 6: 166–173, 1995. [PubMed] [Google Scholar]
- 21.Curley GF, Laffey JG, Zhang H, Slutsky AS. Biotrauma and ventilator-induced lung injury: clinical implications. Chest 150: 1109–1117, 2016. doi: 10.1016/j.chest.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Uhlig S, Ranieri M, Slutsky AS. Biotrauma hypothesis of ventilator-induced lung injury. Am J Respir Crit Care Med 169: 314–315, 2004. doi: 10.1164/ajrccm.169.2.950. [DOI] [PubMed] [Google Scholar]
- 23.Hubmayr RD. Volutrauma and regional ventilation revisited. Am J Respir Crit Care Med 188: 1388–1389, 2013. doi: 10.1164/rccm.201311-1993ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cressoni M, Chiumello D, Algieri I, Brioni M, Chiurazzi C, Colombo A, Colombo A, Crimella F, Guanziroli M, Tomic I, Tonetti T, Luca Vergani G, Carlesso E, Gasparovic V, Gattinoni L. Opening pressures and atelectrauma in acute respiratory distress syndrome. Intensive Care Med 43: 603–611, 2017. doi: 10.1007/s00134-017-4754-8. [DOI] [PubMed] [Google Scholar]
- 25.Halbertsma FJJ, Vaneker M, Scheffer GJ, van der Hoeven JG. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth J Med 63: 382–392, 2005. [PubMed] [Google Scholar]
- 26.Dhanireddy S, Altemeier WA, Matute-Bello G, O’Mahony DS, Glenny RW, Martin TR, Liles WC. Mechanical ventilation induces inflammation, lung injury, and extra-pulmonary organ dysfunction in experimental pneumonia. Lab Invest 86: 790–799, 2006. doi: 10.1038/labinvest.3700440. [DOI] [PubMed] [Google Scholar]
- 27.Dreyfuss D, Saumon G. From ventilator-induced lung injury to multiple organ dysfunction? Intensive Care Med 24: 102–104, 1998. doi: 10.1007/s001340050529. [DOI] [PubMed] [Google Scholar]
- 28.Gattinoni L, Marini JJ, Collino F, Maiolo G, Rapetti F, Tonetti T, Vasques F, Quintel M. The future of mechanical ventilation: lessons from the present and the past. Crit Care 21: 183, 2017. doi: 10.1186/s13054-017-1750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poole J, McDowell C, Lall R, Perkins G, McAuley D, Gao F, Young D. Individual patient data analysis of tidal volumes used in three large randomized control trials involving patients with acute respiratory distress syndrome. Br J Anaesth 118: 570–575, 2017. doi: 10.1093/bja/aew465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villar J, Blanco J, Kacmarek RM. Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care 22: 1–6, 2016. doi: 10.1097/MCC.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 31.Jain V, Duse A, Bausch DG. Planning for large epidemics and pandemics: challenges from a policy perspective. Curr Opin Infect Dis 31: 316–324, 2018. doi: 10.1097/QCO.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8: 475–481, 2020[Erratum inLancet Respir Med8: e26, 2020]. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, Fergusson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the berlin definition. JAMA 307: 2526–2533, 2012. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 34.Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA 323: 2329–2330, 2020. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, Zhang J, Zhao C. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol 92: 1902–1914, 2020. doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323: 1775–1776, 2020. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 37.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46: 846–848, 2020[Erratum inIntensive Care Med46: 1294–1297, 2020]. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deliwala SS, Ponnapalli A, Seedahmed E, Berrou M, Bachuwa G, Chandran A. A 29-year-old male with a fatal case of COVID-19 acute respiratory distress syndrome (CARDS) and ventilator-induced lung injury (VILI). Am J Case Rep 21: e926136, 2020. doi: 10.12659/AJCR.926136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tremblay LN, Slutsky AS. Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians 110: 482–488, 1998. [PubMed] [Google Scholar]
- 40.Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 55: 105954, 2020. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlahakis NE, Hubmayr RD. Response of alveolar cells to mechanical stress. Curr Opin Crit Care 9: 2–8, 2003. doi: 10.1097/00075198-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett 87: 148102, 2001. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 43.Ragazzon MRP, Gravdahl JT, Vagia M. Viscoelastic properties of cells: modeling and identification by atomic force microscopy. Mechatronics 50: 271–281, 2018. doi: 10.1016/j.mechatronics.2017.09.011. [DOI] [Google Scholar]
- 44.Wei M-T, Zaorski A, Yalcin HC, Wang J, Hallow M, Ghadiali SN, Chiou A, Ou-Yang HD. A comparative study of living cell micromechanical properties by oscillatory optical tweezers. Opt Express 16: 8594–8603, 2008. doi: 10.1364/oe.16.008594. [DOI] [PubMed] [Google Scholar]
- 45.Hochmuth RM. Micropipette aspiration of living cells. J Biomech 33: 15–22, 2000. doi: 10.1016/S0021-9290(99)00175-X. [DOI] [PubMed] [Google Scholar]
- 46.Deng L, Trepat X, Butler JP, Millet E, Morgan KG, Weitz DA, Fredberg JJ. Fast and slow dynamics of the cytoskeleton. Nat Mater 5: 636–640, 2006. doi: 10.1038/nmat1685. [DOI] [PubMed] [Google Scholar]
- 47.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Taback NA, Millet EJ, Fredberg JJ. Time scale and other invariants of integrative mechanical behavior in living cells. Phys Rev E Stat Nonlin Soft Matter Phys 68: 41914, 2003. doi: 10.1103/PhysRevE.68.041914. [DOI] [PubMed] [Google Scholar]
- 48.Gardel ML, Kasza KE, Brangwynne CP, Liu J, Weitz DA. Chapter 19: mechanical response of cytoskeletal networks. Methods Cell Biol 89: 487–519, 2008. doi: 10.1016/S0091-679X(08)00619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hadjiantoniou S, Guolla L, Pelling AE. Mechanically induced deformation and strain dynamics in actin stress fibers. Commun Integr Biol 5: 627–630, 2012. doi: 10.4161/cib.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yalcin HC, Hallow KM, Wang J, Wei MT, Ou-Yang HD, Ghadiali SN. Influence of cytoskeletal structure and mechanics on epithelial cell injury during cyclic airway reopening. Am J Physiol Lung Cell Mol Physiol 297: L881–L891, 2009. doi: 10.1152/ajplung.90562.2008. [DOI] [PubMed] [Google Scholar]
- 51.Dailey HL, Ricles LM, Yalcin HC, Ghadiali SN. Image-based finite element modeling of alveolar epithelial cell injury during airway reopening. J Appl Physiol (1985) 106: 221–232, 2009. doi: 10.1152/japplphysiol.90688.2008. [DOI] [PubMed] [Google Scholar]
- 52.Shurbaji S, El-Sherbiny IM, Alser M, Ali IH, Kordi H, Al-Sadi A, Popelka A, Benslimane F, Yacoub M, Yalcin HC. Nitric oxide releasing hydrogel nanoparticles decreases epithelial cell injuries associated with airway reopening. Front Bioeng Biotechnol 8: 579788, 2021. doi: 10.3389/fbioe.2020.579788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charalampopoulos I, Dermitzaki Ε, Vardouli L, Tsatsanis C, Stournaras C, Margioris ΑΝ, Gravanis Α. Dehydroepiandrosterone sulfate and allopregnanolone directly stimulate catecholamine production via induction of tyrosine hydroxylase and secretion by affecting actin polymerization. Endocrinology 146: 3309–3318, 2005. doi: 10.1210/en.2005-0263. [DOI] [PubMed] [Google Scholar]
- 54.Koukouritaki SB, Theodoropoulos PA, Margioris AN, Gravanis A, Stournaras C. Dexamethasone alters rapidly actin polymerization dynamics in human endometrial cells: evidence for nongenomic actions involving cAMP turnover. J Cell Biochem 62: 251–261, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 55.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. ( Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, AngusDC , et.al). Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 324: 1330–1341, 2020. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahase E. Covid-19: low dose steroid cuts death in ventilated patients by one third, trial finds. BMJ 369: m2422, 2020. doi: 10.1136/bmj.m2422. [DOI] [PubMed] [Google Scholar]
- 57.Yalcin HC, Perry SF, Ghadiali SN. Influence of airway diameter and cell confluence on epithelial cell injury in an in vitro model of airway reopening. J Appl Physiol (1985) 103: 1796–1807, 2007. doi: 10.1152/japplphysiol.00164.2007. [DOI] [PubMed] [Google Scholar]
- 58.Shurbaji S, Al-Ruweidi MKAA, Ali FH, Benslimane FM, Yalcin HC. Application of a flow-induced stress wave and investigation of associated injuries on cell monolayers using a parallel plate flow chamber. Methods Protoc 3: 65, 2020. doi: 10.3390/mps3040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Francis LW, Gonzalez D, Ryder T, Baer K, Rees M, White JO, Conlan RS, Wright CJ. Optimized sample preparation for high-resolution AFM characterization of fixed human cells. J Microsc 240: 111–121, 2010. doi: 10.1111/j.1365-2818.2010.03392.x. [DOI] [PubMed] [Google Scholar]
- 60.Magdesian MH, Sanchez FS, Lopez M, Thostrup P, Durisic N, Belkaid W, Liazoghli D, Grütter P, Colman DR. Atomic force microscopy reveals important differences in axonal resistance to injury. Biophys J 103: 405–414, 2012. doi: 10.1016/j.bpj.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]